Introduction
Breast cancer is the most prevalent malignancy in
women (1). Despite a statistically
significant decline in breast cancer incidence and death rate in
recent years, breast cancer is still the leading cause of cancer
death among women worldwide (1,2) and
second cause of cancer death among women in the US (1). Furthermore, ∼30% of patients with
early-stage of breast cancer have recurrent disease (3). One of the major obstacles in the
succesful treatment of breast cancer and the main cause of breast
cancer recurrence is the resistance of cancer cells to therapeutic
agents (3).
Several reports have provided compelling evidence
for the connection between profoundly deregulated cellular iron
metabolism and breast cancer progression (4–7).
More importantly, the results of two recent comprehensive studies
conducted by Pinnix et al (4) and Miller et al (6) demonstrated that iron regulatory gene
signatures may predict breast cancer outcome. This suggests that
characterizing the disturbances in iron metabolism in cancer cells,
in addition to providing diagnostic and prognostic value, may be
also potential therapeutic strategies for breast cancer treatment
(8–10). Additionally, a report by Whitnall
et al (11) has
demonstrated that alterations in cellular iron metabolism may
contribute to the acquisition of a cancer cell drug-resistant
phenotype. The latter was evident from the data showing that
treatment of the human etoposide-resistant MCF-7 breast cancer
cells with iron-chelating agents reversed the resistance of cancer
cells to chemotherapeutic agents (11); however, there is a lack of
conclusive information regarding the dysregulation of iron
metabolism in drug-resistant breast cancer cells.
The present study was undertaken to investigate the
status and role of iron metabolism in drug-resistant breast cancer
cells. We demonstrate that human MCF-7 breast cancer cells with an
acquired resistance to the chemotherapeutic agents doxorubicin and
cisplatin exhibit extensive alterations in the cellular iron
homeostasis as characterized by marked changes in the level of
intracellular ‘free iron’, a high-spin form of iron [Fe(III)]
detectable by electron paramagnetic resonance (EPR) and proteins
responsible for the cellular uptake, storage and export of iron,
especially by profound upregulation of ferritin light chain (FTL)
protein. Furthermore, the results demonstrate that targeted
downregulation of overexpressed FTL protein by microRNA miR-133a
increases the sensitivity of drug-resistant cells to doxorubicin
and cisplatin. This suggests that correcting of intracellular iron
metabolism may be a potential approach to overcome resistance of
breast cancer cells to chemotherapeutic agents.
Materials and methods
Cell lines and cell culture
The human breast adenocarcinoma MCF-7 cell line and
its variants resistant to doxorubicin hydrochloride (MCF-7/DOX;
resistance index, 5.6) or cis-diammineplatinum(II)
dichloride (MCF-7/CDDP; resistant index, 6.0) were cultured in
complete Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich,
St. Louis, MO, USA) containing 10% embryonal calf serum
(Sigma-Aldrich) and 40 μg/ml gentamycin at 37°C in 5%
CO2 atmosphere. The drug-resistant variants of MCF-7
cell lines were established as described in Chekhun et al
(12) and the resistant index was
determined as outlined in Kars et al (13). Cells were seeded at a density of
0.5×106 viable cells per 100-mm plate and the medium was
changed every other day for 4 days.
Low-temperature Fe(III) EPR
After 24 h of culturing in complete DMEM, the cells
were scrapped onto ice, washed in phosphate-buffered saline (PBS),
centrifuged at 1000 g for 10 min at 4°C and the pellet was
re-suspended in PBS. The suspension containing 2×106
cells was transferred into EPR tubes and immediately frozen in
liquid nitrogen. The level of free iron was determined by a
low-temperature EPR method (14).
Briefly, samples were maintained at −196°C during recording of the
spectra using a finger Dewar filled with liquid nitrogen. The
following parameters were used for the low-temperature EPR: sweep
width 1525 G; frequency 9.15 GHz; microwave power 40 mW; modulation
amplitude 10.0 G; and modulation frequency 100 kHz. The g-value was
calculated using the standard formula g = hv/βH, where h is
Planck’s constant, v is the frequency, β is the Bohr magneton and H
is the external magnetic field at resonance.
Immunocytochemistry
The levels of transferrin receptor 1 (TFR1),
ferritin light chain (FTL), ferritin heavy chain (FTH1) and
ferroportin (FPN) were determined by immunocytochemistry. Cells
were cultured on glass coverslips for 24 h and fixed in ice-cold
methanol:acetone (1:1) at −20°C for 10 min. The fixed cells were
then rinsed in PBS and after blocking of non-specific staining with
a 1% BSA solution for 20 min, the cells were incubated with primary
rabbit anti-human antibodies against TFR1 (1:100; BS1620; Bioworld
Technology, Minneapolis, MN, USA), FTL (1:500; ab69090; Abcam,
Cambridge, MA, USA), FTH1 (1:150; GTX62020; GeneTex, Irvine, CA,
USA) and FPN (1:50; ab78066; Abcam) at room temperature for 60 min
followed by incubation with an UltraVision LP Detection System
(Thermo Fisher Scientific, Waltham, MA, USA) for 15 min. Staining
was developed with 3,3′-diaminobenzidine Quanto (Thermo Fisher
Scientific). The cells were counterstained with hematoxylin. The
staining intensity was evaluated by the H-score method as described
in McClelland et al (15).
