Introduction
The survival rates of malignant diseases such as
breast cancer, lymphoma and leukemia, are increasing (1), because of the improvements in
therapeutic techniques. However, premature ovarian failure (POF) is
an established long-term adverse effect of chemotherapy in young
women. The POF can lead to infertility and amenorrhea and typical
climacteric symptoms such as palpitations, heat intolerance, hot
flashes, night sweats, irritability, anxiety, depression, sleep
disturbance, decreased libido, hair coarseness, vaginal dryness,
and fatigue (2), which have a
significant impact on the quality of life of young women.
Younger women of childbearing age are confronted
with the risk of infertility by chemotherapy-induced amenorrhea.
Though a number of options to preserve fertility are available,
such as cryopreservation of oocyte, embryo or ovarian tissue,
ovarian transposition and hormonal protection with GnRH analogs
(3), the latter has an edge over
the others since it is less invasive and more convenient.
Animal studies and phase II clinical research have
suggested that GnRHa can lead to temporary ovarian suppression to
preserve ovarian function (4–6).
Recently, several studies have been conducted on the ovarian
function preservation in young women undergoing gonadotoxic
chemotherapy. But the conclusions drawn from these studies are not
unanimous. There were also some reviews and meta-analysis on the
topic, some of which came up with the results that GnRHa can
benefit the ovarian function following chemotherapy (7,8),
while one of the studies produced the conclusion that the benefit
of using GnRHa for fertility preservation in young breast cancer
patients remains uncertain (9). In
the present study, we conducted a meta-analysis to assess the
efficacy of GnRH agonists in protecting the ovaries against
chemotherapy induced damage in premenopausal women with malignant
diseases.
Materials and methods
Data sources and searches
The electronic medical literature databases
including Pubmed, MELINE, Cochrane library, Embase, CNKI and
Wanfang, were explored for available articles on the topic
published till November, 2013 using crosslinking keywords. Articles
in Chinese and English were included. Only randomized controlled
trials (RCTs) were selected. The keywords included fertility,
ovarian failure, ovarian preservation, chemotherapy and GnRHa.
Reference lists were also searched for additional studies. The
experimental groups were prescribed GnRHa while undergoing
chemotherapy and the control group underwent chemotherapy without
GnRHa.
Study selection
Two investigators reviewed each study. The following
inclusion criteria were used to select the studies for our
meta-analysis: i) premenopausal females under the age of 46
undergoing potentially gonadotoxic chemotherapy; ii) a control
group suffering with similar ailments, who were undergoing
chemotherapy but were not given GnRHa; iii) a clear definition to
evaluate ovarian function, such as recurrence of menses, level of
follicle-stimulating hormone (FSH). If the study have repeated
publications, we took the one with the most complete data. The
following studies were excluded: i) the studies were not RCTs, such
as case-control design, reviews, letters or case reports; ii)
publications in languages other than Chinese and English; and iii)
studies with a dropout rate of greater than 10%.
Data extraction and quality
assessment
The data were extracted by two reviewers
independently, the terms were as follows: i) the basic information
of author, year of publication, country of the study, study type;
ii) patient population, type of disease, age; iii) GnRHa dose,
timing; iv) chemotherapy regimens; v) definition of premature
ovarian failure; and vi) duration of follow-up.
The methodological quality of each study was
assessed in accordance to the guidelines in the Cochrane reviewers’
handbook (Version 5.1.0) (10).
The following trial features were assessed: i) random sequence
generation; ii) allocation concealment; iii) blinding of
participants and personnel; iv) blinding of outcome assessment; v)
incomplete outcome data; vi) selective reporting; vii) other
sources of bias. The evaluations were categorized as ‘low risk’,
‘high risk’ or ‘unclear risk’ of bias. If all the qualities
criteria were judged low risk, the trial was considered to have low
risk of bias (score A); if one or more qualities criteria were
judged unclear risk, but no one were judged high risk, the trial
was considered to have moderate risk of bias (score B); and if one
or more qualities criteria were judged high risk, the trial was
considered high risk of bias (score C). The publication bias was
also assessed using funnel plot.
Statistical methods
In this meta-analysis, the main outcomes were the
post-chemotherapy ovarian function. All analyses were performed
with Review Manager statistical software (Review Manager 5.1).
Dichotomous data were used to calculate the risk ratio (RR) and 95%
confidence interval, the heterogeneity of the RR were also tested.
In the Q test, it was considered heterogeneous if P<0.1, while
in the I2 statistic, 0 to 40% were deemed not important;
30 to 60% might represent moderate heterogeneity; 50 to 90% might
represent substantial heterogeneity; and 75 to 100% considerable
heterogeneity (10). A
fixed-effect model was used when there was no heterogeneity, when
the heterogeneity present could not be readily explained; a
random-effect model was used. In this meta-analysis, our results
showed P=0.0002, I2=72% indicating that there was
substantial heterogeneity, so a random-effect model was employed.
