Introduction
The incidence of oral squamous cell carcinoma (OSCC)
is increasing gradually, and about 300,000 patients are annually
estimated to develop oral cancer worldwide (1–3).
OSCC is the most common malignant neoplasm of the oral cavity and
represents about 90% of all oral malignancies (4). Though the standard treatment for OSCC
is thought to be surgical operation, we often select chemotherapy
for patients with advanced or recurrent OSCC. Among anticancer
drugs, 5-florouracil (5-FU) is a basic drug for cancers of the
digestive organs including OSCC, and it has wide clinical
application. After discovery of 5-FU by Heidelberger in 1957, 5-FU
has been in use for over 50 years (5). However, resistance to 5-FU is a major
problem for successful cancer treatment, and the mechanism of
5-FU-resistance in cancers is still not clear.
Epithelial-to-mesenchymal transition (EMT) causes
profound morphological and phenotypic changes to a cell (6). EMT is a process that enables
epithelial cells to lose their epithelial characteristics and
acquire mesenchymal cell characteristics. Briefly, epithelial cell
becomes spindle-shaped, lose their characteristic polarization,
interacting with each other only through focal points as
mesenchymal cells (7) and
development of pseudopodia is observed. Moreover, epithelial cell
phenotype markers such as E-cadherin is downregulated, and
mesenchymal markers including vimentin, Snail, N-cadherin and Twist
are upregulated during EMT (8). It
has been reported that EMT is important in tumor progression,
metastasis and chemoresistance (9,10).
In addition, it has been demonstrated that the acquisition of EMT
is related to cancer stem cells (CSC) which contributes to the
chemoresistance as well as tumor progression and metastasis
(11,12).
In the present study, we established two
5-FU-resistant OSCC cell lines (HSC2/FU and HSC4/FU) and
investigated the mechanisms of 5-FU resistance in association with
EMT characteristics.
Materials and methods
Cell culture and establishment of
5-FU-resistant cell lines
HSC2 and HSC4 cells were purchased from Cell Bank,
RIKEN BioResource Center (Ibaraki, Japan). Both cell lines were
exposed to step-wise increasing concentrations of 5-FU (Wako,
Osaka, Japan). The cells that survived in culture medium including
5-FU for a year, were cloned by limiting dilution. Cells were
cultured in Dulbecco’s modified Eagle’s medium (DMEM)
(Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal
bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA), 100
μg/ml streptomycin, 100 U/ml penicillin (Invitrogen) in a
humidified atmosphere containing 5% CO2.
In vitro cell growth assay
Cells (5×103 cells per well) were seeded
on 96-well plates (Becton-Dickinson Labware, Franklin lakes, NJ,
USA) in DMEM supplemented with 10% FBS. Twenty-four hours later,
the cells were treated with 5-FU (0, 2, 4 and 8 μg/ml).
After 48 h, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) was added to each well (25 μl/well) and
incubated for 4 h. The blue dye taken up by cells was dissolved in
dimethyl sulfoxide (100 μl/well), and the absorbance was
measured with a spectrophotometer (Bio-Rad Laboratories, Hercules,
CA, USA) at 490 nm. All assays were run in triplicate.
Terminal deoxyribonucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay
Cells (5×103 cells per well) were seeded
on micro-cover glass (Matsunami Glass Ind., Osaka, Japan) in DMEM
containing 10% FBS. After incubation for 24 h, the culture medium
was replaced with DMEM with 10% FBS and 2 μg/ml of 5-FU.
After further incubation for 48 h, the cells on the micro-cover
glass were washed twice with phosphate-buffered saline (PBS), air
dried, and fixed in 4% paraformaldehyde at room temperature for 30
min. TUNEL assay was performed using a DeadEnd™ Colorimetric TUNEL
System according to the manufacturer’s instructions (Promega
Corporation, Madison, WI, USA). Briefly, the cells on the
micro-cover glass were incubated in 20 μg/ml proteinase K
for 15 min. Endogenous peroxidase of cells on the micro-cover glass
was blocked by incubating in a 3% hydrogen peroxide solution for 5
min after cells were rinsed in distilled water. After being washed
with PBS (0.05 M phosphate buffer containing 0.145 M sodium
chloride, pH 7.4), the cells were incubated with equilibration
buffer and then TdT enzyme in a humidified chamber at 37°C for 60
min. They were subsequently put into pre-warmed working strength
stop wash buffer for 10 min. After being rinsed in PBS, the cells
were incubated with antidigoxigenin-peroxidase conjugate for 30
min. Peroxidase activity in each cell was demonstrated by the
application of diaminobenzidine. Hematoxylin was used as a
counterstain. At least 1,000 cells were counted under a microscope
in several random fields of each micro-cover glass. The number of
apoptotic cell was calculated the number of TUNEL positive cells
divided by the total number of counted cells and the result was
expressed as a percentage.
In the same manner as above, TUNEL assay was
performed in 4-μm-thick paraffin sections of tumor using a
DeadEnd Colorimetric TUNEL System according to the manufacturer’s
instructions (Promega).
Western blot analysis
Cells were lysed with RIPA Buffer (Thermo
scientific, Rockford, IL, USA). Whole cell lysates were subjected
to electrophoresis on 10% SDS-polyacrylamide gels, and then
transferred to a PVDF membrane. The membranes were incubated with
anti-E-cadherin rabbit polyclonal antibody (Santa Cruz
Biotechnology, Santa Cruz, CA, USA), anti-N-cadherin rabbit
monoclonal antibody (Epitomics, Burlingame, CA, USA), anti-Twist
mouse monoclonal antibody (Epitomics). The antibody was detected
using a chromogenic immunodetection system, WesternBreeze
(Invitrogen) according to the manufacturer’s instructions. Also,
anti-a-tubulin monoclonal antibody (Santa Cruz Biotechnology) was
used for normalization of western blot analysis.
Nude mice
Female athymic nude mice with CAnN.
Cg-Foxnlnu/CrlCrlj genetic background (CLEA Japan, Inc., Tokyo,
Japan) were purchased at 4 weeks of age and kept under sterile
conditions in a pathogen-free environment. The mice were provided
with sterile water and food ad libitum and all manipulations
were carried out aseptically inside a laminar flow hood. The mice
were maintained and were handled in accordance with the Guidelines
for Animal Experimentation of Yamaguchi University.
In vivo tumor growth assay
The effect of 5-FU treatment was assessed by
inoculation of cells into 5-week-old female athymic nude mice.
Cells (1×106) were suspended in 0.1 ml of serum-free
medium and injected into the subcutaneous tissue of mice (average
weight 15.0 g) using a 27-gauge needle. Tumors at the inoculation
site were monitored and measured. When the tumors reached 100–150
mm3 in volume, they were divided into 4 groups, and
treated with 5-FU for 3 weeks. Briefly, 5-FU (15 mg/kg) was
injected into the peritoneal cavity for 3 weeks (7 times/week).
