Introduction
Colorectal cancer (CRC) is one of the most common
malignancies. The incidence of colon cancer shows an increasing
trend with tendency for younger age (1). The treatments of colon cancer
including surgery resection or radiotherapy combined with
chemotherapy (2). However, these
treatments improved patient survival with the decreasing of life
quality. Thus, there is an urgent clinic need to develop new
treatment regimens for colon cancer.
Natural products are widely accepted as validated
agents for many conditions (3–6).
Many herbs or their components have already been used clinically as
potential candidates for anticancer drugs, including
anthocyanidins, catechins, quercetin and genistein (7,8). A
few studies and clinical epidemiologic studies indicated that red
wine has apparent effect on reducing the risks of cardiovascular
diseases and cancer (9,10). Polyphenolic compounds are present
at high levels in red wine, which may mainly contribute to the
benefits of red wine.
Resveratrol (3,5,4′-trihydroxystilbene, Res), as a
natural polyphenolic compound mostly from beans and grapes, was
discovered in red wine by Siemann and Creasy in 1992 (11), and can be used as inhibitor for
platelet aggregation, cardiac-protection and as anticancer agent
(10,12). It has been reported also that Res
can inhibit diet induced obesity (13) inhibiting proliferation and
promoting apoptosis in various tumor cells, such as colon cancer,
breast cancer and prostate cancer cells in vitro and in
vivo (14–20). For colon cancer cells, the
anti-proliferation and apoptotic inducing effects of Res have
already been validated, but the mechanism underlying these
activities remains unclear.
PI3K/Akt signaling plays a critical role in
modulating cell survival and apoptosis (21), it has been found over-activated in
many cancers reducing apoptosis and promoting proliferation. The
PI3K/Akt signaling was regulated by many other factors, one of the
major negative regulators is phosphatase and tensin homologue
(PTEN). PTEN was identified as a tumor suppressor (22,23),
which is often deleted or mutated in a variety of cancers at high
frequency (24,25). PTEN protein acts as a phosphatase
specifically catalyzing the dephosphorylation of the 3-phosphate of
the inositol ring in phosphatidylinositol (3,4,5)-trisphosphate (PIP3), leading to the
biphosphate product PIP2. The dephosphorylation of PIP3 results in
the inactivation of PI3K/Akt signal pathway because PIP3 is
critical in activation of Akt (26).
Wnt/β-catenin signaling pathway is important for
cell proliferation and differentiation, its aberrant activation is
another major cause of colon cancer. The Wnt/β-catenin signaling
can be upregulated by the phosphorylation of glycogen synthase 3
kinase β (GSK3β), an important negative regulator for Wnt/β-catenin
signaling pathway, when PI3K/Akt signal is activated (27,28).
Herein, we investigated the possible molecular
mechanism underlying the proliferation inhibitory and apoptosis
inducing activities of Res in colon cancer cells. We found that Res
exhibits prominent anti-proliferation and apoptosis inducing
activity in HCT116 cells. Mechanistically, we demonstrated that Res
can upregulate the expression of PTEN and inhibit the activation of
PI3K/Akt signaling pathway, as well as to inhibit the Wnt/β-catenin
signaling transduction, respectively. Our results support that Res
can be used as anticancer agent alone or combined with other agents
in colon cancer treatment.
Materials and methods
Reagents and cell culture
Resveratrol (Res) was purchased from Xi’an Haoxuan
Biotechnology Co. Ltd., (Xi’an, China), and dissolved with dimethyl
sulfoxide (DMSO). The aliquots were kept at −20°C. VO-OHpic was
from Sigma-Aldrich (St. Louis, MO, USA). HCT116 cell line was
kindly provided by Dr Bert Vogelstein (Johns Hopkins Oncology
Center; Baltimore, MD, USA). For in vivo experiment, Res was
prepared with 0.5% carboxymethylcellulose sodium (CMCNa) as
suspension. All antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Cells were maintained in
the Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine
serum (FBS), 100 U/ml of penicillin, and 100 μg/ml of
streptomycin at 37°C in 5% CO2.
Crystal violet viability assay
Crystal violet assay was conducted as previously
described (29). Experimentally,
HCT116 cells were treated with indicated concentrations of Res. At
24, 48 or 72 h after treatment, cells were carefully washed with
cold phosphate-buffered saline (PBS, 4°C) and stained with 0.5%
crystal violet formalin solution at room temperature for 20–30 min.
The stained cells were washed with water and air dried for imaging
and quantification. For quantification, the crystal violet was
dissolved in 20% acetic acid at room temperature for 20 min with
shaking. The absorbance at 570 nm was measured (30).
Flow cytometry analysis for cell cycle
and apoptosis
Cells were seeded into 6-well plates. For cell cycle
analysis, cells were treated with different concentrations of Res
or solvent for 48 h. Then, cells were washed with PBS, collected
and washed with cold (4°C) 70% ethanol followed by washing with
50%, 30% ethanol and PBS; incubated with 1 ml of 20 mg/ml propidium
iodide (PI) containing RNase (1 mg/ml) in PBS for 30 min followed
by fluorescence activated cell sorting (FACS) assay. For apoptosis
measure, cells were harvested after treated with different
concentrations of Res for 48 h. Then, cells were washed with PBS
(4°C), followed by incubating with Annexin V-EGFP and PI as the kit
procedures (KeyGen Biotech Co. Ltd., Nanjing, China). Then, the
cells were subjected to FACS assay.
Western blot assay
Cells were seeded in 6-well plates and treated with
different concentrations of Res or solvent. At the scheduled time
point, cells were lysed and the lysate were denatured by boiling
for 10 min. Total protein were separated by SDS-PAGE, transfered
with polyvinylidene difluoride (PVDF) membrane, blocked in 10%
skimmed milk and probed with antibody against the target proteins.
Finally, the images of target bands were developed with SuperSignal
West Pico Chemiluminescent substrate.
RNA isolation and reverse transcription
polymerase chain reaction (RT-PCR)
Cells were seeded in T25 flask and treated with
different concentrations of Res. The total RNA was extracted with
TRIzol reagents (Invitrogen, Carlsbad, CA, USA), followed by
reverse transcriptional reaction to obtain the cDNA product.
Finally, the cDNA products were used as PCR templates to detect the
target gene expression. The primer sequences are available upon
request.