Briefly, the percentage of slightly (a), moderately (b) and
strongly stained cells (c) was determined and then used in the
formula S = (1 × a) + (2 × b) + (3 × c) to calculate an ‘H-score’.
The S values ranged from 0 (no expression) to 300 (strong
expression in 100% cells).
Drug sensitivity assay
To determine drug sensitivity, MCF-7, MCF-7/DOX and
MCF-7/CDDP cells were plated at a density of 10,000 cells per well
in 96-well plates. Cells were cultured for 24 h and then treated
with doxorubicin, cisplatin, or bleomycin sulfate (Sigma-Aldrich).
After 48- and 72-h incubation, cell survival was analyzed with
sulforhodamine B (16) and MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
assays (17). The IC50
(inhibitory concentration to produce 50% cell death) values were
determined from using the resulting dose-response curves. The
experiments were repeated twice and each cell line was tested in
triplicate.
Transfection of MCF-7/DOX, MCF-7/CDDP and
MDA-MB-231 cells with pre-miR-133a and siRNA-FTL
MCF-7/DOX, MCF-7/CDDP and MDA-MB-231 cells were
seeded in 100-mm dishes at a density of 1×106 cells per
dish and transfected with 20 nM of pre-miR-133a (Life Technologies,
Grand Island, NY, USA) and 10 nM of Silencer® Select
siRNA-FTL (Life Technologies), in three independent replicates,
using Lipofectamine™ 2000 (Life Technologies) transfection reagent
according to the manufacturer’s instructions. MCF-7/DOX, MCF-7/CDDP
and MDA-MB-231 cells transfected with scrambled RNA oligonucleotide
served as controls. At 48 h post-transfection, adherent cells were
harvested by mild trypsinization and the viability of cells was
monitored by a MTT test. The cells were then re-seeded and the
transfection repeated. Forty-eight hours after the second
transfection, adherent cells were harvested by mild trypsinization,
