Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common cancer and the third most common cause of cancer-related
mortality worldwide, which is often associated with longstanding
chronic liver inflammation and cirrhosis (1–3).
Conventional chemotherapy is one of the common strategies in HCC
treatment (4). However,
chemotherapeutic drugs are often accompanied by multiple side
effects. In the tradition of eastern medicine, natural drugs have
made a significant contribution to the treatment of liver diseases.
Chinese herbal medicines are serving as a rich source for
discovering new therapeutic agents in tumor because of their
effectiveness and low side effects (5,6).
Therefore, the search for new effective agents possibly from
natural herbs with the ability of directly inhibit HCC cell
proliferation or to induce HCC cells apoptosis is urgent.
Paeonia lactiflora pall root, a traditional
Chinese herb, was recognized as a component of effective
prescriptions for treatment of liver disease (7). Total glucosides of peony (TGP) which
consists of paeoniflorin, albiflorin, benzoylpaeoni-florin,
oxypaeoniflorin, paeonin, etc., extracted from Paeonia
lactiflora pall root, has been recognized as the effective
herbs which have been used in the treatment of hepatitis and other
chronic liver disease (8). The
anti-inflammatory, anti-oxidative, anti-hepatic injury and
immunoregulatory activities of TGP have been extensively proved by
our laboratory for several years (8,9). Our
previous studies have demonstrated that TGP could significantly
inhibit proliferation of human hepatoma cells (10,11).
In addition, the main bioactive components of TGP, paeoniflorin,
has been reported to exert antitumor activity against a number of
malignancies including non-small cell lung cancer, gastric cancer,
leukemia, and cervical cancer (12–14).
Astragalus membranaceus, another popular traditional Chinese
herb, exhibited significant growth-inhibitory, proapoptotic and
angiogenesis-suppression effects in human cancer cells in
pharmacological experiments and relevant clinical reports (15,16).
In vitro studies of our laboratory demonstrated that
Radix Astragali, its extracts, as well as its active
ingredients such as astragalosides, significantly induced apoptosis
and inhibited invasion of tumor cells (17,18).
In traditional oriental medicine, in order to achieve a variety of
treatments simultaneously, or to enhance a single effect without
causing severe side effects, a combination of herbs is
conventionally used (19). In
order to obtain a more effective remedy for the treatment of HCC
other than exclusively using Paeonia lactiflora or
Astragalus membranaceus, we combined these two herbs based
on both traditional literature and the results of our previous
study mentioned above. The standardized extract from Paeonia
lactiflora and Astragalus membranaceus (PAE), with a
standard ratio of 4:1 of these two plants, was mainly composed of
TGP and the total astragalosides (20). Our previous animal studies have
confirmed that PAE exhibited the most significant hepato-protective
activity compared to exclusively using Paeonia lactiflora or
Astragalus membranaceus (21). Our previous studies have shown that
PAE had obvious protective effects on acute liver injury and
chronic liver fibrosis induced by carbon tetrachloride (CCl4)
(20,22). These above studies have clearly
demonstrated that PAE is an effective and therapeutic agent for
chronic liver disease.
HCC is one of the main complications of chronic
liver diseases. HCC mainly developed in the background of advanced
liver fibrosis and cirrhosis through the malignant transformation
of cirrhotic nodules and premalignant lesions (23). The usual multistep process of
fibrosis-cirrhosis-HCC, has been recognized as the significant
progresses of HCC (24). Previous
research in our laboratory has evidenced that PAE possessed a
distinct inhibitory effect on hepatic fibrosis. The data obtained
from our previous studies created considerable interest in PAE as a
therapeutic agent in HCC.
In the present study, to determine the effect of PAE
on HCC cell proliferation, apoptosis, migration and invasion, in
cultured human HCC cell line HepG2 and SMMC-7721 as a model system
and we analysed the changes after treatment with PAE. The results
of this study show that PAE revealed obviously pro-apoptosis and
inhibitory effect on proliferation, migration and invasion of HCC
cells. These data suggested that PAE may be a novel, efficient
candidate agent for treatment of HCC.
Materials and methods
Preparation of PAE
Radix Paeonia lactiflora and Radix
Astragali were purchased from Hefei Heyitang Pharmacy, China.
Voucher specimens (Pan 2009002 and Pan 2009013) were identified and
deposited in relevant departments of Anhui College of Traditional
Chinese Medicine. The sliced dried roots of Radix Paeonia
lactiflora and Radix Astragali were mixed in the ratio
of 4:1. The mixed roots (25 kg) were extracted two times with 70%
aqueous ethanol, six times 1.5 h each time. Then, the extracts were
combined and evaporated to dry powder under reduced pressure. The
residue obtained from the combined extracts was dissolved in 8 l of
water. After filtration, the aqueous solution was extracted three
times with 8 l water-saturated n-butanol successively each
time, which yielded dry powder after being combined and evaporated
to dryness under reduced pressure. Then, ∼1 kg of the
n-butanol extract was chromatographed on polystryrene resin
(D101, 0.3–1.25 mm, Nankai Chemical Factory, Tianjin, China) with
water, 10% ethanol and 70% ethanol, respectively. Portions of 70%
ethanol were collected and evaporated to dryness under reduced
pressure, which yielded 310 g of yellow dry powder. The result of
chromatometry showed that the dry powder contained 74.7% total
glycoside of paeony (TGP) and 20.6% astragalosides (ASTs). TGP
consist mainly of paeoniflorin, oxypaeoniflorin, benzoyl
paeoniflorin, albiflorin and lactiflorin (25). ASTs consist of astragaloside I–IV
and aoyasapogenoside (20). Before
treatment on HCC cells, PAE was suspended in phosphate-buffered
saline (PBS).
Chemicals and reagent
MTT was purchased from Sigma Chemical Co. (St.
Louis, MO, USA). Cell Apoptosis PI Detection Kit was provided by
Nanjing KeyGen Biotech Co. Ltd. (Nanjing, China). Bcl-2, Bax and
cleaved caspase-3 primary antibody were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Dulbecco’s modified Eagle’s
medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco
Co. (CA, USA). BD Matrigel™ Basement Membrane Matirx was purchased
from BD Biosciences (Bedford, MA, USA). Other chemicals used in the
experiment were analytical grade from commercial sources.
Cell culture
Human HCC cell line HepG2 and SMMC-7721 was
purchased from Shanghai Cell Bank, Chinese Academy of Sciences
(Shanghai, China), cultured in DMEM supplemented with 10% (v/v) FBS
and 100 U/ml of penicillin and 100 μg/ml of streptomycin.
HepG2 and SMMC-7721 cells were maintained at 37°C in a 5%
CO2 atmosphere.
Cell proliferation assay
MTT assay was performed to determine the number of
viable cells. The cells were cultured in 96-well plates at a
density of 1×104 cells per well with fresh medium
containing PAE at the various concentrations (12.5, 25, 50, 100 and
200 mg/l). After treatment for 24, 48 or 72 h, MTT solution was
added (20.0 μl/well) and then the plates were incubated at
37°C in 5% CO2 for another 4 h. The MTT-formazan product
were dissolved in 150 μl dimethyl sulfoxide (DMSO) per well.
After 10 min, the plates were read on BioTek Elxx808 microplate
reader (Winooski, VT, USA) at 490 nm.