Construction of recombinant adenoviruses
for exogenous expression of PTEN, GFP and knockdown siRNA fragments
for PTEN
Recombinant adenoviruses for exogenous expression of
PTEN (Ad-PTEN) or GFP (Ad-GFP) were generated with the AdEasy
system as previously described (31) as well as the recombinant
adenoviruses expressing small interference RNA (siRNA) fragments
for PTEN silence (Ad-siPTEN). Recombinant adenovirus expressing GFP
was used as the vector control.
Xenograft tumor model of human colon
cancer and histological evaluation
All animal experiments followed the guidelines of
Institutional Animal Care and Use Committee of Chongqing Medical
University (Chongqing, China). Athymic nude mice (female, 4–6 weeks
old, 5/group) were from the animal center of Chongqing Medical
University (Chongqing, China). HCT116 cells were cultured and
resuspended in PBS for subcutaneous injection
(1×106/injection) into the flanks of the athymic nude
mice. The mice were treated with Res (50 or 150 mg/kg) or the same
volume of solvent through intragastric administration one week
after cancer cell injection, once a day for four weeks. At the end
of the 4th week, all nude mice were sacrificed, the tumor samples
were retrieved and fixed in 10% formalin, and then embedded in
paraffin. Serial sections of the embedded samples were stained with
hematoxylin and eosin (H&E).
Luciferase reporter assay
Firefly luciferase reporter assay was carried out as
follows. Cells were seeded in T25 flasks and transfected with 3.0
μg per flask of pTOP-Luc (β-catenin/Tcf4 responsive elements
reporter plasmid) using Lipofectamine (Invitrogen). After 12 h,
cells were replated in 24-well plates and treated with different
concentrations of Res or solvent as control. After 24 h, cells were
lysed and subjected to luciferase activity assays following the
manual of the kit (Promega, Madison, WI, USA). Luciferase activity
was normalized with total cellular protein concentrations of the
samples. Each assay was done in triplicate.
Statistical analysis
All experiments were performed in triplicates and
the results were repeated in at least two independent experiments.
Statistical analysis of results was conducted using t-test
(Microsoft Excel). Data are expressed as mean ± standard deviation
(SD). Statistical significance was set at P<0.05.
Results
Resveratrol inhibits the proliferation in
colon cancer cells
It has been reported that Res inhibits the
proliferation of various cancer cells and modulates the processes
of cancer (32). To investigate
whether Res can affect the proliferation of human colon cancer, we
analyzed the effect of Res on proliferation in human colon cancer
cells. The results indicate that Res can inhibit the proliferation
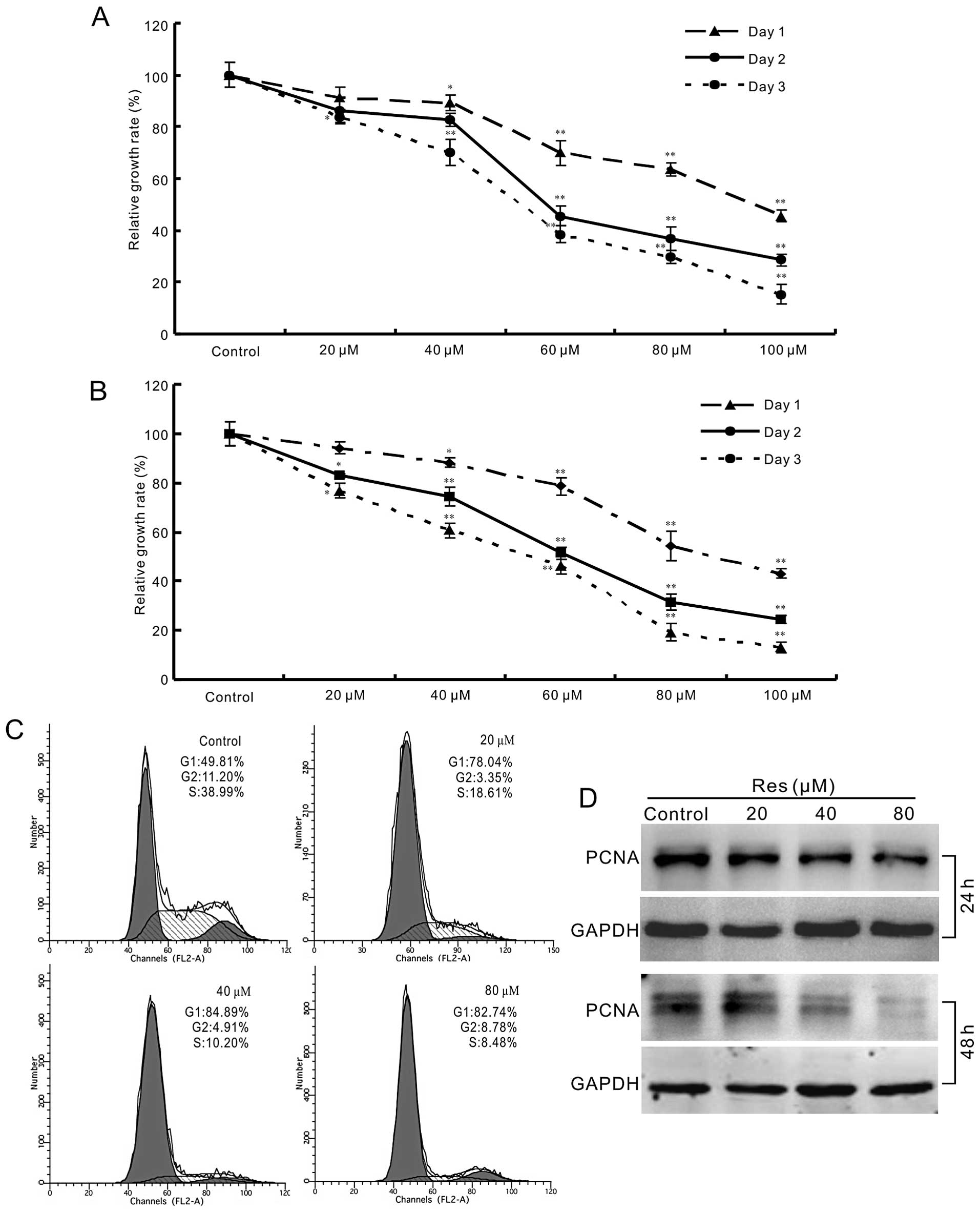
of HCT116 and SW480 cells concentration-dependently (Fig. 1A and B). Cell cycle analysis shows
that Res can arrest the cell cycle at G1 phase in HCT116 cells
(Fig. 1C). For further testing, we
detected the effect of Res on the expression of proliferating cell
nuclear antigen (PCNA) in HCT116 cells. The result shows that Res
decreases the expression of PCNA concentration-dependently in
HCT116 cells (Fig. 1D). These data
demonstrate that Res is able to inhibit the proliferation of HCT116
cells.