washed in PBS and frozen at −80°C for subsequent analyses. The
experiments were repeated twice.
Western blot analysis of protein
expression
The level of FTL protein in the breast cancer cells
was determined by western immunoblot analysis with primary
antibodies against ferritin light chain (FTL; 1:200; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) as described in Shpyleva et
al (18).
Statistical analyses
Statistical analysis was done using Statistica 7.0
software (StatSoft Inc., Tulsa, OK, USA). Results are presented as
mean ± SD. Data were analyzed by one-way analysis of variance
(ANOVA), with pair-wise comparisons being made by the
Student-Newman-Keuls method. P-values <0.05 were considered
statistically significant.
Results
Changes in intracellular Fe(IIl) in the
MCF-7/DOX and MCF-7/CDDP resistant cancer cells
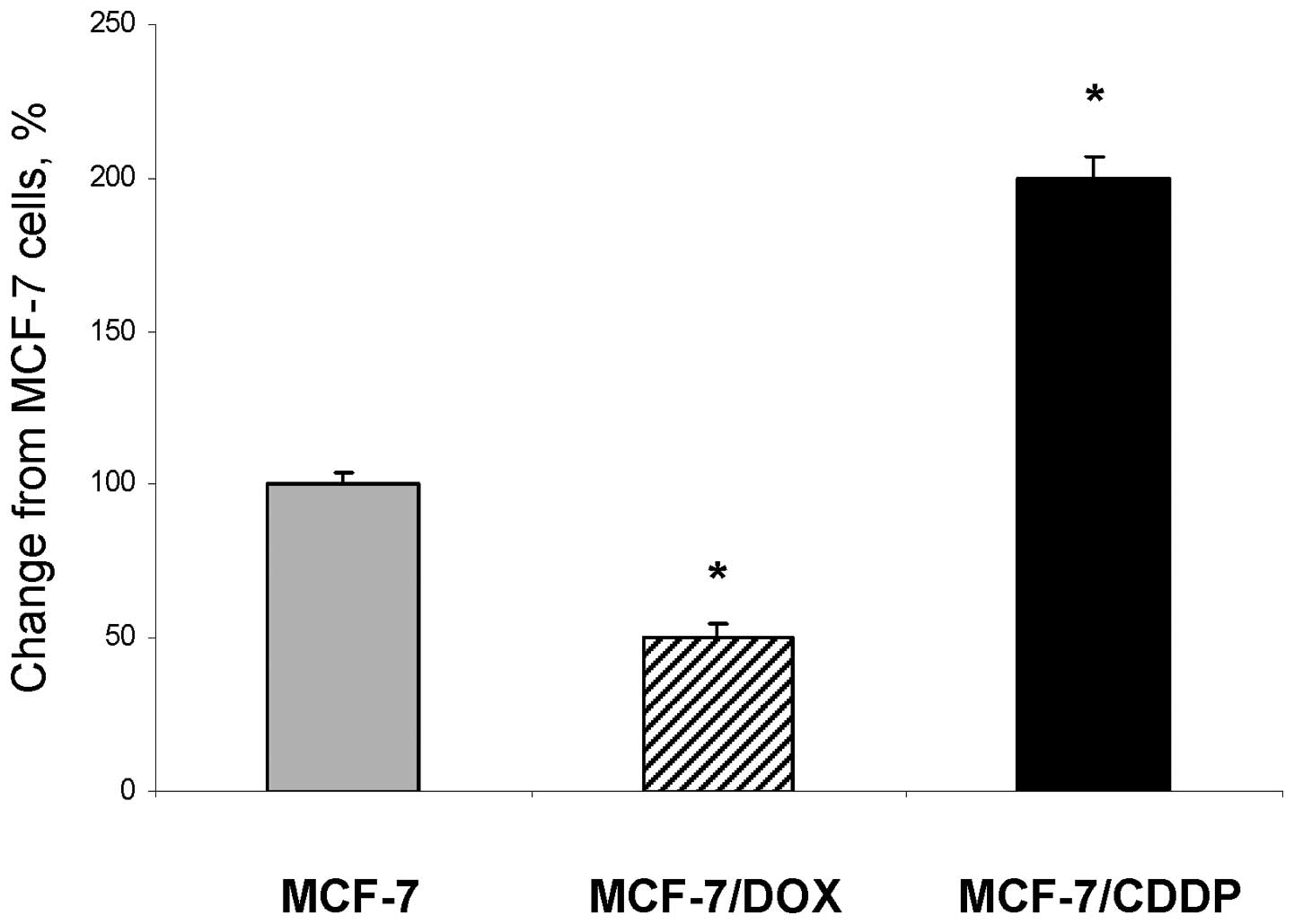
Fig. 1 shows that
drug-resistant MCF-7/DOX and MCF-7/CDDP cells are characterized by
a substantial variation in the ‘free’ intracellular Fe(III) content
as compared to parental MCF-7 cells. Specifically, the level of
intracellular Fe(IIl) in the MCF-7/CDDP cells was 2.0 times greater
than in MCF-7 cells. In contrast, the intracellular level of
Fe(III) in MCF-7/DOX cells was 2.0 times lower than in parental
MCF-7 cells.
Alterations of iron-regulatory proteins
in the MCF-7/DOX and MCF-7/CDDP resistant cancer cells
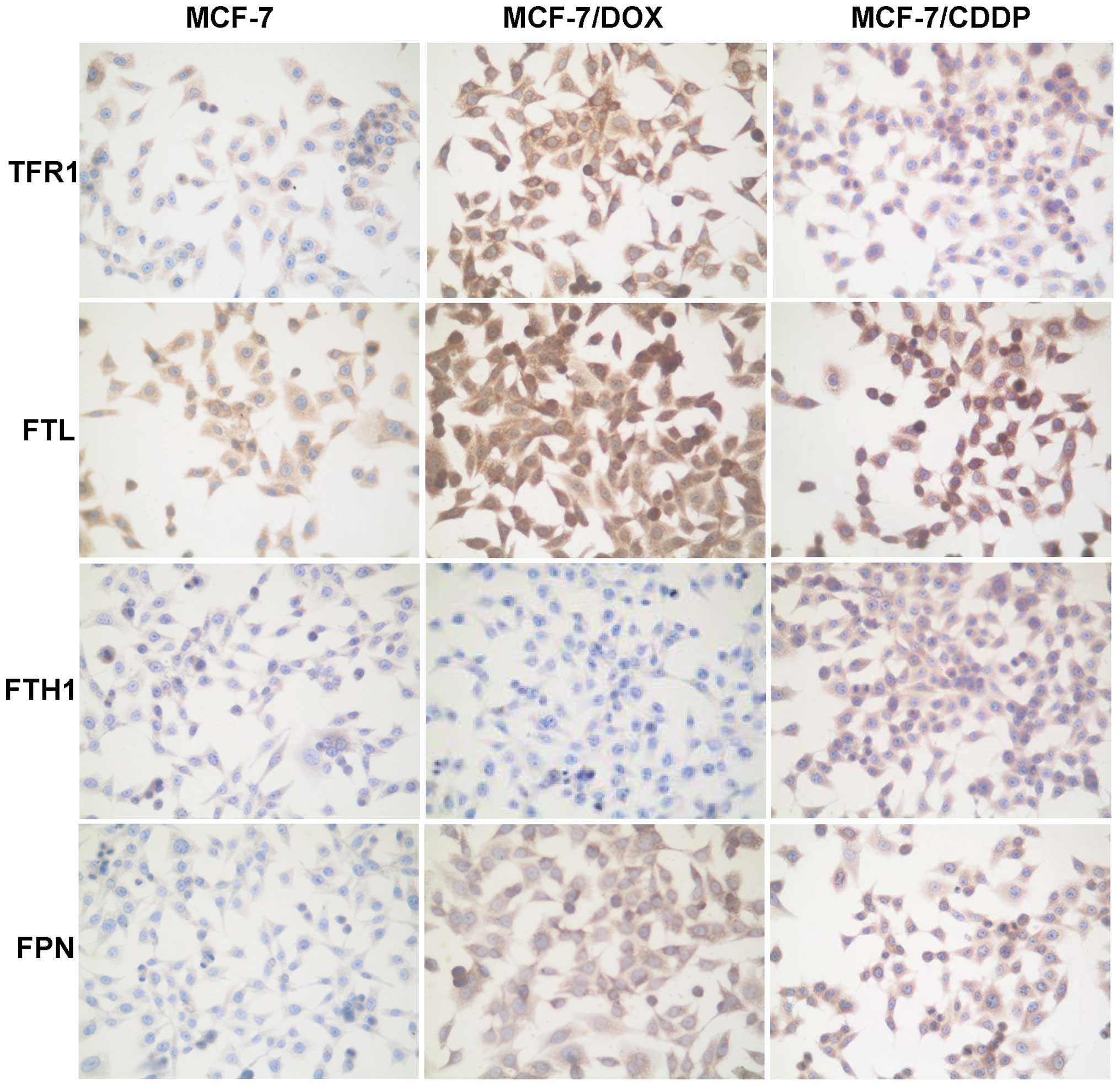
Table I shows
changes in the levels of proteins involved in the cellular uptake
(TFR1), storage (FTL and FTH1) and export (FPN) of iron in MCF-7
cells and the MCF-7/DOX and MCF-7/CDDP drug-resistant variants. In
the MCF-7/DOX and MCF-7/CDDP drug-resistant cells the levels of
TFR1, FTL and FPN proteins were 3.0, 1.5 and 2.5 times greater than
their values in the parental MCF-7 cells (Table I and Fig. 2).
