Introduction
New agents including the small molecule targeted
inhibitors like sunitinib, sorafenib and temsirolimus, for advanced
renal cell carcinoma (RCC) have been developed based on a
biological process in the RCC carcinogenesis. However, they are
often accompanied by adverse effects that occasionally require dose
reduction or discontinuation. Tenacious cancer cells subsequently
become resistant to the same drug treatment. Therefore, development
of new molecular targeting agents as novel targets with minimal
adverse effects is still needed (1–3).
The permeation to basement membrane and the
extracellular matrix of neoplastic cells is regarded as the most
important step in metastatic process. MMP is a group of enzymes
that degenerate various elements of extracellular matrix including
elastin, gelatin, agrican, fibronectin and laminin (4,5).
Further more, an impact of MMP on the early proliferation of tumor
cells of metastatic focus has been reported. In addition, tissue
inhibitor of MMP (TIMP), which is an endogenous MMP inhibitor
participates in the activity of MMP and disproportion between TIMP
and MMP modulates the progression of tumors (6,7).
Among these MMPs, MMP-9 degenerates type IV
collagen, gelatinized types I and II collagen and participates in
the permeation of basement membrane by tumor cells. The expression
of MMP-9 is increased in malignant tumors in comparison with benign
or non-invasive tumors (7,8). In addition, overexpression of MMP-9
is confirmed in renal cell carcinoma (8–12).
Kawada et al reported association of MMP-9 expression and
poor prognosis using clinical specimen of renal cell carcinoma
(13,14). Therefore, we expected the new
therapeutic drug that inhibits activity of MMP-9 may have an
antitumor effect for renal cell carcinoma.
Pyrrole-imidazole polyamides (PIP) are powerful gene
regulating compounds, which can bind to the minor groove of DNA
double strand in a sequence-specific manner. They are composed of
two aromatic amino acids [N-methylpyrrole (Py) and
N-methylimidazole (Im)]. The combination of Im/Py recognizes GC in
the DNA double-helix, Py/Py recognizes TA and AT and arrangements
of these combinations made it possible to bind to a variety of
sequence. Since they can bind DNA with higher affinity and
specificity than the usual DNA binding proteins, PIPs designed to
recognize the binding domain of transcription factors could inhibit
the expression of downstream genes. Precise binding to a known
sequence may make it possible to reduce adverse events, therefore
PIP is expected to become a new molecular targeting agent (15–18).
Previously, we reported that PIP targeting for MMP-9 inhibit the
