Introduction
Each year worldwide there are approximately nine
million new cases of cancer reported, and 30–50% of cancer patients
suffer from different degrees of pain. Cancer-induced bone pain
(CIBP) is a major clinical problem in patients with end-stage
cancer, which affects the quality of life of cancer patients
(1,2). CIBP is usually induced by primary
bone cancer or secondary bone metastasis from breast, prostate and
lung cancer, among others. Although significant advances have been
made in management of CIBP (3), it
is still a difficult issue in clinical practice, because current
treatments for this pain are limited due to inadequate knowledge of
the mechanisms associated with CIBP. Therefore, further
investigations on the mechanisms underlying cancer bone pain may
lead to the development of new effective therapies.
In recent years, neuron-glia interaction has been
considered a driving force for the development and maintenance of
abnormal pain. Glial activation has been widely studied in
different animal models, such as neuropathic pain after injuries to
peripheral nerves (4) or the
spinal cord (5), inflammatory pain
after injection of inflammatory substances (e.g., formalin) into a
hindlimb (6), cancer pain after
inoculation of tumor cells (7), or
orofacial pain after lesion of joint muscle (8). In these pain models, both microglia
and astrocytes are activated, which interact with neurons and are
involved in pain control. Activation of glial cells induces
pro-inflammatory mediators, which trigger neural plasticity,
leading to an increase of neuronal excitability and pain
hypersensitivity. On the other hand, pain could be potentiated by
growth factors such as brain-derived neurotrophic factor (BDNF),
glia cell-derived neurotrophic factor (GDNF) and nerve growth
factor (NGF); these are produced by activation of glial cells. The
studies suggest that CIBP may be a mixture of inflammatory and
neuropathic stimuli (9), and a
variety of cellular signaling pathways are involved in this
process, including the extracellular signal-regulated protein
kinase (ERK)/mitogen activated protein kinase (MAPK) signaling
pathway (10).
Neuregulin 1 (NRG1), a member of the NRG family of
proteins, contains an epidermal growth factor (EGF)-like domain,
which binds to the epidermal growth factor-like receptors ErbB3 and
4. These receptors are also found in microglia, and subsequently
heterodimerize with ErbB2 (11).
NRG1 has been implicated in the development of the central and
peripheral nervous systems, cardiac tissue and tumors (12–14).
The effects of NRG1 on cell proliferation, survival, migration and
synaptic plasticity are associated with dimerization activation of
ErbB2 (15–17). The NRG1-ErbB signaling pathway can
trigger various intracellular signaling pathways including
phosphatidylinositol 3-kinase (PI3K)/Akt and MAPK (18). Recent studies have revealed that
following peripheral nerve injury, the level of NRG1 increased
within the dorsal horn, leading to the activation of ErbB2,
specifically within microglia and the pain-related hypersensitivity
(19). However, whether the
abnormal expression of NRG1-ErbB2 is involved in CIBP has not yet
been reported.
We used a rat model of tibia bone cancer pain to
investigate the effect of NRG1-ErbB2 receptor signaling in
regulation of CIBP, providing insight into the novel mechanisms
underlying CIBP regulation. Elucidating this mechanism may lead to
the development of effective approaches for the management of bone
cancer pain.
Materials and methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM) and fetal
bovine serum (FBS) were obtained from Gibco, USA. PD168393 was
purchased from Calbiochem, and HRG1β (ab50277) was obtained from
Abcam, UK. Rabbit anti-ErbB2 (ab2428) and anti-p-ErbB2 (ab47755)
were bought from Abcam. Mouse anti-NRG1 (SC-57384), rabbit
anti-p-AKT-1 and rabbit anti-p-p38MAPK were purchased from Santa
Cruz, USA. Anti-GAPDH was obtained from Sigma, USA. PVDF membrane
and ECL Western Blotting Substrates were products of Millipore, USA
and Pierce, USA, respectively. SYBR PrimeScript™ RT-PCR kit was
obtained from Takara, Japan. Designed PCR primers were synthesized
by Takara Biotechnology (Dalian) Co., Ltd, China.
Preparation of Walker 256 mammary gland
carcinoma cells
The Walker 256 mammary gland carcinoma cell line was
purchased from Cancer Institute and Hospital, Chinese Academy of
Medical Sciences, Beijing, China. Cells were maintained in DMEM
with 10% fetal bovine serum and penicillin/streptomycin (100 U/ml).
The harvested cells were suspended at a concentration of
2×107 cells/ml. Female Sprague-Dawley (SD) rats,
weighing 80–100 g, were provided by Experimental Animal Center,
Shengjing Hospital of China Medical University, Shenyang, China.
Each rat received intra-abdominal injection of 0.5 ml of the cell
suspension. Ascites formed 6–7 days following tumor cell injection.
Thereafter, 5 to 10 ml of ascites were collected and cell were
harvested by centrifugation, then suspended in phosphate buffer
saline (PBS) at a density of 1×107 cells/ml. Same amount
of cells were taken as a negative control by heat inactive
treatment in a 100°C water bath for 10 min.
Establishment of rat model of
cancer-induced bone pain
Female SD rats as mentioned above, weighing 200–220
g, were routinely housed in light (12 h dark/12 h light) and
temperature-controlled (22–24°C) rooms and had free access to food
and water. Experimental animal use was approved by the Experimental
Ethics Committee of Shengjing Hospital of China Medical University.
Animal experiments were carried out in accordance with the
Guidelines laid down by the NIH in the US regarding the care and
use of animals for experimental procedures. The rat model of CIBP
was established as previously described (20). In brief, rats were anesthetized by
intraperitoneal injection of 10% chloral hydrate (300 mg/kg). The
right leg was shaved and the skin was disinfected with 70% (v/v)
ethanol. A 1-cm incision was made in the skin, and the tibia was
carefully exposed with minimal damage to blood vessels and nerve.
The tibia was pierced using a 23-gauge needle. A total of 10
μl of cell suspension in PBS that contains 0.1 million
(105) Walker 256 mammary gland carcinoma cells were
slowly injected into the right tibia cavity of each rat using a
25-μl microinjection syringe within 2 min. The injection
site was sealed with bone wax, followed by irrigation with sterile
normal saline.
Intrathecal catheterization
Intrathecal catheterization was carried out as
previously described (21). In
brief, animals were anesthetized by 10% chloral hydrate (300 mg/kg,
i.p.). A PE10 intrathecal catheter was inserted 2 mm cephalad into
the rat lumbar subarachnoid space at the level of L3–L4
inter-vertebrae. The tip of the catheter was located near the
lumbar enlargement of the spinal cord for intrathecal
administration of the drugs. The catheter was tunneled
subcutaneously and externalized through the skin in the neck
region. Two days later, 20 μl lidocaine (20 g/l) was
injected intrathecally to rats without impaired movement. If these
animals showed lower limb paralysis within 30 sec of injection
time, it was a successful sign for catheterization.
Experimental design
To verify the effect of ErbB2 signaling on the
regulation of CIBP, PD168393, a structurally related
irreversible-binding ErbB2 inhibitor (22), was used. Animals were randomly
divided into four groups, including: sham operation (n=6 for each
time point), CIBP (n=6 for each time point), CIBP + PD168393
treatment (n=12), and CIBP + vehicle treatment (n=12). In CIBP
group, intact Walker 256 mammary gland carcinoma cells were
injected into the tibia cavity of rat without any treatment,
whereas in the sham operation group, the same amount of
heat-inactivated cells (negative control) were injected. In the
CIBP + PD168393 or CIBP + vehicle groups, 10 μg of PD168393
was dissolved in 5% DMSO (10 μl), or the same volume of DMSO
was administered by intrathecal injection on the 6th day after pain
model establishment, respectively. Drug was injected daily for 9
successive days.
