Introduction
Nasopharyngeal carcinoma (NPC) is a malignancy with
unique geographic distribution and is most common in certain areas
of Asia such as the southern part of China (1). In clinical practice, early detection
of NPC still remains a major challenge and many NPC patients are
diagnosed in the advanced stage with limited treatment options.
This situation contributes to the relatively high mortality of NPC.
Currently, the therapeutic options of NPC include chemotherapy,
radiotherapy and chemoradiotherapy (2). Despite the significant progress in
our understanding of NPC genetics and disease development, the
therapy of NPC still remains to be a great challenge due to drug
resistance, disease relapse and metastasis. The high toxicity of
conventional chemotherapy and radiotherapy also affects the quality
of life of NPC patients (3,4).
Therefore, it is of great importance to develop novel and effective
therapeutic strategies that specifically kill NPC and is tolerated
by normal cells (3). Metabolic
intervention based on the unique metabolic alterations in cancer
cells is one of the attractive therapeutic strategies with high
anticancer activity and selectivity.
One of the major metabolic alterations in cancer
cells is elevation in aerobic glycolysis, a phenomenon known as the
Warburg effect observed many decades ago (5). The increase in glucose uptake by
cancer cells constitute the key biochemical basis of
18fluorodeoxyglucose positron emission tomography
(FDG-PET) for clinical diagnosis of cancer (6). Although the exact reasons for the
preference of cancer cells to use the glycolytic pathway still
remain to be investigated, this altered energy metabolism may
represent a vulnerability of cancer to therapeutic intervention
(7). Similar to many other cancer
types with high FDG-PET signal (6,8), NPC
cells seem to be highly active in glycolysis and can be readily
detected using FDG-PET scan, which in fact has been used for
diagnosis and prognosis evaluation of NPC with high standard uptake
values (SUV) being associated with poor prognosis (9). This is consistent with the recent
study suggesting that active aerobic glycolysis occur in the head
and neck squamous carcinoma (10).
However, it remains unclear if the active glycolytic activity in
NPC cells could be exploited for therapeutic purpose and the key
enzymes in the glycolytic pathway that may be targeted to kill NPC
cells remain to be investigated.
Lactate dehydrogenase (LDH) is an enzyme that
catalyzes the conversion of pyruvate to lactate and thus
significantly affect the glycolytic metabolism. Interestingly, the
expression of LDH has been found to be an independent prognosis
biomarker in different cancers including NPC (11,12).
Furthermore, the level of LDH seemed to be associated with NPC bone
metastasis (13). Knockdown of LDH
has been shown to cause inhibition of tumor growth in vitro
and in vivo in several types of human cancers (14–18),
indicating an association between elevated glycolytic activity and
the malignant behavior of NPC cells. These observations suggest
that LDH may be a key enzyme that plays an essential role in energy
metabolism in NPC cells and that it may be possible to target this
enzyme as a therapeutic strategy for treatment of this malignant
disease. In the present study, we showed that inhibition of LDH by
oxamate led to significant suppression of glucose uptake and
lactate generation and inhibited NPC growth in vitro and
in vivo. We also found a drug combination strategy that is
highly effective in abrogating energy metabolism in NPC cells and
significant therapeutic implications.
Materials and methods
Reagents
Oxamate was purchased from Sigma-Aldrich. LDH
activity detection kit was purchased from Biovision (San Francisco,
CA, USA). Strips for glucose and lactate detection were the product
of Roche (Mannheim, Germany). Annexin V/PI staining reagents were
purchased from Keygene (Nanjing, China). MitoSOX and Rhodamine-123
were from Molecular Probes (OR, USA). ATP detection kit was
purchased from Promega (Madison, WI, USA).
Cell culture
Human nasopharyngeal carcinoma cell lines CNE1 and
CNE2 were cultured in DMEM medium supplemented with 10% FBS. The
immortalized nasopharyngeal epithelial cells (NP69) were cultured
in keratinocyte-SFM medium (Invitrogen) supplemented with bovine
pituitary extract (BD Biosciences). A hypoxic culture condition was
created by incubating cells in a sealed modular incubator chamber
(Billups-Rothenberg, Del Mar, CA, USA) flushed with a gas mixture
containing 5% CO2 and 95% N2. Because the
culture flasks contained ambient oxygen at the beginning of the
experiments, the final oxygen content in the hypoxia chamber was
∼0.5–1% after achieving air equilibrium as described previously
(19).
MTT assay
Cells in logarithmic growing phase were seeded in
96-well plates with 3,000 cells in each well. Various
concentrations of oxamate were added to the wells and the plates
were placed in a cell culture incubator for 72 h. The samples were
then incubated with MTT reagent (20 μl/well) and incubated
for another 4 h. After the culture medium was removed, the cells
were lysed with 200 μl DMSO to dissolve the purple formazan
and the OD values were detected at the wavelength of 570 nm as
described previously (20).
Colony formation assay
Cells in logarithmic phase were seeded in 6-well
plates with 500 cells in each well. Various concentrations of
oxamate were added to the wells. The cells were cultured for two
weeks before fixation and staining with crystal violet. The samples
were photographed and the colonies were counted as described
previously (21).
Annexin V/PI assay
Apoptosis was detected using Annexin V/PI double
staining as described previously (20). After the drug treatment, cells were
harvested, washed and counted. The cells were then stained with
Annexin V and PI in binding buffer for 15 min and cell viability
was analyzed using the FACSCalibur flow cytometer (BD Co.,
USA).
Cell cycle analysis
Cells in logarithmic phase were seeded in 6-well
plates with a density of 2×105 cells per well. Different
concentrations of oxamate were added and the plates were incubated
for 24 h. The cells were harvested and fixed with 75% alcohol at
4°C for 4 h and then incubated with RNase at 37°C for 30 min
followed by addition of PI. Finally, cell cycle distribution were
detected by using the BD FACSCalibur flow cytometer (22).
Glucose uptake and lactate production
assays
Cells were seeded in 6-well plate with the density
of 2×105 cells/well. After the indicated incubation
periods, medium was removed for measurement of glucose and lactate
concentrations using the Roche Accurend Analyzer (Roche) as
previously described (23).
Experiments were repeated for at least three times.
Assay of cellular ATP
Cells in logarithmic growth were seeded in 96-well
opaque plates with the density of 50,000 cells per well. Oxamate
(50 mM) was added and the plates were incubated for 6, 12, 24 or 48
h. Cellular ATP contents were measured using the Promega ATP
detection kits according to the procedures recommended by the
manufacturer. Luminescence (relative light unit) was detected by a
luminescent plate reader as previously described (23). ATP contents in the samples were
calculated according to the standard curve.
Reactive oxygen species (ROS) and
mitochondrial transition potential (MTP) detection
Cells were stained for 30 min with DCF-DA for ROS
detection or Rhodamine-123 for MTP detection. The samples were
harvested and analyzed using a BD Calibur flow cytometry as
described previously (24).
LDH activity assay
LDH activity was analyzed as described previously
(25). Briefly, cells were washed,
homogenized and centrifuged. The supernatant was added into a
96-well plate. After addition of the reaction mix, enzyme reaction
was initiated and OD value was detected at the wavelength of 450
nm. LDH activity was calculated as described previously (26).