Flow cytometry analysis
HepG2 and SMMC-7721 cells (1×106 cells
per well) were plated in 6-well plates and treated with PAE (12.5,
25, 50, 100 and 200 mg/l) for 48 h. After being harvested by
trypsinization, the cells were washed twice with cold PBS and
centrifuged. The cell pellet was resuspended in 1 ml cold PBS and
fixed in 9 ml of 70% ethanol at −20°C for ≥12 h. RNase was added
and the cells incubated at 37°C for 30 min then centrifuged and
resuspended in 500 μl PBS. Next, propidium iodide (PI)
staining buffer was added, and left in the dark at room temperature
for 30 min. The cells were collected and apoptosis was examined by
FC 500 (Beckman Coulter, Brea, CA, USA).
Immunocytochemistry analysis
Cells were seeded onto glass coverslips overnight.
After incubation with various concentrations of PAE for 48 h, the
coverslips were washed twice with PBS and 4% paraformaldehyde
solution was added for 30 min at room temperature.
Immunocytochemistry staining for Bcl-2 and Bax was performed
according to the manufacturer’s instructions (Zhongshan Jinqiao,
Beijing, China). PBS was used as a negative control instead of the
primary antibody. The results were quantitatively analyzed by
Olympus BX53F video microscope (Olympus, Tokyo, Japan) and the
Image-Pro Plus software (Media Cybernetics, Rockville, MD, USA) in
five random fields per slide. The relative intensity of Bcl-2 and
Bax were reflected by optical density value.
Western blot analysis
HepG2 and SMMC-7721 cells were treated with PAE at
various gradient concentrations. Then protein was extracted from
cells in RIPA lysis buffer [50 mmol/l Tris-HCl, pH 7.4, 150 mmol/l
NaCl, 10 mmol/l phenylmethylsulfonyl fluoride (PMSF), 1 mmol/l
ethylene diamine tetraacetic acid (EDTA), 0.1% sodium dodecyl
sulfate (SDS), 1% Triton X-100, 1% sodium deoxycholate]. The
protein concentration was determined with the Bradford assay. A
protein sample was mixed with the 5X sample buffer (4:1) (Bio-Rad,
Hercules, CA, USA) and heated in boiling water for 10 min. The
proteins were resolved by SDS-PAGE, transferred to a polyvinylidene
fluoride (PVDF) membrane (Millipore, Bedford, MA, USA). After
blocking with 5% non-fat milk (blocking solution) at room
temperature, the membrane was incubated with primary antibodies
diluted in blocking solution overnight at 4°C. After washing the
blot in TBST three times, the horseradish peroxidase
(HRP)-conjugated secondary antibodies were applied for 2 h at room
temperature. After extensive washing in TBST, immunodetection was
visualized by enhanced chemiluminescence (Pierce, Rockford, IL,
USA) using hydrogen peroxide and luminol as substrates.
Autoradiographs were scanned using Image Quant LAS 4000 mini (GE
Healthcare Bio-Sciences AB, Uppsala, Sweden). The density of the
specific bands was quantified using ImageJ software (National
Institutes of Health, Bethesda, MD, USA).
Wound healing assay
HepG2 and SMMC-7721 cells were seeded into 24-well
plates, at 80–90% confluency, the cell monolayer was wounded with a
200 μl-pipette. After washing with PBS three times to remove
cell debris, the remaining cells were treated with 12.5, 25, 50,
100 or 200 mg/l PAE and then images were captured by microscope at
0 and 24 h after treatment. Cell motility was evaluated according
to the following formula: Cell motility = (distance 24 h − distance
0 h)/distance 0 h.
Matrigel Transwell assay
The invasion assay was performed using Transwell
co-culture chambers (24 wells, 8-μm pore size, Costar,
Corning Inc., NY, USA). In brief, upper inserts were coated with 30
μl Matrigel (1:4 in dilution; BD Biosciences) and then to
set for 0.5 h at 37°C. SMMC-7721 cells (5×105) were
resuspended in 100 μl serum-free DMEM medium containing 0.1%
BSA and were added into the upper insert, while in the lower
chamber containing DMEM (600 μl) with 10% FBS as a
chemoattractant. Then DMEM medium free of FBS added 12.5, 25, 50,
100 or 200 mg/l PAE in the upper chamber. After 36-h incubation at
37°C, the SMMC-7721 cells in the upper chambers were scraped off
carefully with swabs; the invaded cells were fixed and stained with
0.1% crystal violet. Following air-drying, the invaded SMMC-7721
cells were counted in 5 random visual fields (×400) for each
insert.
Statistical analysis
Data are expressed as the means ± SD and statistical
analysis was performed using one-way ANOVA. All statistical
analyses were performed with the statistical package SPSS 13.0
(SPSS Inc, Chicago, IL, USA). A level of P<0.05 was considered
statistically significant.
Results
Inhibitory activity of PAE on hepatoma
cell proliferation
To determine the role of PAE in the proliferation of
HepG2 and SMMC-7721 cells, cells were incubated with various
concentrations of PAE for 24, 48 or 72 h. As shown in Table I, the inhibitory rate of PAE (12.5,
25, 50, 100 and 200 mg/l) on HepG2 cells was 6.39, 10.66, 24.53,
41.69 and 63.21% and the inhibitory rate of PAE on SMMC-7721 cells
was 2.81, 13.61, 33.66, 41.73 and 68.33% (Table II) after treatment for 48 h. The
MTT experiment confirmed that hepatoma cells were sensitive to PAE
and PAE displayed an inhibitory effect on the proliferation of
human hepatoma cells.
 | Table I.Effect of PAE on the proliferation of
HepG2 cells (x̄±s, n=8). |
Table I.
Effect of PAE on the proliferation of
HepG2 cells (x̄±s, n=8).
| | 24 h | 48 h | 72 h |
|---|
|
|
|
|---|
| Group | Concentration
(mg/l) |
A490nm | Inhibitory rate
(%) |
A490nm | Inhibitory rate
(%) |
A490nm | Inhibitory rate
(%) |
|---|
| Control PAE | - | 0.451±0.025 | - | 0.530±0.019 | - | 0.937±0.012 | - |
| 12.5 | 0.430±0.017 | 4.66 | 0.496±0.035 | 6.39 | 0.824±0.050 | 11.96 |
| 25 | 0.413±0.042 | 8.39 | 0.473±0.021 | 10.66 | 0.738±0.033a | 21.19 |
| 50 | 0.372±0.040a | 17.52 | 0.399±0.051b | 24.53 | 0.724±0.029a | 22.73 |
| 100 | 0.282±0.026b | 37.49 | 0.309±0.023b | 41.69 | 0.559±0.015b | 40.32 |
| 200 | 0.203±0.022b | 55.03 | 0.195±0.048b | 63.21 | 0.445±0.043b | 52.56 |
 | Table II.Effect of PAE on the proliferation of
SMMC-7721 cells (x̄±s, n=8). |
Table II.
