Introduction
Despite important advances in early detection and
treatment, breast cancer continues to be a relevant public health
concern, currently being the second cause of cancer-related deaths
in woman (1,2). The lifetime risk for developing
breast cancer is 1 in 8 for western countries, and resistance to
radiation and drugs constitutes a major cause of mortality
(3).
Although chemotherapy has become a routine in most
anticancer regimens, this therapeutic approach is currently limited
by the ability of cancer cells to develop resistance to
conventional drugs, a phenomenon that is particularly common during
the course of treatment of solid tumors. Therefore, the search for
new compounds with selective anticancer activity continues to be an
important driving force for the development and implementation of
novel anticancer therapies. Ideally, any anticancer drug should
have an acceptable therapeutic index, that is, the drug should
exert a cytotoxic effect on malignant cells with a minimal effect
on normal cells. In this context, plants that for centuries have
been used as part of folk medicine are unique sources of natural
and bioactive compounds with potential anticancer effect (4). Indeed, numerous anticancer drugs in
current use have been extracted from plants, including vinblastine,
an alkaloid extracted from Catharanthus roseus that inhibits
the assembly of the mitotic spindle microtubules (5), and paclitaxel, an alkaloid extracted
from Taxus brevifolia whose activity stabilizes microtubules
during cell division (6). Several
other drugs extracted from plants have recently proven useful as
anticancer therapies, including betolunic acid (7), resveratrol (8) and homoharringtotine (9), among others.
Senecio graveolens, also known as Chachacoma,
is a herb that inhabits the Andes Mountains in South America,
thriving in regions located 3,500 meters above the sea level. It
belongs to the group Senecioneae, Asteraceae comprised of more than
900 species in Chile; Senecio comprises almost all 200
species, the richest gender in Chile. The ethnobotanic resources
are used regularly for their medicinal properties in wound healing,
as antiemetics, as anti-inflammatory agents, and as a means to
ameliorate altitude sickness (principally as a vasodilator
preparations) (10–12).
Pyrrolizidine alkaloids (PA), eremophilanolides and
cacalolides are characteristic compounds isolated from
Senecio species (13).
Although the toxic effects exhibited by these plants have been
largely attributed to their content of PA (14) the development of oxidative stress
and the consequent generation of reactive oxygen species (ROS) and
ROS-mediated cellular damage (15,16),
likely also contribute to toxicity.
Hypoxia is a pathophysiologic feature of nearly half
of all locally advanced tumors (17). In particular, it is believed that
up to one third of breast tumors are chronically subjected to
hypoxic conditions (18). While
the hypoxic microenvironment may serve as a selection barrier that
allows the emergence of tumor cells with enhanced resistance to
anticancer drugs (19,20), hypoxia may also sensitize cancer
cells to the cytotoxic effects of ROS generated by some
chemotherapeutic drugs (20).
Thus, the tumor microenvironment can determine the outcome of the
pre-clinical assessments of potential chemotherapeutic agents.
The main goals of this study were to characterize
the cytotoxic effect induced by an important ethnopharmacology
resource called Chachacoma (S. graveolens), and to explore
the possible mechanisms responsible for the induction of cell
death. The ethanolic extract and its major compound were evaluated
in breast cancer cell lines under normoxic and hypoxic
conditions.
Materials and methods
Plant material and crude extract
The S. graveolens was collected from a region
near the Chungará lake in the North of Chile (18°12′55″S;
69°17′40″O) at 4,500 meters above the sea level. Approximately
180.87 g of dry and ground plant material (principally flowers,
leaves and stems) were macerated in 95% ethanol for 72 h. The
extract was then filtered, concentrated under reduced pressure at
40°C, and subjected to separation with a mixture of ethyl
acetate/water (500 ml each). The resulting organic phase was
finally concentrated under reduced pressure, resulting in a yield
of 54.86 g of crude extract.
Purification and identification of major
compounds
The organic phase (20 g) was separated by column
chromatography using silica gel G-60 as stationary phase. As a
mobile phase, a gradient of increasing polarity based on mixing
hexane: ethyl-acetate applying gradients with increasing polarity
from 49:1 to 1:49 were used. Following analysis with thin layer
chromatography (TLC), samples with similar constitution were pooled
and concentrated under reducing pressure. The resulting crystalline
product was recrystallized using EtO2:MeOH (1:1). A
total of 2.42 g of pure compound (12.1% yield from the crude
extract) was obtained from the original concentrated organic phase
isolated form the crude plant material, mp 94–95°C. In order to
identify major compounds, the 1H, 13C (DEPT 135), sel. 2D HSQC and
2D HMBC spectra were recorded in CDCl3 solutions on a Bruker Avance
400 Digital nuclear magnetic resonance (NMR) spectrometer.
Cell culture and cytotoxic assays
Four human breast cancer cell lines were used in
this study: MCF-7, ZR-75-1 and MDA-MB-231, and the non-tumorigenic
MCF-10F cell lines. All cell lines were obtained from the American
Type Culture Collection (ATCC). Cells were cultured in specific
media according to ATCC recommendations. When required, cells were
incubated under hypoxic conditions (1% O2), thus
mimicking the in vivo tumor microenvironment. In both cases
the incubation condition was established at 37°C, humid atmosphere
and 5% CO2.