Resveratrol induces apoptosis in colon
cancer cells
Most anticancer agents have characteristics of
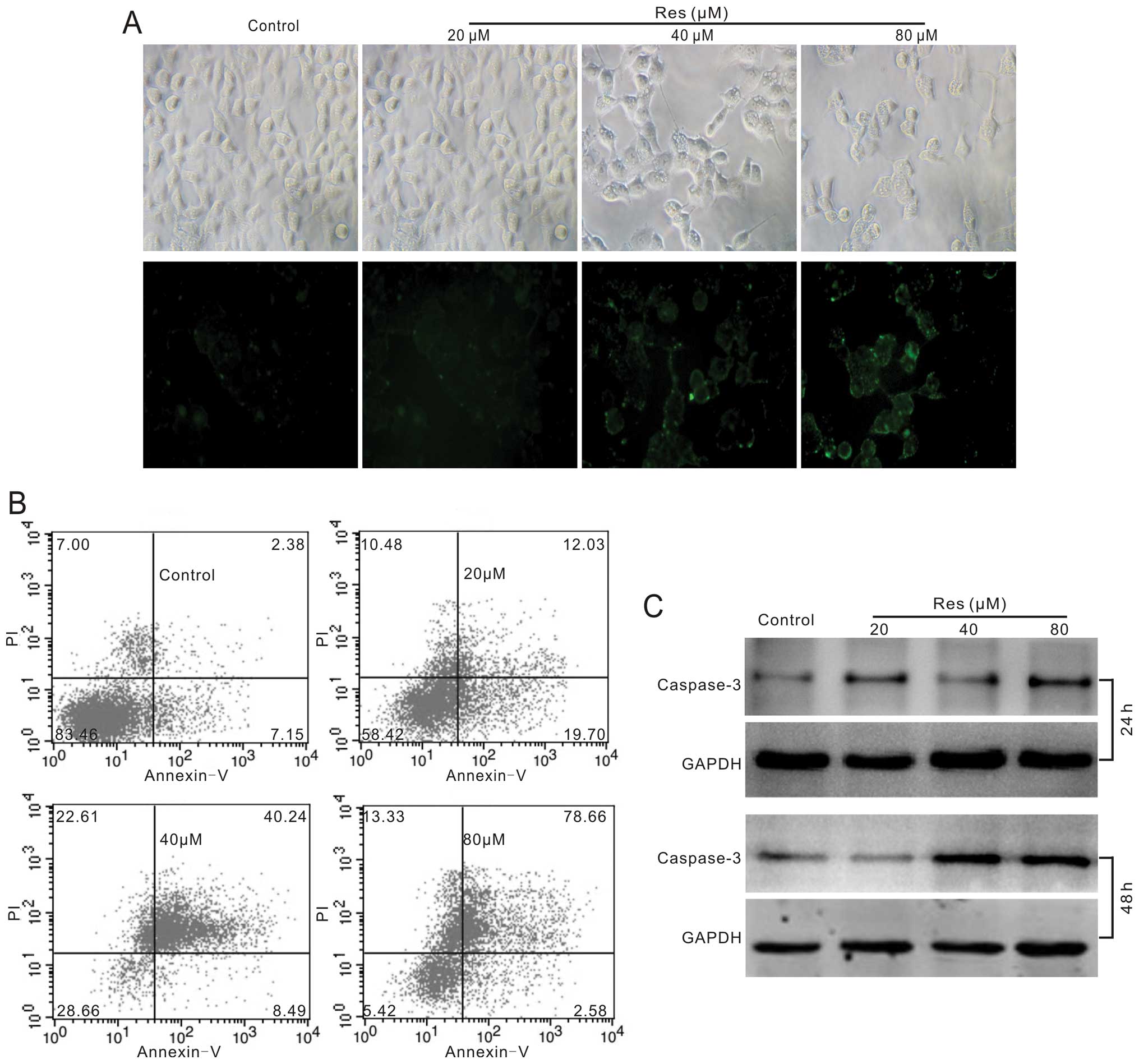
apoptosis induction, so we tested whether Res could induce
apoptosis in HCT116 cells. We employed Annexin V staining, FACS and
western blotting to assay the effect of Res on apoptosis in HCT116
cells. The results show that Res can induce apoptosis clearly and
concentration-dependently (Fig. 2A and
B). Western blot assay results indicate that the protein level
of caspase-3 increased substantially (Fig. 2C). These results strongly suggest
that Res is a potent apoptosis inducer for human colon cancer
cells.
