Introduction
FHL2 is a LIM-only protein that belongs to the four
and a half LIM-only protein family. It consists of four LIM domains
and one N-terminal half LIM domain. The abbreviation LIM represents
the names of three transcription factors (Lin-11, Isl-1 and Mec-3)
in which such a domain was first identified (4). It is strongly expressed in cardiac
and skeletal muscle cells but a much lower level was observed in
other tissues and cell types (1–4). The
LIM domains are double zinc finger motifs that play multiple roles
in protein-protein interaction (5). FHL2 can interact with over 50
different proteins with diverse functions such as receptors,
enzymes, transcription factors, cofactors or splicing factors
(6). Thus, FHL2 is involved in the
regulation of various cellular processes including proliferation,
differentiation, migration, adhesion, motility and contraction.
The role of FHL2 in cancer is particularly
intriguing since FHL2 binds to different proteins and can function
in a cell-type dependent fashion as transcriptional co-activators
of several transcription factors, including androgen receptor,
AP-1, CREB, BRCA1, WT-1 and NF-κB in various transformed cell types
(7–11), or as transcriptional co-repressors
of ERK2, PLZF, SRF and FOXO1 (12–15).
FHL2 was first identified as being downregulated in human
rhabdomyosarcoma cells, suggesting a suppressor role in tumor
development (16). Many recent
studies have reported the differential expression of FHL2 in tumor
tissues. Interestingly, FHL2 is overexpressed in breast cancer
(17), prostate cancer (18), ovarian cancer (4), gastrointestinal cancer (19) and glioma (20) but downregulated in liver cancer
(21), making the role of FHL2 in
cancer development elusive. It is also notable that FHL2 triggers
apoptosis in human RD, monkey kidney COS-1 and normal mouse
fibroblast NIH 3T3 cell lines (22) but plays an anti-apoptotic role in
glioblastoma (20). The intriguing
aspects of FHL2 being as oncoprotein or tumor suppressor may be
related to its interaction with different partner proteins even in
different cell types (23).
Suppression of FHL2 was detected to induce cell differentiation and
inhibit tumorigenesis in FHL2 high expression colon cancer cells,
while in HT29 cells with bare FHL2 expression, attenuated FHL2
suppressed cell growth and differentiation (19,23).
We and other groups have reported that FHL2 was also a potent EMT
inducer by stimulating vimentin and MMP-9 expressions and causing a
loss of E-cadherin in colon DLD1 cells (24) and FHL2 negatively regulated the
transcription of E-cadherin through interaction with Snail1
(25). For the tissue and cellular
function specificity, FHL2 might be the target in cancer biological
therapy.
In our study, we have demonstrated that a novel and
effective way to knockdown FHL2, the rAAV-FHL2-shRNA can induce
apoptosis significantly, inhibit tumorigenesis and tumor growth,
strongly enhance the antitumor activity of 5-FU and markedly
prolonged the survival time of animals bearing tumor xenografts
in vivo. Our results document that rAAV-shRNA-FHL2 is a
promising candidate for gene therapy of colon cancer.
Materials and methods
Cell culture
Culture reagents were purchased from Invitrogen
(Carlsbad, CA, USA). Colon cancer cell line LoVo was obtained from
American Type Culture Collection (Rockville, MD, USA) and cultured
as described (19). Cell was
maintained at 37°C in a 5% CO2 humidified incubator and
subcultured using 0.25% trypsin every 2–3 days before confluence
was reached.
Constructs
As we have described previously (20), the enhanced green fluorescence
protein (EGFP) was constructed by inserting the EGFP gene between
the XhoI and EcoRI sites of the AAV2 vector. Short
hairpin RNA (shRNA) targeting FHL2 (sense:
5′-TCGACGCGAATCTCTCTTTGGCAAGTTCAAGAG
ACTTGCCAAAGAGAGATTCGTTTTTTGGAAT-3′; anti-sense:
5′-CTAGATTCCAAAAAACGAATCTCTCTTTGG
CAAGTCTCTTGAACTTGCCAAAGAGAGATTCGCG-3′) or luciferase (sense:
5′-TCGACGCGTACGCGGAATACT TCGATTCAAGAGATCGAAGTATTCCGCGTACGTTTT
TTGGAAT-3′; antisense: 5′-CTAGATTCCAAAAAACGT
ACGCGGAATACTTCGATCTCTTGAATCGAAGTATTC CGCGTACGCG-3′) was initially
inserted into the SalI and XbaI sites of pAVU6+7
plasmid and then the construct was sub-cloned into the AAV2
expression plasmid in order to substitute the CMV promoter with the
U6 promoter.
Western blot analysis
The whole cell lysates were prepared with lysis
buffer (20 mM Tris-HCl, 1 mM EDTA, 1 mM EGTA, 1 mM sodium vanadate,
0.2 mM phenylmethylsulfonyl fluoride, 0.5% NP-40, 1 μg/ml
leupeptin, 1 μg/ml aprotinin, and 1 μg/ml pepstatin
A). In total, 10 or 30 μg of cell lysate was subjected to
SDS-PAGE, transferred to PVDF membranes, and probed with first
antibodies against CDK6, cyclin D, procaspase 3, cleaved caspase 3,
procaspase 8, cleaved caspase 8 (Santa Cruz Biotechnology, Santa
Cruz, CA, USA), procaspase 9 (Alexis Biochemical, San Diego, CA,
USA), cleaved caspase 9 (Imgenex, San Diego, CA, USA) or FHL2 (MBL
International Incorporation, Woburn, Japan) followed by the
HRP-conjugated secondary antibody. Goat antihuman actin antibody
(I-19, Santa Cruz Biotechnology) was used as internal control.
Antigen-antibody complexes were visualized by the enhanced
chemiluminescence (ECL) system (Amersham Biosciences, Little
Chalfont, UK).
Preparation of rAAV
The rAAV particles were produced using a helper
virus free system as previously described in HEK 293 cells
(20). The viruses were purified
by HiTrap Heparin column chromatography (Sigma Chemical Co., St.
Louis, MO, USA) and viral titer was determined by real-time PCR
using the SYBR-Green I kit (Applied Biosystems, Foster City, CA,
USA) with a forward primer (5′-CGGCTGTTGGGCACTGA-3′) and a reverse
primer (5′-CCGAAGGGACGAAGCAGAAG-3′). Aliquot of viral stocks
(1.5×1012 viral genomes/ml) were stored at −80°C before
use.
