Introduction
Colorectal cancer (CRC) is one of the most common
human malignancies worldwide. Despite recent advances in treatment
with chemotherapy and radiation therapy, CRC remains a major cause
of cancer death (1). Thus, there
is a crucial need to explore novel cancer-related genes that may
serve as diagnostic markers and molecular targets in CRC
therapy.
A hypoxic microenvironment is associated with many
solid tumors, including breast cancer (2), prostate cancer (3,4),
brain tumor (5), malignant
melanomas (6,7), lung cancer (8), liver cancer (9,10),
ovarian cancer (11,12), and CRC (13). Furthermore, intratumoral hypoxia
affects every major aspect of cancer biology, including cell
invasion, metastasis, and determination of cell death (14).
Many molecules in the hypoxia-response pathway are
good candidates for therapeutic targeting (15–17).
The anti-VEGF antibody, bevacizumab, is used clinically for
treating several human cancers (18); this supports the notion that
hypoxia-induced genes are clinically-relevant therapeutic targets.
Therefore, the identification of novel hypoxia-inducible genes
holds great potential for the development of additional cancer
therapies.
We previously reported that liver metastatic tissue
derived from patients with CRC was a useful in vivo model
for identifying novel hypoxia-inducible genes and prognostic
markers. These markers included the mRNA expression levels of
Jumonji domain containing 1A (JMJD1A), adrenomedullin (ADM),
Ephrin-A1 (EFNA1), and procollagen-lysine, 2-oxoglutarate
5-dioxygenase 2 (PLOD2) (19–22).
In those experiments, we also found that the mRNA expression of
secretoglobin, family 2A, member 1 (SCGB2A1) was highly induced in
hypoxic regions of metastasized liver (fold-change, 1.57). Thus, we
hypothesized that SCGB2A1 expression may be a novel prognostic
factor in patients with CRC.
The aim of the present study was to examine the
prognostic impact of SCGB2A1 and the biological significance of
SCGB2A1 in CRC. We reported previously (23–25)
that SCGB2A1 was a useful marker in several cancers for the
molecular detection of minimal residual disease in lymph nodes, but
the mechanism remains unknown (26–29).
Here, we found that SCGB2A1 was an independent
prognostic factor, and expression of SCGB2A1 promoted both
chemoresistance and radioresistance.
Cancer cell stemness is a primary underlying
mechanism that contributes to resistance to chemotherapy and
radiation therapy (30,31). Our data indicated that cancer
stemness was enhanced by forced expression of SCGB2A1. Thus, our
findings may provide clues for the development of a novel
anticancer therapy.
Materials and methods
Cell lines and culture conditions
Human CRC-derived cell lines, DLD1, SW480, and LoVo,
were obtained from the American Type Culture Collection. Cells were
grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented
with 10% fetal bovine serum, 100 U/ml penicillin and 100
μg/ml streptomycin at 37°C in a humidified incubator with 5%
CO2.
Clinical samples and microarray
analysis
For microarray analysis, we prospectively collected
1,978 primary CRC samples from consecutive patients who had
curative operations in 2003 to 2006 from Osaka University Hospital
and its nine associated hospitals (32). Tumor samples were consecutively
collected from a total of 222 CRC patients for microarray analysis.
Microarray analysis was carried out as described previously
(33) using an oligonucleotide
microarray covering 30,000 human probes (AceGene; DNA Chip
Research, Inc. and Hitachi Software Engineering Co. Ltd.). The mean
follow-up times were 42.9±28.9 months for patients with
disease-free survival (DFS) and 56.7±20.4 months for all surviving
patients [overall survival (OS)]. Table I shows the clinicopathological
features of patients from each institute, including gender, tumor
location, extent of wall invasion, lymph node metastasis,
histologic grade, Dukes’ stage, and vessel invasion. In this study,
no patient received preoperative chemotherapy or irradiation. After
surgery, patients with Dukes’ stage C/D tumors were generally
treated with 5-fluorouracil (5-FU)-based chemotherapy.
 | Table I.Relationship between SCGB2A1
expression and clinicopathological factors in patients with
colorectal cancer. |
Table I.
Relationship between SCGB2A1
expression and clinicopathological factors in patients with
colorectal cancer.
| Total n=222 | SCGB2A1 mRNA
expression | P-value |
|---|
|
|---|
| Low (n=111) | High (n=111) |
|---|
| Age (years) | 66.2±10.0 | 65.8±9.7 | 66.7±10.3 | 0.61 |
| Gender | | | | |
| Male/female | 133/89 | 62/49 | 71/40 | 0.27 |
| Tumor location | | | | |
| Colon/rectum | 141/81 | 77/34 | 64/47 | 0.09 |
| Depth of
invasiona | | | | |
| mp/ss | 26/196 | 8/103 | 18/93 | 0.06 |
| Lymph node
metastasis | | | | |
|
Present/absent | 109/113 | 52/59 | 57/54 | 0.59 |
| Histological
grade | | | | |
| Well
differentiated/others | 53/169 | 28 /83 | 25/86 | 0.75 |
| Dukes’ stage | | | | |
| AB/CD | 108/114 | 58/53 | 50/61 | 0.35 |
| Vessel
invasion | | | | |
|
Present/absent | 184/38 | 89/22 | 95/16 | 0.37 |
Transfection of vector
DLD1, SW480 and LoVo were transfected with an
SCGB2A1 expression vector with the FuGENE® 6
transfection reagent (Promega, USA), according to the
manufacturer’s protocol. Control cells were transfected with the
same method, but with an empty control vector.
Proliferation assay
Overexpressing SCGB2A1 cell lines and control cell
lines were seeded in 96-well plates (2,000 cells/well) and grown at
37°C with 5% CO2. After 24 and 72 h, we assayed cell
viability with a Cell Counting Kit-8 (Dojindo, Japan), according to
themanufacturer’s instructions. After 2-h pre-incubations in the
assay solution, the viable cell number in each well was determined
from the absorbance at 450 nm (OD 450), measured with a microplate
reader (Bio-Rad Model 680 XR, USA).