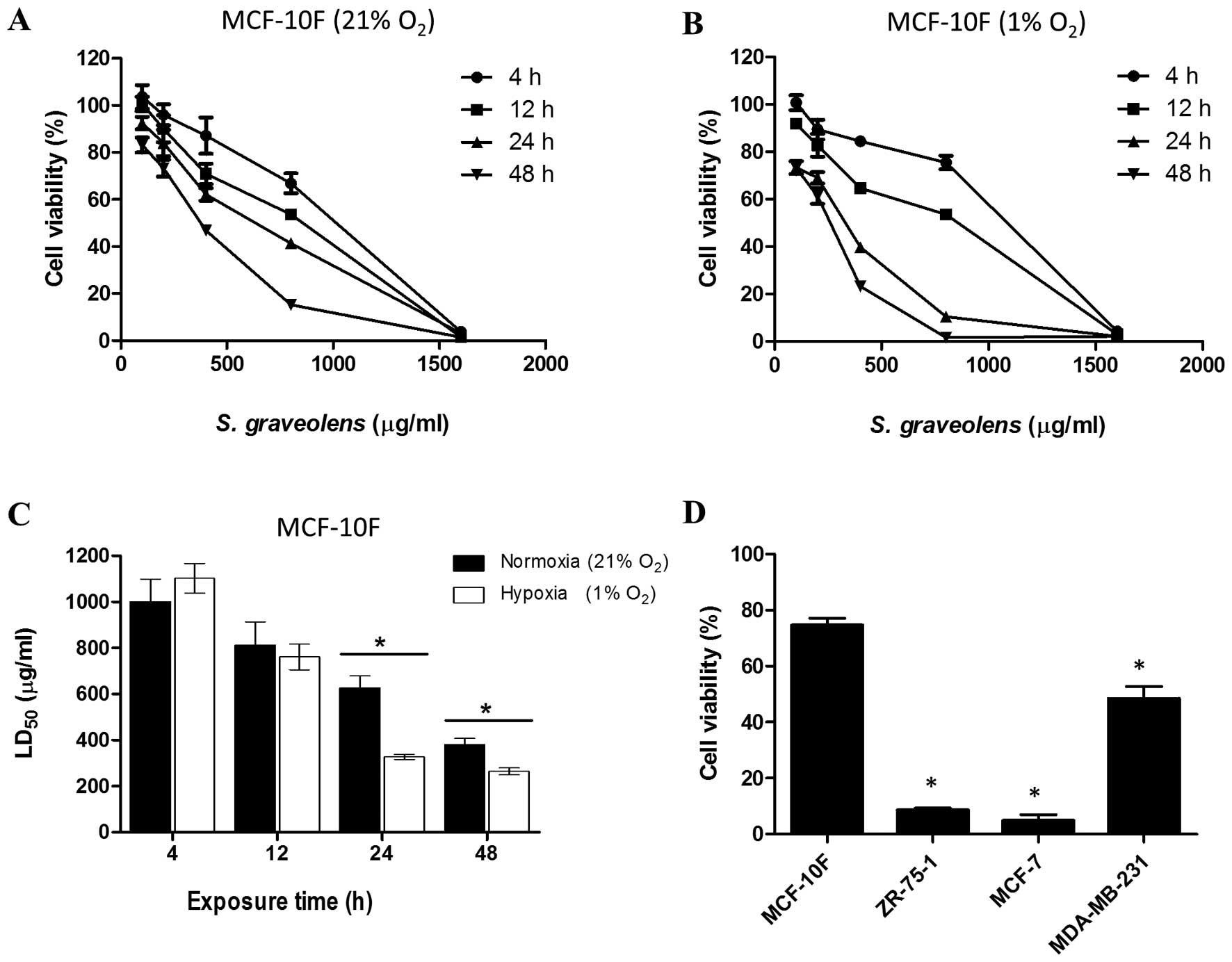
The cytotoxic effect of the S. graveolens
extract was assessed in MCF-10F cells in a dose- and time-dependent
manner. Cells (1×104 and 4×104) cultured
under normoxic and hypoxic conditions, respectively, were seeded in
24-well plates in quadruplicate and then incubated for 4 days until
70% confluence. After this incubation period, cells were exposed to
concentrations of the S. graveolens extract ranging from 0
to 1,600 μg/ml (dissolved in 0.5% DMSO). The effect of each
concentration was assayed for 4, 12, 24 and 48 h. In addition,
MCF-10F cells were exposed to the major compound dissolved in
ethanol (50%) at concentrations ranging from 0 to 96.8
μg/ml. Cell viability was assessed using neutral red uptake
assay after 24 h of treatment.
Western blot analyses
Cell lysates were prepared by using extraction
buffer [50 mM Tris-HCl, pH 8.0, 130 mM NaCl, 1% (w/v) NP40, 1 mM
phenylmethylsulfonyl fluoride, 5 mM MgCl2, and 1 mM
orthovanadate]. Proteins (40 μg) were separated by SDS-PAGE
and electroblotted onto PVDF membrane. The membrane was blocked in
5% non-fat dry milk in TBST for 1 h at room temperature and
incubated overnight with the following primary antibodies: MnSOD
(sc-133134), caspase-8 (sc-7890), caspase-3 (sc-7272), and MAP
LC3α/β (sc-292354) (Santa Cruz Biotechnology, Santa Cruz, CA, USA)
(dilution 1:500-1:1,000). HP-conjugated secondary antibodies
(anti-mouse and anti-rabbit) (Santa Cruz Biotechnology) were used
at a dilution of 1:5,000. Signals were detected using the ECL kit
Super Signal West Pico Chemiluminescent Substrate (Thermo
Scientific, Rockford, IL, USA) according to the company’s protocol
and recorded in Kodak BioMax light film (Sigma-Aldrich, St. Louis,
MO, USA). β-actin (A5318) (Sigma-Aldrich) was used as loading
control (dilution 1:20,000).
Transmission electron microscopy
(TEM)
A cell pellet was collected following scrapping of
adherent cells. The pellet was washed two times with PBS and then
fixed in 2% gluteralde-hyde/50 mM sodium phosphate, pH 7.0, at room
temperature. Sample processing and image capture was carried out at
The Advanced Microscopy Unit of the Pontificia Universidad Católica
de Chile.
Statistical analysis
Numerical data were expressed as the average ±
standard error of the mean (SEM). Comparison between treated groups
and controls was carried out by ANOVA and Dunnet’s test. A
P<0.05 was considered to be significant. The lethal dose at 50%
(LD50) was calculated by a non-linear regression curve
with the use of GraphPad Prism 5.0 for Windows (GraphPad Software,
San Diego, CA, USA).
Results
Differential cytotoxicity effect is
caused by synergism between S. graveolens extract and hypoxia
The viability of MCF-10F cells treated with the
ethanolic extract of S. graveolens was assessed by neutral red
uptake under normoxic and hypoxic conditions in a concentration-
and time-dependent manner (Fig. 1A and
B). As shown in Fig. 1A and B,
the cytotoxic effect was more pronounced in cells subjected to
hypoxia compared to cells maintained under normoxic conditions.