Gene transfection in vitro
LoVo cells were cultured in 6-well plastic plates
and transfected with rAAV-EGFP or rAAV-FHL2-shRNA at different
multiplicity of infection (MOI = 1×104,
1×105, 5×105). From then on, the enhanced
green fluorescent protein (EGFP) was observed under a fluorescent
microscope and the expression of rAAV-FHL2-shRNA was detected by
western blot analysis.
Flow cytometry scan
To analyze cell cycle, cells were collected and
fixed with ice-cold 70% ethanol in PBS and stored at −4°C until
use. After resuspension, cells were incubated with 100 μl of
RNase I (1 μg/ml) and 100 μl of propidium iodide (PI)
(400 μg/ml) at 37°C and analyzed by flow cytometry
(Becton-Dickinson, Franklin Lakes, NJ, USA). The cell cycle phase
distribution was calculated from the resultant DNA histogram using
Multicycle AV software (Phoenix Flow Systems, San Diego, CA, USA).
Apoptosis was detected using the Annexin V-FITC kit according to
the manufacturer’s instructions (Trevigen Inc., Gaithersburg, MD,
USA). Briefly, cells with various treatments were collected and
stained with Annexin V-FITC and PI in the dark for 15 min at room
temperature. After addition of binding buffer, cells were analyzed
by flow cytometry and analyzed using Winmdi 2.8.
Cell viability assay
The colorimetric WST-1 assay was performed to assess
the effect of rAAV-FHL2-shRNA on cell proliferation (26). The measurement was based on the
ability of viable cells to cleave the sulfonated tetrazolium salt
WST-1
(4-(3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio)-1,3-benzene
disulfonate) by mitochondrial dehydrogenases. LoVo cells (5,000
cells/well) were plated in a 96-well plate in regular growth
medium, and after 16 h the medium was replaced with 2% FBS
containing medium. After 72 h, 10 μl WST-1 reagent was added
in each well followed by additional incubation for 2 h. The
absorbance at 450 nm was measured using a microplate reader.
Morphological detection of apoptosis
Morphological evaluation of apoptotic cell death was
performed as previously described with some modification (27). Cells were fixed for 5 min in 3%
paraformaldehyde in phosphate-buffered saline (PBS). After
air-drying, cells were stained for 10 min in Hoechst 33258 (10
μg/ml), mounted in 50% glycerol containing 20 mM citric acid
and 50 mM orthophosphate, and stored at −20°C before analysis,
nuclear morphology was evaluated using a Zeiss IM 35
fluorescent.
Caspase 3, 8, 9 activity assay
Caspase 3, 8, 9 activity was determined using the
ApoAlert caspase colorimetric assay kit according to the
manufacturer’s instructions (Clontech, Moutain View, CA, USA)
(28). Briefly, assays were
performed on 96-well microtiter plates by incubating 10 μl
protein of cell lysate per sample in 80 μl reaction buffer
[1% NP-40, 20 mmol/l Tris-HCl (pH 7.5), 137 mmol/l Nad, and 10%
glycerol] containing 10 μl caspase 3, 8, 9 substrate
(Ac-DEVDpNA) (2 mmol/l). Lysates were incubated at 37°C for 4 h.
Samples were measured with an enzyme-linked immunosorbent assay
reader at an absorbance of 405 nm. All of experiments were
performed at least 4 times.
Experimental animal model and
tumorigenesis assay
Five to six-weeks-old female BALB/c nude mice were
bred under pathogen-free conditions at the Southern Medical
University (Guangzhou, China). All animal studies were approved by
the Southern Medical University Animal Care and Use Committee. LoVo
cells in exponential growth phase were harvested and washed twice
in PBS. The cells were resus-pended in PBS at a density of
5×107 cells/ml and 0.1 ml (5×106 cells) of
the cell suspension was then injected subcutaneously into the right
flank of each nude mouse (29).
Seven days after subcutaneous tumor cell injection, mice were
anesthetized and 1.5×1011 v.g. of rAAV-Luc-shRNA,
rAAV-FHL2-shRNA, rAAV-Luc-shRNA + 5-FU or rAAV-FHL2-shRNA + 5-FU
was injected directly into the tumors at three different locations
by a 10-ml micro-syringe (Hamilton, Reno, NV, USA). Four mice (4
injections) were included in each group (each transduced cell
line). The volumes of tumor were calculated as follows: V=(4/3)
R12R2, where R1 is radius 1 and R2 is radius 2 and
R1<R2. Mice were sacrificed and tumors were dissected and
weighed on day 35 after inoculation.
Statistical analysis
Data are presented as the mean ± standard error of
mean (SEM). The significance of the difference between groups was
evaluated with the Student’s t-test or one-way ANOVA test.
P<0.05 was considered significant difference.
