Introduction
Hepatocellular carcinoma (HCC) is a common
malignancy, the third cause of cancer-related mortality worldwide
(1). Frequent intrahepatic and
extrahepatic metastases are the main factors contributing to the
high mortality of HCC patients (2). Previously, bone metastases were
considered less common in patients with HCC. However, due to the
improved duration of intrahepatic primary tumors, bone metastases
from HCC seem to be increasing and frequently recorded (3,4).
Thus, early diagnosis of bone metastasis plays a pivotal role in
the therapeutic regimen and the assessing prognosis (5). It is generally considered that cancer
is a dynamic exchange between tumor cells and surrounding host
cells, as first proposed in 1889 by Stephen Paget who indicated
that the seeding of metastatic cancer cells depended on the host
organ microenvironment (the ‘seed and soil’ concept) (6). To our knowledge, soluble factors
secreted by host cells and direct cell-to-cell interactions are
deemed to contribute to the preferential metastasis and growth of
cancer cells in bone (7,8), however, the underlying mechanism of
metastasis of HCC in the bone is poorly understood.
Marrow/mesenchymal stromal cells (MSCs) play a major
role of tumor stroma in bone microenvironment and have an effect on
growth and metastasis of human malignancies. However, the exact
effect of MSCs on tumor growth and progress is still under debate
(9). The contradiction exists in
some cancer cells, including melanoma (10,11),
breast cancer (12), colon cancer
(13), Kaposi’s sarcoma (14), and prostate cancer (15). In HCC, some studies demonstrated
that MSCs contributed to tumor progression (16–18),
several other studies demonstrated that MSCs could inhibit
metastasis (19–21) and tumorigenesis (22). MSCs in HCC metastasis remains
controversial. MSCs secrete various cytokines that have both
paracrine and autocrine functions, besides MSCs are able to
generate a direct effect through intercellular signaling via
physical contact with tumor cells (23).
CCL5 (also known as regulated upon activation,
normally T cell expressed and secreted, or RANTES) belongs to the
CC family of inflammatory chemokines and is expressed by many liver
and infiltrating cells (24), and
interacts with the G-protein coupled receptors CCR1, CCR3 and CCR5.
CCL5 is also secreted from tumor or stromal cells, and may act in
an autocrine or a paracrine manner on cancer cells to enhance their
migration and invasion (12,25,26).
It was shown that CCL5 exhibited in vitro migratory and
invasive stimulus on HCC cells (27,28),
but some other studies reported that increased CCL5 expression
might be connected with favorable outcomes in some cancer diseases
(29,30). However, the effects of CCL5
mediated by HS-5 cells and detailed mechanisms of Huh7 cell
progress are largely unknown, thus, there is an urgent need for
increased understanding.
Based on the studies above, we aimed to investigate
the effects of CCL5 from HS-5 cells on Huh7 cells, as well as the
underlying mechanisms. Our investigation found that HS-5-CM could
promote the proliferation, migration and invasion of Huh7 cells,
and inhibited apoptosis. CCL5 down-regulation inhibited the effects
of HS-5 cells on Huh7 cells migration and invasion via PI3K-Akt
signaling pathway and reduced MMP-2 expression. These results
suggest that MSCs mediated CCL5 promoting migration and invasion of
Huh7 cells and it may offer a novel strategy to efficiently inhibit
metastasis.
Materials and methods
Cell culture and reagents
The human hepatocellular carcinoma cell lines Huh7,
SMMC-7721, HepG2 and normal human liver cell lines LO2 were kindly
provided by Dr Tongchuan He (University of Chicago Medical Center),
and bone marrow stromal cell lines HS-5 were purchased from
American Type Culture Collection (ATCC, Manassas, VA). Cells were
maintained in Dulbecco’s modified Eagle’s medium (DMEM, HyClone,
USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA) and
100 U/ml streptomycin/penicillin at 37°C in 5% CO2. The
primary antibodies used in this study were: rabbit anti-Akt
monoclonal antibody, rabbit anti-phosphor-Akt monoclonal antibody,
rabbit anti-ERK1/2 monoclonal antibody and rabbit
anti-phosphor-ERK1/2 monoclonal antibody and were obtained from
Cell Signaling Technology. Rabbit anti-MMP-2 polyclonal antibody
was purchased from Immunoway (Immunoway, USA). Mouse anti-β-actin
monoclonal antibody was purchased from Santa Cruz Biothechnology.
Secondary antibodies included HRP-conjugated goat anti-mouse IgG
antibody and anti-rabbit IgG antibody were purchased from Zhongshan
Goldenbridge Biotechnology. Specific inhibitors of PI3K (LY294002)
and ERK1/2 (PD98059) were obtained from Cell Signaling Technology.
CCL5 neutralization antibody was purchased from Peprotech.
Collection of HS-5 cell conditioned
medium (HS-5-CM)
HS-5 cells were plated in a 100 mm2
culture dish at a density of 80%, for 6 h, for cells adherence,
culture medium was changed to serum-free DMEM (SF DMEM). The
culture supernatant was harvested every day for 3 days and then all
medium were pooled, and sterile filtered. The HS-5-CM was stored at
−20°C.
Co-culture experiment
The co-culture experiment was set up in duplicates
using 6-well Transwell inserts (Millipore, USA) with 0.4 μm
pore size. In Transwell chamber, HS-5 cells were plated at the
density of 105 cells/well in 1 ml, whereas Huh7 or
SMMC-7721 or LO2 cells were plated in the 6-well plates at the
density of 2×105 cells/well in 2 ml. Cells were left to
adhere for 6 h before being put together in the SF DMEM for 3 days,
and cells alone were used as control.
Cell viability assay
MTT [3-(4, 5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide] assay was used to detect cell
viability. A total of 2×103 cells/well were cultured in
96-well plates and treated with fresh DMEM, 50% HS-5-CM
(CM:DMEM=1:1) or 100% HS-5-CM containing 1% FBS for 24, 48 and 72
h. After the indicated hours of culture, the MTT reagent (Sigma,
St. Louis, MO, USA) was added (20 μl/well), and incubated
for 4 h at 37°C. Dimethyl sulfoxide was added to dissolve the
formazan product for 10 min at room temperature. Finally, the
absorbance was measured daily for the next three days at 492 nm
using a microplate reader. Each group was done in sextuplicate, and
the overall experiment was repeated three times.