Chemosensitivity assay
We tested cell sensitivity to 5-FU and oxaliplatin
(L-OHP), which are generally used in chemo-therapy for CRC. We
seeded 2,000 cells/well in 96-well plates and incubated at 37°C
with 5% CO2. After 24 h, the culture medium was replaced
with fresh medium in the presence of 5-FU or L-OHP at predetermined
IC50 concentrations. After 24 and 48 h of treatment with
chemotherapy, we assayed cell viability with same method used in
the proliferation assay.
Radiation sensitivity assay
To measure radiation sensitivity, each cell line was
seeded at 2,000 cells/well into 96-well plates and incubated at
37°C with 5% CO2. After 24 h, a 137Cs Gamma
Cell 40 Exactor (MDS Nordion, Canada) was used to irradiate DLD1
and SW480 cells at 8 Gy and LoVo cells at 6 Gy. After 24 and 72 h
of treatment with radiation therapy, we assayed cell viability with
same method used in the proliferation assay.
Sphere formation assay
The sphere formation assay was performed essentially
as described previously (34). In
brief, single cells were plated in 96-well ultralow attachment
plates (Corning Inc., USA) at a density of 100 cells/well and grown
in tumorspheric culture medium (Dulbecco’s modified Eagle’s medium,
DMEM/F-12), supplemented with 20 ng/ml human platelet growth factor
(Sigma-Aldrich, USA), 20 ng/ml epidermal growth factor (Invitrogen,
USA) and 1% antibiotic-antimycotic solution (Invitrogen) at 37°C in
a humidified atmosphere of 95% air and 5% CO2. We
counted the number of spheres ≥100 μm in all wells and
evaluated differences in the average number/well.
Statistical analysis
Statistical analysis was performed with the StatView
5.0 program (Abacus Concepts, Inc., USA). The Kaplan-Meier method
was used to examine DFS and OS, and the log-rank test was used to
determine statistical significance. A Cox proportional hazard model
was used to assess the risk ratio with simultaneous contributions
from several covariates. Statistical analysis was performed with
the Student’s t-test or Fisher’s exact test for categorical data
and with the Mann-Whitney U test for non-parametric data.
Correlation significance was assessed with Pearson’s correlation
coefficient-test. Values of p<0.05 denoted a statistically
significant difference.
Results
Relationship between SCGB2A1 expression
and clinicopathological factors
Patients were divided into two groups (high or low)
according to whether the SCGB1A1 mRNA expression level was above or
below the median SCGB2A1 expression value. The relationships
between SCGB2A1 mRNA expression and clinicopathological factors
were examined (Table I). There
were no significant differences between groups in age, gender,
tumor location, depth of tumor invasion, lymph node metastasis,
histological grade, Dukes’ stage or vessel invasion.
Impact of SCGB2A1 mRNA expression on
disease-free survival
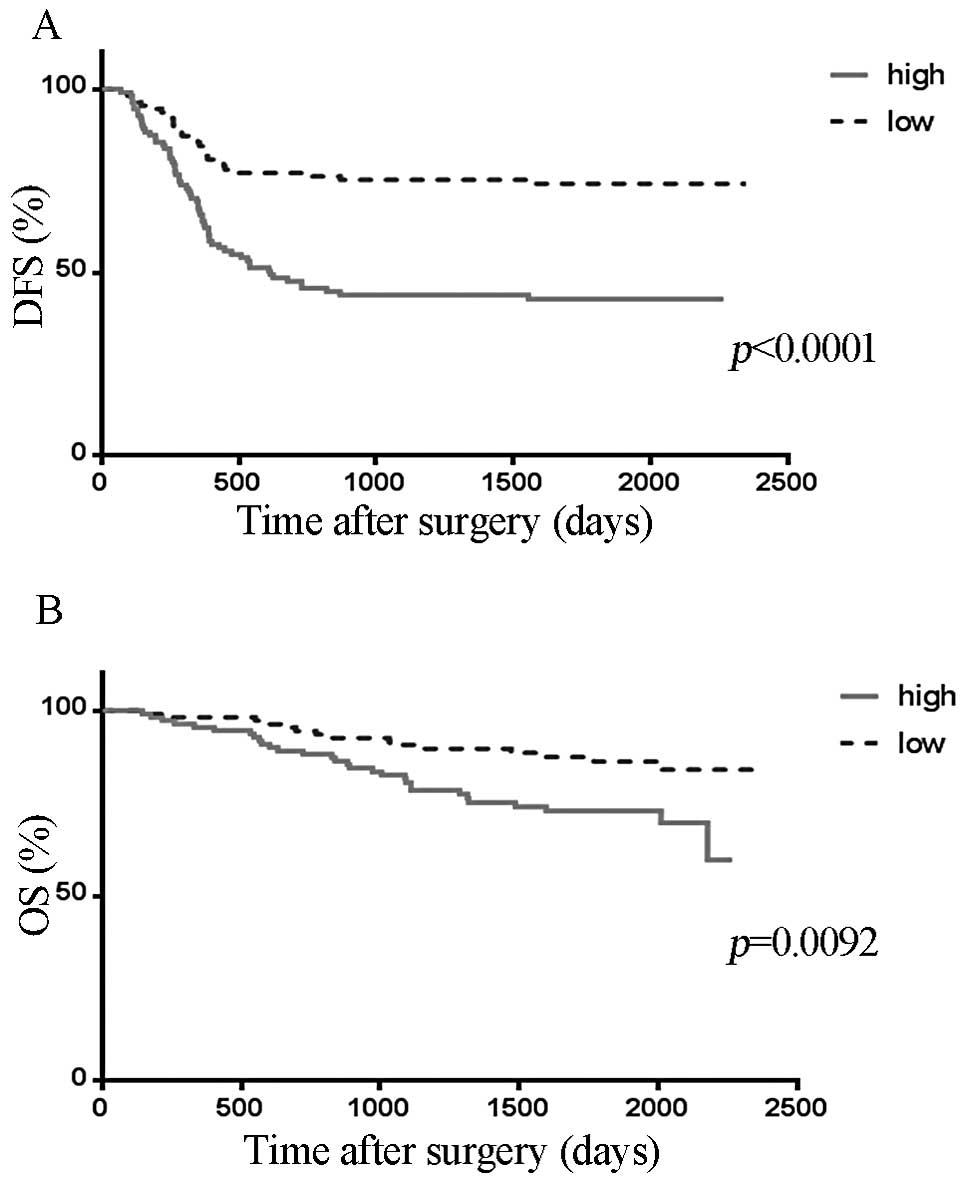
The DFS curves were stratified according to SCGB2A1
expression levels. The DFS was significantly longer in the low
SCGB2A1 expression group compared to that in the high SCGB2A1
expression group (p<0.0001; Fig.