To observe the effect of NRG1 on the induction of
cancer-induced bone pain, animals were randomly divided into three
groups, sham operation, HRG1β and HRG1β + PD168393. Six animals
were used for each time point. In the sham operation group, 10
μl of normal saline was administered by intrathecal
injection; while in the HRG1β group, 10 μl of exogenous NRG1
(HRG1β, 0.4 ng/μl) was administered by intrathecal
injection. In the HRG1β + PD168393 group, 1 h post-injection of 10
μg PD168393, the same dose of HRG1β was injected. Drug
injection was performed once a day for three successive days.
Behavior tests
Thermal hyperalgesia was determined by measuring paw
withdrawal latency (PWL) during heat stimulation, using a thermal
stimulator manufactured by Biomedical Engineering Institute of
Chinese Academy of Medical Science (BME-410A). As described
previously (23,24), rats were placed in a container (22
cm × 12 cm × 12 cm; length × width × height) with a smooth glass
floor. All rats were given 30 min to acclimate to the testing
environment. A heat source was focused on a portion of the hindpaw,
which was flush against the glass and a radiant thermal stimulus
was delivered to that site. The stimulus shut off automatically
when the hindpaw moved, or after 20 sec to avoid tissue damage.
Thermal stimuli were delivered three times to each hindpaw at
10-min intervals. Baseline thermal withdrawal latency for each rat
was measured one day prior to experiment.
Mechanical paw withdrawal thresholds (PWT) were
measured with von Frey filaments using a modification of the
up-and-down method (25). Rats
were placed in transparency organic glass cages with wire mesh
bases. A total of 30 min of time for the acclimation of testing
environment was given. A series of von Frey filaments with bending
pressures ranging from 0.6 to 26 g were used. Those von Frey hairs
were pressed perpendicularly against the plantar surface of the
hind paw and held for 3–4 sec. Each von Frey filament was applied 5
times with 15-sec intervals. A positive response was regarded in
presence of sharp withdrawal of the paw, licking of the paw or
flinching upon removal of the von Frey filament. Baseline PWT for
each rat was measured one day prior to experiment.
Radiobioassay analysis
To examine tibial destruction from the inoculated
tumor, animals were radiographed at 6, 14 and 21 days following
tumor cell inoculation. Animal right hind limbs were placed on
X-ray film and exposed to an X-ray source for 1/20 sec at 40 kV (p)
at day 12 post-inoculation. The radiographic images were visually
quantified using the scale developed by Schwei et al
(26).
Histological examination
Animals were euthanized under anesthesia three weeks
following establishment of the CIBP model. The right tibia and
surrounding tissues were carefully removed, fixed in 4%
paraformaldehyde (PFA), decalcified in PFA solution containing 5%
formic acid, embedded in paraffin and sectioned. Sections were
deparaffinized by immersing the tissues in dimethylbenzene,
followed by rehydration. Haematoxylin and eosin (H&E) staining
was performed according to standard procedures.
Real-time quantitative reverse
transcription-polymerase chain reaction (qRT-PCR)
Animals were sacrificed and the L4–L6 lumbar spinal
cord segments were dissected. Total RNA was extracted using TRIzol
Reagent according to the manufacturer’s protocol (Invitrogen, USA).
cDNA was then transcribed using PrimeScript™ RT Enzyme Mix I, Oligo
(dT) primer, Random 6 mers and 5xPrimeScript™Buffer (Takara). The
sequences of specific primers are listed in Table I. PCR reactions with 25 μl
volumes was carried out using a LightCycler Quantitative PCR
instrument (Roche, USA) in the following conditions: a preheating
cycle at 95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec,
specific annealing temperatures are indicated in Table I for 15 sec, and 72°C for 10 sec.
The relative expression from amplified RNA samples was calculated
using the 2−ΔΔCT method (27).
 | Table I.Specific primers for qRT-PCR. |
Table I.
Specific primers for qRT-PCR.
| Target gene | Primer
sequences | Annealing
temperature (°C) |
|---|
| NRG-1 | Forward:
5′-GGCAGTCAGCCCCTTTGTG-3′
Reverse: 5′-TGCAGGGTTGTGATGAAAGGA-3′ | 58 |
| ERBB2 | Forward:
5′-CCTGCCTCCACTTCAATCAT-3′
Reverse: 5′-CAGGATCCCACTTCCGTAGA-3′ | 59 |
| Akt-1 | Forward:
5′-CTCTGCATTGCCGAGTCC-3′
Reverse: 5′-GGTTCCTCCCCAGCACAT-3′ | 58 |
| p38MAPK | Forward:
5′-AGTGGCTGACCCTTATGAC-3′
Reverse: 5′-CACAGTGAAGTGGGATGGA-3′ | 58 |
| GAPDH | Forward:
5′-ATGACTCTACCCACGGCAAG-3′
Reverse: 5′-GGAAGATGGTGATGGGTTTC-3′ | 60 |
Western blot analysis
Protein was extracted from L4–L6 lumbar spinal cord
segments by homogenization with radio immunoprecipitation assay
(RIPA) lysis buffer and protein concentration was measured using a
bicinchoninic acid (BCA) Protein Assay reagent kit (Santa Cruz)
according to the manufacturer’s instructions. Equal amounts of
protein extracts were then separated by 6 or 10% sodium dodecyl
sulfate-polyacryl-amide gel electrophoresis (SDS-PAGE), transferred
to a PVDF membrane, then blocked with 5% (w/v) non-fat dry milk in
tris buffered saline plus Tween-20 (TBS-T; 0.1% Tween-20), followed
by overnight incubation with primary antibodies at 4°C. The applied
primary antibodies were diluted with TBS-T as follows: anti-ErB2
(1:200), anti-p-ErB2 (1:200), anti-NRG1 (1:200), anti-p-Akt-1
(1:500), anti-p-p38MAPK (1:500), and anti-GAPDH (1:1,000). After
washing with TBS-T, the membranes were incubated with HRP labeled
secondary antibodies (1:5,000). The expression level of target
proteins was determined by analysis using an Image Acquisition and
Analysis System (GDS8000, UVP, Inc., USA). The densitometric values
were semiquantified by Bio-Rad and Quantity One imaging analysis
software. The housekeeping protein, GAPDH, was used as an internal
control.
Statistical analysis
Statistical analysis was performed using SPSS 16.0
software. Data were presented as the mean ± standard deviation
(SD). P-value <0.05 was considered to be significant. Data were
analyzed using a one-way and repeated measures analysis of variance
(ANOVA) followed by Bonferroni-corrected post-hoc tests.
