Introduction
Ovarian cancer is the most lethal gynecologic
malignancy and the fifth leading cause of cancer death in women
(1–3). The estimated newly diagnosed cases of
ovarian cancer are 204,000 with 125,000 deaths annually worldwide
(1,4). In human, there are three major types
of ovarian cancer: epithelia, stromal and germ cell (5). Among these, epithelial ovarian cancer
(EOC) accounts for about 85–90% of total ovarian cancers and occurs
most commonly in postmenopausal women (2,6). The
5-year survival of patients diagnosed with ovarian cancer at an
early stage is significantly different from that of patients with
ovarian cancer at an advanced stage. Studies over the past 3
decades show that the 5-year disease-free survival and overall
survival of patients with the early stage (stage I) ovarian cancer
are 91 and 94%, respectively, with surgery alone (7). However, the 5-year survival of
patients with advanced stage disease is <30% (8) and has not enhanced significantly,
despite the improvement of various treatments. EOC is highly lethal
because it tends to be at an advanced stage upon diagnosis. The low
survival rate for women with EOC results in part from an inability
to detect the disease at an early curable stage, the lack of
effective treatment for the advanced cancer, and our incomplete
understanding of how the EOC develops. It is, therefore, essential
to identify potential biomarkers and their regulation by the
signaling pathways which are possibly altered in the early stages
of ovarian cancer. To this effect, we have applied a proteomics
study to identify potential biomarkers by comparing BRCA1-mutated
immortalized ovarian surface epithelial (OSE) cells, genetically
predisposed to ovarian cancer due to the expression of a mutated
BRCA1, to wild-type immortalized OSE cells, since women with a
germ-line mutation in the BRCA1 gene have a cumulative lifetime
risk of 40–50% for ovarian cancer (9). We observed a set of differentially
expressed proteins (unpublished data). Among these proteins,
cystatin B (CSTB) was found to be increased in BRCA1-mutated
cells.
CSTB, also known as stefin B, belongs to the
cystatins superfamily which has primarily been explored with
respect to its capacity to inhibit intracellular cysteine proteases
leaking from lysosomes (10) and
has been implicated in several types of cancers, such as breast,
lung and colorectal cancers, glioblastoma, squamous cell carcinoma
of the head and neck, laryngeal, esophageal and hepatocellular
carcinomas, and prostatic adenocarcinoma (11–20).
The dysregulated expression of CSTB appears to be associated with
tumorigenesis and may be mediated by a variety of cytokines and
growth factors, including transforming growth factor-β (TGF-β).
TGF-β has been implicated in many developmental,
physiologic and pathologic processes (21), and plays a key role in
tumorigenesis of many tissues, including the ovary (22). TGF-β signals through type I and
type II serine/threonine kinase receptors and intracellular
signaling Smad proteins (23,24).
Following receptor activation upon TGF-β stimulation,
receptor-regulated Smads, such as Smad2 and Smad3, are
phosphorylated and activated, and subsequently form complexes with
Smad4 that translocate into the nucleus where they interact with
other transcription factors to regulate the transcription of target
genes (25), thereby activating
downstream signaling cascades. However, accumulated evidence shows
that TGF-β signaling is impaired in ovarian cancer. TGF-β1, its
receptors (TβR-I and TβR-II), and its signaling proteins (Smad2 and
Smad4) have been found to be mutated in ovarian cancer (26,27).
Loss-of-function mutations can lead to the disruption of
TGF-β-signaling pathways and subsequent loss of cell cycle control
(28).
The current study was undertaken to explore the
expression of CSTB in human ovarian benign, borderline, and
malignant tumors and investigate whether CSTB expression is
associated with clinicopathological features of human EOC.
Furthermore, we examined for the first time the regulation of CSTB
expression mediated by the TGF-β signaling pathway in ovarian
cancer cells.
Materials and methods
Patients and ovarian tissue samples
The study on human subjects was approved by the
Ethics Committee of Jinshan Hospital, Fudan University. The ovarian
tissue samples from 27 patients with ovarian tumor and 6 patients
with non-ovarian tumor at Jinshan Hospital, Fudan University from
January, 2005 to December, 2012 were retrieved for the present
study. All patients underwent cytoreductive surgery. None of the
patients had received chemotherapy or radiotherapy before surgery.
The 10% formalin-fixed paraffin-embedded ovarian tissue specimens
were collected and pathologically diagnosed by the Department of
Pathology, Jinshan Hospital. The histological examination and
classification were based on the current criteria of tumor, node,
metastasis (TNM) classification method from the American Joint
Committee on Cancer (AJCC) and from the World Health Organization
(WHO). Final diagnosis with tumor stage and grade was performed by
experienced gynaecologists and pathologists according to the FIGO
(International Federation of Gynaecological Oncologists) system.
Among the patients, thirteen patients aged 39–66, four patients
aged 37 to 50, and ten patients aged 39 to 70 were diagnosed with
malignant, borderline and benign tumors, respectively. The normal
ovaries from six patients aged 41 to 67 who were diagnosed with
non-ovarian diseases were removed and used as controls.
Immunohistochemical staining and
analysis
Four micrometer tissue section was heated at 60°C
for 2 h, followed by deparaffinizing in xylene and rehydrating with
graded alcohols. For antigen retrieval, the section was immersed in
0.1 M sodium citrate buffer (pH 6.0) and heated in a microwave oven
at 100°C for 1.5 min. Endogenous peroxide activity was quenched by
3% hydrogen peroxide in methanol for 15 min. After blocking with
10% normal goat serum (Maixin Bio, Fuzhou, China) for 40 min at
room temperature, the section was incubated with a polyclonal
rabbit anti-CSTB antibody (1:1,000 dilution, Abcam, Hong Kong,
China) at 4°C overnight, followed by incubation with biotinylated
anti-rabbit secondary antibody (Maixin Bio) at room temperature for
1 h. After washing, the signal was detected using a DAB Kit (Maixin
Bio). Finally, the section was counterstained with hematoxylin and
photographed under a light microscope (Nikon, Tokyo, Japan). A
normal ovarian tissue without a primary antibody was used as a
negative control.