1A).
In a univariate analysis, the level of SCGB2A1 mRNA
expression and various other clinicopathological parameters were
evaluated for their impact on DFS (Table II). The DFS was significantly
associated with the depth of invasion (p=0.0082), lymph node
metastasis (p<0.0001), histological grade (p=0.0193), vessel
invasion (p=0.0001) and the expression of SCGB2A1
(p<0.0001).
 | Table II.Analysis of associations between
disease-free survival and clinicopathological factors, including
SCGB2A1 mRNA expression. |
Table II.
Analysis of associations between
disease-free survival and clinicopathological factors, including
SCGB2A1 mRNA expression.
| Variable | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|---|
| P-value | Relative risk | 95 % CI | P-value |
|---|
| Tumor location
(colon/rectum) | 0.0655 | | | |
| Depth of
invasiona
(mp/ss) | 0.0082 | 0.327 | 0.118–0.909 | 0.0321 |
| Lymph node
metastasis (+/−) | <0.0001 | 1.592 | 1.025–2.473 | 0.0386 |
| Histological grade
(well differentiated/others) | 0.0193 | 1.276 | 0.711–2.293 | 0.4141 |
| Dukes’ stage
(AB/CD) | <0.0001 | | | |
| Vessel invasion
(yes/no) | 0.0001 | 3.207 | 1.121–9.177 | 0.0298 |
| SCGB2A1
(high/low) | <0.0001 | 2.784 | 1.775–4.369 | <0.0001 |
A multivariate Cox regression analysis demonstrated
that the mRNA expression of SCGB2A1 was a significant prognostic
factor for DFS (p<0.0001; Table
II). Among the other covariates, the depth of invasion, lymph
node metastasis and vessel invasion were significant prognostic
factors (p=0.0321, 0.0386 and 0.0298, respectively; Table II).
Impact of SCGB2A1 expression on overall
survival
The OS curves were stratified according to SCGB2A1
expression levels. The OS was significantly longer in the low
SCGB2A1 expression group compared to that in the high SCGB2A1
expression group (p=0.0092; Fig.
1B).
In a univariate analysis, mRNA expression of SCGB2A1
and various clinicopathological parameters were evaluated for their
impact on OS. The OS was significantly associated with lymph node
metastasis (p<0.0001), vessel invasion (p=0.0201) and the
expression of SCGB2A1 (p=0.0092) (Table III).
 | Table III.Analysis of associations between
overall survival and clinicopathological factors, including SCGB2A1
mRNA expression. |
Table III.
Analysis of associations between
overall survival and clinicopathological factors, including SCGB2A1
mRNA expression.
| Variable | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|---|
| P-value | Relative risk | 95 % CI | P-value |
|---|
| Tumor location
(colon/rectum) | 0.8824 | | | |
| Depth of
invasiona
(mp/ss) | 0.2763 | | | |
| Lymph node
metastasis (+/−) | <0.0001 | 4.065 | 1.911–8.643 | 0.0003 |
| Histological grade
(well differentiated/others) | 0.0632 | | | |
| Dukes’ stage
(AB/CD) | <0.0001 | | | |
| Vessel invasion
(yes/no) | 0.0201 | 2.192 | 0.506–9.493 | 0.2939 |
| SCGB2A1
(high/low) | 0.0092 | 1.988 | 1.067–3.704 | 0.0305 |
A multivariate Cox regression analysis demonstrated
that the mRNA expression of SCGB2A1 was a significant prognostic
factor for OS (p=0.0305; Table
III). Among the other covariates, only lymph node metastasis
was a significant prognostic factor (p=0.0003; Table III).
