Copy number increase involving chromosome 8q belong
to the most frequent aberrations in human solid cancers, including
for example tumors of the breast, ovary, endometrium, lung, colon,
head and neck, urinary bladder, kidney and prostate (1,2).
Whereas gains often affect large portions of 8q or even the entire
q-arm, high-level amplifications are typically focused on the
chromosomal bands 8q21 (3–5) and 8q24 (6,7),
thus highlighting the loci of putative oncogenes, including MYC at
8q24. Several candidate oncogenes have been suggested to reside
inside the 8q21 region, including tumor protein D52 (TPD52)
(3,8–13).
TPD52 has been suggested to play a role for vesicle
trafficking and Ca2+ dependent exocytotic secretion, and
has been shown to facilitate cytokinesis in rapidly proliferating
cells (14,15).
In line with an oncogenic role, TPD52 overexpression
has been described from a multitude of cancer types, including
breast (9), lung prostate
(2), ovarian (8), pancreatic cancer (16), multiple myeloma (17,18),
Burkitt’s lymphoma (19), melanoma
(20) and testicular germ cell
tumors (21), and has been linked
to poor prognosis in breast, medulloblastoma, lung and prostate
cancer patients (22). Moreover,
cell line experiments and in vivo analysis in mice support
the implication of TPD52 in tumorigenesis and progression to
metastasis (23,24). Accordingly, TPD52 has been
suggested as a promising target for antitumor therapies in breast
(25) and prostate cancer
(24), and it seems likely that
also other tumor types showing TPD52 overexpression could
profit from a TPD52 specific therapy.
A systematic analysis of TPD52 expression in human
cancers in order to identify tumor types that might benefit from
potential anti-TPD52 therapies is lacking. In this study, we have
employed quantitative real-time PCR in more than 900 tumor samples
to compare the prevalence and expression levels of TPD52
across 29 important human cancer types and corresponding normal
tissues.
Formalin-fixed, paraffin embedded tissues were
selected from the archive of the Institute of Pathology, University
Medical Center Hamburg-Eppendorf (Hamburg, Germany). A total of 999
cancer samples and 40 normal tissue samples were included into the
study. A detailed list of all samples is given in Table I. One pathologist reviewed all
hematoxylin and eosin stained sections of all tissues and selected
one block per tumor for RNA isolation. For tumor samples areas with
high tumor cell content (≥60% tumor cells) were marked on the
slide. A hollow needle was used to take two tissue cylinders
(diameter from 0.5 mm) from each tissue block for nucleic acid
isolation. The local ethics committee approved usage of the human
tissue samples for research purposes.
Punched tissue material was deparaffinized with
xylene and 80% ethanol. After digestion with proteinases K at 56°C
overnight, total RNA was isolated using the RNeasy FFPE kit
(Qiagen) in a full- automated nucleic acid isolation device
(QIAcube, Qiagen). cDNA was synthesized in a 96-well plate format
using the high-capacity cDNA reverse transcription kit (Applied
Biosystems) following the manufacturer’s instructions. Total RNA (1
μg) was used for reverse transcription of all samples.
Real-time PCR was performed using the LightCycler
LC480 (Roche) detection system, and the QuantiTect SYBR-Green PCR
Kit (Qiagen). For specific amplification of TPD52 and the
housekeeping gene TBP the QuantiTect Primer Assay (Qiagen) was
used. The following conditions were used for PCR: i) initial
denaturation step at 95°C for 10 min; and ii) 40 cycles at 95°C for
20 sec and 55°C for 40 sec. Relative quantity of TPD52
expression in tumor samples was calculated by the 2-ΔΔCt
method standardized to TPD52 expression in corresponding
normal tissue. A fold change of 2 was used to determine the
frequency of significant TPD52 regulated cancers.
A total of 894 cancer samples from 29 different
tumor types and 40 normal tissue samples from 20 different normal
tissue types were included in the analysis (Table I). A total of 105 (10.5%) tumor
samples and 3 (7.5%) normal tissue samples were excluded from
analysis because either the reference gene TBP or the target
gene TPD52 showed a Ct value exceeding 35, indicating that
too little cDNA was generated for reliable TPD52 expression
analysis.
