Introduction
Bladder cancer is one of the most lethal diseases in
developed countries. The major type of bladder cancer leading to
death is transitional cell carcinoma (TCC), which is characterized
by muscle invasive bladder cancer (MIBC) (1,2).
Most of the MIBC found in TCC is deeply involved in metastasis,
migration and invasion (3). An
understanding of the molecular mechanisms involved in the migration
and invasion of MIBC could lead to identification of therapeutic
targets for effective treatment.
Members of the matrix metalloproteinase (MMP) family
are capable of degrading the extracellular matrix, and have been
associated with essential roles in the migration and invasion of
tumor cells (3–5). This has been correlated with
gelatinase activity by MMP-2 (72 kDa) and MMP-9 (92 kDa) (4,5).
Several studies have examined the MMP-9 association with the
progression of bladder cancer that involves tumor grade, invasion,
migration and metastasis (3). In
addition, the expression of MMP-9 is strongly increased through the
release of several cytokines and growth factors in different types
of cells (6–9). Transcription factors including NF-κB,
Sp-1 and AP-1 have been identified as essential mediators for the
induction of MMP-9 in cancer cells (6–10).
Cumulative studies have demonstrated that signaling pathways such
as MAPK and AKT contribute to the MMP-9 expression that is
regulated by transcription factors (8,9,11–13).
Interleukin-7 (IL-7) is an essential survival factor
of T and B cells (14,15). It has been demonstrated that IL-7
exerts proliferative activity on murine B-cells within the bone
marrow culturing system (14,15),
and studies have suggested regulatory roles for IL-7 that involve
in the proliferation, differentiation, and survival in the
development of lymphocytes (14,15).
Recent studies have shown the effect of IL-7 in anti-apoptotic
function and in cell cycle progression during T-cell proliferation
(14,15). The binding of IL-7 to the IL-7
receptor α chain (IL-7Rα) stimulated the activation of Jak/Stat and
PI3K/AKT signaling in T-cells (14,15).
Although many studies have demonstrated the biological function of
IL-7 in the context of T- and B-cell homeostasis, its exact
regulatory mechanism in the migration and invasion of tumor cells
has yet to be fully explained.
Herein we demonstrate the novel molecular mechanisms
underlying the migration and invasion of bladder cancer cells
induced by cytokine IL-7. The present study is the first to focus
on the function of IL-7 as it applies to the migration and invasion
of tumor cells. Our results demonstrated that p27KIP1 is required
for the IL-7-induced migration and invasion of bladder cancer 5637
cells via ERK1/2-mediated MMP-9 expression and the activation of
the NF-κB binding motif.
Materials and methods
Materials
Polyclonal antibodies to ERK, phospho-ERK, p38MAPK,
phospho-p38MAPK, JNK, and phospho-JNK were obtained from Cell
Signaling (Danvers, MA, USA). Polyclonal antibodies to cyclin E,
CDK2, CDK4, cyclin D1, p53, p21WAF1, p27KIP1 and GAPDH were
obtained from Santa Cruz (Santa Cruz, CA, USA). U0126 was obtained
from Calbiochem (San Diego, CA, USA). The polyclonal MMP-9 antibody
was obtained from Chemicon. Small interfering RNA (siRNA)
oligonucleotides targeting p27KIP1 (5′-CAA ACGUGCGAGUGUCUAAUU-3′)
and scramble (5′-CUGUCA GUCAGUCGUAGUAUU-3′) were designed and
synthesized by Genolution (Seoul, Korea).
Cell cultures
A human bladder carcinoma cell line (5637) was
obtained from the American Type Culture Collection. The cells were
maintained in DMEM (4.5 g glucose/liter) supplemented with 10%
fetal calf serum, L-glutamine, and antibiotics (Biological
Industries, Beit Haemek, Israel) at 37°C in a 5% CO2
humidified incubator.
Cell proliferation
Cells were placed in 12-well culture plates at
4×104 cells/ml with DMEM containing 10% FBS. Cells were
incubated at 37°C for 24 h. The medium was then exchanged for
serum-free medium. After 24 h, the cells were stimulated with IL-7
and then trypsinized with trypsin-EDTA. Cells were counted using a
coulter counter chamber (Coulter Corp., FL, USA).
Immunoblot analysis
Growth-arrested cells were treated with IL-7 in the
absence of 10% FBS for various durations at 37°C. The cells were
then washed twice with cold PBS and freeze-thawed in 250 μl
lysis buffer [containing, in mmol/l, HEPES (pH 7.5) 50, NaCl 150,
EDTA 1, EGTA 2.5, DTT 1, β-glycerophosphate 10, NaF 1,
Na3VO4 0.1, and phenylmethylsulfonyl fluoride
0.1 and 10% glycerol, 0.1% Tween-20, 10 μg/ml of leupeptin,
and 2 μg/ml of aprotinin], and then scraped into 1.5 ml
tubes. The lysates were placed on ice for 15 min and then
centrifuged at 12,000 rpm for 20 min at 4°C. The protein
concentration of the supernatant was determined using a Bradford
reagent method (Bio-Rad). Equal amounts of cellular proteins were
resolved by electrophoresis on a 0.1% SDS-10% polyacrylamide gel
(SDS-PAGE) under denaturing conditions. The proteins were
transferred electrophoretically to nitrocellulose membranes
(Hybond, Amersham Corp.). After blocking in 10 mmol/l Tris-HCl (pH
8.0), 150 mmol/l NaCl, and 5% (wt/vol) non-fat dry milk, the
membranes were treated with primary antibodies for 90 min, followed
by incubation with peroxidase-conjugated secondary antibodies for
45 min. The immunocomplexes were detected using a chemiluminescence
reagent kit (Amersham Corp.). For the immunoblotting studies, the
experiments were repeated at least 3 times.
Wound-healing migration assay
Cells were plated on 6-well dishes and grown to 90%
confluence in 2 ml of growth medium. A wound was created in cells
using a 2-mm-wide tip and then the cells were treated with IL-7.
They were allowed to migrate, and photographs were taken through an
inverted microscope (×40 magnification).
Invasion assay
Cells (2.5×104) were resuspended with
IL-7 in 100 μl of medium and placed in the upper part of a
transwell plate. The cells were then incubated for 24 h. The cells
had to pass through a polycarbonate membrane with 8 μm-sized
pores and a thin layer of an ECM Matrix-like material. The ability
of the cells to invade the ECM Matrix-like material was determined
using a commercial cell invasion assay kit (Chemicon International,
Billerica, MA, USA).