Scoring of CSTB immunoreactive staining was
performed by two independent pathologists without any prior
knowledge of patient’s clinical data. The proportion of positive
cells was scored by the extent of immunoreactive staining to one of
the following categories as described previously (29): 0, 0% no positive cells; 1, ≤25%
positive cells; 2, 26–50% positive cells; 3, 51–75% positive cells;
and 4, >75% positive cells. The intensity of immunoreactive
staining was assigned as 0, no staining; 1, weak staining; 2,
moderate staining; and 3, strong staining. A final immunoreactive
score, also known as the staining index (SI), was determined by the
sum of the positive extent and staining intensity. SI was clustered
into four groups: ‘0’, ≤2 sum points; ‘1’, 3–4 sum points; ‘2’, 5–6
sum points; and ‘3’, 7 sum points. Finally, for this study, we
defined the cases with grades equal to 0 and 1 as CSTB negativity
and those with grades equal to 2 and 3 as CSTB positivity.
Cell culture and TGF-β1 treatment
Human serous ovarian cancer cell lines were
purchased from American Type Culture Collection (ATCC, Manassas,
VA, USA). OVCAR-3 cells were cultured in RPMI-1640 medium (HyClone,
Thermo Fisher Scientific Inc., Beijing, China) supplemented with
20% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA, USA).
SK-OV-3 cells were cultured in McCoy’s 5A medium (Sigma, Saint
Louis, MO, USA) supplemented with 10% FBS. After seeding for 24 h,
the cells were treated with recombinant human TGF-β1 (R&D
Systems, Minneapolis, MN, USA) at different doses (0, 0.1, 1 and 10
ng/ml) for 24 h. For a time-course study, OVCAR-3 and SK-OV-3 cells
were treated with 10 and 1 ng/ml of TGF-β1, respectively, for 1, 6
and 24 h. A TGF-β type I receptor kinase inhibitor, SB-431542
(30), was obtained from Sigma and
dissolved at a concentration of 10 mM in dimethyl sulfoxide (DMSO).
For blocking the TGF-β signaling pathway, cells were pre-treated
with 10 μM SB-431542 for 30 min and then treated with TGF-β1
for 24 h. DMSO was used as a vehicle control.
RNA extraction and quantitative real-time
PCR
Total RNA in the cells was extracted using TRIzol
(Invitrogen), according to the manufacturer’s instruction. One
microgram of total RNA was reversely transcribed using a reverse
transcription kit (Takara Biotechnology Co., Ltd., Dalian, China).
The primer sequences were 5′-CTGTGTTTAAGGCCGTGTCA-3′ (forward) and
5′-AGGTCAGCTCATCATGCTTG-3′ (reverse) for human CSTB and
5′-ACAATGTGGCCGAGGACTTT-3′ (forward) and 5′-GCACGAAGGCTCATCATTCA-3′
(reverse) for human β-actin (synthesized by Sangon Biotech Co.,
Ltd., Shanghai, China). PCR amplification was performed at 95°C, 5
sec and 60°C, 31 sec for 40 cycles using Takara SYBR Premix
Taq™ II (Tli RNaseH Plus) kit (Takara Biotechnology Co.,
Ltd.) with an initial step of denaturing RNA at 95°C for 30 sec.
Assays were conducted in triplicate and repeated three times. The
amount of target (CSTB) normalized to an endogenous control
(β-actin) given by 2ΔΔCt, in which threshold cycle (Ct)
was obtained using Sequence Detection Software v1.4 (7300 Real Time
PCR System, Applied Biosystems, Foster City, CA, USA).
Western blot analysis
OVCAR-3 and SK-OV-3 cells were lysed in SDS lysis
buffer (Beyotime, Haimen, China) with 1% PMSF (Beyotime), followed
by sonication. Equal amount of protein was separated on 15%
SDS-PAGE and transferred to a PVDF membrane (Millipore, Billerica,
MA, USA). After blocking with 5% non-fat milk in Tris-buffered
saline with Tween-20 (TBS-T) for 1 h, the membrane was incubated
with a primary antibody at 4°C overnight and subsequently incubated
with horseradish peroxidase-conjugated goat anti-rabbit or
anti-mouse IgG (1:3,000 dilution, Cell Signaling Technology, Inc.,
Danvers, MA, USA) for 1 h at room temperature. The following
primary antibodies were used: rabbit anti-CSTB (1:10,000 dilution,
Abcam), mouse anti-Smad 2 (1:2,000 dilution), rabbit
anti-phospho-Smad2 (1:2,000 dilution), and rabbit anti-β-actin
(1:5,000 dilution) (Cell Signaling Technology, Inc.). Signals were
detected using Immobilon™ Western Chemiluminescent HRP Substrate
(Millipore) and quantified using Tanon-4500 Gel Imaging System with
GIS ID Analysis Software v4.1.5 (Tanon Science & Technology
Co., Ltd., Shanghai, China).
Statistical analyses
All analyses were performed with SPSS Statistics
19.0 for Windows (SPSS, Chicago, IL, USA). For comparison between
two groups of positivity and correlation between CSTB expression
and histological types or the clinicopathological characteristics,
a Fisher’s exact test was performed. For comparison between the two
scoring groups of immunostaining, a Wilcoxon rank-sum test was
applied. For comparison between two groups in the treatment
experiments, a Student’s t-test was used. Results are presented as
the mean ± standard error of mean (SEM). Significant difference was
considered at the value of P<0.05.