 | Table I.The levels of TFR1, FTL, FTH1 and FPN
proteins in parental MCF-7 breast cancer cells and its
drug-resistant variants MCF-7/DOX and MCF-7/CDDP. |
Table I.
The levels of TFR1, FTL, FTH1 and FPN
proteins in parental MCF-7 breast cancer cells and its
drug-resistant variants MCF-7/DOX and MCF-7/CDDP.
| The level of protein
expression, H-score
|
|---|
| Protein | MCF-7 | MCF-7/DOX | MCF-7/CDDP |
|---|
| TFR1 | 72±1.8 | 205±5.4a | 215±5.5a |
| FTL | 195±4.5 | 298±2.1a | 296±5.8a |
| FTH1 | 125±2.7 | 79±0.8a | 220±6.1a |
| FPN | 83±1.1 | 200±3.4a | 203±5.2a |
In contrast to the cell-type-independent changes in
TFR1, FTL and FPN proteins in MCF-7/DOX and MCF-7/CDDP cells,
alterations in the levels of FTH1 protein were cell-specific. This
was evidenced by a 37% downregulation of FTH1 in the MCF-7/DOX
cells as compared to the parental MCF-7 cells, while in the
MCF-7/CDDP, FTH1 was substantially (by 176%) upregulated.
Intracellular localization of FTL protein
in the MCF-7/DOX and MCF-7/CDDP resistant cancer cells
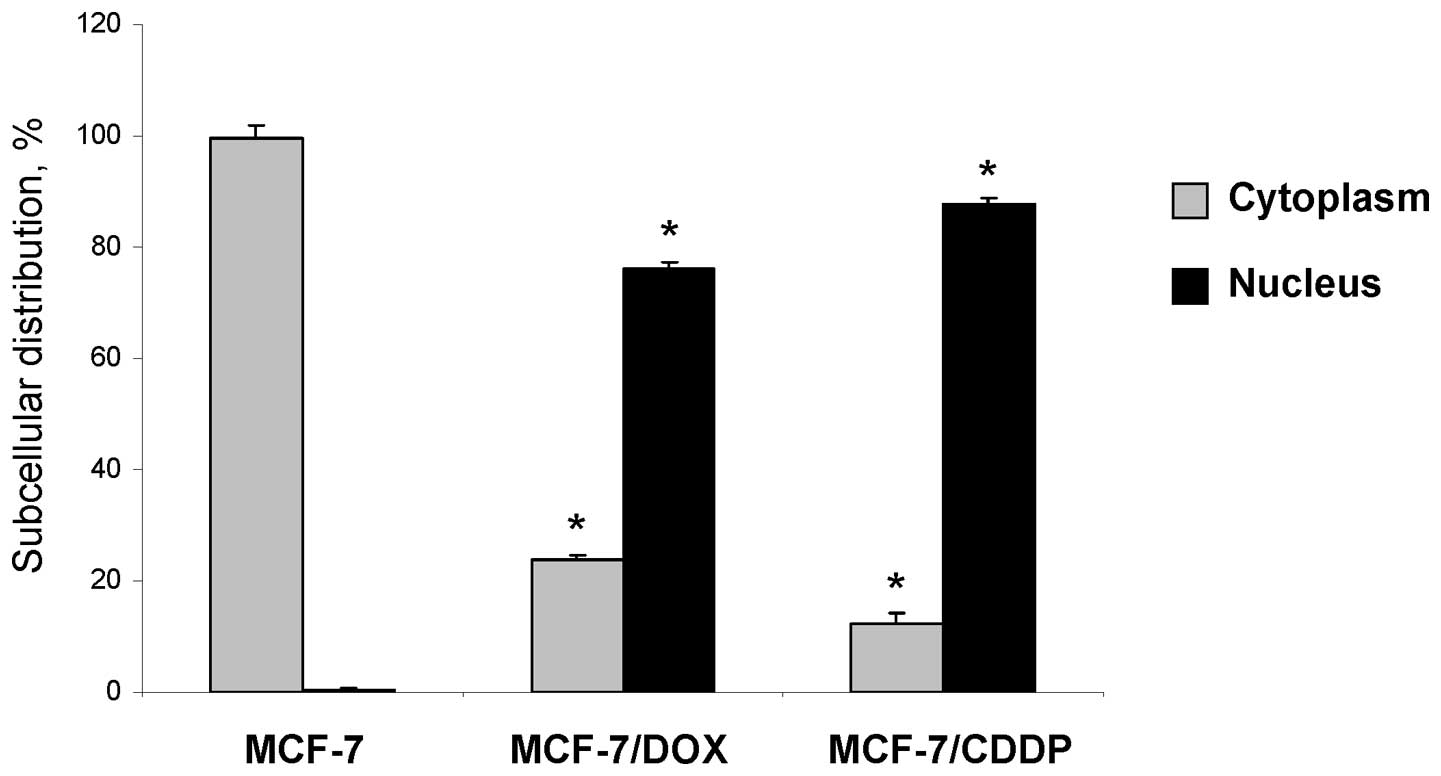
In our previous study we demonstrated that
MDA-MB-231 breast cancer cells, which exhibit an advanced and
intrinsic drug-resistant phenotype, were characterized by an
upregulation of FTH1 and, especially, FTL proteins (18). Additionally, MDA-MB-231 breast
cancer cells were characterized by increased levels of these
proteins in their nuclei (18).