migration and invasion of colon cancer cells in vitro and
also inhibits their metastasis in vivo (19).
In the present study, we examined MMP-9
immunore-activity in clinical specimens and analyzed the degree of
association and matched cancer-specific survival. Furthermore, we
investigated effects of PIP on the inhibition of MMP-9 expression
and resulting inhibition of cellular invasion by using human renal
cell carcinoma cell line Caki-2.
Materials and methods
Tissue samples
Two hundred and forty-nine surgical samples with
informed consent were obtained at Surugadai Nihon University
Hospital from 1990 to 2003 (Table
I). All of the studies using these specimens were performed
under the approval of Nihon University School of Medicine Ethics
Review Board (IRB no. 106-1).
 | Table I.Patient background (n=249, age range
24–85. with an average of 60 years). |
Table I.
Patient background (n=249, age range
24–85. with an average of 60 years).
| Category | N | % |
|---|
| Gender | | |
| Male | 185 | 74 |
| Female | 64 | 26 |
| Cell type | | |
| Clear | 203 | 82 |
| Non-clear | 46 | 18 |
| Fuhrman’s grade | | |
| 1 | 90 | 36 |
| 2 | 108 | 43 |
| 3+4 | 51 | 21 |
| Stage | | |
| 1 | 135 | 54 |
| 2 | 39 | 16 |
| 3 | 41 | 16 |
| 4 | 34 | 14 |
Immunohistochemistry
Immunostaining for MMP-9 were performed and the
degree of immunoreactivity was analyzed to find the association
between cancer-specific survival by using Kaplan-Meier method with
log-rank test. For immunostaining, formalin-fixed,
paraffin-embedded 4-μm thick samples placed on silane coat
slide glass (Dako, Carpinteria, CA, USA) were used. After,
retrieval with 500 W microwave, slides were processed with 3%
hydrogen peroxide and 5% non-fat milk phosphate buffered saline
(PBS) to block non-specific antibodies. Rabbit polyclonal matrix
metalloproteinase-9 antibody (Cell Signaling Technology, USA) was
used as a primary antibody. Afterwards, samples were visualized by
simple stain MAX-PO (Nichirei, Tokyo, Japan). The staining
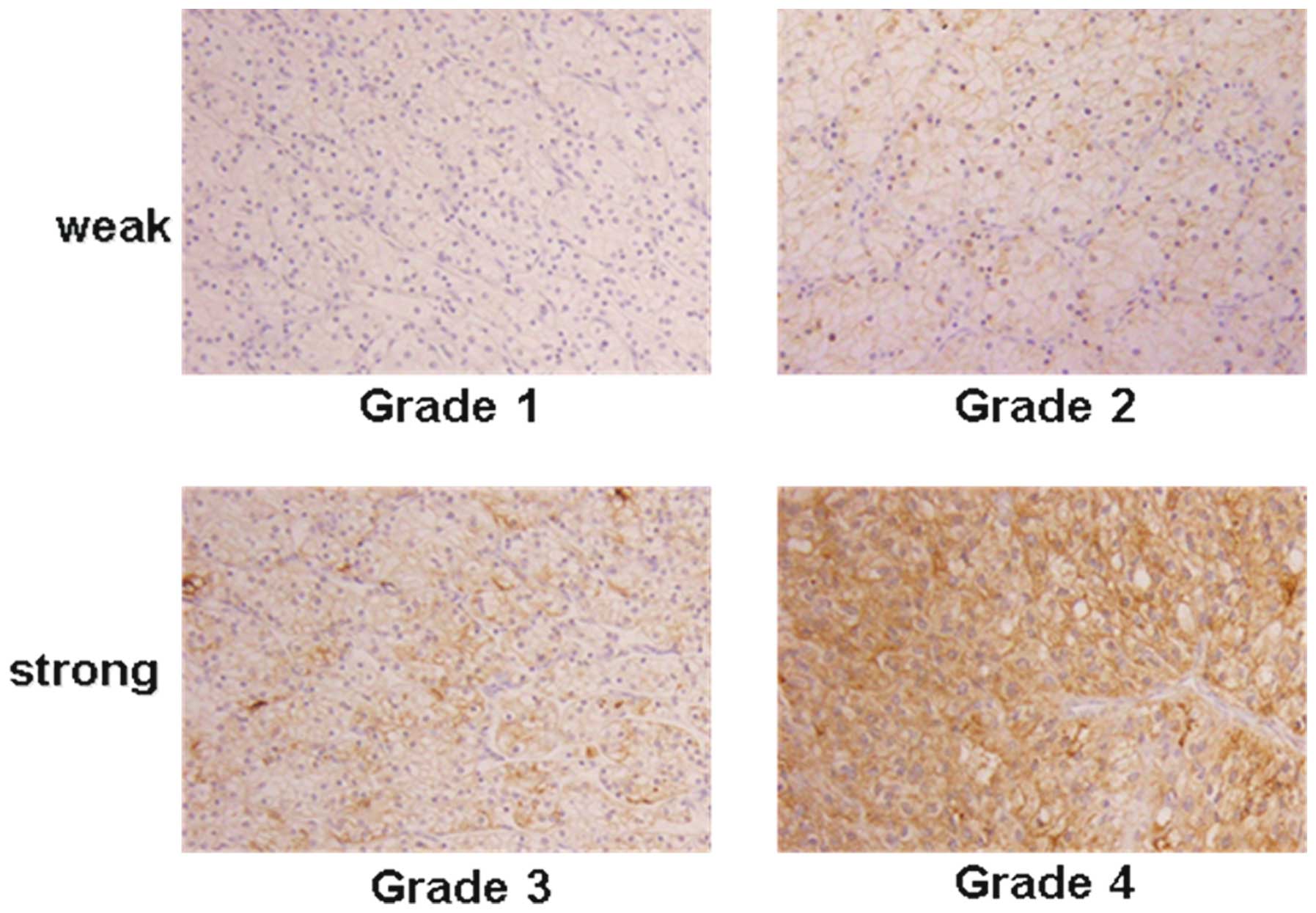
intensity of MMP-9 was stratified using a 4-grade scale, with grade
1 indicating the absence of immunostaining or faint membranous
staining of rare tumor cells, grade 2 indicating membranous
staining in most tumor cells, grade 3 indicating diffuse membranous
and/or cytoplasmic staining in groups of tumor cells and grade 4
indicating significant cytoplasmic staining in most tumor cells
(Fig. 1). For the evaluation of
immunohistochemical staining, intensities of grades 1 and 2 were
considered weak expressions of each protein, whereas grades 3 and 4
were considered strong.