Resveratrol inhibits tumor growth in a
xenograft tumor model
The above evidence has proven that Res is a potent
proliferation inhibitor agent for colon cancer cells. We next
investigated the in vivo anticancer activity of Res with a
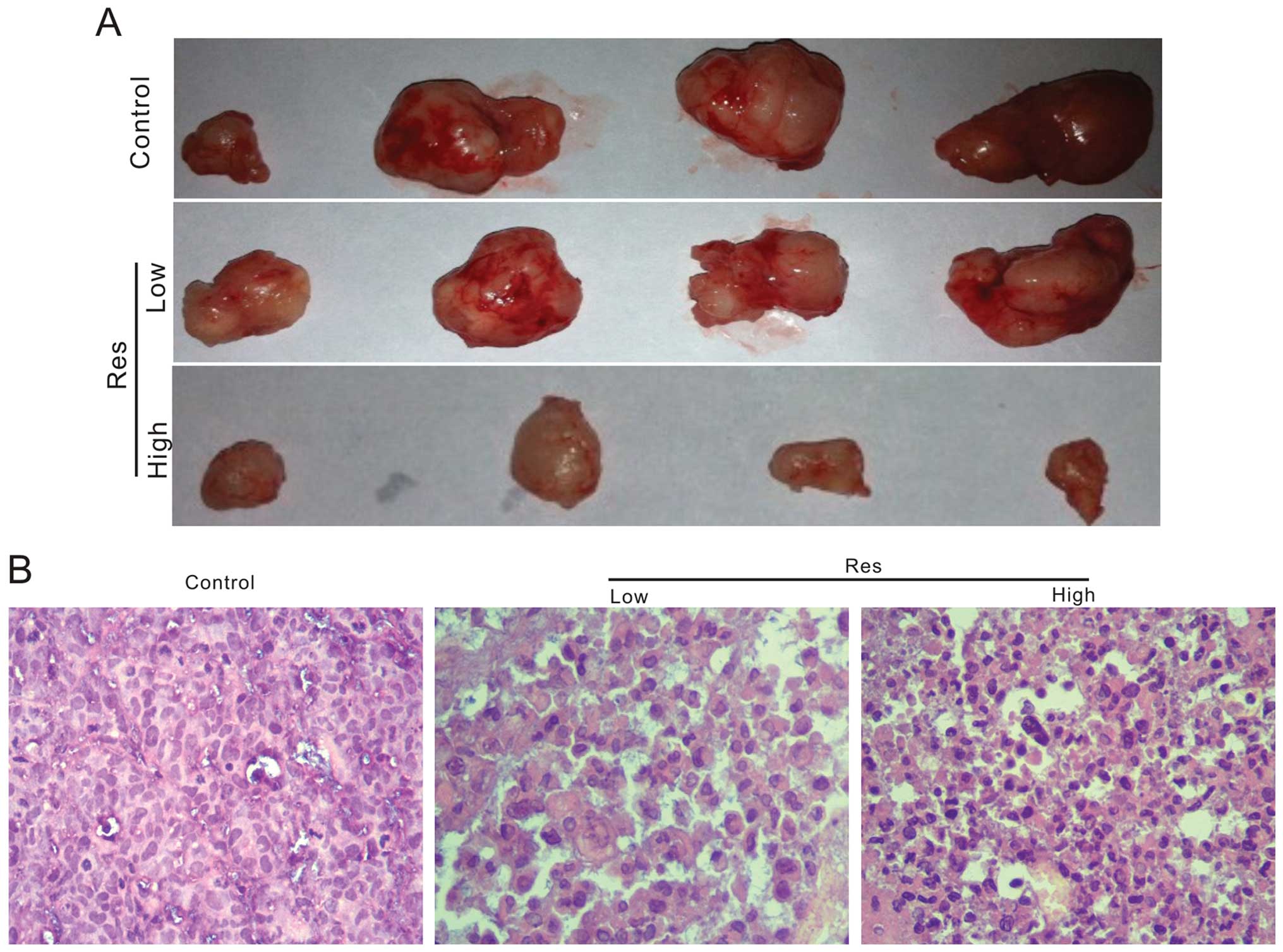
xenograft tumor model for colon cancer. We injected
1×106 HCT116 cells into flanks of athymic nude mice. One
week after injection, we treated the mice with intragastric
administration of Res (50 or 150 mg/kg), once a day for four weeks.
The results show that tumor masses from mice treated with Res are
smaller than those of the control group (Fig. 3A). The H&E staining results
indicate that Res treated groups exhibited a decreased cellularity
in tumor masses (Fig. 3B)
suggesting that Res can inhibit tumor growth in vivo,
although it can not eliminated tumors completely.
PTEN is involved in the
anti-proliferation effect of resveratrol in colon cancer cells
PTEN, as a tumor suppressor gene, has been found
damaged or deficient in many cancer (33–35).
It may be one of the targets for anticancer treatment. Thus, we
investigated whether PTEN is involved in the anti-proliferation
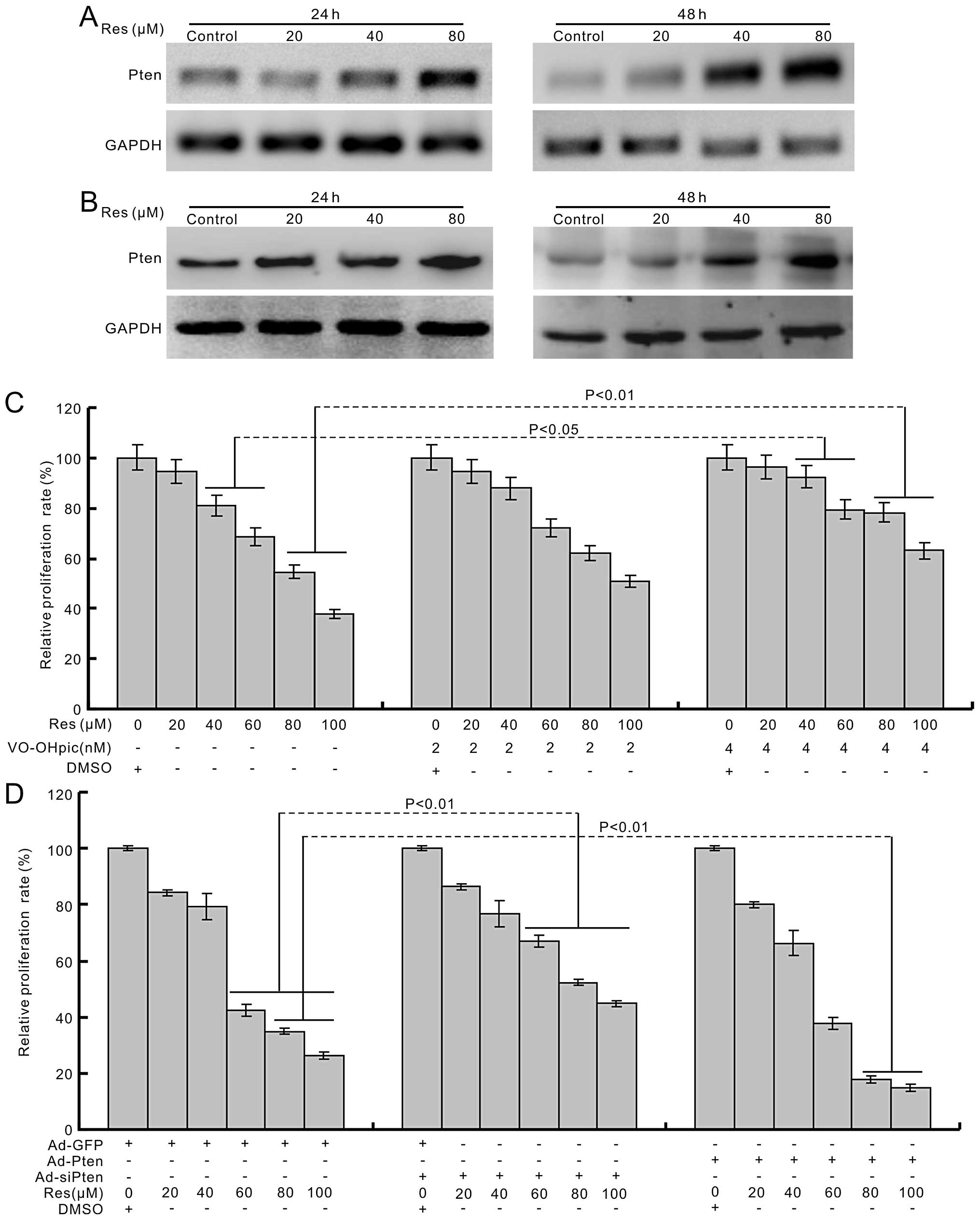
effect of Res in human colon cancer cells. The PCR and western blot
results show that Res can induce the expression of PTEN
concentration-dependently (Fig. 4A and
B). The PTEN inhibitor attenuates the anti-proliferation effect
of Res in HCT116 cells (Fig. 4C).