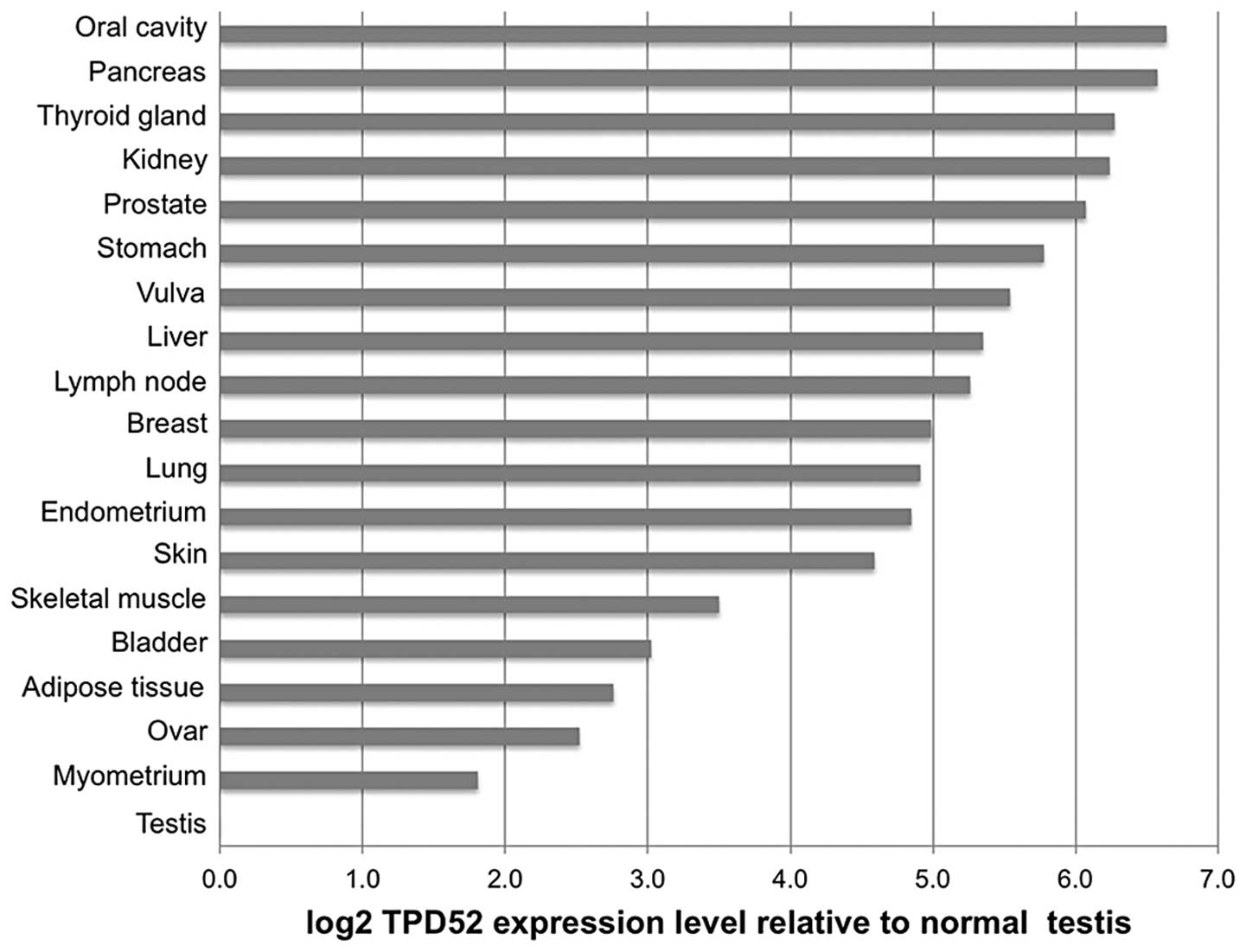
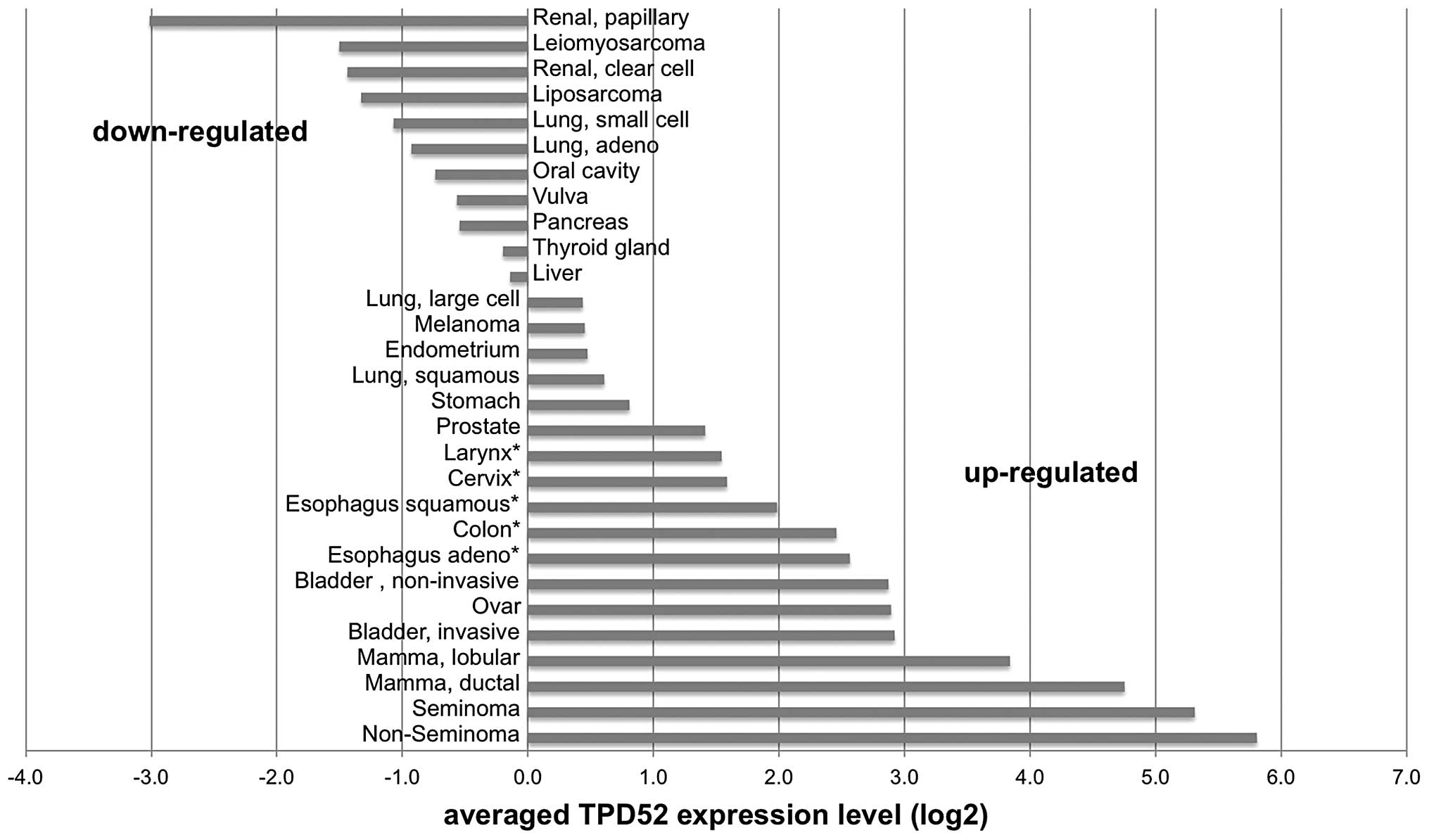
To compare TPD52 expression across the different
tumor types, average expression levels in the cancer samples were
normalized to the corresponding normal tissue. For cancer types
without available corresponding normal tissues, the average
expression level of all normal tissues (ΔCt=2.4±0.4) was used for
normalization. These cancer types included tumors of the larynx,
cervix, esophagus and colon. This analysis revealed ≥1.5-fold TPD52
overexpression in 18/29 (62%) analyzed tumor types, with highest
levels in non-seminoma (56-fold overexpression compared to
corresponding normal tissue), seminoma (42-fold), ductal breast
cancer (28-fold) and lobular breast cancer (14-fold).
Downregulation as compared to the corresponding normal tissues was
found in 11 (38%) of the analyzed tumor types, including papillary
renal cell cancer (-8-fold), leiomyosarcoma (-6-fold), clear cell
renal cell cancer (-5-fold), liposarcoma (-5-fold) and lung cancer
(-4-fold) as the tumor types with the strongest downregulation. All
data are summarized in Fig. 2.
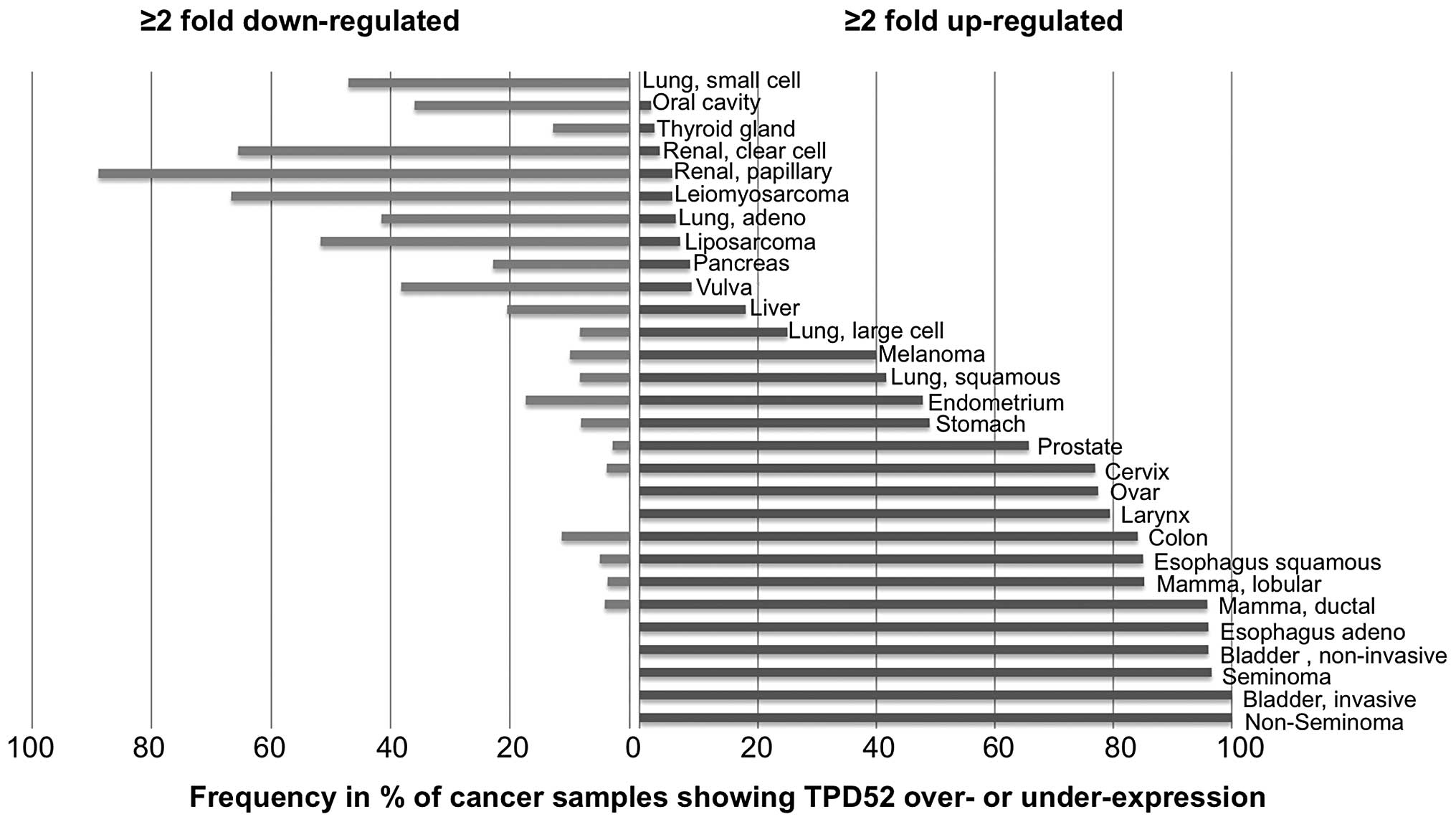
In order to estimate the variability of TPD52
expression in the analyzed tumor types, we determined the fraction
of samples showing at least 2-fold up- or downregulation. This
analysis revealed that TPD52 overexpression was particular
frequent (80% of samples showing ≥2-fold overexpression) in germ
cell tumors (non-seminoma and seminoma), bladder cancer, esophageal
carcinoma and mamma carcinoma, whereas cancer types typically
showing TPD52 downregulation included renal cell carcinoma