Also, mice in control group were received saline (100 μl) by
peritumoral injection. The tumors were measured every two days and
the tumor volumes were calculated. At 21 days, mice were sacrificed
by cervical dislocation and the tumors were dissected out, fixed in
neutral-buffered formalin and embedded in paraffin for further
study.
Statistical analysis
All statistical significance was set at P<0.05.
Statistical analyses were performed using StatView software
(version 5.0J, SAS Institute Inc., Cary, NC, USA).
Results
Cell growth
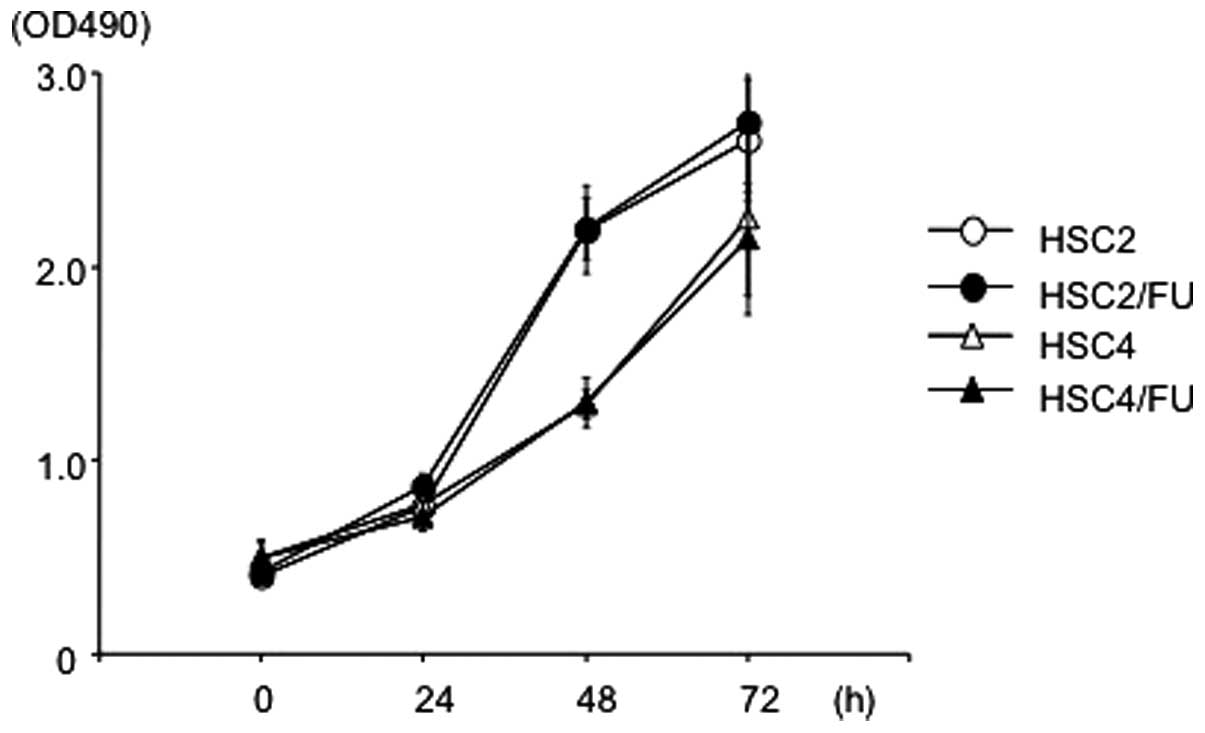
Both HSC2 and HSC4 cells were treated with
increasing concentrations of 5-FU up to 10 μg/ml to obtain
5-FU-resistant cells. In vitro cell growth inhibition assay
was performed occasionally to determine the resistance nature of
the cells and HSC2/FU and HSC4/FU were established almost one year
later. There were no significant differences in cellular
proliferation between HSC2 and HSC2/FU, and between HSC4 and
HSC4/FU (Fig. 1).
Effects of 5-FU on the growth of parental
and 5-FU-resistant OSCC cells in vitro
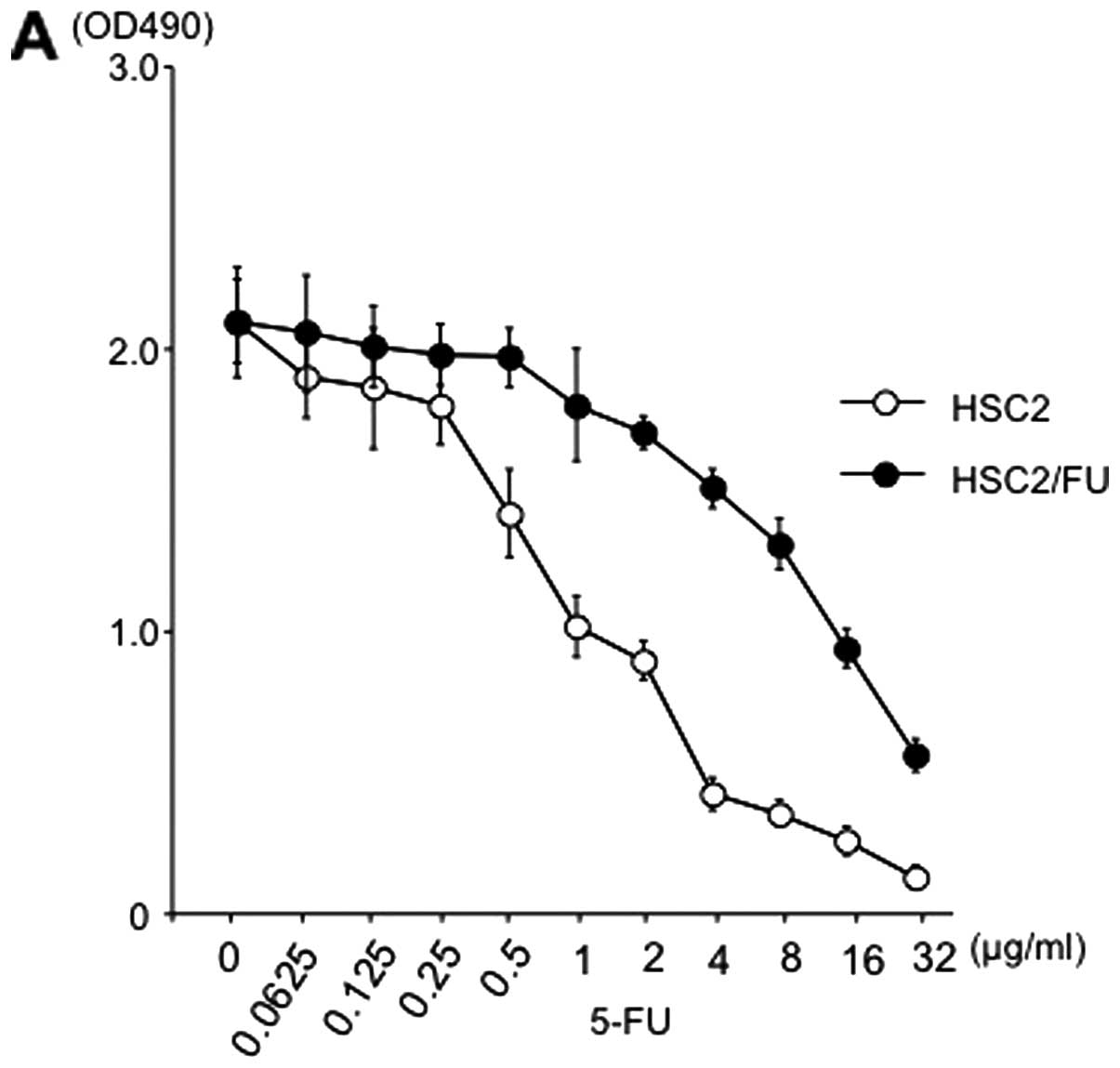
To compare the growth inhibitory effect of 5-FU
between parental and resistant cell lines, we treated the cells
with various concentrations of 5-FU. The inhibitory concentration
(IC50) data indicated that the 5-FU-resistance levels of
HSC2/FU (IC50, 14.0 μg/ml) were 14-fold greater
than that of the HSC2 (IC50, 1.0 μg/ml) (Fig. 2A), and the 5-FU-resistance levels
of HSC4/FU cells (IC50, 7.0 μg/ml) were 5-fold
greater than that of the HSC4 (IC50, 1.4 μg/ml)
(Fig. 2B).