Results
Time course of pain development in the
rat model of CIBP
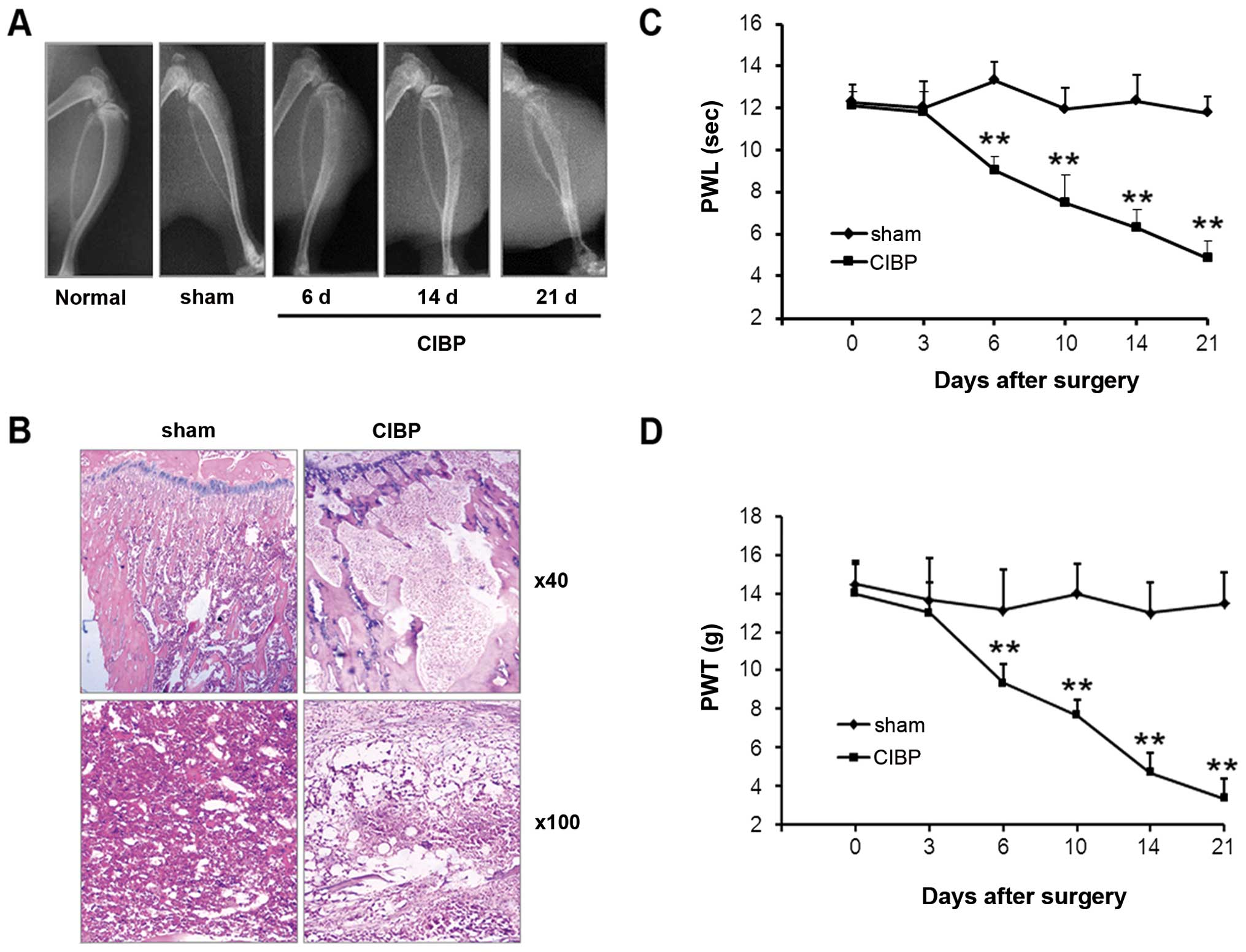
Radiological and histological examinations were used
to evaluate bone destruction in the rat model of CIBP. As shown by
X-ray images, no obvious alternation of tibia structure was found
at either the 6th, 14th, or 21st day after inoculation of
heat-inactivated Walker 256 mammary gland carcinoma cells (sham)
(Fig. 1A; bone destruction score:
0; n=6). However, 6 days following inoculation of Walker 256 tumor
cells, defects within the proximal tibial epiphysis were detected.
These defects became more prominent at the 14th day (bone
destruction score: day 6, 1.66±0.52; day 14, 2.66±0.54; n=6 for
each). Further deterioration was found at 21 days post-injection,
with the occurrence of cortical destruction, tumor dissemination
and soft tissue tumors (bone destruction score: 4.33±0.43; n=6).
Histological examination showed that bone marrow spaces were
infiltrated with tumor cells at the 21st day following tumor cell
inoculation (Fig. 1B). Moreover,
bone trabecula defects, loss of normal bone structure, cortical
destruction, and soft tissue tumors were observed in the CIBP model
group. Bone destruction was not observed in animals within the sham
operation group. A progressive decrease in both PWL and PWT,
corresponding to thermal and mechanical stimulation of the
inoculated hind paw, occurred at the 6th day after tumor cell
injection (Fig. 1C and D).
Compared with the sham operation group, significant differences in
PWT and PWL values in the CIBP group were found at days 6, 10, 14
and 21 following tumor cell inoculation (P<0.01).
Sustained activation of NRG1 and ErbB2
signaling pathway in the spinal dorsal tissues of the rat CIBP
model
Next, we examined the expression level of NRG1 and
ErbB2 in the tissues of L4–L6 spinal cord space from rats
inoculated with intact or heat-inactivated Walker 256 mammary gland
carcinoma cells. Compared with sham operation control, the
expression levels of mRNA and protein of NRG1, ErbB2 and p-ErbB2
were significantly elevated at day 6 after tumor cell inoculation
(P<0.05) (Fig. 2).
Time-dependent upregulation of NRG1, ErbB2 and p-ErbB2 expression
was observed within three weeks following tumor cell injection.
These observations suggest that sustained activation of NRG1 and
ErbB2 signaling in the spinal dorsal tissues after intra-tibia
inoculation with tumor cells might be involved in development of
CIBP in the rat model.