Zymography
Conditioned medium was electrophoresed in a
polyacrylamide gel containing 1 mg/ml gelatin. The gel was then
washed at room temperature for 2 h with 2.5% Triton X-100 and
subsequently at 37°C overnight in a buffer containing 10 mM
CaCl2, 150 mM NaCl and 50 mM Tris-HCl, pH 7.5. The gel
was stained with 0.2% Coomassie blue and photographed using a light
box. Proteolysis was detected as a white zone in a dark blue field
(8,9).
Transfection
Cells were transfected with siRNA using
Lipofectamine 2000 transfection reagent according to the
manufacturer’s protocols (Invitrogen). After the indicated
incubation with IL-7, the cells were studied via immunoblot,
zymography, EMSA, invasion and wound healing migration.
Nuclear extracts and electrophoretic
mobility shift assay (EMSA)
Cultured cells were collected by centrifugation,
washed and suspended in a buffer containing 10 mM HEPES (pH 7.9),
10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT and 0.5 mM PMSF.
After 15 min on ice, the cells were vortexed in the presence of
0.5% Nonidet NP-40. The nuclear pellet was then collected by
centrifugation and extracted in a buffer containing 20 mM HEPES pH
7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, and 1 mM PMSF for
15 min at 4°C.
The nuclear extract (10–20 μg) was
preincubated at 4°C for 30 min with the 100-fold excess of an
unlabeled oligonucleotide spanning the -79 MMP-9 cis-element of
interest. The sequences were as follows: AP-1,
CTGACCCCTGAGTCAGCACTT; NF-κB, CAGTGGAATTCCCCAGCC; and, Sp-1,
GCCCATTCCTTCCGCCCCCAGATGAAGCAG. The reaction mixture was then
incubated at 4°C for 20 min in a buffer (25 mM HEPES buffer pH 7.9,
0.5 mM EDTA, 0.5 mM DTT, 0.05 M NaCl, and 2.5% glycerol) with 2
μg of poly dI/dC and 5 fmol (2×104 cpm) of a
Klenow end-labeled (32P-ATP) 30-mer oligonucleotide,
which spanned the DNA binding site in the MMP-9 promoter. The
reaction mixture was electro-phoresed at 4°C in a 6% polyacrylamide
gel using a TBE (89 mM Tris, 89 mM boric acid and 1 mM EDTA)
running buffer. The gel was rinsed with water, dried and exposed to
X-ray film overnight (8,9).
Statistical analysis
Where appropriate, data were expressed as the mean ±
SE. Data were analyzed by factorial ANOVA and a Fisher’s least
significant difference test where appropriate. Statistical
significance was set at P<0.05.
Results
IL-7 induces wound-healing migration and
invasion of bladder cancer 5637 cells
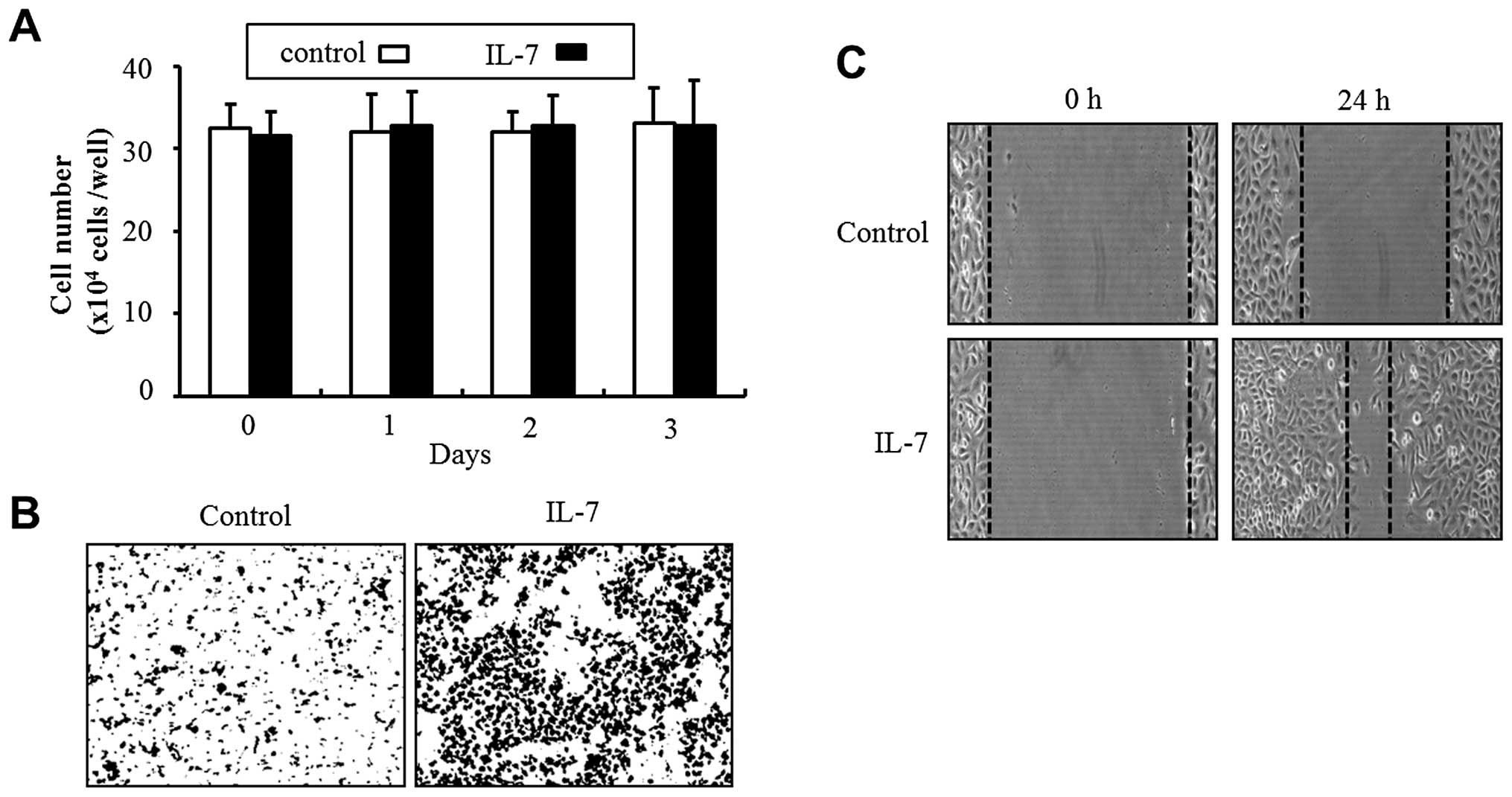
To investigate whether IL-7 would induce the
migration and invasion of bladder cancer 5637 cells, a
wound-healing migration and Matrigel invasion assay was performed.