In the overall results, P<0.05 signifies that GnRHa has a
positive effect in preserving the ovarian function.
The publication bias was also assessed by funnel
plot, which was plotted by Review Manager 5.1. Using RR as abscissa
and standard error of RR napierian logarithm as ordinate, bias was
considered to be under control if the funnel plot was
symmetrical.
Results
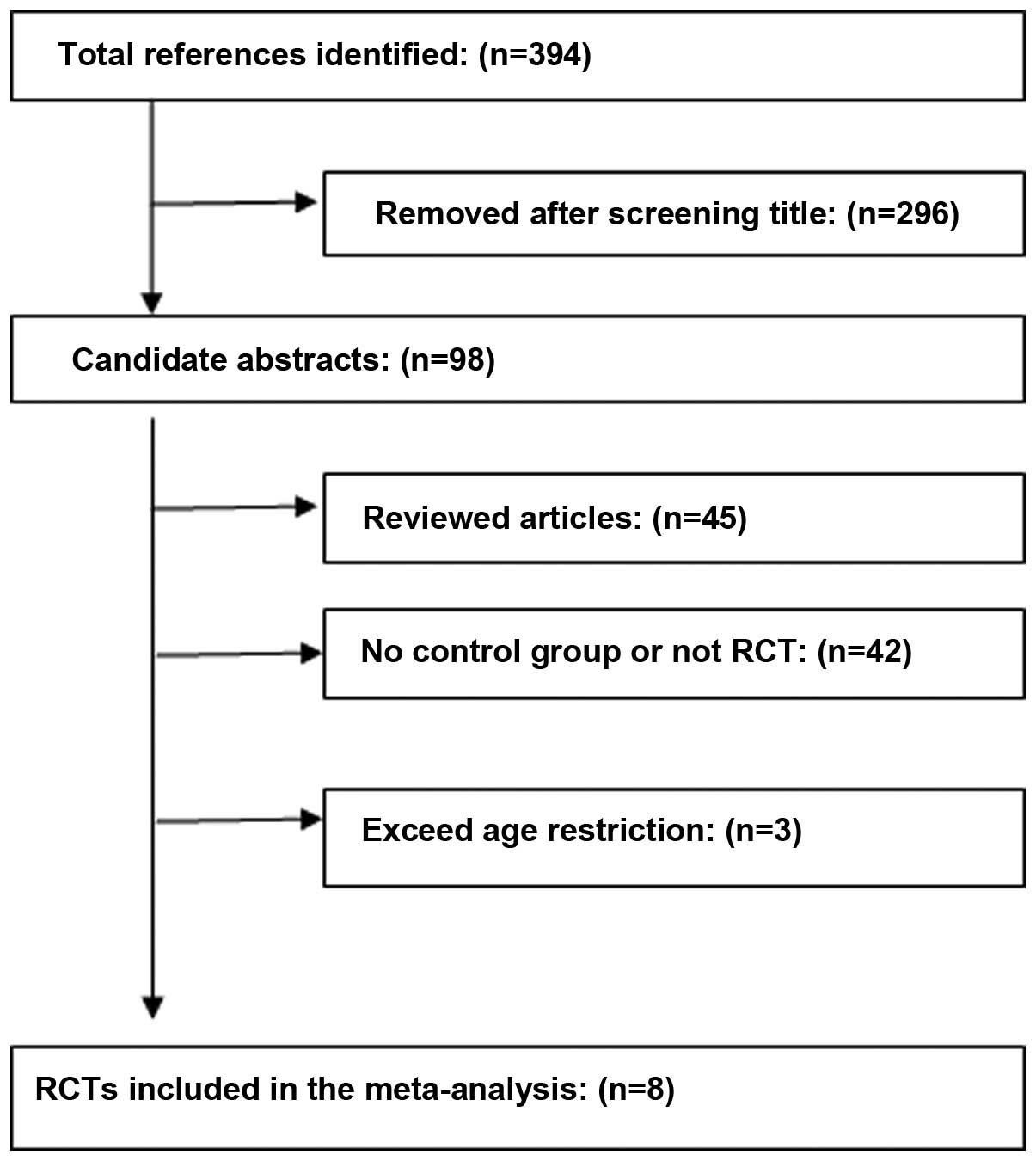
After the databases were thoroughly investigated,
394 articles were selected, then applying the inclusion and
exclusion criteria, eight RCTs composed of 621 patients were
included (Fig. 1) (11–18).
A total of 321 women were treated with GnRHa during their
chemotherapy, while 300 women underwent chemotheray without GnRHa.