Flow cytometry
Cell cycle and cell apoptosis analysis were assessed
by flow cytometry. Cells were divided into 3 groups: i) control
group, Huh7 cells; ii) co-culture group, Huh7 cells were
co-cultured with HS-5 cells; and iii) HS-5-CM group, Huh7 cells
were treated with HS-5-CM. In brief, cells were seeded into 6-well
plates at a density of 2×105 cells/well. Every
experiment was repeated twice in each group. Cells were harvested,
re-suspended in 1 ml cold PBS. Samples were fixed with pre-cold 75%
enthanol for 1 h at 4°C by cell cycle assay, and other samples were
added using propidium iodide (PI) and FITC-Annexin V for cell
apoptosis analysis according to the manufacturer’s protocol
(Invitrogen, USA). Respectively, data were analyzed using FACsorter
(Becton Dickinson, CA, USA).
Wound-healing assay, Transwell chamber
migration and invasion assay
For wound-healing assay, cells were grown to 95%
confluence and the monolayer was scratched with a pipette tip, and
then culture medium was replaced with fresh DMEM or 50% HS-5-CM or
100% HS-5-CM containing 1% FBS. Cells that migrated into the
scratched area were compared using bright field microscopy at 48 h.
Cell migration and invasion assay were performed by 24-well
Transwell chambers (8 μm pore size, Millipore) without or
with ECM gel (Sigma). Briefly, cells (5×104/400
μl) in SF DMEM were seeded onto the upper chambers, and
fresh DMEM or 50% HS-5-CM or 100% HS-5-CM (600 μl/well)
containing 20% FBS added to the lower chambers. After 24 h, cells
that migrated to the underside of the filter were fixed with
methanol and stained with hematoxylin and eosin (H&E), and
counted by brightfield microscopy. All the experiments were
repeated three times.
Animal studies
The in vivo experiments were performed in
accordance with the guidelines established by the Animal Care and
Use Committee, of Chongqing University Laboratory Animal Research.
The 4-6-week old male NOD/SCID mice were randomly divided into 2
groups (n=4/group), respectively, and were injected subcutaneously
with a mixture of equal number of HS-5 cells (3×106) and
Huh7 cells (3×106) or with Huh7 cells (6×106)
alone. Tumor volume was measured every 10 days with vernier
calipers, and calculated in mm3 as ab2/2,
where a and b represent the largest and smallest tumor diameters,
respectively. The mice were sacrificed after 70 days, and tumor
tissues, livers and lungs were collected and stained using H&E
in order to examine the histopathology.
Total RNA isolation, RT-PCR, and
real-time quantitative PCR (qRT-PCR) analysis
Huh7 cells were co-cultured with HS-5 cells or alone
in SF DMEM for 3 days, total RNA was isolated using TRIzol Reagents
(Invitrogen) according to the manufacturer’s instructions. Total
RNA (2 μg) was used to generate cDNA templates for reverse
transcriptase-PCR. Touchdown PCR analysis determined the gene
expression levels and was performed by using the following program:
95°C × 2 min for one cycle, 10 cycles at 92°C × 20 sec, 65°C × 30
sec, and 70°C × 40 sec with a decrease of one degree per cycle, and
25 cycles at 92°C × 20 sec, 55°C × 30 sec, and 70°C × 40 sec. 70°C
× 5 min for one cycle. Real-time PCR was run in the Rotor-Gene 6000
Real-Time PCR machine (Corbett Research, Australia) using SYBR
Premix Ex Taq (Takara) with the following protocol: initial
activation of HotStar Taq DNA polymerase at 95°C for 10 min, then
45 cycles of 95°C for 5 sec and 60°C for 20 sec. GAPDH was used as
an internal control. The primer efficiency was confirmed to be high
(>90%) and comparable (Table
I). Data were analyzed according to the 2−ΔΔCt
method. The expression levels of mRNA were normalized to GAPDH.
 | Table I.The sequence of PCR primers for each
gene. |
Table I.
The sequence of PCR primers for each
gene.
| Genes | Forward primers
(5′→3′) | Reverse primers
(5′→3′) |
|---|
| CXCL12 |
TGAGCTACAGATGCCCATGC |
TTCTCCAGGTACTCCTGAATCC |
| CXCR4 |
ACGCCACCAACAGTCAGA |
ACAACCACCCACAAGTCA |
| CXCR7 |
TTATCGCTGTCTTCTACTTCC |
CTTGGTGAGCCCTGTTTT |
| CX3CL1 |
AAGGAGCAATGGGTCAAGG |
ACGGGAGGCACTCGGAAA |
| CX3CR1 |
CACCGACATTTACCTCCT |
TTGGCTTTCTTGTGGTTCTT |
| CXCL8 |
ACTCCAAACCTTTCCACC |
CTTCTCCACAACCCTCTG |
| CXCR1 |
AAAATGGCGGATGGTGTT |
GACGAAGAAGTGTAGGAGGT |
| CCL5 |
TTGCTACTGCCCTCTGCG |
CACTTGGCGGTTCTTTCG |
| CCR1 |
CCCTTGGAACCAGAGAGAAG |
CAAACTCTGTGGTCGTGTCA |
| CCR3 |
TCGTTCTCCCTCTGCTCG |
AGATGCTTGCTCCGCTCA |
| CCR5 |
CTGCTACTCGGGAATCCTAAA |
CATAGCTTGGTCCAACCTGTTA |
| MMP-2 |
CTACGATGATGACCGCAAGT |
CAGTCCGCCAAATGAACC |
| MMP-7 |
GGAGGAGATGCTCACTTCGAT |
AGGAATGTCCCATACCCAAAGA |
| MMP-9 |
GGGACGCAGACATCGTCATC |
TCGTCATCGTCGAAATGGGC |
| GAPDH |
CAGCGACACCCACTCCTC |
TGAGGTCCACCACCCTGT |
Enzyme-linked immunosorbent assay
(ELISA)
To determine quantification of the levels of CCL5
secreted in the supernatants from co-culture or non co-culture, the
culture medium was collected and detected by using ELISA Kits
(RayBiotech, ELH-RANTES-001), according to the manufacturer’s
protocol. Briefly, samples were added to the coated wells with CCL5
mAb of 96-well plates and incubated for 2.5 h at room temperature,
washed four times and then incubated with an HRP-linked
streptavidin solution for 30 min at room temperature in the dark.