Results
Overexpression of CSTB in human ovarian
tumors
By immunohistochemistry staining, the overexpression
of CSTB was observed in human ovarian tumors, including benign,
borderline and malignant tumors, albeit at different levels
(Fig. 1A, B and C), compared with
the normal ovarian tissues that showed negative staining (Fig. 1D). The positive staining (brown
color) was mainly distributed in the cytoplasm of the epithelial
cells of ovarian tumor and the expression of CSTB protein was weak
in benign tumors, moderate in borderline tumors, and strong in
malignant tumors. Indeed the highest intensity of staining was
found in ovarian serous, mucinous and clear cell malignant tumors
(Fig. 1A, B and C). Based on the
SI system as indicated before, we classified CSTB expression into
positive and negative categories (Table I). By comparison of ovarian tumors
with the normal ovarian tissue, we found that the positive rate of
CSTB expression was different. The positive rate of CSTB expression
was significantly higher in tumors, including benign, borderline
and malignant tumors, than that in normal tissue (P<0.01),
though there was no difference in the CSTB-positive rate between
benign, borderline and malignant tumors (P>0.05) (Table I). However, statistical analysis of
the immunoreactive scores of CSTB protein between the types of
tissues indicated that the expression of CSTB protein was different
between the four groups: the normal ovarian tissue, ovarian benign
tumor, ovarian borderline tumor and ovarian malignant tumor. The
immunoreactive score of CSTB protein is significantly higher in the
tumors than that in the normal tissue (all P<0.01) (Table II). Furthermore, by comparison of
benign, borderline and malignant tumors, we observed that the
immunoreactive score of CSTB protein was significantly higher in
borderline and malignant tumors than that in benign tumors (both
P<0.05). The expression of CSTB protein tended to be high in
malignant tumors, but there was no significant difference of
immunoreactive score of CSTB protein between borderline and
malignant tumors (P>0.05). These data suggest that CSTB is an
ovarian tumor marker and an increase in the expression of CSTB in
ovarian tissue represents tumor progression.
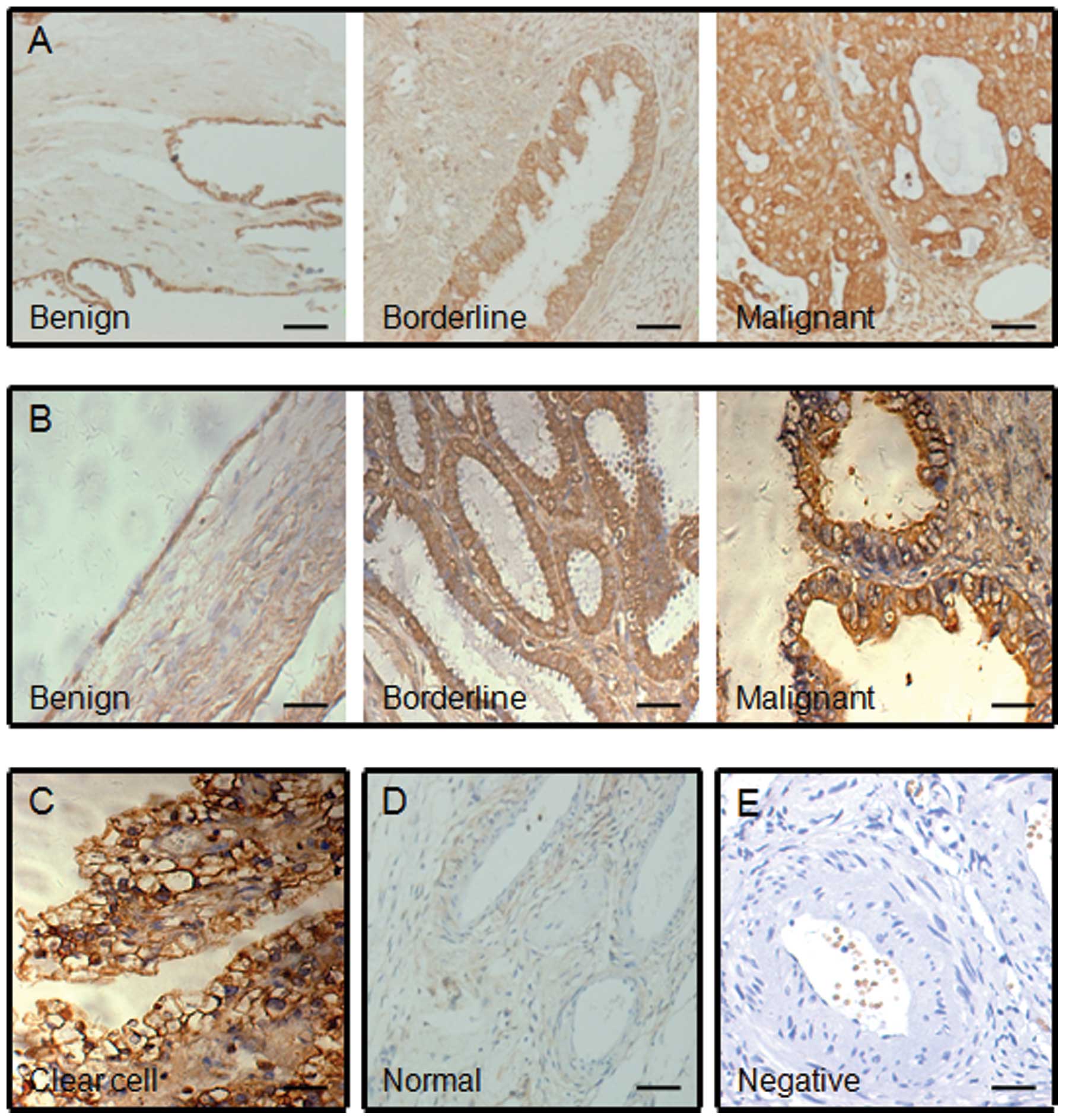 | Figure 1.Immunohistochemical staining of CSTB
protein in human ovarian tissues. Representative images of CSTB
expression in (A) ovarian serous, (B) mucinous and (C) clear cell
tumors, and (D) the normal ovarian tissue are shown. Benign, benign
tumor; borderline, borderline tumor; malignant, malignant tumor;
clear cell, clear cell malignant tumor; normal, normal ovarian
tissue; negative, negative control without first antibody in the
(E) normal ovarian tissue. A brown color in epithelial cell is
considered as a positive staining. Original magnification, ×200.
Scale bar, 100 μm. |
 | Table I.CSTB protein expression in human
ovarian tissues. |
Table I.
CSTB protein expression in human
ovarian tissues.
| n | CSTB expression
| CSTB positive rate
(%) |
|---|
| Positive | Negative |
|---|
| Normal | 6 | 0 | 6 | 0.00 |
| Benign | 10 | 8 | 2 | 80.00 |
| Borderline | 4 | 4 | 0 | 100.00 |
| Malignant | 13 | 12 | 1 | 92.31 |
 | Table II.Comparison of CSTB immunostaining in
the ovarian tissues. |
Table II.