Effect of PAE on the proliferation of
SMMC-7721 cells (x̄±s, n=8).
| | 24 h | 48 h | 72 h |
|---|
|
|
|
|---|
| Group | Concentration
(mg/l) |
A490nm | Inhibitory rate
(%) |
A490nm | Inhibitory rate
(%) |
A490nm | Inhibitory rate
(%) |
|---|
| Control PAE | - | 0.467±0.022 | - | 0.498±0.021 | - | 0.992±0.052 | - |
| 12.5 | 0.437±0.027 | 6.28 | 0.484±0.047 | 2.81 | 0.975±0.050 | 1.65 |
| 25 | 0.419±0.035 | 10.27 | 0.430±0.042a | 13.61 | 0.843±0.048a | 14.96 |
| 50 | 0.389±0.027a | 16.64 | 0.330±0.030b | 33.66 | 0.672±0.015b | 32.23 |
| 100 | 0.248±0.032b | 46.94 | 0.290±0.036b | 41.73 | 0.612±0.041b | 38.26 |
| 200 | 0.170±0.022b | 63.68 | 0.158±0.025b | 68.33 | 0.492±0.033b | 50.41 |
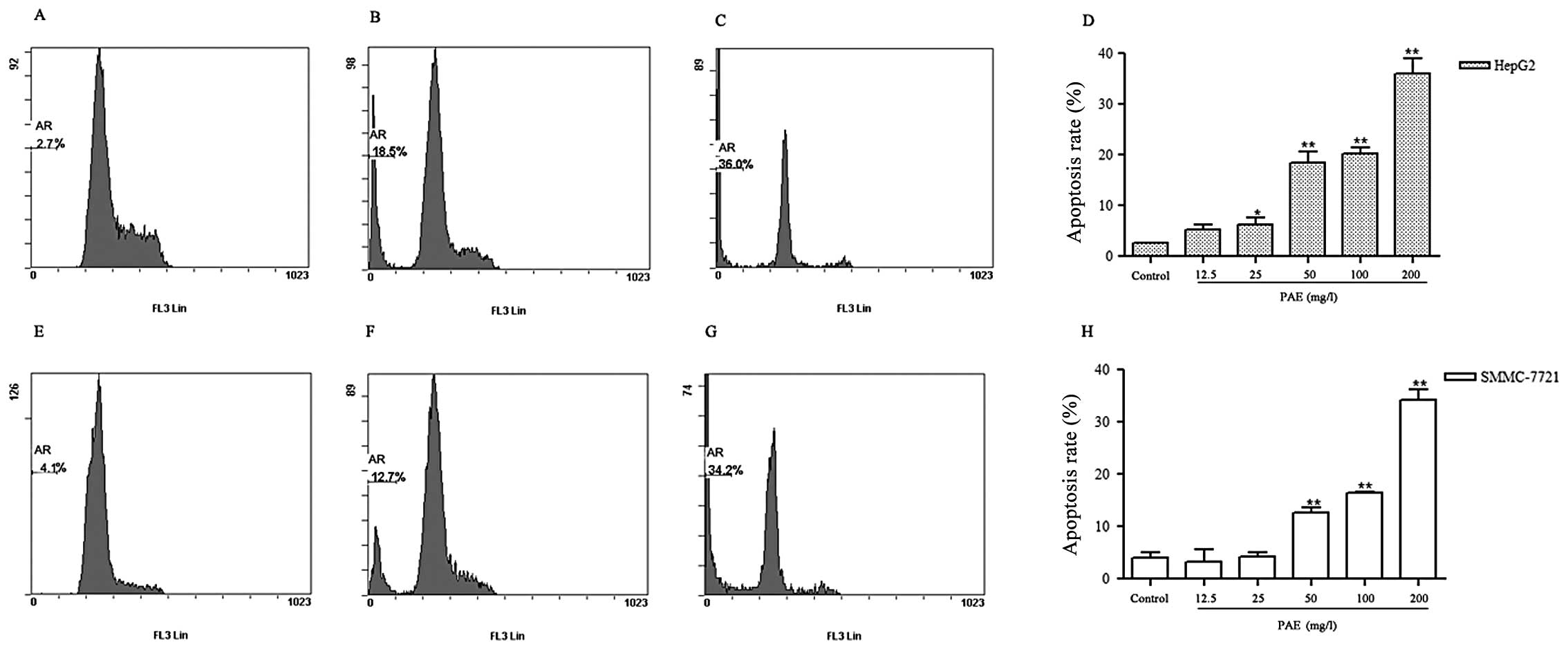
PAE induces apoptosis of HepG2 and
SMMC-7721 cells
To determine whether PAE induced apoptosis of HepG2
and SMMC-7721 cells, a Cell Apoptosis PI Detection kit was applied
by flow cytometry. As shown in Fig.
1, the percentage of apoptotic cells increased from 2.7% in
control treated cells to 36.0% in PAE 200 mg/l treatment group of
HepG2 cells (Fig. 1C). Similar
results were observed in SMMC-7721 cells. PAE 200 mg/l treatment
increased the percentage of apoptotic cells by 34.2% in SMMC-7721
cells (Fig. 1G). These results
showed that PAE significantly induced apoptosis of hepatoma
cells.
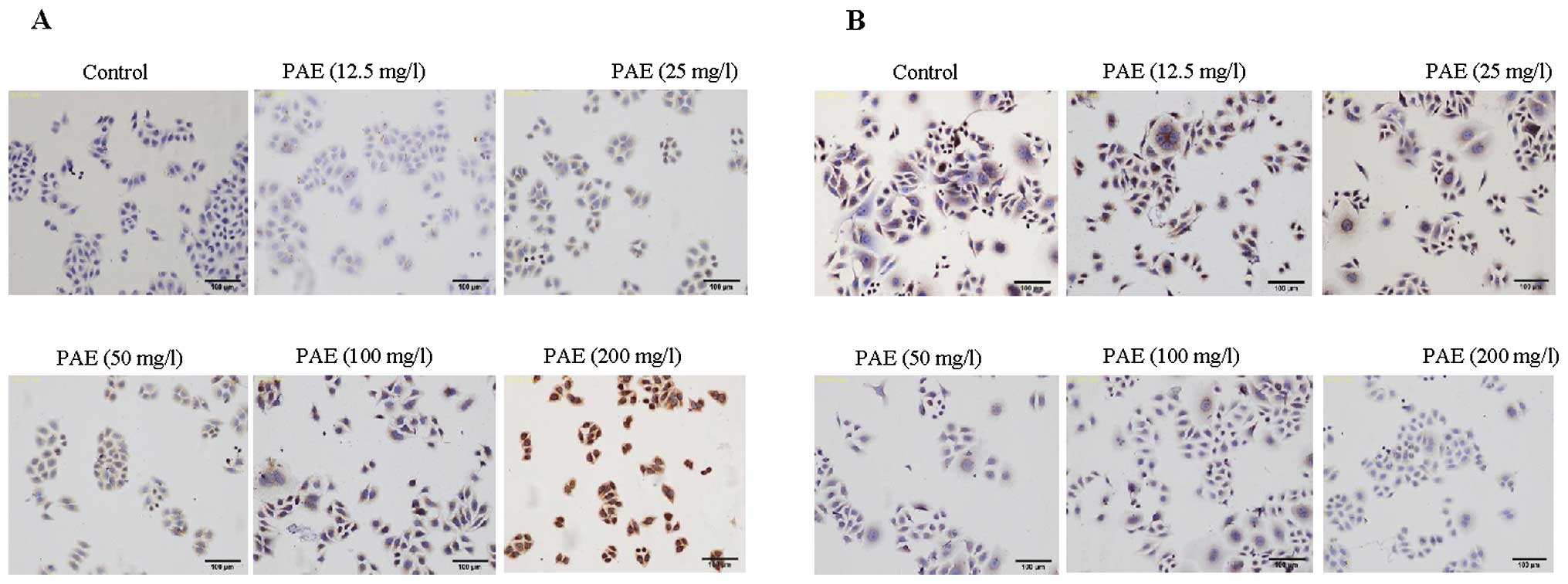
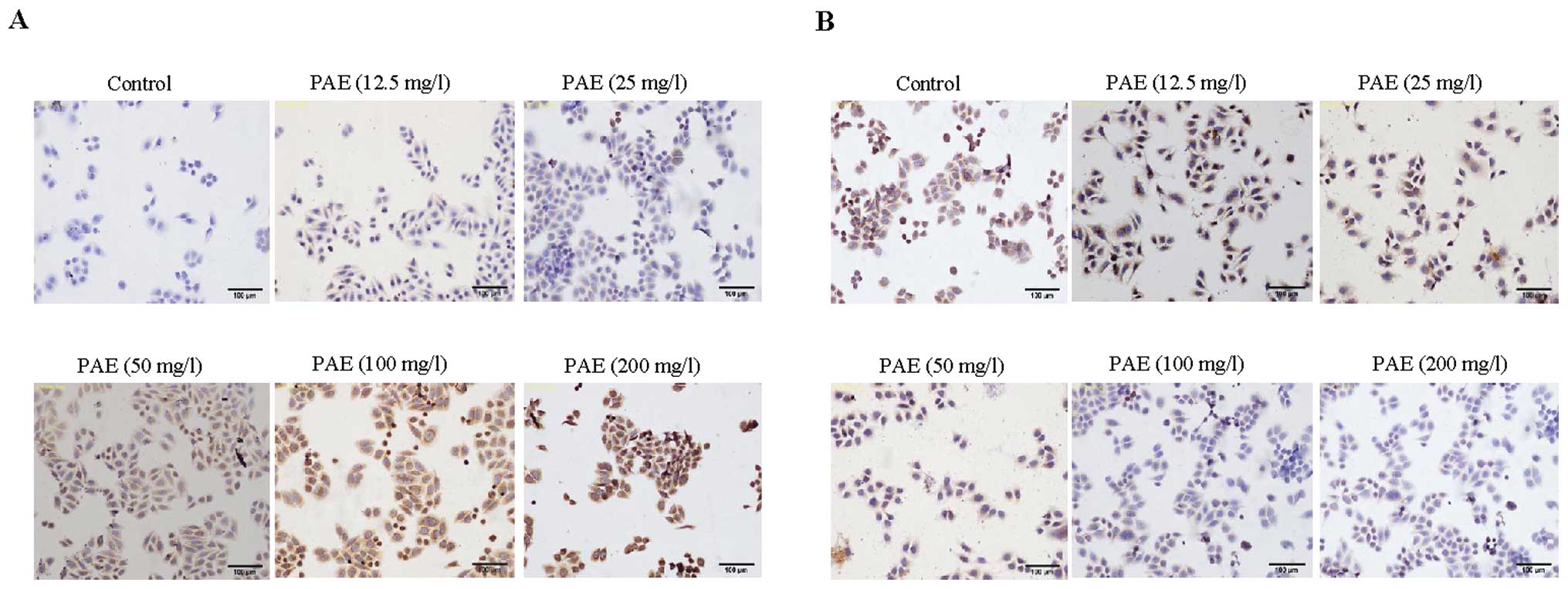
PAE modulates the expression of Bcl-2 and