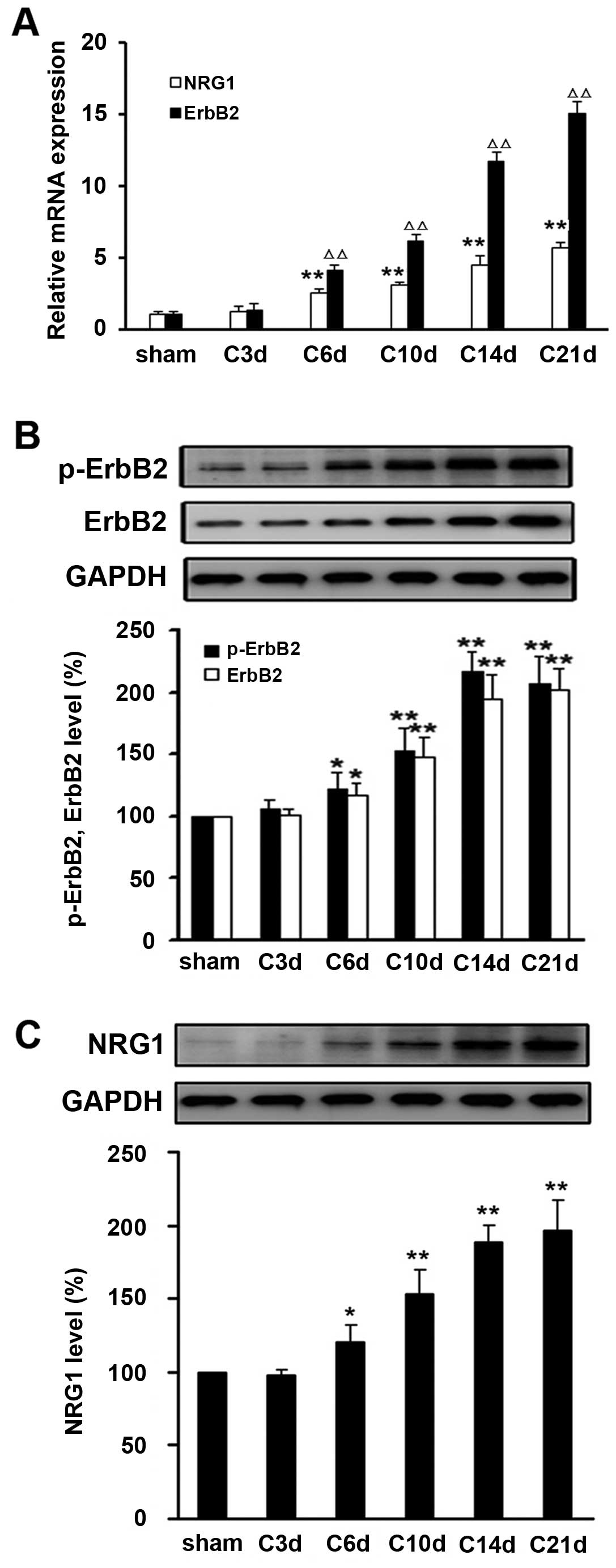 | Figure 2.Sustained activation of NRG1 and ErbB2
in the spinal dorsal tissues after intra-tibia inoculation with
Walker 256 mammary gland carcinoma cells. (A) As indicated day
after Walker 256 inoculation (day 3, 6, 10, 14 and 21), the mRNA
levels of ErbB2 and NRG1 were determined by real-time qRT-PCR.
Relative mRNA expression (target gene/GAPDH) was quantified from
six animal samples. NRG1 vs. sham, *P<0.05,
**P<0.01; ErbB2 vs. sham, ΔP<0.05,
ΔΔP<0.01. (B) Protein expressions of ErbB2, (B)
p-ErbB2 and (C) NRG1 were examined by western blot analysis.
Relative protein expression (target protein/GAPDH) was quantified
from six animal samples. *P<0.05,
**P<0.01 compared with sham (day 0); n=6. |
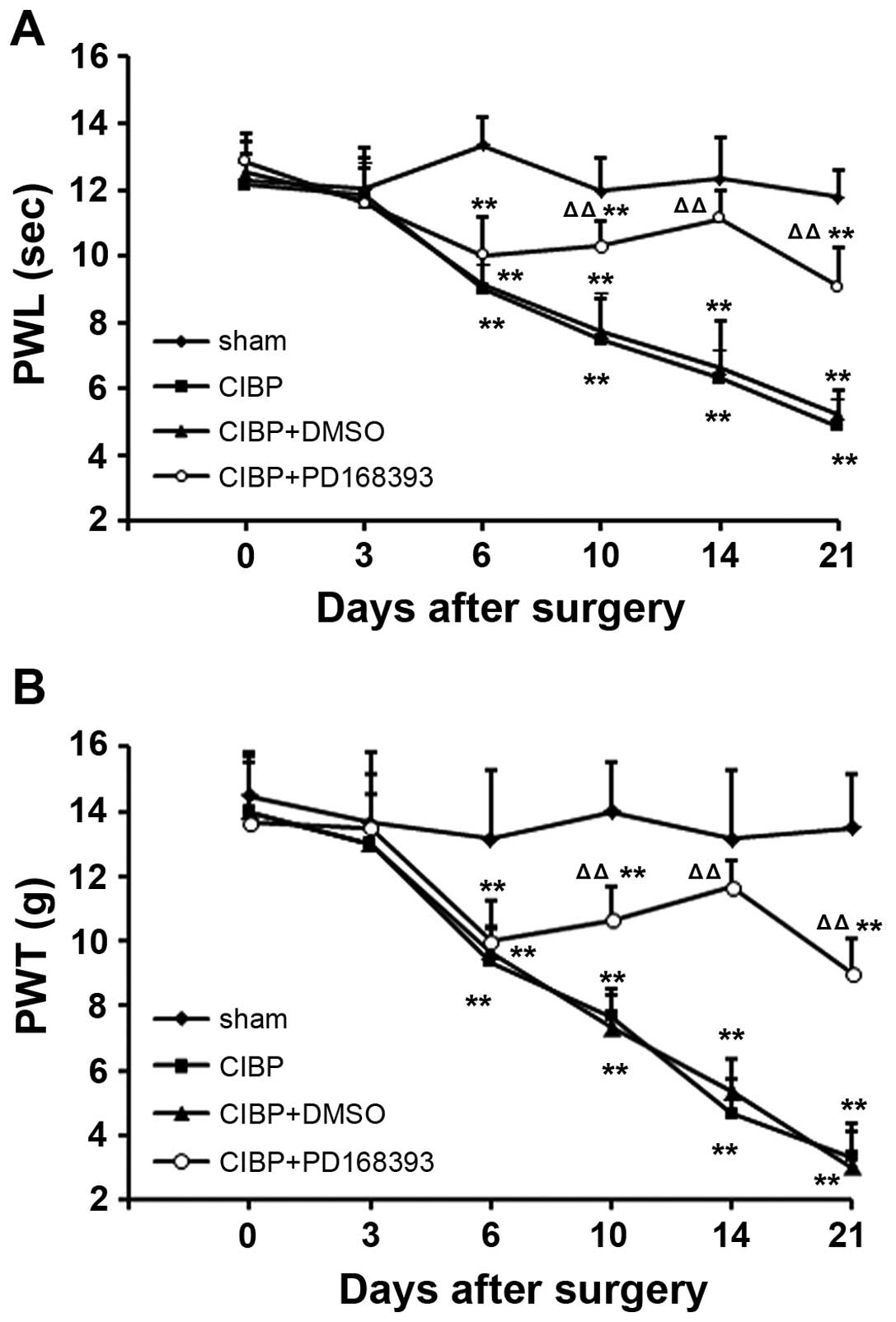
Inhibition of ErbB2 signaling attenuates
pain-like behavior induced in the rat model of CIBP
To clarify the role of ErbB signaling pathway in
CIBP, PD168393, a selective tyrosine kinase inhibitor of ErbB2 was
used in this study. The inhibitor was administered daily,
intrathecally, beginning 6 days after tumor cell inoculation. As
shown in Fig. 3, after 4 days of
treatment with the ErbB2 inhibitor, CIBP-induced thermal
hyperalgesia and mechanical allodynia were significantly attenuated
10 days after tumor cell inoculation (P<0.01, compared to CIBP
group). These protective effects were sustained until 14th day
after tumor cell inoculation, afterwards gradually decreasing until
day 21, likely due to drug withdrawal at day 15. The expression of
NRG1 was not impacted by the ErbB2 inhibitor.