(90% papillary and 68% clear cell), leiomyosarcoma (69%) and
liposarcoma (52%). All data are shown in Fig. 3.
The good concordance of our results as compared to
published studies underlines the validity of our analysis. Only few
tumor types analyzed in our study showed discrepant results as
compared to the literature, including pancreatic cancer, melanoma
and colon cancer. It is possible, that these discrepancies are due
to technical differences in the first place. For example,
Loukopoulos et al (16)
demonstrated upregulation of TPD52 in all 42 analyzed tumors, but
analyzed xenografts instead of primary tumors in order to
artificially maximize the fraction of tumor cells in the sample.
Riker et al (60),
Skrzypczak et al (61) and
Hong et al (62) reported
downregulation of TPD52 expression in colon cancer, whereas
upregulation was found in our analysis. We did not include normal
colon as a reference in our study but used an average expression
value across all analyzed normal tissues as a surrogate. It is
possible, that this strategy resulted in bias. The same may also
apply for our results obtained from larynx, cervix and esophageal
carcinomas, where we used the same ‘average’ reference.
The usage of matched normal tissues for reference in
the vast majority of our samples enabled us to estimate the
relative impact of TPD52 in the individual tumor types. Testicular
germ cell cancers showed the highest levels and frequency of
TPD52 expression, suggesting that TPD52 upregulation
might play a particularly important role in this tumor type.
Comparative genomic hybridization data suggest that large fractions
of chromosome 8q, including the TPD52 locus, can be