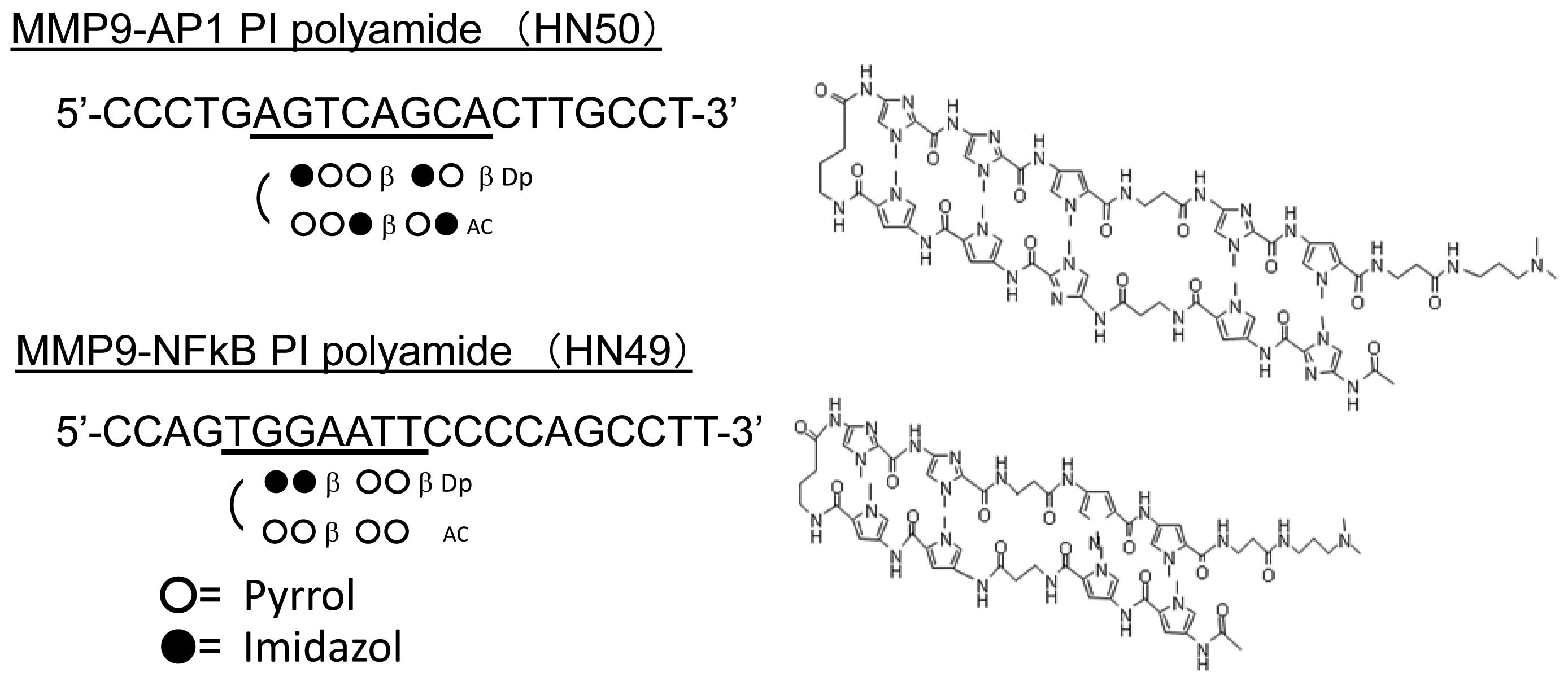
Synthesis of PIP targeting human
MMP-9
The expression of MMP-9 gene is known to be
regulated by the transcription factors NF-κB, AP-1, Sp1, whose
binding sites are located within 2.2 kbp upstream from
transcription start site. We designed and synthesized two PIPs
targeting MMP-9-NF-κB binding site (−600 to −605) and MMP-9-AP-1
binding site (−70 to −77) (Fig.
3). In each experiment, we diluted them in dimethyl sulfoxide
(DMSO) to make 10 mM stock solutions.
Cell lines and culture conditions
The human renal cell carcinoma cell line Caki-2 was
kindly transferred from Dr Cowell (Roswell Park Cancer Institute,
NY, USA) and cultured in McCoy’s 5A (Invitrogen Life Technologies,
CA, USA) supplemented with 10% FBS (McCoy’s5A FBS PS), 100 U/ml
penicillin, 100 μg/ml streptomycin and non-essential amino
acids (0.1 mM) under conditions of 5% CO2, 37°C.
Real-time RT-PCR
Cells/well (5.0×103) of Caki-2 cells were
plated in 6-well plates and cultured for 24 h. Then PIPs were
applied to the wells at the final concentration of 3 or 10
μM. No compound was applied to the negative control cells.
After 48-h culture, the cells were washed with PBS and dissolved in
TRIzol reagent (Invitrogen Life Technologies), then RNA was
extracted following the manufacturer’s instructions. After the
treatment with DNase, first-strand cDNA was synthesized using
iScript (Bio-Rad, Hercules, CA, USA) and real-time RT-PCR was
carried out using SYBR Premix Ex Taq, Perfect Real-Time (Takara
Bio, Otsu, Japan). The primers used in the real-time PCR to detect
human MMP-9 were 5′-GAGACCGGTGAGCTGGATAG-3′ (forward);
5′-TACACGCGAGTGAAGGTGAG-3′ (reverse) and for human GAPDH were
5′-GCACCGTCAAGGCTGAGAAC-3′ (forward); 5′-TGGTGAAGACGCCAGTGGA-3′
(reverse). The relative quantity of the MMP-9 expression level was
normalized by the expression level of GAPDH.
In vitro cell proliferation
Cells were seeded on 96-well micro-plates at the
concentration of 3.0×103 cells per well and cultured at
37°C in 5% CO2. Afterwards, cells were incubated for 72
h in the presence or absence of PIPs. Cell viability was evaluated
by WST-8 (Nacalai Tesque, Japan) assay, in which absorbance at OD
450 nm was measured using with Wallac 1420 counter (Amersham
Bioscience, Piscataway, NJ, USA).
Matrigel invasion assay
Invasion assay was carried out using BioCoat
Matrigel invasion chambers (Becton-Dickinson Labware Co., MA, USA).
Cells were suspended in culture medium at the concentration of
6.0×103 cells/ml and 500 μl of the suspension was
seeded on the upper chamber. The lower chamber was filled with 500
μl of the culture medium without cells. Cells were incubated
for 48 h with or without PIPs. After removing non-invasive cells
with a cotton swab, invasive cells adhering to membrane of the
upper chamber were fixed and stained with Diff-Quick solution.
Number of the cells on the membrane was counted under a light
microscope at ×200 magnification. The counting was done for 10
fields per each membrane.