Results
Concentration and time kinetics of rAAV
transduction in LoVo cell
LoVo cells were infected with rAAV-EGFP at different
multiplicity of infection (MOIs) ranging from 1×104 to
5×105. Forty-eight hours after infection, expression of
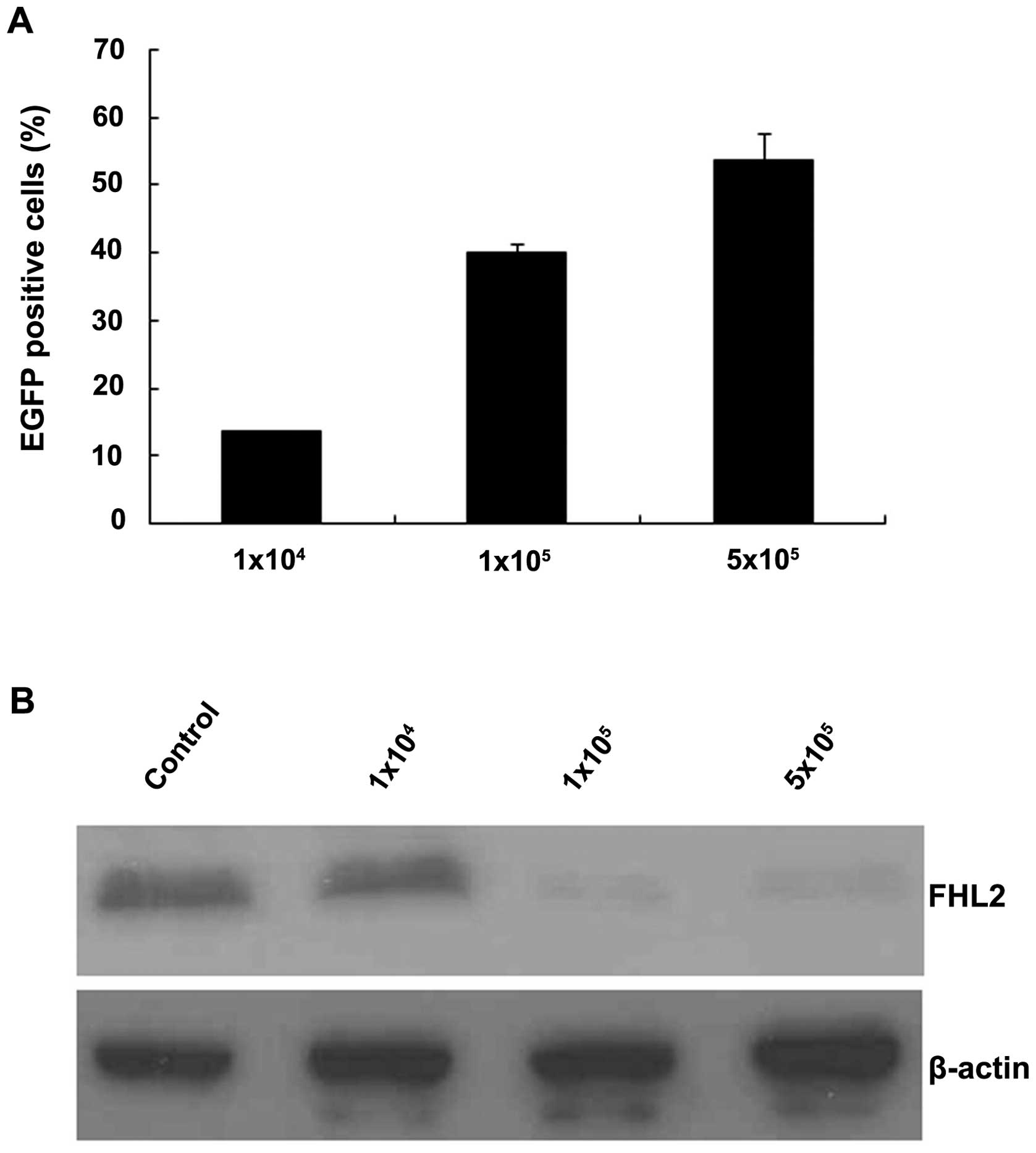
EGFP was analyzed for by flow cytometry (30). At a MOI of 1×104, an
average of 13.6% of cells (SEM= 0.2%) was EGFP positive. At a MOI
of 1×105, a significant increase of positive cells up to
40.1% (SEM=1.2%) was observed. At a MOI of 5×105, a
slight but insignificant increase of EGPF cells was detected
(53.7%; SEM=4.0%; Fig. 1A, one-way
ANOVA analysis). As shown in Fig.
1B, FHL2 expressed at high level in LoVo cells, while with
different dosage rAAVFHL2-shRNA transduction, FHL2 expression
decreased dose-dependently.
With this efficient gene interference by rAAV, FHL2
was specifically and highly inhibited. No significance decrease in
5×105 titer, suggested 1×105 as the most
appropriate MOI in the future experiments.
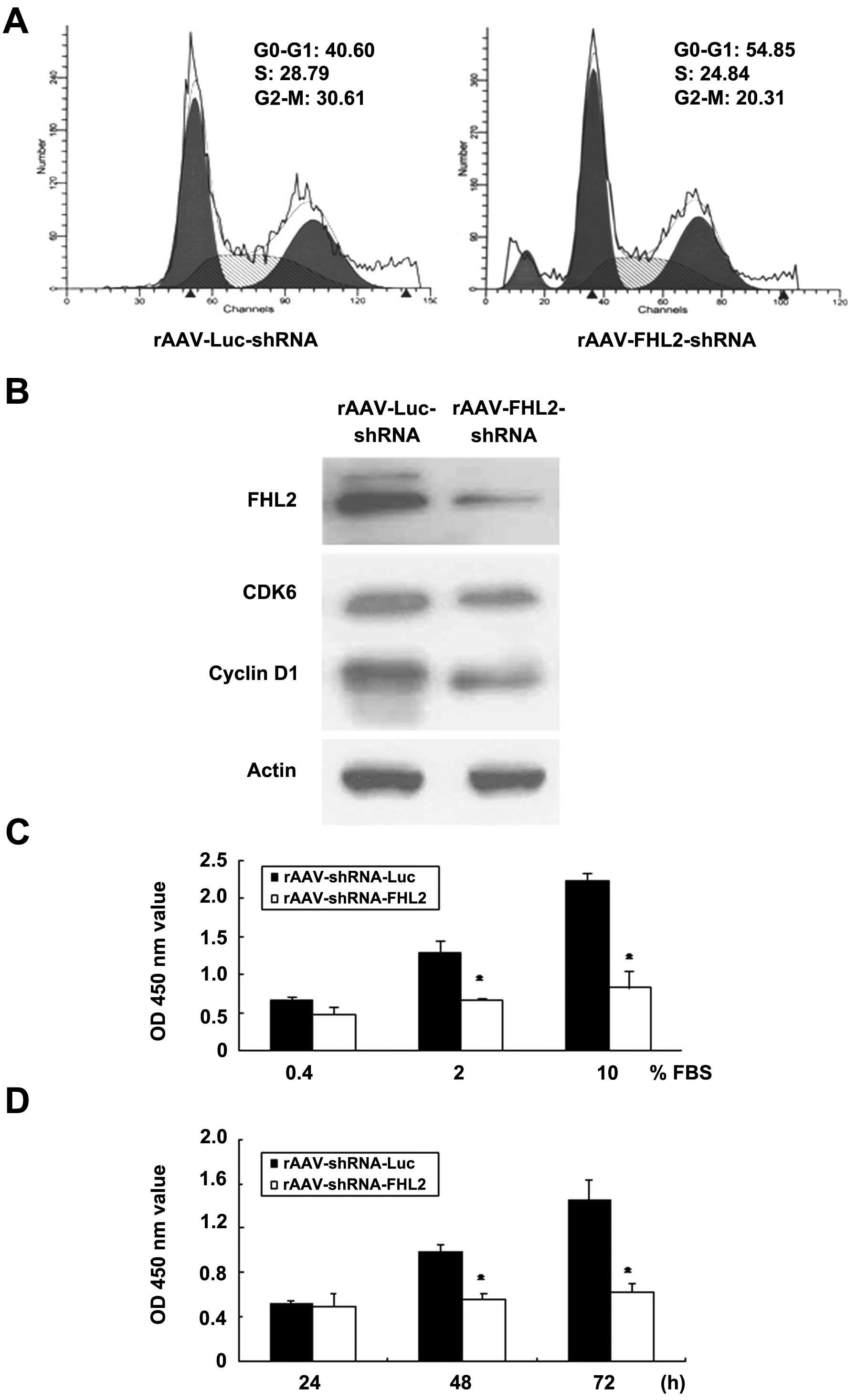
rAAV-FHL2-shRNA induces G0/G1 cell cycle
arrest
To further address effect of FHL2 on the cell cycle,
we employed rAAV vectors expressing either FHL2-shRNA or Luc-shRNA
to infect LoVo cells at a dose of 1×105 MOI. Flow
cytometry revealed that rAAV-FHL2-shRNA transduction led to
significant G0/G1 phase accumulation in LoVo cells (Fig. 2A).
