Introduction
Acquired resistance to antiestrogen or aromatase
inhibitor therapy affects ∼40–50% of patients whose breast tumors
are estrogen receptor α (ERα)-positive (1). Multiple mechanisms contribute to
endocrine resistance and new therapies are needed to prevent
endocrine resistance and treat these patients (2).
(1–3)β-D-glucans are diverse polysaccharides
derived from plant cell walls composed of D-glucose monomers linked
by (1–3)β-glycosidic bonds. The activities of
β-glucans have been studied in vivo and in vitro
(3). When ingested in plant
materials, β-glucans are absorbed in the small intestine and taken
up by macrophages. β-glucans are considered to be ‘biological
response modifiers’ since they exhibit immunomodulatory,
wound-healing, antiviral, antibacterial, anti-coagulatory and
antitumoral activities (4).
Because of their size, β-glucans work by binding to cell surface
receptors (5). β-glucans act on
several immune receptors, e.g., Dectin-1, complement receptor
(CR3), scavenger receptors (SR), lactosylceramide (LacCer), and
toll-like receptors, e.g., TLR-2/6, and trigger responses in
macrophages, neutrophils, monocytes, natural killer cells, and
dendritic cells in vitro (5,6).
β-glucans themselves had no direct cytotoxic effects on a panel of
common cancer cell lines including carcinoma, sarcoma and blastoma
cells (6).
Cell inhibitory activities of β-glucans in cancer
cells in vitro have also been reported. A water-soluble
β-glucan extract from the mycelia of Poria cocos was
reported to inhibit the viability (MTT assay) of MCF-7 breast
cancer cells with an IC50 of 400 μg/ml and to
decrease cyclin D1 and cyclin E protein expression (7). The goal of this study was to examine
the effect of a purified preparation of (1–3)β-D-glucan on the growth of
endocrine-sensitive, estrogen receptor α (ERα)+ MCF-7
cells compared to normal breast epithelial (ERα−)
MCF-10A cells; estradiol (E2)-independent, tamoxifen
(TAM) and fulvestrant-resistant, ERα+ LCC9 (8,9) and
LY2 (10,11) cells; and ‘triple
negative/basal-like’ MDA-MB-231 (12) cells. Additionally, we examined the
effect of β-D-glucan on the expression of a set of genes implicated
in breast cancer in MCF-7 and LCC9 cells using a PCR array. While
not affecting MCF-10A normal breast epithelial cell proliferation,
our results indicate that β-D-glucan inhibits breast cancer cell
proliferation and modulates gene expression independent of ERα
activity and may be useful for inhibiting endocrine-resistant
breast cancer cell proliferation.
Materials and methods
Cells
MCF-7 and MDA-MB-231 human breast cancer cells were
purchased from ATCC (Manassas, VA, USA) and maintained in IMEM
supplemented with 5% fetal bovine serum (Atlanta Biologicals,
Lawrenceville, GA, USA) and 1% penicillin/streptomycin (Mediatech,
Manassas, VA, USA) (13). LCC9
(8) and LY2 (10) cell lines were derived from MCF-7
cells by cultivation with the anti-estrogens ICI 182,780
(Fulvestrant) and LY 117018 respectively, and were graciously
provided as a gift by Dr Robert Clarke, Georgetown University.
MCF-10A cells are immortalized normal human breast epithelial cells
that were also purchased from ATCC and grown in DMEM/F12
supplemented with 5% horse serum, 20 ng/ml epidermal growth factor
(EGF), 16.67 μg/ml insulin and 0.1% hydrocortisone
(Sigma-Aldrich, St. Louis, MO, USA). Prior to treatment, the medium
was replaced with phenol red-free IMEM supplemented with 5%
dextran-coated charcoal-stripped FBS (DCC-FBS) and 1%
penicillin/streptomycin for 48 h (referred to as
‘serum-starving’).
Chemicals
Estradiol (E2) and 4-hydroxytamoxifen
(4-OHT) were purchased from Sigma-Aldrich. ICI 182,780 was from
Tocris (Ellisville, MO, USA). β-D-glucan was purchased from Sigma
(cat. no. G6513, purity ∼97%). β-D-glucan was dissolved either in
water or in DMSO (Sigma) by heating in a waterbath at 90°C for 4–5
min. Once dissolved, the β-D-glucan stocks were stored at −20°C
until use. For all experiments, any effect(s) of DMSO were
corrected in the calculations.
Cell proliferation and cell death
assays
The cells were seeded at a density of 5,000
cells/well in 96-well plates and were incubated for 24 h in growth
medium prior to treatment. To initiate the experiment, the medium
was removed and cells were treated with different concentrations of
β-D-glucan (1–400 μg/ml, as indicated in the Figures) and
incubated for 72 h. Medium and treatments were changed after the
first 48 h of incubation. For certain experiments, the cells were
also treated with 100 nM or 1 μM 4-OHT and β-D-glucan (10,
50 and 100 μg/ml) to examine the potential synergistic
effect of β-D-glucan with 4-OHT. Cell proliferation was determined
after 72 h by measuring BrdU incorporation using an ELISA kit from
Roche Applied Science (cat. 11647229001, Indianapolis, IN, USA)
according to the manufacturer’s instructions. IC50
values were calculated using Excel.
Cell death was examined using the Live/Dead
Viability/Cytotoxicity assay (Invitrogen), which determines
intracellular esterase activity and plasma membrane integrity. In
brief, 3×105 cells were incubated with DMSO or
increasing concentrations of β-D-glucan for 72 h. Cells were
stained with the live and dead reagent [2 μmol/l ethidium
homodimers-1 (Eth-1) and 1 μmol/l calcein-AM] and incubated
at room temperature for 30 min. Fluorescence was read at 530 and
645 nM. The Live/Dead cell assay controls and calculations for %
dead cells followed the manufacturer’s protocol.
PCR arrays
MCF-7 or LCC9 cells were serum-starved, as above,
for 48 h and then treated with DMSO (vehicle control), 10 or 50
μg/ml β-D-glucan, 10 nM E2, or 100 nM 4-OHT.
Total RNA was extracted using RNeasy Mini kit (Qiagen, Valencia,
CA, USA). RNA quality was examined by NanoDrop Spectroscopy and
cDNA synthesis was performed using the RT2 PCR Array First Strand
kit (SABiosciences, Qiagen). RT2 Profiler PCR Array Breast Cancer
SABiosciences cat no. PAHS-131ZA-12 contains 84 genes commonly
involved in the dysregulation of signal transduction and other
biological processes during breast carcinogenesis and in breast
cancer cell lines plus 5 housekeeping genes http://www.sabiosciences.com/rt_pcr_product/HTML/PAHS-131Z.html.
Breast cancer PCR arrays were performed according to the
manufacturer’s instructions. Data analysis was performed using the
web-based analysis tool (www.sabiosciences.com/pcrarraydataanalysis.php),
including fold change and cluster analyses.
Quantitative real-time PCR (qRT-PCR)
analysis of mRNA expression
Total RNA was isolated from MCF-7 or LCC9 cells
after 24-h treatment with DMSO (vehicle control), 10 nM
E2, 100 nM 4-OHT, or 10 or 50 μg/μl
β-D-glucan with RNeasy Mini kit (Qiagen) according to the
manufacturer’s instructions. The quality and quantity of the
isolated RNA was analyzed using NanoDrop spectroscopy. RNA (1
μg) was reverse-transcribed using the High Capacity cDNA
Reverse Transcription kit (Applied Biosystems, Carlsbad, CA, USA)
and quantitation was performed using TaqMan primers and probes sets
with TaqMan Gene Expression Master Mix (Applied Biosystems) and 18S
was used for normalization. qRT-PCR was run using a ViiA7 Real-time
PCR system (Applied Biosystems) with each reaction run in
triplicate. Analysis and fold change were determined using the
comparative threshold cycle (Ct) method. The change mRNA expression
was calculated as fold-change, i.e., relative to DMSO-treated cells
(control).
Western blot analysis
Whole cell lysates were prepared from MCF-7 and LCC9
cells grown in phenol red-free IMEM + 5% DCC-stripped FBS for 48 h
prior to addition of DMSO (vehicle control) or 10 or 50
μg/ml β-D-glucan dissolved in DMSO for 24 h. Whole cell
extracts (30 μg protein) were separated on 10% SDS-PAGE gels
and the resulting western blot was probed with HC-20 ERα antibody
(Santa Cruz Biotechnology, Santa Cruz, CA, USA). The PVDF membrane
was stripped and re-probed for β-actin (Sigma) for normalization.
Chemiluminescent bands were visualized on a Carestream Imager using
Carestream Molecular Imaging software (New Haven, CT, USA).
Statistical analysis
Values are reported as ± SEM. Student’s t-test was
used for comparisons between control and treatment. One-way ANOVA
was used for multiple comparisons followed by Student-Newman-Keuls
or Dunnett’s post hoc tests using GraphPad Prism. Values
with p<0.05 were considered statistically significant.