Comparison of CSTB immunostaining in
the ovarian tissues.
| Comparison | Z score | P-value |
|---|
| Normal vs.
benign | −3.422 | 0.001 |
| Normal vs.
borderline | −2.631 | 0.009 |
| Normal vs.
malignant | −3.211 | 0.001 |
| Benign vs.
borderline | −2.286 | 0.022 |
| Benign vs.
malignant | −2.319 | 0.020 |
| Borderline vs.
malignant | 0.238 | 0.812 |
Correlation of CSTB expression with
clinicopathological features
Next we examined whether the expression of CSTB is
correlated with the clinicopathological features of patients with
epithelial ovarian tumors. The histopathological characteristics
are listed in Table III. All
information was gathered by reviewing medical charts and pathology
records. By comparison of CSTB protein expression associated with
age, we found that CSTB expression was not significantly different
between younger (≤45 years) and older (>45 years) patients with
ovarian tumor (P>0.05). The positive rate of CSTB expression was
100% in mucinous and clear cell tumors and 66.67% in serous tumor,
including benign, borderline and malignant tumors (Table III). However, multiple comparisons
of the histological types showed that there was no difference of
CSTB expression between serous, mucinous and clear cell tumors
(P>0.05). By comparison of CSTB expression associated with tumor
size, the positivity of CSTB expression was not significantly
different between small size (≤2 cm) and large size (>2 cm) of
tumors (total and malignant, both P>0.05). We examined the
involvement of lymph nodes on CSTB expression and found that the
CSTB protein was not associated with lymph node metastasis
(P>0.05). Multiple comparison of clinical stages revealed that
there was no difference in the positivity of CSTB expression.
Because the sample number of malignant tumors was relatively small
(total 13 cases), an increase in sample number must be considered
in future study. Overall, these data indicate that CSTB is a tumor
marker and there is no correlation of CSTB expression with
clinicopathological features of ovarian cancer patients, such as
age, histological types, tumor size, lymph node metastasis and
clinical stages.
 | Table III.Clinicopathological features of
patients with epithelial-type ovarian tumors correlated with CSTB
expression detected by immunohistochemistry. |
Table III.
Clinicopathological features of
patients with epithelial-type ovarian tumors correlated with CSTB
expression detected by immunohistochemistry.
|
Clinico-pathological features | n | CSTB expression
| P-value |
|---|
| Positive (%) | Negative (%) |
|---|
| Age at
diagnosis | | | | 0.669 |
| ≤45 | 8 | 7 (87.50) | 1 (12.50) | |
| >45 | 19 | 17 (89.47) | 2 (10.53) | |
| Histological
type | | | | 0.492a |
| Serous tumor | | | | |
| Benign | 6 | 4 (66.67) | 2 (33.33) | |
| Borderline | 2 | 2 (100.00) | 0 (0.00) | |
| Malignant | 7 | 6 (85.71) | 1 (14.29) | |
| Mucinous
tumor | | | | |
| Benign | 4 | 4 (100.00) | 0 (0.00) | |
| Borderline | 2 | 2 (100.00) | 0 (0.00) | |
| Malignant | 3 | 3 (100.00) | 0 (0.00) | |
| Clear cell
tumor | 3 | 3 (100.00) | 0 (0.00) | |
| Tumor size | | | | 0.308b |
| Benign | | | | |
| ≤2 cm | 0 | 0 (0.00) | 0 (0.00) | |
| >2 cm | 10 | 8 (80.00) | 2 (20.00) | |
| Borderline | | | | |
| ≤2 cm | 0 | 0 (0.00) | 0 (0.00) | |
| >2 cm | 4 | 4 (100.00) | 0 (0.00) | |
| Malignant | | | | 0.231 |
| ≤2 cm | 3 | 2 (66.67) | 1 (33.33) | |