The cyclin-dependent kinases together with the
cyclin D proteins are specifically involved in the progression of
cells through the G1 phase and the entry into the S phase. Here,
the protein expression of cyclin D1 and CDK6 was analyzed in total
protein lysates after transduced with FHL2-shRNA. Cyclin D1 and
CDK6 were decreased in rAAV-FHL2-shRNA compared with rAAV-Luc-shRNA
(Fig. 2B).
Ectopic expression of FHL2-shRNA
suppresses cell growth
WST-1 assay is commonly used to estimate cell
survival, growth, and differentiation (26). The cells with rAAV-Luc-shRNA was
considered as control, cultured in 0.4, 2.0 and 10% FBS for 72 h.
The OD 450 values of control group were 0.672±0.031, 1.284±0.162
and 2.236±0.091; whereas those of rAAV-FHL2-shRNA were 0.482±0.083,
0.662±0.028 and 0.826±0.209, respectively (Fig. 2C). Ectopic expression of
rAAV-FHL2-shRNA suppressed cell growth in a serum-dependent manner
(P<0.05).
We assessed the proliferation of rAAV-FHL2-shRNA
cells cultured in complete medium (5% FBS) for various time points.
The OD 450 of rAAV-Luc-shRNA were 0.522±0.022, 0.986±0.062 and
1.456±0.152% with 24, 48 and 72 h culture individually, whereas
those of rAAV-FHL2-shRNA cells were inhibited as 0.496±0.124,
0.562±0.041 and 0.62±0.084%, respectively (Fig. 2D, P<0.05).
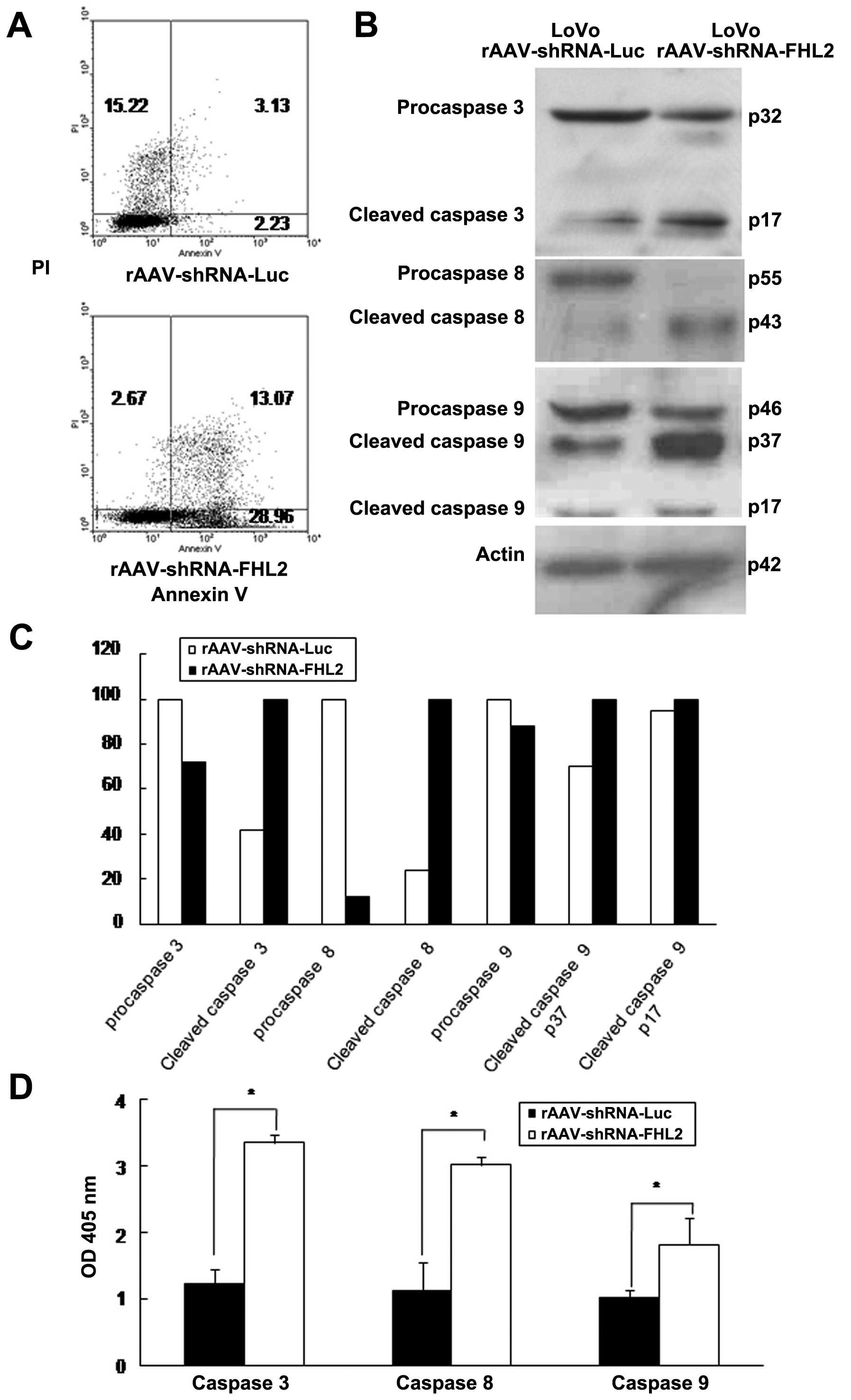
rAAV-FHL2-shRNA activates intrinsic and
extrinsic apoptotic pathways
To investigate the mechanism of rAAV-FHL2-shRNA
induced growth suppression, apoptosis was assayed by flow
cytometry. As shown in Fig. 3A,
rAAV-FHL2-shRNA induced more apoptosis than rAAV-Luc-shRNA in LoVo
cells, indicating that rAAV-FHL2-shRNA inhibits cell growth by
inducing apoptosis.