Bax in HepG2 and SMMC-7721 cells
To identify a possible mechanism by which PAE
induced apoptosis in hepatoma cells, immunocytochemistry was
performed. Our data demonstrated that morphological changes
occurred during PAE exposure in HepG2 and SMMC-7721 cells (Figs. 2 and 3). PAE exposure-induced apoptosis in the
hepatoma cells was identified by cell shrinkage and pyknosis. Cell
shrinkage leads to smaller cells and the cytoplasm was dense and
the organelles were more tightly packed. In addition, Bax staining
was observed with PAE treatment at 12.5, 25, 50, 100 and 200 mg/l.
Bax expression was the most prominent after treatment with 200 mg/l
PAE. The expression of Bax in the control group presented weak
staining in the cytoplasm (Figs.
2A and 3A). However, the
expression of Bcl-2 presented strong dark brown staining in the
control group (Figs. 2B and
3B). With concentrations of 50,
100 and 200 mg/l PAE the expression of Bcl-2 presented weak brown
staining or lack of staining.
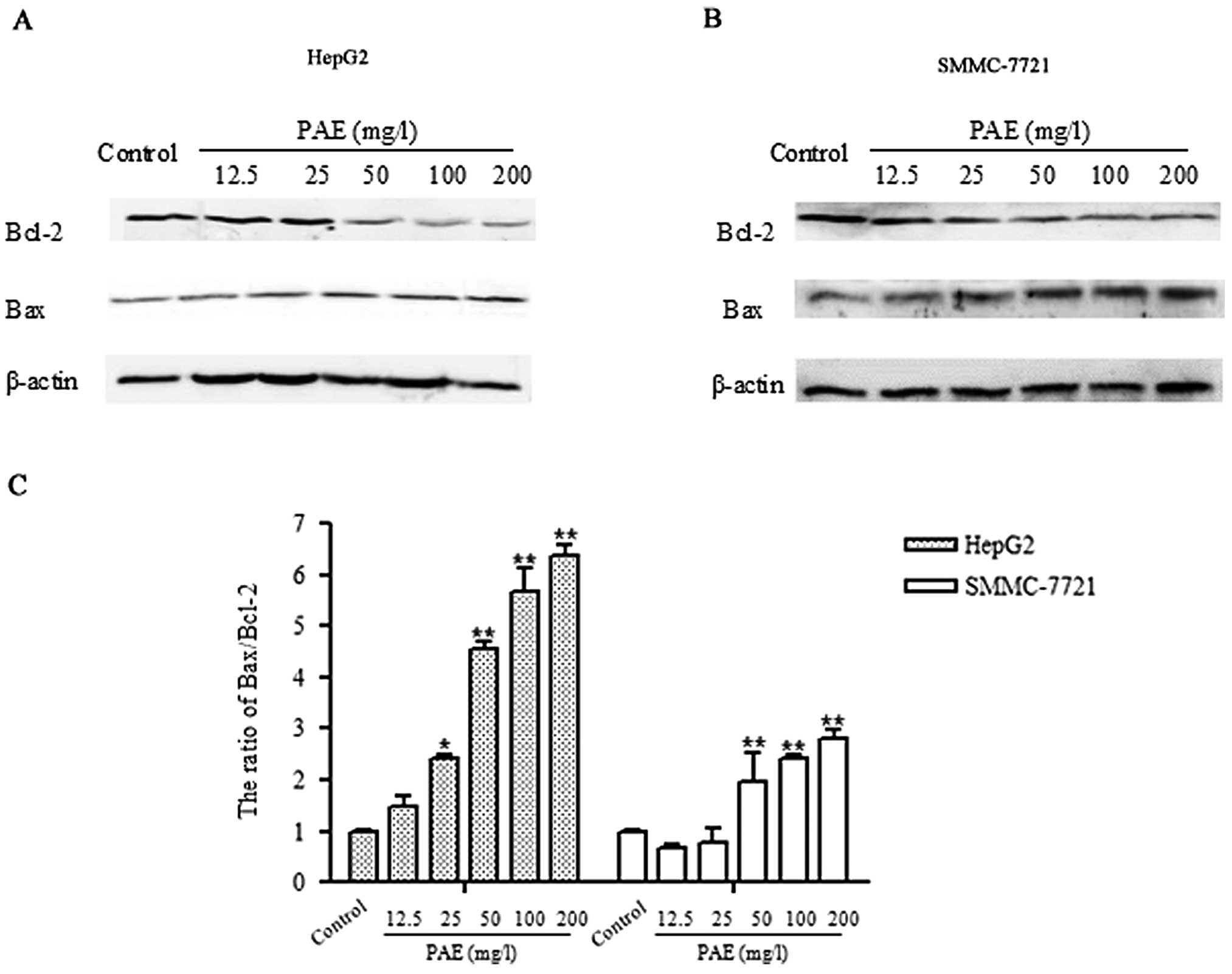
Consistent with immunocytochemistry results, western
blot analysis showed decreased expression of Bcl-2 in PAE-treated
hepatoma cells compared to control group. PAE at concentrations of
50, 100 and 200 mg/l markedly inhibited Bcl-2 expression. However,
the expression of Bax was elevated in various concentrations of PAE
(50, 100 and 200 mg/l) treated cells compared to the untreated
control cells. As shown in Fig.