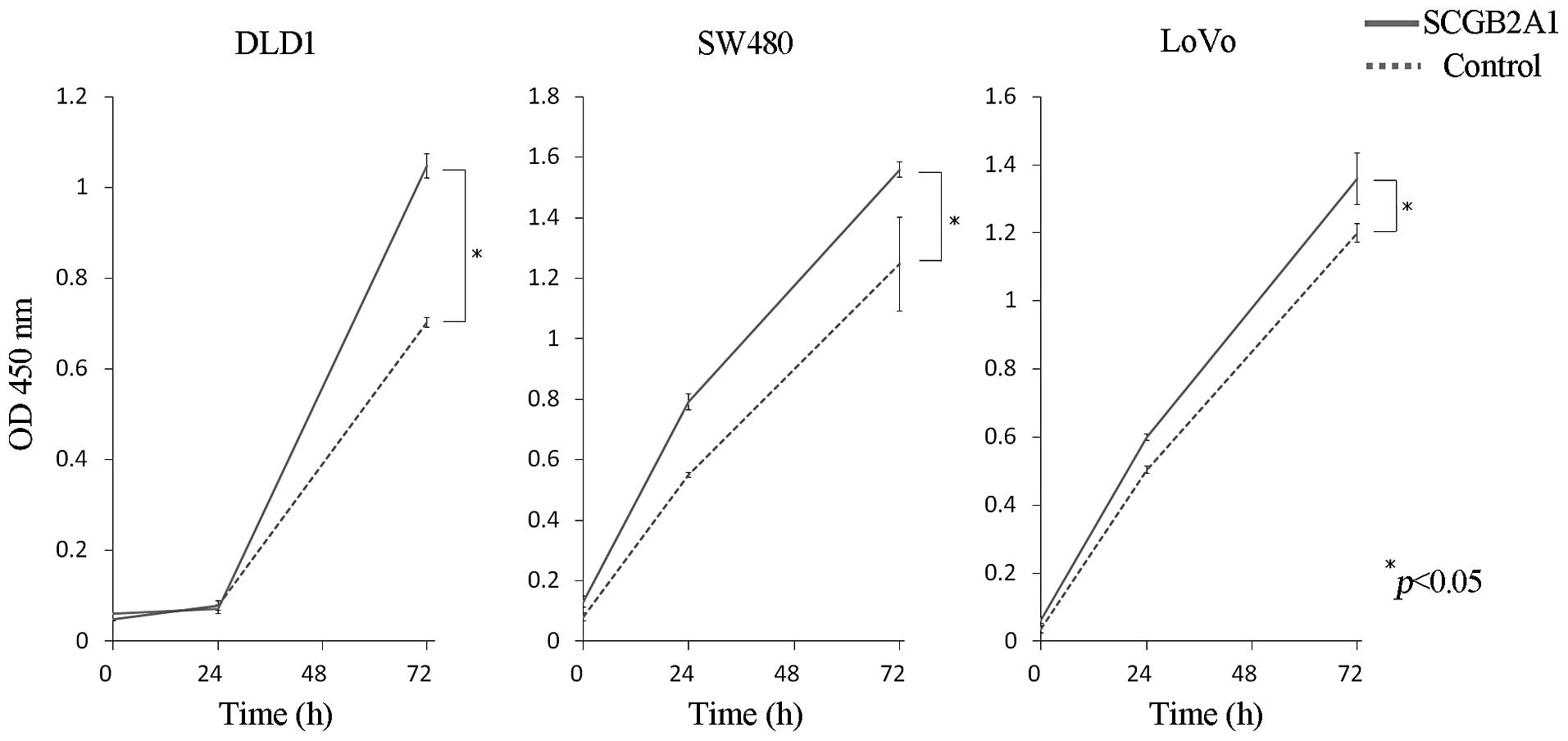
Proliferation assay
To explore SCGB2A1 gene function, we first
transfected a plasmid that encoded SCGB2A1 or the empty control
vector into CRC-derived cell lines, DLD1, SW480 and LoVo. Our
results showed that upregulation of SCGB2A1 elicited significant
cell proliferation compared to cells transfected with the empty
control vector at 72 h (p<0.05) (Fig. 2).
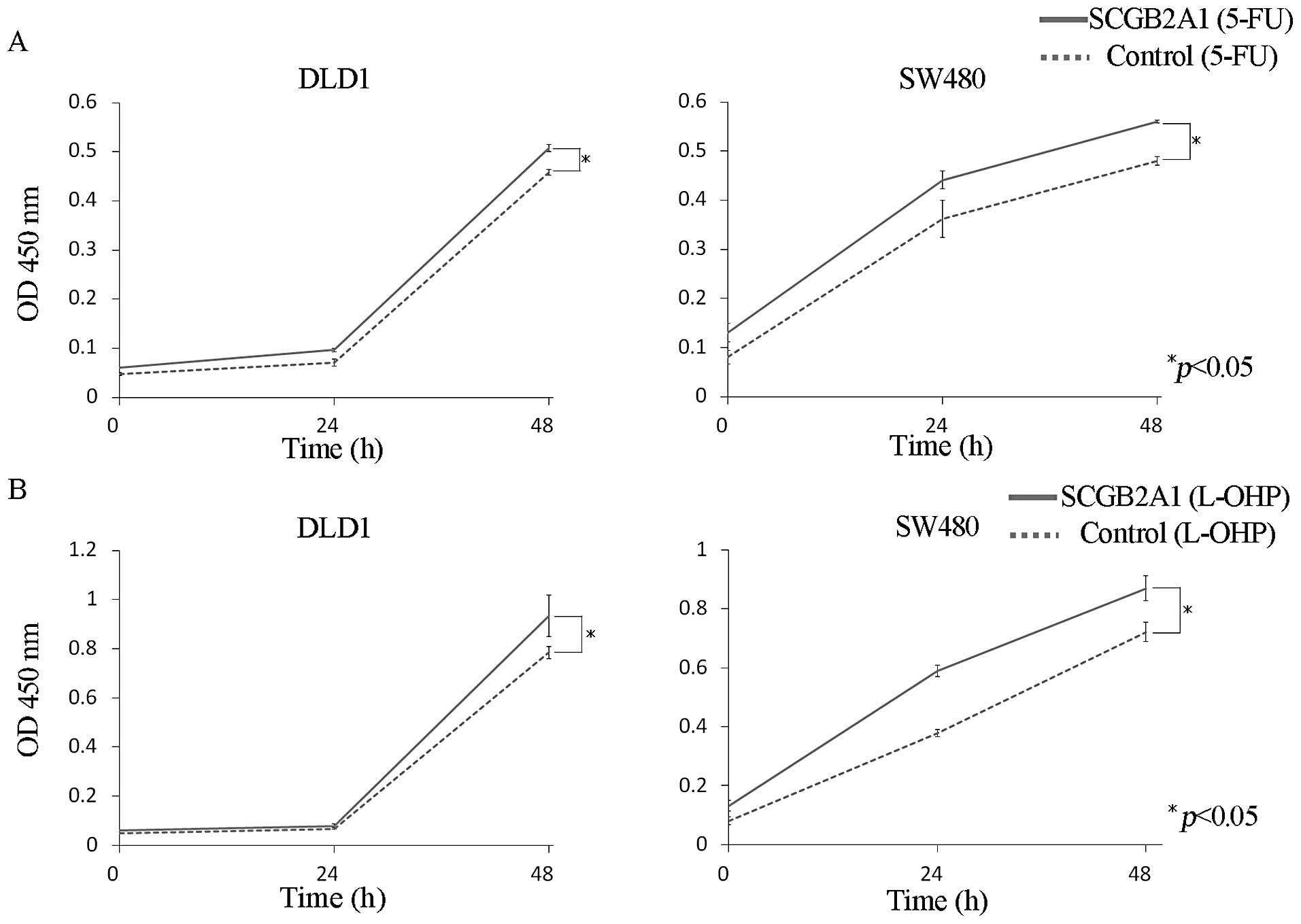
Chemoresistance and radioresistance
conferred by SCGB2A1 in CRC cells
CRC-derived cells (DLD1 and SW480) transfected with
SCGB2A1 exhibited much greater resistance to the anticancer drugs,
5-FU and L-OHP, than cells transfected with empty control vector at
48 h (p<0.05) (Fig. 3).