Caspases are essential in cells for apoptosis and
have been termed ‘executioner’ proteins for their roles (28,31–33).
rAAV-FHL2-shRNA activated caspases 3, 8 and 9 as evident by the
increasing protein level of cleaved caspases (Fig. 3B and C). Moreover, the activity of
caspases 3, 8 and 9 was analyzed after rAAV-shRNA-FHL2
transduction. The ratio between OD 405 nm of transfected cells and
OD 405 nm of parental cells was calculated. The activity of
caspases 3, 8 and 9 was significantly increased in rAAV-FHL2-shRNA
compared with rAAV-Luc-shRNA (Fig.
3D).
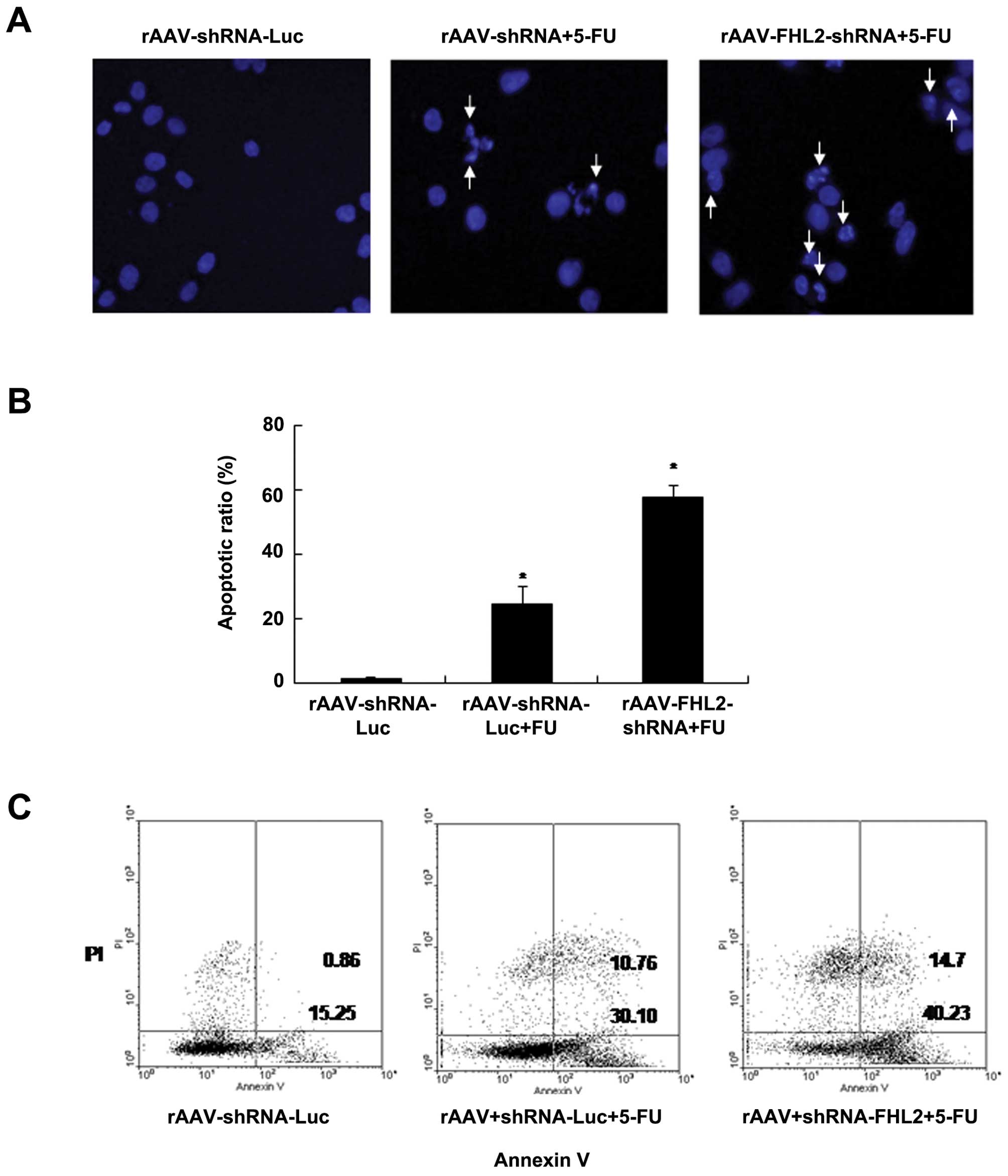
Suppression of rAAV-FHL2-shRNA increases
cell susceptibility to apoptotic stimuli
To evaluate the role of rAAV-FHL2-shRNA in
chemotherapy-induced apoptosis, the cells were transduced with
rAAV, treated or not with 5-FU (50 μg/ml in NS) (34), apoptotic morphological changes was
analyzed by staining with Hoechst 33258 blue fluorescence; a
brightly blue-fluorescent condensed nuclei and chromatin
fragmentation was considered as apoptosis by fluorescence
microscopy (Fig. 4A). Similarly,
the ratios of condensed nuclei positive cells were higher in
rAAV-FHL2-shRNA + 5-FU of LoVo cells comparing with the
rAAV-Luc-shRNA and rAAV-Luc-shRNA + 5-FU controls (P<0.05
comparing with rAAV-Luc-shRNA control) (Fig. 4B). Moreover, rAAV-Luc-shRNA and
rAAV-FHL2-shRNA cells were treated with 5-FU for 48 h (NS was used
as vehicle), double stained with Annexin V-FITC and PI, followed by
flow cytometry analysis to determine the apoptosis. As shown in
Fig. 4C, the apoptotic index of
rAAV-Luc-shRNA + 5-FU and rAAV-FHL2-shRNA + 5-FU was significantly
increased relative to rAAV-Luc-shRNA controls.