The comprehensive characteristics are shown in Table I. Women in five of the trials had
breast cancer, two had lymphoma and one had ovarian malignancy. The
GnRHa used in the RCTs included triptorelin, goserelin, buserelin
and dipherelin. The standard dose was maintained and most of the
GnRHa were administered by subcutaneous or intramuscular injection,
but in one of the cases, it was given intranasally (13). In all trials, the first dose was
administered at least one week prior to chemotherapy in order to
avoid chemotherapy during the expected ovarian flare. We found that
there were certain inconsistencies in the ways POF were defined in
the trials; five (11–14,16,17)
of them were defined as amenorrhea only, while two trials had
considered its association with the follicle-stimulating hormone
(FSH), estrodiol (E2). The types of chemotherapy regimen
used and the number of cycles were also taken into account. Two
trials (11,18) had a follow-up duration less than 12
months, others had maintained follow-up for more than 12 months.
All the trials included were evaluated to have low or moderate risk
of bias according to the methodological quality assessments in the
Cochrane reviewers’ handbook (version 5.1.0) (10).
 | Table I.Review of the studies in the
meta-analysis. |
Table I.
Review of the studies in the
meta-analysis.
| Authors/(Refs.)
country, year | Patient
population | Study type | GnRH, dose,
timing | Chemotherapy | Definition of
premature ovarian failure (POF) | Duration of
follow-up | Quality
assessment |
|---|
| Del Mastro et
al (15) Italy, 2011 | Breast cancer age
21–45 (premenopausal) | Parallel, randomized,
open-label trial | Triptorelin, 3.75 mg,
q.4wk, at least 1 week before 1st chemotherapy | Anthracycline-based;
anthracycline plus taxane-based; CMF-based | No menses within 12
months and post-menopausal FSH and E2 | 12 months | A |
| Munster et al
(12) USA, 2012 | Breast cancer age
21–43 | Prospective
randomized | Triptorelin, 3.75 mg,
q.28-30d, 1st injection is between 7 days to 4 weeks before
chemotherapy | AC×4; AC→T×4; FEC×6;
FAC×6 | Amenorrhea | Median follow-up