Animal study
Balb/c nude mice (age 4–6 weeks) were purchased from
the Laboratory Animal Center of Guangdong Province. Animal study
was performed according to a research protocol approved by the
ethics committee of the laboratory animal research of Sun Yat-Sen
University Cancer Center. Two million CNE-2 cells were inoculated
into the right flank of the mice. When the average tumor volume
reached 100 mm3, the mice were divided into two groups,
a control group and the oxamate-treatment group (with 5 mice in
each group). Oxamate was given by i.p. injection (750 mg/kg, daily)
as described previously (25). The
control group was administered with PBS. The mouse body weights and
tumor volumes were measured twice weekly. At the end of the
experiment, mice were sacrificed and the tumors were excised for
pathological examination. The tumor volume was calculated according
to the following formula: Tumor volume (mm3) =
ab2/2, where a and b represent the long and short
diameter of the tumor, respectively.
Statistical analysis
Data are presented as the means and SD of at least
three independent measurements. Statistical analysis (one way ANOVA
or unpaired t-test) was used to determine differences between means
of different experimental groups. Significance level for all
statistical comparisons was set at P<0.05. Graph pad Prism 5.0
software (US) was used to draw pictures. Canvas X (US) was used to
align and process pictures.
Results
Selective anti-proliferative effect of
oxamate against NPC cells
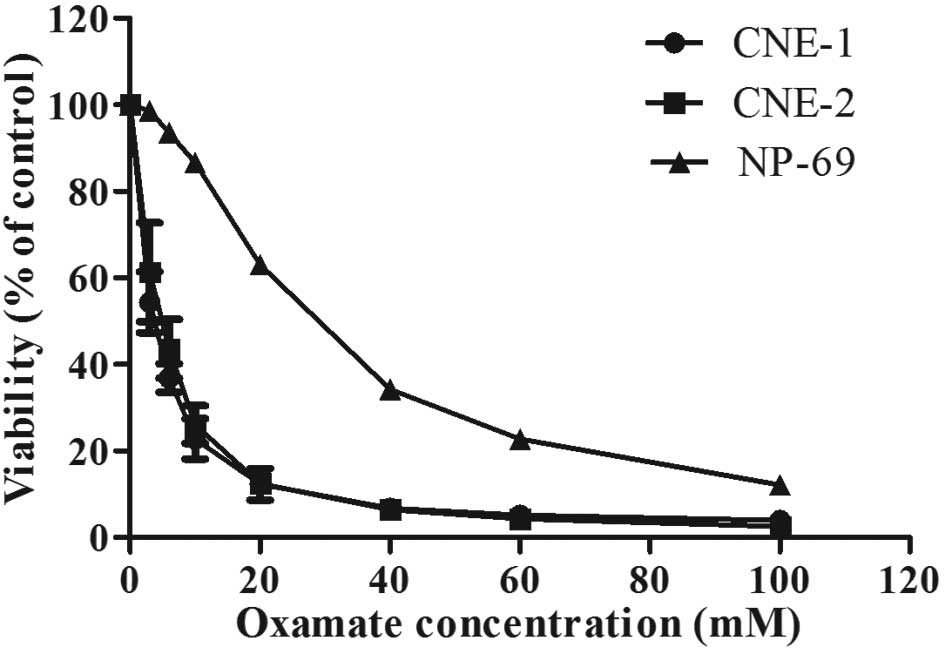
We first used MTT assay to evaluate the effect of
oxamate treatment on the proliferation of two human NPC cell lines
(CNE-1 and CNE-2). The results showed that oxamate caused a strong
inhibition of proliferation in nasopharyngeal carcinoma cell lines
in a dose-dependent manner (Fig.
1). CNE-1 and CNE-2 exhibited similar sensitivity to oxamate
with the IC50 values of 3.6±0.75 and 4.80±0.95 mM,
respectively. Intriguingly, the cytotoxicity of oxamate against
immortalized human normal nasopharyngeal epithelium NP-69 cells was
markedly less (higher IC50 value, 30 mM) compared to
malignant NPC cell lines (P<0.05), indicating a preferential
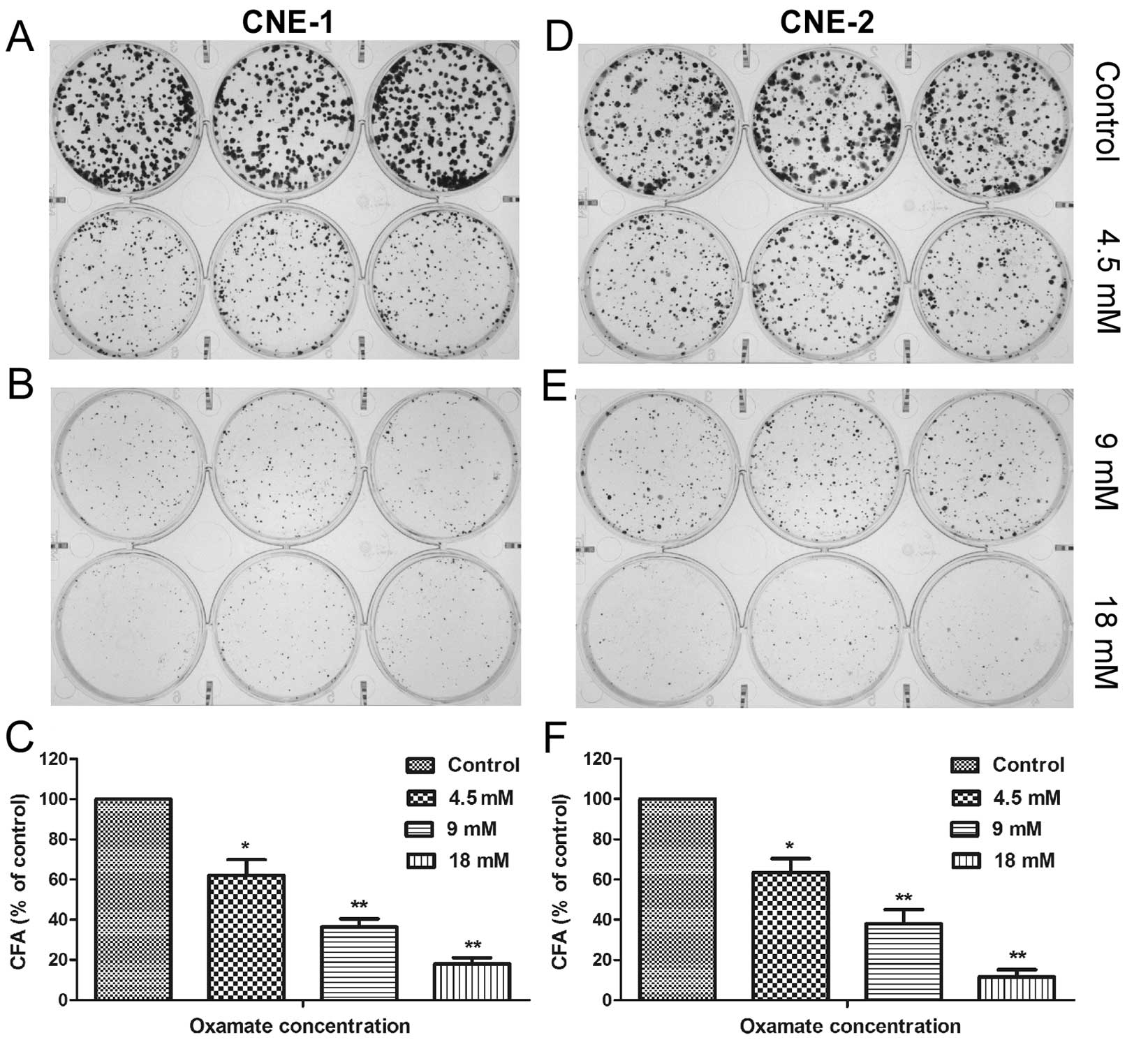
inhibitory effect of oxamate against NPC cells (Fig. 1). We also used colony formation
assay to further evaluate the effect of oxamate on the colony
forming ability of NPC cells. The results showed that oxamate was
able to effectively suppress colony formation of NPC cells in a
concentration-dependent manner, with >80% of colonies lost when
treated with 18 mM oxamate in both NPC cell lines (Fig. 2, P<0.01).