A treatment of 5637 cells with IL-7 for 24 h significantly
increased migration into the wound area, compared with the control
cells (Fig. 1C). In addition, the
results from the invasion assay showed that IL-7 treatment produced
strong invasiveness through Matrigel by comparison with non-treated
cells (Fig. 1B). However, exposure
to IL-7 had no effect on cell proliferation (Fig. 1A).
IL-7 promotes MMP-9 expression via
binding activation of the NF-κB and AP-1 motifs
To define the involvement of MMP expression in the
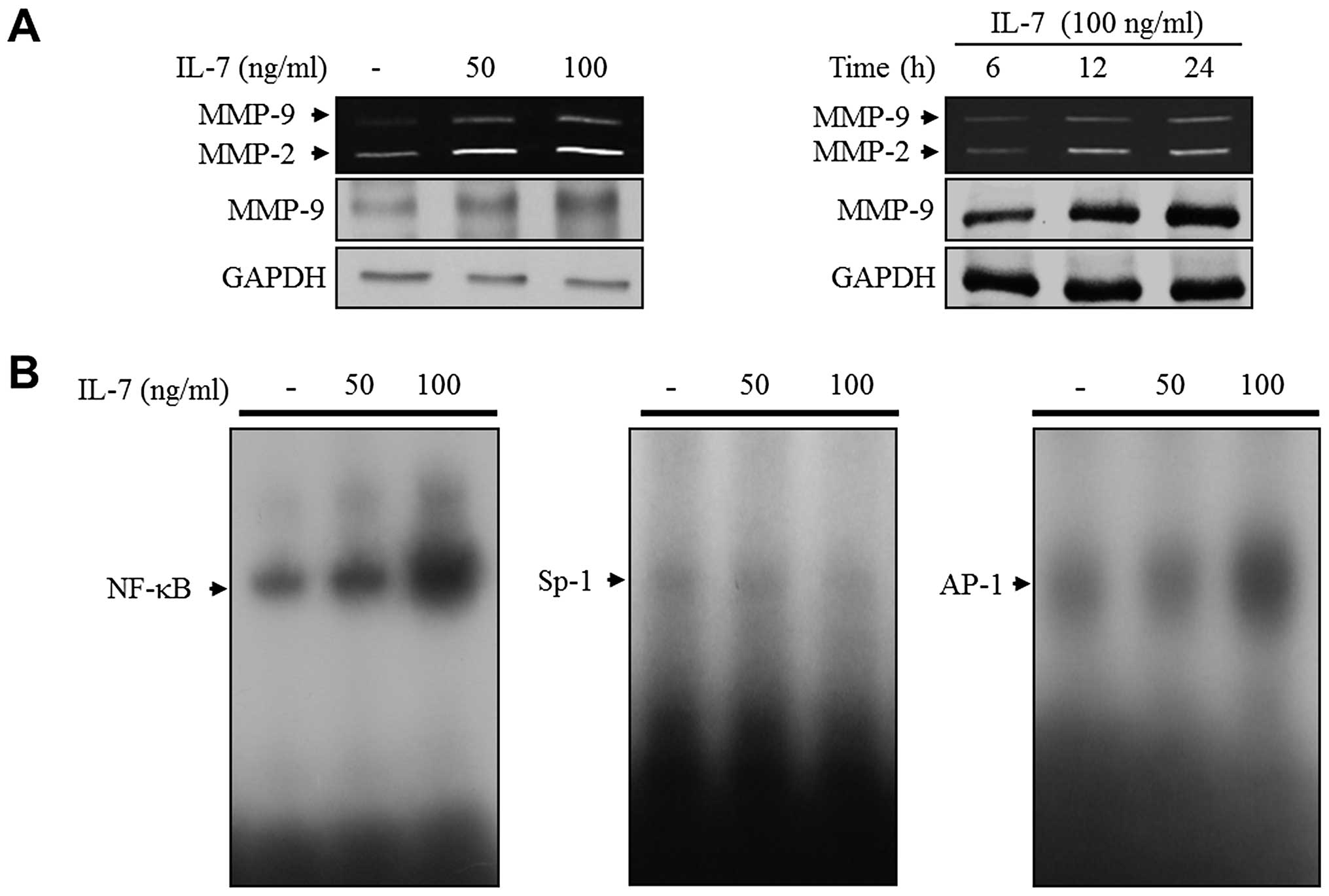
IL-7-induced migration and invasion of 5637 cells, we examined MMP
expression using a zymographic assay. IL-7 was used to treat with
5637 cells at the indicated concentrations in the serum-starved
medium for 24 h. IL-7 treatment showed a dose-dependent increase in
the expressions of both MMP-2 and MMP-9 (Fig. 2A). In addition, the level of both
MMP-2 and MMP-9 expression was increased in IL-7-treated cells in a
time-dependent manner (Fig. 2A).
Similar results were observed in immunoblot analysis (Fig. 2A). Previous studies have implicated
the expression of MMP-9 in the migration and invasion of bladder
cancer cells (3). Therefore, we
next focused on the MMP-9 expression in IL-7-treated 5637 cells. To
define the regulatory mechanism of MMP-9 expression, an EMSA
experiment was conducted. The 5637 cells were treated with IL-7 at
indicated concentrations for 24 h, and the binding activity of the
transcription factors was investigated using nuclear extracts,
which are a part of several DNA binding elements of bladder cancer
cells. As shown in Fig. 2B, IL-7
treatment significantly induced the binding activity of NF-κB and
AP-1 motifs in 5637 cells. However, there was no binding activity
of Sp-1 in IL-7-treated 5637 cells (Fig. 2B). These results indicate that IL-7
may induce MMP-9 expression by activating the binding function of
transcription factor NF-κB and that of AP-1 in bladder cancer 5637
cells.