| >2 cm | 10 | 10 (100.00) | 0 (0.00) | |
| LN metastasis | | | | 0.769 |
| Yes | 3 | 3 (100.00) | 0 (0.00) | |
| No | 10 | 9 (90.00) | 1 (10.00) | |
| FIGO stage | | | | 0.154c |
| I | 5 | 5 (100.00) | 0 (0.00) | |
| II | 1 | 0 (0.00) | 1 (100.00) | |
| III | 6 | 6 (100.00) | 0 (0.00) | |
| IV | 1 | 1 (100.00) | 0 (0.00) | |
Regulation of CSTB expression by TGF-β1
in ovarian cancer cells
Since TGF-β plays a key role in ovarian
tumorigenesis and since CSTB was overexpressed in ovarian cancer,
we subsequently investigated whether TGF-β1 affects CSTB protein
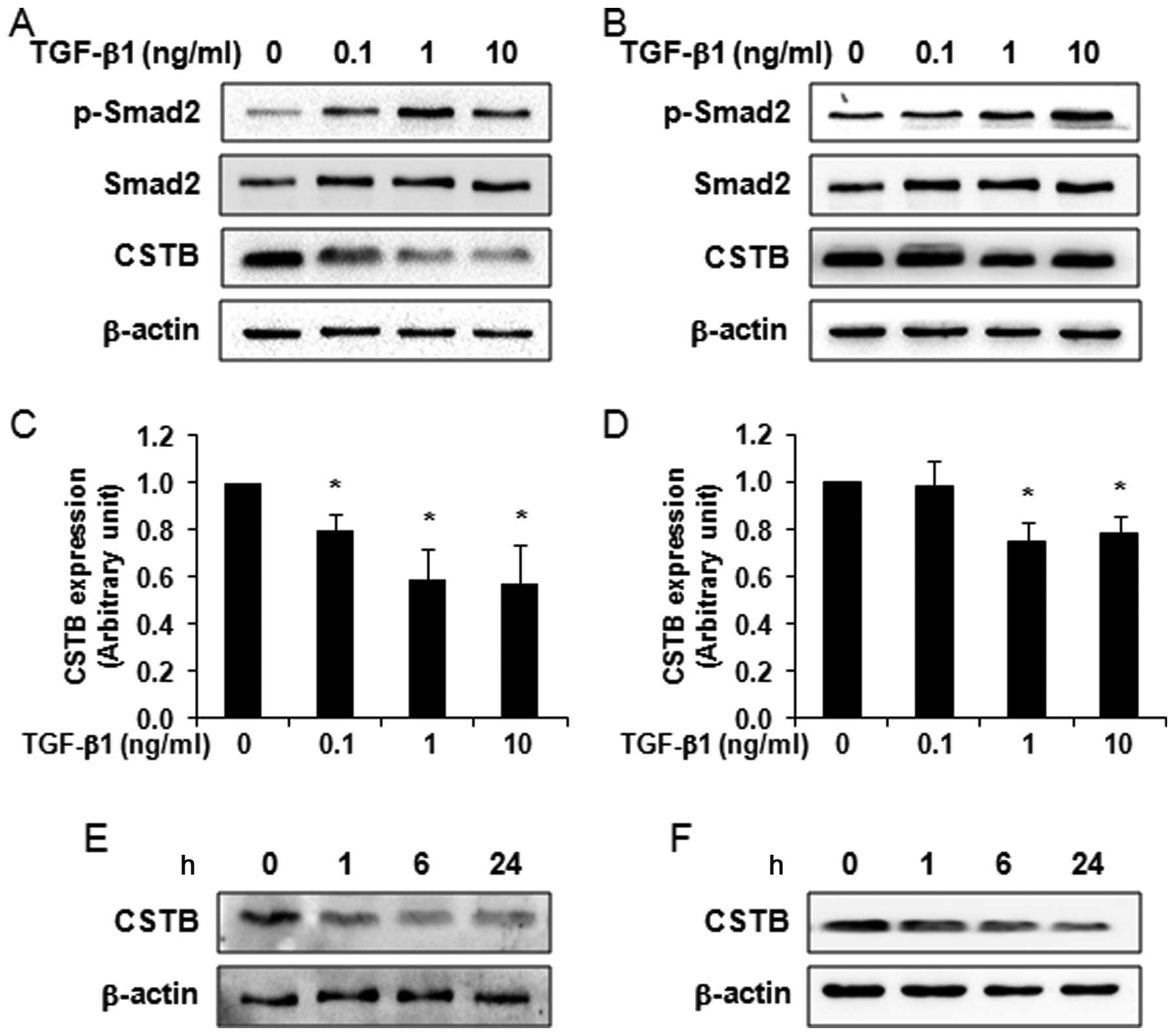
expression. The dose-dependent and time-course studies of TGF-β1 in
two epithelial ovarian cancer cell lines, OVCAR-3 and SK-OV-3, were
applied. Because Smad2 is a TGF-β signaling protein and activated
upon TGF-β1 treatment (25), first
of all we detected the phosphorylation of Smad2 by western blot
analysis to confirm the responsiveness of tested cells to TGF-β1.
Indeed we found that TGF-β1 at different doses (ranged 0.1–10
ng/ml) increased the phosphorylation of Smad2 in OVCAR-3 (Fig. 2A) and SK-OV-3 (Fig. 2B) cells. After stripping, we
reprobed the same blot with anti-CSTB antibody and found that
TGF-β1 significantly decreased CSTB in OVCAR-3 (Fig. 2A and C) and SK-OV-3 (Fig. 2B and D) cells in a dose-dependent
manner. The time-course study also showed that CSTB expression was
decreased in OVCAR-3 (Fig. 2E) and
SK-OV-3 (Fig. 2F) cells after 10
and 1 ng/ml of TGF-β1 treatment, respectively.
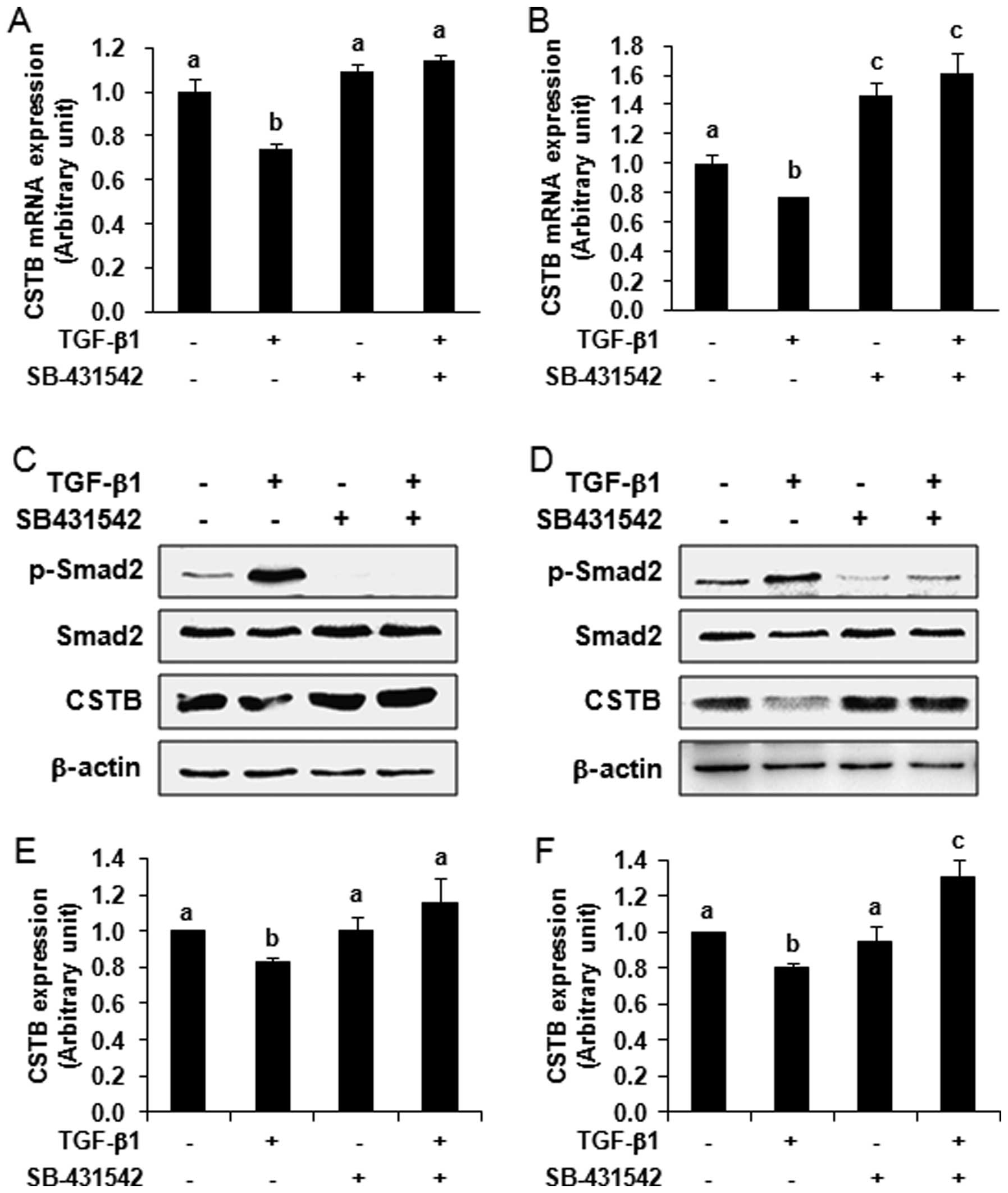
Next, we blocked the TGF-β signaling pathway by
treating ovarian cancer cells with a special TGF-β type I receptor
kinase inhibitor, SB-431542, to see if the regulation of CSTB
expression is mediated by the TGF-β signaling pathway. By comparing
with the vehicle control group, we found that TGF-β1 had an
inhibitory effect on the expression of CSTB mRNA. The expression of
CSTB mRNA was significantly decreased after TGF-β1 treatment in
OVCAR-3 (Fig. 3A) and SK-OV-3
(Fig. 3B) cells detected by qPCR
(P<0.05). This downregulation of CSTB mRNA expression by TGF-β1
was abolished after pre-treatment of cells with 10 μM
SB-431542 for 30 min. In SK-OV-3 cells, we observed an increase of
CSTB expression after SB-431542 pre-treatment, which may be due to
the elimination of the inhibitory effect of endogenous TGF-β on
CSTB expression. At the protein level, we observed the
phosphorylation of Smad2 in OVCAR-3 (Fig. 3C) and SK-OV-3 (Fig. 3D) cells upon 10 and 1 ng/ml of
TGF-β1 treatment, respectively, for 24 h. This phosphorylation was
abolished in the cells pre-treated with 10 μM SB-431542 for
30 min, indicating the responsiveness of the cells to this
inhibitor. After stripping, the same blots were reprobed with
anti-CSTB antibody. We found that the expression of CSTB protein
was significantly decreased after TGF-β1 treatment (P<0.05) and
the decrease of CSTB by TGF-β1 was blocked by the pre-treatment of
SB-431542 in OVCAR-3 (Fig. 3C and
E) and SK-OV-3 (Fig. 3D and F)
cells. Thus, our data indicate that the regulation of CSTB
expression is mediated by the TGF-β signaling pathway.
Discussion
In the present study we revealed the expression of
CSTB associated with clinicopathological features in human
epithelial ovarian cancer and examined for the first time the
expression of CSTB regulated by the TGF-β signaling pathway in