Thus, compared to cells exposed to 21% oxygen, treatment with the
phytochemical extract led to a significant reduction in
LD50 values (P<0.05) in cells that were undergoing
hypoxia (1% oxygen). This reduction in cell viability was more
pronounced at 24 h of treatment, reaching 48% of the values
obtained under normoxia (Fig.
1C).
We next tested the effect of 200 μg/ml of A
S. graveolens, a dose that corresponds to the LD25 for
MCF-10F cells, on three tumorigenic mammary epithelial cells lines.
Cells were maintained under hypoxic conditions and their viability
measured 24 h after initiating treatment. Such conditions were
considered the most effective and less harmful for MCF-10F, which
was set as our control. This treatment led to a significant
reduction in cell viability (P<0.05) of all the malignant cell
lines in comparison to its non-tumorigenic counterpart. As shown in
Fig. 1D, the viability of ZR-75-1
and MCF-7 cells was reduced to 9 and 6%, respectively. However, the
viability of MDA-MB-231 cells diminished only by 50%.
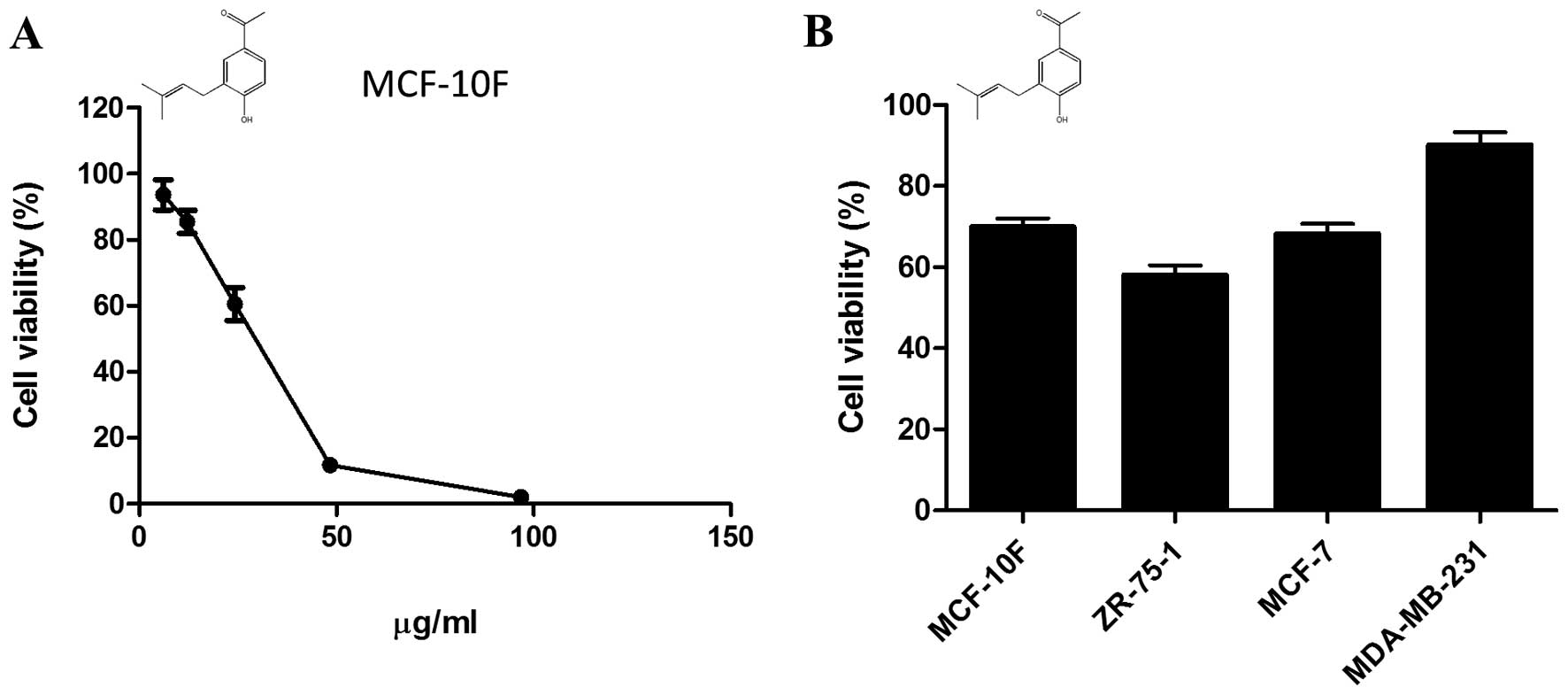
Cytotoxicity elicited by
1-[4-hydroxy-3-(3-methylbut-2-enyl) phenyl]ethanone
Using H-NMR, we have previously identified
1-[4-hydroxy-3-(3-methylbut-2-enyl)phenyl]ethanone
(C13H16O2; MW: 204,26884 g/mol), also known
as prenilated acetophenone, as the major compound present at the
organic phase of extracts from S. graveolens. The working
dose of prenilated acetophenone, corresponding to LD25
for MCF-10F (13.8 μg/ml), was estimated by a dose-response
curve (Fig. 2A). The compound was
then added to the media of breast cancer cells growing under
hypoxic conditions and the viability was measured 24 h later.
Overall, the prenilated acetophenone was more cytotoxic than the
crude extract. However, the compound was unable to reduce cell
viability of malignant cells under hypoxic conditions (1% oxygen)
in a proportion similar to that shown by the extract (Fig. 2B).