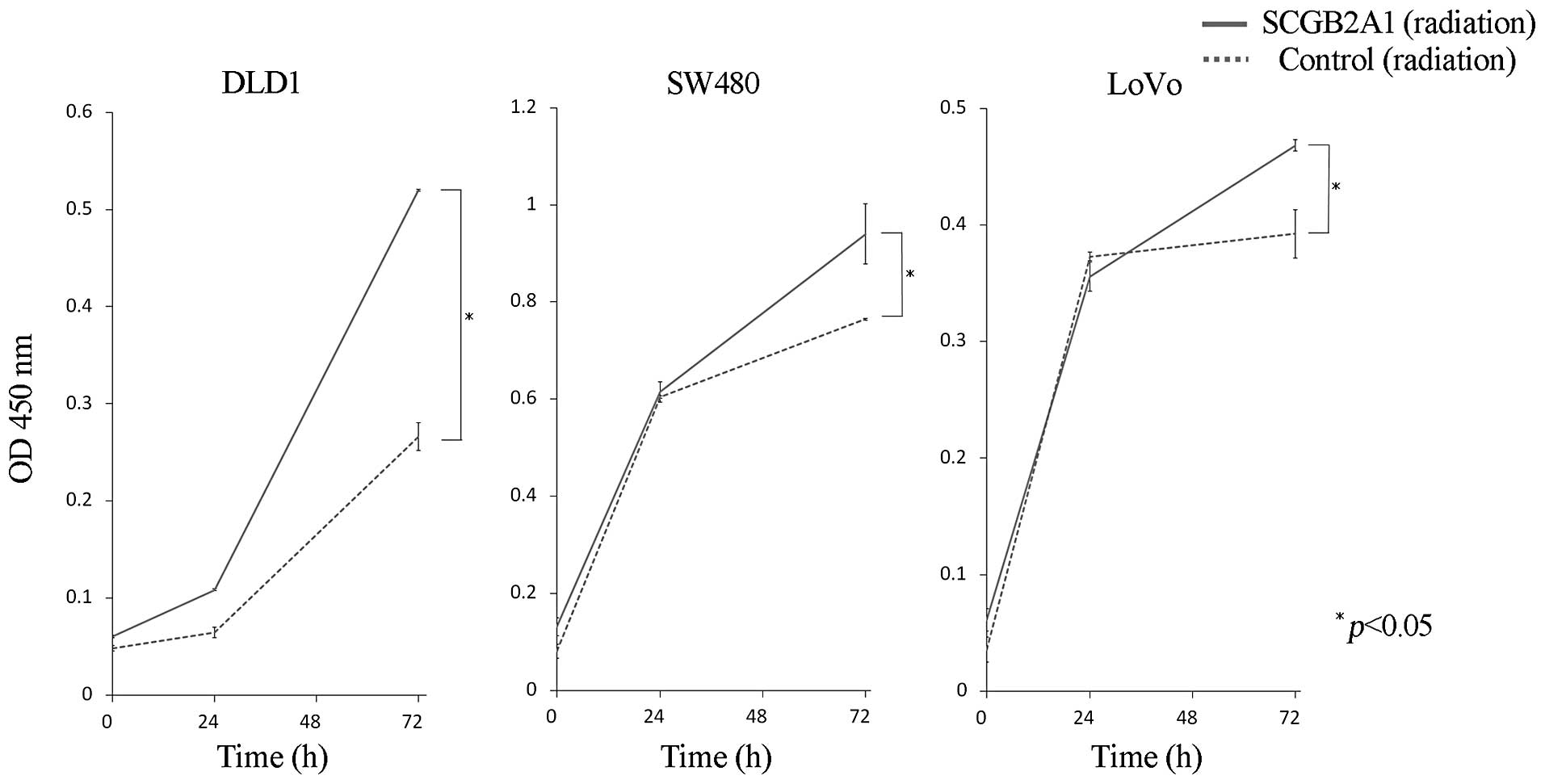
Likewise, upregulation of SCGB2A1 in DLD1, SW480 and LoVo cells
conferred stronger radioresistance compared to cells transfected
with empty control vector at 72 h (p<0.05) (Fig. 4).