The absorbance was then measured at 450 nm on the microplate reader
(Sunrise Remote, Switzerland) immediately. All the experiments were
repeated for three times.
Gelatin zymography of MMP-2 and MMP-9
activity
Equal amount of protein from Huh7 cells co-cultured
with HS-5 cells or alone were mixed with SDS buffer, and were added
onto a 5% (w/v) stacking polyacrylamide gel and separated on a 10%
(w/v) polyacrylamide gel containing 1.0 mg/ml porcine gelatin
(Sigma) for the detection of MMP-2 and MMP-9 activity. After
electrophoresis, gels were washed twice for 40 min in 2.5% Triton
X-100 (Sigma) to remove SDS, and incubated for 42 h at 37°C in 50
mM Tris-HCl (pH 7.6) containing 150 mM NaCl, 10 mM CaCl2
and 0.02% Brij-35, and gels were stained for 3 h in 45%
methanol/10% acetic acid containing 0.5% Coomassie brilliant Blue
R-250 (w/v). Finally, destained with a solution containing 5%
acetic acid until clear bands of gelatinolysis appeared on a dark
background, proteolytic activity was detected as clear bands on a
blue backgroup of the Coomassie Blue staining gel.
Western blot analysis
Western blot was done using standard protocols. In
brief, Huh7 cells co-cultured or non co-cultured were washed three
times with cold PBS and lysed in RIPA lysis buffer, and cell lysate
was denatured after boiling. The protein concentration was measured
according to NanoDrop100 UV-Vis Spectrophotometer (Thermo
Scientific, USA). Equal amount of proteins were loaded onto 10%
SDS-PAGE gels, and transferred subsequently onto PVDF membranes.
After blocking with 5% BSA (Bovine Serum Albumin, Solarbio) in
TBST, the membranes were probed with the primary antibody, and
followed by incubation with a secondary antibody conjugating with
horseradish peroxidase. Protein levels were quantified with