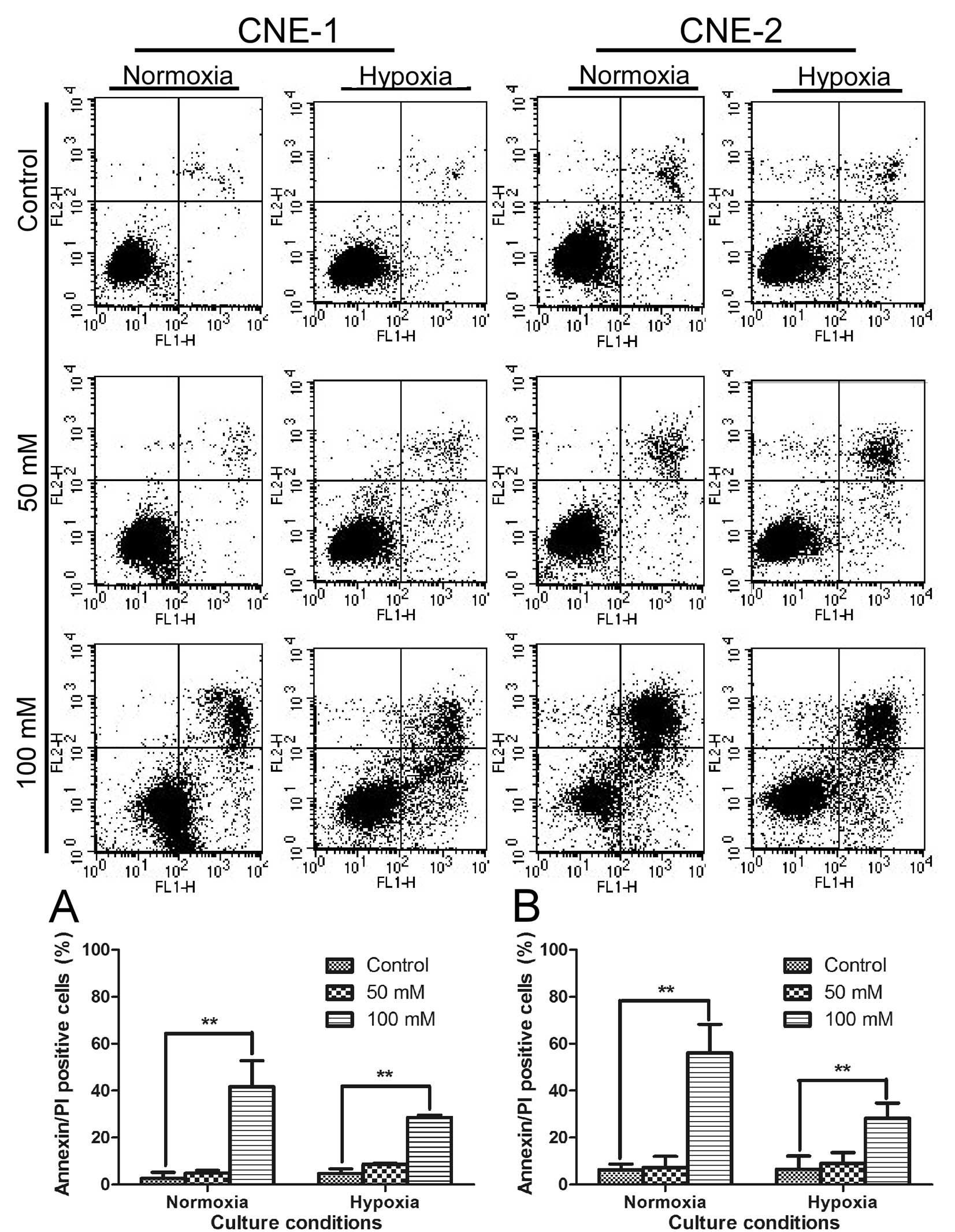
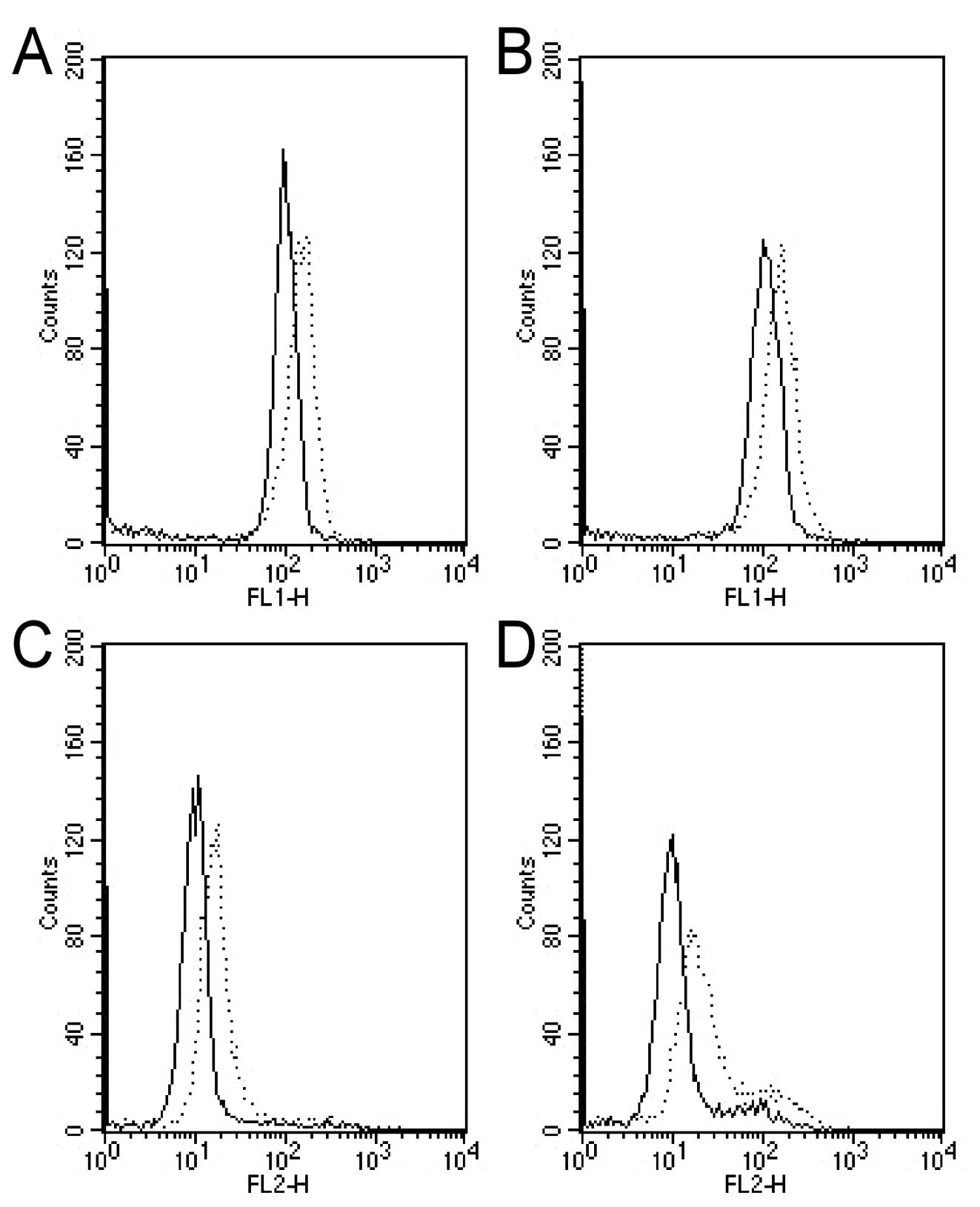
Induction of apoptosis by oxamate in NPC
cells
We then further tested if the strong
anti-proliferative activity of oxamate was due to cytotoxic effect
or cytostatic effect. Annexin V/PI double-staining assay was first
used to test if oxamate can directly induce apoptotic cell death in
NPC cells under normaxic and hypoxic conditions. The results
indicated that oxamate at high concentrations (50–100 mM) was able
to induce apoptosis in both CNE-1 and CNE-2 under normoxia
(Fig. 3, P<0.01).