frequently [42–70%, (21,63,64)]
gained or amplified (21) in
testicular germ cell cancers, supporting a role of genomic gains
for the overexpression also in this cancer type. This is consistent
with overexpression of TPD52 in testicular germ cell tumors
with CGH-confirmed 8q copy number gains reported by Scotheim et
al (21).
We thank Christina Koop, Inge Brandt,
Jannette Lütgens and Bianca Kelp for excellent technical
assistance.
|
1.
|
Schraml P, Kononen J, Bubendorf L, et al:
Tissue microarrays for gene amplification surveys in many different
tumor types. Clin Cancer Res. 5:1966–1975. 1999.PubMed/NCBI
|
|
2.
|
Rubin MA, Varambally S, Beroukhim R, et
al: Overexpression, amplification, and androgen regulation of TPD52
in prostate cancer. Cancer Res. 64:3814–3822. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Choschzick M, Lassen P, Lebeau A, et al:
Amplification of 8q21 in breast cancer is independent of MYC and
associated with poor patient outcome. Mod Pathol. 23:603–610. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Richter J, Jiang F, Gorog JP, et al:
Marked genetic differences between stage pTa and stage pT1
papillary bladder cancer detected by comparative genomic
hybridization. Cancer Res. 57:2860–2864. 1997.
|
|
5.
|
Sauter GH, Munzing W, von Ritter C and
Paumgartner G: Bile acid malabsorption as a cause of chronic
diarrhea: diagnostic value of 7alpha-hydroxy-4-cholesten-3-one in
serum. Dig Dis Sci. 44:14–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
El Gedaily A, Bubendorf L, Willi N, et al:
Discovery of new DNA amplification loci in prostate cancer by
comparative genomic hybridization. Prostate. 46:184–190.