ovarian cancer cells. CSTB was initially found in ascites fluid
from patients with ovarian carcinoma by Lah et al in 1992
(31) and so far this is the only
group to show the expression of CSTB in ovarian cancer. Here we
demonstrated that CSTB protein was indeed not only overexpressed in
epithelial ovarian malignant tumor, but also expressed in benign
and borderline tumors; the latter was not reported previously.
Serous carcinoma, arising from the ovarian surface
epithelium (OSE) and/or fallopian tube epithelium (FTE), is the
most frequent ovarian cancer. Although the detection of CSTB in
ovarian serous malignant tumor has been reported (31,32),
this is the first report showing that CSTB was also expressed in
mucinous and clear cell tumors. Furthermore, we observed the
overexpression of CSTB in benign and borderline tumors, comparing
with normal tissue counterparts which appeared negative, suggesting
that CSTB is tumor tissue-specific. However, the function and the
role of CSTB in ovarian tumorigenesis remain unclear. CSTB is one
of the endogenous inhibitors of lysosomal cysteine proteases and
thought to play a role in protecting against the proteases leaking
from lysosomes. Alterations in CTSB expression have been found at
various diseases, including epilepsy and cancer. CSTB mutations are
responsible for progressive myoclonus epilepsy type 1 (EPM1)
(33). CSTB-null mice can develop
symptoms that mimic EPM1 (34). In
cancer research, CSTB deficiency reduces tumor growth via the
sensitization of tumor cells to oxidative stress in a breast cancer
model (35). CSTB deficiency in
these mice results in enhanced cathepsin B and D activities,
indicating lysosomal dysfunction. On the other hand, increased CSTB
has been observed in various cancers such as lung, hepatocellular
and colorectal cancers (17–19).
It has been reported that CSTB, derived from serous ovarian
carcinomas, strongly inhibits papain and cathepsin L and moderately
inhibits cathepsin B (32). These
results imply an in vivo role for CSTB in tumorigenesis. An
imbalance between intracellular cathepsins and CSTB may facilitate
the progression of ovarian epithelial cell transformation.
By comparing the clinicopathological features of
patients with epithelial-type tumors of the ovary, we found that
CSTB was not correlated with age, histological types, tumor size
and stage, and lymph node metastasis. Although the number of cases
in this study was relatively small (total 27 patients with ovarian
tumor), our data were similar to the results obtained from a lung
cancer study that the high concentration of CSTB in human lung
tumor tissue specimen is not correlated with TNM stages, but
positively correlated with survival probability (17). However, in bladder cancer, urine
levels of CSTB are positively correlated with tumor grade, stage
and shorter time to disease recurrence and progression (36). During the preparation of this
manuscript, a group from Russia reported the elevation of serum and
ascites CSTB in ovarian cancer patients (37). Overall, these studies indicate that
CSTB may be useful as an ovarian tumor marker and a target protein
for diagnosis, prognosis and therapy in cancer. Therefore, the
follow-up of patients with an ovarian tumor and the measurement of
the serum and urine levels of CSTB in patients may be of great
interest and should be proposed as the next investigation.