Exogenous expression of PTEN potentiates the proliferation
inhibitory effect of Res, while knockdown of PTEN inhibits this
effect of Res in HCT116 cells (Fig.
4D). These results indicate that PTEN is involved in the
anti-proliferation of Res in human colon cancer cells.
Resveratrol downregulates PI3K/Akt
signaling in HCT116 cells through upregulating PTEN
One major function of PTEN is to negatively regulate
PI3K/Akt signaling. The PI3K/Akt signaling pathway is one of the
essential pathways for cell survival and proliferation, which is
over-activated in many human cancers, such as breast, colon and
prostate cancers (10,14). Thus, we investigated whether
PI3K/Akt signaling is involved in the anti-proliferation effect of
Res in HCT116 cells. The results indicated that Res has no apparent
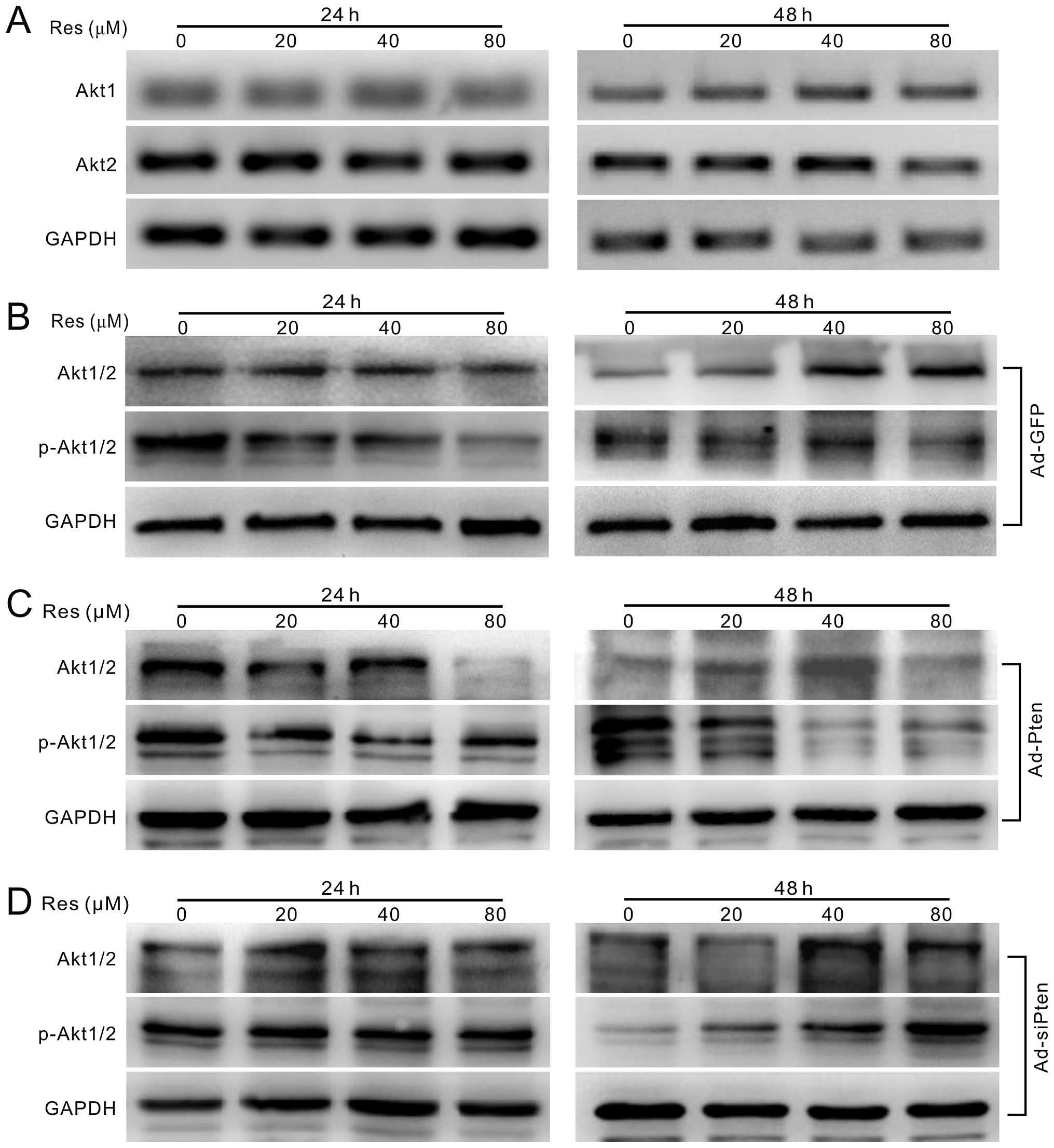
effect on the mRNA expression of Akt1 and Akt2 (Fig. 5A), but can reduce the
phosphorylation level of Akt1/2 concentration-dependently (Fig. 5B). Res combined with adenovirus
mediated exogenous expression of PTEN decreases the phosphorylation
of Akt1/2 substantially (Fig. 5C),
while knockdown of PTEN reverses the effect of Res on the
phosphorylation of Akt1/2 (Fig.
5D). These results suggest that Res can inhibit PI3K/Akt
signaling activation in HCT116 cells through the upregulation of
PTEN.
Resveratrol inhibits Wnt/β-catenin
signaling transduction independent of PTEN/PI3K/Akt in HCT116
cells
The abnormal activation of Wnt/β-catenin signaling
is one of the major causes of cancer, which can be regulated by
PTEN/PI3K/Akt signaling through the phosphorylation of GSK-3β.