2001.PubMed/NCBI
|
|
7.
|
Al-Kuraya K, Schraml P, Torhorst J, et al:
Prognostic relevance of gene amplifications and coamplifications in
breast cancer. Cancer Res. 64:8534–8540. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Byrne JA, Balleine RL, Schoenberg Fejzo M,
et al: Tumor protein D52 (TPD52) is overexpressed and a gene
amplification target in ovarian cancer. Int J Cancer.
117:1049–1054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Balleine RL, Fejzo MS, Sathasivam P,
Basset P, Clarke CL and Byrne JA: The hD52 (TPD52) gene is a
candidate target gene for events resulting in increased 8q21 copy
number in human breast carcinoma. Genes Chromosomes Cancer.
29:48–57. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Van Duin M, van Marion R, Vissers K, et
al: High-resolution array comparative genomic hybridization of
chromosome arm 8q: evaluation of genetic progression markers for
prostate cancer. Genes Chromosomes Cancer. 44:438–449.
2005.PubMed/NCBI
|
|
11.
|
Hicks J, Krasnitz A, Lakshmi B, et al:
Novel patterns of genome rearrangement and their association with
survival in breast cancer. Genome Res. 16:1465–1479. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Rodriguez V, Chen Y, Elkahloun A, Dutra A,
Pak E and Chandrasekharappa S: Chromosome 8 BAC array comparative
genomic hybridization and expression analysis identify
amplification and overexpression of TRMT12 in breast cancer. Genes
Chromosomes Cancer. 46:694–707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kim JH, Dhanasekaran SM, Mehra R, et al:
Integrative analysis of genomic aberrations associated with
prostate cancer progression. Cancer Res. 67:8229–8239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Boutros R, Fanayan S, Shehata M and Byrne
JA: The tumor protein D52 family: many pieces, many puzzles.
Biochem Biophys Res Commun. 325:1115–1121. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Thomas DD, Frey CL, Messenger SW, August
BK and Groblewski GE: A role for tumor protein TPD52
phosphorylation in endo-membrane trafficking during cytokinesis.
Biochem Biophys Res Commun. 402:583–587. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Loukopoulos P, Shibata T, Katoh H, et al:
Genome-wide array-based comparative genomic hybridization analysis
of pancreatic adenocarcinoma: identification of genetic indicators
that predict patient outcome. Cancer Sci. 98:392–400. 2007.
View Article : Google Scholar
|
|
17.
|
Largo C, Alvarez S, Saez B, et al:
Identification of overexpressed genes in frequently
gained/amplified chromosome regions in multiple myeloma.
Haematologica. 91:184–191. 2006.PubMed/NCBI
|
|
18.
|
Tiacci E, Orvietani PL, Bigerna B, et al:
Tumor protein D52 (TPD52): a novel B-cell/plasma-cell molecule with
unique expression pattern and Ca(2+)-dependent association with
annexin VI. Blood. 105:2812–2820. 2005.PubMed/NCBI
|
|
19.
|
Dave SS, Fu K, Wright GW, et al: Molecular
diagnosis of Burkitt’s lymphoma. N Engl J Med. 354:2431–2442.
2006.
|
|
20.
|
Roesch A, Becker B, Bentink S, et al:
Ataxia telangiectasia-mutated gene is a possible biomarker for
discrimination of infiltrative deep penetrating nevi and metastatic
vertical growth phase melanoma. Cancer Epidemiol Biomarkers Prev.
16:2486–2490. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Skotheim RI, Autio R, Lind GE, et al:
Novel genomic aberrations in testicular germ cell tumors by
array-CGH, and associated gene expression changes. Cell Oncol.
28:315–326. 2006.PubMed/NCBI
|
|
22.
|
Liu R, Wang X, Chen GY, et al: The
prognostic role of a gene signature from tumorigenic breast-cancer
cells. N Engl J Med. 356:217–226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Lewis JD, Payton LA, Whitford JG, et al:
Induction of tumorigenesis and metastasis by the murine orthologue
of tumor protein D52. Mol Cancer Res. 5:133–144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Ummanni R, Teller S, Junker H, et al:
Altered expression of tumor protein D52 regulates apoptosis and
migration of prostate cancer cells. FEBS J. 275:5703–5713. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Shehata M, Bieche I, Boutros R, et al:
Nonredundant functions for tumor protein D52-like proteins support
specific targeting of TPD52. Clin Cancer Res. 14:5050–5060. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Porter D, Lahti-Domenici J, Keshaviah A,
et al: Molecular markers in ductal carcinoma in situ of the breast.