These findings suggested that rAAV-FHL2-shRNA
enhanced the susceptibility of cancer cells to apoptotic triggers
induced by 5-FU.
In vitro transduction of rAAV-FHL2-shRNA
inhibits tumor formation in vivo
Moreover, the antitumor effect of rAAV-FHL2-shRNA
and/or 5-FU in vivo was demonstrated by a xenograft model in
nude mice. LoVo cells were subcutaneously injected in the right
flanks of the nude mice. When the tumor nodules became visible
(about 3–5 mm in diameter), rAAV-Luc-shRNA or rAAV-FHL2-shRNA was
injected directly into the tumor and 5-FU was intraperitoneally
injected as a co-treatment. The tumor sizes were continuously
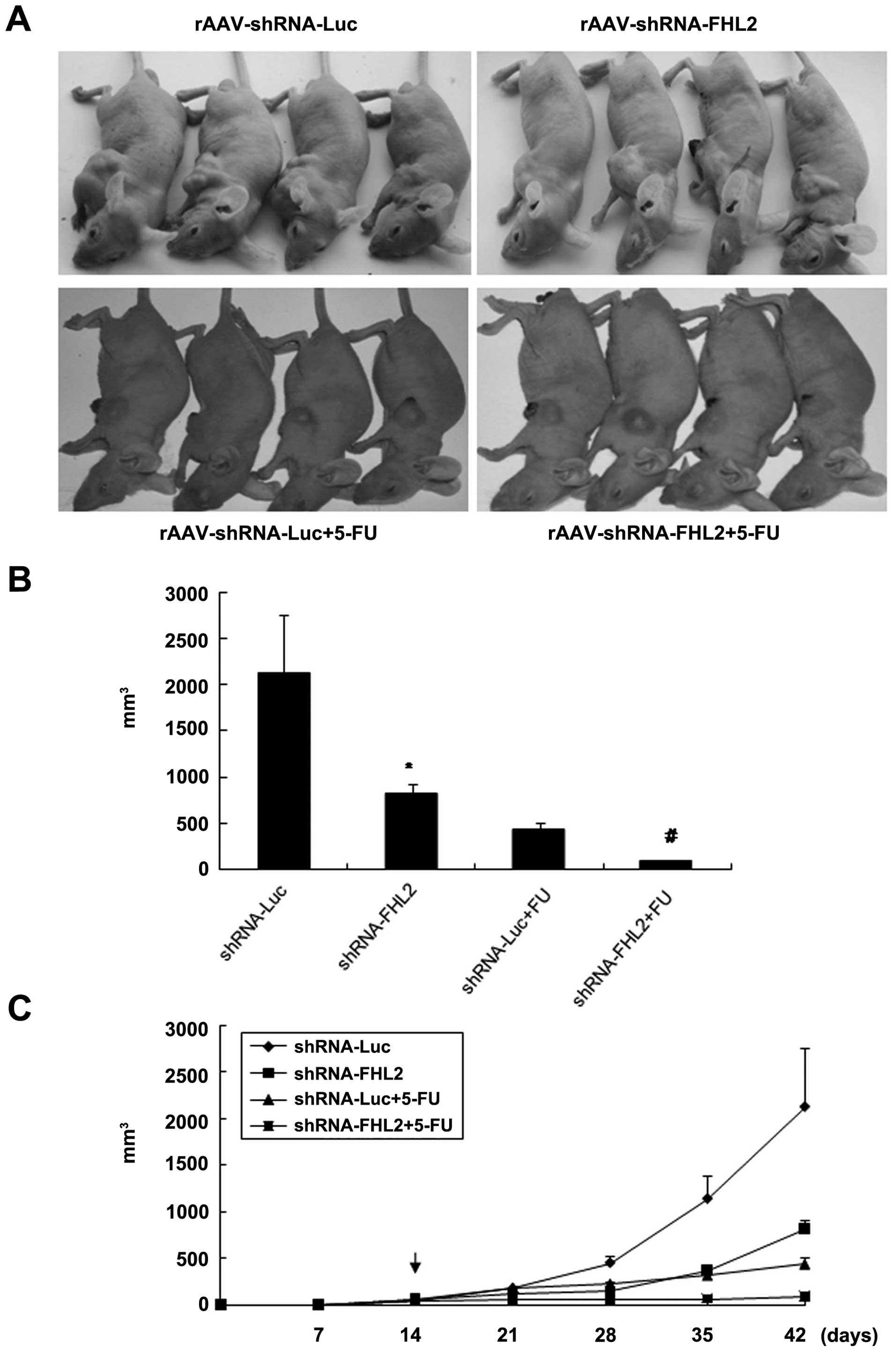
monitored on a weekly basis. As shown in Fig. 5, the tumor volumes of the
rAAV-FHL2-shRNA and rAAV-FHL2-shRNA + 5-FU treated mice were
markedly smaller than those of the rAAV-Luc-shRNA treated mice 4
weeks after rAAV injection. These data suggest that
rAAV-shRNA-FHL2-transduction with 5-FU treatment is sufficient to
suppress tumorigenesis.
Discussion
Identification of the differences in the genetics of
cancer cells with normal cells and exploration of cancer-associated
genes are important for the development of targeted therapies in
cancer treatment. Nude mice have been used extensively in cancer
research because they do not reject allografts and often do not
reject xenografts, making them an invaluable animal model to assess
the therapeutic effect of novel molecules before human clinical
trials. Here, we have demonstrated the anti-tumor efficacy of FHL2
inhibition by rAAV-shRNA in cells and nude mouse xenograft models
through inducing cell cycle arrest and apoptosis, yielding that
rAAV-shRNA-FHL2 might be a novel and potent therapeutic or 5-FU
co-therapeutic agent for colon cancer.
As shown in our previous study, FHL2 was an oncogene
in gastrointestinal cancers and inhibited cell differentiation and
induced tumorigenesis (19),
methods targeting FHL2 might be a promising strategy for the
treatment of GI cancers. AAV is a non-enveloped, single-strand DNA
virus, which belongs to the family Parvoviridae. AAV was used as
our gene delivery vector because it offered several advantages over
other delivery methods. First, it can infect a wide range of host
cells irrespective of their cell cycle stages. Second, it mediates
long-term gene expression. Third, when coupled to a strong promoter
like the hybrid CMV enhancer/chicken β-actin (CAG) promoter used in
this study, it is capable of delivering high levels of transgene
expression in a wide variety of cell types. For these reasons, AAV
carrying therapeutic genes like VEGF-Trap, AAV-hTERT-TRAIL and
endostatin have been employed in the treatment of malignant
glioblastoma, hepatic carcinoma and pancreatic cancer with varying
degree of success.