Results
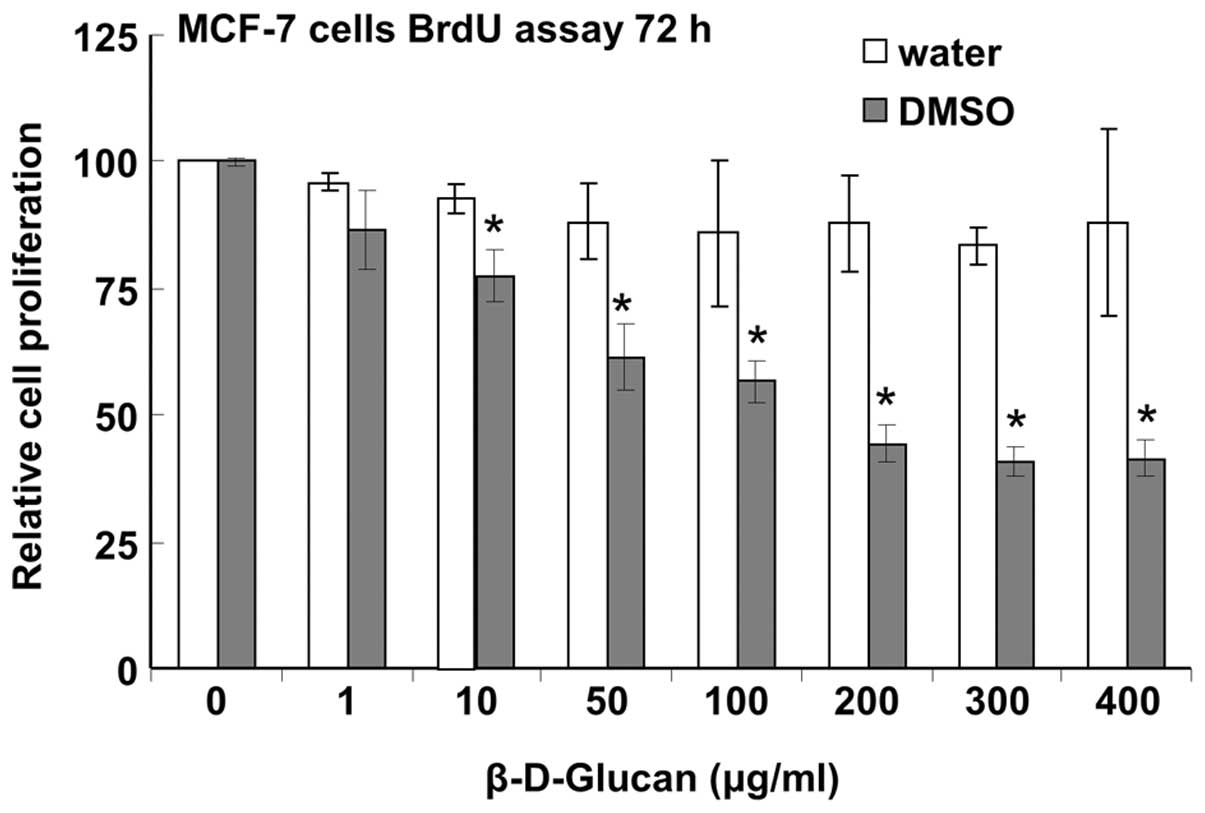
β-D-glucan dissolved in DMSO but not
water inhibits MCF-7 cell proliferation
Batch-to-batch variability of extracts of β-glucans
leads to problematic heterogeneity of effects and controversy
regarding their significance as potential anticancer agents
(14). To obviate this issue, we
purchased β-D-glucan purified from barley from Sigma and tested its
activity in breast cancer cells. There was no inhibition of MCF-7
cell proliferation when cells were treated with β-glucan dissolved
in boiling water, but cells were inhibited with an IC50
of ∼164±12 μg/ml with β-glucan dissolved in DMSO (Fig. 1).
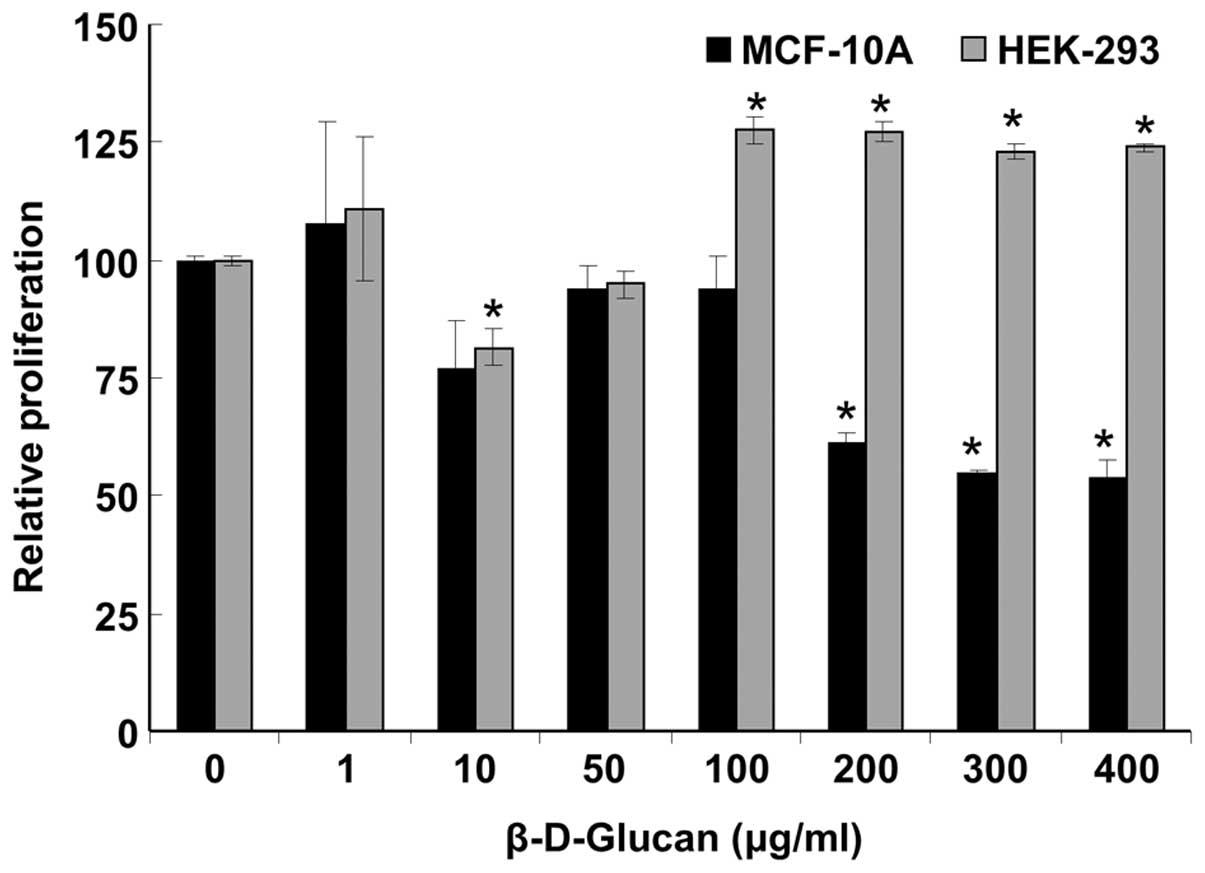
β-D-glucan inhibits MCF-10A, but not
HEK-293 cell proliferation
Next, we examined if DMSO-solubilized β-D-glucan
affected the growth of ‘normal’ cells using MCF-10A immortalized
breast epithelial cells and HEK-293 cells (Fig. 2). β-D-glucan inhibited MCF-10A
proliferation with an IC50 of ∼464 μg/ml, but had
no significant inhibitory effect on HEK-293 cells, although a
somewhat ‘U-shaped’ response was detected, i.e., apparent
inhibition at 10 μg/ml and stimulation at 100–400
μg/ml.
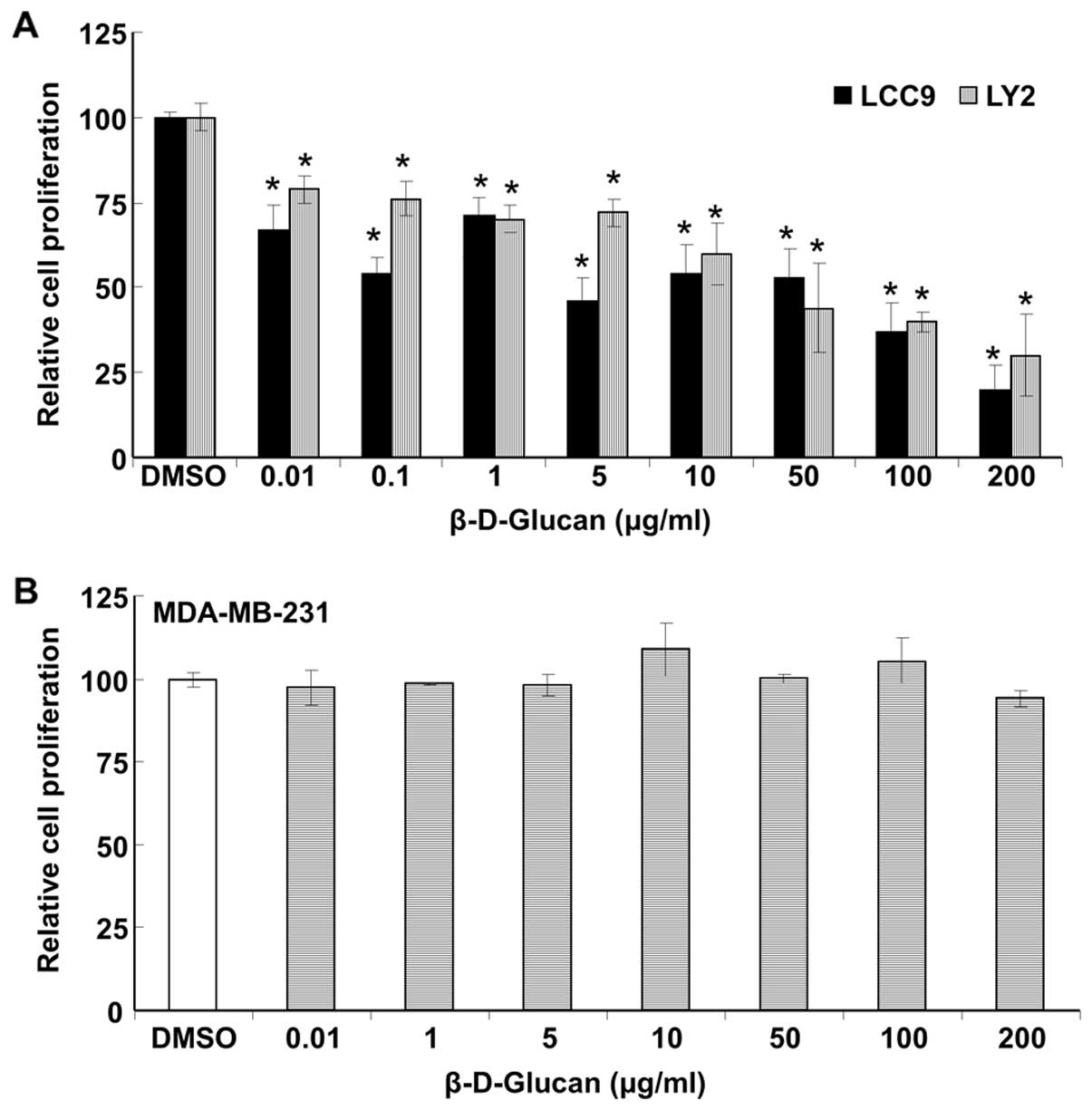
β-D-glucan inhibits the proliferation of
endocrine-resistant cells
The development of acquired resistance to tamoxifen
and other endocrine agents is a major concern in breast cancer
patients. We examined if DMSO-solubilized β-D-glucan inhibited the
growth of LCC9 and LY2 endocrine-resistant breast cancer cells
(Fig. 3A). β-D-glucan inhibited
the proliferation of each cell line, with IC50 values of
4.6±0.3 and 24.2±1.4 μg/ml for LCC9 and LY2, respectively.