This observation prompted us to investigate whether or not the
upregulation of FTL in the MCF-7/DOX and MCF-7/CDDP resistant
cancer cells is also accompanied by an altered subcellular
distribution. It is well established that both FTH1 and FTL are
primarily cytosolic proteins (19). Indeed, in parental MCF-7 cells, FTL
is located only in the cytoplasm (Figs. 2 and 3). In contrast, in the MCF-7/DOX and
MCF-7/CDDP resistant cancer cells, FTL was located primarily in the
nucleus.
Sensitivity of the MCF-7/DOX and
MCF-7/CDDP cells to chemotherapeutic agents
Having found significant changes in the levels of
iron-regulatory proteins and intracellular Fe(III) content in the
MCF-7/DOX and MCF-7/CDDP cells compare to MCF-7 cells, we
investigated whether these changes were accompanied by a different
resistance to other chemotherapeutic drugs, especially those in
which the mechanism of action is linked to iron metabolism.
Table II shows that the MCF-7/DOX
cells, which are characterized by a low level of Fe(IIl), were the
most resistant to bleomycin, a model chemotherapeutic agent whose
anticancer activity is associated with iron (20). In contrast, the MCF-7/CDDP
resistant cancer cells that have an increased level of
intracellular Fe(III) showed the same sensitivity to bleomycin
treatment as MCF-7 cells.
 | Table II.Drug sensitivity of parental MCF-7
breast cancer cells and its drug-resistant variants MCF-7/DOX and
MCF-7/CDDP. |
Table II.
Drug sensitivity of parental MCF-7
breast cancer cells and its drug-resistant variants MCF-7/DOX and
MCF-7/CDDP.
| The half maximal
inhibitory concentration IC50, μM
|
|---|
| Drug | MCF-7 | MCF-7/DOX | MCF-7/CDDP |
|---|
| DOX | 4.1±0.3 | 23.3±2.1a | 12.4±1.2a |
| CDDP | 15.3±1.3 | 16.0±1.0 | 93.3±7.0a |
| Bleomycin | 6.4±0.5 | 11.3±0.6a | 4.1±0.4 |
MiR-133a targets FTL and increases
sensitivity of breast cancer cells to chemotherapeutic agents
Recent reports demonstrating the critical role of
iron-regulatory proteins in breast cancer progression (4–7)
suggest that targeting these proteins may be a potential
therapeutic approach to improve clinical management of breast
cancer and overcome resistance of breast cancer cells to
chemotherapeutic agents (11).
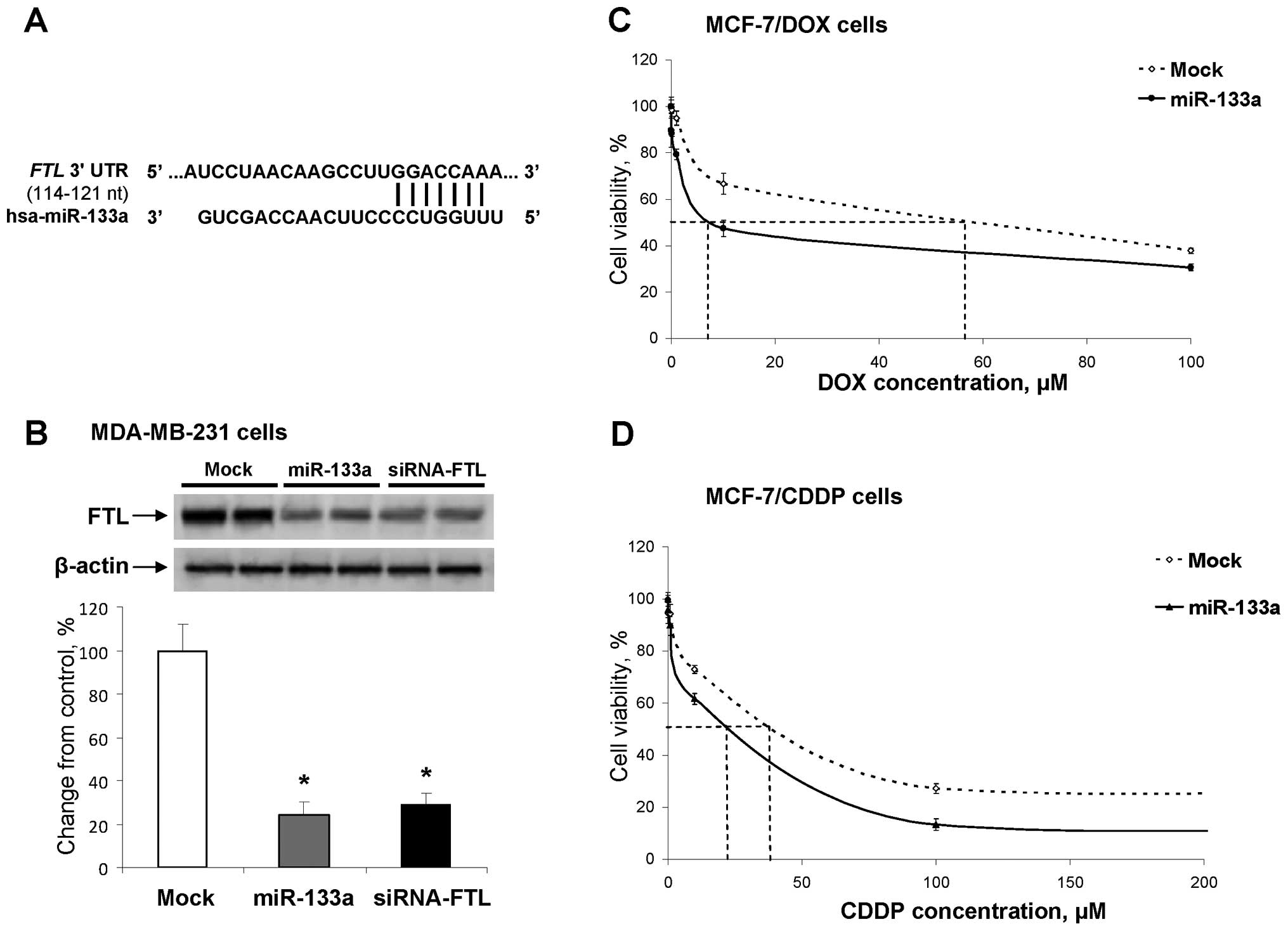
Computational analysis of the 3′-UTR of FTL gene, using the
TargetScanHuman, version 6.2, database (www.targetscan.org), revealed the presence of putative