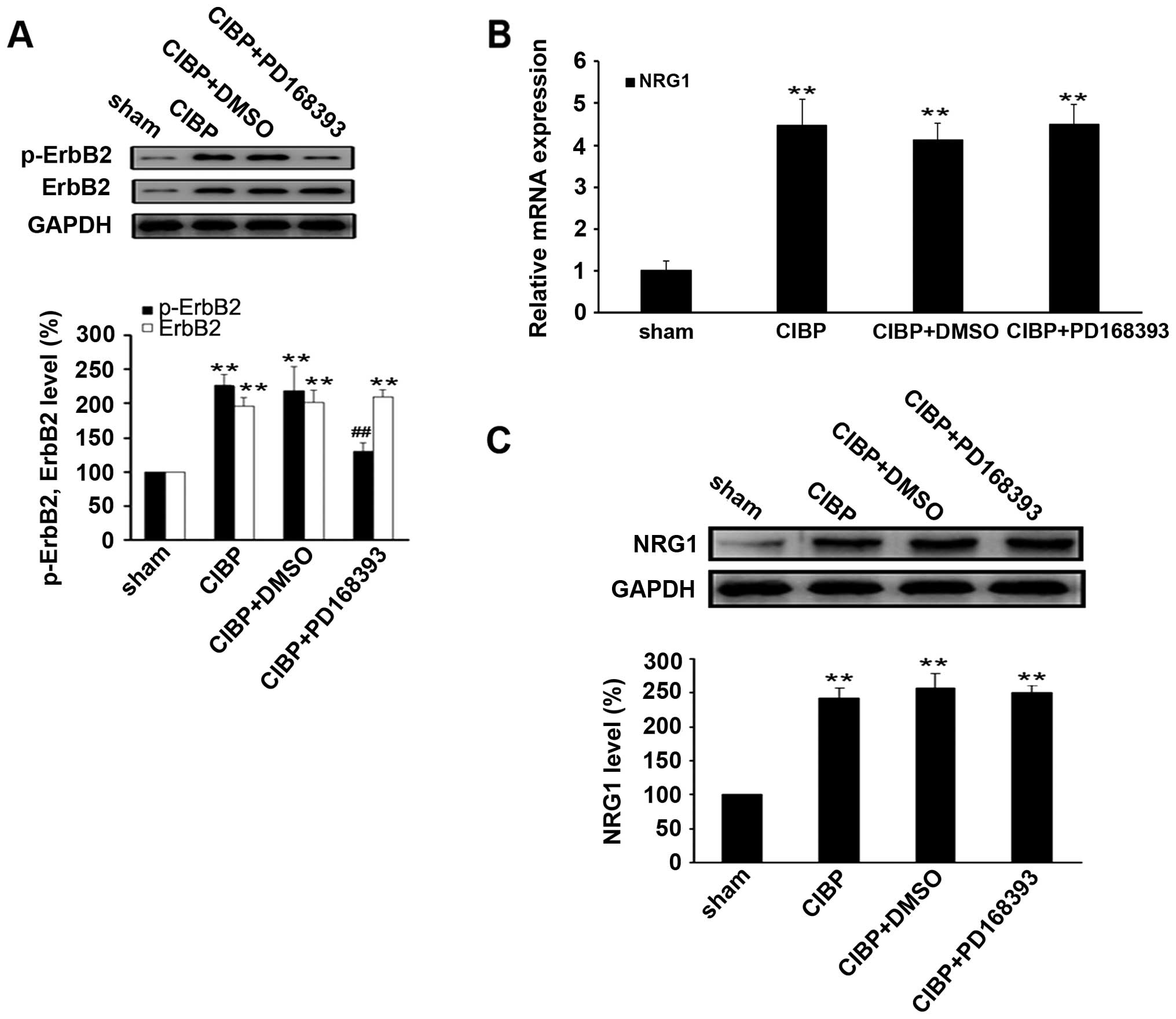
To confirm the inhibitory effect of PD168393 on
ErbB2 signaling activity, we examined the CIBP-induced
phosphorylation of ErbB2 in spinal cord tissues of rats. The
results showed that the level of phosphor-ErbB2 was significantly
reduced in spinal cord tissues of rats in CIBP + PD168393 group,
compared with CIBP or CIBP + DMSO (P<0.01) (Fig. 4A). However, treatment with PD168393
had no effect on the expression levels of NRG1 mRNA and protein, as
compared with CIBP group (Fig. 4B and
C), suggesting NRG1 may act as an upstream regulator of ErbB2
signaling pathway.
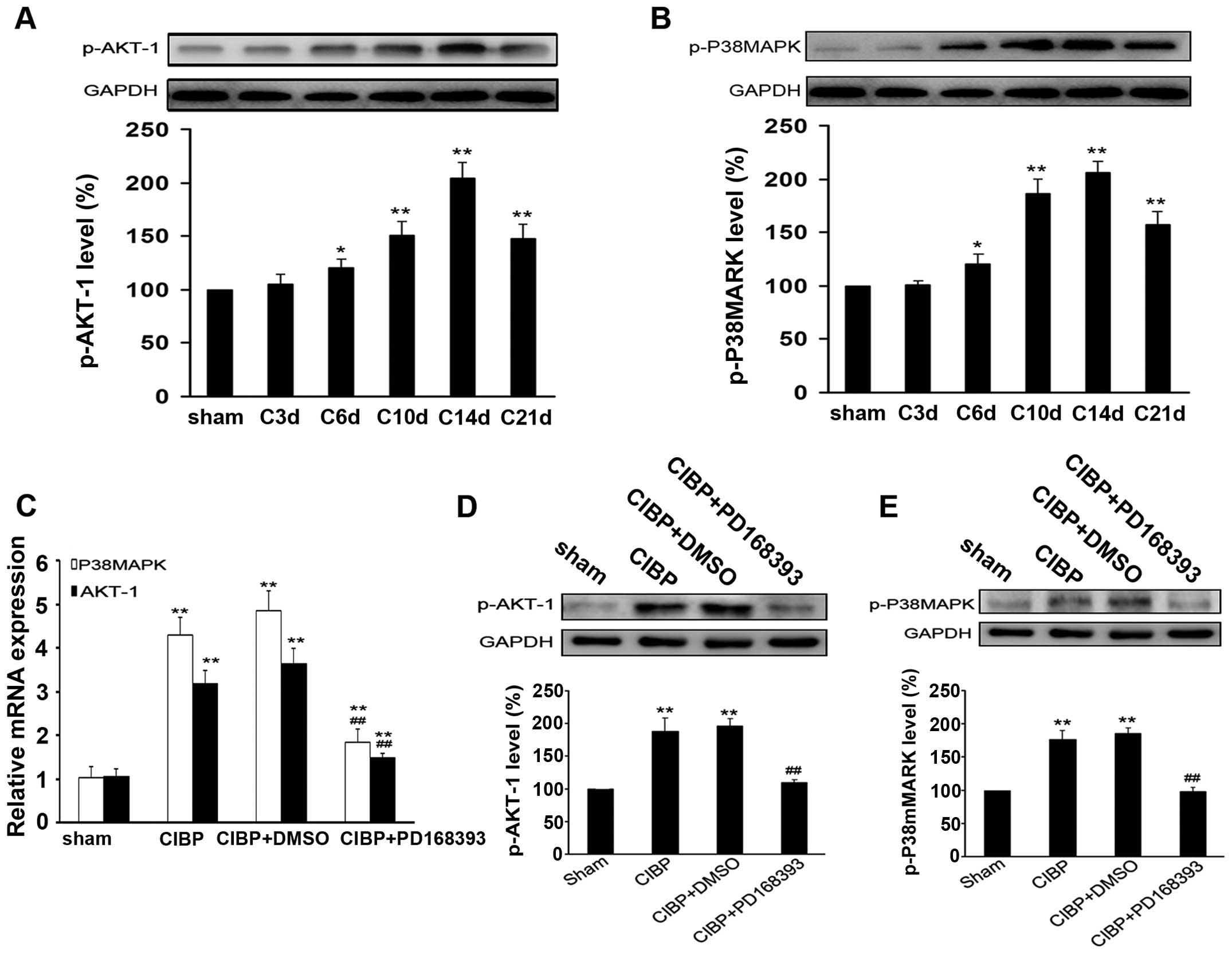
Inhibition of ErbB2 signaling suppresses
the activation of Akt-1 and p38MAPK induced by tumor inoculation in
the rat model of CIBP
As shown in Fig. 5A and
B, activation of Akt-1 and P38MAPK was detected in rats with
intratibia injection of tumor cells. The phosphorylation level of
Akt-1 and P38MAPK gradually increased from the 6th day after tumor
inoculation, and reached a peak at the 14th day, then decreased
until day 21. After 9 days of treatment with PD168393, the mRNA
expressions of Akt-1 and P38MAPK were greatly reduced and the
protein levels of p-Akt-1 and p-P38MAPK were significantly reduced
in spinal cord tissues of rats, as compared with those in CIBP
group (P<0.01) (Fig. 5C–E).