Statistical analysis
All values are expressed as the mean ± SE and the
statistical significance was analyzed using the Student’s t-test. A
P-value of <0.05 was considered statistically significant.
Results
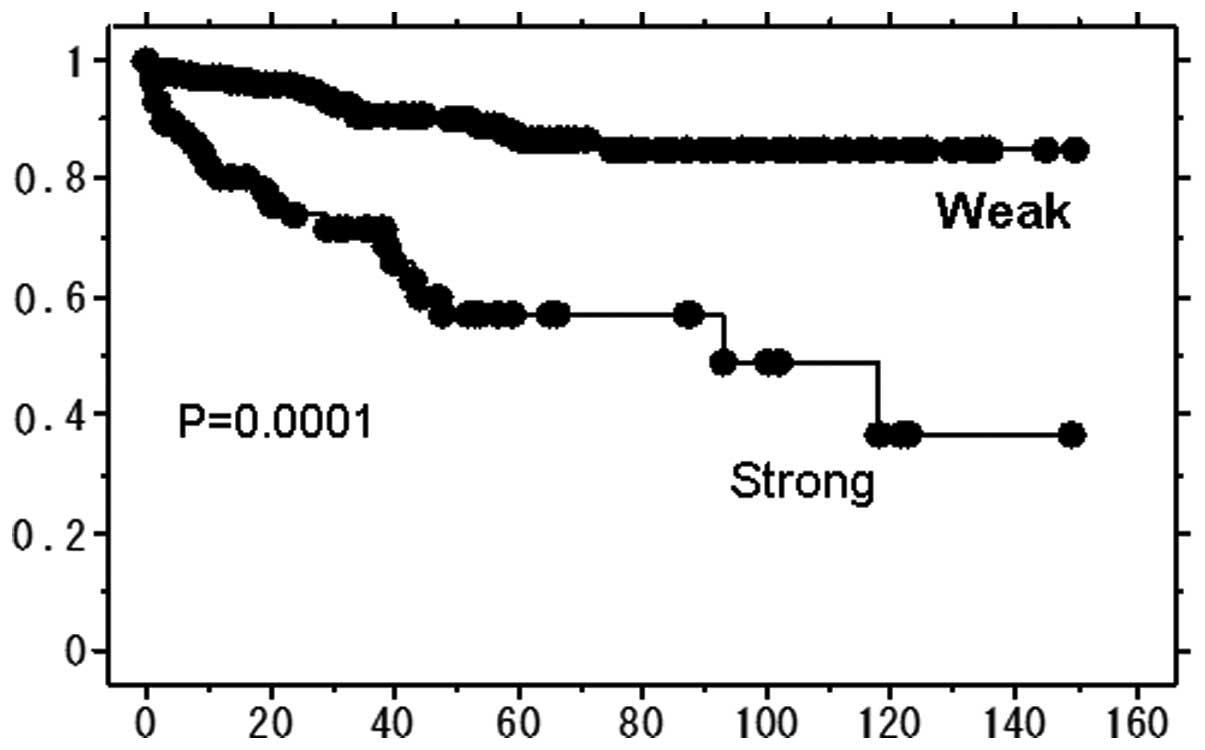
Immunohistochemistry
Two hundred and forty-nine of RCC specimens were
subjected to immunohistochemical analysis to detect MMP-9
expression. One hundred and ninety-four out of 249 specimens (78%)
were classified to weak and 55 (22%) to strong MMP-9 expression.
Significant association was seen in a cancer-specific survival rate
with the strength of the staining (Fig. 2). Patients in the strong stain
group showed shorter survival than those in the weak group.