Effects of 5-FU on apoptosis of parental
and 5-FU-resistant OSCC cells
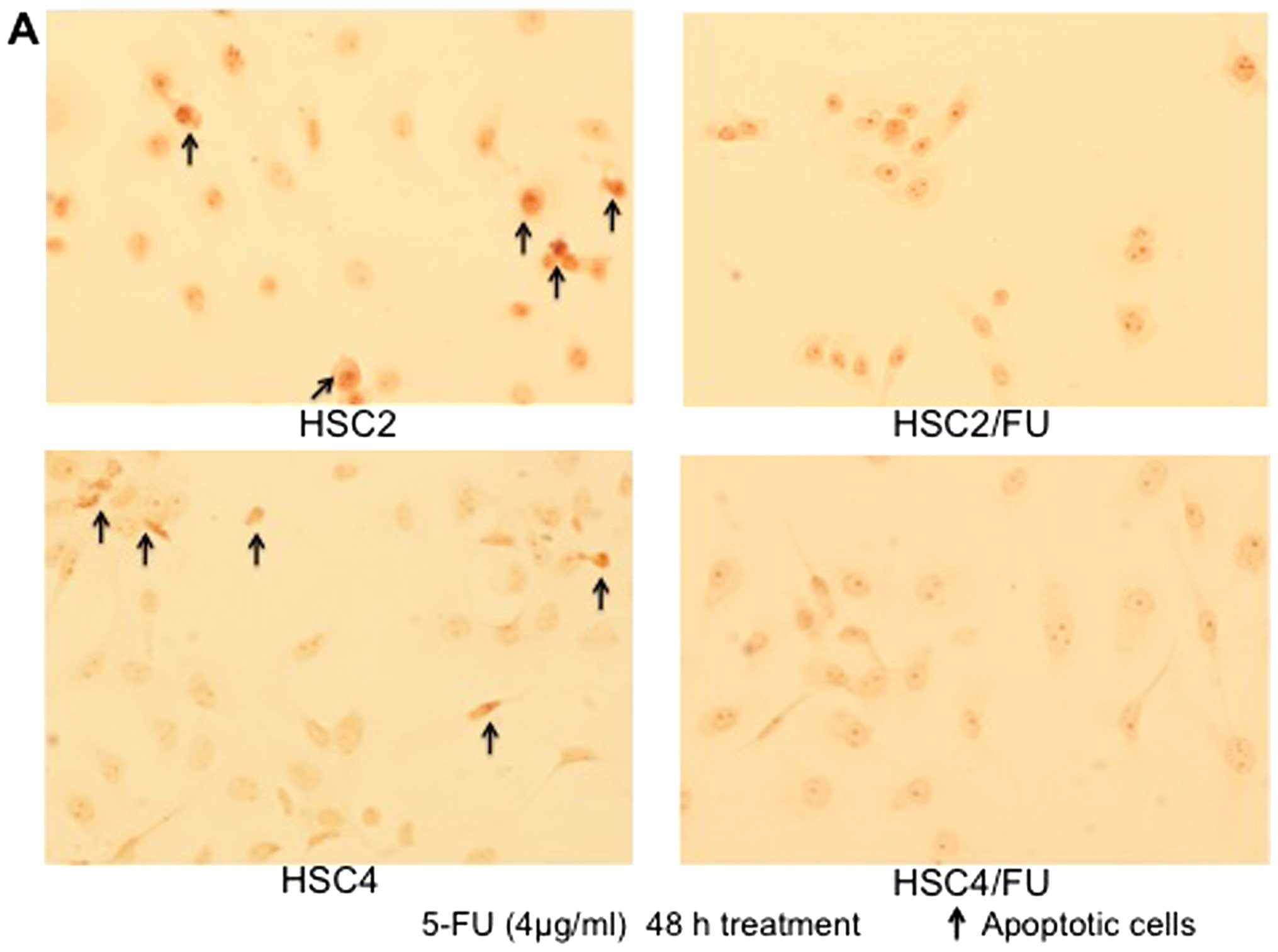
TUNEL staining showed a remarkable increase in the
number of HSC2 cells exposed to 5-FU that were stained brown, which
indicated the occurrence of apoptosis; whereas 5-FU-induced
apoptosis was less evident in the HSC2/FU cells. Similarly,
5-FU-induced apoptosis occurred at an increased rate in HSC4
compared to HSC4/FU cells (Fig.
3A). In both cases, the number of apoptotic cells were
significantly less in the 5-FU-resistant OSCC cells compared to the
parental cell lines (Fig. 3B).