4C, the Bax to Bcl-2 ratio was significantly increased in the
PAE treated cells compared to the control group. The results
indicate that PAE induces apoptosis in HepG2 and SMMC-7721 cells
mainly through regulating the Bcl-2 family.
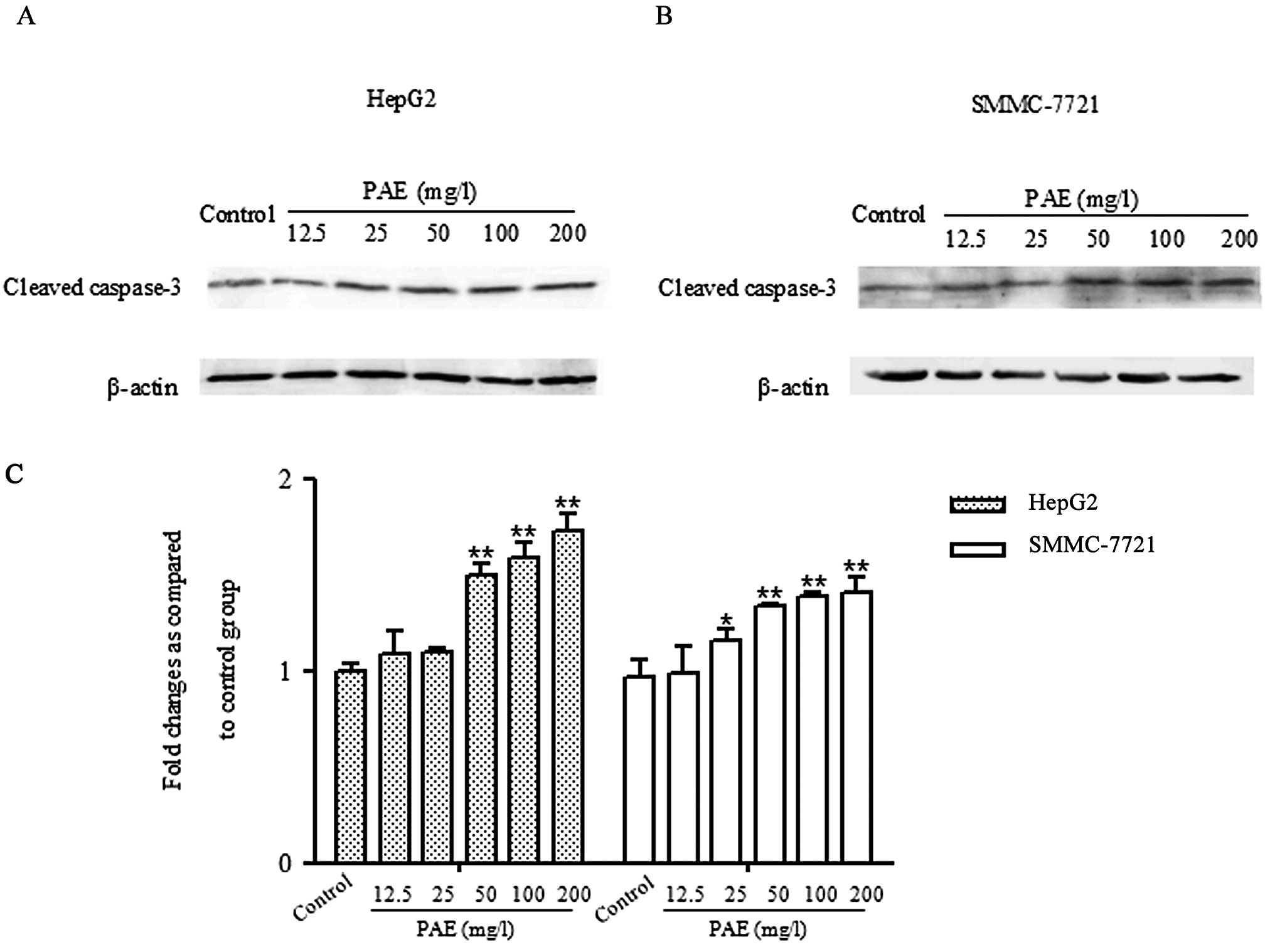
PAE increases caspase-3 activation in
HepG2 and SMMC-7721 cells
To determine whether PAE effects the activation of
caspase-3 in HepG2 and SMMC-7721 cells, the activation of caspase-3
was quantified through western blotting. As shown in Fig. 5, low levels of cleaved caspase-3
were observed in control treated cells. Treatment with various
concentrations of PAE (50, 100 and 200 mg/l) markedly increased the
levels of cleaved caspase-3 in HepG2 and SMMC-7721 cells.