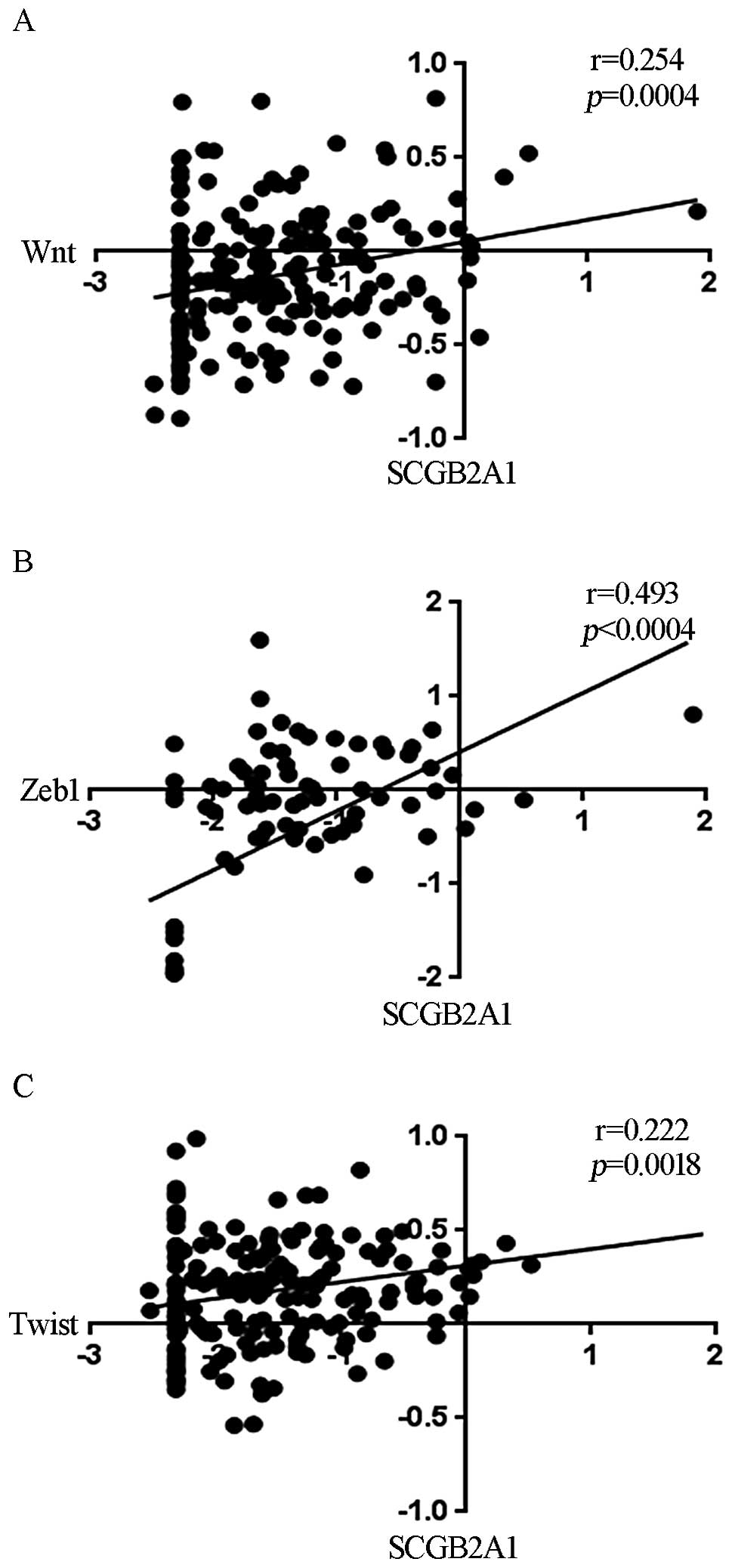
Correlation of SCGB2A1 with cancer
stemness-related genes and sphere formation
For elucidation of the chemoresistance and
radioresistance mechanism, we analyzed the microarray data to
identify genes that were correlated with SCGB2A1 expression.
We found that SCGB2A1 was correlated with the expression of
wingless and INT-1 (Wnt; Fig. 5A), zinc finger E-box binding
homeobox 1 (Zeb1; Fig.
5B) and Twist (Fig.
5C).
Recently, the development of cancer stem cells
(CSCs) was proposed to be one of the major mechanisms underlying
treatment resistance. One study demonstrated a relationship between
colon CSCs and Wnt activity (35).
Another study showed that Zeb1 induction was associated with
the epithelialmesenchymal transition, and it was also related to
CSCs (36).
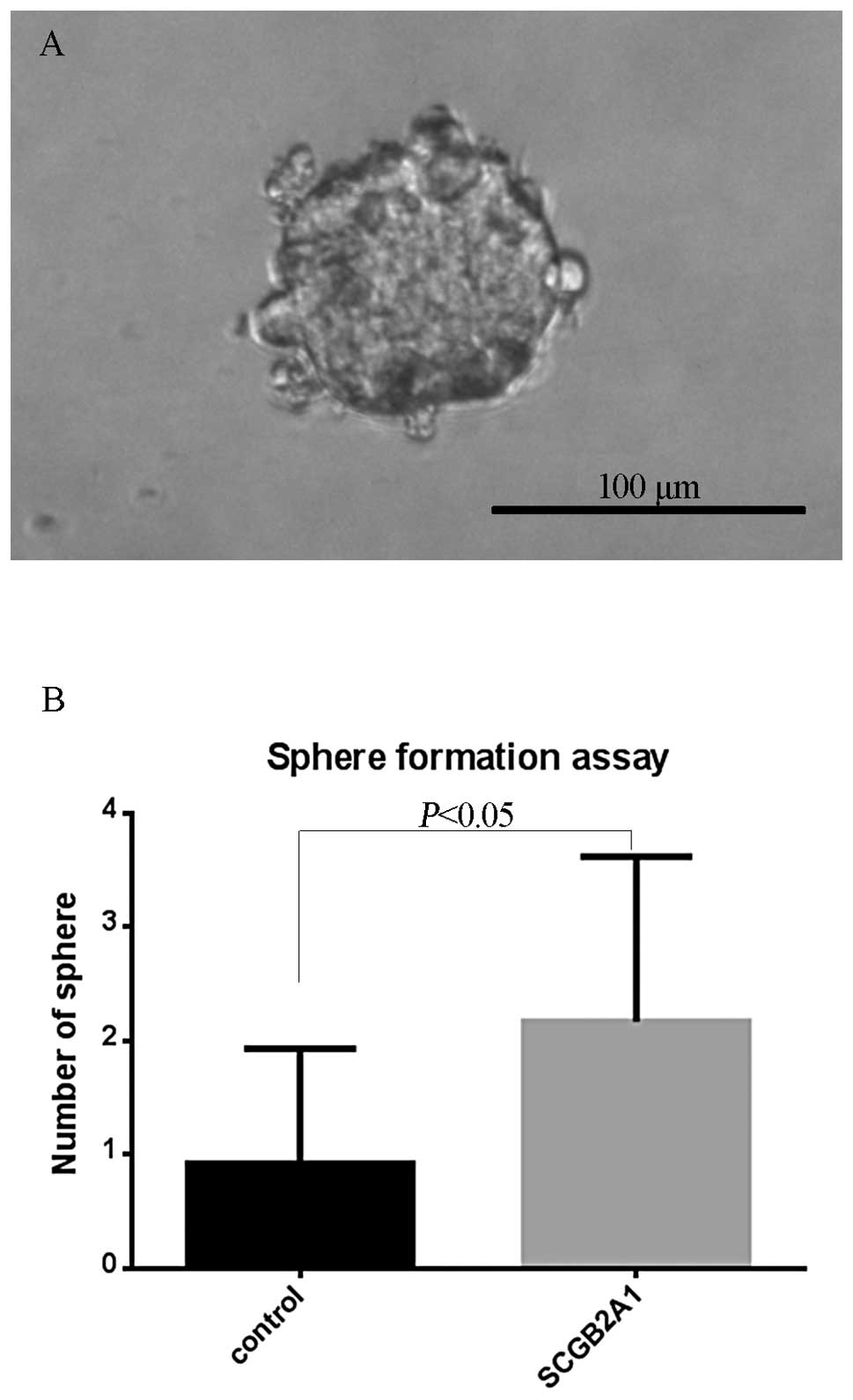
We conducted a sphere formation assay to evaluate
whether SCGB2A1 overexpressing cells acquired cancer cell stemness.