time: 18 months | A |
| Loverro et al
(14) Italy, 2007 | Hodgkin’s lymphoma
mean age 20–38 | Prospective
randomized | Triptorelin, 3.25 mg
q.mth or triptorelin 11.25 mg q.3mth | 13 patients: ABVD×6,
13 patients: ABVD×5 alternating with C(M)OPP, 3 patients: C(M)OPP
alternative ABV, then DHAP | No menses within 12
months | Mean time: 4.2±2.8
years | B |
| Waxman et al
(13) England, 1987 | Advanced Hodgkin’s
disease age 17–46 | Prospective
randomized | Buserelin 200
μg tid intranasal starting 1 week before chemotherapy | Up to 6 cycles of
MVPP | Amenorrhea | Mean (years): GnRHa,
2.3; no GnRHa, 2.0 | B |
| Elgindy et al
(16) Egypt, 2013 | Breast cancer
(hormone-insensitive) age 18–40 | Randomized
controlled | Triprelin 3.75mg
q.4wk, the 1st injection was 1 week before chemotherapy | FAC×6 | No menses within 12
months | 12 months | A |
| Gerber et al
(17) Germany, 2011 | Breast cancer
(hormone-insensitive) age 18–45 | Randomized
open-label, controlled multicenter trial | Goserelin 3.6 mg
q.4k, 1st injection at least 2 weeks before chemotherapy |
Anthracycline/cyclophosphamide-based
chemotherapy | No regular
menses | 24 months | B |
| Badawy et al
(11) Egypt, 2009 | Breast cancer age
18–40 | Randomized, control
trial | Goserelin 3.6 mg
q.4wk, for 6 months | FAC×6 | Early cessation of
menstruation, ovulation and increased serum FSH level | 8 months | B |
| Gilani et al
(18) Iran, 2007 | Ovarian malignancy
age 12–40 (post-menarche) | Prospective,
randomized, controlled | Diphereline 3.75 mg
q.28d, 1st injection 7–10 days before chemotherapy | VAC, BEP, TC, CP, for
less than 6 months | Early, permanent
cessation of menstruation, FSH ≤20 mlu/ml | 6 months | B |
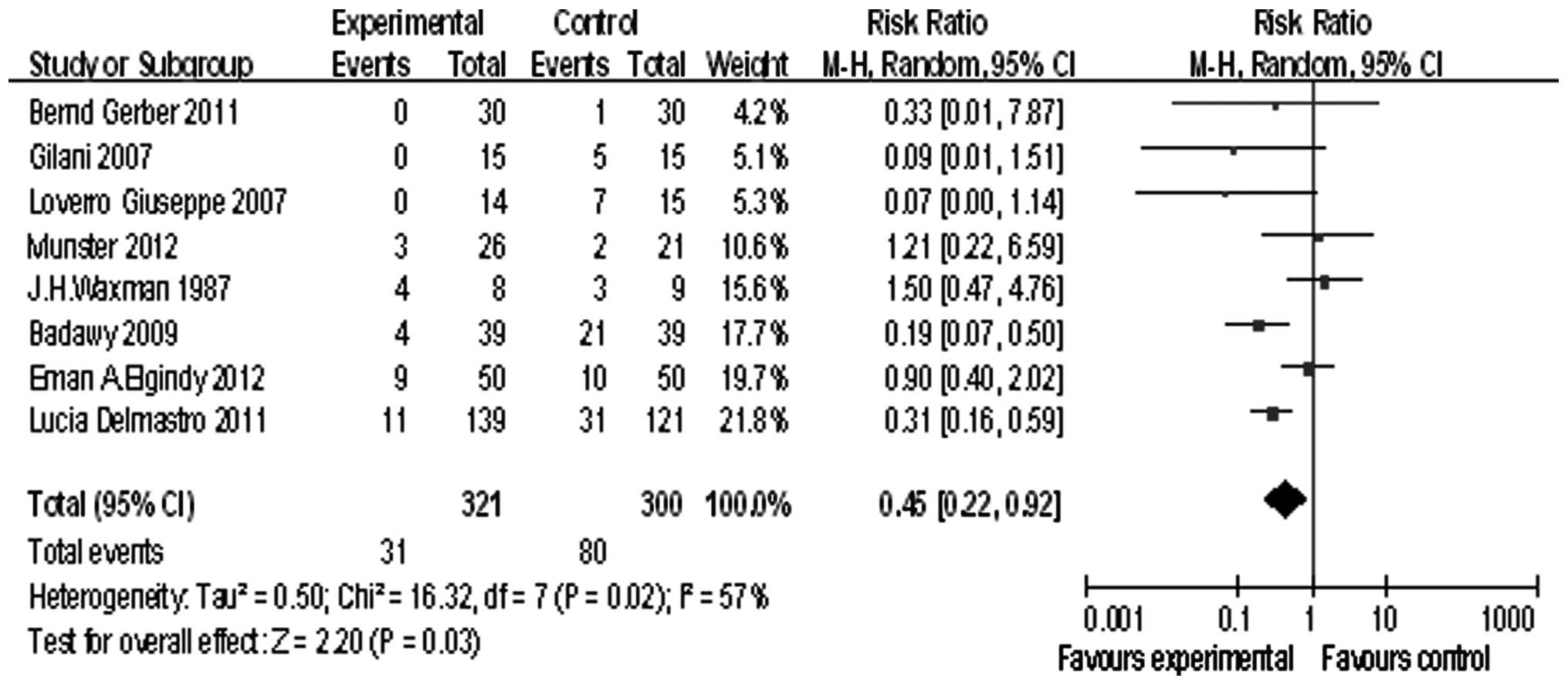
Eight studies reported POF, we tested the
heterogeneity of the RR [τ2=0. 5; χ2=16.32,
df=7 (P=0.02); I2=57%], and because of the heterogeneity
of the RR, a randomized model was used. The summary RR was 0.45,
and the 95% confidence interval was (0.22, 0.92), P=0.03 (Fig. 2). This demonstrated that GnRHa
exerted its positive effects in preserving the ovarian function by
preventing POF.