However, no significant difference in levels of p-Akt-1 and
p-P38MAPK was found between CIBP group and CIBP + DMSO group
(Fig. 5D and E).
The administration of exogenous NRG1
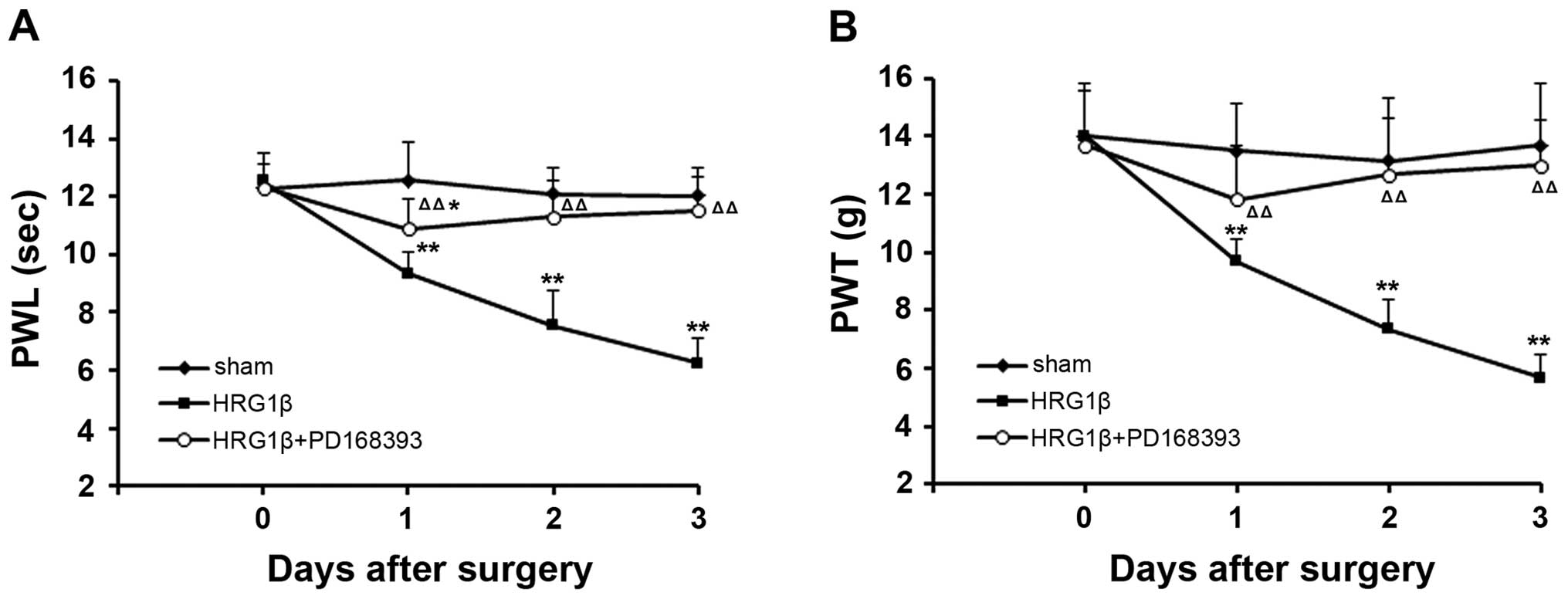
provokes pain-like behavior in the rat model of CIBP
To validate the effect of NRG1 on pain, we observed
the pain-like behavior of rats with intrathecal injection of
exogenous NRG1. We found that intrathecal administration of
exogenous NRG1 (HRG1β) could trigger thermal hyperalgesia and
mechanical allodynia in rats immediately after injection. In our
observation, the values of PWL and PWT were significantly reduced
after an administration of HRG1β in a time-dependent pattern
(P<0.01, compared with sham group) (Fig. 6). However, co-treatment with HRG1β
and PD168393 relieved the exogenous NRG1-induced thermal
hyperalgesia and mechanical allodynia (P<0.01 compared to
treatment with HRG1β alone) (Fig.
6).
The administration of exogenous NRG1
induces the activation of ErbB2, Akt-1 and p38MAPK, which could be
blocked by ErbB2 inhibitor
Our study also found that administration of
exogenous NRG1 induced an increase in mRNA and protein expression
of ErbB2, as well as the level of p-ErbB2 (Fig. 7A, C and D). Interestingly, the
activation of ErbB2 could be inhibited by co-administration of
PD168393 (Fig. 7D). Nevertheless,
the co-treatment with PD168393 and HRG1β had no impact on the
expression of NRG1, as compared to the treatment with HRG1β alone
(P>0.05) (Fig. 7C). Moreover,
co-treatment with PD168393 and HRG1β also suppressed HRG1β-induced
Akt-1, p38MAPK transcription and activation (Fig. 7B, E and F), implying that ErbB2 may
serve as downstream factor of NRG1 in the regulation of signaling
pathways of both Akt-1 and p38MAPK.