In contrast, β-D-glucan had no effect on MDA-MB-231
triple-negative/basal-like breast cancer cells (Fig. 3B).
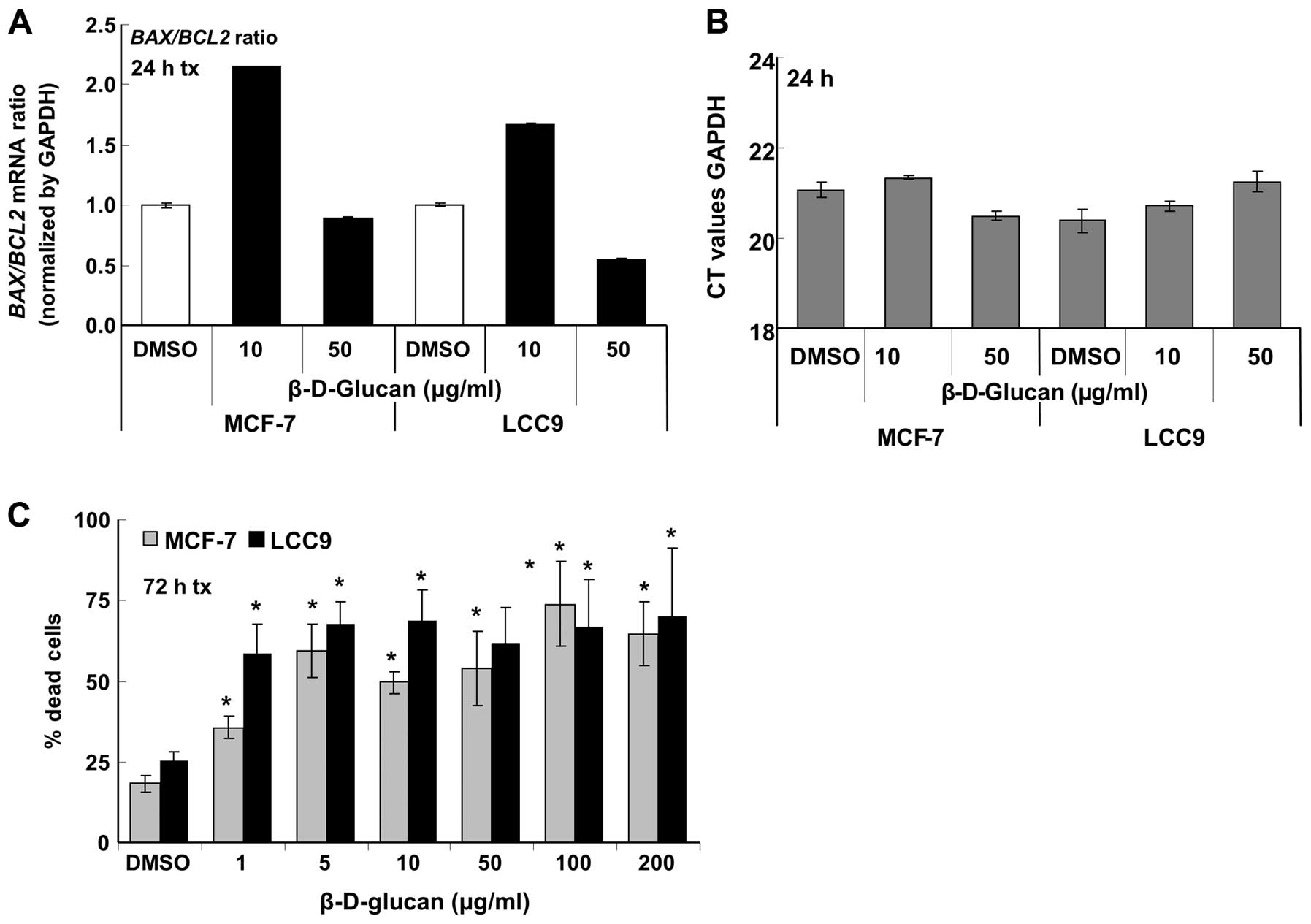
To examine the possible contribution of apoptosis to
the observed decrease in MCF-7 and LCC9 cell viability with
β-D-glucan treatment, we measured the expression of BAX
(pro-apoptotic) and BCL2 (anti-apoptotic) in MCF-7 and LCC9
cells treated with vehicle (DMSO), 10 or 50 μg/ml β-D-glucan
(Fig. 4A). GAPDH mRNA
transcript levels were not affected by β-D-glucan (Fig. 4B). An increased BAX/BCL2 is
an indicator of apoptosis (15).
As reported previously (16),
basal BCL2 expression was higher in the endocrine-resistant
LCC9 cells compared to parental, endocrine-sensitive MCF-7 cells
(data not shown). β-D-glucan (10 μg/ml) increased the
BAX/BCL2 ratio in both cell lines, but that increase was not
sustained at 50 μg/ml β-D-glucan.
Live/Dead cell assays were performed to examine cell
death through determination of intracellular esterase activity and
plasma membrane integrity (Fig.
4C). The data show that β-D-glucan increases cell death in both
MCF-7 and LCC9 cells with more death in LCC9 versus MCF-7 cells at
1 μg/ml β-D-glucan. There appears to be a saturation, with
maximal ∼70% cell death in both cell lines.
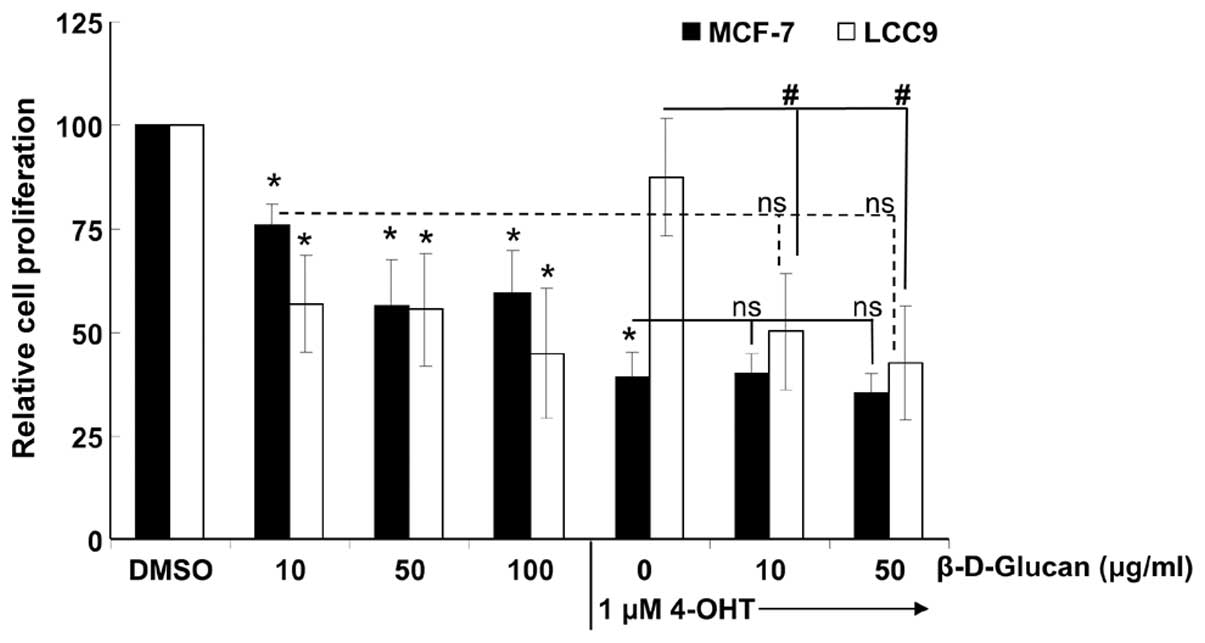
β-D-glucan has no effect on
TAM-sensitivity of MCF-7 or LCC9 cells
A β-D-glucan extract called schizophyllan that was
extracted from the mushroom Schizophyllum commune by boiling
in water showed no additive effect with TAM treatment in
suppressing PCNA staining in DMBA-induced mouse mammary tumors, but
inhibited TAM-induced PCNA staining in liver tumors of the same
mice (17). We tested if
β-D-glucan synergized with 4-OHT to inhibit MCF-7
endocrine-sensitive and LCC9 endocrine-resistant cell growth. There
was no effect of β-D-glucan on the inhibition of MCF-7 cell growth
by 4-OHT, nor was there any effect of 4-OHT on the inhibition of
LCC9 cell proliferation by β-D-glucan (Fig. 5).