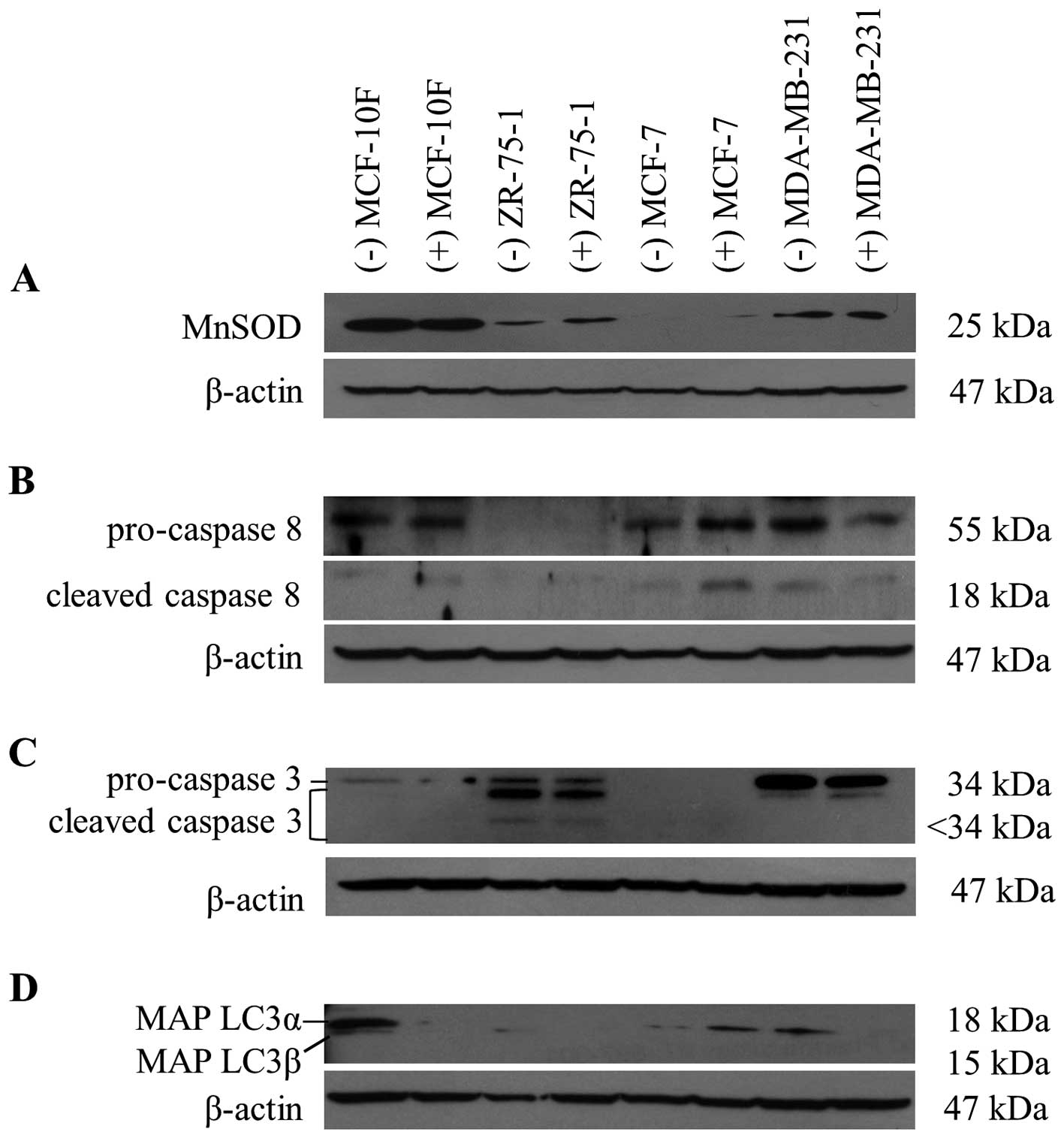
Low MnSOD expression is associated with
sensitivity to the cytotoxic effect of the extract
The cytotoxicity elicited by certain phytochemicals
from Senecio species, in synergism with hypoxia, could be
dependent on the ability of the cell to respond to the cellular
damage induced by oxidative stress. Therefore, we set out to
examine the relationship between the cytotoxic induced by S.
graveolens and MnSOD expression, an important mitochondrial
antioxidant enzyme recognized as relevant in detoxification system
from free radicals. As shown in Fig.
3A, the protein levels of MnSOD did not change in response to
the phytochemical treatment. However, the basal expression of MnSOD
correlated well with the degree of cytotoxicity observed in the
different cell lines, with the highest levels of cytotoxicity
observed in cells with reduced expression of MnSOD (Figs. 1D and 3A).
Caspases and MAP LC3 expression after S.
graveolens treatment
We next studied the cellular processes that might be
responsible for the cytotoxic effects of S. graveolens.
Apoptosis is the result of the recruitment and activation of
cysteine-aspartic acid proteases (caspases) and caspase-8 is the
most upstream protease involved in the extrinsic apoptotic pathway.
Activated caspase-8 (subunit p18, 18 kDa) and its inactive
precursor pro-caspase-8 (55 kDa) were overexpressed in MCF-7 cells
following exposure to S. graveolens extract (Fig. 3B). In contrast, caspase-8 levels
were reduced in MDA-MB-231 cells in response to the extract. The
expression of caspase-3 is known to be abrogated in MCF-7 cells, as
confirmed in Fig. 3C. On the other
hand, cleaved caspase-3 (<34 kDa) was observed in ZR-75-1 cells,
indicating initiation of the apoptotic process. In contrast,
MDA-MB-231 cells displayed overexpression of pro-caspase-3 (34
kDa), but not the cleaved isoform.
We next checked the levels of MAP LC3α and β
proteins, which are indicative of autophagosome formation and
widely used as markers of autophagy. Autophagy is a cellular
catabolic degradation response to starvation or stress whereby
cellular components are engulfed, digested and recycled to sustain
cellular metabolism (11). Most
evidence supports a role for autophagy in sustaining cell survival,
but it is possible to observe cell death as a result from
progressive cellular consumption attributed to a continued
autophagy (21). As shown in
Fig. 3C, both MAP LC3α and MAP
LC3β were downregulated in MCF-10F and MDA-MB-231 cell lines.
However, MAP LC3β was overexpressed in MCF-7 cells after extract
exposure. Under these conditions, we could not detect MAP LC3 in
ZR-75-1 cells. Taken together, the cytotoxic effects induced by
S. graveolens in MCF-7 cells can be mechanistically linked
to autophagic and apoptotic initiation. Further work will be
necessary to define the relative contribution of these processes to
the final cytotoxic effect elicited by S. graveolens.