IL-7 stimulates the phosphorylation of
ERK1/2 and increases the expression of p27KIP1 in 5637 cells
To determine the signaling pathways underlying
molecular events in the bladder cancer cells that are induced by
IL-7, we examined the MAPK signaling molecules ERK1/2, JNK and
p38MAPK. In the present study, IL-7 induced the maximum level of
ERK1/2 phosphorylation within 5 min (Fig. 3A). This effect was inhibited by
adding the ERK1/2 inhibitor U0126 (Fig. 3B). However, IL-7 did not induce the
phosphorylation of either JNK or p38MAPK in 5637 cells (Fig. 3A). IL-7 is known to modulate cell
cycle progression in lymphocytes (14,15).
Therefore, the expression levels of cell cycle proteins were
examined in bladder cancer cells in response to IL-7. The 5637
cells were treated with IL-7 for 24 h, and the levels of cell cycle
regulatory proteins were determined using immunoblot analysis. The
results of the present study demonstrated that the expression level
of p27KIP1 was induced by IL-7 treatment dose-dependently (Fig. 3C). However, no significant increase
was observed for the expression levels of other proteins (cyclin
D1, cyclin E, CDK2, CDK4, p21WAF1, and p53) in IL-7-treated 5637
cells (Fig. 3C).
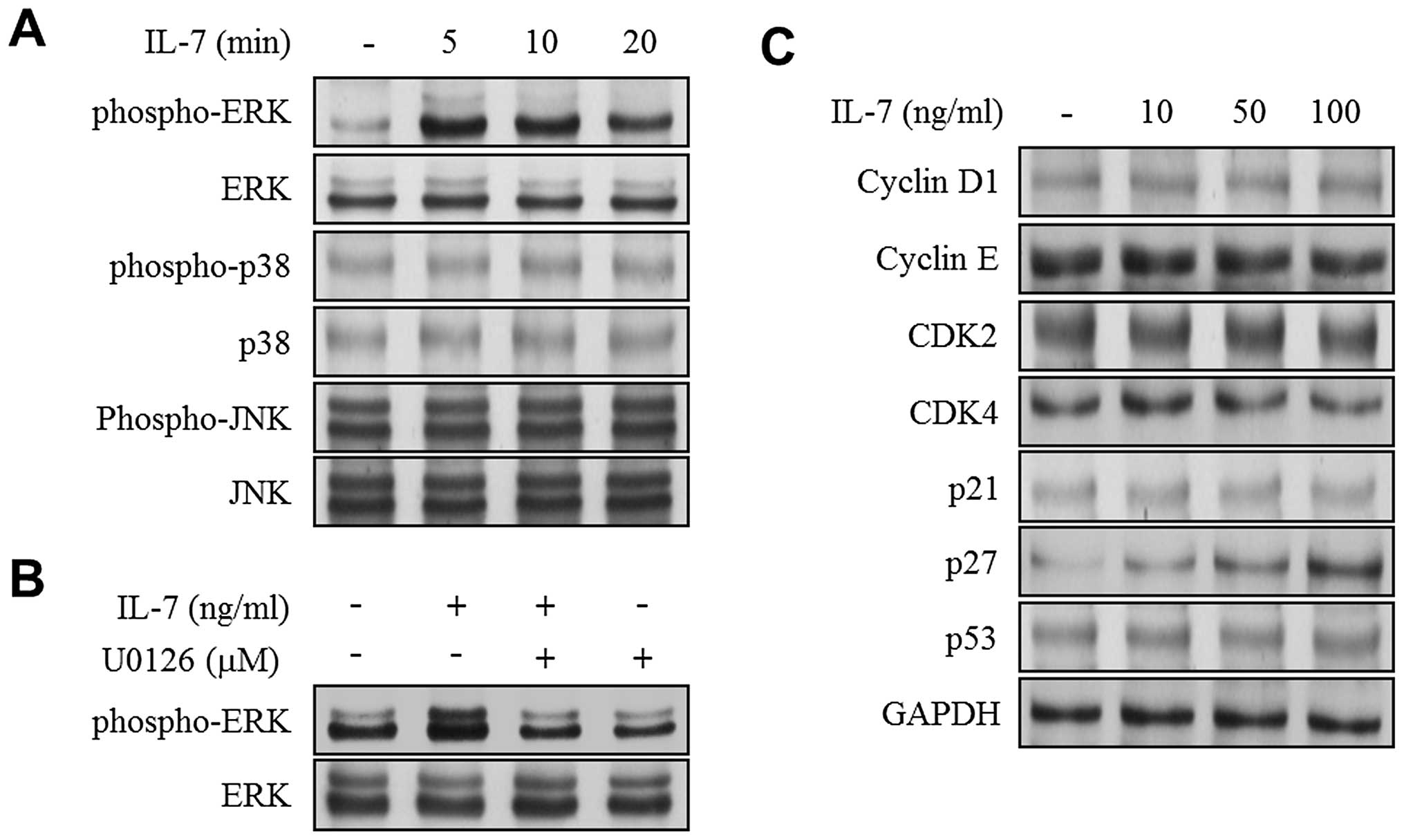 | Figure 3.IL-7 induces phosphorylation of ERK1/2
and expression of cell cycle inhibitor p27KIP1. (A and C)
Serum-starved cells were treated with IL-7 at indicated times and
concentrations. Immunoblot analysis was performed with specific
antibodies using phopho-ERK1/2, ERK1/2, phospho-p38, p38,
phospho-JNK, JNK, cyclin D1, cyclin E, CDK2, CDK4, p21WAF1, p27KIP1
and p53. GAPDH was used as an internal control. (B) Serum-starved
cells were pre-treated with U0126 (10 μM) for 40 min and