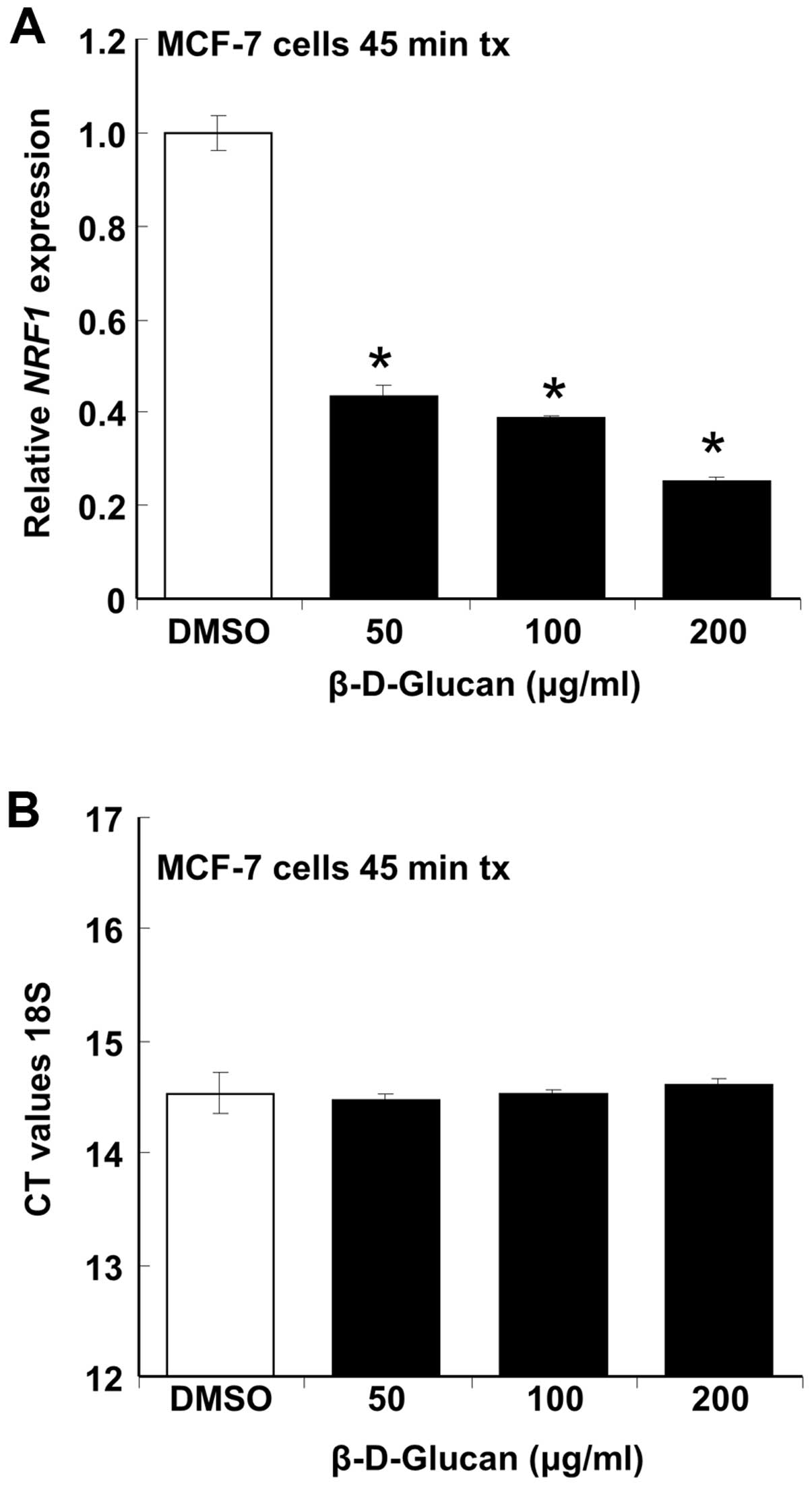
β-D-glucan inhibits NRF-1 expression in
MCF-7 cells
Nuclear respiratory factor-1 (NRF-1) is a master
transcription factor regulating the transcription of nuclear genes
controlling many aspects of mitochondrial function including
respiration (18). Knockdown of
NRF-1 in MCF-7 breast cancer cells using siRNA increases apoptosis
and overexpression of NRF-1 inhibits 4-OHT-mediated apoptosis
(19). We tested the hypothesis
that the inhibition of MCF-7 cell proliferation and viability by
β-D-glucan (Figs. 1 and 4) would be reflected in inhibition of
NRF-1 expression. β-D-glucan rapidly inhibited NRF-1 transcription
in a concentration-dependent manner without affecting 18S rRNA
expression (Fig. 6). The rapid
inhibition of NRF-1 transcription is commensurate with plasma
membrane effects of β-D-glucan.
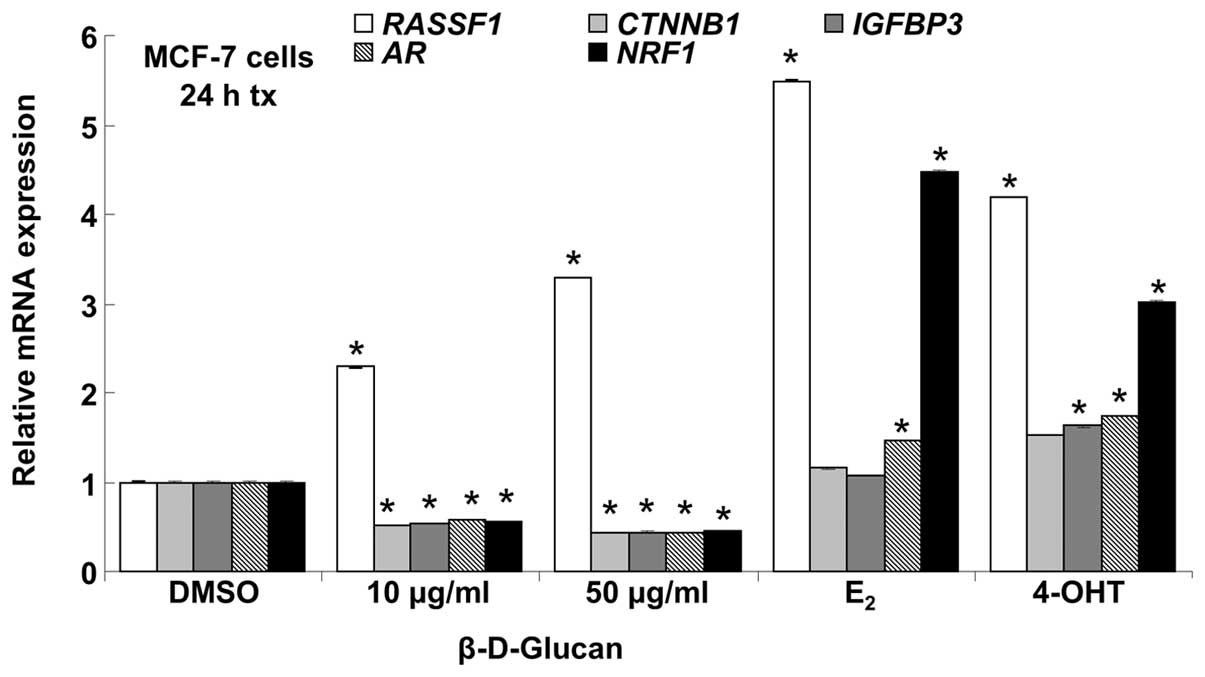
β-D-glucan affects breast cancer gene
expression in a cell type-dependent manner
To identify other potential breast cancer-associated
genes regulated by β-D-glucan, we performed PCR array analysis on
84 genes commonly dysregulated during breast carcinogenesis and in
breast cancer cell lines (Breast Cancer PCR array PAHS-131Z,
SABiosciences). For these experiments, MCF-7 or LCC9 cells were
serum-starved for 48 h in phenol red-free medium and then treated
in duplicate with DMSO (vehicle control), 10 nM E2, 100
nM 4-OHT, or 10 or 50 μg/ml β-D-glucan for 24 h. Using a
2-fold cut off, β-D-glucan altered the expression of 17 genes in
MCF-7 cells: 8 downregulated and 9 upregulated. Some, but not all,
genes showed a dose-dependent effect of β-D-glucan, e.g.,
IGFBP3 showed a greater decrease with 50 than 10
μg/ml β-D-glucan (Table I).
In the group of genes with increased expression in MCF-7 with
β-D-glucan, some β-D-glucan regulated genes had a similar
expression pattern as with E2 treatment and others
showed similarity with 4-OHT treatment (Table II). β-D-glucan altered the
expression of 8 genes in LCC9: 3 downregulated and 5 upregulated
(Tables III and IV). E2 altered the expression
of 17 genes in MCF-7: 9 downregulated and 8 upregulated. 4-OHT
altered the expression of 8 genes in MCF-7: 1 downregulated and 7
upregulated. E2 altered the expression of 5 genes in
LCC9: 2 downregulated and 3 upregulated. 4-OHT altered the
expression of 10 genes in LCC9: 5 downregulated and 5 upregulated.
We note that 17 genes showed lower expression in LCC9 than MCF-7
cells (Table V). Conversely, 31
genes showed higher expression in LCC9 than MCF-7 cells (Table VI).
 | Table I.Genes with decreased expression
following treatment with β-D-glucan, E2 and/or 4-OHT in
MCF-7 endocrine-sensitive breast cancer cells. |
Table I.