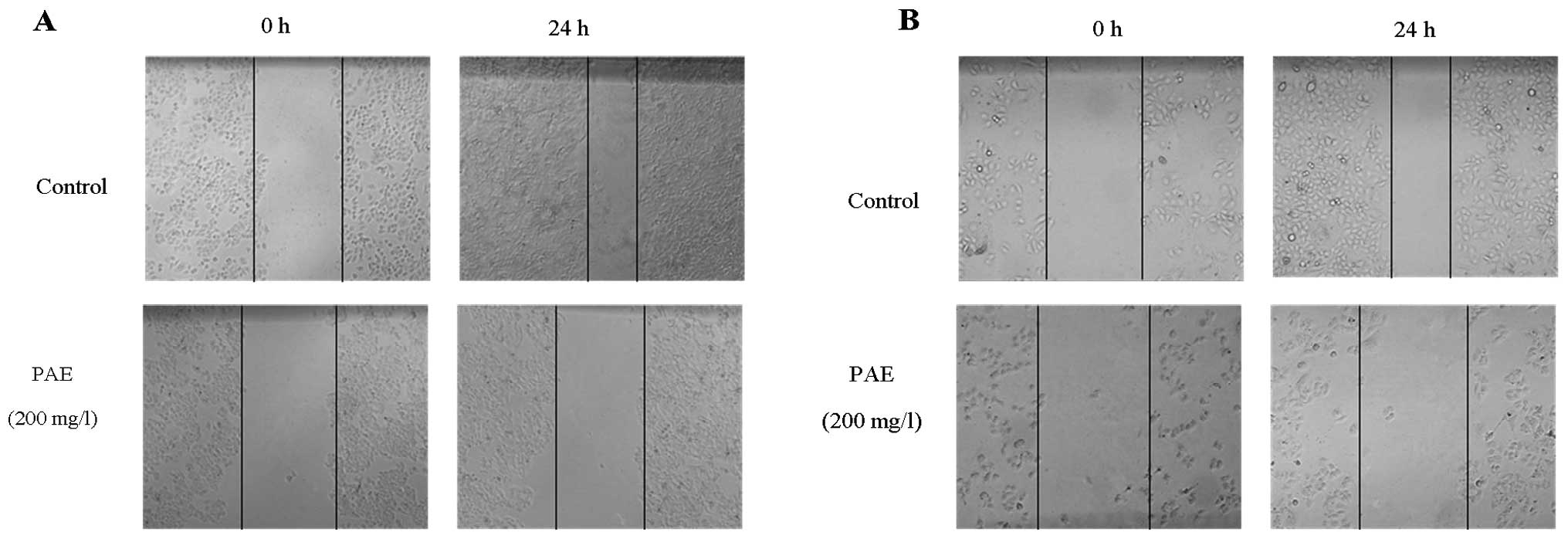
PAE inhibits migration and invasion of
HCC cells
As HCC cell migration and invasion were associated
with metastatic potential, a cell scratch migration and a Matrigel
invasion assays were performed to corroborate the PAE effects.
Wound healing assay was performed after treatment with PAE at
concentrations of 12.5, 25, 50, 100 and 200 mg/l at 0 and 24 h. The
results showed that PAE (50, 100 and 200 mg/l) significantly
suppressed the migration ability of HepG2 cells and SMMC-7721 cells
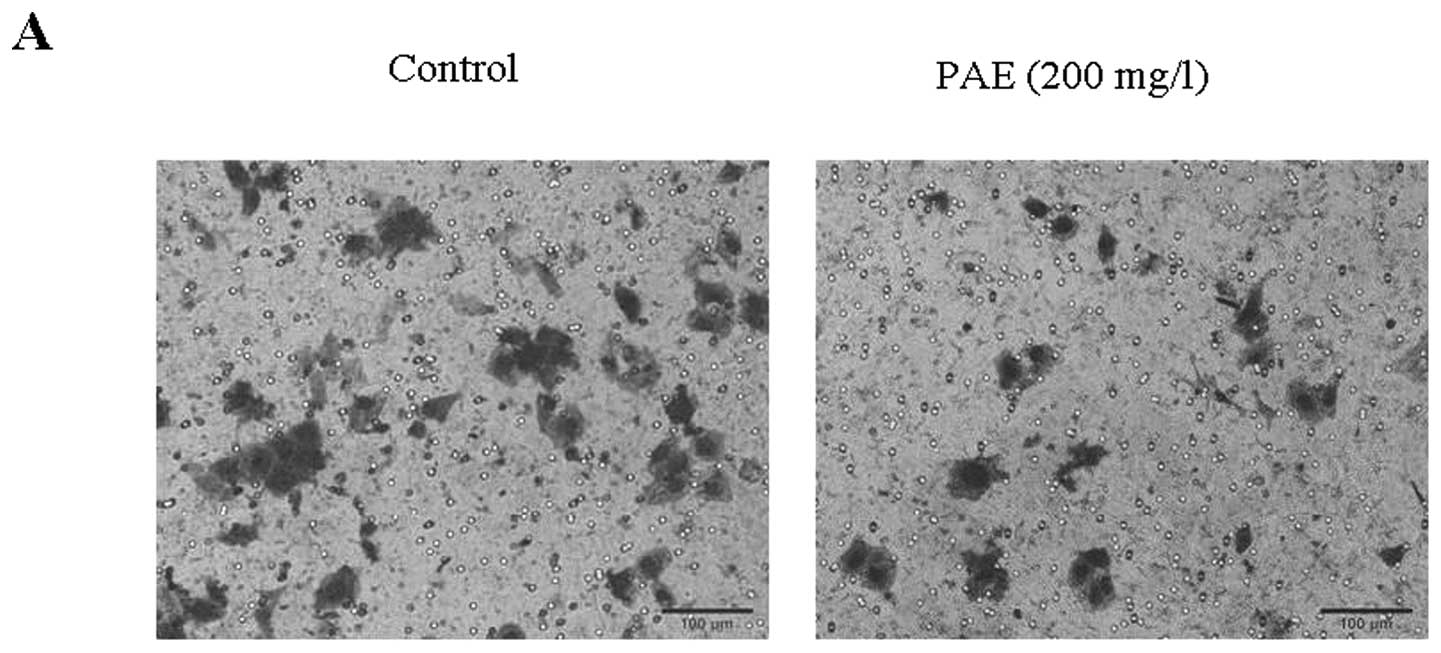
(Fig. 6). As shown in Fig. 7A, transwell invasion assay revealed
a significant reduction in invasion of SMMC-7721 cells treated with
PAE 200 mg/l in comparison to control cells. These two observations
emphasized the inhibitive role of PAE in HCC cell migration and
invasion.
Discussion
Despite many years of intensive research, HCC
remains a devastating malignancy (26). Conventional chemotherapy is one of
the common strategies for HCC treatment (27,28).
Although chemotherapeutic drugs have been administered to patients
with HCC, the curative effect was often accompanied by various side
effects (6). Therefore, the
development of new drugs with the ability to directly inhibit
cancer cell proliferation and induce cell apoptosis for the
treatment of HCC is urgent needed. Recently, chemotherapy and other
intervention strategies using extracts of Chinese herbal medicines
have emerged as a promising alternative option to improve the
quality of life for HCC patients (5,29–31).
For example, swain-sonine (an extract from Sphaerophysa
salsula or Astragalus membranaceus) and paeoniflorin
(extracted from the root of Paeonia lactiflora), have been
confirmed to inhibit cell growth, induce apoptosis and potentiate
the cytotoxic effect on HCC cells and cervical cancer cells
(12,32,33).
PAE was designed according to the principles of Chinese traditional
treatment on liver disease, as well as applying with the
contemporary extraction and purification technology (20). PAE in vivo was confirmed to
exhibit obvious hepatoprotective effects on the acute liver injury
and chronic liver fibrosis (20,22).
Moreover, in vitro studies indicated the primary mechanisms
of its anti-liver injury effects may be associated with inhibiting
proliferation of hepatic stellate cells and MAPK activation
(34). In addition, these previous
results have proved that PAE exerted conspicuously higher
efficiency than the use of the extracts TGP and total
astragalosides exclusively.