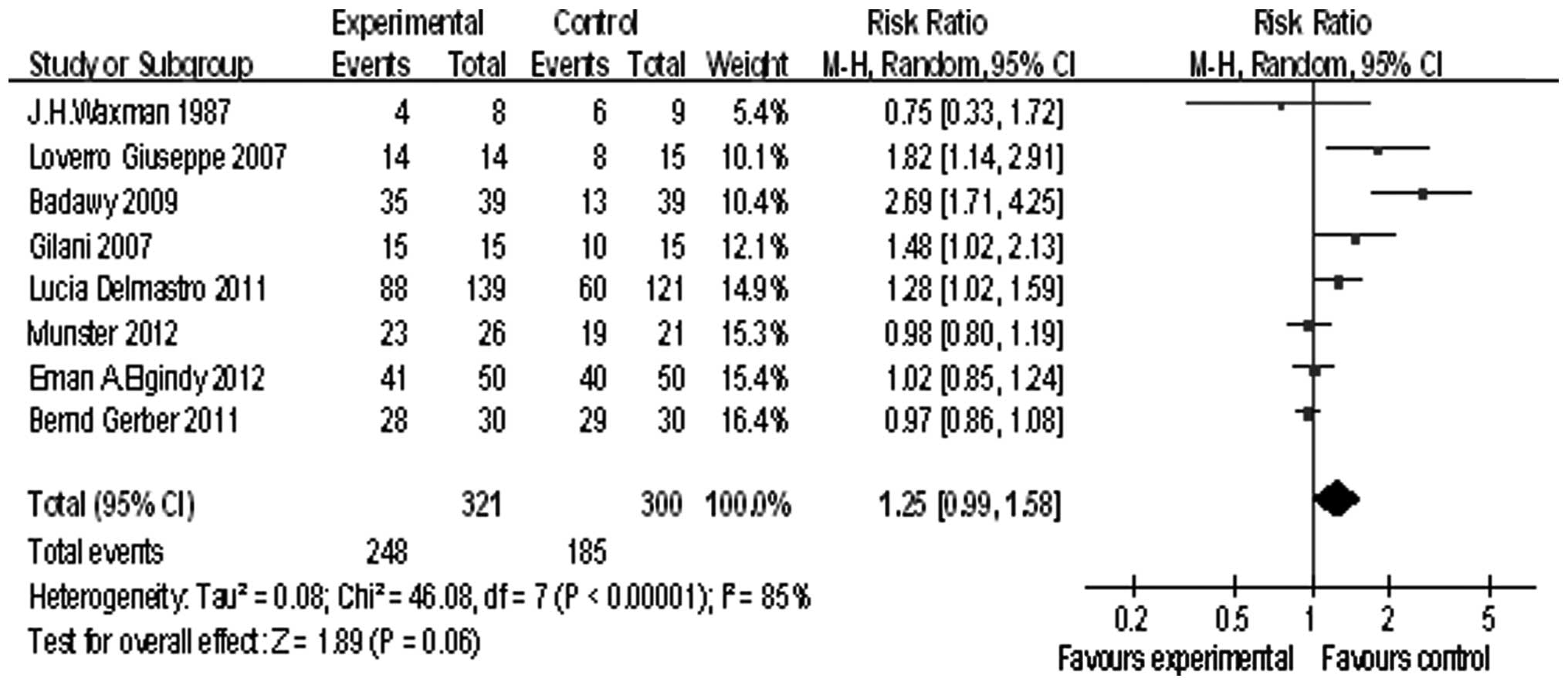
Eight studies reported the rate of menstruation
recovery, the summary RR was 1.25, and the 95% confidence interval
was (0.99, 1.58), P=0.06 (Fig. 3).
This result showed GnRHa can improve the menstruation recovery
rate.
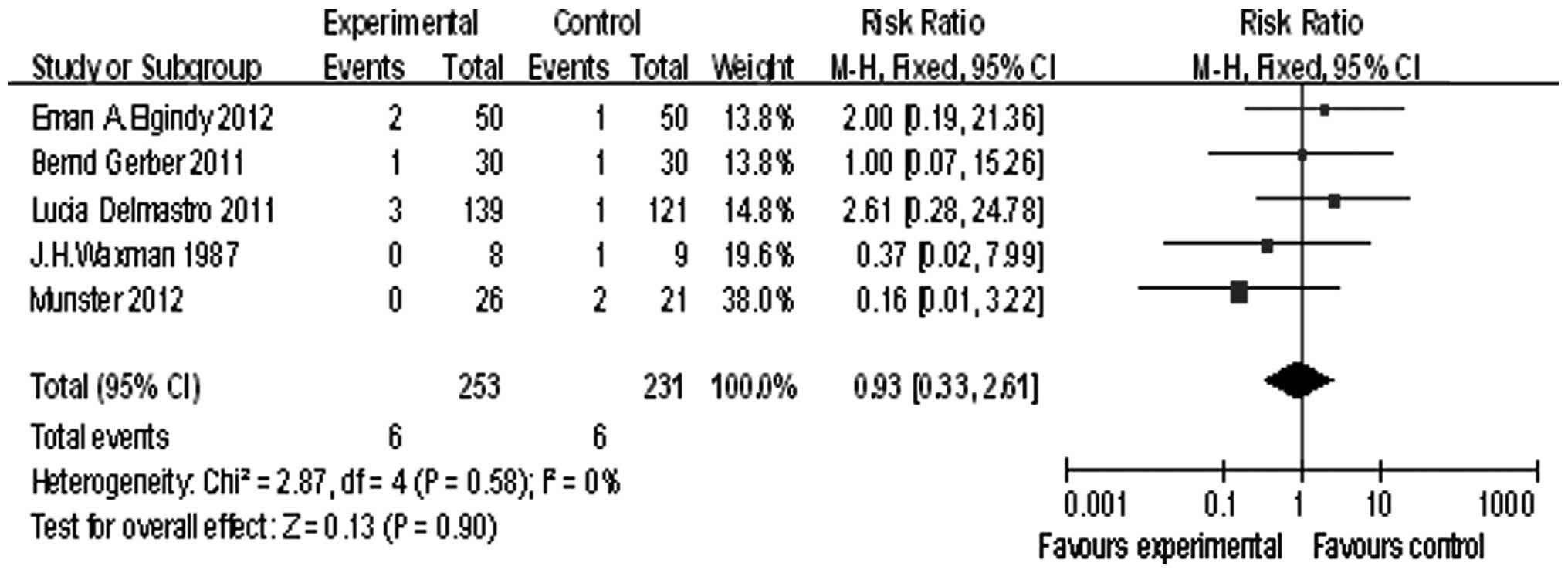
Five of the studies reported pregnancy, the summary
RR was 0.93, and the 95% confidence interval was (0.33, 2.61), P=
0.90 (Fig. 4). The pregnancy rate
showed no significant difference between the GnRHa group and the
chemotherapy alone group.