Genes with decreased expression
following treatment with β-D-glucan, E2 and/or 4-OHT in
MCF-7 endocrine-sensitive breast cancer cells.
| Symbol | 10 μg/ml
β-D-glucan | 50 μg/ml
β-D-glucan | E2 | 4-OHT |
|---|
| AR | 1.1 | −1.2 | −2.1 | −1.2 |
| ATM | −1.3 | −1.6 | −4.3 | −1.9 |
| CCND2 | −1.4 | 1.0 | −2.1 | −1.0 |
| CDKN1C | −2.2 | −2.4 | −2.0 | −1.6 |
| CSF1 | −1.1 | −1.2 | −2.4 | 1.1 |
| CTNNB1 | −1.0 | −4.3 | −1.2 | 1.0 |
| ERBB2 | −1.6 | −1.9 | −2.2 | 1.4 |
| GRB7 | −1.6 | −1.7 | −2.4 | 1.0 |
| IGFBP3 | −1.7 | −2.2 | −1.7 | 1.2 |
| MUC1 | −2.1 | −1.4 | −1.4 | −1.0 |
| NOTCH1 | −1.6 | −1.0 | −2.3 | −1.5 |
| PLAU | −2.3 | −2.4 | −1.5 | 1.1 |
| RARB | −3.5 | −2.2 | −3.3 | −2.3 |
| SLIT2 | −1.5 | −2.7 | −3.0 | 1.5 |
| SNAI2 | −2.4 | −1.5 | −1.1 | 1.9 |
 | Table II.Genes with increased expression
following treatment with β-D-glucan, E2 and/or 4-OHT in
MCF-7 endocrine-sensitive breast cancer cells. |
Table II.
Genes with increased expression
following treatment with β-D-glucan, E2 and/or 4-OHT in
MCF-7 endocrine-sensitive breast cancer cells.
| Symbol | 10 μg/ml
β-D-glucan | 50 μg/ml
β-D-glucan | E2 | 4-OHT |
|---|
| BIRC5 | 2.6 | 3.0 | 2.2 | 1.0 |
| BRCA1 | 2.7 | 2.6 | 2.5 | −1.6 |
| BRCA2 | 2.3 | 2.7 | 2.5 | −1.3 |
| CCNA1 | 2.1 | 2.6 | 5.3 | 1.2 |
| CTSD | 2.2 | 1.7 | 1.8 | 1.0 |
| EGF | 1.2 | −1.2 | 1.3 | 2.5 |
| GLI1 | 1.7 | 1.2 | −1.6 | 2.3 |
| GSTP1 | 1.8 | 1.1 | −1.2 | 3.5 |
| KRT5 | 1.7 | −1.0 | −1.2 | 2.2 |
| MKI67 | 1.9 | 2.6 | 2.2 | 1.1 |
| NME1 | 1.7 | 1.8 | 2.1 | −1.2 |
| PGR | 3.8 | 2.9 | 2.9 | −1.2 |
| PTGS2 | 2.5 | 1.8 | 1.9 | 1.1 |
| RASSF1 | 4.0 | 3.8 | 2.2 | 3.2 |
|
SERPINE1 | 1.3 | 1.4 | 2.0 | 2.4 |
| TP73 | 1.2 | −1.2 | −1.3 | 6.5 |
 | Table III.Genes with decreased expression
following treatment with β-D-glucan, E2 and/or 4-OHT in
LCC9 endocrine-resistant breast cancer cells. |
Table III.
Genes with decreased expression
following treatment with β-D-glucan, E2 and/or 4-OHT in
LCC9 endocrine-resistant breast cancer cells.
| Symbol | 10 μg/ml
β-D-glucan | 50 μg/ml
β-D-glucan | E2 | 4-OHT |
|---|
| ADAM23 | 1.2 | −1.6 | 1.2 | −2.3 |
| BRCA2 | −1.4 | −1.6 | −2.3 | −2.4 |
| CDH13 | −2.5 | −1.8 | −2.6 | −1.8 |
| CDKN1C | −2.4 | −1.4 | −1.6 | −3.0 |
| CTNNB1 | −1.4 | −2.1 | −1.7 | −2.1 |
| ID1 | 1.6 | 1.2 | −1.7 | −2.4 |
 | Table IV.Genes with increased expression
following treatment with β-D-glucan, E2 and/or 4-OHT in
LCC9 endocrine-resistant breast cancer cells. |
Table IV.
Genes with increased expression
following treatment with β-D-glucan, E2 and/or 4-OHT in
LCC9 endocrine-resistant breast cancer cells.
| Symbol | 10 μg/ml
β-D-glucan | 50 μg/ml
β-D-glucan | E2 | 4-OHT |
|---|
| EGF | 1.8 | 5.1 | 4.6 | 2.1 |
| GLI1 | 4.9 | 7.4 | 3.3 | 8.3 |
| HIC1 | 1.4 | 1.4 | 1.6 | 2.5 |
| IGF1 | 5.6 | 1.4 | 1.7 | 2.9 |
| IGFBP3 | 2.0 | 2.0 | 2.9 | 1.1 |
| PTGS2 | 2.2 | 1.2 | 1.7 | 1.1 |
| TWIST1 | −1.2 | −1.1 | 1.5 | 3.7 |
 | Table V.Genes with lower expression in LCC9
endocrine-resistant vs. MCF-7 endocrine-sensitive breast cancer
cells. |
Table V.
Genes with lower expression in LCC9
endocrine-resistant vs. MCF-7 endocrine-sensitive breast cancer
cells.
| Symbol | Fold |
|---|
| ABCG2 | −35.2 |
| BCL2 | −2.1 |
| CCND2 | −2.0 |
| CDKN1A | −5.0 |
| EGF | −2.6 |
| EGFR | −9.1 |
| GATA3 | −4.1 |
| ID1 | −2.2 |
| IGF1R | −2.5 |
| IGFBP3 | −25.7 |
| JUN | −5.5 |
| KRT18 | −3.9 |
| KRT8 | −4.6 |
| MGMT | −2.5 |
| PLAU | −2.0 |
| SLC39A6 | −3.7 |
| THBS1 | −14.1 |
 | Table VI.Genes with higher expression in LCC9
endocrine-resistant vs. MCF-7 endocrine-sensitive breast cancer
cells. |
Table VI.
Genes with higher expression in LCC9
endocrine-resistant vs. MCF-7 endocrine-sensitive breast cancer
cells.
| Symbol | Fold |
|---|
| ABCB1 | 2.1 |
| ADAM23 | 11.4 |
| BIRC5 | 5.9 |
| BRCA1 | 2.8 |
| BRCA2 | 7.2 |
| CCNA1 | 78.0 |
| CDH13 | 4.3 |
| CDK2 | 4.1 |
| CDKN1C | 2.3 |
| CDKN2A | 2.1 |
| CST6 | 2.1 |
| ESR2 | 2.1 |
| GLI1 | 2.2 |
| GSTP1 | 28.2 |
| HIC1 | 4.0 |
| IGF1 | 2.1 |
| IL6 | 2.1 |
| KRT5 | 2.1 |
| MAPK1 | 4.4 |
| MMP2 | 2.1 |
| MMP9 | 3.0 |
| MYC | 3.6 |
| PGR | 4.5 |
| PRDM2 | 2.2 |
| PTEN | 5.2 |
| RASSF1 | 2.1 |
|
SERPINE1 | 85.4 |
| SFRP1 | 2.1 |
| TWIST1 | 5.1 |
| VEGFA | 2.6 |
| XBP1 | 3.7 |
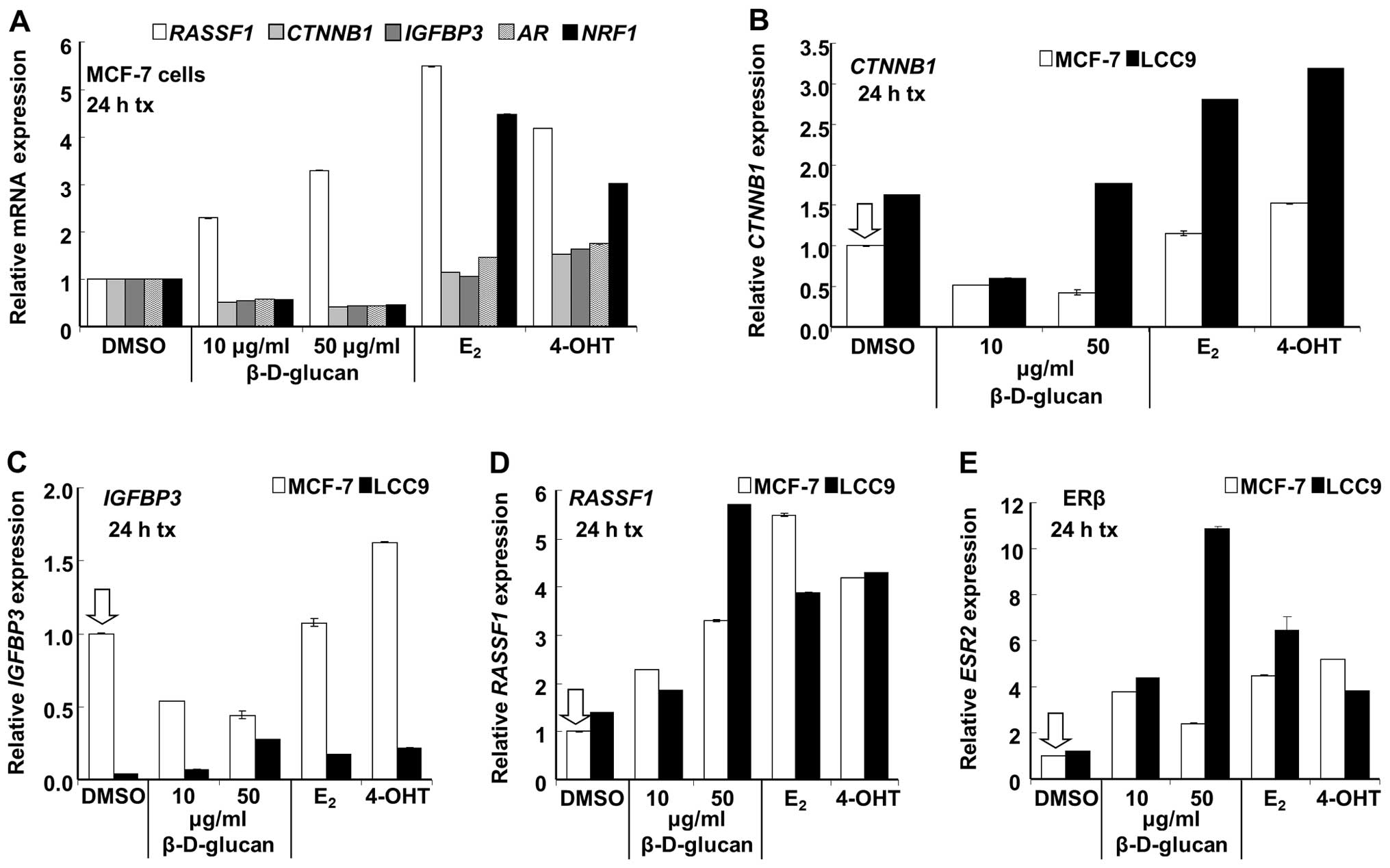
Confirmation of select changes in breast
cancer gene expression by qRT-PCR
To determine if the changes detected in the PCR
array after treatment of MCF-7 and LCC9 cells with β-D-glucan were
reproducible by qRT-PCR, five gene targets were selected for
verification: RASSF1, CTNNB1, IGFBP3, ESR2 (ERβ), and
AR (Tables I–III, V
and VI). 18S was used for
normalization and was not significantly different between the two
cell lines or with β-D-glucan treatment (data not shown). The
rationale for selecting these genes for follow-up is based on their
regulation by β-D-glucan in the PCR array: RASSF1 was
increased in MCF-7 (E2 and 4-OHT also increased
RASSF1, Table II);
CTNNB1 was decreased in MCF-7 and LCC9; IGFBP3 and
ESR2 were increased in LCC9; AR was decreased in
MCF-7. Further rationale is based on their roles in breast cancer.