SuperSignal West Pico Chemiluminescent Substrate kit.
Statistical analysis
Results are presented as mean ± standard deviation
(SD). Comparisons were made using independent sample Student’s
t-test in GraphPad prism 5. Statistical significance is indicated
as p<0.05.
Results
HS-5 cells stimulate proliferation and
suppress apoptosis of Huh7 cells in vitro
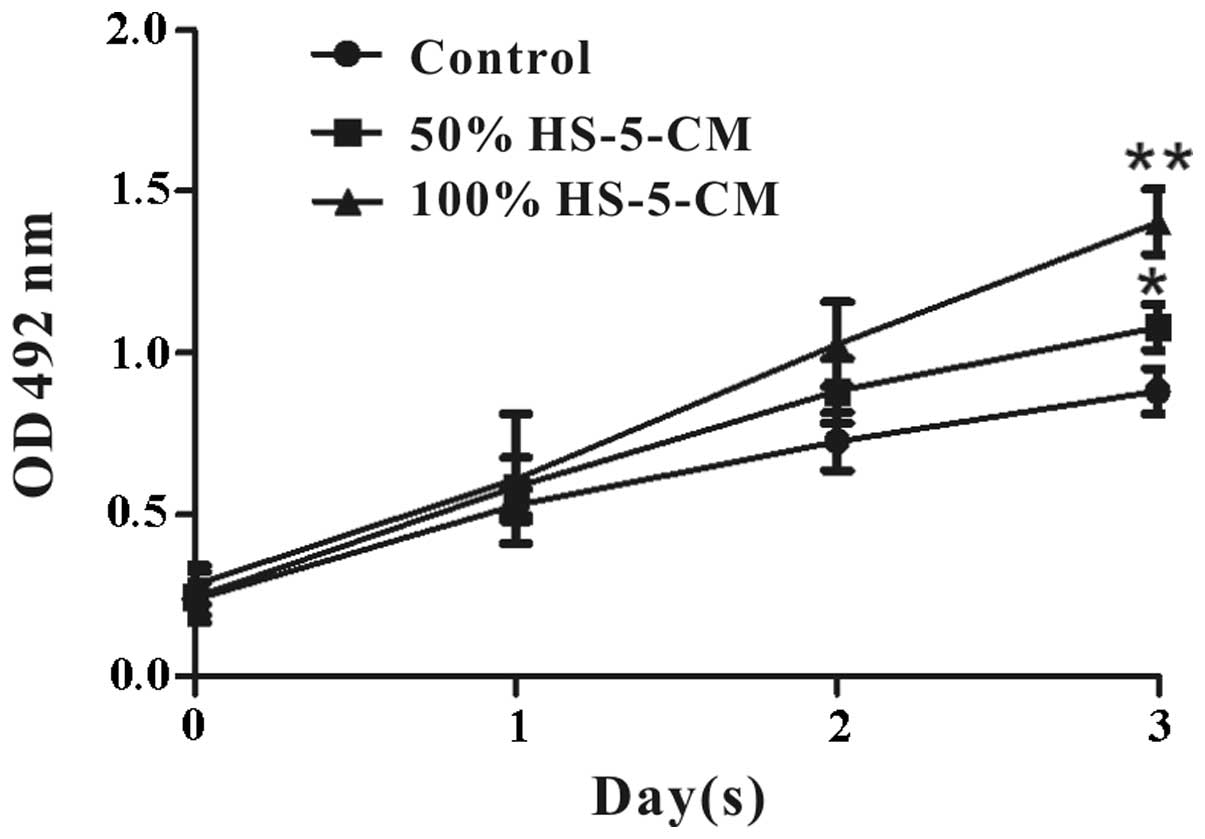
The effects of HS-5 cells on Huh7 cell viability
in vitro were evaluated by MTT assay. It revealed that Huh7
cell viability treated with HS-5-CM increased obviously and
time-dependently compared with control (Fig. 1). To further investigate the
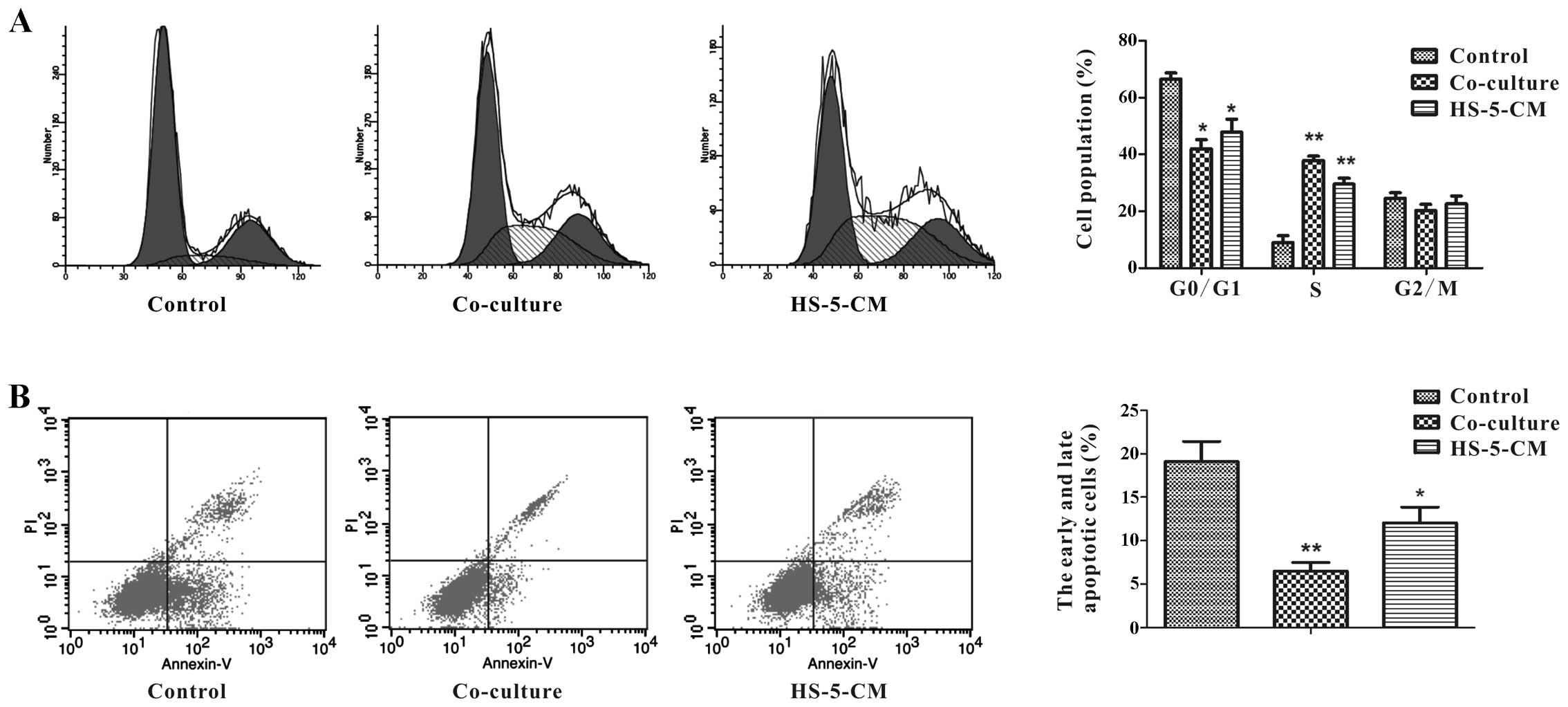
mechanisms of HS-5 cell-induced proliferation of Huh7 cells, flow
cytometry was performed to measure the cell cycle. In order to
study how HS-5 cells affect Huh7 cell growth in tumor
microenvironment, we adopted two ways to culture Huh7 cells:
co-cultured with HS-5 cells, and treatment with HS-5-CM, subsequent
experiments were done using 100% HS-5-CM. Respectively, Huh7 cells
in co-culture group (37.85±1.52%) and in HS-5-CM group
(29.56±2.03%) were at S phase of cell cycle at day 3, which was
significantly higher than the control (8.96±2.34%) (Fig. 2A). These two ways had different
degrees of increase in S phase, but the data demonstrated that HS-5
cells could promote Huh7 cell proliferation.
The proportion of Huh7 cells undergoing apoptosis
was determined by flow cytometry analysis. After 3 days, the data
showed the apoptosis ratio (early + late) of Huh7 cells in
co-culture group was approximately 6.50%, and it was significantly
decreased compared to the control group, although HS-5-CM was not
distinct in decreasing the apoptotic percentage of Huh7 cells, the
small change implied that HS-5 cells could suppress Huh7 cell
apoptosis (Fig. 2B).