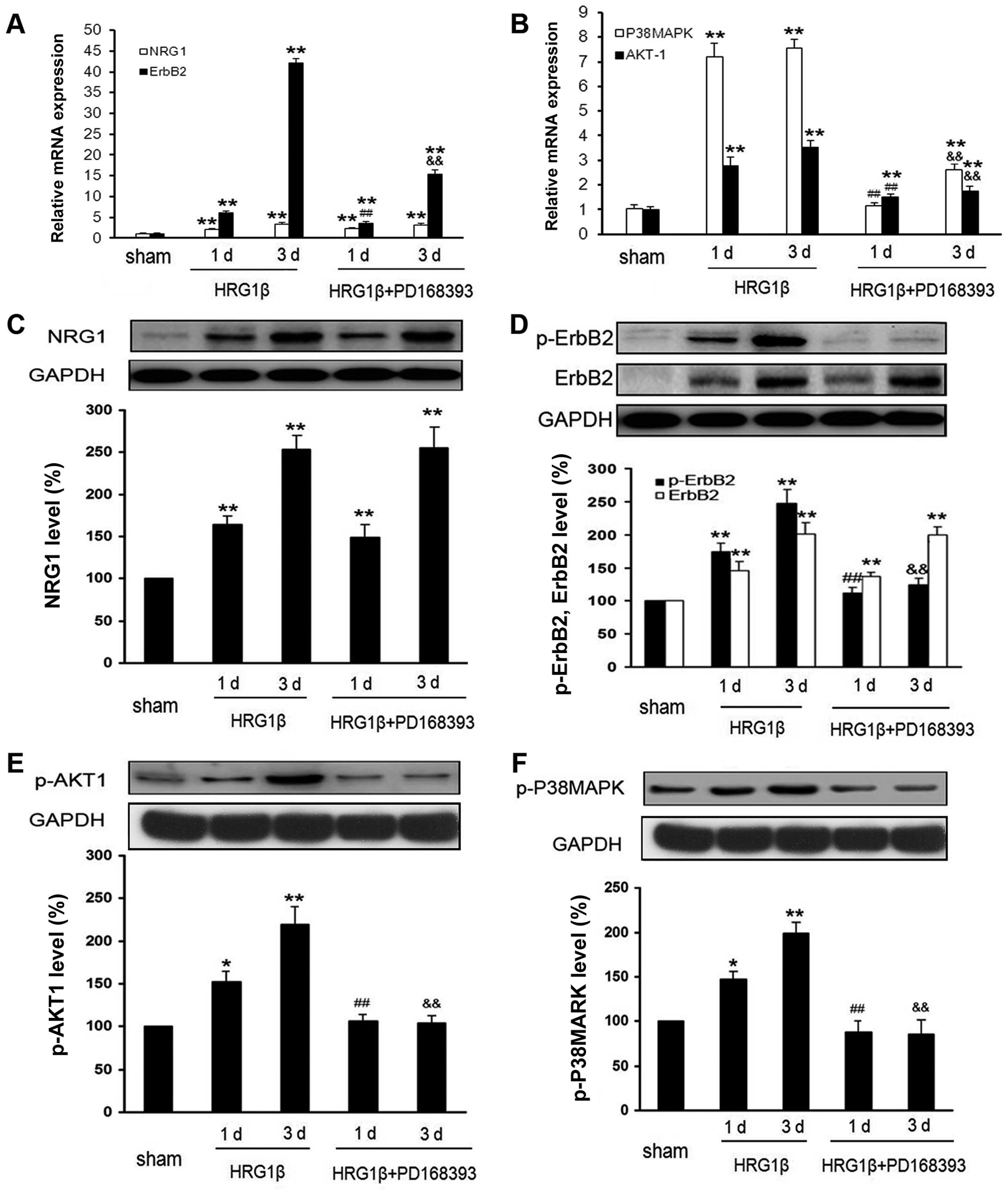 | Figure 7.Administration of exogenous NRG1
induces the activation of ErbB2, Akt-1 and p38MAPK, which could be
blocked by ErbB2 inhibitor. In sham operation group, 10 μl
of normal saline was injected into the tibia cavity and 10
μl of neuregulin-β1 EGF domain (HRG1β, 0.4 ng/μl) was
injected into the tibia cavity in HRG1β group. In HRG1β + PD168393
group, the same dose of HRG1β was injected after 1-h treatment with
10 μg PD168393. Drug injection was performed once a day for
three successive days. (A and B) The mRNA expressions of NRG1,
ErbB2, Akt-1 and p38MAPK were analyzed by q-RT-PCR. (C–F) The
protein expressions were determined by western blot analysis.
*P<0.05, **P<0.01 compared with sham;
#P<0.05, ##P<0.01 compared with HRG1β
1d; &P<0.05, &&P<0.01
compared with HRG1β 3d; n=6. |
Discussion
Emerging lines of evidence show that astrocytes and
microglia in the spinal cord play essential roles in the initiation
and maintenance of persistent pain induced by tissue inflammation,
nerve injury and cancer (28–30).
NRG1 and ErbB have been found to be expressed in the spinal cord
glia (31,32). The NRG1-ErbB signaling pathway has
been shown to promote microglial proliferation and chemotaxis,
contributing to microgliosis and pain after peripheral nerve injury
(19), but its role in CIBP
remains largely unknown. In the present investigation, we
determined the involvement of NRG1-ErbB signaling using a rat CIBP
model which was established by inoculation of Walker 256 tumor
cells (33). Consistent with a
previous report (34), bone
destruction, as well as the initiation of pain-like behavior
occurred 6 days after tumor cell inoculation. The incidence of
cortical destruction, tumor dissemination and soft tissue tumors
was elevated days after tumor cell inoculation. The results of
radiobioassay, histological and behavior analysis confirmed the
successful establishment of a CIBP rat model.
Previous studies have shown that NRG1 activates the
ErbB2 signaling pathway and promotes growth of microglial cells in
the spinal cord, which may contribute to neuropathic pain following
injury (35,36). In accordance with those reports, we
found an increase in the expression levels of NRG1 and ErbB2 in the
spinal cord of rats inoculated with tumor cells. Moreover, the
intrathecal administration of PD168393, an inhibitor of ErbB2
signaling, greatly reduced the CIBP-induced thermal hyperalgesia as
well as mechanical allodynia the rat model of CIBP. Furthermore,
LaCroix-Fralish et al reported that intrathecal
administration of recombinant NRG1-β1 protein significantly
decreased the hindpaw tactile withdrawal threshold in rats
(36). Consistent with their
finding, our study demonstrated that intrathecal administration of
exogenous NRG1 could trigger thermal hyperalgesia and mechanical
allodynia through upregulation of ErbB2 and p-ErbB2 mRNA and
protein levels.
Calvo et al demonstrated that in
vitro, NRG1 induces activation of the ErbB2 receptor and
enhances the phosphorylation of ERK1/2 and Akt, rather than the
activation of p38MAPK in micoglial cells (35), however, in our study, we found the
enhanced phosphorylation of both Akt-1 and p38MAPK in spinal cord
tissues from the rat model of CIBP. This discrepancy may be due to
the difference in animal models used between studies. Similarly,
another study found an increase in phosphorylated p38 in the dorsal
root ganglia (DRG) and spinal dorsal horn neurons on day 14 after
an inoculation of osteosarcoma cells into the femur medullary canal
(37). In that study, treatment
with the p38MAPK inhibitor SCIO-469, led to a decrease in
osteosarcoma-induced clinical score, with no impact on allodynia
(37). Hence, the necessity of
p38MAPK in CIBP needs to be further clarified. In addition,
PI3K/Akt activation in the periphery has been suggested to
contribute to pain behavior that was induced by capsaicin in rats
(38). Intradermal administration
of the PI3K inhibitor, wortmannin, dose-dependently inhibited the
changes in exploratory behavior evoked by capsaicin injection
(38). Here, we show that Akt
signaling pathway is also activated and serves as a downstream
factor of NRG1-ErbB2 signaling, as intrathecal administration of
exogenous NRG1 promoted the activation of Akt-1, but inhibition of
ErbB2 signaling reduced the level of p-Akt-1.
Collectively, our current study demonstrates that
the NRG1-ErbB2 signaling pathway is involved in the regulation of
CIBP in an experimental rat model. Akt-1 and p38MAPK kinases are
also involved, and serve as downstream modulators of the NRG1-ErbB2
pathway. The roles of Akt-1 and p38MAPK in NRG1-ErbB2-mediated CIBP
need to be further investigated.
Acknowledgements
This study was supported by a grant
from Liaoning Science and Technology Plan Projects (no.
2012225016).
References
|
1.
|
Bruera E and Kim HN: Cancer pain. JAMA.