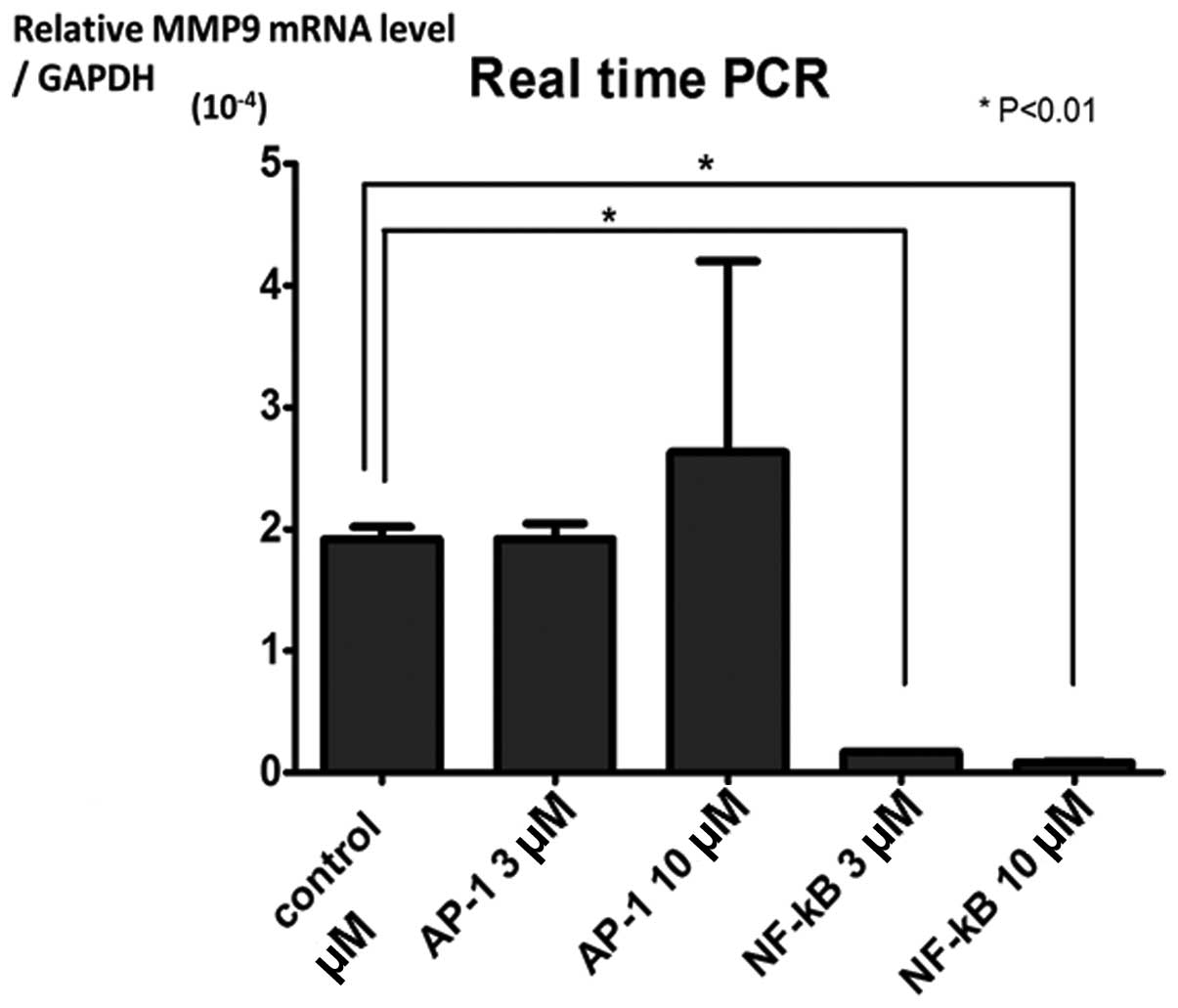
Expression of MMP-9 in MMP-9-PIP treated
cells
We tested the expression level of MMP-9 in the cells
treated with or without MMP-9 PIPs. The cells treated with 3 or 10
μM of MMP-9-NF-κB PIP showed significantly lower expression
level than that of control cells (P<0.01) (Figs. 3 and 4). Even though the tendency of
dose-dependency of MMP-9-NF-κB PIP in the suppression of MMP-9
expression was observed, no significance was attained between 3 and
10 μM (Fig. 4).
Cell proliferation in MMP-9-PIP treated
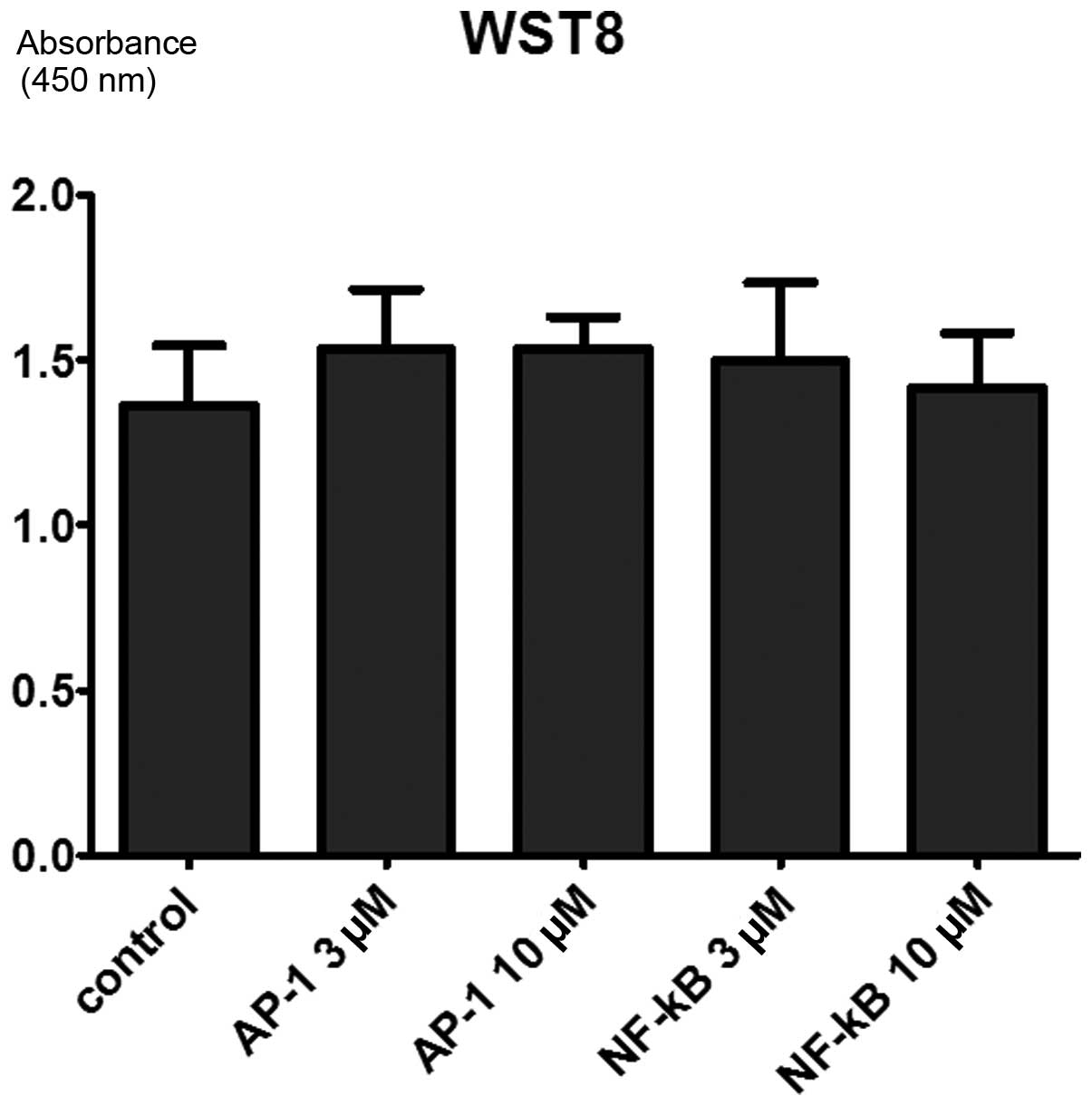
cells
Cell viability was tested by WST8 assay 72 h after
PIPs administration, however, no significant difference was found