The appearance of spheres, which form after several weeks, is
considered indicative of the ability to self-renew. This phenomenon
would be consistent with development of a CSC phenotype (34). After 3 weeks in culture, sphere
formation was observed in the SW480 cell line, but not the other
cell lines (Fig. 6A). Also, we
found that upregulation of SCGB2A1 could generate many more
spheres compared to baseline SCGB2A1 levels in cells
transfected with the empty control vector (p<0.0001) (Fig. 6B).
Discussion
The SCGB2A1 (mammaglobin B) gene
encodes a small secreted protein of the uteroglobin superfamily.
This super-family includes nine human secretoglobins that are
localized on chromosome 11q12.2 (37). SCGB2A1 was first isolated by
Becker et al in 1998 (38).
SCGB2A1 is considered a candidate marker for the
molecular detection of several minimal cancers in lymph nodes
(23–29) and for the diagnosis of occult tumor
cells in effusions from patients with various malignancies,
including gynecological cancers (39). However, the biological function of
SCGB2A1 has not yet been clarified in CRC.
The present report was the first to show that
SCGB2A1 could be an important prognostic factor for patients with
CRC. We showed that enhanced expression of SCGB2A1 in CRC cells
might confer the property of treatment resistance.
We previously established a method for finding
clinically important, hypoxia-inducible genes from samples of liver
that had metastasized from CRC (19–22).
In a chronically hypoxic environment, cancer cells undergo genetic
and adaptive changes that allow them to become more clinically
aggressive, and they develop resistance to irradiation and
chemotherapy (15,16,40).
An efficient therapeutic strategy for combating those cell types is
essential for overcoming cancer. However, it is difficult to
identify important hypoxia-inducible genes that are related to
clinical cancer biology in vitro, because cancer cells
typically exist in chronically hypoxic conditions in vivo.
Therefore, cancer cells develop complex interactions that affect
several pathways.
The most important finding in this study was the
reciprocal relationship between SCGB2A1 expression and treatment
resistance. We hypothesized that treatment resistance was caused by
the development of cancer stemness, because our data showed that
the expression of SCGB2A1 was correlated with the expression
of Wnt, Zeb1 and Twist. The sphere formation assay is
commonly used to detect CSCs in vitro. As we expected,
SCGB2A1-expressing cells showed more abundant sphere formation than
control cells. This cancer-stemness property may partly explain why
SCGB2A1 expression was associated with chemoresistance and
radioresistance in CRC. These findings suggested that
SCGB2A1-expressing cells have enhanced malignancy potential. Our
data also suggested that SCGB2A1 may represent a novel therapeutic
target.
In conclusion, we showed that SCGB2A1 represents a
novel prognostic factor for CRC. SCGB2A1 correlated with
chemoresistance, radioresistance, and cancer cell stemness.
Acknowledgements
This study was supported by a
Grant-in-Aid for Cancer Research from the Ministry of Education,
Science, Sports and Culture Technology, Japan (grant 21390360).
References
|
1.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2.
|
Vaupel P, Briest S and Hockel M: Hypoxia
in breast cancer: pathogenesis, characterization and
biological/therapeutic implications. Wien Med Wochenschr.
152:334–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Sooriakumaran P and Kaba R: Angiogenesis
and the tumour hypoxia response in prostate cancer: a review. Int J
Surg. 3:61–67. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Higgins LH, Withers HG, Garbens A, et al:
Hypoxia and the metabolic phenotype of prostate cancer cells.
Biochim Biophys Acta. 1787:1433–1443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Jensen RL: Brain tumor hypoxia:
tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a
therapeutic target. J Neurooncol. 92:317–335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
O’Connell MP, Marchbank K, Webster MR, et
al: Hypoxia induces phenotypic plasticity and therapy resistance in
melanoma via the tyrosine kinase receptors ROR1 and ROR2. Cancer
Discov. Oct 8–2013.(Epub ahead of print). View Article : Google Scholar : 2013.
|
|
7.
|
Zeng W, Yang D, Long T, et al: CD147
promotes melanoma progression through hypoxia-induced MMP2
activation. Curr Mol Med. Oct 3–2013.(Epub ahead of print).
|
|
8.
|
Lee GW, Go SI, Cho YJ, et al:
Hypoxia-inducible factor-1alpha and excision repair
cross-complementing 1 in patients with small cell lung cancer who
received front-line platinum-based chemotherapy: a retrospective
study. J Thorac Oncol. 7:528–534. 2012. View Article : Google Scholar
|
|
9.
|
Zhang L, Huang G, Li X, et al: Hypoxia
induces epithelial-mesenchymal transition via activation of SNAI1
by hypoxia-inducible factor -1alpha in hepatocellular carcinoma.