Mol Cancer Res. 1:362–375. 2003.PubMed/NCBI
|
|
27.
|
Sorlie T, Perou CM, Tibshirani R, et al:
Gene expression patterns of breast carcinomas distinguish tumor
subclasses with clinical implications. Proc Natl Acad Sci USA.
98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Gruvberger S, Ringner M, Chen Y, et al:
Estrogen receptor status in breast cancer is associated with
remarkably distinct gene expression patterns. Cancer Res.
61:5979–5984. 2001.PubMed/NCBI
|
|
29.
|
Wang R, Xu J, Saramaki O, et al: PrLZ, a
novel prostate-specific and androgen-responsive gene of the TPD52
family, amplified in chromosome 8q21.1 and overexpressed in human
prostate cancer. Cancer Res. 64:1589–1594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Dhanasekaran SM, Barrette TR, Ghosh D, et
al: Delineation of prognostic biomarkers in prostate cancer.
Nature. 412:822–826. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Welsh JB, Sapinoso LM, Su AI, et al:
Analysis of gene expression identifies candidate markers and
pharmacological targets in prostate cancer. Cancer Res.
61:5974–5978. 2001.PubMed/NCBI
|
|
32.
|
Bhattacharjee A, Richards WG, Staunton J,
et al: Classification of human lung carcinomas by mRNA expression
profiling reveals distinct adenocarcinoma subclasses. Proc Natl
Acad Sci USA. 98:13790–13795. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Garber ME, Troyanskaya OG, Schluens K, et
al: Diversity of gene expression in adenocarcinoma of the lung.
Proc Natl Acad Sci USA. 98:13784–13789. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Beer DG, Kardia SL, Huang CC, et al:
Gene-expression profiles predict survival of patients with lung
adenocarcinoma. Nat Med. 8:816–824. 2002.PubMed/NCBI
|
|
35.
|
Dyrskjot L, Thykjaer T, Kruhoffer M, et
al: Identifying distinct classes of bladder carcinoma using
microarrays. Nat Genet. 33:90–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Pomeroy SL, Tamayo P, Gaasenbeek M, et al:
Prediction of central nervous system embryonal tumour outcome based
on gene expression. Nature. 415:436–442. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Huang Y, Prasad M, Lemon WJ, et al: Gene
expression in papillary thyroid carcinoma reveals highly consistent
profiles. Proc Natl Acad Sci USA. 98:15044–15049. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Risinger JI, Maxwell GL, Chandramouli GV,
et al: Microarray analysis reveals distinct gene expression
profiles among different histologic types of endometrial cancer.
Cancer Res. 63:6–11. 2003.PubMed/NCBI
|
|
39.
|
Mutter GL, Baak JP, Fitzgerald JT, et al:
Global expression changes of constitutive and hormonally regulated
genes during endometrial neoplastic transformation. Gynecol Oncol.
83:177–185. 2001. View Article : Google Scholar
|
|
40.
|
Giordano TJ, Thomas DG, Kuick R, et al:
Distinct transcriptional profiles of adrenocortical tumors
uncovered by DNA microarray analysis. Am J Pathol. 162:521–531.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Chen X, Cheung ST, So S, et al: Gene
expression patterns in human liver cancers. Mol Biol Cell.
13:1929–1939. 2002. View Article : Google Scholar PubMed/NCBI
|
|
42.
|
Pollack JR, Sorlie T, Perou CM, et al:
Microarray analysis reveals a major direct role of DNA copy number
alteration in the transcriptional program of human breast tumors.
Proc Natl Acad Sci USA. 99:12963–12968. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Adelaide J, Finetti P, Bekhouche I, et al:
Integrated profiling of basal and luminal breast cancers. Cancer
Res. 67:11565–11575. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Pache M, Glatz-Krieger K, Sauter G and
Meyer P: Expression of sex hormone receptors and cell cycle
proteins in melanocytic lesions of the ocular conjunctiva. Graefes
Archive Clin Exp Ophthalmol. 244:113–117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Jonsson G, Staaf J, Olsson E, et al:
High-resolution genomic profiles of breast cancer cell lines
assessed by tiling BAC array comparative genomic hybridization.
Genes Chromosomes Cancer. 46:543–558. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Bulavin DV, Demidov ON, Saito S, et al:
Amplification of PPM1D in human tumors abrogates p53
tumor-suppressor activity. Nat Genet. 31:210–215. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Parris TZ, Danielsson A, Nemes S, et al:
Clinical implications of gene dosage and gene expression patterns
in diploid breast carcinoma. Clin Cancer Res. 16:3860–3874. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Hawthorn L, Luce J, Stein L and Rothschild
J: Integration of transcript expression, copy number and LOH
analysis of infiltrating ductal carcinoma of the breast. BMC
Cancer. 10:4602010. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Melchor L, Alvarez S, Honrado E, et al:
The accumulation of specific amplifications characterizes two
different genomic pathways of evolution of familial breast tumors.