among the cells treated with MMP-9-NF-κB PIP, MMP-9-AP-1 PIP and
the control cells (Fig. 5). This
result indicates that MMP-9 PIPs do not affect cell viability and
proliferation activity.
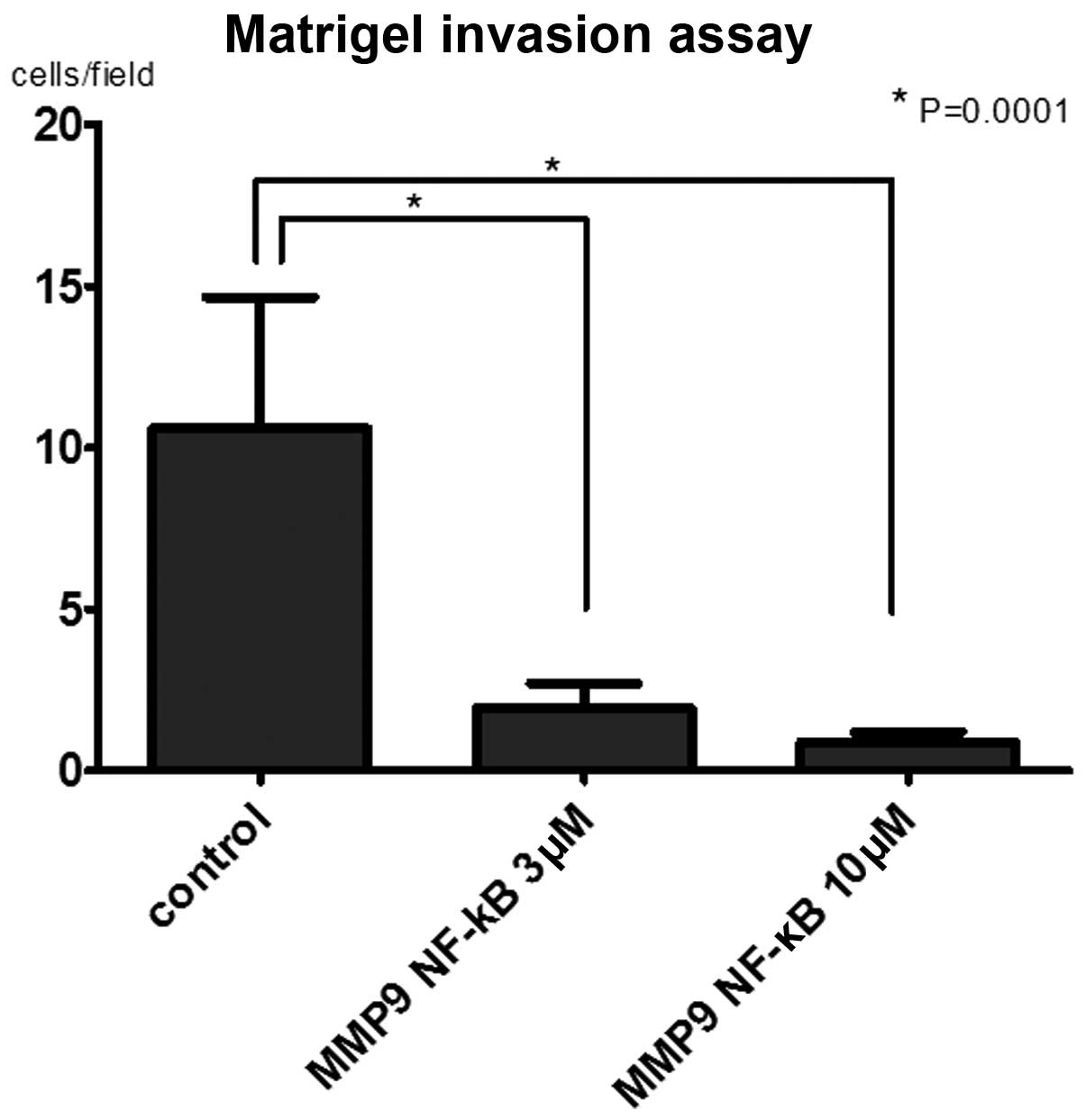
Invasion assay for MMP-9-PIP treated
cells
To evaluate the effect of PIPs on cell invasion
activity, matrigel invasion assay was performed. The number of
invaded cells in MMP-9-NF-κB PIP treated group were significantly
lower than in control group (P<0.0001). Even though the tendency
of dose dependency of MMP-9-NF-κB PIP in the suppression of MMP-9
expression was observed, there was no significant difference
between 3 and 10 μM (Fig.