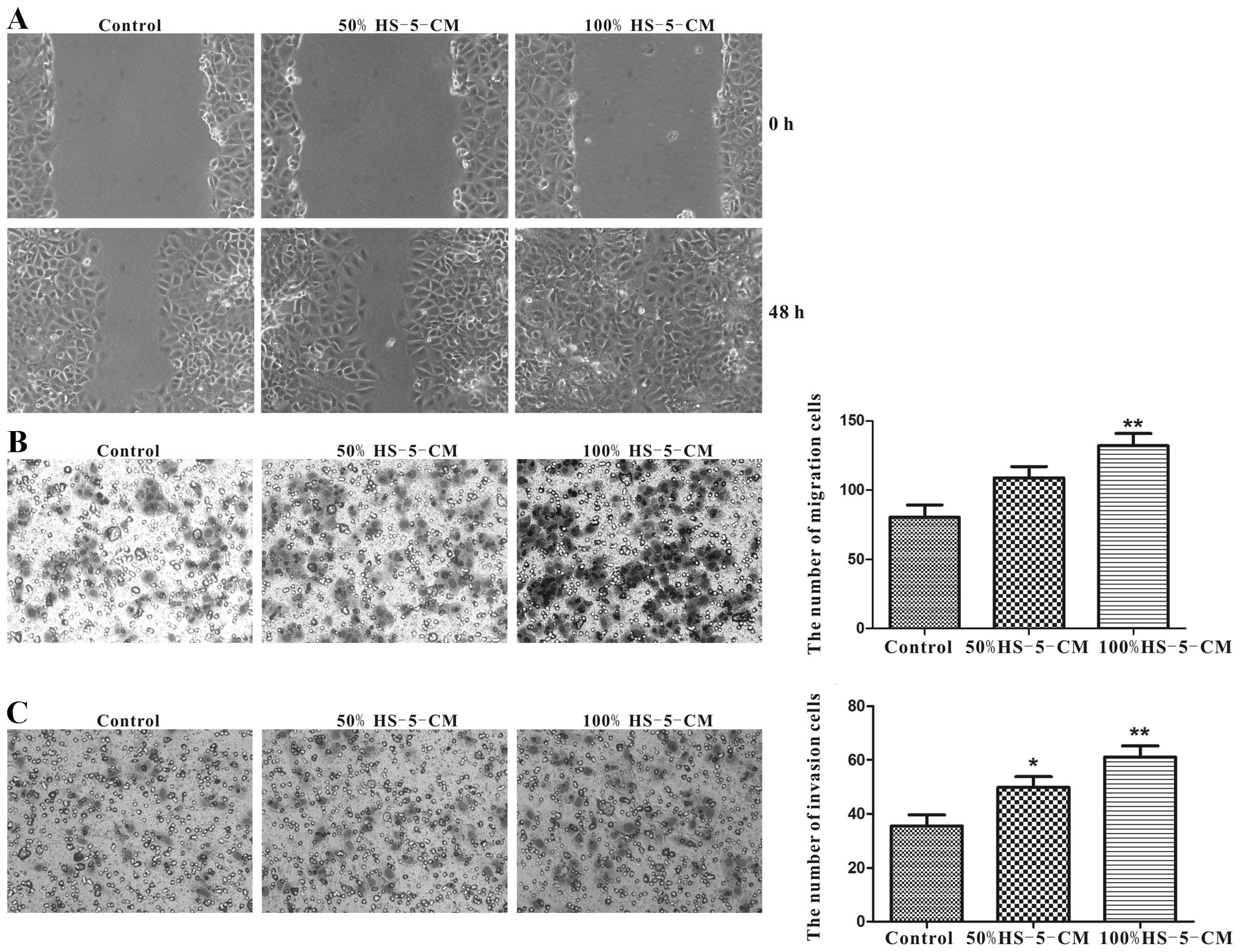
HS-5 cells significantly promote
migration and invasion of Huh7 cells in vitro
As cell migration and invasion abilities are
important determinants in the progression of HCC metastasis, the
potential to migrate was tested in the commonly used wound-healing
assay. After cultured with HS-5-CM for 48 h, the motility of Huh7
cells was significantly accelerated compared with control,
especially in 100% HS-5-CM (Fig.
3A). Transwell assay was carried out to verify the migration
potency. In line with the wound-healing experiment, it demonstrated
that 100% HS-5-CM prominently increased the migration potency of
Huh7 cells compared to the control (Fig. 3B). Applying Transwell assay to test
the invasion property of Huh7 cells for 24 h, respectively, the
number of transmembrane cells in 50% HS-5-CM group and 100% HS-5-CM
group was remarkably increased compared to the control group
(Fig. 3C).
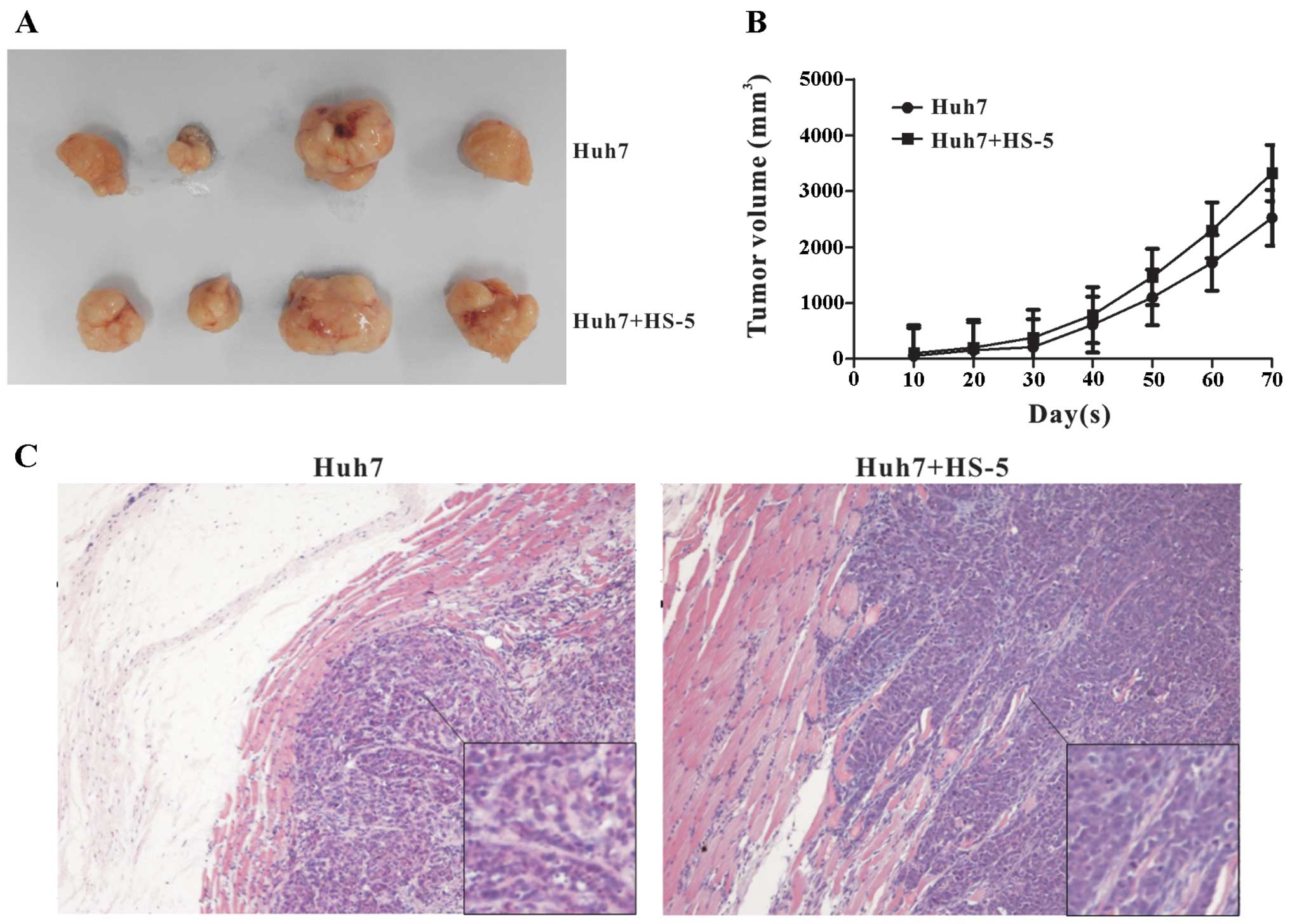
HS-5 cells promote tumor growth of Huh7
cells in vivo
A critical experiment for determining whether HS-5
cells affect tumor growth of HCC is the in vivo
tumorigenicity assay. Therefore, we sought to validate our in
vitro findings by using an in vivo model. It found that