Clin Cancer Res. 11:8577–8584. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Hernandez L, Wilkerson PM, Lambros MB, et
al: Genomic and mutational profiling of ductal carcinomas in situ
and matched adjacent invasive breast cancers reveals intra-tumour
genetic heterogeneity and clonal selection. J Pathol. 227:42–52.
2012. View Article : Google Scholar
|
|
51.
|
Valk PJ, Verhaak RG, Beijen MA, et al:
Prognostically useful gene-expression profiles in acute myeloid
leukemia. N Engl J Med. 350:1617–1628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Maia S, Haining WN, Ansen S, et al: Gene
expression profiling identifies BAX-delta as a novel tumor antigen
in acute lymphoblastic leukemia. Cancer Res. 65:10050–10058. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Andersson A, Ritz C, Lindgren D, et al:
Microarray-based classification of a consecutive series of 121
childhood acute leukemias: prediction of leukemic and genetic
subtype as well as of minimal residual disease status. Leukemia.
21:1198–1203. 2007. View Article : Google Scholar
|
|
54.
|
Basso K, Margolin AA, Stolovitzky G, Klein
U, Dalla-Favera R and Califano A: Reverse engineering of regulatory
networks in human B cells. Nat Genet. 37:382–390. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Storz MN, van de Rijn M, Kim YH,
Mraz-Gernhard S, Hoppe RT and Kohler S: Gene expression profiles of
cutaneous B cell lymphoma. J Invest Dermatol. 120:865–870. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Bredel M, Bredel C, Juric D, et al:
High-resolution genome-wide mapping of genetic alterations in human
glial brain tumors. Cancer Res. 65:4088–4096. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Sun L, Hui AM, Su Q, et al: Neuronal and
glioma-derived stem cell factor induces angiogenesis within the
brain. Cancer Cell. 9:287–300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Murat A, Migliavacca E, Gorlia T, et al:
Stem cell-related ‘self-renewal’ signature and high epidermal
growth factor receptor expression associated with resistance to
concomitant chemoradiotherapy in glioblastoma. J Clin Oncol.
26:3015–3024. 2008.
|
|
59.
|
Detwiller KY, Fernando NT, Segal NH, Ryeom
SW, D’Amore PA and Yoon SS: Analysis of hypoxia-related gene
expression in sarcomas and effect of hypoxia on RNA interference of
vascular endothelial cell growth factor A. Cancer Res.
65:5881–5889. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Riker AI, Enkemann SA, Fodstad O, et al:
The gene expression profiles of primary and metastatic melanoma
yields a transition point of tumor progression and metastasis. BMC
Med Genomics. 1:132008. View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Skrzypczak M, Goryca K, Rubel T, et al:
Modeling oncogenic signaling in colon tumors by multidirectional
analyses of microarray data directed for maximization of analytical
reliability. PLoS One. 5:e130912010. View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Hong Y, Downey T, Eu KW, Koh PK and Cheah
PY: A ‘metastasis-prone’ signature for early-stage mismatch-repair
proficient sporadic colorectal cancer patients and its implications
for possible therapeutics. Clin Exp Metastasis. 27:83–90. 2010.
|
|
63.
|
Korkola JE, Heck S, Olshen AB, et al: In
vivo differentiation and genomic evolution in adult male germ cell
tumors. Genes Chromosomes Cancer. 47:43–55. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
McIntyre A, Summersgill B, Lu YJ, et al:
Genomic copy number and expression patterns in testicular germ cell
tumours. Br J Cancer. 97:1707–1712. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65.
|
Payton LA, Lewis JD, Byrne JA and Bright
RK: Vaccination with metastasis-related tumor associated antigen
TPD52 and CpG/ODN induces protective tumor immunity. Cancer Immunol
Immunother. 57:799–811. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66.
|
Lewis J, Sullivan L, Byrne J, de Riese W
and Bright R: Memory and cellular immunity induced by a DNA vaccine
encoding self antigen TPD52 administered with soluble GM-CSF.
Cancer Immunol Immunother. 58:1337–1349. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67.
|
Talantov D, Mazumder A, Yu JX, et al:
Novel genes associated with malignant melanoma but not benign
melanocytic lesions. Clin Cancer Res. 11:7234–7242. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
68.
|
Zhu H, Lam DC, Han KC, et al: High
resolution analysis of genomic aberrations by metaphase and array
comparative genomic hybridization identifies candidate tumour genes
in lung cancer cell lines. Cancer Lett. 245:303–314. 2007.
View Article : Google Scholar
|
|
69.
|
Yamagata N, Shyr Y, Yanagisawa K, et al: A
training-testing approach to the molecular classification of
resected non-small cell lung cancer. Clin Cancer Res. 9:4695–4704.
2003.PubMed/NCBI
|
|
70.
|
Wachi S, Yoneda K and Wu R:
Interactome-transcriptome analysis reveals the high centrality of
genes differentially expressed in lung cancer tissues.
Bioinformatics. 21:4205–4208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
71.
|
Su LJ, Chang CW, Wu YC, et al: Selection
of DDX5 as a novel internal control for Q-RT-PCR from microarray
data using a block bootstrap re-sampling scheme. BMC Genomics.