binding sites for two microRNAs, miR-22 and miR-133. Recently, Wu
et al (21) showed that the
level of miR-133a is markedly reduced in breast cancer and is
associated with breast cancer progression. The expression of
miR-133a was also lower in breast cancer cell lines and displayed
Ct values of >33 cycles in MCF-7/DOX and MCF-7/CDDP cells
suggesting that this miRNA is not expressed in drug resistant cells
(data not shown). In contrast, the expression of miR-22 was
substantially increased in MCF-7 cells resistant to doxorubicin and
cisplatin (22,23).
To determine whether or not upregulation of miR-133a
affects the FTL levels and increases the sensitivity of
drug-resistant cells to chemotherapeutic agents, we transfected
MCF-7/DOX and MCF-7/CDDP cells with pre-miR-133a. First, we
confirmed experimentally that miR-133a targets human FTL mRNA. This
was accomplished by transfecting human MDA-MB-231 breast cancer
cells, which are characterized by high levels of FTL, with the
miR-133a precursor or a siRNA directed against FTL. Fig. 4B shows a reduction in the levels of
FTL by 76 and 71%, respectively, in the MDA-MB-231 cells
transfected with pre-miR-133a precursor and siRNA, as compared to
mock-transfected cells. Then, we transfected MCF-7/DOX and
MCF-7/CDDP drug-resistant cells with pre-miR-133a and found an
ectopic upregulation of miR-133a that resulted in a substantially
increased cancer cell sensitivity to doxorubicin and cisplatin.
This was evidenced by the fact the IC50 concentration
for doxorubicin and cisplatin in MCF-7/DOX and MCF-7/CDDP cells
decreased 7.9- and 2.0-fold, respectively (Fig. 4C and D).
Discussion
Iron is required for normal cell function and is
finely regulated by extracellular and intracellular mechanisms
responsible for iron homeostasis. In contrast, in cancer cells, the
intracellular iron metabolism is profoundly disturbed (5). A number of previous studies have
demonstrated a fundamental role of an altered intracellular iron
homeostasis in breast cancer. Specifically, breast tumors have
aberrant levels of iron-regulatory proteins, including TFR1,
ribonucleotide reductase, FTL, FTH1, FPN and hepcidin (4–7,24–27).
It is believed that these changes may accelerate tumor growth
leading to a more aggressive tumor behavior, metastasis, drug
resistance and high recurrence of the disease (9,17).