Although the overexpression of CSTB in various
cancers is observed, the mechanisms underlying the regulation of
CSTB in cancer progression are unknown. Because the growth
inhibitory effect of TGF-β prevents overproliferation of OSE during
wound healing after ovulation, the dysregulation of TGF-β signaling
is thought to be crucial to the development of EOC (28,38).
Ovarian cancer at early stage is refractory to TGF-β-mediated
growth inhibition, whereas at later stage TGF-β promotes tumor
proliferation and epithelial-mesenchymal transition (EMT) (22,38–40).
However, whether the expression of CSTB in ovarian tumor is
regulated by the TGF-β signaling pathway remains unclear. Our in
vitro study showed that CSTB expression in two epithelial
ovarian cancer cell lines was decreased after TGF-β1 treatment. By
blocking the TGF-β signaling pathway using an inhibitor to TGF-β
type I receptor, we demonstrated for the first time that the
inhibition of TGF-β signaling resulted in the abolishment of CSTB
suppression, suggesting that the regulation of CSTB is mediated by
the TGF-β signaling pathway. It has been reported that alteration
of TGF-β components is crucial for ovarian tumorigenesis (26–28,41,42).
We speculate that in in vivo circumstance the loss of TGF-β
responsiveness or the defectiveness of TGF-β signaling may lead to
the upregulation of CSTB, while the TGF-β-mediated CSTB-inhibitory
pathway can be interrupted in the ligand-receptor-Smad axis, such
as insufficient secretion and activation of TGF-β1, mutational
inactivation of its receptors or Smad proteins, and misconducted
signal transduction.
In conclusion, CSTB was overexpressed in human
epithelial ovarian tumors, including serous, mucinous and clear
cell tumors. The immunoreactive staining of CSTB was negative in
normal tissue, weak in benign tumor and strong in borderline and
malignant tumors, which may represent tumor progression. CSTB at
mRNA and protein levels was regulated by the TGF-β signaling
pathway. Our data suggest that CSTB is tumor tissue-specific and a
potential diagnostic marker for ovarian cancer. Gaining better
understanding of how TGF-β regulates the CSTB expression during
ovarian tumorigenesis may lead to better therapeutic strategies by
targeting CSTB for this devastating disease. The follow-up of
patients and the examination of the serum and urine CSTB in a
larger population of patients with ovarian tumor may be of great
interest in subsequent studies.
Abbreviations:
|
CSTB
|
cystatin B;
|
|
TGF-β
|
transforming growth factor-β;
|
|
EOC
|
epithelial ovarian cancer;
|
|
OSE
|
ovarian surface epithelial;
|
|
TNM
|
tumor, node, metastasis;
|
|
IHC
|
immunohistochemistry;
|
|
qPCR
|
quantitative polymerase chain
reaction;
|
|
SI
|
staining index
|
Acknowledgements
This study was supported by grants
from National Natural Science Foundation of China (81272880), the
Shanghai Committee of Science and Technology (124119b1300),
Shanghai Municipal Health Bureau (2012-186), and a start-up fund of
research from Jinshan Hospital (2012-2) to G.X.
References
|
1.
|
Rauh-Hain JA, Krivak TC, Del Carmen MG and
Olawaiye AB: Ovarian cancer screening and early detection in the
general population. Rev Obstet Gynecol. 4:15–21. 2011.PubMed/NCBI
|
|
2.
|
Auersperg N, Wong AS, Choi KC, Kang SK and
Leung PC: Ovarian surface epithelium: biology, endocrinology, and
pathology. Endocr Rev. 22:255–288. 2001.PubMed/NCBI
|
|
3.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
4.
|
Sankaranarayanan R and Ferlay J: Worldwide
burden of gynaecological cancer: the size of the problem. Best
Pract Res Clin Obstet Gynaecol. 20:207–225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Burger HG, Fuller PJ, Chu S, et al: The
inhibins and ovarian cancer. Mol Cell Endocrinol. 180:145–148.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Jelovac D and Armstrong DK: Recent
progress in the diagnosis and treatment of ovarian cancer. CA
Cancer J Clin. 61:183–203. 2011. View Article : Google Scholar
|
|
7.
|
Jelovac D and Armstrong DK: Role of
farletuzumab in epithelial ovarian carcinoma. Curr Pharm Des.
18:3812–3815. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Van Nagell JR Jr and Pavlik EJ: Ovarian
cancer screening. Clin Obstet Gynecol. 55:43–51. 2012.
|
|
9.
|
Prat J, Ribe A and Gallardo A: Hereditary
ovarian cancer. Hum Pathol. 36:861–870. 2005. View Article : Google Scholar
|
|
10.
|
Turk V, Stoka V and Turk D: Cystatins:
biochemical and structural properties, and medical relevance. Front
Biosci. 13:5406–5420. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Smid L, Strojan P, Budihna M, et al:
Prognostic value of cathepsins B, D and steffins A and B in
laryngeal carcinoma. Eur Arch Otorhinolaryngol. 254(Suppl 1):
S150–S153. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Kos J and Lah TT: Cysteine proteinases and
their endogenous inhibitors: target proteins for prognosis,
diagnosis and therapy in cancer (Review). Oncol Rep. 5:1349–1361.
1998.PubMed/NCBI
|
|
13.
|
Levicar N, Kos J, Blejec A, et al:
Comparison of potential biological markers cathepsin B, cathepsin
L, stefin A and stefin B with urokinase and plasminogen activator
inhibitor-1 and clinicopathological data of breast carcinoma
patients. Cancer Detect Prev. 26:42–49. 2002. View Article : Google Scholar
|
|
14.
|
Zhang R, Tremblay TL, McDermid A, Thibault
P and Stanimirovic D: Identification of differentially expressed
proteins in human glioblastoma cell lines and tumors. Glia.