followed by IL-7 (100 ng/ml) treatment for 5 min. Immunoblot
analysis was performed to determine the phosphorylation level of
ERK1/2. |
ERK1/2 signaling is involved in
NF-κB-mediated MMP-9 expression during the migration and invasion
of bladder cancer 5637 cells induced by IL-7
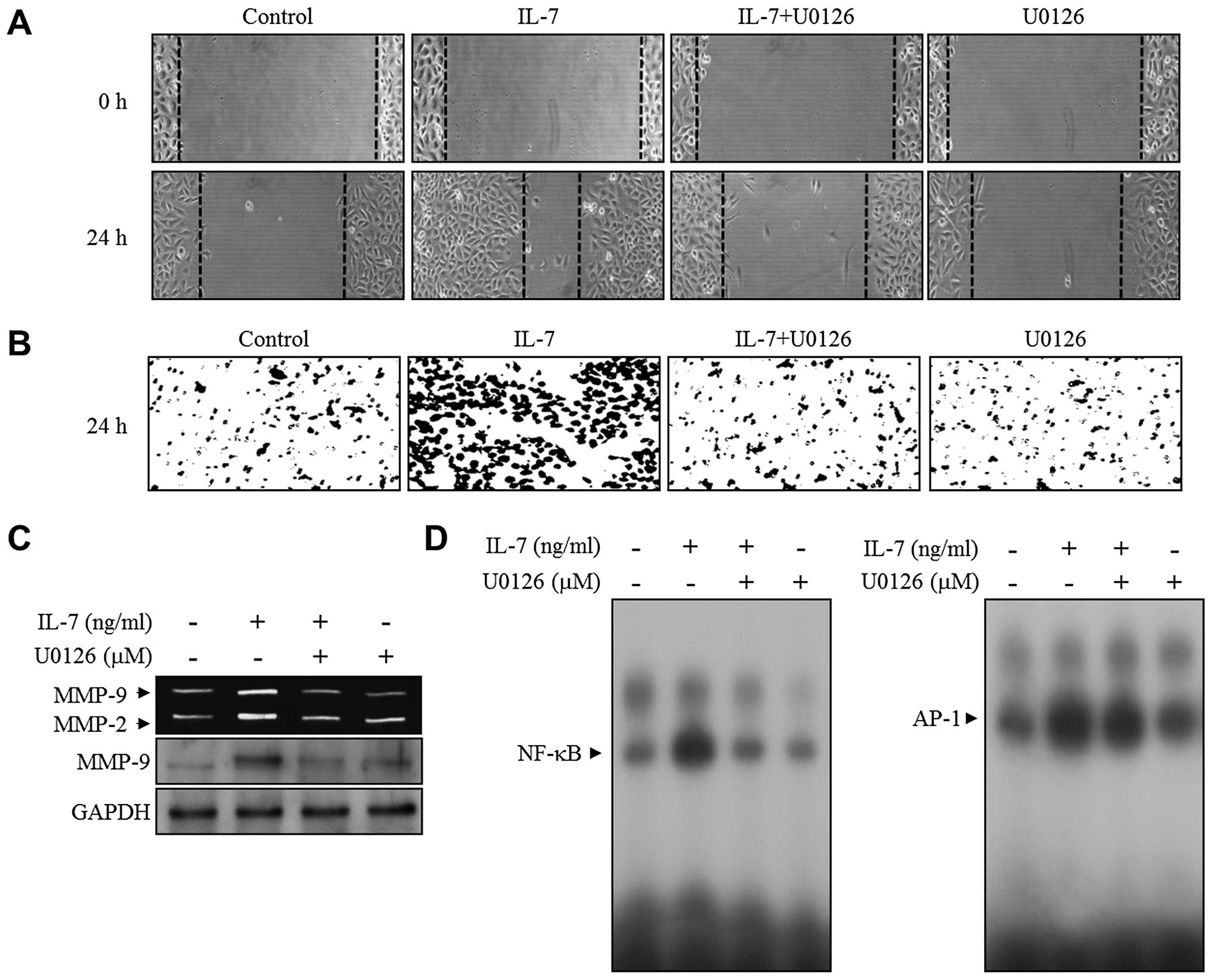
To determine the potential role of ERK1/2 signaling,
the effect of ERK1/2 inhibitor U0126 was investigated in the
IL-7-induced migration and invasion of bladder cancer cells. The
5637 cells were pre-treated with U0126 followed by IL-7 treatment.
Inhibition of ERK1/2 signaling blocked the wound-healing migration
and invasion of the 5637 cells induced by IL-7 (Fig. 4A and B). A subsequent study was
designed to elucidate the ERK1/2 signaling, which induces the
expression of the MMP-9 in IL-7-treated bladder cancer 5637 cells.
The effect of U0126 on MMP-9 expression was analyzed. Zymographic
and immunoblot analysis showed that IL-7-induced MMP-9 expression
was suppressed by the addition of U0126 (Fig. 4C). To further define the functional
role of ERK1/2 signaling in the transcriptional regulation
associated with the upregulation of MMP-9 expression in
IL-7-treated bladder cancer 5637 cells, an EMSA assay was performed
in both the presence and absence of U0126 using NF-κB and AP-1
binding motifs. The induction of NF-κB binding activation by IL-7
was attenuated in the presence of U0126 to the control level,
whereas U0126 had no effect on the binding activation of AP-1 in
IL-7-stimulated 5637 cells (Fig.
4D). These results showed that ERK1/2 signaling is associated
with MMP-9 expression via the NF-κB binding activity in
IL-7-stimulated bladder cancer cells.