Interestingly, this compound seemed also effective in causing
apoptosis under hypoxia (1% oxygen), although at a slightly lower
rate (Fig. 3, P<0.01).
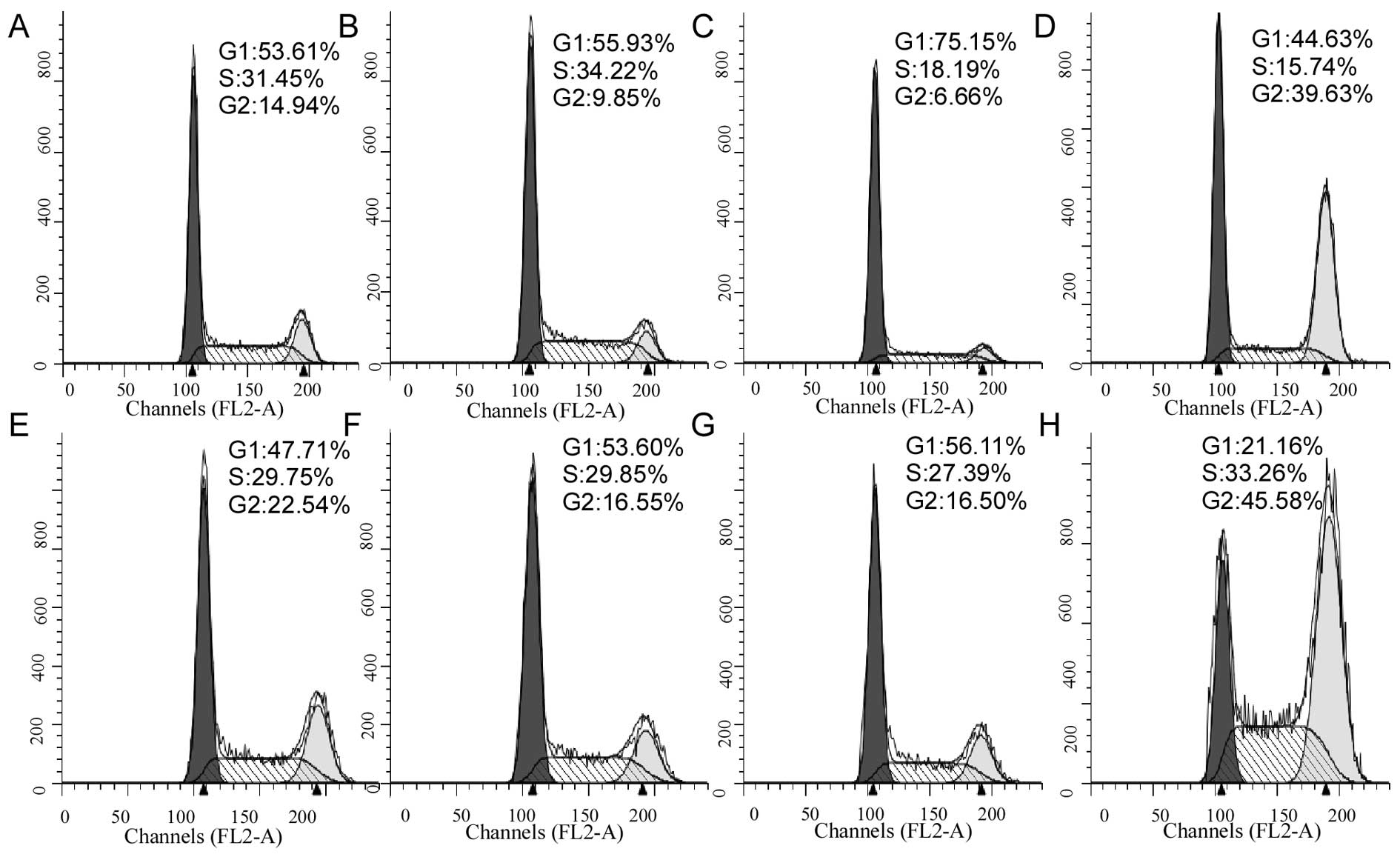
Cell cycle analysis to test the potential
effect of oxamate on cell cycle progression
As shown in Fig. 4,
oxamate at the concentrations of 25–50 mM did not significantly
affect cell cycle distributions (Fig.
4C–G), while a higher concentration (100 mM) induced cell cycle
arrest at G2 phase in both CNE-1 cells (Fig. 4D) and CNE-2 cell (Fig. 4H).