6).
Discussion
In the present study, we confirmed an association of
MMP-9 immunoreactivity and the cancer-specific survival rate by
using clinical specimens. The patients with stronger
immuno-reactivity of MMP-9 showed shorter survival than patients
with weaker immunoreactivity. The result suggests the importance of
MMP-9 in survival of RCC patients. Moreover, Kawata et al
suggested an association of MMP-9 expression between high nuclear
grade (13) and systemic symptoms
(14) in clinical cases of RCC. We
also confirmed a similar tendency in this study (data not
shown).
Some reports concerned an antitumor effect by
reducing activity of MMP-9. They reduced MMP-9 expression using
shRNA against co-regulator of the nuclear receptor for ovarian
cancer proline-, glutamic acid-, leucine-rich protein-1 (PELP1)
(20), or a monoclonal antibody
against heat shock protein 90 (HSP90) of breast cancer cells
(21). These reports confirmed an
antitumor effect of MMP-9 in animal experiments. Our result and
those reported encouraged us to develop PIPs targeting MMP-9.
We found suppressed expression of MMP-9 mRNA in
Caki-2 cells after the PIP administration possibly by inhibiting
transcription factor binding at the promoter domain. Though
MMP-9-AP-1 PIP is reported to have suppressive effect in the cell
lines of breast cancer, colon cancer and cervical cancer (19), only MMP-9-NF-κB PIP showed
suppression in the Caki-2 cell line. We presumed that mechanisms of
the expression control vary among the cell types. Therefore, when
designing PIP, detailed analysis of target gene transcription
regulation is necessary to select an appropriate transcription
factor for inhibition in each tumor and tumor type. Effective PIP
may be decided from a candidate regulation domain of the targeting
gene promoter.
For the evaluation of invasiveness of tumor in
vitro, we performed Matrigel invasion assay, which has been
commonly used to test cell invasion activity (22–24).
We found that both 3 and 10 μM of MMP-9-NF-κB PIP restrained
cell invasiveness significantly in comparison to control. These
results were compatible with the result of the analysis of MMP-9
mRNA expression level, suggesting that MMP-9-NF-κB PIP reduce the
cell invasiveness by suppressing MMP-9 expression. Since Matrigel
includes two of the major components of basement membrane, laminin
and collagen IV, this result suggested the possibility to control
cell invasion by administration of MMP-9-NF-κB PIP in the
tissue.
The cell proliferation ability showed no significant
difference between PIP treated group and control. The result does
not contradict the fact that the main role of MMP-9 is degeneration
of basement membrane and it is not directly participating in cell
proliferation. However, Bauvois reported MMP-9 is not only involved
in cell invasion but in cell proliferation and vascularization by
an interaction of growth factor, cytokine and vascularization
factor (25). Therefore, future
studies are necessary including experiments concerning effects of
MMP-9-PIP other than suppression of invasion.
We reported previously the in vitro and in
vivo effect of MMP-9-PIP on breast cancer and colon cancer cell
line (19). In the mouse model,
intravenous injection of MMP-9 PIP clearly inhibited colon cancer
metastasis to the liver. In that study, we also found that PIP
remains in the cell nuclei for at least 6 days suggesting a
continuous PIP effect. Antisense DNA or RNAi requires an adequate
drug delivery system because it is easily disintegrated by nucleic
acid degrading enzymes. However, PIP is consisted of
N-methylpyrrole(Py) and N-methylimidazole (Im) and is uptaken by
individual cells without special delivery system. It is stable
without being a target of nucleic acid degrading enzymes. This
should be a strong advantage for PIP.
Tyrosine kinase inhibitor (TKI) targeting mainly
VEGF and the mTOR inhibitor are widely used for advanced renal cell
carcinoma. However, most of them have adverse effects including
skin reaction, hypertension, myeloablation, interstitial lung
disease and sudden exacerbation at the time of withdrawal. Complete
response is rarely seen and main effect of these molecular
targeting agents at present is extension of progression-free
survival and maintenance of the QOL of RCC patients. PIP may act
supplementary for the effect of these molecular targeting agents
and for reduction of its dosage because the mechanism of PIP is
distinct from existing standard therapy (24). This is considered to be the second
advantage.
In conclusion, MMP-9-NF-κB PIP in renal cell
carcinoma cell line caki-2 suppressed its expression of MMP-9 and
invasiveness. A possibility is suggested of MMP-9-NF-κB PIP as a
new therapeutic agent for renal cell carcinoma.
Acknowledgements
The authors thank Dr Yusuke Nagane and
Dr Xiaofei Wang for their technical advice and valuable
discussions.