siRNA-mediated knockdown of p27KIP1
restores migration, invasion, MMP-9 expression, ERK1/2 activation,
and binding activity of NF-κB in IL-7-stimulated bladder cancer
cells
Our results showed that IL-7 induces p27KIP1
expression (Fig. 3C). Moreover,
IL-7 promoted cellular responses, such as ERK1/2 phosphorylation,
MMP-9 expression, NF-κB binding activity, migration, and invasion,
in bladder cancer cells. Thus, the results reported above prompted
us to examine whether p27KIP1 is involved in IL-7-mediated bladder
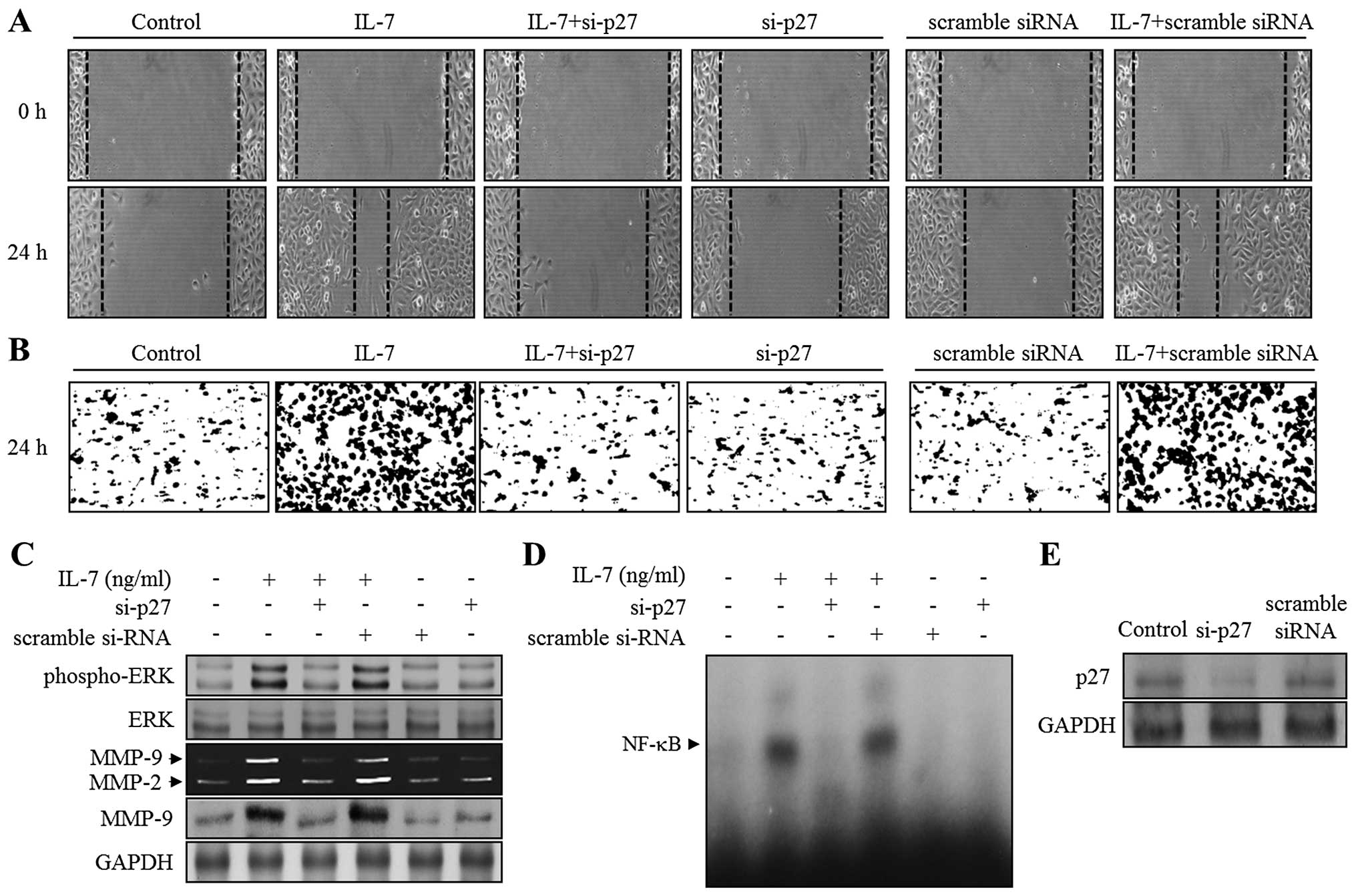
cancer cellular responses. To confirm this hypothesis, either a
p27KIP1-specific siRNA (si-p27) or a scrambled siRNA was
transfected into bladder cancer cells, which were then stimulated
with IL-7. The knockdown of p27KIP1 was characterized by immunoblot
analysis (Fig. 5E). IL-7 treatment
of 5637 cells, and of cells transfected with scrambled siRNA,
promoted both wound healing and invasion (Fig. 5A and B). This enhancement was
almost abolished in si-p27 transfected cells (Fig. 5A and B). IL-7-induced
phosphorylation of ERK1/2 was recovered in cells transfected with
si-p27 (Fig. 5C). In addition,
zymography and immunoblot analysis showed that IL-7 treatment of
5637 cells transfected with si-p27 resulted in the reduction of
MMP-9 expression (Fig. 5C).
Finally, as shown in Fig. 5D,
NF-κB DNA binding activity induced by IL-7 was almost completely
suppressed by the transfection of si-p27. These results suggest
that p27KIP1 might contribute to the migration and invasion of
bladder cancer cells through ERK1/2-associated MMP-9 expression by
activating the NF-κB binding ability in IL-7-stimulated bladder
cancer cells.
Discussion
Several studies have demonstrated the essential role
of cytokine IL-7 in the development and survival of lymphocytes
(14,15). Many studies have suggested that
IL-7 is an immune responsive cytokine responsible for the
proliferation of T-cells and the growth of murine B-cell precursors
(14,15). At least two studies have suggested
a vital role for IL-7 in the induction of anti-apoptotic factors
and the regulation of cell cycle progression in lymphocytes
(14,15). However, to date, the molecular
events underlying the migration and invasion of tumor cells that is
induced by IL-7 remain unclear. In the present study, we
demonstrated the roles and molecular mechanisms that integrate
migration, invasion, MMP-9 regulation, signaling pathway, and
cell-cycle regulation in IL-7-stimulated bladder cancer cells.
The progression of tumors has long been associated
with the migration and invasion of cancer cells, which is an
essential step in degradation of the extracellular matrix (ECM) by
proteolytic enzymes including MMP-2 and MMP-9 (4,5).
Based on these reports, we postulated that IL-7 might have a
pivotal role in the development and progression of tumor cells.
Initially, we examined the ability of bladder cancer 5637 cells to
migrate and invade when treated with IL-7. The result from the
present study showed that IL-7 promoted the induction of
wound-healing migration. Consistent with this result, invasive
capacity through Matrigel was also enhanced by IL-7 treatment. In
addition, the treatment of bladder cancer 5637 cells with IL-7
induced the expression of MMP-2 and MMP-9. Cumulative studies have
suggested that MMP-9 expression is an important element in tumor
grade, invasion, migration, and metastasis in bladder cancer
(3). In in vivo orthotopic
xenograft models, the role of MMP-9 has been documented in bladder
tumors (3,16). Previous reports have used
validation studies associated with tumor grade and metastasis to
document preclinical evidence that shows a key role for MMP-9 in
bladder cancer (17–21). Therefore, we next focused on the
identification of the transcription factors regulating MMP-9 in
IL-7-stimulated bladder cancer cells. Several transcription factors
that regulate MMP-9 promoters have been identified in tumor cells:
NF-κB, Sp-1, and AP-1 (6–10). The results from our EMSA data
identified NF-κB and AP-1 as main regulatory factors of
IL-7-mediated MMP-9 expression in 5637 bladder cancer cells. These
results are the first to show that IL-7 induces MMP-9 expression
through the activation of transcription factors NF-κB and AP-1 in
bladder cancer cells, which results in the breakdown of the ECM and
contributes to migration and invasion.