Before in vivo study, the antitumor effect of
rAAV-FHL2-shRNA was evaluated in vitro. Two key classes of
regulatory molecules, cyclins and cyclin-dependent kinases (CDKs),
determine a cell’s progress through the cell cycle. Consistent with
studies from other groups (17,23),
we found that rAAV-FHL2-shRNA inhibited cell cycle progression and
caused significant G0/G1 arrest by inhibiting cyclin D1 and CDK6 in
colon cancer cells (Fig. 2A and
B); rAAV-FHL2-shRNA induced G0/G1 arrest was also contributed
to the inhibition of cell proliferation time- and serum-dependently
(Fig. 2C and D). Next,
rAAV-FHL2-shRNA induced apoptosis was revealed by western blot
analysis and caspase activity assay (Fig. 3). In general, there are two
well-characteristic pathways of apoptosis that are termed
‘extrinsic pathway’ and ‘intrinsic pathway’. The former is
triggered by the interaction between the membrane death receptors
such as Fas, TNF-RI, DR3, DR4, DR5 and caspase 8 and their
respective ligands. Regarding the intrinsic pathway, also known as
the mitochondrial pathway, involves the increase of the
mitochondrial permeability and the release of apoptogenic molecules
such as cytochrome c and Smac/DIABLO from mitochondria into
cytosol, resulting in the activation of pro-caspase 9 and the
downstream caspases. Activity of caspases 3, 8 and 9 were
significantly higher in rAAV-FHL2-shRNA group, indicating that
apoptosis induced by FHL2 inhibition was through both intrinsic and
extrinsic pathway (31–33).
The effect of rAAV-FHL2-shRNA with or without
chemo-therapy 5-FU treatment on colon cancer was investigated in
vitro and in vivo (Figs.
4 and 5) (29,34).
Although few studies have shown the antitumor effect of FHL2
inhibition in different cancers, to the best of our knowledge there
are no reports regarding therapeutic value of rAAV-FHL2-shRNA in
colon cancer. Inhibition of FHL2 by rAAV-FHL2-shRNA, as a long-term
effective and specific targeting method, inhibited colon
tumorigenesis and enhanced 5-FU-induced apoptosis in vitro. In
vivo, it inhibited xenograft tumorigenesis and resulted in near
eradication of established colon cancer xeno-graft when combined
with 5-FU.
In conclusion, we have demonstrated that suppression
of FHL2 by rAAV-FHL2-shRNA decreases colon cancer cell growth and
cell cycle in vitro, and hinder tumor progression and
outright regression in combination with 5-FU in vivo.
Therefore, rAAV-FHL2-shRNA is potentially an important molecular
target for the design of novel anti-colon cancer therapy.
Abbreviations:
|
FHL2
|
four and a half LIM-only protein
2;
|
|
AAV
|
adeno-associated virus;
|
|
EGFP
|
enhanced green fluorescence
protein;
|
|
PI
|
propidium iodide
|
Acknowledgements
This study was supported the National
Natural Science Foundation of China (81172057 and 81272761),
‘President Foundation of Nanfang Hospital, Southern Medical
University’ (2012B009), a high-level topic matching funds of
Nanfang Hospital (2010036 and G201227).
References
|
1.
|
Samson T, Smyth N, Janetzky S, Wendler O,
Müller JM, Schüle R, von der Mark H, von der Mark K and Wixler V:
The LIM-only proteins FHL2 and FHL3 interact with alpha- and
beta-subunits of the muscle alpha7beta1 integrin receptor. J Biol
Chem. 279:28641–28652. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Labalette C, Nouet Y, Sobczak-Thepot J,
Armengol C, Levillayer F, Gendron MC, Renard CA, Regnault B, Chen
J, Buendia MA and Wei Y: The LIM-only protein FHL2 regulates cyclin
D1 expression and cell proliferation. J Biol Chem. 283:15201–15208.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Chan KK, Tsui SK, Lee SM, Luk SC, Liew CC,
Fung KP, Waye MM and Lee CY: Molecular cloning and characterization
of FHL2, a novel LIM domain protein preferentially expressed in
human heart. Gene. 210:345–350. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Sanchez-Garcia I, Osada H, Forster A and
Rabbitts TH: The cysteine-rich LIM domains inhibit DNA binding by
the associated homeodomain in Isl-1. EMBO J. 12:4243–4250.
1993.PubMed/NCBI
|
|
5.
|
Bach I: The LIM domain: regulation by
association. Mech Dev. 91:5–17. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Johannessen M, Moller S, Hansen T, Moens U
and Van Ghelue M: The multifunctional roles of the
four-and-a-half-LIM only protein FHL2. Cell Mol Life Sci.
63:268–284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Morlon PA and Sassone-Corsi P: The
LIM-only protein FHL2 is a serum inducible transcriptional
coactivator of AP-1. Proc Natl Acad Sci USA. 100:3977–3982. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Fimia GM, Cesare DDe and Sassone-Corsi P:
A family of LIM-only transcriptional coactivators: tissue-specific
expression and selective activation of CREB and CREM. Mol Cell
Biol. 20:8613–8622. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Yan J, Zhu J, Zhong H, Lu Q, Huang C and
Ye Q: BRCA1 interacts with FHL2 and enhances FHL2 transactivation
function. FEBS Lett. 553:183–189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Du X, Hublitz P, Günther T, Wilhelm D,
Englert C and Schüle R: The LIM-only coactivator FHL2 modulates WT1
transcriptional activity during gonadal differentiation. Biochim
Biophys Acta. 1577:93–101. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Stilo R, Leonardi A, Formisano L, Jeso B
Di, Vito P and Liguoro D: TUCAN/CARDINAL and DRAL participate in a
common pathway for modulation of NF-kappaB activation. FEBS Lett.