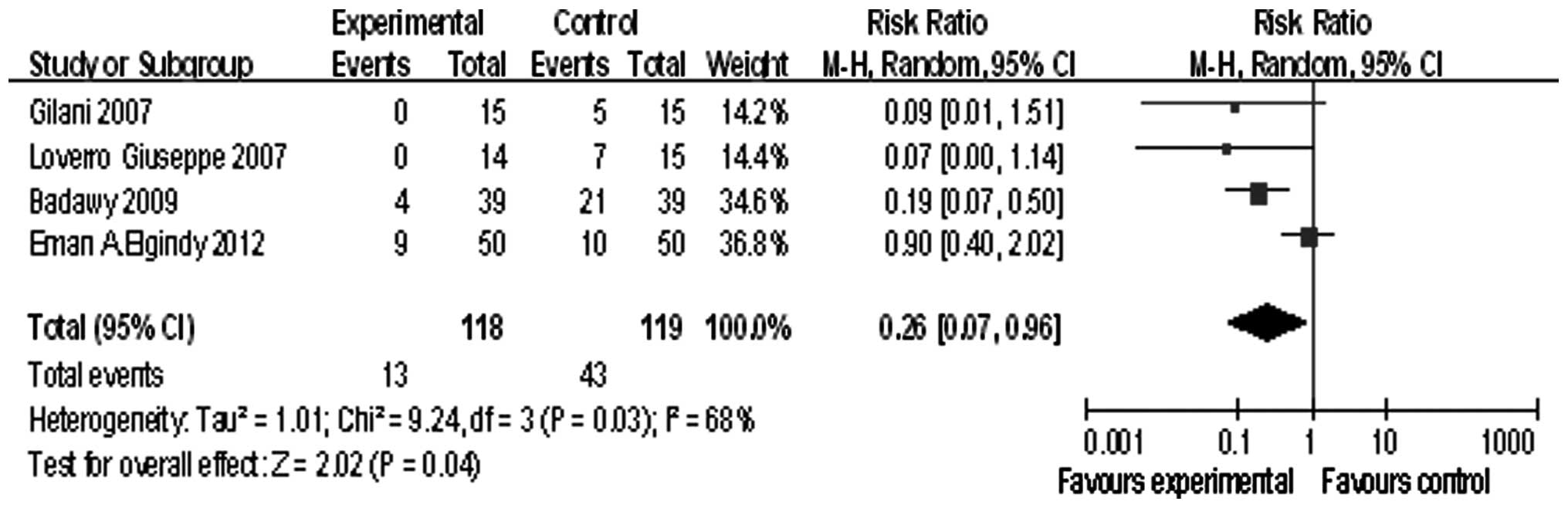
Four studies involved patients younger than 40
years, the summary RR was 0.26, and the 95% confidence interval was
(0.07, 0.96), P=0.04 (Fig. 5).
Publication bias was assessed by the use of a funnel
plot, the plot was basically symmetrical and the distribution of
most of the points was in an inverted funnel manner.
Discussion
The mechanism of action by which GnRHa preserves
ovarian function is not clear, but may include: i) the interruption
of FSH secretion and inhibition of follicles from entering the
growing stage; ii) reduction of ovarian perfusion; iii) protection
of ovarian germline stem cells directly; and iv) upregulation of
phingosine-I-phosphate (19).
Based on the meta-analysis we conducted, there was
evidence that GnRHa could preserve the ovarian function following
chemotherapy in young pre-menpausal women. POF has been referred to
as a form of hypogonadism that manifests as premature menopause or
development of amenorrhea due to cessation of ovarian function,
only two trials considered the level of the FSH, E2.