A number of studies have identified signal
transduction pathways that are correlated with the biological
responses of lymphocytes in response to IL-7 (14,15).
Previous studies have shown that cytokine IL-7 induces the
phosphorylation of Jak2/Stat3, PI3/AKT, and Src in T-cells and
B-cells (14,15). In addition, the binding of IL-7 to
IL-7Rα triggers the phosphorylation of MAPKs, such as ERK1/2 and
p38MAPK, in T-cells and B-cells (22,23).
Consistent with earlier studies, the present results show the
phosphorylation of ERK1/2 in IL-7-treated bladder cancer 5637
cells. Previously, the involvement of ERK1/2 signaling has been
suggested in the regulation of the migration and expression of
MMP-9 in different types of cells (8,9,12,13,24).
However, the role of the signaling pathway in IL-7-mediated cell
migration and invasion remains unclear. In the present study, we
showed that ERK1/2 kinase inhibitor U0126 inhibited both
wound-healing migration and Matrigel invasion in IL-7-stimulated
5637 bladder cancer cells. In addition, the inhibition of ERK1/2 by
pre-incubation with U0126 suppressed the MMP-9 expression and NF-κB
binding activity induced by IL-7 in 5637 cells without alteration
of the AP-1 binding activity. The results of the present study
clearly suggest that ERK1/2 signaling is an essential factor in the
transcription factor NF-κB-mediated MMP-9 expression in
IL-7-stimulated migration and invasion of bladder cancer cells.
In addition to its role in modulating the
development, survival, and proliferation of lymphocytes (14,15),
several studies have suggested that IL-7 plays a role in the cell
cycle regulation of T-cells (14,15).
IL-7 is known to promote cell proliferation through the decreased
level of p27KIP1 and increased activation of CDK2 and CDK4 in
T-cells (25,26). A previous study has shown that the
p27KIP1 expression is intensely associated with IL-7Rα in the T
cell lineage (27). However, the
effect of IL-7 on cancer cell response has not been previously
reported. In the present study, we examined the expression level of
p27KIP1 in the presence of IL-7, and found an increase in treated
bladder cancer cells. In an attempt to understand the involvement
of p27KIP1 in the IL-7-induced migration and invasion of bladder
cancer cells, we conducted a p27KIP1-specific siRNA knockdown
experiment. The results from the present study demonstrated that
p27KIP1 is a crucial factor for the migration and invasion of
bladder cancer cells induced by IL-7. Our data also showed that
p27KIP1 regulated the ERK1/2-mediated expression of MMP-9 via the
binding activation of the NF-κB motif in the migration and invasion
of bladder cancer cells induced by IL-7. These studies suggest that
p27KIP1 may be involved in the cascade of migration and invasion of
cancer cells, which leads to bladder cancer progression.
Many studies have shown that inflammatory cytokines
have a dual role that includes the induction of tumor progression
as well as an inhibitory effect of tumor growth (28–30).
The results of the present study showed a novel role for IL-7, as
it proved to be responsible for the migration and invasion of
bladder cancer cells without altering the cell growth. A previous
study showed that the decreased expression of p27KIP1 resulted in a
disease progression in patients with colorectal and breast cancer
(31,32). In addition, p27KIP1 status has been
associated with recurrence and disease-specific mortality in
patients with bladder tumors (33). However, the exact mechanism of the
cell cycle regulators underlying the migration and invasion in
IL-7-treated cancer cells remains to be elucidated. The results
from the present study revealed that p27KIP1 is an essential factor
in the migration and invasion of bladder cancer cells induced by
IL-7.
In conclusion, the present study presents novel
evidence demonstrating that p27KIP1 is associated with
ERK1/2-mediated MMP-9 expression via the binding activity of the
NF-κB motif in IL-7-stimulated bladder cancer cells, which
subsequently leads to migration and invasion. These results form a
theoretical basis for the progression of bladder cancer, and future
study is required to examine the efficacy of the IL-7 gene using
animal models.
Acknowledgements
This study was supported by the Basic
Science Research Program through the National Research Foundation
of Korea (NRF), funded by the Ministry of Education, Science and
Technology (2008-0062611).
References
|
1.
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar
|
|
2.
|
Levi F, La Vecchia C, Randimbison L and
Franceschi S: Incidence of infiltrating cancer following
superficial bladder carcinoma. Int J Cancer. 55:419–554. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Black PC and Dinney CP: Bladder cancer
angiogenesis and metastasis - translation from murine model to
clinical trial. Cancer Metastasis Rev. 26:623–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Matrisian LM: Metalloproteinases and their
inhibitors in matrix remodeling. Trends Genet. 6:121–125. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Liotta LA: Tumor invasion and
metastasis-role of extracellular matrix: Rhoads Memorial Award
Lecture. Cancer Res. 46:1–7. 1986.PubMed/NCBI
|
|
6.
|
Bond M, Rosalind P, Fabunmi P, Baker AH
and Newby AC: Synergistic upregulation of metalloproteinase-9 by
growth factors and inflammatory cytokines: an absolute requirement
for transcription factor NF-kappa B. FEBS Lett. 435:29–34. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Sato H and Seiki M: Regulatory mechanism
of 92 kDa type IV collagenase gene expression which is associated
with invasiveness of tumor cells. Oncogene. 8:395–405.
1993.PubMed/NCBI
|
|
8.
|
Lee SJ, Cho SC, Lee EJ, Kim S, Lee SB, Lim
JH, Choi YH, Kim WJ and Moon SK: Interleukin-20 promotes migration
of bladder cancer cells through extracellular signal-regulated
kinase (ERK)-mediated MMP-9 protein expression leading to nuclear
factor (NF-κB) activation by inducing the up-regulation of
p21(WAF1) protein expression. J Biol Chem. 288:5539–5552.
2013.PubMed/NCBI
|
|
9.
|
Moon SK, Cha BY and Kim CH: ERK1/2
mediates TNF-alpha-induced matrix metalloproteinase-9 expression in
human vascular smooth muscle cells via the regulation of NF-kappaB
and AP-1: involvement of the ras dependent pathway. J Cell Physiol.