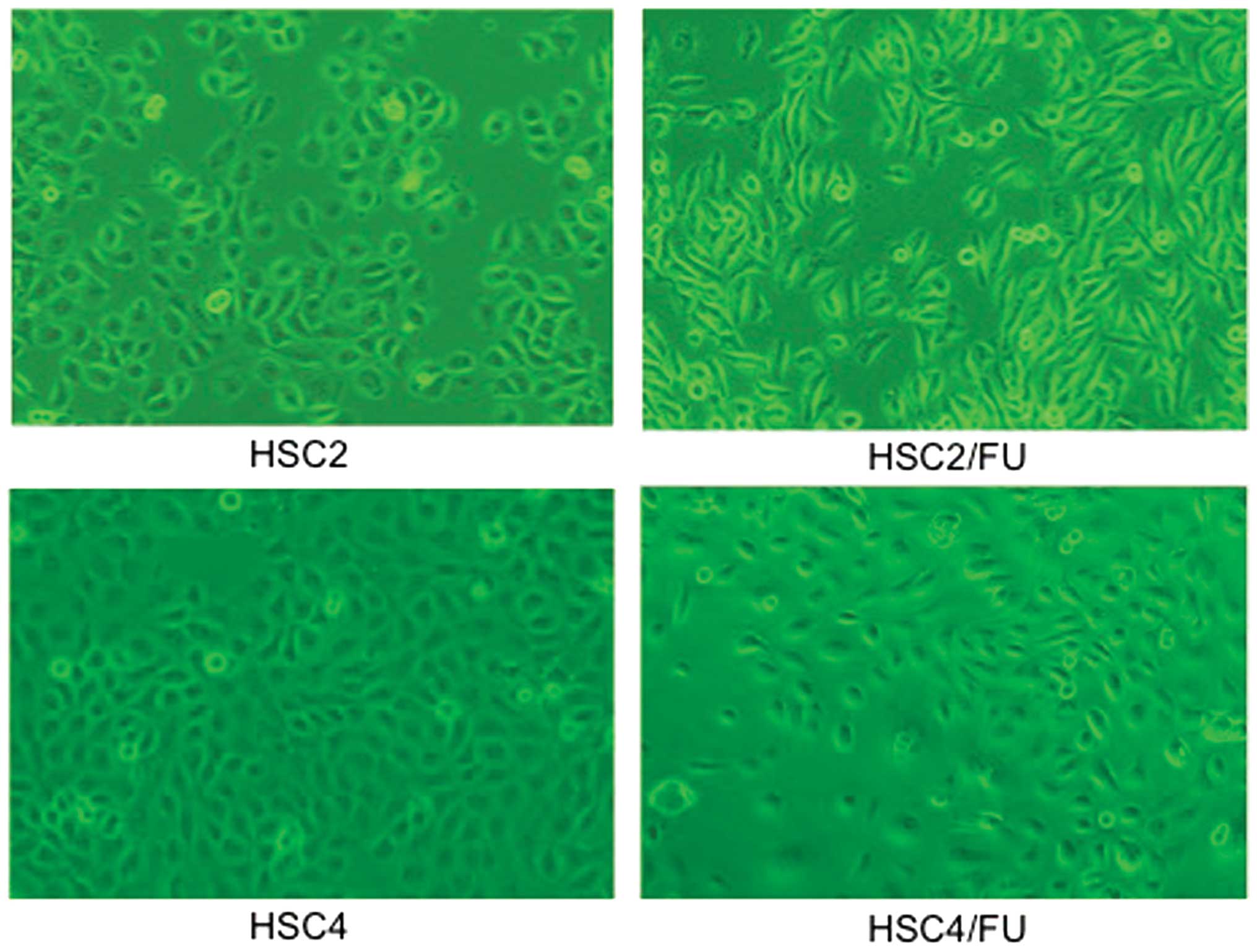
Cell morphology of 5-FU-resistant OSCC
cell lines
The 5-FU-resistant cells, HSC2/FU and HSC4/FU, were
morphologically distinct from their parental cell lines (Fig. 4). The resistant cells had lost the
characteristics of cell-cell adhesion, developed spindle-shaped
morphology and showed pseudopodia; which are typical phenotypes of
EMT.
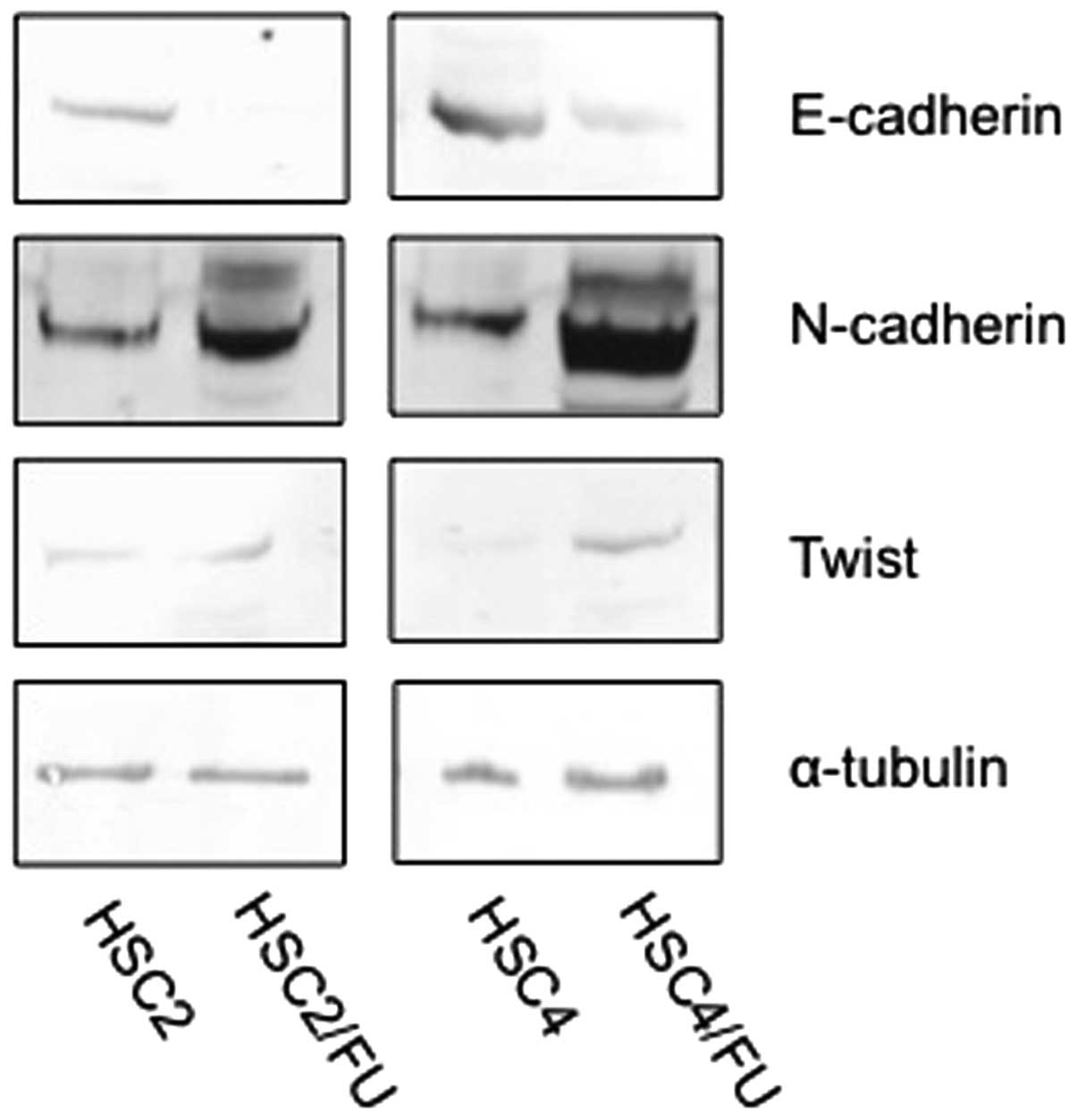
Expression of E-cadherin, N-cadherin and
Twist in parental and 5-FU-resistant OSCC cells in vitro
Unlike epithelial cells that express high levels of
E-cadherin, mesenchymal cells express N-cadherin. Our western blot
analysis showed decreased E-cadherin and increased N-cadherin and
Twist in the HSC2/FU and HSC4/FU cells in vitro compared to
the parental cell lines (Fig.