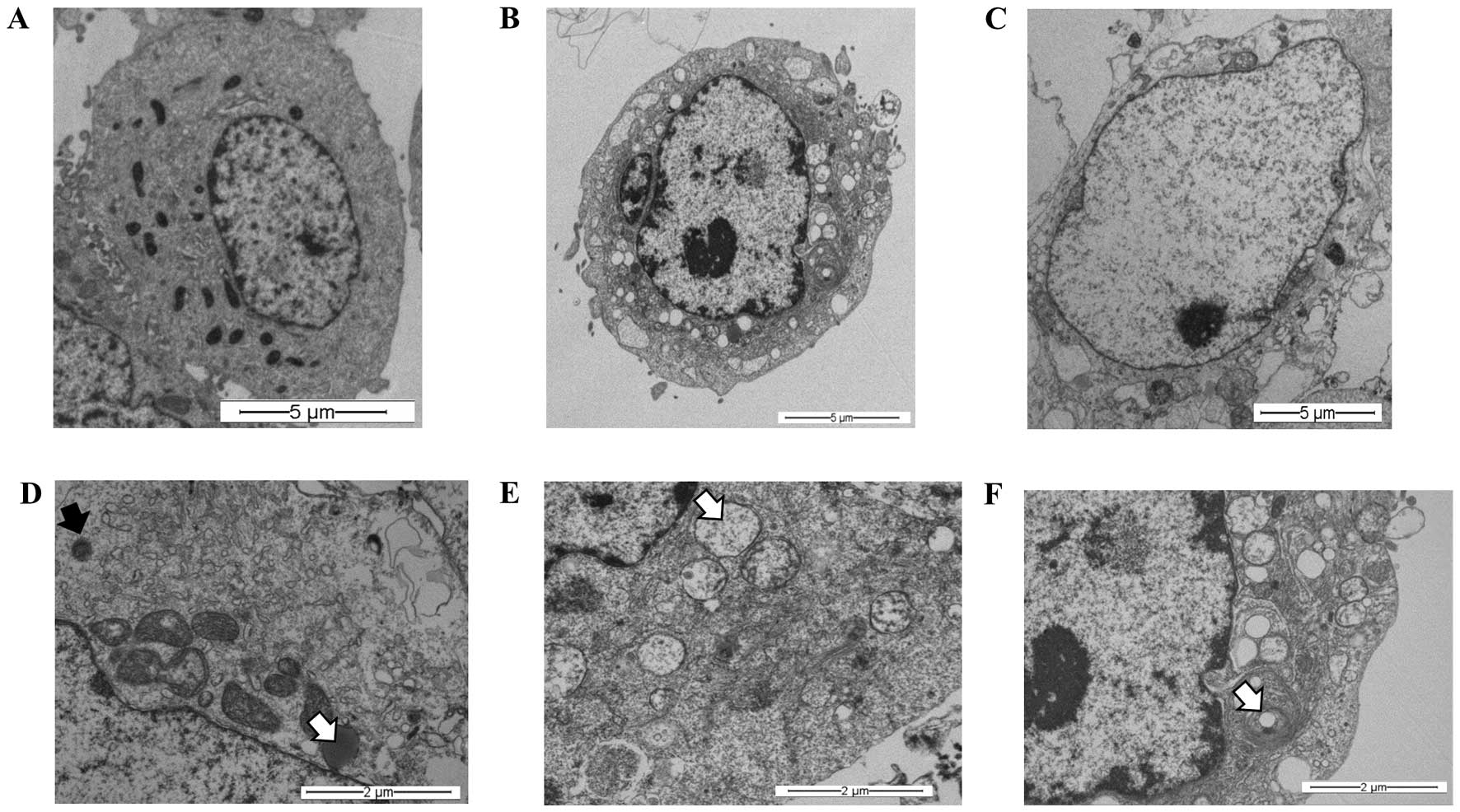
Synergism between S. graveolens
and hypoxia leads to cell death in MCF-7 cells
Our findings (Fig.
3C) suggest that the extract might trigger the autophagic
process in MCF-7 cells subjected to hypoxia. To confirm the
presence of a digestive process, and specifically the presence of
autophagy, an ultra-structural analysis using transmission electron
microscopy was carried out. At first, these experiments were
performed under normoxic conditions (21% O2) to rule out
any cellular change induced by oxygen concentration. MCF-7 cells
were treated with 100 μg/ml of the S. graveolens
extract (half LD25 dose). TEM analysis of treated cells
revealed ultrastructural signs suggestive of cellular digestion
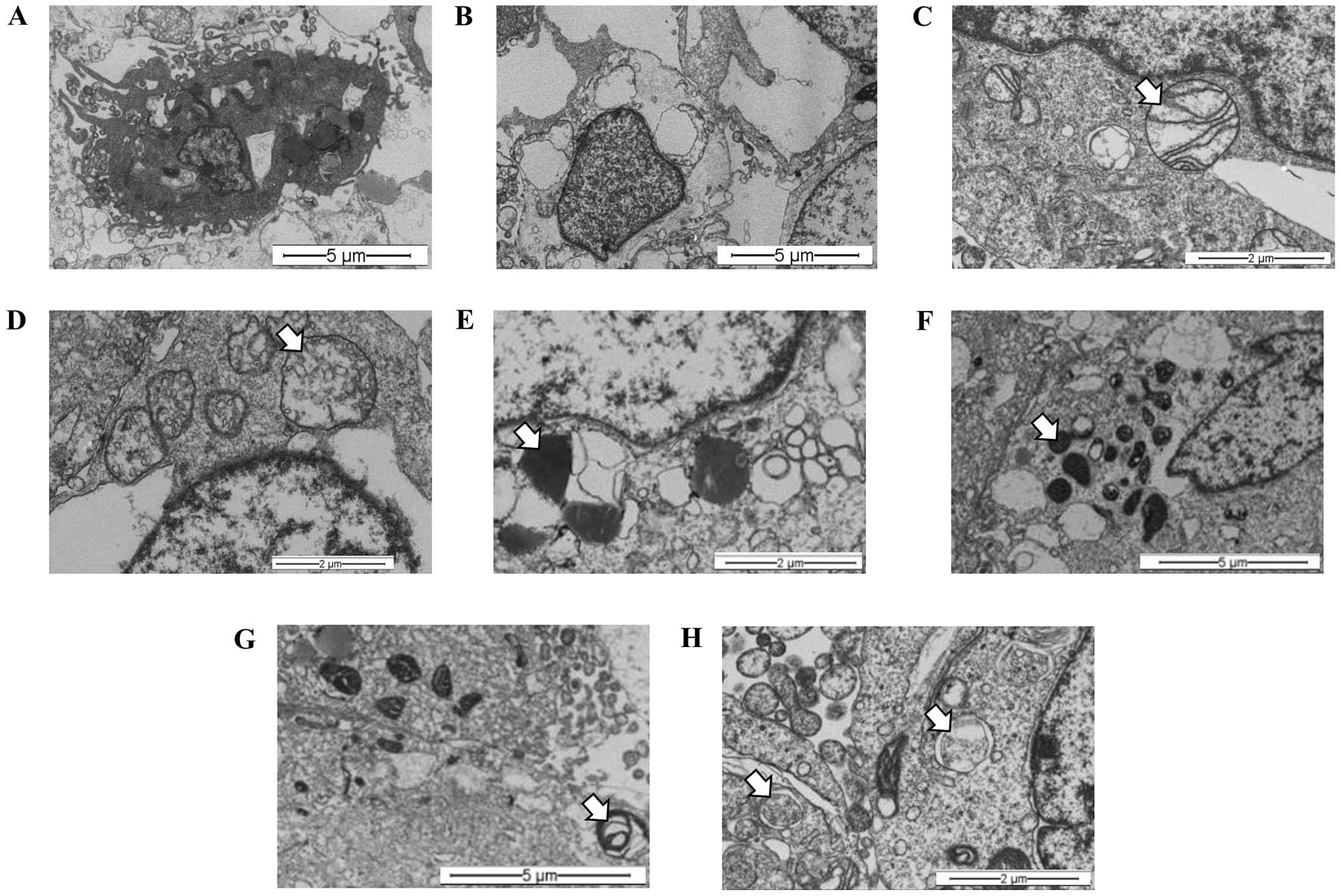
(Fig. 4B) and necrosis (Fig. 4C), when compared with controls
(Fig. 4A). These changes were
characterized by the presence of lysosomes in different stages of
maturation, including primary lysosomes and residual bodies
(Fig. 4D), vacuoles (Fig. 4E) and lamellar bodies (white arrow)
(Fig. 4F).
The synergistic effects of hypoxia (1%
O2) and the S. graveolens extract were then
studied at the ultra-structural level. Representative images
revealed apoptotic components (Fig.
5A) coexisting with necrosis (Fig.
5B) in almost all the samples analyzed, regardless of the
presence of extract. Nonetheless, cells treated with S.
graveolens triggered necrosis to a greater extent compared with
non-treated cells, the latter showing features of apoptotic cell
death. Overall, necrotic cells were characterized by the presence
of swollen mitochondria and disruption of mitochondrial crests
(Fig. 5C), including swollen
vacuole morphology (Fig. 5D). In
spite of this complex morphologic scenario, it was still possible
to observe signs of intracellular digestion in treated cells,
including lysosomes in different stages of maduration. Thus,
primary lysosomes (Fig. 5E),
autolysosomes (Fig. 5F) and
residual bodies (Fig. 5G) were
frequent findings in these cells. Moreover, autophagosomes were
also observed in treated cells in major frequency in comparison to
non-treated cells (Fig. 5H).
Discussion
To our knowledge, this is the first report
describing the cytotoxic effects induced by the ethanolic extract
of S. graveolens on breast cancer cell lines. This
phytochemical extract induced a cytotoxic effect against breast
cancer cells, but failed to have similar effects on non-tumorigenic
cells.
The cytotoxic effect induced by S. graveolens
extract in malignant cells was prominent when these cells were
maintained under hypoxia in comparison with the same cells
maintained under normoxic conditions.
Our study takes advantage of intrinsic differences
in response between non-tumorigenic and malignant cells exposed to