198:417–427. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Sato H, Kita M and Seiki M: v-Src
activates the expression of 92-kDa type IV collagenase gene through
the AP-1 site and the GT box homologous to retinoblastoma control
elements. A mechanism regulating gene expression independent of
that by inflammatory cytokines. J Biol Chem. 268:23460–23468.
1993.PubMed/NCBI
|
|
11.
|
Nicosia SV, Bai W, Cheng JQ, Coppola D and
Kruk PA: Oncogenic pathways implicated in ovarian epithelial
cancer. Hematol Oncol Clin North Am. 17:927–943. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Cho A, Graves J and Reidy MA:
Mitogen-activated protein kinases mediate matrix
metalloproteinase-9 expression in vascular smooth muscle cells.
Arterioscler Thromb Vasc Biol. 20:2527–2532. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Yao J, Xiong S, Klos K, Nguyen N, Grijalva
R, Li P and Yu D: Multiple signaling pathways involved in
activation of matrix metalloproteinase-9 (MMP-9) by heregulin-beta1
in human breast cancer cells. Oncogene. 20:8066–8074. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kittipatarin C and Khaled AR: Interlinking
interleukin-7. Cytokine. 39:75–83. 2007. View Article : Google Scholar
|
|
15.
|
Jiang Q, Li WQ, Aiello FB, Mazzucchelli R,
Asefa B, Khaled AR and Durum SK: Cell biology of IL-7, a key
lymphotrophin. Cytokine Growth Factor Rev. 16:513–533. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Mian BM, Dinney CP, Bermejo CE, Sweeney P,
Tellez C, Yang XD, Gudas JM, McConkey DJ and Bar-Eli M: Fully human
anti-interleukin 8 antibody inhibits tumor growth in orthotopic
bladder cancer xenografts via down-regulation of matrix
metal-loproteases and nuclear factor-kappaB. Clin Cancer Res.
9:3167–3175. 2003.PubMed/NCBI
|
|
17.
|
Sier CF, Casetta G, Verheijen JH, Tizzani
A, Agape V, Kos J, Blasi F and Hanemaaijer R: Enhanced urinary
gelatinase activities (matrix metalloproteinases 2 and 9) are
associated with early-stage bladder carcinoma: a comparison with
clinically used tumor markers. Clin Cancer Res. 6:2333–2340.
2000.
|
|
18.
|
Davies B, Waxman J, Wasan H, Abel P,
Williams G, Krausz T, Neal D, Thomas D, Hanby A and Balkwill F:
Levels of matrix metalloproteases in bladder cancer correlate with
tumor grade and invasion. Cancer Res. 53:5365–5369. 1993.PubMed/NCBI
|
|
19.
|
Nutt JE, Durkan GC, Mellon JK and Lunec J:
Matrix metalloproteinases (MMPs) in bladder cancer: the induction
of MMP9 by epidermal growth factor and its detection in urine. BJU
Int. 91:99–104. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Moses MA, Wiederschain D, Loughlin KR,
Zurakowski D, Lamb CC and Freeman MR: Increased incidence of matrix
metalloproteinases in urine of cancer patients. Cancer Res.
58:1395–1399. 1998.PubMed/NCBI
|
|
21.
|
Di Carlo A, Terracciano D, Mariano A and
Macchia V: Urinary gelatinase activities (matrix metalloproteinases
2 and 9) in human bladder tumors. Oncol Rep. 15:1321–1326.
2006.PubMed/NCBI
|
|
22.
|
Fleming HE and Paige CJ: Pre-B cell
receptor signaling mediates selective response to IL-7 at the pro-B
to pre-B cell transition via an ERK/MAP kinase-dependent pathway.
Immunity. 15:521–531. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Crawley JB, Rawlinson L, Lali FV, Page TH,
Saklatvala J and Foxwell BM: T cell proliferation in response to
interleukins 2 and 7 requires p38MAP kinase activation. J Biol
Chem. 272:15023–15027. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Reddy KB, Nabha SM and Atanaskova N: Role
of MAP kinase in tumor progression and invasion. Cancer Metastasis
Rev. 22:395–403. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Li WQ, Jiang Q, Aleem E, Kaldis P, Khaled
AR and Durum SK: IL-7 promotes T cell proliferation through
destabilization of p27Kip1. J Exp Med. 203:573–582. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Barata JT, Cardoso AA, Nadler LM and
Boussiotis VA: Interleukin-7 promotes survival and cell cycle
progression of T-cell acute lymphoblastic leukemia cells by
down-regulating the cyclin-dependent kinase inhibitor p27(kip1).
Blood. 98:1524–1531. 2001. View Article : Google Scholar
|
|
27.
|
Hofmeister R, Khaled AR, Benbernou N,
Rajnavolgyi E, Muegge K and Durum SK: Interleukin-7: physiological
roles and mechanisms of action. Cytokine Growth Factor Rev.
10:41–60. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Dranoff G: Cytokines in cancer
pathogenesis and cancer therapy. Nat Rev Cancer. 4:11–22. 2004.
View Article : Google Scholar
|
|
30.
|
Goswami B, Rajappa M, Sharma M and Sharma
A: Inflammation: its role and interplay in the development of
cancer, with special focus on gynecological malignancies. Int J
Gynecol Cancer. 18:591–599. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Loda M, Cukor B, Tam SW, Lavin P,
Fiorentino M, Draetta GF, Jessup JM and Pagano M: Increased
proteasome-dependent degradation of the cyclin-dependent kinase
inhibitor p27 in aggressive colorectal carcinomas. Nat Med.
3:231–234. 1997. View Article : Google Scholar
|
|
32.
|
Catzavelos C, Bhattacharya N, Ung YC,
Wilson JA, Roncari L, Sandhu C, Shaw P, Yeger H, Morava-Protzner I,
Kapusta L, Franssen E, Pritchard KI and Slingerland JM: Decreased
levels of the cell-cycle inhibitor p27Kip1 protein: prognostic
implications in primary breast cancer. Nat Med. 3:227–230. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Shariat SF, Zlotta AR, Ashfaq R,
Sagalowsky AI and Lotan Y: Cooperative effect of cell-cycle
regulators expression on bladder cancer development and biologic
aggressiveness. Mod Pathol. 20:445–459. 2007. View Article : Google Scholar : PubMed/NCBI
|