5).
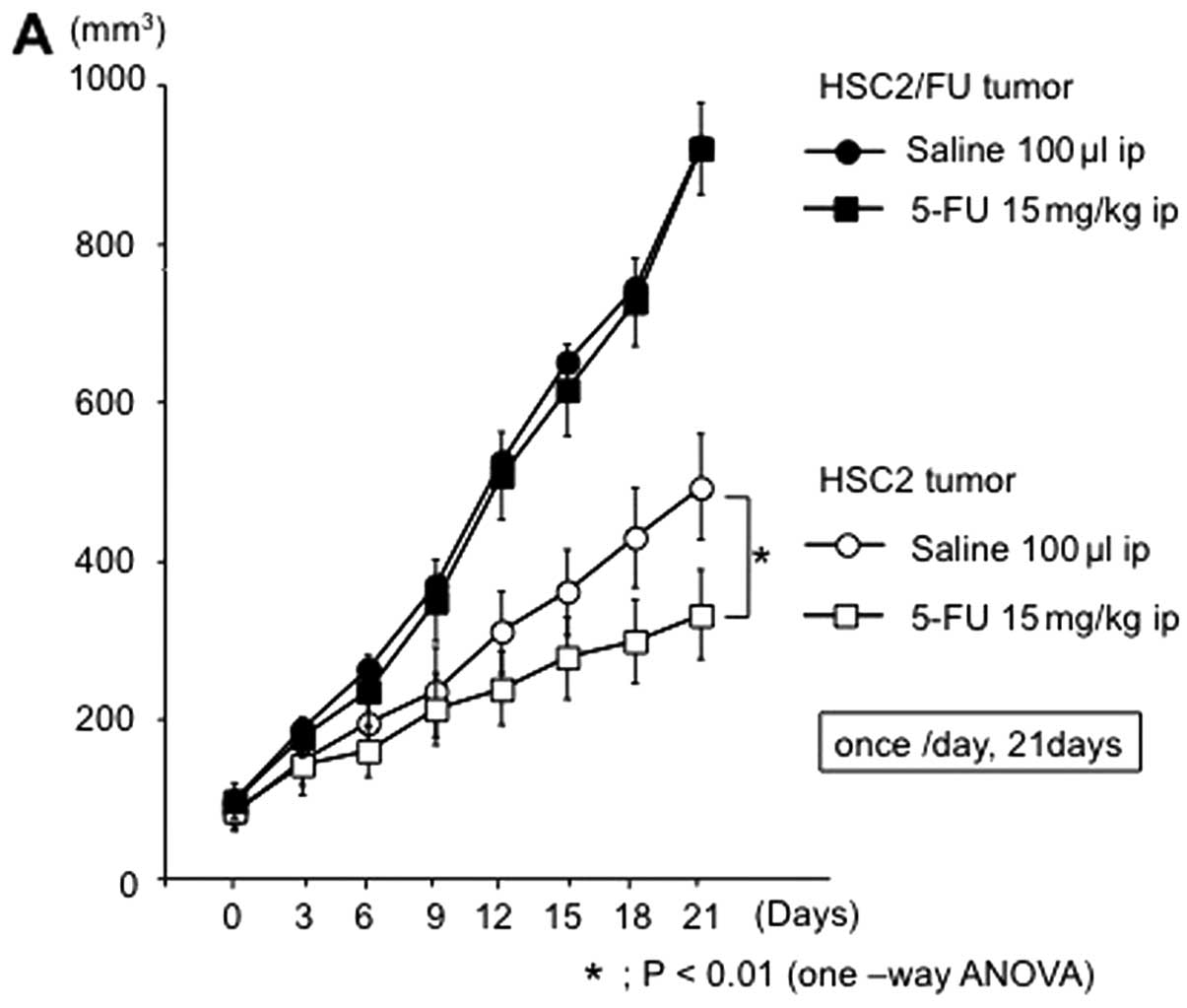
Effects of 5-FU on the growth and
apoptosis of parental and 5-FU-resistant OSCC tumors in vivo
Tumor xenograft studies demonstrated that HSC2/FU
and HSC4/FU tumors are resistant to 5-FU when compared to HSC2 and
HSC4 tumors (Fig. 6). Briefly,
tumor volume of HSC2 and HSC4 was significantly decreased by 5-FU
administration though HSC2/FU and HSC4/FU tumors were not affected
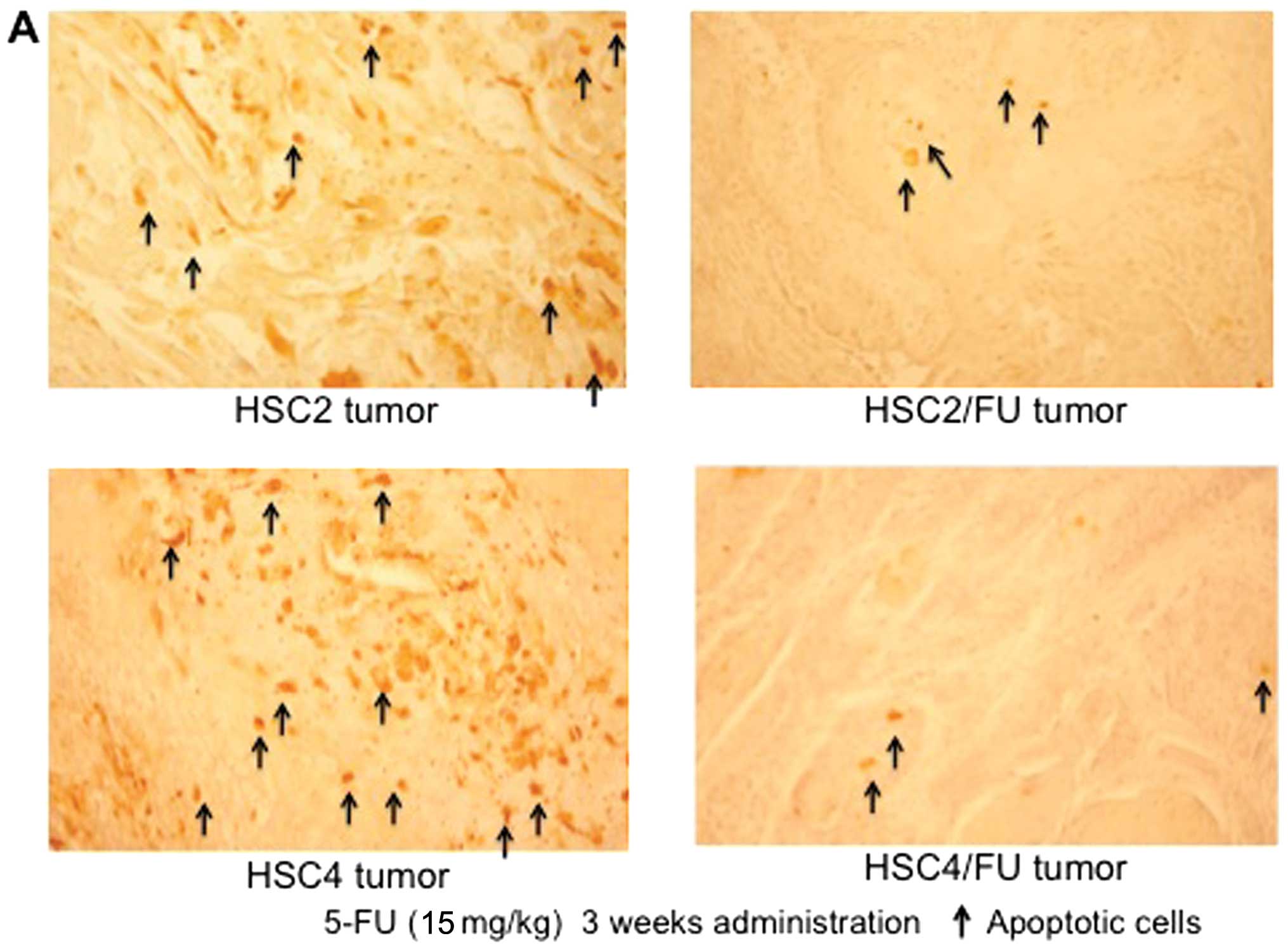
by 5-FU injection. Moreover, TUNEL assay showed a significant
decreased number of apoptotic cells in 5-FU-resistant tumors after