RASSF1A is a tumor suppressor gene that is downregulated by
hypermethylation in various human cancers including breast cancer
(20,21). CTNNB1 encodes β-catenin, an
adherens junction protein that plays a critical role in cellular
adhesion and intercellular communication which also translocates to
the nucleus to activate genes whose promoters contain binding sites
for Tcf/Lef (22). Activation of
the Wnt/β-catenin pathway plays a role in breast tumorigenesis
(23,24). Insulin-like growth factor (IGF)
binding protein 3 (IGFBP3) is a major carrier of IGF1 and IGF2 in
circulation and IGFBP3 levels are reduced in breast cancer
patients, giving rise to higher free IGF1 levels and poor prognosis
(25,26). IGFBP3 also stimulates or inhibits
normal and neoplastic breast cell proliferation by stimulating EGFR
activation or stimulating apoptotic effector proteins (27,28).
E2 stimulates IGFBP3 expression in MCF-7 cells (29) and both E2 and 4-OHT
increased IGFBP transcript expression in MDA-MB-231 triple
negative breast cancer cells transfected with ERα (30). When IGFBP3 was transfected into
LCC9 endocrine-resistant breast cancer cells, it was shown, by
co-immunoprecipitation, to interact with the 78-kDa glucose
regulated protein (GRP78), which is highly expressed in LCC9 and
other endocrine-resistant breast cancer cells (31), and to dissociate caspase 7 from
GRP78, thus sensitizing LCC9 cells to growth inhibition by
fulvestrant (ICI 182,780) (32).
Increased AR expression is found in tamoxifen-resistant breast
tumors and overexpression of AR in MCF-7 cells caused the cells to
become resistant to growth inhibition by tamoxifen (33).
The increase in RASSF1 transcript expression
(Table II) was reproducibly
increased by treatment with β-D-glucan, E2 and 4-OHT in
MCF-7 cells (Fig. 7). The
inhibition of CTNNB1, IGFBP3 and AR (Table I) by β-D-glucan in MCF-7 cells was
confirmed; however, E2 and 4-OHT did not significantly
inhibit the expression of these genes (Fig. 7), a result different from that
detected in the PCR array. Since qRT-PCR is the accepted standard
to compare transcript levels, these data suggest that E2
and 4-OHT may not significantly inhibit CTNNB1, IGFBP3 and
AR in MCF-7 cells with 24 h of treatment. In fact,
CTNNB1 (β-catenin) transcript expression was statistically
increased by 4-OHT in MCF-7, although only by 0.5-fold (Fig. 7).
CTNNB1 (β-catenin) expression was decreased
by β-D-glucan in a concentration-dependent manner in LCC9 in the
PCR array (Table III) and by 10
μg/ml β-D-glucan as assessed by qRT-PCR (Fig. 7). However, 50 μg/ml
β-D-glucan, E2 and 4-OHT increased CTNNB1 in LCC9
cells. CTNNB1 basal expression was ∼63% higher in LCC9 than
MCF-7 (Fig. 8A), although this was
not detected in the PCR array (Table
VI). β-catenin mRNA and protein expression is increased in
another tamoxifen-resistant cell line derived from MCF-7 cells
(34) and in breast tumors
(35). The increase in
CTNNB1 transcript expression with E2 and 4-OHT
was significant in both MCF-7 and LCC9 cells, although the
fold-response, 1.7- and 2-fold respectively, compared to basal
(DMSO), was higher in LCC9 cells. This increase in β-catenin
expression would be expected to interact with and increase TCF/LEF1
target gene expression in these cells, a pathway contributing to
breast cancer progression (23).
AR (androgen receptor, AR, NR3C4) expression
was reduced by β-D-glucan in MCF-7 cells while E2 and
4-OHT slightly increased AR expression.
We confirmed that β-D-glucan inhibited NRF-1
transcription in MCF-7 cells (Figs.
6 and 7) whereas E2
and 4-OHT increased NRF-1 expression, as previously reported
(19,36). Basal NRF-1 transcript expression
was higher in LCC9 cells and was increased by β-D-glucan and
inhibited by E2 and 4-OHT (Fig. 8B).
Expression of IGFBP3 (insulin-like growth
factor binding protein 3) was 25.7-fold lower in LCC9 than MCF-7
(Table V). This result was
confirmed by qRT-PCR (Fig. 8C).
This is consistent with a previous report that IGFBP3 protein
secretion was reduced in tamoxifen-resistant LY2 and ZR-75-9a1
cells (37). IGFBP3 (which
sequesters IGF) was increased by β-D-glucan and E2 in
LCC9 cells (Table IV). These
results were confirmed by qRT-PCR. 4-OHT also increased
IGFBP3 in LCC9 cells. In MCF-7 cells, β-D-glucan inhibited
IGFBP3 transcript expression whereas 4-OHT increased
IGFBP3 expression.
ESR2 (ERβ) and RASSF1 (Ras-association
domain family protein 1) showed higher expression in LCC9 than
MCF-7 cells in the PCR array (Table
VI). This may be surprising since ERβ inhibits the
proliferative activity of ERα (38) and RASFF1 is a tumor
suppressor gene whose inactivation by hypermethylation of a CpG
island in the gene promoter (39)
has been implicated in a wide variety of sporadic human cancers,
including breast cancer (20).
Results were confirmed by qRT-PCR (Fig. 8D and E). As in MCF-7 cells,
β-D-glucan, E2 and 4-OHT increased RASSF1
expression in LCC9 cells (Fig.