HCT116 cells are predisposed to the mutation of β-catenin, thus, it
can not be degradated by the degradative complex in the canonical
Wnt signaling pathway. We measured whether Res could still decrease
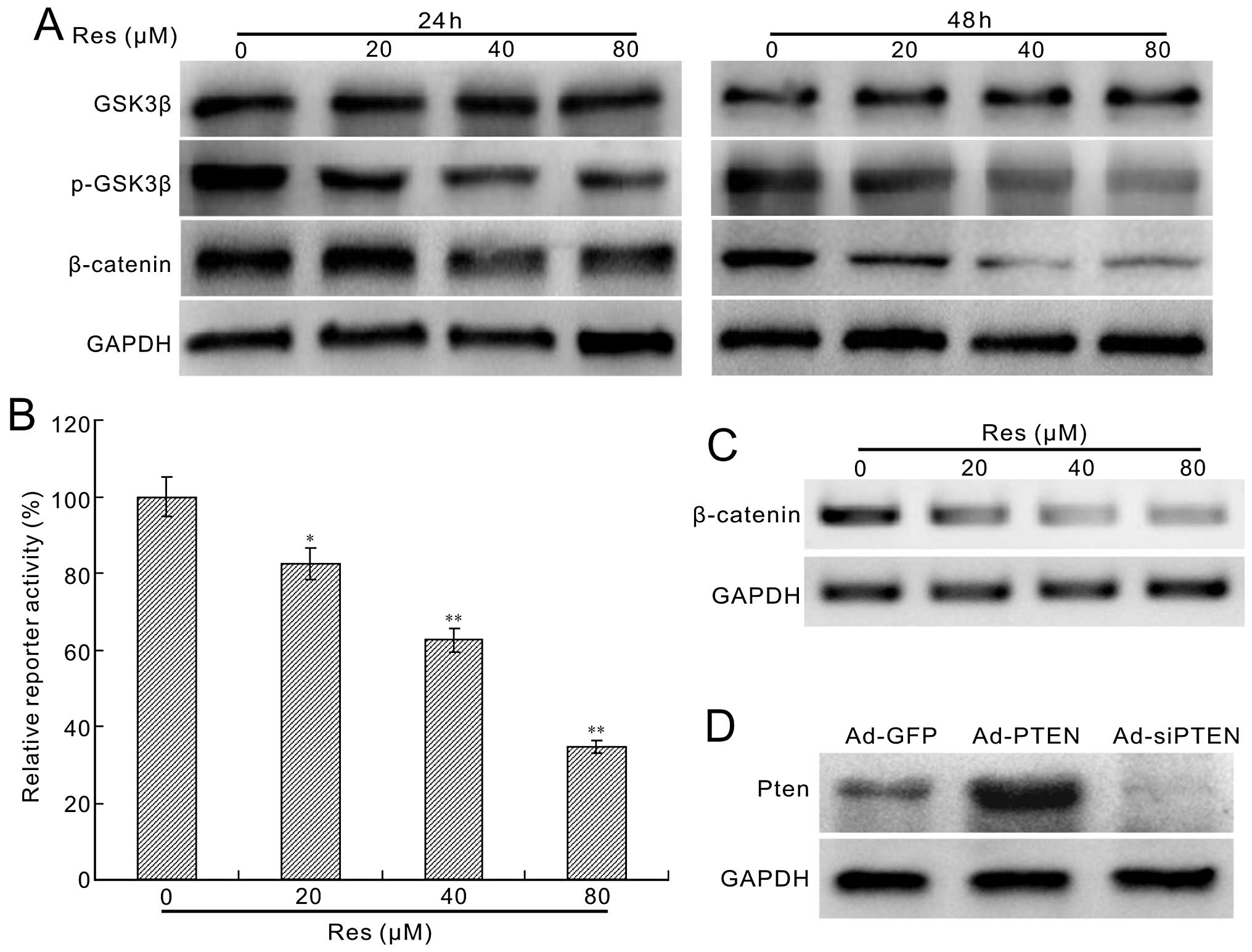
the level of β-catenin in HCT116 cells. We found that Res has no
apparent effect on the total level of GSK-3β, but decreases the
phosphorylation of GSK-3β substantially, as well as the protein
level of β-catenin (Fig. 6A). The
β-catenin/Tcf4 reporter assay results indicate that Res inhibited
the reporter activity concentration-dependently (Fig. 6B). Moreover, the β-catenin mRNA
expression was also decreased by Res (Fig. 6C). These results suggest that the
canonical Wnt signaling pathway is involved in the
anti-proliferation effect of Res in HCT116 cells.
Discussion
Colon cancer is one of the most frequently diagnosed
malignancies, with high incidence in western countries (1). Although the treatment for colon
cancer has advanced substantially, the prognosis is still more
modest than had been hoped (36).
There is a great clinical need to explore new treatment regimens
for colon cancer. In this investigation, we demonstrated that Res
has potent anti-proliferation activity in human colon cancer cells,
and the anti-proliferation effect of Res may be mediated by
inhibiting PI3K/Akt signaling through upregulating the expression
of PTEN and blocking Wnt/β-catenin signaling transduction,
respectively.
Res, as a natural polyphenolic compound, is found in
the skin of red grapes and other fruits as well as in Japanese
knotweed roots (11). Several
studies have proved that Res can inhibit proliferation and induce
apoptosis of breast cancer cells, prostate cancer cells and colon
cancer cells (10,13,14).
No clinical trial has yet reported the cancer prevention effect of
Res (37). Moreover, the
bioavailability of Res is very low, so even a high dose of Res may
not reach the sufficient concentration required for systemic
treatment for cancers (38),
however, this may be a benefit for digestive tract cancer
treatment. Our in vitro results show that Res can inhibit
the proliferation of HCT116 cells, even at the concentration of 20
μM. These data confirmed that Res has the potential to be an
anticancer agent. Interestingly, our investigation indicates that
Res shows no apparent proliferation inhibitory effect in HEK-293
cells, even at the concentration of 100 μM (data not shown).
This result implies that the anti-proliferation effect of Res may
be more specific to cancer cells.
The above studies, and our new results validate the
proliferation inhibitory effect of Res on colon cancer cells, but
the exact molecular mechanism underlying this remains unknown.
Vanamala et al reported that Res could induce apoptosis
through the suppression of Wnt pathway and activation of p53
signaling pathways in human colon cancer (39). The expression of MicroRNA-21
participates in the inhibition of prostate cancer growth and
metastasis initialized by Res (16). Recent studies indicate that p38 and
PI3K signaling pathways are involved in the anticancer activity of
Res (40). Wnt, PI3K/Akt and p38
signaling pathways are all essential for cell proliferation and
differentiation, and found to be aberrant in many cancers. However,
the exact molecular mechanism of how Res regulates these signaling
pathways remains unclear.