treatment with 5-FU (Fig. 7).
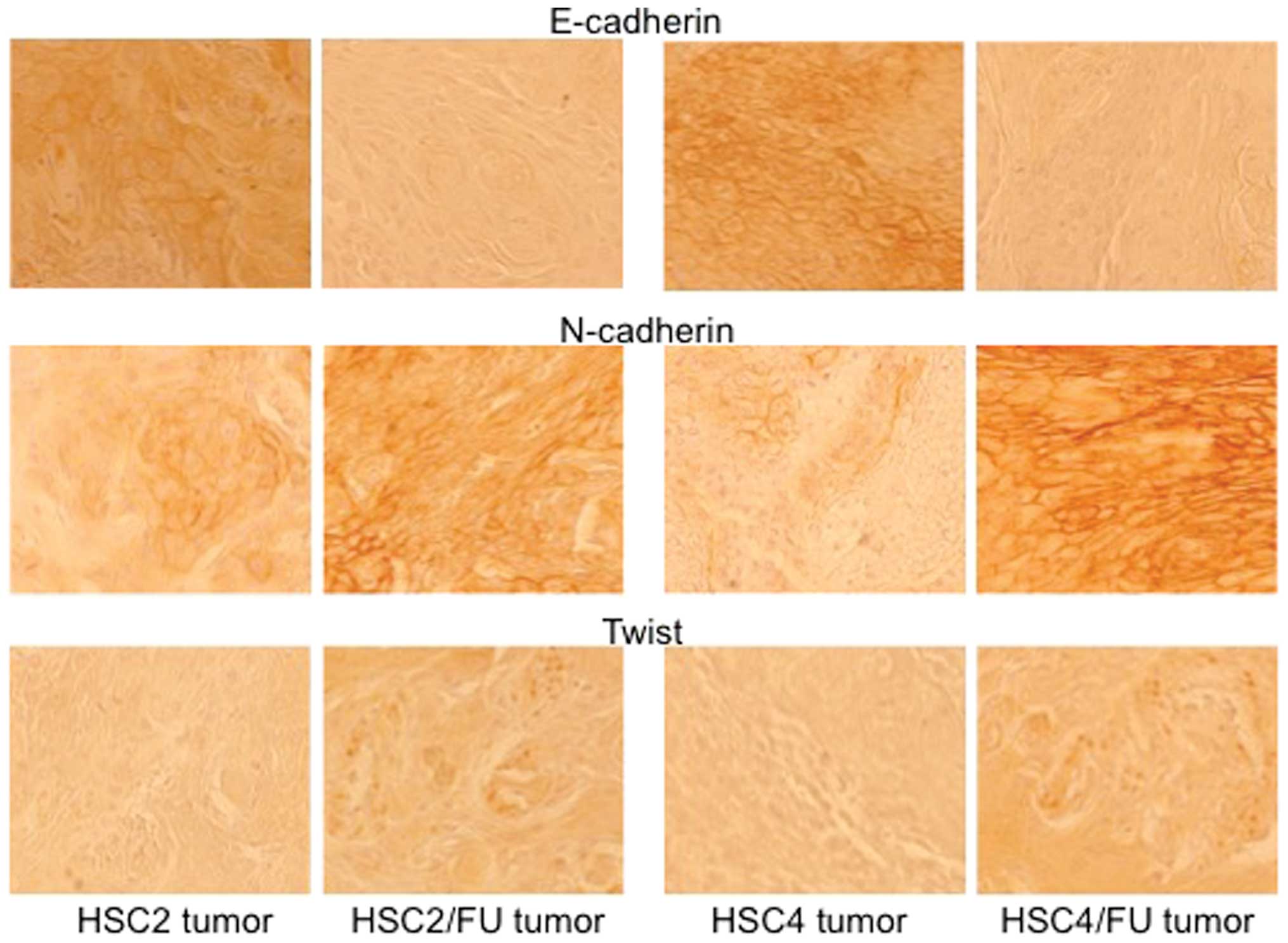
Expression of E-cadherin, N-cadherin and
Twist in parental and 5-FU-resistant OSCC tumors in vivo
We carried out immunohistochemistry experiments to
evaluate the expression pattern of EMT-related factors (E-cadherin,
N-cadherin and Twist) between 5-FU-sensitive tumors and
5-FU-resistant tumors. The expression of E-cadherin was markedly
decreased in 5-FU-resistant tumors, and the expression of
N-cadherin and Twist was increased in 5-FU-resistant tumors
(Fig. 8).