8E). In contrast to the increase in ERβ, β-D-glucan reduced
ESR1 (ERα) mRNA transcript levels in MCF-7 cells and
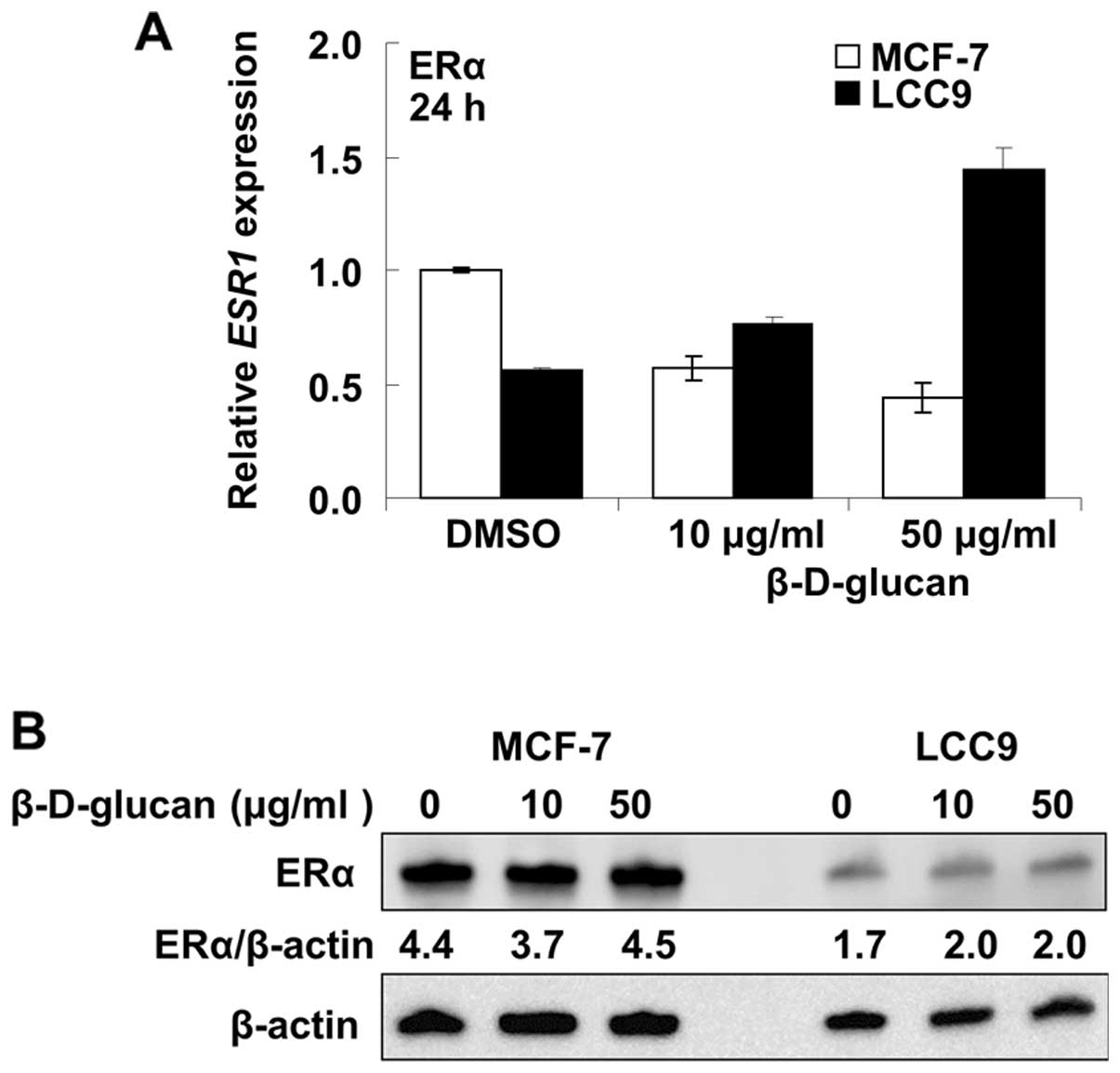
increased ERα mRNA expression in LCC9 cells (Fig. 9A). ERα protein expression was
unaffected (change <10%) by β-D-glucan treatment in MCF-7 cells
and increased ∼17% in LCC9 cells (Fig.
9B).
Discussion
Multiple mechanisms contribute to acquired endocrine
resistance in breast cancer and new therapies are needed to prevent
disease recurrence (2). Here we
report that DMSO-solubilized β-D-glucan inhibited the proliferation
of endocrine-sensitive MCF-7 and endocrine-resistant LCC9 and LY2
breast cancer cell lines, but did not inhibit the proliferation of
MDA-MB-231 TNBC cells. Notably the IC50 values for the
breast cancer cell lines were significantly lower than that for
MCF-10A immortalized breast epithelial cells. We also report that
β-D-glucan increases cell death in both MCF-7 and LCC9 cells with
more death in LCC9 versus MCF-7 cells at 1 μg/ml β-D-glucan.
We found that 10 μg/ml β-D-glucan increased the
BAX/BCL2 ratio in both MCF-7 and LCC9 cells, but that
increase was not sustained at 50 μg/ml β-D-glucan. Given the
decrease in NRF-1 transcription with β-D-glucan, it is possible
that β-D-glucan is inhibiting mitochondrial function due to
toxicity at the 50 μg/ml β-D-glucan concentration. Further
studies will be required to probe mechanisms of cell death in
response to β-D-glucan. We had hoped that β-D-glucan would
synergize with 4-OHT to inhibit breast cancer cell proliferation,
but it did not. These findings agree with the lack of effect of
β-D-glucan and TAM in DMBA-induced mammary tumors (17).
A previous study reported that water-soluble
β-glucan extract from the mycelia of Poria cocos inhibited
MCF-7 cell viability with an IC50 of 400 μg/ml
(7). Others reported no inhibition
of cell viability, measured by MTT assay, using Clitocybe
alexandri and Lepista inversa mushroom extracts
dissolved in boiling water, but when dissolved in methanol or
ethanol, MCF-7 cells were inhibited with an IC50 of
20–80 μg/ml, depending on which solvent and which mushroom
extract was tested (40). Our data
are in agreement with a lack of inhibitory activity of β-glucan
dissolved in water, and indicate that anti-proliferative activity
in MCF-7 cells β-glucan depends on solubilization in an organic
solvent. Future studies are needed to identify the active
components of the DMSO-solubilized β-D-glucan.
HEK-293 cells showed a non-monotonic or possible
‘U-shaped’ dose response to β-D-glucan in which there was a slight
inhibition at a low concentration (10 μg/ml), but increasing
concentrations resulted in stimulation of cell proliferation.
U-shaped or other non-monotonic dose-responses, referred to as
‘hormesis’, have been reported in studies of a variety of
chemotherapeutics, cytokines, rosiglitazone and other clinically
used drugs (41),
endocrine-disruptors (42), and
phytoestrogens indicating that ‘compounds in a cellular context,
can have opposite effects at different concentrations’ (43). Mechanistically, the mechanism for
the lack of linear response in HEK-293 cells is unknown, but we may
speculate that at lower β-D-glucan concentrations a higher affinity
antiproliferative response is triggered whereas at higher
concentrations, i.e., a lower affinity response, there is an
increase in cell proliferation which appears to reach
saturation.
A PCR array identified potential breast
cancer-associated genes regulated by β-D-glucan and selected genes
were verified by qRT-PCR. AR (NR3C4) expression was reduced by
β-D-glucan in MCF-7 cells. There is one report that AR
overexpression in MCF-7 cells reduced ERα and caused cells to
become tamoxifen-resistant (33).
Chromatin immunoprecipitation sequencing (ChIP-seq) and microarray
expression profiling has revealed significant cross-talk in gene
regulation between AR and ERα in ZR-75-1 human breast cancer cells
(44). The role of AR suppression
by β-D-glucan on the β-D-glucan inhibition of cell proliferation in
MCF-7 cells is unknown and may be investigated in future
studies.
β-D-glucan, E2 and 4-OHT increased
RASSF1 expression in MCF-7 and LCC9 cells. A reduction in
RASSF1 has been shown to correlate with tamoxifen resistance
(45) and the ability of
β-D-glucan to increase RASSF1 expression may correspond to
the observed inhibition of cell proliferation and increase in cell
death. Further studies will be required to determine the downstream
targets regulated by β-D-glucan-induced RASSF1. Likewise,
the increase in ERβ mRNA in LCC9 cells treated with β-D-glucan is
another logical follow-up for this study to further characterize
the mechanisms by which β-D-glucan inhibits breast cancer cell
proliferation in vitro.
Acknowledgements
This study was performed in the lab
of C.M.K. and was supported by National Institute of Health (NIH)
R01 DK053220 to C.M.K. Z.M.T.J. was supported by a fellowship from
the Iraq Science Fellowship Program for her time in the USA. L.M.L.
was supported by National Institute of Environmental Health
Sciences (NIEHS) T32 ES011564.
References
|
1.
|
Ring A and Dowsett M: Mechanisms of
tamoxifen resistance. Endocr Relat Cancer. 11:643–658. 2004.
View Article : Google Scholar
|
|
2.
|
Martin L-A, Pancholi S, Farmer I, Guest S,
Ribas R, Weigel M, Thornhill A, Ghazoui Z, A’Hern R, Evans D, Lane
H, Johnston S and Dowsett M: Effectiveness and molecular
interactions of the clinically active mTORC1 inhibitor everolimus
in combination with tamoxifen or letrozole in vitro and in vivo.
Breast Cancer Res. 14:R1322012. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Saraswat-Ohri S, Vashishta A, Vetvicka V,
Descroix K, Jamois F, Yvin JC and Ferrieres V: Biological
properties of (1→3)-beta-D-glucan-based synthetic oligosaccharides.
J Med Food. 14:369–376. 2011.
|
|
4.
|
Bohn JA and BeMiller JN: (1→3)-β-d-Glucans
as biological response modifiers: a review of structure-functional
activity relationships. Carbohydr Polym. 28:3–14. 1995.
|
|
5.
|
Kim HS, Hong JT, Kim Y and Han SB:
Stimulatory effect of beta-glucans on immune cells. Immune Netw.
11:191–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Chan G, Chan W and Sze D: The effects of
beta-glucan on human immune and cancer cells. J Hematol Oncol.
2:252009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Zhang M, Chiu LC, Cheung PC and Ooi VE:
Growth-inhibitory effects of a beta-glucan from the mycelium of
Poria cocos on human breast carcinoma MCF-7 cells: cell
cycle arrest and apoptosis induction. Oncol Rep. 15:637–643.
2006.
|
|
8.
|
Brunner N, Boulay V, Fojo A, Freter CE,
Lippman ME and Clarke R: Acquisition of hormone-independent growth
in MCF-7 cells is accompanied by increased expression of
estrogen-regulated genes but without detectable DNA amplifications.
Cancer Res. 53:283–290. 1993.
|
|
9.
|
Brunner N, Boysen B, Jirus S, Skaar TC,
Holst-Hansen C, Lippman J, Frandsen T, Spang-Thomsen M, Fuqua SA
and Clarke R: MCF7/LCC9: an antiestrogen-resistant MCF-7 variant in
which acquired resistance to the steroidal antiestrogen ICI 182,780
confers an early cross-resistance to the nonsteroidal antiestrogen
tamoxifen. Cancer Res. 57:3486–3493. 1997.
|
|
10.
|
Bronzert DA, Greene GL and Lippman ME:
Selection and characterization of a breast cancer cell line
resistant to the anti-estrogen LY 117018. Endocrinology.