PTEN, a tumor suppressor often mutated or lost in
many cancers, acts as a phosphatase to specifically catalyse PIP3
dephosphorylation at the 3-phosphate of the inositol ring and turn
PIP3 to PIP2 through which it negatively regulates PI3K/Akt
signaling. PI3K/Akt signaling has been associated with many
cellular functions, including proliferation, differentiation,
motility and survival. Previous studies indicated that PTEN may
play an important role in early stages of sporadic colorectal
carcinogenesis and reduced or lost PTEN expression is more frequent
in colon cancer (25); colon
cancer cells with high expression of PTEN is correlated with
chemosensitivity (41). Our
results show that Res can up regulate the expression of PTEN in
HCT116 cells. Either PTEN specific inhibitor or knockdown of PTEN
can attenuate the anti-proliferation effect of Res in HCT116 cells,
while exogenous expression of PTEN can potentiate the proliferation
inhibitory effect of Res in these cells. These data indicate that
PTEN may be important for the anti-proliferation effect of Res in
HCT116 cells. Further analysis confirmed that the up regulated PTEN
by Res is correlated with the inactivation of PI3K/Akt signaling by
decreasing the phosphorylation of Akt1/2 in HCT116 cells.
Therefore, our results suggest that PTEN/PI3K/Akt is involved in
the proliferation inhibitory effect of Res in HCT116 cells.
Another major cause of colon cancer is the over
activated Wnt/β-catenin signaling pathway. Most colon cancer cells
are predisposed to the mutation of β-catenin or APC (42). Wnt signaling, including canonical
and noncanonical Wnt signaling, plays an important role in
embryogenesis and development. The β-catenin plays a pivotal role
in canonical Wnt signaling. In the absence of Wnt proteins, Axin,
GSK-3β and APC assemble as a complex to promote the proteolytic
degradation of β-catenin. When Wnt proteins bind with the frizzled
receptor, the degradation complex will be destroyed and the
β-catenin can be accumulated in the cytoplasm and translocate to
the nucleus. Eventually, β-catenin interacts with TCF/LEF
transcription factors to regulate the downstream gene
expression.
The mutation of APC, β-catenin or phosphorylation of
GSK-3β cause β-catenin not to be degraded normally by the
destruction complex and accumulate in the cytoplasm. The activation
of PI3K/Akt can activate the canonical Wnt signaling through the
phosphorylation of GSK-3β by the phosphorylated Akt1/2, blocking
the formation of β-catenin destroying complex (28). Hence, the upregulation of PTEN may
inhibit the canonical Wnt signaling by promoting the degradation of
β-catenin. Although our results show that Res can decrease the
phosphorylation of GSK-3β, the protein level of β-catenin, and the
β-catenin/Tcf4 reporter activity, but it may not result from the
decreased phosphorylation of GSK-3β by PTEN/PI3K/Akt signaling. As
it is predisposed to mutation of β-catenin in HCT116 cells, the
β-catenin can not be degraded by the destruction complex in this
colon cancer cells. With additional investigation, we unveiled that
Res can inhibit the mRNA expression of β-catenin. Thus, these
results suggest that Res can inhibit the Wnt/β-catenin signaling
transduction, but it may not result from the upregulation of PTEN
in HCT116 cells.
Taken together, Our data strongly suggest that Res
can inhibit the proliferation and promote apoptosis in colon cancer
cells. These activities of Res may be mediated by PI3K/Akt
signaling through upregulating the expression of PTEN and reducing
the Wnt/β-catenin signaling transduction through inhibiting the
expression of β-catenin, respectively. However, the detailed
molecular mechanism of how Res regulate the expression of PTEN and
β-catenin need to be further deciphered.
Acknowledgements
We thank Dr Bert Vogelstein of the
Johns Hopkins Oncology Center (Baltimore, MD, USA) for his kind
provision of HCT116 cells. We thank Professor Qi-Xin Zhou of
Chongqing Medical University (Chongqing, China) for his critical
reading of the manuscript. This study was supported in part by
research grants from Natural Science Foundation of China (Grants:
NSFC 81071462 and 81372120), Chongqing Science & Technology
Commission of China (Grant: CSTC 2011BB5129), and the National
Basic Research Program of China (Grant: 2011CB70790).
References
|
1.
|
Brunagel G, Vietmeier BN, Bauer AJ, Schoen
RE and Getzenberg RH: Identification of nuclear matrix protein
alterations associated with human colon cancer. Cancer Res.
62:2437–2442. 2002.PubMed/NCBI
|
|
2.
|
Fey MF: Adjuvant therapy for colon cancer.
Schweiz Med Wochenschr. 130:1760–1765. 2000.(In German).
|
|
3.
|
Cragg GM, Grothaus PG and Newman DJ:
Impact of natural products on developing new anti-cancer agents.
Chem Rev. 109:3012–3043. 2009. View Article : Google Scholar
|
|
4.
|
Mishra BB and Tiwari VK: Natural products:
an evolving role in future drug discovery. Eur J Med Chem.
46:4769–4807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Zou DM, Brewer M, Garcia F, et al: Cactus
pear: a natural product in cancer chemoprevention. Nutr J.
4:252005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Konkimalla VB and Efferth T: Anti-cancer
natural product library from traditional Chinese medicine. Comb
Chem High Throughput Screen. 11:7–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Khan N, Afaq F and Mukhtar H: Cancer
chemoprevention through dietary antioxidants: progress and promise.
Antioxid Redox Signal. 10:475–510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Korkina LG, De Luca C, Kostyuk VA and
Pastore S: Plant polyphenols and tumors: from mechanisms to
therapies, prevention, and protection against toxicity of
anti-cancer treatments. Curr Med Chem. 16:3943–3965. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Das S, Santani DD and Dhalla NS:
Experimental evidence for the cardioprotective effects of red wine.
Exp Clin Cardiol. 12:5–10. 2007.
|
|
10.
|
Jang M, Cai L, Udeani GO, et al: Cancer
chemopreventive activity of resveratrol, a natural product derived
from grapes. Science. 275:218–220. 1997. View Article : Google Scholar
|
|
11.
|
Gehm BD, McAndrews JM, Chien PY and
Jameson JL: Resveratrol, a polyphenolic compound found in grapes
and wine, is an agonist for the estrogen receptor. Proc Natl Acad
Sci USA. 94:14138–14143. 1997. View Article : Google Scholar
|
|
12.
|
Bhat KPL, Kosmeder JW II and Pezzuto JM:
Biological effects of resveratrol. Antioxid Redox Signal.