hypoxia. In these settings, hypoxia seems to enhance the
sensitivity of malignant cells to S. graveolens treatment.
By itself, hypoxia is able to stimulate release of ROS bursts
through mitochondria (10,22), which in turn could explain the
greater sensitivity of cells to certain drugs with the resulting
enhancement of the cytotoxic effects. On the other hand, a dose of
200 μg/ml was able to reduce the cell viability by more than
90% in MCF-7 and ZR-75-1 cell lines. Other methanolic extracts,
e.g. from Senecio stabianus, have the ability of inhibit the
cell viability on MCF-7 cell line (IC50 91.1
μg/ml) under normoxic environment, but was not compared with
a normal counterpart (23).
Previous reports have shown that the highly
aggressive MDA-MB-231 cells, which do not express estrogen
receptor, progesterone receptor, and do not have HER-2/Neu
amplification (triple-negative), growing under hypoxic conditions,
are able to control the tumor pH, as well as the hypoxia-induced
extracellular acidosis, thus allowing aggressive tumor cells to
survive the hostile environment imposed by hypoxia (24,25).
Since restriction of oxygen may increase superoxide metabolism
(26) and phytochemicals extracts
belong from Senecio species produce reactive oxygen species
(ROS) (15), we also investigated
whether hypoxia and S. graveolens extract may influence
superoxide dismutase expression, enzyme that removes superoxide
radicals (O2•−) and produces hydrogen
peroxide (H2O2), as the first step to
detoxify the environment from radical oxygen species (27). The expression of MnSOD was not
altered by hypoxia or S. graveolens. However, the cellular
response to these treatments was dependent on the basal levels of
this enzyme. Thus, cells with higher levels of basal MnSOD were
able to better survive S. graveolens exposure, compared to
those showing lower levels of MnSOD. These findings are in line
with the fact that a high MnSOD content in tumor cells is
associated with a more aggressive and metastatic phenotype
(28). Moreover, cytotoxicity
studies have revealed that MCF-10F and MDA-MB-231 cells are
resistant to the drug metformin, and this resistance is accompanied
by increased levels of MnSOD. In contrast, MCF-7 cells, which are
sensitive to metformin, display low MnSOD expression (29). Moreover, tumor cells with high
MnSOD content are associated with invasive and meta-static profiles
(30,31). Based on these findings, the authors
(32) proposed that inhibition of
superoxide dismutase may provide a potential alternative to
eliminate cancer cells.
Another novel finding was the synergistic effect of
hypoxia and S. graveolens extract in triggering apoptosis,
necrosis, as well as the self-digestion process known as autophagy.