117:1409–1417. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Davidson NE, Bronzert DA, Chambon P,
Gelmann EP and Lippman ME: Use of two MCF-7 cell variants to
evaluate the growth regulatory potential of estrogen-induced
products. Cancer Res. 46:1904–1908. 1986.PubMed/NCBI
|
|
12.
|
Chen J-Q and Russo J: ER[alpha]-negative
and triple negative breast cancer: molecular features and potential
therapeutic approaches. Biochim Biophys Acta. 1796:162–175.
2009.
|
|
13.
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Vetvicka V and Vetvickova J:
beta1,3-Glucan: silver bullet or hot air? Open Glycosci. 3:1–6.
2010.
|
|
15.
|
Wyrębska A, Gach K, Lewandowska U,
Szewczyk K, Hrabec E, Modranka J, Jakubowski R, Janecki T,
Szymański J and Janecka A: Anticancer activity of new synthetic
α-methylene-δ-lactones on two breast cancer cell lines. Basic Clin
Pharmacol Toxicol. 2013 Aug 19–2013.Epub ahead of print. View Article : Google Scholar
|
|
16.
|
Crawford AC, Riggins RB, Shajahan AN,
Zwart A and Clarke R: Co-inhibition of BCL-W and BCL2 restores
antiestrogen sensitivity through BECN1 and promotes an
autophagy-associated necrosis. PLoS One. 5:e86042010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Mansour A, Daba A, Baddour N, El-Saadani M
and Aleem E: Schizophyllan inhibits the development of mammary and
hepatic carcinomas induced by 7,12 dimethylbenz(alpha) anthracene
and decreases cell proliferation: comparison with tamoxifen. J
Cancer Res Clin Oncol. 138:1579–1596. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Scarpulla RC: Nuclear control of
respiratory chain expression by nuclear respiratory factors and
PGC-1-related coactivator. Ann NY Acad Sci. 1147:321–334. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Ivanova MM, Luken KH, Zimmer AS, Lenzo FL,
Smith RJ, Arteel MW, Kollenberg TJ, Mattingly KA and Klinge CM:
Tamoxifen increases nuclear respiratory factor 1 transcription by
activating estrogen receptor β and AP-1 recruitment to adjacent
promoter binding sites. FASEB J. 25:1402–1416. 2011.PubMed/NCBI
|
|
20.
|
Dammann R, Schagdarsurengin U, Seidel C,
Strunnikova M, Rastetter M, Baier K and Pfeifer GP: The tumor
suppressor RASSF1A in human carcinogenesis: an update. Histol
Histopathol. 20:645–663. 2005.PubMed/NCBI
|
|
21.
|
Shukla S, Mirza S, Sharma G, Parshad R,
Gupta SD and Ralhan R: Detection of RASSF1A and RARbeta
hypermethylation in serum DNA from breast cancer patients.
Epigenetics. 1:88–93. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Lin S, Xia W, Wang J, Kwong K, Spohn B,
Wen Y, Pestell R and Hung M: Beta-catenin, a novel prognostic
marker for breast cancer: its roles in cyclin D1 expression and
cancer progression. Proc Natl Acad Sci USA. 97:4262–4266. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Howe LR and Brown AM: Wnt signaling and
breast cancer. Cancer Biol Ther. 3:36–41. 2004. View Article : Google Scholar
|
|
24.
|
Paul S and Dey A: Wnt signaling and cancer
development: therapeutic implication. Neoplasma. 55:165–176.
2008.PubMed/NCBI
|
|
25.
|
Espelund U, Cold S, Frystyk J, Orskov H
and Flyvbjerg A: Elevated free IGF2 levels in localized,
early-stage breast cancer in women. Eur J Endocrinol. 159:595–601.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Duggan C, Wang CY, Neuhouser ML, Xiao L,
Smith AW, Reding KW, Baumgartner RN, Baumgartner KB, Bernstein L,
Ballard-Barbash R and McTiernan A: Associations of insulin-like
growth factor and insulin-like growth factor binding protein-3 with
mortality in women with breast cancer. Int J Cancer. 132:1191–1200.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Martin JL, Lin MZ, McGowan EM and Baxter
RC: Potentiation of growth factor signaling by insulin-like growth
factor-binding protein-3 in breast epithelial cells requires
sphingosine kinase activity. J Biol Chem. 284:25542–25552. 2009.
View Article : Google Scholar
|
|
28.
|
McIntosh J, Dennison G, Holly JMP, Jarrett
C, Frankow A, Foulstone EJ, Winters ZE and Perks CM: IGFBP-3 can
either inhibit or enhance EGF-mediated growth of breast epithelial
cells dependent upon the presence of fibronectin. J Biol Chem.
285:38788–38800. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Levenson AS, Svoboda KM, Pease KM, Kaiser
SA, Chen B, Simons LA, Jovanovic BD, Dyck PA and Jordan VC: Gene
expression profiles with activation of the estrogen receptor
alpha-selective estrogen receptor modulator complex in breast
cancer cells expressing wild-type estrogen receptor. Cancer Res.
62:4419–4426. 2002.
|
|
30.
|
Wang D-Y, Fulthorpe R, Liss SN and Edwards
EA: Identification of estrogen-responsive genes by complementary
deoxyribonucleic acid microarray and characterization of a novel
early estrogen-induced gene: EEIG1. Mol Endocrinol. 18:402–411.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Cook KL, Shajahan AN, Wärri A, Jin L,
Hilakivi-Clarke LA and Clarke R: Glucose-regulated protein 78
controls cross-talk between apoptosis and autophagy to determine
antiestrogen responsiveness. Cancer Res. 72:3337–3349. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Li C, Harada A and Oh Y: IGFBP-3
sensitizes antiestrogen-resistant breast cancer cells through
interaction with GRP78. Cancer Lett. 325:200–206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
De Amicis F, Thirugnansampanthan J, Cui Y,
Selever J, Beyer A, Parra I, Weigel NL, Herynk MH, Tsimelzon A,
Lewis MT, Chamness GC, Hilsenbeck SG, Ando S and Fuqua SA: Androgen
receptor overexpression induces tamoxifen resistance in human
breast cancer cells. Breast Cancer Res Treat. 121:1–11.
2010.PubMed/NCBI
|
|
34.
|
Hiscox S, Jiang WG, Obermeier K, Taylor K,
Morgan L, Burmi R, Barrow D and Nicholson RI: Tamoxifen resistance
in MCF7 cells promotes EMT-like behaviour and involves modulation
of beta-catenin phosphorylation. Int J Cancer. 118:290–301. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Fodde R and Brabletz T: Wnt/β-catenin
signaling in cancer stemness and malignant behavior. Curr Opin Cell
Biol. 19:150–158. 2007.
|
|
36.
|
Mattingly KA, Ivanova MM, Riggs KA,
Wickramasinghe NS, Barch MJ and Klinge CM: Estradiol stimulates
transcription of Nuclear Respiratory Factor-1 and increases
mitochondrial biogenesis. Mol Endocrinol. 22:609–622. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Maxwell P and van den Berg HW: Changes in
the secretion of insulin-like growth factor binding proteins -2 and
-4 associated with the development of tamoxifen resistance and
estrogen independence in human breast cancer cell lines. Cancer
Lett. 139:121–127. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Thomas C and Gustafsson J-Å: The different
roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer.
11:597–608. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Pfeifer GP and Dammann R: Methylation of
the tumor suppressor gene RASSF1A in human tumors. Biochemistry.
70:576–583. 2005.PubMed/NCBI
|
|
40.
|
Vaz JA, Heleno SA, Martins A, Almeida GM,
Vasconcelos MH and Ferreira ICFR: Wild mushrooms Clitocybe
alexandri and Lepista inversa: in vitro antioxidant
activity and growth inhibition of human tumour cell lines. Food
Chem Toxicol. 48:2881–2884. 2010.PubMed/NCBI
|
|
41.
|
Doñate F, Parry GC, Shaked Y, Hensley H,
Guan X, Beck I, Tel-Tsur Z, Plunkett ML, Manuia M, Shaw DE, Kerbel
RS and Mazar AP: Pharmacology of the novel antiangiogenic peptide
ATN-161 (Ac-PHSCN-NH2): observation of a U-shaped dose-response
curve in several preclinical models of angiogenesis and tumor
growth. Clin Cancer Res. 14:2137–2144. 2008.PubMed/NCBI
|
|
42.
|
Vandenberg LN, Colborn T, Hayes TB,
Heindel JJ, Jacobs DR, Lee D-H, Shioda T, Soto AM, vom Saal FS,
Welshons WV, Zoeller RT and Myers JP: Hormones and
endocrine-disrupting chemicals: low-dose effects and nonmonotonic
dose responses. Endocr Rev. 33:378–455. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Almstrup K, Fernandez MF, Petersen JH,
Olea N, Skakkebaek NE and Leffers H: Dual effects of phytoestrogens
result in u-shaped dose-response curves. Environ Health Perspect.
110:743–748. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Need EF, Selth LA, Harris TJ, Birrell SN,
Tilley WD and Buchanan G: Research resource: interplay between the
genomic and transcriptional networks of androgen receptor and
estrogen receptor α in luminal breast cancer cells. Mol Endocrinol.
26:1941–1952. 2012.PubMed/NCBI
|
|
45.
|
Fiegl H, Millinger S, Mueller-Holzner E,
Marth C, Ensinger C, Berger A, Klocker H, Goebel G and
Widschwendter M: Circulating tumor-specific DNA: a marker for
monitoring efficacy of adjuvant therapy in cancer patients. Cancer
Res. 65:1141–1145. 2005. View Article : Google Scholar : PubMed/NCBI
|