521:165–169. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Purcell NH, Darwis D, Bueno OF, Müller JM,
Schüle R and Molkentin JD: Extracellular signal-regulated kinase 2
interacts with and is negatively regulated by the LIM-only protein
FHL2 in cardiomyocytes. Mol Cell Biol. 24:1081–1095. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
McLoughlin P, Ehler E, Carlile G, Licht JD
and Schafer BW: The LIM only protein DRAL/FHL2 interacts with and
is a corepressor for the promyelocytic leukemia zinc finger
protein. J Biol Chem. 277:37045–37053. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Philippar U, Schratt G, Dieterich C,
Müller JM, Galgóczy P, Engel FB, Keating MT, Gertler F, Schüle R,
Vingron M and Nordheim A: The SRF target gene Fhl2 antagonizes
RhoA/MAL-dependent activation of SRF. Mol Cell. 16:867–880. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yang Y, Hou H, Haller EM, Nicosia SV and
Bai W: Suppression of FOXO1 activity by FHL2 through SIRT1-mediated
deacetylation. EMBO J. 24:1021–1032. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Genini M, Schwalbe P, Scholl FA, Remppis
A, Mattei MG and Schafer BW: Subtractive cloning and
characterization of DRAL, a novel LIM-domain protein down-regulated
in rhabdomyosarcoma. DNA Cell Biol. 16:433–442. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Martin BT, Kleiber K, Wixler V, Raab M,
Zimmer B, Kaufmann M and Strebhardt K: FHL2 regulates cell
cycle-dependent and doxorubicininduced p21Cip1/Waf1 expression in
breast cancer cells. Cell Cycle. 6:1779–1788. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Kinoshita M, Nakagawa T, Shimizu A and
Katsuoka Y: Differently regulated androgen receptor transcriptional
complex in prostate cancer compared with normal prostate. Int J
Urol. 12:390–397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Wang J, Yang Y, Xia HH, Gu Q, Lin MC,
Jiang B, Peng Y, Li G, An X, Zhang Y, Zhuang Z, Zhang Z, Kung HF
and Wong BC: Suppression of FHL2 expression induces cell
differentiation and inhibits gastric and colon carcinogenesis.
Gastroenterology. 132:1066–1076. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Li M, Wang J, Ng SS, Chan CY, Chen AC, Xia
HP, Yew DT, Wong BC, Chen Z, Kung HF and Lin MC: The
four-and-a-half-LIM protein 2 (FHL2) is overexpressed in gliomas
and associated with oncogenic activities. Glia. 56:1328–1338. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ding L, Wang Z, Yan J, Yang X, Liu A, Qiu
W, Zhu J, Han J, Zhang H, Lin J, Cheng L, Qin X, Niu C, Yuan B,
Wang X, Zhu C, Zhou Y, Li J, Song H, Huang C and Ye Q: Human
four-and-a-half LIM family members suppress tumor cell growth
through a TGFbeta-like signaling pathway. J Clin Invest.
119:349–361. 2009.PubMed/NCBI
|
|
22.
|
Scholl FA, McLoughlin P, Ehler E, de
Giovanni C and Schafer BW: DRAL is a p53-responsive gene whose four
and a half LIM domain protein product induces apoptosis. J Cell
Biol. 151:495–506. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Amann T, Egle Y, Bosserhoff AK and
Hellerbrand C: FHL2 suppresses growth and differentiation of the
colon cancer cell line HT-29. Oncol Rep. 23:1669–1674.
2010.PubMed/NCBI
|
|
24.
|
Zhang W, Jiang B, Guo Z, Sardet C, Zou B,
Lam CS, Li J, He M, Lan HY, Pang R, Hung IF, Tan VP, Wang J and
Wong BC: Four and a half LIM protein 2 (FHL2) promotes invasive
potential and epithelial-mesenchymal transition in colon cancer.
Carcinogenesis. 31:1220–1229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Zhang W, Wang J, Zou B, Sardet C, Li J,
Lam CS, Ng L, Pang R, Hung IF, Tan VP, Jiang B and Wong BC: Four
and a half LIM protein 2 (FHL2) negatively regulates the
transcription of E-cadherin through interaction with Snail1. Eur J
Cancer. 47:121–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Takada M, Nakamura Y, Koizumi T, Toyama H,
Kamigaki T, Suzuki Y, Takeyama Y and Kuroda Y: Suppression of human
pancreatic carcinoma cell growth and invasion by
epigallocatechin-3-gallate. Pancreas. 25:45–48. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Ewald JA, Desotelle JA, Church DR, Yang B,
Huang W, Laurila TA and Jarrard DF: Androgen deprivation induces
senescence characteristics in prostate cancer cells in vitro and in
vivo. Prostatex. 73:337–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Kar S, Palit S, Ball WB and Das PK:
Carnosic acid modulates Akt/IKK/NF-κB signaling by PP2A and induces
intrinsic and extrinsic pathway mediated apoptosis in human
prostate carcinoma PC-3 cells. Apoptosis. 7:35–47. 2012.PubMed/NCBI
|
|
29.
|
Chao TC and Greager JA: Experimental
pulmonary sarcoma metastases in athymic nude mice. J Surg Oncol.
65:123–126. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Wendtner CM, Kofler DM, Theiss HD,
Kurzeder C, Buhmann R, Schweighofer C, Perabo L, Danhauser-Riedl S,
Baumert J, Hiddemann W, Hallek M and Büning H: Efficient gene
transfer of CD40 ligand into primary B-CLL cells using recombinant
adeno-associated virus (rAAV) vectors. Blood. 100:1655–1661.
2002.PubMed/NCBI
|
|
31.
|
Chatfield K and Eastman A: Inhibitors of
protein phosphatases 1 and 2A differentially prevent intrinsic and
extrinsic apoptosis pathways. Biochem Biophys Res Commun.
323:1313–1320. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Mühlethaler-Mottet A, Bourloud KB,
Auderset K, Joseph JM and Gross N: Drug-mediated sensitization to
TRAIL-induced apoptosis in caspase-8-complemented neuroblastoma
cells proceeds via activation of intrinsic and extrinsic pathways
and caspase-dependent cleavage of XIAP, Bcl-xL and RIP. Oncogene.
23:5415–5425. 2004.
|
|
33.
|
Obexer P, Geiger K, Ambros PF, Meister B
and Ausserlechner MJ: FKHRL1-mediated expression of Noxa and Bim
induces apoptosis via the mitochondria in neuroblastoma cells. Cell
Death Differ. 14:534–547. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Guo XL, Li D, Hu F, Song JR, Zhang SS,
Deng WJ, Sun K, Zhao QD, Xie XQ, Song YJ, Wu MC and Wei LX:
Targeting autophagy potentiates chemotherapy-induced apoptosis and
proliferation inhibition in hepatocarcinoma cells. Cancer Lett.
320:171–179. 2012. View Article : Google Scholar
|