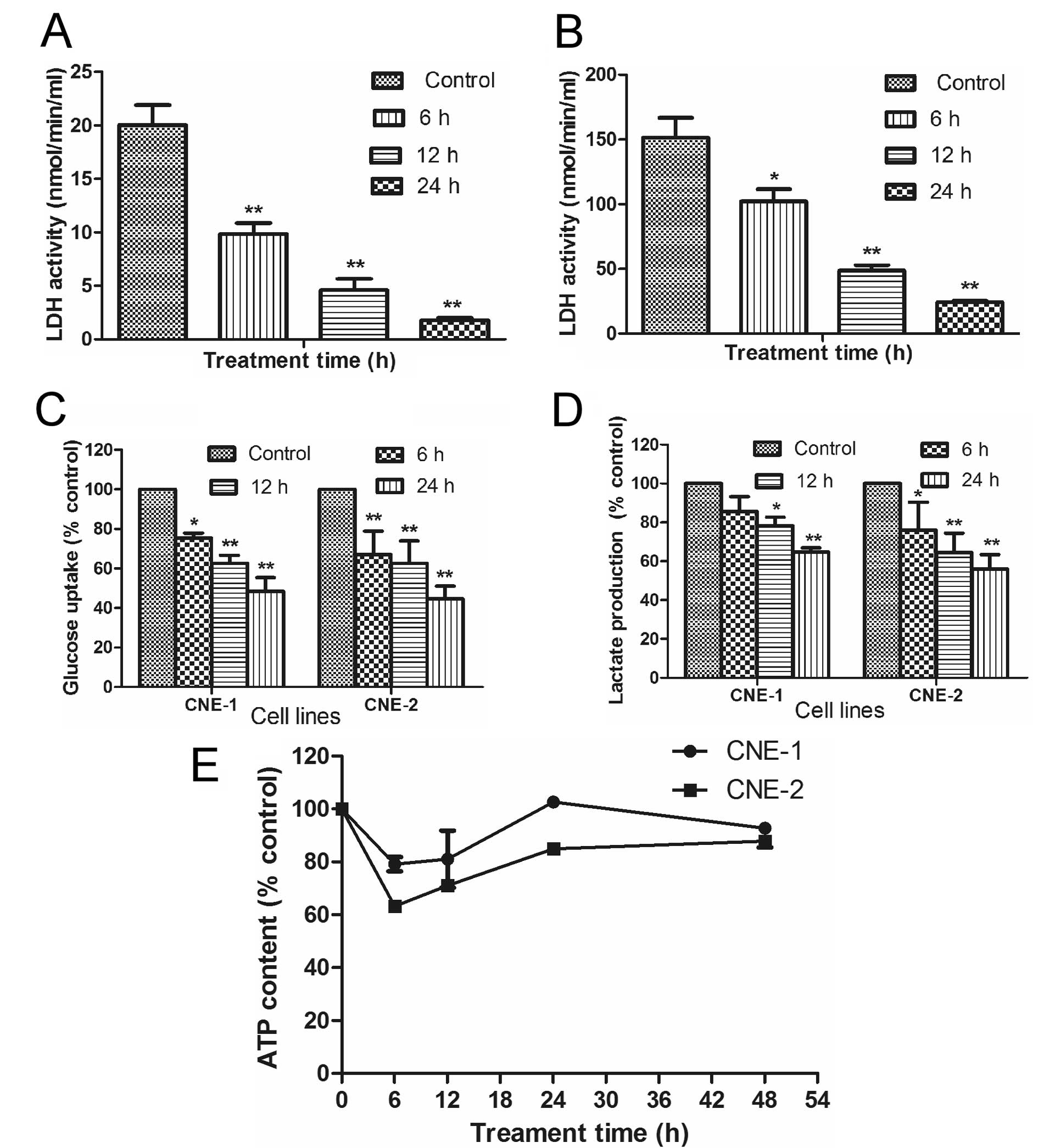
Oxamate inhibits glycolytic activity in
NPC
Since oxamate is an inhibitor of lactate
dehydrogenase (LDH), we tested if this compound could affect
glucose uptake and lactate generation, two key indicators of
glycolytic activity, in NPC cells. The ability of oxamate to
inhibit LDH activity was first confirmed in two NPC cell lines. As
shown in Fig. 5A and B, incubation
of CNE-1 and CNE-2 cells with 50 mM oxamate resulted in a
time-dependent inhibition of LDH in both cell lines (P<0.01).
Interestingly, the basal LDH activity in CNE-2 cells (150 U) was
significantly higher than that of CNE-1 (20 U), which could be a
reason for the higher sensitivity of CNE-2 cells to oxamate in
apoptotic response (Fig. 3) and
cell cycle arrest (Fig. 4), since
this cell line might be more dependent on LDH.
To further evaluate the impact of oxamate on glucose
energy metabolism in NPC cells, we analyzed the effect of this
compound on glucose uptake, lactate production and cellular ATP
contents. Incubation of NPC cells with oxamate caused a
time-dependent decrease in glucose uptake (Fig. 5C, P<0.05) and lactate production
(Fig. 5D, P<0.05) in both CNE-1
and CNE-2 cell lines. Such decrease was consistent with the
time-dependent inhibition of LDH enzyme activity (Fig. 5A and B). Interestingly, inhibition
of glycolysis by oxamate only caused a transient decrease in
cellular ATP by 20–40% during the first 6–12 h and cellular ATP
levels restore to the control levels by 24–48 h (Fig. 5E, P>0.05). These data together
suggest that NCP cells were able to compensate the loss of ATP due
to glycolytic inhibition by upregulation of another energy
production pathway such as oxidation phosphorylation in the
mitochondria.
Oxamate induces an increase in
mitochondrial transmembrane potential and ROS
The potential effect of oxamate on mitochondria
functional status was tested in two NPC cell lines. As shown in
Fig. 6, we used two fluorescent
probes the Rhodamine-123 and Mito-SOX to evaluate the effect of
oxamate on mitochondrial transmembrane potential (MTP) and reactive
oxygen species (ROS), respectively, and showed that treatment of
NPC cells with 50 mM oxamate induces a significant increase in MTP
and ROS levels both in CNE-1 and CNE-1 cells (Fig. 6, P<0.05). These changes seem to
suggest that the mitochondrial metabolic activity might be
increased in response to inhibition of glycolysis in the cytosol to
compensate ATP generation. This possibility was further confirmed
by drug combination study in which inhibition of mitochondrial
respiration by rotenone significantly enhances the effect of
oxamate in terms of ATP depletion and cytotoxicity.
Inhibition of mitochondrial respiration
in combination with oxamate leads to synergistic ATP depletion and
cytotoxicity in NPC cells
Mitochondrial oxidative phosphorylation and
glycolysis in cytosol are two major pathways that produce cellular
ATP. Based on the observations that oxamate inhibited glycolysis
and caused an increase in mitochondrial ROS and MTP, we postulated
that glycolytic inhibition by oxamate might cause a compensatory
increase in mitochondrial ATP generation, which prevented ATP
depletion and massive cell death. To investigate this possibility,
we used rotenone, a specific inhibitor of mitochondrial respiratory
chain complex I, in combination with oxamate and tested their
combined effect on cellular ATP and cell viability. As shown in
Fig. 7, a sub-toxic concentration
of oxamate (3.15 mM) or a sub-toxic concentration of rotenone (10
nM) alone did not cause any significant depletion of ATP in CNE-1
cells (Fig. 7A) or CNE-2 cells
(Fig. 7B, P<0.01). Combination
of both compounds at the same concentrations led to a synergistic
depletion of cellular ATP and massive loss of cell viability by
>80% in both cell lines (Fig. 7C
and D, P<0.01). These results together suggest that NCP
cells might be able to utilize glycolysis and oxidative
phosphorylation to compensate for the loss of ATP if one of these
metabolic pathways is inhibited and that a simultaneous inhibition
of both pathways would be highly effective in killing NPC
cells.