In this study, we report that human MCF-7 breast
cancer cells with an acquired resistance to the chemotherapeutic
drugs doxorubicin and cisplatin exhibited substantial alterations
in the intracellular iron content and levels of iron-regulatory
proteins involved in the cellular uptake, storage and export of
iron. The results demonstrate that the levels of intracellular
‘free iron’ in drug-resistant MCF-7/DOX and MCF-7/CDDP cells were
distinctively different. This was evidenced by the fact that the
level of intracellular iron in MCF-7/DOX cells was substantially
reduced as compared to parental MCF-7 cells, which may be explained
by a direct iron-chelating ability of doxorubicin (28,29)
and by ability of doxorubicin to interact with iron response
elements of FTH1 and FTL mRNAs (30). In contrast, the level of
intracellular iron was profoundly increased in MCF-7/CDDP
drug-resistant cells.
It is well-established that changes in the cellular
iron levels are one of the major causes of the upregulation of
iron-regulatory proteins and ferritins and the translocation of
ferritins to the nucleus (19,31).
One of the key findings in this study was profoundly increased
levels of FTL protein in MCF-7/DOX and MCF-7/CDDP drug-resistant
cells, in general and in the nuclei, in particular. Similarly, in
our previous study, increased levels of FTL and FTH1 were found in
the aggressive and drug-resistant human MDA-MB-231 breast cancer
cells (18). In view of this, we
hypothesize that increased levels of FTL may be one of the factors
associated with drug-resistance. This suggestion is supported by
evidence showing that nuclear ferritins protect DNA from DNA
damage-inducing compounds, including DNA-alkylating
chemotherapeutic drugs (32).
The increased levels of FTH1 and FTL proteins in
breast cancer indicate that these proteins may be used as
diagnostic and, more importantly, as prognostic markers for breast
cancer (4,6,26,27).
Additionally, several reports have shown that FTH1 and FTL may be
potential targets in cancer and that their downregulation may
substantially increase the efficiency of cancer therapy (33,34).
Moreover, it has been suggested that targeted correction of
upregulated ferritins may also be a potential approach to overcome
resistance of breast cancer cells to chemotherapeutic agents
(11). The results of the present
study, showing that targeted downregulation of FTL protein by
miR-133a increases sensitivity of MCF-7/DOX and MCF-7/CDDP cells to
doxorubicin and cisplatin support this suggestion. Additionally,
these findings are in good agreement with a recent report by Liu
et al (34) demonstrating
that silencing of FTH1 by siRNA substantially sensitized tumors to
chemotherapy.
In conclusion, the data presented herein point to
dysregulated iron metabolism as one of the main factors associated
with drug-resistant phenotype of breast cancer cells and indicate
that correction of these alterations may substantially improve the
efficiency of breast cancer treatment.
Acknowledgements
The views expressed in this paper do
not necessarily represent those of the U.S. Food and Drug
Administration.
References
|
1.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2011. View Article : Google Scholar
|
|
2.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
3.
|
Gonzalez-Angulo AM, Morales-Vasquez F and
Hortobagyi GN: Overview of resistance to systemic therapy in
patients with breast cancer. Adv Exp Med Biol. 608:1–22. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Pinnix ZK, Miller LD, Wang W, D’Agostino R
Jr, Kute T, Willingham MC, Hatcher H, Tesfay L, Sui G, Di X, Torti
SV and Torti FM: Ferroportin and iron regulation in breast cancer
progression and prognosis. Sci Transl Med. 2:43ra562010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Torti SV and Torti FM: Ironing out cancer.
Cancer Res. 71:1511–1514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Miller LD, Cofman LG, Chou JW, Black MA,
Bergh J, D’Agostino R Jr, Torti SV and Torti FM: An iron gene
signature predicts outcome in breast cancer. Cancer Res.
71:6728–6737. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kwok JC and Richardson DR: The iron
metabolism of neoplastic cells: alterations that facilitate
proliferation? Crit Rev Oncol Hematol. 42:65–78. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yu Y, Gutierrez E, Kovacevic Z, Saletta F,
Obeidy P, Rahmanto YS and Richardson DR: Iron chelators for the
treatment of cancer. Curr Med Chem. 19:2689–2702. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Hoke EM, Maylock CA and Shacter E:
Desferal inhibits tumor growth and does not interfere with
tumoricidal activity of doxorubicin. Free Radic Biol Med.
39:403–411. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Rao VA, Klein SR, Agama KK, Toyoda E,
Adaci N, Pommier Y and Shacter EB: The iron chelator Dp44mT causes
DNA damage and selective inhibition of topoisomerase II alpha in
breast cancer cells. Cancer Res. 69:948–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Whitnall M, Howard J, Ponka P and
Richardson DR: A class of iron chelators with a wide spectrum of
potent antitumor activity that overcomes resistance to
chemotherapeutics. Proc Natl Acad Sci USA. 103:14901–14906. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Chekhun VF, Lukyanova NYu, Kovalchuk O,
Tryndyak VP and Pogribny IP: Epigenetic profiling of
multidrug-resistant human MCF-7 breast adenocarcinoma cells reveals
novel hyper- and hypomethylated targets. Mol Cancer Ther.
6:1089–1098. 2007. View Article : Google Scholar
|
|
13.
|
Kars MD, Iseri OD, Gündüz U, Ural AU,
Arpaci F and Molnár J: Development of rational in vitro models for
drug resistance in breast cancer and modulation of MDR by selected
compounds. Anticancer Res. 26:4559–4568. 2006.PubMed/NCBI
|
|
14.
|
Pate KT, Randel NA, Fraser B, Clement MH
and Srinivasan C: Measuring ‘free’ iron levels in Caenorhabditis
elegans using low-temperature Fe(III) electron paramagnetic
resonance spectroscopy. Anal Biochem. 358:199–207. 2006.
|
|
15.
|
McClelland RA, Wilson D and Leake R: A
multicentre study into the reliability of steroid receptor
immunocytochemical assay quantification. Eur J Cancer. 27:711–715.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Skehan P, Storeng R, Scudiero D, Monks A,
McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S and Boyd MR:
New colorimetric cytotoxicity assay for anticancer-drug screening.