3:1041–1064. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Gulvady AA, Ciolino HP, Cabrera RM and
Jolly CA: Resveratrol inhibits the deleterious effects of
diet-induced obesity on thymic function. J Nutr Biochem.
24:1625–1633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Fang JY, Li ZH, Li Q, Huang WS, Kang L and
Wang JP: Resveratrol affects protein kinase C activity and promotes
apoptosis in human colon carcinoma cells. Asian Pac J Cancer Prev.
13:6017–6022. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Lu R and Serrero G: Resveratrol, a natural
product derived from grape, exhibits antiestrogenic activity and
inhibits the growth of human breast cancer cells. J Cell Physiol.
179:297–304. 1999. View Article : Google Scholar
|
|
16.
|
Sheth S, Jajoo S, Kaur T, et al:
Resveratrol reduces prostate cancer growth and metastasis by
inhibiting the Akt/MicroRNA-21 pathway. PLoS One. 7:e516552012.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Fouad M, Agha A, Merzabani MA and Shouman
S: Resveratrol inhibits proliferation, angiogenesis and induces
apoptosis in colon cancer cells: Calorie restriction is the force
to the cytotoxicity. Hum Exp Toxicol. 32:1067–1080. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Yang HL, Chen WQ, Cao X, et al: Caveolin-1
enhances resveratrol-mediated cytotoxicity and transport in a
hepatocellular carcinoma model. J Transl Med. 7:222009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Miura D, Miura Y and Yagasaki K:
Hypolipidemic action of dietary resveratrol, a phytoalexin in
grapes and red wine, in hepatoma-bearing rats. Life Sci.
73:1393–1400. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Liu HS, Pan CE, Yang W and Liu XM:
Antitumor and immunomodulatory activity of resveratrol on
experimentally implanted tumor of H22 in Balb/c mice. World J
Gastroenterol. 9:1474–1476. 2003.PubMed/NCBI
|
|
21.
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and Gonzalez-Baron M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004.PubMed/NCBI
|
|
22.
|
Mahimainathan L and Choudhury GG:
Inactivation of platelet-derived growth factor receptor by the
tumor suppressor PTEN provides a novel mechanism of action of the
phosphatase. J Biol Chem. 279:15258–15268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Takeda K, Kanekura T and Kanzaki T:
Negative feedback regulation of phosphatidylinositol 3-kinase/Akt
pathway by over-expressed cyclooxygenase-2 in human epidermal
cancer cells. J Dermatol. 31:516–523. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Chalhoub N and Baker SJ: PTEN and the
PI3-kinase pathway in cancer. Annu Rev Pathol. 4:127–150. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Waniczek D, Snietura M, Mlynarczyk-Liszka
J, et al: PTEN expression profiles in colorectal adenocarcinoma and
its precancerous lesions. Pol J Pathol. 64:15–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Franke TF, Kaplan DR, Cantley LC and Toker
A: Direct regulation of the Akt proto-oncogene product by
phosphatidylinositol-3,4-bisphosphate. Science. 275:665–668. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Zhang W, Zhang H, Wang N, et al:
Modulation of β-catenin signaling by the inhibitors of MAP kinase,
tyrosine kinase, and PI3-kinase pathways. Int J Med Sci.
10:1888–1898. 2013.
|
|
28.
|
Robertson BW and Chellaiah MA: Osteopontin
induces beta-catenin signaling through activation of Akt in
prostate cancer cells. Exp Cell Res. 316:1–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
He BC, Chen L, Zuo GW, et al: Synergistic
antitumor effect of the activated PPARgamma and retinoid receptors
on human osteosarcoma. Clin Cancer Res. 16:2235–2245. 2010.
View Article : Google Scholar
|
|
30.
|
Stepanovic S, Vukovic D, Dakic I, Savic B
and Svabic-Vlahovic M: A modified microtiter-plate test for
quantification of staphylococcal biofilm formation. J Microbiol
Methods. 40:175–179. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
He TC, Zhou S, da Costa LT, Yu J, Kinzler
KW and Vogelstein B: A simplified system for generating recombinant
adenoviruses. Proc Natl Acad Sci USA. 95:2509–2514. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Joe AK, Liu H, Suzui M, Vural ME, Xiao D
and Weinstein IB: Resveratrol induces growth inhibition, S-phase
arrest, apoptosis, and changes in biomarker expression in several
human cancer cell lines. Clin Cancer Res. 8:893–903.
2002.PubMed/NCBI
|
|
33.
|
Pal I and Mandal M: PI3K and Akt as
molecular targets for cancer therapy: current clinical outcomes.
Acta Pharmacol Sin. 33:1441–1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Lin MS, Huang JX, Chen WC, et al:
Expression of PPARgamma and PTEN in human colorectal cancer: An
immunohistochemical study using tissue microarray methodology.
Oncol Lett. 2:1219–1224. 2011.PubMed/NCBI
|
|
35.
|
Garcia JM, Silva JM, Dominguez G, et al:
Allelic loss of the PTEN region (10q23) in breast carcinomas of
poor pathophenotype. Breast Cancer Res Treat. 57:237–243. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Aggarwal S and Chu E: Current therapies
for advanced colorectal cancer. Oncology. 19:589–595.
2005.PubMed/NCBI
|
|
37.
|
Athar M, Back JH, Tang X, et al:
Resveratrol: a review of preclinical studies for human cancer
prevention. Toxicol Appl Pharmacol. 224:274–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Boocock DJ, Faust GE, Patel KR, et al:
Phase I dose escalation pharmacokinetic study in healthy volunteers
of resveratrol, a potential cancer chemopreventive agent. Cancer
Epidemiol Biomarkers Prev. 16:1246–1252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Vanamala J, Reddivari L, Radhakrishnan S
and Tarver C: Resveratrol suppresses IGF-1 induced human colon
cancer cell proliferation and elevates apoptosis via suppression of
IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer.
10:2382010. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Gweon EJ and Kim SJ: Resveratrol induces
MMP-9 and cell migration via the p38 kinase and PI-3K pathways in
HT1080 human fibrosarcoma cells. Oncol Rep. 29:826–834.
2013.PubMed/NCBI
|
|
41.
|
Hsu CP, Kao TY, Chang WL, Nieh S, Wang HL
and Chung YC: Clinical significance of tumor suppressor PTEN in
colorectal carcinoma. Eur J Surg Oncol. 37:140–147. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
He BC, Gao JL, Luo X, et al: Ginsenoside
Rg3 inhibits colorectal tumor growth through the down-regulation of
Wnt/β-catenin signaling. Int J Oncol. 38:437–445. 2011.PubMed/NCBI
|