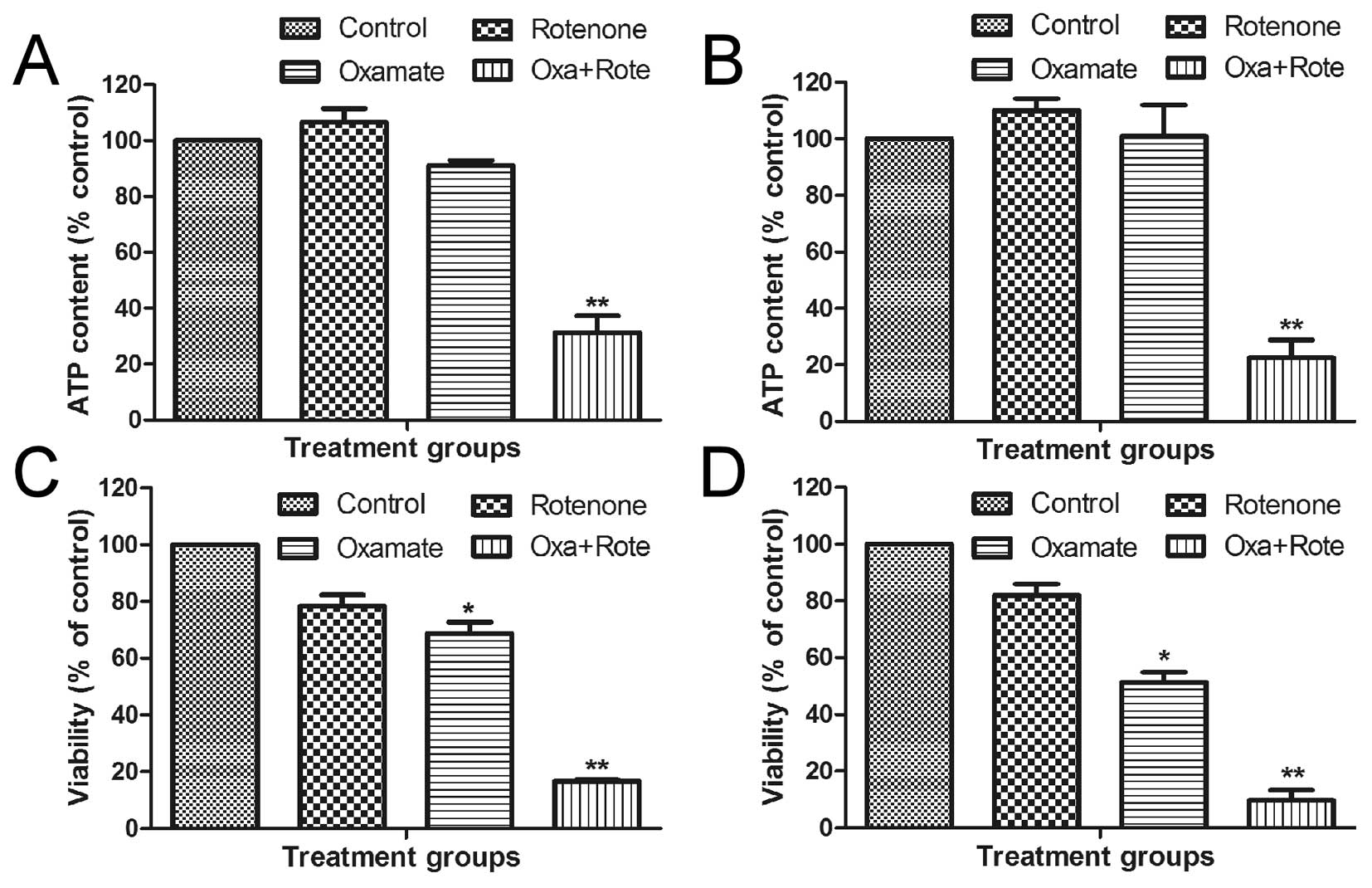 | Figure 7.Effect of oxamate and rotenone on
cellular ATP and viability in NPC cells. (A) CNE-1 cells were
incubated with oxamate (3.15 mM), rotenone (0.01 μM), or
their combination for 24 h as indicated, ATP content was measured.
(B) CNE-2 cells were incubated with oxamate (3.15 mM), rotenone
(0.01 μM), or their combination for 24 h as indicated, ATP
content was measured. (C) CNE-1 cells were incubated with oxamate
(3.15 mM), rotenone (0.01 μM), or their combination for 72 h
as indicated, cell viability was then measured. (D) CNE-2 cells
were incubated with oxamate (3.15 mM), rotenone (0.01 μM),
or their combination for 72 h as indicated, cell viability was then
measured; *P<0.05; **P<0.01. |
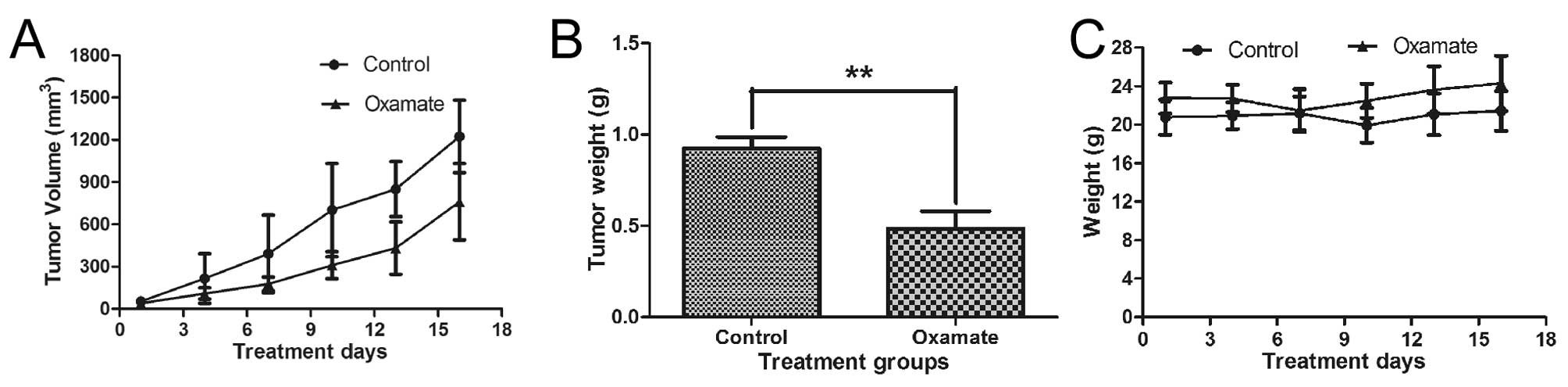
Antitumor activity of oxamate in
vivo
Animal experiments were performed to test the
potential therapeutic activity of oxamate against NPC. CNE-2 cells
were inoculated subcutaneously (2 million cells/injection) into the
right flanks of nude mice. When the tumor volume reached
approximately 100 mm3, the mice were divided into two
groups (5 mice/each). The treatment group received oxamate
injection (750 mg/kg, i.p.) daily, while the control group received
PBS treatment. As shown in Fig.
8A, oxamate treatment substantially inhibited tumor growth. At
the end of the experiment, tumors were removed for weighing. The
average tumor weights in the oxamate-treated group were
significantly less than that of the control group (P<0.01,
Fig. 8B, P<0.01). The daily
oxamate treatment seemed well-tolerated by the animals and there
was no significant loss of body weights (Fig. 8C, P>0.05). These data together
suggest that oxamate may have potential for treatment of NPC.