42:194–208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Strojan P, Anicin A, Svetic B, Pohar M,
Smid L and Kos J: Stefin A and stefin B: markers for prognosis in
operable squamous cell carcinoma of the head and neck. Int J Radiat
Oncol Biol Phys. 68:1335–1341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Shiraishi T, Mori M, Tanaka S, Sugimachi K
and Akiyoshi T: Identification of cystatin B in human esophageal
carcinoma, using differential displays in which the gene expression
is related to lymph-node metastasis. Int J Cancer. 79:175–178.
1998. View Article : Google Scholar
|
|
17.
|
Ebert E, Werle B, Julke B, et al:
Expression of cysteine protease inhibitors stefin A, stefin B, and
cystatin C in human lung tumor tissue. Adv Exp Med Biol.
421:259–265. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ji NY, Kang YH, Park MY, et al:
Development of a fluorescent microsphere immunoassay for cystatin B
(CSTB) in serum of patients with hepatocellular carcinoma. Clin
Chem Lab Med. 49:151–155. 2011.PubMed/NCBI
|
|
19.
|
Kos J, Krasovec M, Cimerman N, Nielsen HJ,
Christensen IJ and Brunner N: Cysteine proteinase inhibitors stefin
A, stefin B, and cystatin C in sera from patients with colorectal
cancer: relation to prognosis. Clin Cancer Res. 6:505–511.
2000.PubMed/NCBI
|
|
20.
|
Mirtti T, Alanen K, Kallajoki M, Rinne A
and Soderstrom KO: Expression of cystatins, high molecular weight
cytokeratin, and proliferation markers in prostatic adenocarcinoma
and hyperplasia. Prostate. 54:290–298. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Massague J: TGFbeta in cancer. Cell.
134:215–230. 2008. View Article : Google Scholar
|
|
22.
|
Nilsson EE and Skinner MK: Role of
transforming growth factor beta in ovarian surface epithelium
biology and ovarian cancer. Reprod Biomed Online. 5:254–258. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Attisano L and Wrana JL: Signal
transduction by the TGF-beta superfamily. Science. 296:1646–1647.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Derynck R, Zhang Y and Feng XH: Smads:
transcriptional activators of TGF-beta responses. Cell. 95:737–740.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Massague J, Seoane J and Wotton D: Smad
transcription factors. Genes Dev. 19:2783–2810. 2005. View Article : Google Scholar
|
|
26.
|
Cardillo MR, Yap E and Castagna G:
Molecular genetic analysis of TGF-beta1 in ovarian neoplasia. J Exp
Clin Cancer Res. 16:49–56. 1997.PubMed/NCBI
|
|
27.
|
Wang D, Kanuma T, Mizunuma H, et al:
Analysis of specific gene mutations in the transforming growth
factor-beta signal transduction pathway in human ovarian cancer.
Cancer Res. 60:4507–4512. 2000.PubMed/NCBI
|
|
28.
|
Berchuck A, Rodriguez G, Olt G, et al:
Regulation of growth of normal ovarian epithelial cells and ovarian
cancer cell lines by transforming growth factor-beta. Am J Obstet
Gynecol. 166:676–684. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Zeng F, Xu G, Zhou T, et al: Reduced
expression of activin receptor-like kinase 7 in breast cancer is
associated with tumor progression. Med Oncol. 29:2519–2526. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Inman GJ, Nicolas FJ, Callahan JF, et al:
SB-431542 is a potent and specific inhibitor of transforming growth
factor-beta superfamily type I activin receptor-like kinase (ALK)
receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 62:65–74. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Lah TT, Kokalj-Kunovar M, Kastelic L, et
al: Cystatins and stefins in ascites fluid from ovarian carcinoma.
Cancer Lett. 61:243–253. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Kastelic L, Turk B, Kopitar-Jerala N, et
al: Stefin B, the major low molecular weight inhibitor in ovarian
carcinoma. Cancer Lett. 82:81–88. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Pennacchio LA, Lehesjoki AE, Stone NE, et
al: Mutations in the gene encoding cystatin B in progressive
myoclonus epilepsy (EPM1). Science. 271:1731–1734. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Kaur G, Mohan P, Pawlik M, et al: Cystatin
C rescues degenerating neurons in a cystatin B-knockout mouse model
of progressive myoclonus epilepsy. Am J Pathol. 177:2256–2267.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Butinar M, Prebanda MT, Rajkovic J, et al:
Stefin B deficiency reduces tumor growth via sensitization of tumor
cells to oxidative stress in a breast cancer model. Oncogene. Aug
19–2013.Epub ahead of print. View Article : Google Scholar
|
|
36.
|
Feldman AS, Banyard J, Wu CL, McDougal WS
and Zetter BR: Cystatin B as a tissue and urinary biomarker of
bladder cancer recurrence and disease progression. Clin Cancer Res.
15:1024–1031. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Gashenko EA, Lebedeva VA, Brak IV,
Tsykalenko EA, Vinokurova GV and Korolenko TA: Evaluation of serum
procathepsin B, cystatin B and cystatin C as possible biomarkers of
ovarian cancer. Int J Circumpolar Health. 72:212152013. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Wong AS and Leung PC: Role of endocrine
and growth factors on the ovarian surface epithelium. J Obstet
Gynaecol Res. 33:3–16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Akhurst RJ and Derynck R: TGF-beta
signaling in cancer - a double-edged sword. Trends Cell Biol.
11:S44–S51. 2001.PubMed/NCBI
|
|
40.
|
Derynck R, Akhurst RJ and Balmain A:
TGF-beta signaling in tumor suppression and cancer progression. Nat
Genet. 29:117–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Lynch MA, Nakashima R, Song H, et al:
Mutational analysis of the transforming growth factor beta receptor
type II gene in human ovarian carcinoma. Cancer Res. 58:4227–4232.
1998.PubMed/NCBI
|
|
42.
|
Chen T, Triplett J, Dehner B, et al:
Transforming growth factor-beta receptor type I gene is frequently
mutated in ovarian carcinomas. Cancer Res. 61:4679–4682.
2001.PubMed/NCBI
|