8:1402007. View Article : Google Scholar : PubMed/NCBI
|
|
72.
|
Hou J, Aerts J, den Hamer B, et al: Gene
expression-based classification of non-small cell lung carcinomas
and survival prediction. PLoS One. 5:e103122010. View Article : Google Scholar : PubMed/NCBI
|
|
73.
|
Yu K, Lee CH, Tan PH and Tan P:
Conservation of breast cancer molecular subtypes and
transcriptional patterns of tumor progression across distinct
ethnic populations. Clin Cancer Res. 10:5508–5517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
74.
|
Scanlan MJ, Gout I, Gordon CM, et al:
Humoral immunity to human breast cancer: antigen definition and
quantitative analysis of mRNA expression. Cancer Immun.
1:42001.PubMed/NCBI
|
|
75.
|
Byrne JA, Tomasetto C, Garnier JM, et al:
A screening method to identify genes commonly overexpressed in
carcinomas and the identification of a novel complementary DNA
sequence. Cancer Res. 55:2896–2903. 1995.PubMed/NCBI
|
|
76.
|
Byrne JA, Maleki S, Hardy JR, et al: MAL2
and tumor protein D52 (TPD52) are frequently overexpressed in
ovarian carcinoma, but differentially associated with histological
subtype and patient outcome. BMC Cancer. 10:4972010. View Article : Google Scholar : PubMed/NCBI
|
|
77.
|
Tothill RW, Tinker AV, George J, et al:
Novel molecular subtypes of serous and endometrioid ovarian cancer
linked to clinical outcome. Clin Cancer Res. 14:5198–5208. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
78.
|
Hendrix ND, Wu R, Kuick R, Schwartz DR,
Fearon ER and Cho KR: Fibroblast growth factor 9 has oncogenic
activity and is a downstream target of Wnt signaling in ovarian
endometrioid adenocarcinomas. Cancer Res. 66:1354–1362. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
79.
|
Petrova DT, Asif AR, Armstrong VW, et al:
Expression of chloride intracellular channel protein 1 (CLIC1) and
tumor protein D52 (TPD52) as potential biomarkers for colorectal
cancer. Clin Biochem. 41:1224–1236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80.
|
Buffart TE, Coffa J, Hermsen MA, et al:
DNA copy number changes at 8q11-24 in metastasized colorectal
cancer. Cell Oncol. 27:57–65. 2005.PubMed/NCBI
|
|
81.
|
Kaiser S, Park YK, Franklin JL, et al:
Transcriptional recapitulation and subversion of embryonic colon
development by mouse colon tumor models and human colon cancer.
Genome Biol. 8:R1312007. View Article : Google Scholar : PubMed/NCBI
|
|
82.
|
Roessler S, Jia HL, Budhu A, et al: A
unique metastasis gene signature enables prediction of tumor
relapse in early-stage hepatocellular carcinoma patients. Cancer
Res. 70:10202–10212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
83.
|
Wang R, Xu J, Mabjeesh N, et al: PrLZ is
expressed in normal prostate development and in human prostate
cancer progression. Clin Cancer Res. 13:6040–6048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
84.
|
Cho-Vega JH, Tsavachidis S, Do KA,
Nakagawa J, Medeiros LJ and McDonnell TJ: Dicarbonyl/L-xylulose
reductase: a potential biomarker identified by laser-capture
microdissection-micro serial analysis of gene expression of human
prostate adenocarcinoma. Cancer Epidemiol Biomarkers Prev.
16:2615–2622. 2007. View Article : Google Scholar
|
|
85.
|
Hendriksen PJ, Dits NF, Kokame K, et al:
Evolution of the androgen receptor pathway during progression of
prostate cancer. Cancer Res. 66:5012–5020. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
86.
|
Bismar TA, Demichelis F, Riva A, et al:
Defining aggressive prostate cancer using a 12-gene model.
Neoplasia. 8:59–68. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
87.
|
Rhodes DR, Barrette TR, Rubin MA, Ghosh D
and Chinnaiyan AM: Meta-analysis of microarrays: interstudy
validation of gene expression profiles reveals pathway
dysregulation in prostate cancer. Cancer Res. 62:4427–4433.
2002.PubMed/NCBI
|
|
88.
|
Luo J, Duggan DJ, Chen Y, et al: Human
prostate cancer and benign prostatic hyperplasia: molecular
dissection by gene expression profiling. Cancer Res. 61:4683–4688.
2001.PubMed/NCBI
|
|
89.
|
Dyrskjot L, Zieger K, Real FX, et al: Gene
expression signatures predict outcome in non-muscle-invasive
bladder carcinoma: a multicenter validation study. Clin Cancer Res.
13:3545–3551. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
90.
|
Sanchez-Carbayo M, Socci ND, Lozano J,
Saint F and Cordon-Cardo C: Defining molecular profiles of poor
outcome in patients with invasive bladder cancer using
oligonucleotide microarrays. J Clin Oncol. 24:778–789. 2006.
View Article : Google Scholar : PubMed/NCBI
|