Discussion
5-FU has widespread clinical use for digestive organ
cancer treatments (13–16) and it is also a key drug for OSCC
treatments. 5-FU has been preferentially used in combination with
other chemotherapeutic drugs such as cisplatin and/or docetaxel for
head and neck cancer treatment including OSCC (17–19).
The detail mechanism of the clinical response to 5-FU chemotherapy
is still unclear as cancer cells gain resistance to 5-FU overtime,
which is a serious problem for 5-FU based chemotherapy. Although
several mechanisms of 5-FU resistance have been investigated
(20–22), its definitive mechanism in OSCC is
still unclear. In order to understand 5-FU resistance in OSCC, we
must establish 5-FU-resistant cancer cell lines and compare them
with the parental cancer cells. Several 5-FU-resistant cancer cells
have been established (23–26),
however, the only OSCC cell line that has been reported to be
resistant to 5-FU is SAS (27). In
the present report, we describe the establishment of two new
5-FU-resistant OSCC cell lines, HSC2/FU and HSC4/FU that show
different EMT characteristics than the parental cell lines.
There was no significant difference in cellular
proliferation between HSC2 and HSC2/FU; however, HSC2/FU tumors
grew faster than HSC2 tumors. In the same manner, the cell
proliferation speed of HSC4 was equal to HSC4/FU, though the growth
of HSC4/FU tumor was faster than HSC4 tumor (Figs. 1 and 6). Our cell/tumor growth inhibition assay
and TUNEL assay results clearly showed the resistant nature of
HSC2/FU and HSC4/FU cells. This resistance to 5-FU may involve many
factors and a number of signaling pathways, but in this study we
only emphasize the EMT characteristics of the resistant cell
lines.
Loss of E-cadherin is considered to be essential for
EMT. There are many transcription factors that can inhibit
E-cadherin activity directly (i.e. Twist, Goosecoid, TCF4, SIX1 and
FOXC2) or indirectly [i.e. SNAI1/Snail 1, SNAI2/Snail 2, ZEB1,
ZEB2, E47 and KLF8 (Kruppel-like factor 8)] (28). Our 5-FU-resistant cells (HSC2/FU
and HSC4/FU) showed decreased E-cadherin expressions and increased
N-cadherin and Twist expressions as well as typical morphologic
phenotypes of EMT, which may reflect an important process of
developing resistance to 5-FU. Though further investigations are
needed to clarify the mechanisms of 5-FU resistance, recovery of
EMT changes in 5-FU-resistant cells may be a potential therapeutic
target for development of the new strategy for advanced and/or
recurrent OSCC treatment.
In conclusion, we found EMT changes in HSC2/FU and
HSC4/FU. However, multiple mechanisms may lead to 5-FU resistance
in OSCC. It is reported that, 5-FU metabolism and activity of 5-FU
transport are closely linked to 5-FU resistance (29,30).
Uchibori et al (26) showed
that, downregulated RNRs (a key enzyme in 5-FU metabolism) and
upregulated MRP5 (an energy-dependent ATP-binding cassette
transporter protein) might confer 5-FU resistance in hepatocellular
carcinoma. Nagata et al reported that overexpression of
cellular inhibitor of apoptosis proteins 2 (cIAP2) contributes to
5-FU resistance in OSCC (27). In
their report, the 5-FU-resistant OSCC cells (SAS/FR2) were not
morphologically distinct from the parental cell line (SAS) though
SAS/FR2 and SAS had spindle-shaped morphology. (it was unclear
whether SAS/FR2 showed EMT changes). Our 5-FU-resistant OSCC cell
line associated with EMT may show high degree of resistance to
radiation as well as chemotherapy. HSC2/FU demonstrated stronger
resistance to radiation as well as cisplatin than HSC2 (data not
shown). Further investigations are required; however, the
5-FU-resistant OSCC cells (HSC2/FU and HSC4/FU) might be useful
in vitro and in vivo models for understanding the
5-FU-resistant mechanisms in OSCC.
Acknowledgements
This study was supported in part by a
Grant-in-Aid from the Japanese Ministry of Education, Science and
Culture.
References
|
1.
|
Rautava J, Luukkaa M, Heikinheimo K, Alin
J, Grenman R and Happonen RP: Squamous cell carcinomas arising from
different types of oral epithelia differ in their tumor and patient
characteristics and survival. Oral Oncol. 43:911–919. 2007.
View Article : Google Scholar
|
|
2.
|
Funk GF, Karnell LH, Robinson RA, Zhen WK,
Trask DK and Hoffman HT: Presentation, treatment, and outcome of
oral cavity cancer: a national cancer data base report. Head Neck.
24:165–180. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Mehrotra R, Singh MK, Pandya S and Singh
M: The use of an oral brush biopsy without computer - assisted
anaylsis in the oral lesions: a study of 94 patients. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 106:246–253. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Lawoyin JO, Lawoyin DO and Aderinokun G:
Intra-oral squamous cell carcinoma in Ibadan: a review of 90 cases.
Afr J Med Med Sci. 26:187–188. 1997.PubMed/NCBI
|
|
5.
|
Chaudhuri NK, Montag BJ and Heidelberger
C: Studies on fluorinated pyrimidines. III The metabolism of
5-fluorouracil-2-C14 and 5-fluoroorotic-2-C14 acid in vivo. Cancer
Res. 18:318–328. 1958.PubMed/NCBI
|
|
6.
|
Greenburg G and Hay ED: Epithelia
suspended in collagen gels can lose polarity and express
characteristics of migrating mesenchymal cells. J Cell Biol.