Discussion
Accumulating evidence suggests that metabolic
alterations are one of the important hallmarks of cancer and that
such biochemical changes may be exploited for therapeutic purpose
(26). Increase in aerobic
glycolysis is perhaps the most prominent metabolic alteration
observed in cancer cells with potentially significant therapeutic
implications in clinical cancer treatment (27). Several small molecular weight
compounds such as 2-deoxyglucose, 3-bromopyruvate, lonidamine and
oxamate have been shown to inhibit glycolysis with significant
anticancer activity in vitro and in vivo (28). Owing to the promising anticancer
effects of glycolytic inhibitors observed in cell culture and in
animal tumor models, it would be important to evaluate glycolytic
inhibitors in clinical setting and to further develop new
generation of compounds with better therapeutic potential. In this
research area, several metabolic targets and inhibitors are
emerging (29,30).
Among the small compounds that inhibit cancer energy
metabolism, oxamate was initially identified over 40 years ago as
an inhibitor of LDH (31).
Inhibition of LDH by oxamate causes a decrease in lactate
production and suppression of cancer cell proliferation. In our
study, we showed that oxamate was able to kill NPC cells both in
normoxic and hypoxic conditions (Fig.
3). This is important because many conventional anticancer
drugs become less effective in hypoxia and thus compounds capable
of killing cancer cells in hypoxia would potentially be useful to
overcome hypoxia-induced drug resistance. Mechanistically, hypoxic
cells are more dependent on glycolysis to generate ATP due to
limited availability of oxygen for oxidative phosphorylation. As
such, glycolytic inhibition is expected to be effective in killing
hypoxic cells. Previous study indeed suggested that hypoxia
rendered cancer cells sensitive to glycolytic inhibitors (32). It is possible, however, that
induction of HIF-1α by hypoxia and activation of Akt and other
survival molecules may protect tumor cells from the cytotoxic
effect of oxamate to some degree.
Previous study showed that high expression of LDH in
breast cancer cells was associated with active glycolysis and
resistance to taxol and that inhibition of LDH by oxamate
sensitized the tumor cells to taxol (33). Interestingly, it was shown that the
high expression of ErbB2 in breast cancer cells promoted LDH
expression and that oxamate was able to effectively kill these
breast cancer cells with Erb2 overexpression (34). Furthermore, breast cancer cells
resistant to trastuzumad (a drug that suppresses Erb2 signaling)
was found to overexpress LDH and inhibition of LDH by oxamate or by
RNAi sensitized these cancer cells to trastuzumad (25). The important role of glycolytic
inhibition in sensitization of cancer cells to chemotherapy and
radiotherapy has also been demonstrated in other experimental
systems (35,36). These data, together with our
observations that oximate was effective in killing NPC cells in
vitro and suppressing NPC tumor growth in vivo, show the
promising therapeutic potential of using oxamate in cancer
treatment. However, it should be noted that the effective
concentrations of oxamate required to kill cancer cells are very
high (in mM range), which might limit its clinical use. The
development of more effective LDH inhibitors such as Galloflavin
with potent anticancer activity (37) may provide a solution.
In this study, oxamate exhibited selective cytotoxic
effect against NPC cells with less cytotoxicity toward immortalized
human nasopharyngeal epithelial cells (NP-69) in vitro and
was well-tolerated in animals, suggesting that normal cells are
less sensitive to glycolytic inhibition, likely due to their normal
mitochondrial function and less dependence on glycolysis. We showed
that oxamate could inhibit the glucose uptake and lactate
production and that such glycolytic inhibition could be observed as
early as 6 h, which was much earlier than the appearance of
apoptosis. These observations together suggest that inhibition of
glycolysis was a primary event leading to cell death. In addition
to glycolytic inhibition, we also observed that oxamate caused
changes in mitochondria as evidenced by an increase in
mitochondrial transmembrane potential and ROS generation (Fig. 6). The mechanisms responsible for
oxamate-induced mitochondrial changes and their role or
contributions to the cytotoxic action of oxamate remain unclear and
require to further investigation. A recent study showed that
oxamate was able to inhibit aspartate aminotransferase (34), suggesting that this compound may
have multiple cellular targets.
Interestingly, we observed that inhibition of
glycolysis by oxamate caused a short-term decrease in cellular ATP
by 20–40% during early drug exposure time period (6–12 h), but
cellular ATP contents recovered to the normal level by 24–48 h,
suggesting that NPC cells (CNE-1 and CNE-2) were able to compensate
the loss of ATP due to glycolytic suppression by oxamate. The
mechanism responsible for this metabolic compensation was likely
through activation of mitochondrial oxidative phosphorylation,
since inhibition of mitochondrial respiratory chain by rotenone in
combination with oxamate caused a severe depletion of cellular ATP
and massive loss of cell viability (Fig. 7). These data suggest that such
mechanism-based drug combination might have potential to improve
therapeutic activity in NPC treatment. This is consistent with the
recent findings that a combination of mitochondria-targeted drugs
and the glycolytic inhibitor 2-deoxyglucose exhibit synergistic
effect against breast cancer cells (38).
In conclusion, we showed that oxamate was able to
inhibit glucose uptake and lactate generation in NPC cells. This
compound exhibited significant anticancer activity against NPC
cells in vitro under normoxia and hypoxia and suppressed
tumor growth in vivo. Activation of mitochondrial oxidative
phosphorylation seems to be a compensatory mechanism that may
compromise the ability of oxamate to kill NPC cells. Combination of
oxamate with an inhibitor of mitochondrial respiration showed
strong synergistic activity. It is important to further test the
promising therapeutic activity of this novel drug combination in
animals. It is also critical to test the potential cytotoxic effect
of such drug combination in normal cells and in animal toxicology
study to evaluate the therapeutic selectivity of this novel drug
combination.
Acknowledgements
The authors thank Mingli Peng of Sun
Yat-Sen University Cancer Centre for assistance with mouse
husbandry. This study was supported in part by a grant from the
major science and technology project of the National Basic Research
Program of China (973 Program grant no. 2012CB967004).
References
|
1.
|
Huang TR, Zhang SW, Chen WQ, et al: Trends
in nasopharyngeal carcinoma mortality in China, 1973–2005. Asian
Pac J Cancer Prev. 13:2495–2502. 2012.PubMed/NCBI
|
|
2.
|
Cheng SH, Jian JJ, Tsai SY, et al:
Prognostic features and treatment outcome in locoregionally
advanced nasopharyngeal carcinoma following concurrent chemotherapy
and radiotherapy. Int J Radiat Oncol Biol Phys. 41:755–762. 1998.
View Article : Google Scholar
|
|
3.
|
Toh HC, Ha TC and Wee J: Personalised
medicine in nasopharyngeal cancer. Lancet Oncol. 13:568–569. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Ayyanathan K, Kesaraju S, Dawson-Scully K
and Weissbach H: Combination of sulindac and dichloroacetate kills
cancer cells via oxidative damage. PLoS One. 7:e399492012.
View Article : Google Scholar
|
|
5.
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Jadvar H: Imaging evaluation of prostate
cancer with (18)F-fluorodeoxyglucose PET/CT: utility and
limitations. Eur J Nucl Med Mol Imaging. 40(Suppl 1): 5–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Schulze A and Harris AL: How cancer
metabolism is tuned for proliferation and vulnerable to disruption.