It has been well accepted that the development
process of HCC mostly connected with the chronic liver disease,
such as liver fibrosis (24), and
the usual pattern of fibrosis-cirrhosis-HCC, has been revealed for
many years. Combined with the previous studies, we hypothesized
that PAE could also affect the follow-up development of fibrosis,
the liver cancer. Therefore, the present study was designed to
firstly investigate whether PAE inhibits proliferation or induces
apoptosis. Our proliferation and flow cytometry assays revealed
that PAE significantly inhibits proliferation and induces apoptosis
of HepG2 and SMMC-7721 cells. Then, to further determine the
possible mechanisms of pro-proliferation and anti-apoptosis which
were induced by PAE, the expression of the Bcl-2 family proteins
and caspase-3 which are acknowledged apoptosis-related regulators,
was examined. The immunocytochemistry analysis and western blotting
results showed decreased expression of Bcl-2 and elevated levels of
Bax and caspase-3 in HepG2 and SMMC-7721 cells treated with PAE
compared with the control groups. These results suggest that PAE
may mediate apoptosis by modulating the expression of Bcl-2 and
caspase family proteins.
Apoptosis is a physiological cell death program that
is a vital pathway in maintaining tissue homeostasis (35). Several factors contribute to
apoptosis, but the pivotal ones are classified into two main
families of proteins including Bcl-2 family and caspase enzymes
(36). The Bcl-2 gene (B-cell
lymphoma/leukemia-2) is one of members of Bcl-2 family, which is
closely related to apoptosis (37). Certain Bcl-2 family proteins have
been recognized as a critical checkpoint within apoptotic pathways
(38). The Bcl-2 family is
composed of a number of genes, incuding the anti-apoptotic and
pro-apoptotic members, such as Bcl-2 and Bax, which play critical
roles in the control of mitochondrial integrity, have been
considered to the crucial switch of the apoptotic process (39,40).
A large body of research indicates that elevated expression of
Bcl-2 inhibits apoptosis in various cell types, inducing cancer
cells (12). The excessive
expression of Bcl-2 leads to the resistance of cells against
cellular toxins, which may connect a mutual pathway or crosstalk in
the signal transduction pathway regulated by Bcl-2 (41). The results of our present studies
showed by immunocytochemistry analysis and western blotting
indicated that PAE reduced the expression of Bcl-2 and enhanced the
expression of Bax, comparing to the non-treated groups, along with
an increased expression of caspase-3.
Caspases are known for playing an important role in
the execution of apoptosis, which frequently activates death
protease, catalyzing the specific cleavage of many pivotal cellular
proteins (42). During apoptosis,
active caspase-3 leads to a self-amplifying cascade of proteolysis
and cleavage of many effect or proteins. Caspase-3 is essential for
cellular DNA damage and apoptosis (43). Certain studies have shown that
overexpression of Bcl-2 prevents the mitochondrial release of cyt
c, thereby inhibiting the activation of caspases cascade and
apoptosis (44). The increase in
caspase-3 activation is synchronized with an increased expression
of pro-apoptotic protein Bax and a decreased expression of
anti-apoptotic protein Bcl-2 (45). The western blot analysis in this
study demonstrated that PAE enhanced the expression of cleaved
caspase-3 both in HepG2 and SMMC-7721 cell lines. In agreement with
the data of the Bcl-2 family, our study indicated PAE may induce
apoptosis through the mechanisms of modulating the Bcl-2 family
protein and the caspase-3 activity.
Due to invasion and intrahepatic metastasis, the
prognosis for patients with HCC is poor. Invasive growth and
metastatic potential is the basic reason which leads to stubborn
and refractory liver cancer (46).
The essential steps for metastases, invasion and migration require
the action of tumor-associated proteases that dissolve the
surrounding tumor matrix and basement membrane (47). Therefore, wound healing and
Matrigel transwell assays were designed to detect the migratory and
invasive abilities of HepG2 and SMMC-7721 cells after treatment
with PAE. The results showed that PAE significantly inhibited the
migration and invasion of HCC cells and provided new insights into
the potential use of PAE in controlling HCC invasion and
metastasis.
In conclusion, our study demonstrated that PAE
caused significant inhibition of cell proliferation, migration and
invasion and pro-apoptotic effect in HCC cells. Our data also
indicated the antitumor effect of PAE is due to the upregulation of
the Bax-to-Bcl-2 ratio, as well as activation of the
caspase-dependent signaling pathway. As far as we know, the study
on association between PAE and HCC cell proliferation, migration,
invasion and apoptosis has not been investigated before. However,
further studies are required to elucidate the detailed mechanisms
of PAE effect on apoptosis and metastases. In view of the present
results, PAE may be considered as a potential therapeutic candidate
in treatment of HCC.
Acknowledgements
This study was supported by grants
from the Specialized Research Fund for the Doctoral Program of
Higher Education of China (no. 20113420120002), the Natural Science
Foundation of the Higher Education Institutions of Anhui Province
(no. KJ2012A153), the Natural Science Foundation of China (no.
81300332). The authors acknowledge the help of the staff members of
the Institute of Clinical Pharmacology, Anhui Medical University,
in conducting the study.
References
|
1.
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Yang JD and Roberts LR: Hepatocellular
carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 7:448–458.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
He J, Gu D, Wu X, et al: Major causes of
death among men and women in China. N Engl J Med. 353:1124–1134.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Villanueva A and Llovet JM: Targeted
therapies for hepatocellular carcinoma. Gastroenterology.
140:1410–1426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ma L, Wen S, Zhan Y, He Y, Liu X and Jiang
J: Anticancer effects of the Chinese medicine matrine on murine
hepatocellular carcinoma cells. Planta Med. 74:245–251. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Wang S, Zheng Z, Weng Y, et al:
Angiogenesis and anti-angiogenesis activity of Chinese medicinal
herbal extracts. Life Sci. 74:2467–2478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Dai LM, Chen MZ and Xu SY: Protective
effects of total glucocides of paeony on experimental hepatitis.
Chinese Pharmacol Bull. 9:449–453. 1993.
|
|
8.
|
Wang H, Wei W, Wang NP, et al: Effects of
total glucosides of peony on immunological hepatic fibrosis in
rats. World J Gastroenterol. 11:2124–2129. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Zheng YQ and Wei W: Total glucosides of
paeony suppresses adjuvant arthritis in rats and intervenes
cytokine-signaling between different types of synoviocytes. Int
Immunopharmacol. 5:1560–1573. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Wang SH, Wei W, Xu DJ and Cui Y:
Proliferation inhibition and mechanism of TGP on HepG2 cell in
vitro. Acta Universitatis Medicinalis Anhui. 41:547–549. 2006.
|
|
11.
|
Wang SH, Wei W, Xu DJ, Xu SP and Cui Y:
Proliferation inhibition of TGP on SMMC-7721 Cell in vitro. Anhui
Med Pharmaceut J. 10:8–9. 2006.
|
|
12.
|
Zhang L and Zhang S: Modulating Bcl-2
family proteins and caspase-3 in induction of apoptosis by
paeoniflorin in human cervical cancer cells. Phytother Res.
25:1551–1557. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Wu H, Li W, Wang T, Shu Y and Liu P:
Paeoniflorin suppress NF-kappaB activation through modulation of I
kappaB alpha and enhances 5-fluorouracil-induced apoptosis in human
gastric carcinoma cells. Biomed Pharmacother. 62:659–666. 2008.