However, POF is rather an ovarian insufficiency than ovarian
failure since the women with POF have been reported to conceive
despite limited ovarian function. Ravdin et al showed that
young breast cancer patients retain their ovarian function
following chemotherapy despite the absence of menstruation
(21). However, infertility
typically proceeds to menopause by within ten years (25). Thus we consider that the former
definition has certain limitation since amenorrhea is not the best
standard for determination of fertility. The diagnosis is based on
detection of elevated FSH that is approaching menopausal level
(usually above 40 IU/l) at least two measurements conducted a few
weeks apart (20). Apart from FSH,
other hormonal indicators such as anti-mullerian hormone (AMH),
estradiol (E2), inhibin-A and B have also been
implicated with the accurate determination of ovarian functions.
Anderson et al came up with an effective method for
determining the residual ovarian function after chemotherapy which
involved the measurement of AMH along with ultrasound-guided antral
follicle count (22).
Among the trials in our analysis, four (11,14,16,18)
were conducted on patients younger than 40 years. Because of the
limited data, we could not estimate the precise function of GnRHa
in this patient group. This leads to a bias in our analysis since
ovarian function has a natural tendency to decline along with age.
Also there is a possibility of data contamination due to the
involvement of the environmental factors. The data analyzed have a
wide geographical variation thus we can not exclude the possibility
of a varied susceptibility of women towards POF due to the
environment they were exposed to.
Tamoxifen is a known independent risk factor which
can affect menstrual cycles. One study (23) showed that the rate of amenorrhea in
patients treated with goserelin alone was 64% compared to 93% in
patients treated with goserelin combined with tamoxifen. However,
of the five trials including patients with breast cancer, there was
a lack of control for confounding effects of tamoxifen.
Only three of the trials were followed up for more
than 2 years. For those women who recovered menstruation, there is
an additional long-term risk of POF (24). The long-term POF reports do not
exist; and one-year follow-up can not evaluate the ability of
fertility well. Thus, long-term follow-up is needed.
The first injection of the GnRHa was generally one
or two weeks before chemotherapy, only two trials (16,17)
proved ovarian suppression before chemotherapy. Ovarian suppression
was not confirmed on chemotherapy administration and ovaries could
be in ‘flare-up’ interval. In this condition, the GnRHa might not
perform well.
Some heterogeneity existed in the trials we
analyzed, which presumably creates a potential bias on the results
we obtained. For instance the heterogeneity in the chemotherapy
regimen could affect our results. Similarly the variation in the
length of follow-up duration also has certain impact in determining
the actual results. On the contrary, one of the strength of our
study was the inclusion of bilingual literatures in English and
Chinese. The study criteria were determined carefully and the
methodological quality of each trial included in this analysis was
assessed following the standard protocol in the Cochrane reviewers’
handbook.
In this meta-analysis, we have deduced the
beneficial role of GnRHa in preserving the ovarian function of the
young women undergoing chemotherapy, but the ability to preserve
fertility was not proved. However, more well-designed trials with
appropriate age limitation (<40 years old) and a more sensitive
marker of ovarian reserve are needed before it is established as a
standard therapy.
Acknowledgements
The study was conducted at Qilu
Hospital, Shandong Medical University and was supported by the
National Natural Science Foundation of China (81072121, 81372808,
JJ and 81173614, LQT) and the science and technology developing
planning (2012G0021823, JJ), (2011GSF12122, ZX) and also by science
and technology developing planning of Jinan (201303035). We would
like to acknowledge Mr Binglin Lv for his support for statistical
analysis.
References
|
1.
|
Surveillance, Epidemiology and End Results
(SEER) Web site: Available online: http://www.seer.cancer.govApril2010
based on the November 2009 submission.
|
|
2.
|
Beck-Peccoz P and Persani L: Premature
ovarian failure. Orphanet J Rare Dis. 1:92006. View Article : Google Scholar
|
|
3.
|
Sonmezer M and Oktay K: Fertility
preservation in female patients. Hum Reprod Update. 10:251–266.
2004. View Article : Google Scholar
|
|
4.
|
Bokser L, Szende B and Schally AV:
Protective effects of D-Trp6-luteinizing hormone-releasing hormone
microcapsules against cyclophosphamide-induced gonadotoxicity in
female rats. Br J Cancer. 61:861–865. 1990. View Article : Google Scholar
|
|
5.
|
Ataya K, Rao LV, Lawrence E and Kimmel R:
Luteinizing hormone-releasing hormone agonist inhibits
cyclophosphamide-induced ovarian follicular depeletion in rhesus
monkeys. Biol Reprod. 52:365–372. 1995. View Article : Google Scholar
|
|
6.
|
Del Mastro L, Catzeddu T, Boni L, et al:
Prevention of chemotherapy-induced menopause by temporary ovarian
suppression with goserelin in young, early breast cancer patients.
Ann Oncol. 17:74–78. 2006.PubMed/NCBI
|
|
7.
|
Clowse ME, Behera MA, Anders CK, Copland
S, et al: Ovarian preservation by GnRH agonists during
chemotherapy: A meta-analysis. J Women’s Health. 18:311–319.