This catabolic process is characterized by the sequestration of
bulk cytoplasm, proteins and organelles in double-membrane
vesicles, known as autophagosomes, which are carried to lysosomes
for degradation (33). Autophagy
plays a pivotal role in countless processes, including cellular
starvation, cellular differentiation, cell death, aging, innate
immunity and as a mechanism of cell death during embryonic
development (34). Although
primarily considered a mechanism of survival (35), several reports have suggested that
autophagic cell death may precede apoptosis in some cancer cells
treated with antitumor agents (36). The distinction between apoptosis
and autophagy as mechanisms of programmed cell death is not always
clear, with several cases of autophagy (also known referred to as
type II cell death) showing also traits of apoptosis (37). Presently, it is not clear whether
the autophagic events observed in MCF-7 cells have a protective
role or, alternatively, they represent the initial steps of an
apoptotic process. Furthermore, the apoptotic process observed by
electron microscopy in MCF-7 cells may be the consequence of
increased expression of caspase-8, a fact reported in cases in
which ROS-dependent activation of caspase-8 contributed to
apoptosis initiation (22).
However, based on the expression levels of cleaved caspase-3
observed in ZR-75-1 cells, the S. graveolens extract might
trigger apoptosis but is not clear if autophagic events exist.
The coexistence of necrosis together with variable
levels of apoptosis and autophagy has been reported in other
studies in which the effect of an anticancer drug seemed to depend
on ROS production (11). It is
then possible that hypoxia by itself triggers necrosis through ROS
formation, an effect that is further enhanced by the use of
Senecio species (38).
The resistance to cell death observed in MDA-MB-231
cells seems to be related to the diminished expression in cleaved
caspase-8 after phytochemical treatment, an observation that was
corroborated by the absence of cleaved caspase-3. Likewise, the
reduced expression of markers of autophagy in MDA-MB-231 cells
following S. graveolens treatment indicates a loss in the
ability of these cells to upregulate autophagy. Thus, the
mechanisms responsible for the cytotoxicity exerted by the plant
extract are presently unclear. It is very likely that the relative
contribution of apoptosis and autophagy to the cytotoxic effects of
S. graveolens is chiefly dependent on the genetic profile of
the cells tested, as alterations in known oncogenic pathways (e.g.,
p53) modify the way in which a cell responds to external
stimuli.
It has been reported that some members of the
Senecio genus produce pyrrolizidine alkaloids as the main
metabolites (39). Since these
substances can cause important hepatotoxic effects under certain
conditions (38,40,41),
there is a growing necessity to identify and isolate active
cytotoxic compounds from Senecio that can have anticancer
effects without affecting normal tissues.
Our results suggest that the cytotoxic activity of
the ethanolic extract obtained from S. graveolens is
specific towards human breast cancer cells and can be enhanced by
hypoxia, an effect that might be explained in part by its ability
to induce a variety of processes, including autophagy, apoptosis
and necrosis in tumor cells. Overall, this study supports the
potential use of S. graveolens as a source of anticancer
drugs. Efforts to isolate the biologically active compound from
these extracts are currently under way in our laboratory.
Acknowledgements
We are grateful to Dr Mario Valenzuela
from Universidad de Tarapacá for the laboratory facilities. We also
appreciate the Emilio Gutierrez advice in ethnopharmacological
plants. This study was supported by grant from FONDECYT (no
11110284) (CECH). We also thank CODECITE-CIHDE.
References
|
1.
|
Greenlee RT, Hill-Harmon MB, Murray T and
Thun M: Cancer statistics, 2001. CA Cancer J Clin. 51:15–36. 2001.
View Article : Google Scholar
|
|
2.
|
Choi S, Lim MH, Kim KM, Jeon BH, Song WO
and Kim TW: Cordycepin-induced apoptosis and autophagy in breast
cancer cells are independent of the estrogen receptor. Toxicol Appl
Pharmacol. 257:165–173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ward C, Langdon SP, Mullen P, et al: New
strategies for targeting the hypoxic tumour microenvironment in
breast cancer. Cancer Treat Rev. 39:171–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Mishra BB and Tiwari VK: Natural products:
an evolving role in future drug discovery. Eur J Med Chem.
46:4769–4807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Makarov AA, Tsvetkov PO, Villard C, et al:
Vinflunine, a novel microtubule inhibitor, suppresses calmodulin
interaction with the microtubule-associated protein STOP.
Biochemistry. 46:14899–14906. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Cisternino S, Bourasset F, Archimbaud Y,
Semiond D, Sanderink G and Scherrmann JM: Nonlinear accumulation in
the brain of the new taxoid TXD258 following saturation of
P-glycoprotein at the blood-brain barrier in mice and rats. Br J
Pharmacol. 138:1367–1375. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Fulda S: Betulinic acid for cancer
treatment and prevention. Int J Mol Sci. 9:1096–1107. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Garcia-Zepeda SP, Garcia-Villa E,
Diaz-Chavez J, Hernandez-Pando R and Gariglio P: Resveratrol
induces cell death in cervical cancer cells through apoptosis and
autophagy. Eur J Cancer Prev. 22:577–584. 2013. View Article : Google Scholar
|
|
9.
|
Zhou DC, Zittoun R and Marie JP:
Homoharringtonine: an effective new natural product in cancer
chemotherapy. Bull Cancer. 82:987–995. 1995.PubMed/NCBI
|
|
10.
|
Guzy RD and Schumacker PT: Oxygen sensing
by mitochondria at complex III: the paradox of increased reactive
oxygen species during hypoxia. Exp Physiol. 91:807–819. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kim Y, Jeong IG, You D, et al: Sodium
meta-arsenite induces reactive oxygen species-dependent apoptosis,
necrosis, and autophagy in both androgen-sensitive and
androgen-insensitive prostate cancer cells. Anticancer Drugs.