HS-5 cells accelerated the growth of Huh7 tumors, up to 20 days,
there was no significant difference in the two groups, while the
tumor volumes became palpable after 20 days, the Huh7 group grew
from 150 to 2,522.4 mm3 and the co-culture group grew
from 200 to 3,321.8 mm3 (Fig. 4A and B). These results were
consistent with the in vitro observation that HS-5 cells
markedly promoted Huh7 cells growth. H&E staining showed no
variance in heterogeneity between the two groups (Fig. 4C). Tumor cells were not found in
the liver and lung tissues between the two groups (data not
shown).
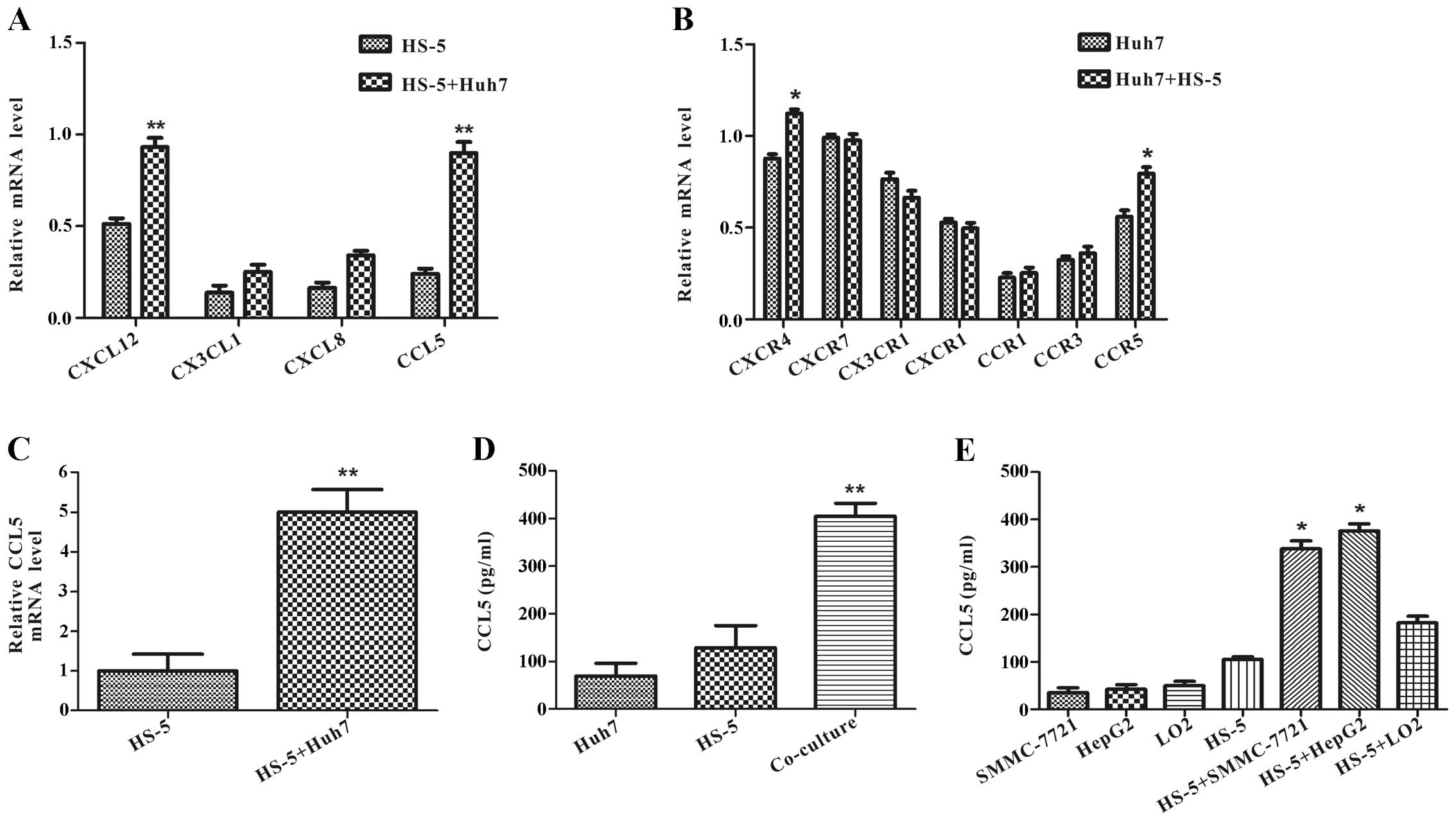
Co-culture with Huh7 cells increases CCL5
expression in HS-5 cells
The molecular mechanisms of these pro-metastatic
impacts in tumor microenvironment are still unclear. To investigate
whether it is mediated by soluble factors secreted from HS-5 cells
in the capacity of migration and invasion, we identified several
chemokines by RT-PCR using RNA prepared from HS-5 cells co-cultured
with Huh7 cells or alone at day 3. It was found that CXCL12,
CX3CL1, CXCL8, CCL5 mRNA levels were upregulated in HS-5 cells
after co-culture, among which CCL5 showed the highest level of
increase (Fig. 5A). We also
examined these chemokine special receptor mRNAs in Huh7 cells.