290:2476–2479. 2003. View Article : Google Scholar
|
|
2.
|
Coleman RE: Metastatic bone disease:
clinical features, pathophysiology and treatment strategies. Cancer
Treat Rev. 27:165–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Laird B, Colvin L and Fallon M: Management
of cancer pain: basic principles and neuropathic cancer pain. Eur J
Cancer. 44:1078–1082. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Colburn RW, DeLeo JA, Rickman AJ, Yeager
MP, Kwon P and Hickey WF: Dissociation of microglial activation and
neuropathic pain behaviors following peripheral nerve injury in the
rat. J Neuroimmunol. 79:163–175. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Hains BC and Waxman SG: Activated
microglia contribute to the maintenance of chronic pain after
spinal cord injury. J Neurosci. 26:4308–4317. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Fu KY, Light AR, Matsushima GK and Maixner
W: Microglial reactions after subcutaneous formalin injection into
the rat hind paw. Brain Res. 825:59–67. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Wang XW, Li TT, Zhao J, et al:
Extracellular signal-regulated kinase activation in spinal
astrocytes and microglia contributes to cancer-induced bone pain in
rats. Neuroscience. 217:172–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Sessle BJ: Glia: non-neural players in
orofacial pain. J Orofac Pain. 21:169–170. 2007.PubMed/NCBI
|
|
9.
|
Urch C: The pathophysiology of
cancer-induced bone pain: current understanding. Palliat Med.
18:267–274. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Wang LN, Yao M, Yang JP, et al:
Cancer-induced bone pain sequentially activates the ERK/MAPK
pathway in different cell types in the rat spinal cord. Mol Pain.
7:482011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Carraway KL III and Cantley LC: A neu
acquaintance for erbB3 and erbB4: a role for receptor
heterodimerization in growth signaling. Cell. 78:5–8. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Pentassuglia L and Sawyer DB: The role of
Neuregulin-1beta/ErbB signaling in the heart. Exp Cell Res.
315:627–637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Esper RM, Pankonin MS and Loeb JA:
Neuregulins: versatile growth and differentiation factors in
nervous system development and human disease. Brain Res Rev.
51:161–175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hynes NE and MacDonald G: ErbB receptors
and signaling pathways in cancer. Curr Opin Cell Biol. 21:177–184.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Huang YZ, Won S, Ali DW, et al: Regulation
of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS
synapses. Neuron. 26:443–455. 2000. View Article : Google Scholar
|
|
16.
|
Canoll PD, Musacchio JM, Hardy R, Reynolds
R, Marchionni MA and Salzer JL: GGF/neuregulin is a neuronal signal
that promotes the proliferation and survival and inhibits the
differentiation of oligodendrocyte progenitors. Neuron. 17:229–243.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Rio C, Rieff HI, Qi P, Khurana TS and
Corfas G: Neuregulin and erbB receptors play a critical role in
neuronal migration. Neuron. 19:39–50. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Mei L and Xiong WC: Neuregulin 1 in neural
development, synaptic plasticity and schizophrenia. Nat Rev
Neurosci. 9:437–452. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Calvo M, Zhu N, Tsantoulas C, et al:
Neuregulin-ErbB signaling promotes microglial proliferation and
chemotaxis contributing to microgliosis and pain after peripheral
nerve injury. J Neurosci. 30:5437–5450. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Medhurst SJ, Walker K, Bowes M, et al: A
rat model of bone cancer pain. Pain. 96:129–140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Zhang YQ, Ji GC, Wu GC and Zhao ZQ:
Excitatory amino acid receptor antagonists and electroacupuncture
synergetically inhibit carrageenan-induced behavioral hyperalgesia
and spinal fos expression in rats. Pain. 99:525–535. 2002.
View Article : Google Scholar
|
|
22.
|
Fry DW, Bridges AJ, Denny WA, et al:
Specific, irreversible inactivation of the epidermal growth factor
receptor and erbB2, by a new class of tyrosine kinase inhibitor.
Proc Natl Acad Sci USA. 95:12022–12027. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Song XJ, Wang ZB, Gan Q and Walters ET:
cAMP and cGMP contribute to sensory neuron hyperexcitability and
hyperalgesia in rats with dorsal root ganglia compression. J
Neurophysiol. 95:479–492. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Wang ZB, Gan Q, Rupert RL, Zeng YM and
Song XJ: Thiamine, pyridoxine, cyanocobalamin and their combination
inhibit thermal, but not mechanical hyperalgesia in rats with
primary sensory neuron injury. Pain. 114:266–277. 2005. View Article : Google Scholar
|
|
25.
|
Tal M and Bennett GJ: Extra-territorial
pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia
and mechano-allodynia in the territory of an uninjured nerve. Pain.
57:375–382. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Schwei MJ, Honore P, Rogers SD, et al:
Neurochemical and cellular reorganization of the spinal cord in a
murine model of bone cancer pain. J Neurosci. 19:10886–10897.
1999.PubMed/NCBI
|
|
27.
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Ye J, Wang T, Han LS, et al: Diagnosis,
treatment, follow-up and gene mutation analysis in four Chinese
children with biotinidase deficiency. J Inherit Metab Dis.
32:S295–S302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Inoue K and Tsuda M: Microglia and
neuropathic pain. Glia. 57:1469–1479. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Hald A, Nedergaard S, Hansen RR, Ding M
and Heegaard AM: Differential activation of spinal cord glial cells
in murine models of neuropathic and cancer pain. Eur J Pain.
13:138–145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Gerecke KM, Wyss JM, Karavanova I,
Buonanno A and Carroll SL: ErbB transmembrane tyrosine kinase
receptors are differentially expressed throughout the adult rat
central nervous system. J Comp Neurol. 433:86–100. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Dimayuga FO, Ding Q, Keller JN, Marchionni
MA, Seroogy KB and Bruce-Keller AJ: The neuregulin GGF2 attenuates
free radical release from activated microglial cells. J
Neuroimmunol. 136:67–74. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Simpkins H, Lehman JM, Mazurkiewicz JE and
Davis BH: A morphological and phenotypic analysis of Walker 256
cells. Cancer Res. 51:1334–1338. 1991.PubMed/NCBI
|
|
34.
|
Mao-Ying QL, Zhao J, Dong ZQ, et al: A rat
model of bone cancer pain induced by intra-tibia inoculation of
Walker 256 mammary gland carcinoma cells. Biochem Biophys Res
Commun. 345:1292–1298. 2006. View Article : Google Scholar
|
|
35.
|
Calvo M, Zhu N, Grist J, Ma Z, Loeb JA and
Bennett DL: Following nerve injury neuregulin-1 drives microglial
proliferation and neuropathic pain via the MEK/ERK pathway. Glia.
59:554–568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Lacroix-Fralish ML, Tawfik VL,
Nutile-McMenemy N and Deleo JA: Neuregulin 1 is a pronociceptive
cytokine that is regulated by progesterone in the spinal cord:
implications for sex specific pain modulation. Eur J Pain.
12:94–103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Svensson CI, Medicherla S, Malkmus S, et
al: Role of p38 mitogen activated protein kinase in a model of
osteosarcoma-induced pain. Pharmacol Biochem Behav. 90:664–675.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Sun R, Yan J and Willis WD: Activation of
protein kinase B/Akt in the periphery contributes to pain behavior
induced by capsaicin in rats. Neuroscience. 144:286–294. 2007.
View Article : Google Scholar : PubMed/NCBI
|