25:53–62. 2014. View Article : Google Scholar
|
|
12.
|
Villagrán C and Castro V: Ciencia indigena
de los andes del norte de Chile - Senecio nutans. Editorial
Universitaria S.A; Santiago, Chile: 2004
|
|
13.
|
Burgueno-Tapia E, Hernandez LR,
Resendiz-Villalobos AY and Joseph-Nathan P: Conformational
evaluation and detailed 1H and 13C NMR assignments of
eremophilanolides. Magn Reson Chem. 42:887–892. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Burgueno-Tapia E, Gonzalez-Coloma A,
Martin-Benito D and Joseph-Nathan P: Antifeedant and phytotoxic
activity of cacalolides and eremophilanolides. Z Naturforsch C.
62:362–366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Bondan C, Soares JC, Cecim M, Lopes ST,
Graca DL and da Rocha RX: Oxidative stress in the erythrocytes of
cattle intoxicated with Senecio sp. Vet Clin Pathol.
34:353–357. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kizaki M, Xian M, Sagawa M and Ikeda Y:
Induction of apoptosis via the modulation of reactive oxygen
species (ROS) production in the treatment of myeloid leukemia. Curr
Pharm Biotechnol. 7:323–329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Vaupel P, Mayer A and Hockel M: Tumor
hypoxia and malignant progression. Methods Enzymol. 381:335–354.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Vaupel P, Briest S and Hockel M: Hypoxia
in breast cancer: pathogenesis, characterization and
biological/therapeutic implications. Wien Med Wochenschr.
152:334–342. 2002. View Article : Google Scholar
|
|
19.
|
Dong XL, Xu PF, Miao C, et al: Hypoxia
decreased chemosensitivity of breast cancer cell line MCF-7 to
paclitaxel through cyclin B1. Biomed Pharmacother. 66:70–75. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Li F, Huang L, Su XL, Gu QH and Hu CP:
Inhibition of nuclear factor-kappaB activity enhanced
chemosensitivity to cisplatin in human lung adeno-carcinoma A549
cells under chemical hypoxia conditions. Chin Med J. 126:3276–3282.
2013.PubMed/NCBI
|
|
21.
|
Reef S, Zalckvar E, Shifman O, et al: A
short mitochondrial form of p19ARF induces autophagy and
caspase-independent cell death. Mol Cell. 22:463–475. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Shi DY, Xie FZ, Zhai C, Stern JS, Liu Y
and Liu SL: The role of cellular oxidative stress in regulating
glycolysis energy metabolism in hepatoma cells. Mol Cancer.
8:322009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Tundis R, Loizzo MR, Bonesi M, et al: In
vitro cytotoxic effects of Senecio stabianus Lacaita
(Asteraceae) on human cancer cell lines. Nat Prod Res.
23:1707–1718. 2009.
|
|
24.
|
Chen CL, Chu JS, Su WC, Huang SC and Lee
WY: Hypoxia and metabolic phenotypes during breast carcinogenesis:
expression of HIF-1alpha, GLUT1, and CAIX. Virchows Arch.
457:53–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Lou Y, McDonald PC, Oloumi A, et al:
Targeting tumor hypoxia: suppression of breast tumor growth and
metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res.
71:3364–3376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Kaewpila S, Venkataraman S, Buettner GR
and Oberley LW: Manganese superoxide dismutase modulates
hypoxia-inducible factor-1 alpha induction via superoxide. Cancer
Res. 68:2781–2788. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Allen RG and Balin AK: Effects of oxygen
on the antioxidant responses of normal and transformed cells. Exp
Cell Res. 289:307–316. 2003. View Article : Google Scholar
|
|
28.
|
Ennen M, Minig V, Grandemange S, et al:
Regulation of the high basal expression of the manganese superoxide
dismutase gene in aggressive breast cancer cells. Free Rad Biol
Med. 50:1771–1779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Zhuang Y and Miskimins WK: Metformin
induces both caspase-dependent and poly(ADP-ribose)
polymerase-dependent cell death in breast cancer cells. Mol Cancer
Res. 9:603–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Nelson KK, Ranganathan AC, Mansouri J, et
al: Elevated sod2 activity augments matrix metalloproteinase
expression: evidence for the involvement of endogenous hydrogen
peroxide in regulating metastasis. Clin Cancer Res. 9:424–432.
2003.
|
|
31.
|
Kattan Z, Minig V, Leroy P, Dauca M and
Becuwe P: Role of manganese superoxide dismutase on growth and
invasive properties of human estrogen-independent breast cancer
cells. Breast Cancer Res Treat. 108:203–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Hileman EA, Achanta G and Huang P:
Superoxide dismutase: an emerging target for cancer therapeutics.
Expert Opin Ther Targets. 5:697–710. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Klionsky DJ: Autophagy revisited: a
conversation with Christian de Duve. Autophagy. 4:740–743. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Bowen ID, Mullarkey K and Morgan SM:
Programmed cell death during metamorphosis in the blow-fly
Calliphora vomitoria. Microsc Res Tech. 34:202–217. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nat Rev Cancer. 7:961–967. 2007.
View Article : Google Scholar
|
|
36.
|
Codogno P and Meijer AJ: Autophagy and
signaling: their role in cell survival and cell death. Cell Death
Differ. 12(Suppl 2): 1509–1518. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Motyl T, Gajkowska B, Zarzynska J,
Gajewska M and Lamparska-Przybysz M: Apoptosis and autophagy in
mammary gland remodeling and breast cancer chemotherapy. J Physiol
Pharmacol. 57(Suppl 7): 17–32. 2006.PubMed/NCBI
|
|
38.
|
Steenkamp V, Stewart MJ, van der Merwe S,
Zuckerman M and Crowther NJ: The effect of Senecio
latifolius a plant used as a South African traditional
medicine, on a human hepatoma cell line. J Ethnopharmacol.
78:51–58. 2001.
|
|
39.
|
Loizzo MR, Tundis R, Statti GA, Menichini
F and Houghton PJ: In-vitro antiproliferative effects on human
tumour cell lines of extracts and jacaranone from Senecio
leucanthemifolius Poiret. J Pharm Pharmacol. 57:897–901. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Hammond GB, Fernandez ID, Villegas LF and
Vaisberg AJ: A survey of traditional medicinal plants from the
Callejon de Huaylas, Department of Ancash, Peru. J Ethnopharmacol.
61:17–30. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Liu F and Ng TB: Antioxidative and free
radical scavenging activities of selected medicinal herbs. Life
Sci. 66:725–735. 2000. View Article : Google Scholar : PubMed/NCBI
|