RT-PCR assay showed that CXCR4 and CCR5 mRNA expression was
increased, and CX3CR1 mRNA was decreased in Huh7 cells co-cultured
with HS-5 cells compared with Huh7 cells alone at day 3 (Fig. 5B).
To confirm the change on CCL5 expression by qRT-PCR
and ELISA assay. qRT-PCR analysis demonstrated that CCL5 mRNA
expression had an approximate 5-fold increase in HS-5 cells
co-cultured with Huh7 cells compared with HS-5 cells alone at day 3
(Fig. 5C). In accordance with CCL5
mRNA level, ELISA assay revealed that co-culture specifically
increased CCL5 protein level more than 3-fold compared with the
secretion from Huh7 cells or HS-5 cells (Fig. 5D).
ELISA assay also verified that the change of CCL5
upregulation was not an accidental event with SMMC-7721 or HepG2 or
LO2 cells. It found that CCL5 protein levels were increased in HS-5
cells co-cultured with SMMC-7721 or HepG2 cells in different
degrees, but not with normal liver cells LO2 (Fig. 5E). The aforementioned data
illustrated that interaction with HCC and HS-5 cells could increase
CCL5 secretion in HS-5 cells.
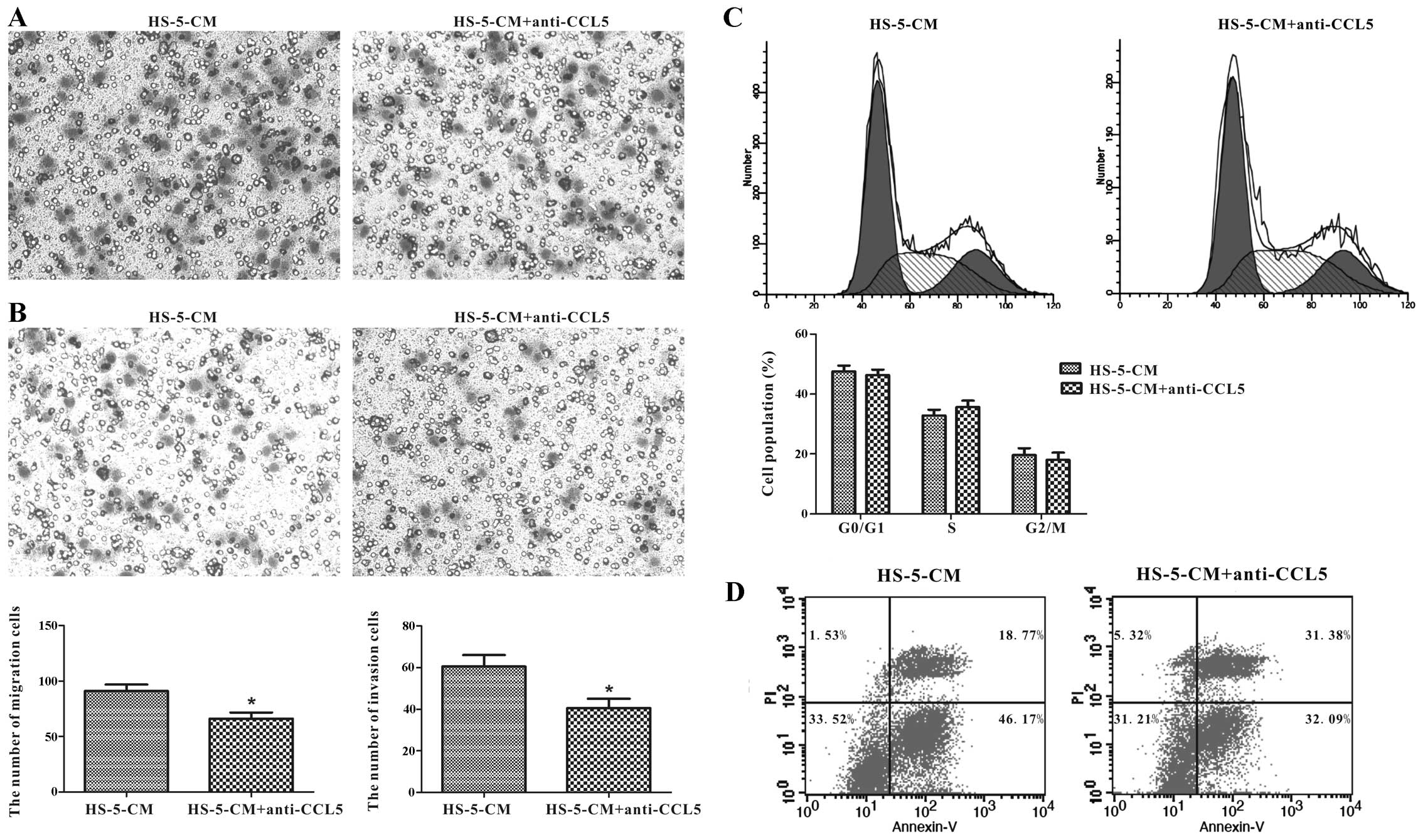
CCL5 secreted from HS-5 cells promotes
Huh7 cell migration and invasion in vitro
Whether HS-5 cells can modulate Huh7 cells
progression via CCL5 secretion remains unreported. We took a
loss-of-function approach to confirm its impact on Huh7 cells,
utilizing anti-CCL5 neutralizing antibody to HS-5-CM which was
cultured in Huh7 cells for 24 h. We tested the migration and
invasion abilities by Transwell assay, and it illustrated that
anti-CCL5 neutralizing antibody caused a decrease of 27.47% in
HS-5-CM-induced migration activity of Huh7 cells compared with the
control (Fig. 6A). Consistently,
Huh7 cell invasion ability was inhibited by 33.06% compared to the
control (Fig. 6B). However, not as
imagined, CCL5 downregulation in HS-5-CM did not overtly change
proliferation and apoptosis of Huh7 cells (Fig. 6C and D). It was thus possible that
CCL5 from HS-5-CM did not play a leading role in Huh7 cell
proliferation and apoptosis impact, but in migration and invasion
potency.