J Natl Cancer Inst. 82:1107–1112. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ni J and Hollander D: Application of the
MTT-assay to functional studies of mouse intestinal intraepithelial
lymphocytes. J Clin Lab Anal. 10:42–52. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Shpyleva SI, Tryndyak VP, Kovalchuk O,
Starlard-Davenport A, Chekhun VF, Beland FA and Pogribny IP: Role
of ferritin alterations in human breast cancer cells. Breast Cancer
Res Treat. 126:63–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Arosio P, Ingrassia and Cavadini P:
Ferritins: a family of molecules for iron storage, antioxidation
and more. Biochim Biophys Acta. 1790:589–599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Dorr RT: Bleomycin pharmacology: mechanism
of action and resistance and clinical pharmacokinetics. Semin
Oncol. 19(Suppl 5): 3–8. 1992.PubMed/NCBI
|
|
21.
|
Wu Z, Wang C, Xiang R, Liu X, Ye S, Yang
X, Zhang G, Xu X, Zhu T and Wu Q: Loss of miR133a expression is
associated with poor survival of breast cancer and restoration of
miR-133a expression inhibited breast cancer growth and invasion.
BMC Cancer. 12:512012. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kovalchuk O, Filkowski J, Meservy J,
Ilnytskyy Y, Tryndyak VP, Chekhun VF and Pogribny IP: Involvement
of microRNA-451 in resistance of the MCF-7 breast cancer cells to
chemotherapeutic drug doxorubicin. Mol Cancer Ther. 7:2152–2159.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Pogribny IP, Filkowski JN, Tryndyak VP,
Golubov A, Shpyleva SI and Kovalchuk O: Alterations of microRNAs
and their targets are associated with acquired resistance of MCF-7
breast cancer cells to cisplatin. Int J Cancer. 127:1785–1794.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Kanojia D, Zhou W, Zhang J, Jie C, Lo PK,
Wang Q and Chen H: Proteomic profiling of cancer stem cells from
primary tumors of HER2/Neu transgenic mice. Proteomics.
12:3407–3415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Eswaran J, Cyanam D, Mudravi P, Reddy SD,
Pakala SB, Nair SS, Florea L, Fuqua SA, Godbole S and Kumar R:
Transcriptomic landscape of breast cancers through mRNA sequencing.
Sci Rep. 2:2642012. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Descotes F, Jézéquel P, Spyratos F,
Campion L, Grenot C, Lerebours F, Campone M, Guérin-Charbonnel C,
Lanoë D, Adams M, andré J, Carlioz A, Martin PM, Chassevent A,
Jourdan ML, Guette C, Zanella-Cleon I and Ricolleau G:
Identification of potential prognostic biomarkers for node-negative
breast tumours by proteomic analysis: a multicentric 2004 national
PHRC study. Int J Oncol. 41:92–104. 2012.PubMed/NCBI
|
|
27.
|
Dong X, Yang M, Sun H, Lü J, Zheng Z, Li Z
and Zhong L: Combined measurement of CA 15-3 with novel
autoantibodies improves diagnostic accuracy for breast cancer. Onco
Targets Ther. 6:273–279. 2013.PubMed/NCBI
|
|
28.
|
Xu X, Persson HL and Richardson DR:
Molecular pharmacology of the interaction of anthracyclines with
iron. Mol Pharmacol. 68:261–271. 2005.PubMed/NCBI
|
|
29.
|
Xu X, Sutak R and Richardson DR: Iron
chelation by clinically relevant anthracyclines: alteration in
expression of iron-regulated genes and atypical changes in
intracellular iron distribution and trafficking. Mol Pharmacol.
73:833–844. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Canzoneri JC and Oyelere AK: Interaction
of anthracyclines with iron responsive elements mRNAs. Nucleic
Acids Res. 36:6825–6834. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Thompson KJ, Fried MG, Ye Z, Boyer P and
Connor JR: Regulation, mechanisms and proposed function of ferritin
trans-location to cell nuclei. J Cell Sci. 114:2165–2177.
2002.PubMed/NCBI
|
|
32.
|
Alkhateeb AA and Connor JR: Nuclear
ferritin: a new role for ferritin in cell biology. Biochim Biophys
Acta. 1800:793–797. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Yang DC, Jiang X, Elliot RL and Head JF:
Antisense ferritin oligonucleotides inhibit growth and induce
apoptosis in human breast carcinoma cells. Anticancer Res.
22:1513–1524. 2002.
|
|
34.
|
Liu X, Madhankumar AB, Slagle-Webb B,
Sheehan JM, Surguladze N and Connor JR: Heavy chain ferritin siRNA
delivered by cationic liposomes increases sensitivity of cancer
cells to chemotherapeutic agents. Cancer Res. 71:2240–2249. 2011.
View Article : Google Scholar
|