View Article : Google Scholar
|
|
14.
|
Tsuboi H, Hossain K, Akhand AA, et al:
Paeoniflorin induces apoptosis of lymphocytes through a
redox-linked mechanism. J Cell Biochem. 93:162–172. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zhang Q, Gao WY and Man SL: Chemical
composition and pharmacological activities of astragali radix.
Zhongguo Zhong Yao Za Zhi. 37:3203–3207. 2012.(In Chinese).
|
|
16.
|
Law PC, Auyeung KK, Chan LY and Ko JK:
Astragalus saponins downregulate vascular endothelial growth
factor under cobalt chloride-stimulated hypoxia in colon cancer
cells. BMC Complement Altern Med. 12:1602012. View Article : Google Scholar
|
|
17.
|
Hu XY, Xia RX, Cheng CB, et al: Mechanism
of apoptosis in human leukemia NB4 cells induced by total
astragalosides. Zhonghua Zhong Liu Za Zhi. 33:345–348. 2011.(In
Chinese).
|
|
18.
|
Liu X, Yang Y, Zhang X, et al: Compound
Astragalus and Salvia miltiorrhiza extract inhibits
cell invasion by modulating transforming growth factor-beta/Smad in
HepG2 cell. J Gastroenterol Hepatol. 25:420–426. 2010.
|
|
19.
|
Nishiyama N, Wang YL and Saito H:
Beneficial effects of S-113m, a novel herbal prescription, on
learning impairment model in mice. Biol Pharm Bull. 18:1498–1503.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Sun WY, Wei W, Wu L, Gui SY and Wang H:
Effects and mechanisms of extract from Paeonia lactiflora
and Astragalus membranaceus on liver fibrosis induced by
carbon tetrachloride in rats. J Ethnopharmacol. 112:514–523.
2007.PubMed/NCBI
|
|
21.
|
Shao FR, Wei W, Liu H, et al: Comparison
of protective effect of Shaoqiduogan extracted by different methods
on immunological liver injury in mice. J Anhui TCM College.
26:21–24. 2007.
|
|
22.
|
Sun WY, Wei W, Gui SY, Wu L and Wang H:
Protective effect of extract from Paeonia lactiflora and
Astragalus membranaceus against liver injury induced by
bacillus Calmette-Guerin and lipopolysaccharide in mice. Basic Clin
Pharmacol Toxicol. 103:143–149. 2008.
|
|
23.
|
Colombo M, de Franchis R, Del Ninno E, et
al: Hepatocellular carcinoma in Italian patients with cirrhosis. N
Engl J Med. 325:675–680. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Paradis V: Histopathology of
hepatocellular carcinoma. Recent Results Cancer Res. 190:21–32.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Zhang XY and Wang J: A study on the
chemical constituents of Paeonia lactiflora Pall. J Shenyang
Pharmaceutl Univ. 18:30–32. 2001.
|
|
26.
|
Rahbari NN, Mehrabi A, Mollberg NM, et al:
Hepatocellular carcinoma: current management and perspectives for
the future. Ann Surg. 253:453–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Alves RC, Alves D, Guz B, et al: Advanced
hepatocellular carcinoma. Review of targeted molecular drugs Ann
Hepatol. 10:21–27. 2011.
|
|
28.
|
Yu YL, Lu Y, Tang X and Cui FD:
Formulation, preparation and evaluation of an intravenous emulsion
containing Brucea javanica oil and Coix Seed oil for
antitumor application. Biol Pharm Bull. 31:673–680. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Li WY, Chiu LC, Lam WS, et al: Ethyl
acetate extract of Chinese medicinal herb Sarcandra glabra
induces growth inhibition on human leukemic HL-60 cells, associated
with cell cycle arrest and up-regulation of pro-apoptotic Bax/Bcl-2
ratio. Oncol Rep. 17:425–431. 2007.PubMed/NCBI
|
|
30.
|
Luqman S and Pezzuto JM: NFkappaB: a
promising target for natural products in cancer chemoprevention.
Phytother Res. 24:949–963. 2010.PubMed/NCBI
|
|
31.
|
Karikas GA: Anticancer and chemopreventing
natural products: some biochemical and therapeutic aspects. J BUON.
15:627–638. 2010.PubMed/NCBI
|
|
32.
|
You N, Liu W, Wang T, et al: Swainsonine
inhibits growth and potentiates the cytotoxic effect of paclitaxel
in hepatocellular carcinoma in vitro and in vivo.
Oncol Rep. 28:2091–2100. 2012.PubMed/NCBI
|
|
33.
|
Hu S, Sun W, Wei W, et al: Involvement of
the prostaglandin E receptor EP2 in paeoniflorin-induced human
hepatoma cell apoptosis. Anticancer Drugs. 24:140–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Sun WY, Wang L, Liu H, Li X and Wei W: A
standardized extract from Paeonia lactiflora and
Astragalus membranaceus attenuates liver fibrosis induced by
porcine serum in rats. Int J Mol Med. 29:491–498. 2012.
|
|
35.
|
Thompson HJ, Strange R and Schedin PJ:
Apoptosis in the genesis and prevention of cancer. Cancer Epidemiol
Biomarkers Prev. 1:597–602. 1992.PubMed/NCBI
|
|
36.
|
Giannattasio A, Angeletti G, De Rosa M, et
al: RNA expression bcl-w, a new related protein Bcl-2 family and
caspase-3 in isolated sertoli cells from pre-pubertal rat testes. J
Endocrinol Invest. 25:C23–C25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Cory S and Adams JM: The Bcl2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Barille-Nion S, Bah N, Vequaud E and Juin
P: Regulation of cancer cell survival by BCL2 family members upon
prolonged mitotic arrest: opportunities for anticancer therapy.
Anticancer Res. 32:4225–4233. 2012.PubMed/NCBI
|
|
40.
|
Zeng H, Kong X, Peng H, et al: Apoptosis
and Bcl-2 family proteins, taken to chronic obstructive pulmonary
disease. Eur Rev Med Pharmacol Sci. 16:711–727. 2012.PubMed/NCBI
|
|
41.
|
Kinoshita M, Eguchi Y and Hynynen K:
Activation of Bak in ultrasound-induced, JNK- and p38-independent
apoptosis and its inhibition by Bcl-2. Biochem Biophys Res Commun.
353:515–521. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Galluzzi L, Kepp O and Kroemer G:
Caspase-3 and prostaglandins signal for tumor regrowth in cancer
therapy. Oncogene. 31:2805–2808. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Porter AG and Janicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Zhu S, Cohen MB, Bjorge JD, Mier JW and
Cho DC: PI3K inhibition potentiates Bcl-2-dependent apoptosis in
renal carcinoma cells. J Cell Mol Med. 17:377–385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Lin HI, Lee YJ, Chen BF, et al:
Involvement of Bcl-2 family, cytochrome c and caspase 3 in
induction of apoptosis by beauvericin in human non-small cell lung
cancer cells. Cancer Lett. 230:248–259. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Tao YM, Liu Z and Liu HL: Dickkopf-1
(DKK1) promotes invasion and metastasis of hepatocellular
carcinoma. Dig Liver Dis. 45:251–257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Korpi JT, Hagstrom J, Lehtonen N, et al:
Expression of matrix metalloproteinases-2, -8, -13, -26 and tissue
inhibitors of metalloproteinase-1 in human osteosarcoma. Surg
Oncol. 20:e18–e22. 2011. View Article : Google Scholar : PubMed/NCBI
|