95:333–339. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Yang AD, Fan F, Camp ER, van Buren G, Liu
W, Somcio R, Gray MJ, Cheng H, Hoff PM and Ellis LM: Chronic
oxali-platin resistance induces epithelial-to-mesenchymal
transition in colorectal cancer cell lines. Clin Cancer Res.
12:4147–4153. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar
|
|
10.
|
Thiery JP and Chopin D: Epithelial cell
plasticity in development and tumor progression. Cancer Metastasis
Rev. 18:31–42. 1999. View Article : Google Scholar
|
|
11.
|
Kong B, Michalski CW and Kleeff J: Tumor
initiating cells in pancreatic cancer: A critical view. World J
Stem Cells. 1:8–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Gupta PB, Chaffer CL and Weinberg RA:
Cancer stem cells: mirage or reality? Nat Med. 15:1010–1012. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lee JH, Kim MC, Oh SY, Kwon HC, Kim SH,
Kwon KA, Lee S, Jeong JS, Choi SR and Kim HJ: Predictive value of
in vitro adenosine triphosphate-based chemotherapy response assay
in advanced gastric cancer patients who received oral
5-fluoro-uracil after curative resection. Cancer Res Treat.
43:117–123. 2011. View Article : Google Scholar
|
|
14.
|
Gamelin EC, Danquechin-Dorval EM, Dumesnil
YF, Maillart PJ, Goudier MJ, Burtin PC, Delva RG, Lortholary AH,
Gesta PH and Larra FG: Relationship between 5-fluorouracil (5-FU)
dose intensity and therapeutic response in patients with advanced
colorectal cancer receiving infusional therapy containing 5-FU.
Cancer. 77:441–451. 1996. View Article : Google Scholar
|
|
15.
|
Murakami Y, Uemura K, Sudo T, Hayashidani
Y, Hashimoto Y, Ohge H and Sueda T: Impact of adjuvant gemcitabine
plus S-1 chemotherapy after surgical resection for adenocarcinoma
of the body or tail of the pancreas. J Gastrointest Surg. 13:85–92.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Huang CL, Yokomise H, Kobayashi S,
Fukushima M, Hitomi S and Wada H: Intratumoral expression of
thymidylate synthase and dihydropyrimidine dehydrogenase in
non-small cell lung cancer patients treated with 5-FU-based
chemotherapy. Int J Oncol. 17:47–54. 2000.
|
|
17.
|
Furusaka T, Asakawa T, Tanaka A, Matsuda H
and Ikeda M: Efficacy of multidrug superselective intra-arterial
chemotherapy (docetaxel, cisplatin, and 5-fluorouracil) using the
Seldinger technique for tongue cancer. Acta Otolaryngol.
132:1108–1114. 2012. View Article : Google Scholar
|
|
18.
|
Lorch JH, Goloubeva O, Haddad RI, Cullen
K, Sarlis N, Tishler R, Tan M, Fasciano J, Sammartino DE and Posner
MR; TAX 324 Study Group: Induction chemotherapy with cisplatin and
fluorouracil alone or in combination with docetaxel in locally
advanced squamous-cell cancer of the head and neck: long-term
results of the TAX 324 randomised phase 3 trial. Lancet Oncol.
12:153–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Blanchard P, Bourhis J, Lacas B, Posner
MR, Vermorken JB, Hernandez JJ, Bourredjem A, Calais G, Paccagnella
A, Hitt R and Pignon JP; Meta-Analysis of Chemotherapy in Head and
Neck Cancer, Induction Project, Collaborative Group:
Taxane-cisplatin-fluorouracil as induction chemotherapy in locally
advanced head and neck cancers: an individual patient data
meta-analysis of the meta-analysis of chemotherapy in head and neck
cancer group. J Clin Oncol. 31:2854–2860. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Peters GJ, Backus HH, Freemantle S, van
Triest B, Codacci-Pisanelli G, van der Wilt CL, Smid K, Lunec J,
Calvert AH, Marsh S, McLeod HL, Bloemena E, Meijer S, Jansen G, van
Groeningen CJ and Pinedo HM: Induction of thymidylate synthase as a
5-fluorouracil resistance mechanism. Biochim Biophys Acta.
1587:194–205. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Longley DB, Harkin DP and Johnston PG:
5-Fluorouracil: mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Harada K, Kawashima Y, Yoshida H and Sato
M: Thymidylate synthase expression in oral squamous cell carcinoma
predicts response to S-1. Oncol Rep. 15:1417–1423. 2006.PubMed/NCBI
|
|
23.
|
Johnston PG, Drake JC, Trepel J and
Allegra CJ: Immunological quantitation of thymidylate synthase
using the monoclonal antibody TS 106 in 5-fluorouracil-sensitive
and -resistant human cancer cell lines. Cancer Res. 52:4306–4312.
1992.
|
|
24.
|
Plasencia C, Rooney PH, Taron M,
Martinez-Balibrea E, McLeod HL and Abad A: Chromosomal imbalance
maps of human 5FU-resistant colorectal cancer cell lines:
implications in the analysis of 5FU-acquired resistance mechanisms.
Int J Oncol. 22:945–953. 2003.
|
|
25.
|
Tanaka T, Bai T and Toujima S:
Establishment and characterization of monoclonal
5-fluorouracil-resistant cell lines derived from human endometrial
adenocarcinoma. Int J Oncol. 37:731–736. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Uchibori K, Kasamatsu A, Sunaga M, Yokota
S, Sakurada T, Kobayashi E, Yoshikawa M, Uzawa K, Ueda S, Tanzawa H
and Sato N: Establishment and characterization of two
5-fluorouracil-resistant hepatocellular carcinoma cell lines. Int J
Oncol. 40:1005–1010. 2012.PubMed/NCBI
|
|
27.
|
Nagata M, Nakayama H, Tanaka T, Yoshida R,
Yoshitake Y, Fukuma D, Kawahara K, Nakagawa Y, Ota K, Hiraki A and
Shinohara M: Overexpression of cIAP2 contributes to 5-FU resistance
and a poor prognosis in oral squamous cell carcinoma. Br J Cancer.
105:1322–1330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: an alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Pratt S, Shepard RL, Kandasamy RA,
Johnston PA, Perry W III and Dantzig AH: The multidrug resistance
protein 5 (ABCC5) confers resistance to 5-fluorouracil and
transports its mono-phosphorylated metabolites. Mol Cancer Ther.
4:855–863. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Nakano Y, Tanno S, Koizumi K, Nishikawa T,
Nakamura K, Minoguchi M, Izawa T, Mizukami Y, Okumura T and Kohgo
Y: Gemcitabine chemoresistance and molecular markers associated
with gemcitabine transport and metabolism in human pancreatic
cancer cells. Br J Cancer. 96:457–463. 2007. View Article : Google Scholar : PubMed/NCBI
|