2009.PubMed/NCBI
|
|
8.
|
Yang B, Shi W, Yang J, Liu H, Zhao H, et
al: Concurrent treatment with gonadotropin-releasing hormone
agonists for chemotherapy-induced ovarian damage in premenopausal
women with breast cancer: A meta-analysis of randomized controlled
trials. Breast. 22:150–157. 2013. View Article : Google Scholar
|
|
9.
|
Turner NH, Partridge A, Sanna G, Di Leo A
and Biganzoli L: Utility of gonadotropin-releasing hormone agonists
forfertility preservation in young breast cancer patients: the
benefit remains uncertain. Ann Oncol. 24:2224–2235. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Higgins JPT and Green S: Cochrane Handbook
for Systematic Reviews of Interventions. Version 5.1.0. The Cohran
Collaboration (updated March 2011).
|
|
11.
|
Badawy A, Elnashar A, El-Ashry M and
Shahat M: Gonadotropin-releasing hormone agonists for prevention of
chemotherapy-induced ovarian damage: prospective randomized study.
Fertil Steril. 91:694–697. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Munster PN, Moore AP, et al: Randomized
trial using gonadotropin-releasing hormone agonist triptorelin for
the preservation of ovarian function during (neo)adjuvant
chemotherapy for breast cancer. J Clin Oncol. 30:533–538. 2012.
View Article : Google Scholar
|
|
13.
|
Waxman JH, Ahmed R, Smith D, Wrigley PEM,
et al: Failure to preserve fertility in patients with Hodgkin’s
disease. Cancer Chemother Pharmacol. 19:159–162. 1987.
|
|
14.
|
Loverro G, Guarini A, Di Naro E, et al:
Ovarian function after cancer treatment in young women affected by
Hodgkin disease (HD). Hematology. 12:141–147. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Del Mastro L, Boni L, Michelotti A,
Gamucci T, Olmeo N, et al: Effect of the gonadotropin-releasing
hormone analogue triptorelin on the occurrence of
chemotherapy-induced early menopause in premenopausal women with
breast cancer: a randomized trial. JAMA. 306:269–276. 2011.
|
|
16.
|
Elgindy EA, El-Haieg DO, Khorshid OM, et
al: Gonadatrophin suppression to prevent chemotherapy-induced
ovarian damage: a randomized controlled trial. Obstet Gynecol.
121:78–86. 2013.PubMed/NCBI
|
|
17.
|
Gerber B, von Minlkwitz G, Stehle H,
Reimer T, et al: Effect of luteining hormone-releasing hormone
agonist on ovarian function after modern adjuvant breast cancer
therapy: The GBG 37 ZORO study. J Clin Oncol. 29:2334–2341. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Gilani MM, Hasanzadeh M, Ghaemmaghami F,
et al: Ovarian preservation with gonadotrop in releasing hormone
analog during chemotherapy. Asia Pac J Clin Oncol. 3:79–83. 2007.
View Article : Google Scholar
|
|
19.
|
Blumenfeld Z: How to preserve fertility in
young women exposed to chemotherapy? The role of GnRH agonist
cotreatment in addition to cryopreservation of embryo, oocytes, or
ovaries. Oncologist. 12:1044–1054. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Goswami D and Conway GS: Premature ovarian
failure. Hum Reprod Update. 11:391–410. 2005. View Article : Google Scholar
|
|
21.
|
Ravdin PM, Fritz NF, Tormey DC and Jordan
VC: Endocrine status of premenopausal node-positive breast cancer
patients following adjuvant chemotherapy and long-term tamoxifen.
Cancer Res. 48:1026–1029. 1989.PubMed/NCBI
|
|
22.
|
Anderson RA, Themmen AP, Al-Qahtani A,
Groome NP and Cameron DA: The effects of chemotherapy and long-term
gonadotropin suppression on the ovarian reserve in premenopausal
women with breast cancer. Hum Reprod. 21:2583–2592. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Sverrisdottir A, Nystedt M, Johansson H
and Fornander T: Adjuvant goserelin and ovarian preservation in
chemotherapytreated patients with early breast cancer: results from
a randomized trial. Breast Cancer Res Treat. 117:561–567. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Partridge A, Gelber S, Gerber RD and
Castiglione-Certsch M: Age of menopause among women who remain
premenopausal following treatment for early breast cancer:
long-term results from International Breast Cancer Study Group
Trials V and VI. Eur J Cancer. 43:1646–1653. 2007. View Article : Google Scholar
|
|
25.
|
Te Velde ER and Pearson PL: The
variability of female reproductive ageing. Hum Reprod Upadate.
8:141–154. 2002.PubMed/NCBI
|