BMC Cancer. 13:1082013. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Liu Y, Zhang JB, Qin Y, et al: PROX1
promotes hepatocellular carcinoma metastasis by way of
up-regulating hypoxia-inducible factor 1alpha expression and
protein stability. Hepatology. 58:692–705. 2013. View Article : Google Scholar
|
|
11.
|
Selvendiran K, Bratasz A, Kuppusamy ML,
Tazi MF, Rivera BK and Kuppusamy P: Hypoxia induces chemoresistance
in ovarian cancer cells by activation of signal transducer and
activator of transcription 3. Int J Cancer. 125:2198–2204. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Liang D, Ma Y, Liu J, et al: The hypoxic
microenvironment upgrades stem-like properties of ovarian cancer
cells. BMC Cancer. 12:2012012. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Chang LH, Chen CH, Huang DY, Pai HC, Pan
SL and Teng CM: Thrombin induces expression of twist and cell
motility via the hypoxia-inducible factor-1alpha translational
pathway in colorectal cancer cells. J Cell Physiol. 226:1060–1068.
2011. View Article : Google Scholar
|
|
14.
|
Semenza GL: Hypoxia and cancer. Cancer
Metastasis Rev. 26:223–224. 2007. View Article : Google Scholar
|
|
15.
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Kizaka-Kondoh S, Inoue M, Harada H and
Hiraoka M: Tumor hypoxia: a target for selective cancer therapy.
Cancer Sci. 94:1021–1028. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar
|
|
18.
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Uemura M, Yamamoto H, Takemasa I, et al:
Jumonji domain containing 1A is a novel prognostic marker for
colorectal cancer: in vivo identification from hypoxic tumor cells.
Clin Cancer Res. 16:4636–4646. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Uemura M, Yamamoto H, Takemasa I, et al:
Hypoxia-inducible adrenomedullin in colorectal cancer. Anticancer
Res. 31:507–514. 2011.PubMed/NCBI
|
|
21.
|
Yamamoto H, Tei M, Uemura M, et al:
Ephrin-A1 mRNA is associated with poor prognosis of colorectal
cancer. Int J Oncol. 42:549–555. 2012.
|
|
22.
|
Noda T, Yamamoto H, Takemasa I, et al:
PLOD2 induced under hypoxia is a novel prognostic factor for
hepatocellular carcinoma after curative resection. Liver Int.
32:110–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Aihara T, Fujiwara Y, Ooka M, Sakita I,
Tamaki Y and Monden M: Mammaglobin B as a novel marker for
detection of breast cancer micrometastases in axillary lymph nodes
by reverse transcription-polymerase chain reaction. Breast Cancer
Res Treat. 58:137–140. 1999. View Article : Google Scholar
|
|
24.
|
Okami J, Dohno K, Sakon M, et al: Genetic
detection for micro-metastasis in lymph node of biliary tract
carcinoma. Clin Cancer Res. 6:2326–2332. 2000.PubMed/NCBI
|
|
25.
|
Aihara T, Fujiwara Y, Miyake Y, et al:
Mammaglobin B gene as a novel marker for lymph node micrometastasis
in patients with abdominal cancers. Cancer Lett. 150:79–84. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Ouellette RJ, Richard D and Maicas E:
RT-PCR for mamma-globin genes, MGB1 and MGB2, identifies breast
cancer micrometastases in sentinel lymph nodes. Am J Clin Pathol.
121:637–643. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Tassi RA, Bignotti E, Falchetti M, et al:
Mammaglobin B expression in human endometrial cancer. Int J Gynecol
Cancer. 18:1090–1096. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Tassi RA, Calza S, Ravaggi A, et al:
Mammaglobin B is an independent prognostic marker in epithelial
ovarian cancer and its expression is associated with reduced risk
of disease recurrence. BMC Cancer. 9:2532009. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Bellone S, Tassi R, Betti M, et al:
Mammaglobin B (SCGB2A1) is a novel tumour antigen highly
differentially expressed in all major histological types of ovarian
cancer: implications for ovarian cancer immunotherapy. Br J Cancer.
109:462–471. 2013. View Article : Google Scholar
|
|
30.
|
Mohrin M, Bourke E, Alexander D, et al:
Hematopoietic stem cell quiescence promotes error-prone DNA repair
and mutagenesis. Cell Stem Cell. 7:174–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Adikrisna R, Tanaka S, Muramatsu S, et al:
Identification of pancreatic cancer stem cells and selective
toxicity of chemotherapeutic agents. Gastroenterology. 143:234–245.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Miyake M, Takemasa I, Matoba R, et al:
Heterogeneity of colorectal cancers and extraction of discriminator
gene signatures for personalized prediction of prognosis. Int J
Oncol. 39:781–789. 2011.PubMed/NCBI
|
|
33.
|
Takeno A, Takemasa I, Doki Y, et al:
Integrative approach for differentially overexpressed genes in
gastric cancer by combining large-scale gene expression profiling
and network analysis. Br J Cancer. 99:1307–1315. 2008. View Article : Google Scholar
|
|
34.
|
Takaishi S, Okumura T, Tu S, et al:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Vermeulen L, De Sousa EMF, van der Heijden
M, et al: Wnt activity defines colon cancer stem cells and is
regulated by the microenvironment. Nat Cell Biol. 12:468–476. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: an emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Ni J, Kalff-Suske M, Gentz R, Schageman J,
Beato M and Klug J: All human genes of the uteroglobin family are
localized on chromosome 11q12.2 and form a dense cluster. Ann NY
Acad Sci. 923:25–42. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Becker RM, Darrow C, Zimonjic DB, Popescu
NC, Watson MA and Fleming TP: Identification of mammaglobin B, a
novel member of the uteroglobin gene family. Genomics. 54:70–78.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Fiegl M, Haun M, Massoner A, et al:
Combination of cytology, fluorescence in situ hybridization for
aneuploidy, and reverse-transcriptase polymerase chain reaction for
human mammaglobin/mammaglobin B expression improves diagnosis of
malignant effusions. J Clin Oncol. 22:474–483. 2004. View Article : Google Scholar
|
|
40.
|
Brennan DJ, Jirstrom K, Kronblad A, et al:
CA IX is an independent prognostic marker in premenopausal breast
cancer patients with one to three positive lymph nodes and a
putative marker of radiation resistance. Clin Cancer Res.
12:6421–6431. 2006. View Article : Google Scholar
|