References
|
1.
|
Srinivasan R, Armstrong AJ, Dahut W, et
al: Anti-angiogenic therapy in renal cell cancer. BJU Int.
99:1296–1300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Board RE, Thistlethwaite FC and Hawkins
RE: Anti-angiogenic therapy in the treatment of advanced renal cell
cancer. Cancer Treat Rev. 33:1–8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Figlin RA: Anti-angiogenic therapy in
renal cell carcinoma: alone, in combination, or sequentially. Clin
Adv Hematol Oncol. 7:662–665. 2009.PubMed/NCBI
|
|
4.
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Klein T and Bischoff R: Physiology and
pathophysiology of matrix metalloproteases. Amino Acids.
41:271–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lukaszewicz-Zajac M, Mroczko B and
Szmitkowski M: Gastric cancer - the role of matrix
metalloproteinases in tumor progression. Clin Chim Acta.
412:1725–1730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Cho NH, Shim HS, Rha SY, et al: Increased
expression of matrix metalloproteinase 9 correlates with poor
prognostic variables in renal cell carcinoma. Eur Urol. 44:560–566.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Awakura Y, Ito N, Nakamura E, et al:
Matrix metalloproteinase-9 polymorphisms and renal cell carcinoma
in a Japanese population. Cancer Lett. 241:59–63. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Zhang X, Yamashita M, Uetsuki H, et al:
Angiogenesis in renal cell carcinoma: evaluation of microvessel
density, vascular endothelial growth factor and matrix
metalloproteinases. Int J Urol. 9:509–514. 2002. View Article : Google Scholar
|
|
11.
|
Sherief MH, Low SH, Miura M, et al: Matrix
metalloproteinase activity in urine of patients with renal cell
carcinoma leads to degradation of extracellular matrix proteins:
possible use as a screening assay. J Urol. 169:1530–1534. 2003.
View Article : Google Scholar
|
|
12.
|
Kallakury BV, Karikehalli S, Haholu A, et
al: Increased expression of matrix metalloproteinases 2 and 9 and
tissue inhibitors of metalloproteinases 1 and 2 correlate with poor
prognostic variables in renal cell carcinoma. Clin Cancer Res.
7:3113–3119. 2001.PubMed/NCBI
|
|
13.
|
Kawata N, Nagane Y, Hirakata H, et al:
Significant relationship of matrix metalloproteinase 9 with nuclear
grade and prognostic impact of tissue inhibitor of
metalloproteinase 2 for incidental clear cell renal cell carcinoma.
Urology. 69:1049–1053. 2007. View Article : Google Scholar
|
|
14.
|
Kawata N, Nagane Y, Igarashi T, et al:
Strong significant correlation between MMP-9 and systemic symptoms
in patients with localized renal cell carcinoma. Urology.
68:523–527. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Lai YM, Fukuda N, Ueno T, et al: Synthetic
pyrrole-imidazole polyamide inhibits expression of the human
transforming growth factor-beta1 gene. J Pharmacol Exp Ther.
315:571–575. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kageyama Y, Sugiyama H, Ayame H, et al:
Suppression of VEGF transcription in renal cell carcinoma cells by
pyrrole-imidazole hairpin polyamides targeting the hypoxia
responsive element. Acta Oncol. 45:317–324. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Matsuda H, Fukuda N, Ueno T, et al:
Development of gene silencing pyrrole-imidazole polyamide targeting
the TGF-beta1 promoter for treatment of progressive renal diseases.
J Am Soc Nephrol. 17:422–432. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Minoshima M, Sasaki S, Fujimoto J, et al:
Synthesis and biological properties of pyrrole-imidazole polyamide
conjugates. In: Nucleic Acids Symp Ser (Oxf); pp. 35–36. 2007,
PubMed/NCBI
|
|
19.
|
Wang X, Nagase H, Watanabe T, et al:
Inhibition of MMP-9 transcription and suppression of tumor
metastasis by pyrroleimidazole polyamide. Cancer Sci. 101:758–766.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Chakravarty D, Roy SS, Babu CR, et al:
Therapeutic targeting of PELP1 prevents ovarian cancer growth and
metastasis. Clin Cancer Res. 17:2250–2259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Stellas D, El Hamidieh A and Patsavoudi E:
Monoclonal antibody 4C5 prevents activation of MMP2 and MMP9 by
disrupting their interaction with extracellular HSP90 and inhibits
formation of metastatic breast cancer cell deposits. BMC Cell Biol.
11:512010. View Article : Google Scholar
|
|
22.
|
Yan L, Kumagai SG, McGuire MH, et al:
Protease activity and invasion of matrigel by the
osteosarcoma-derived OSPR cell line. Biochem Soc Trans.
22:S181994.PubMed/NCBI
|
|
23.
|
Hall DMS and Brooks SA: In vitro invasion
assay using matrigel(R). Methods Mol Med. 58:61–70. 2001.
|
|
24.
|
Deryugina EI, Luo GX, Reisfeld RA, et al:
Tumor cell invasion through matrigel is regulated by activated
matrix metalloproteinase-2. Anticancer Res. 17:3201–3210.
1997.PubMed/NCBI
|
|
25.
|
Bauvois B: New facets of matrix
metalloproteinases MMP-2 and MMP-9 as cell surface transducers:
Outside-in signaling and relationship to tumor progression. Biochim
Biophys Acta. 1825:29–36. 2011.
|