Nature. 491:364–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Erdem V, Selimoglu Sen H, Komek H, et al:
Prognostic factors in non-small cell lung cancer patients and
prognostic importance of PET/CT SUV max value. Tuberk Toraks.
60:207–217. 2012.(In Turkish).
|
|
9.
|
Chan WK, Kwong DL, Yeung DW, Huang B and
Khong PL: Prognostic impact of standardized uptake value of F-18
FDG PET/CT in nasopharyngeal carcinoma. Clin Nucl Med.
36:1007–1011. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Sandulache VC, Ow TJ, Pickering CR, et al:
Glucose, not glutamine, is the dominant energy source required for
proliferation and survival of head and neck squamous carcinoma
cells. Cancer. 117:2926–2938. 2011.PubMed/NCBI
|
|
11.
|
Zhou GQ, Tang LL, Mao YP, et al: Baseline
serum lactate dehydrogenase levels for patients treated with
intensity-modulated radiotherapy for nasopharyngeal carcinoma: a
predictor of poor prognosis and subsequent liver metastasis. Int J
Radiat Oncol Biol Phys. 82:e359–365. 2012. View Article : Google Scholar
|
|
12.
|
Brown JE, Cook RJ, Lipton A and Coleman
RE: Serum lactate dehydrogenase is prognostic for survival in
patients with bone metastases from breast cancer: a retrospective
analysis in bisphosphonate-treated patients. Clin Cancer Res.
18:6348–6355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Zen HG, Jame JM, Chang AY, et al:
Nasopharyngeal carcinoma with bone marrow metastasis. Am J Clin
Oncol. 14:66–70. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Fantin VR, St-Pierre J and Leder P:
Attenuation of LDH-A expression uncovers a link between glycolysis,
mitochondrial physiology, and tumor maintenance. Cancer Cell.
9:425–434. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kim SH and Lee GM: Down-regulation of
lactate dehydrogenase-A by siRNAs for reduced lactic acid formation
of Chinese hamster ovary cells producing thrombopoietin. Appl
Microbiol Biotechnol. 74:152–159. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Sheng SL, Liu JJ, Dai YH, Sun XG, Xiong XP
and Huang G: Knockdown of lactate dehydrogenase A suppresses tumor
growth and metastasis of human hepatocellular carcinoma. FEBS J.
279:3898–3910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Yao F, Zhao T, Zhong C, Zhu J and Zhao H:
LDHA is necessary for the tumorigenicity of esophageal squamous
cell carcinoma. Tumour Biol. 34:25–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Zhang Y, Zhang X, Wang X, et al:
Inhibition of LDH-A by lentivirus-mediated small interfering RNA
suppresses intestinal-type gastric cancer tumorigenicity through
the downregulation of Oct4. Cancer Lett. 321:45–54. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Xu RH, Pelicano H, Zhou Y, et al:
Inhibition of glycolysis in cancer cells: a novel strategy to
overcome drug resistance associated with mitochondrial respiratory
defect and hypoxia. Cancer Res. 65:613–621. 2005.
|
|
20.
|
Tang Z, Yuan S, Hu Y, et al:
Over-expression of GAPDH in human colorectal carcinoma as a
preferred target of 3-bromopyruvate propyl ester. J Bioenerg
Biomembr. 44:117–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Lu W, Hu Y, Chen G, et al: Novel role of
NOX in supporting aerobic glycolysis in cancer cells with
mitochondrial dysfunction and as a potential target for cancer
therapy. PLoS Biol. 10:e10013262012. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Zeng ZL, Wu MW, Sun J, et al: Effects of
the biological clock gene Bmal1 on tumour growth and anti-cancer
drug activity. J Biochem. 148:319–326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Hu Y, Lu W, Chen G, et al: K-ras(G12V)
transformation leads to mitochondrial dysfunction and a metabolic
switch from oxidative phosphorylation to glycolysis. Cell Res.
22:399–412. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Chen G, Chen Z, Hu Y and Huang P:
Inhibition of mitochondrial respiration and rapid depletion of
mitochondrial glutathione by beta-phenethyl isothiocyanate:
mechanisms for anti-leukemia activity. Antioxid Redox Signal.
15:2911–2921. 2011. View Article : Google Scholar
|
|
25.
|
Zhao Y, Liu H, Liu Z, et al: Overcoming
trastuzumab resistance in breast cancer by targeting dysregulated
glucose metabolism. Cancer Res. 71:4585–4597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Hammoudi N, Ahmed KB, Garcia-Prieto C and
Huang P: Metabolic alterations in cancer cells and therapeutic
implications. Chin J Cancer. 30:508–525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Chen Z, Lu W, Garcia-Prieto C and Huang P:
The Warburg effect and its cancer therapeutic implications. J
Bioenerg Biomembr. 39:267–274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Fanciulli M, Bruno T, Giovannelli A, et
al: Energy metabolism of human LoVo colon carcinoma cells:
correlation to drug resistance and influence of lonidamine. Clin
Cancer Res. 6:1590–1597. 2000.
|
|
29.
|
Sun W, Liu Y, Glazer CA, et al: TKTL1 is
activated by promoter hypomethylation and contributes to head and
neck squamous cell carcinoma carcinogenesis through increased
aerobic glycolysis and HIF1alpha stabilization. Clin Cancer Res.
16:857–866. 2010. View Article : Google Scholar
|
|
30.
|
Seo M, Kim JD, Neau D, Sehgal I and Lee
YH: Structure-based development of small molecule PFKFB3
inhibitors: a framework for potential cancer therapeutic agents
targeting the Warburg effect. PLoS One. 6:e241792011. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Elwood JC: Effect of oxamate on glycolysis
and respiration in sarcoma 37 ascites cells. Cancer Res.
28:2056–2060. 1968.PubMed/NCBI
|
|
32.
|
Liu H, Savaraj N, Priebe W and Lampidis
TJ: Hypoxia increases tumor cell sensitivity to glycolytic
inhibitors: a strategy for solid tumor therapy (Model C). Biochem
Pharmacol. 64:1745–1751. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Zhou M, Zhao Y, Ding Y, et al: Warburg
effect in chemosensitivity: targeting lactate dehydrogenase-A
re-sensitizes taxol-resistant cancer cells to taxol. Mol Cancer.
9:332010. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Zhao YH, Zhou M, Liu H, et al:
Upregulation of lactate dehydrogenase A by ErbB2 through heat shock
factor 1 promotes breast cancer cell glycolysis and growth.
Oncogene. 28:3689–3701. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Liu H, Hu YP, Savaraj N, Priebe W and
Lampidis TJ: Hyper-sensitization of tumor cells to glycolytic
inhibitors. Biochemistry. 40:5542–5547. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Aghaee F, Pirayesh Islamian J and
Baradaran B: Enhanced radiosensitivity and chemosensitivity of
breast cancer cells by 2-deoxy-d-glucose in combination therapy. J
Breast Cancer. 15:141–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Manerba M, Vettraino M, Fiume L, et al:
Galloflavin (CAS 568-80-9): a novel inhibitor of lactate
dehydrogenase. Chem Med Chem. 7:311–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Cheng G, Zielonka J, Dranka BP, et al:
Mitochondria-targeted drugs synergize with 2-deoxyglucose to
trigger breast cancer cell death. Cancer Res. 72:2634–2644. 2012.
View Article : Google Scholar : PubMed/NCBI
|