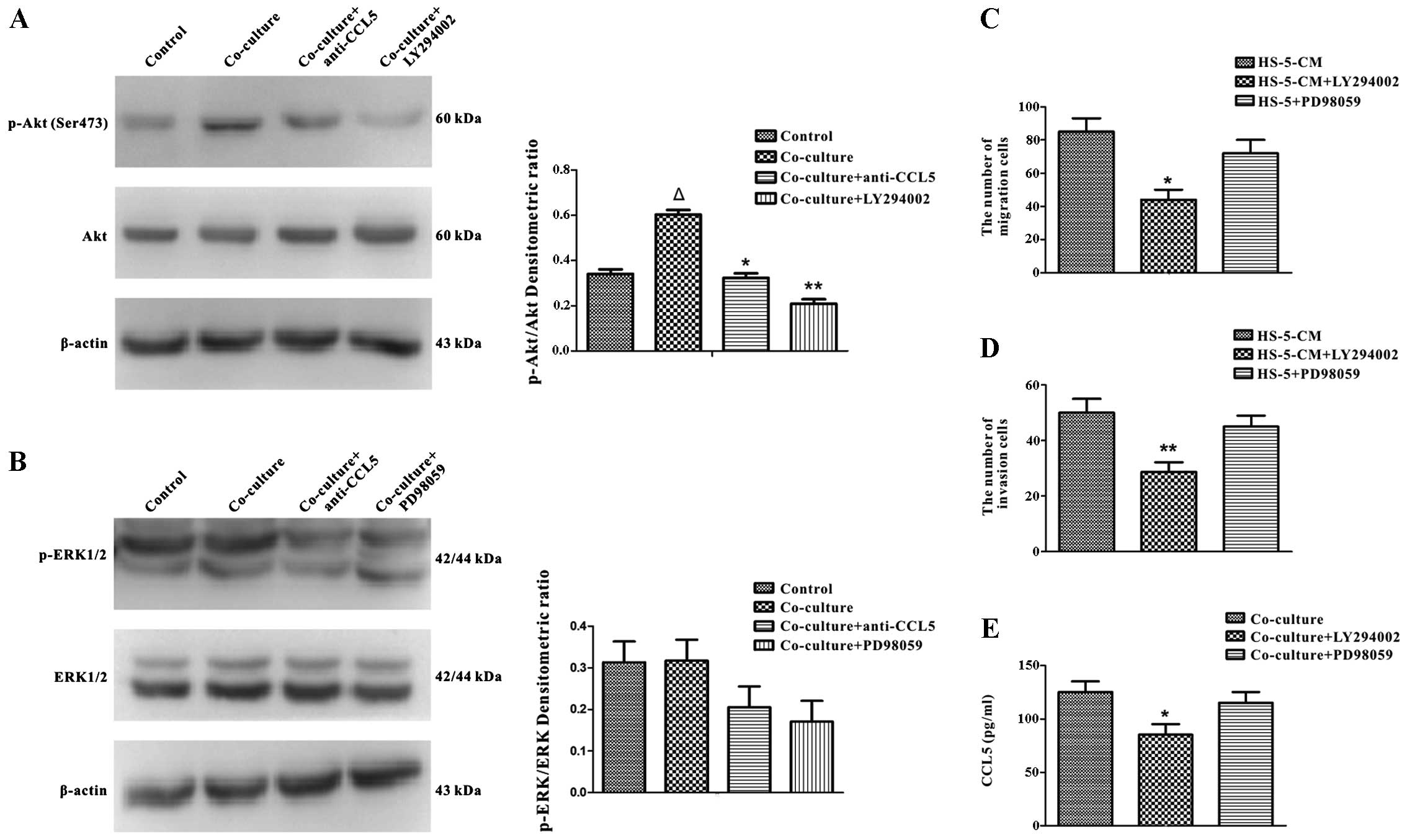
Effects of CCL5-secreted from HS-5 cells
on Huh7 cells via PI3K-Akt signaling pathway
The underlying mechanisms of the interaction with
MSCs and HCC within HCC micro-environment remain an enigma, so we
concentrated on the mechanisms of HS-5 cells inducing migration and
invasion of Huh7 cells. It has been reported that stimulation of
MSCs with certain chemokines causes the activation of Akt kinase
and ERK1/2 MAP kinase (31).
Therefore, we examined the potential changes of Huh7 cells in the
presence of HS-5 cells further. The transwell co-culture with HS-5
cells for 3 days, demonstrated by western blot analysis, revealed
that Huh7 cells had enhanced expression of p-Akt (Ser473) (Fig. 7A), but not p-ERK1/2 (Fig. 7B). The aforementioned data showed
that CCL5 expression was upregulated in HS-5 cells after co-culture
(Fig. 5C and D). Previous research
showed that CCL5 activated Gαi-PI3K-Akt and Gαi-MEK-ERK signaling
pathways (32). So it was
postulated that CCL5 is associated with the activation of p-Akt
(Ser473) and p-ERK1/2 in co-culture system. We applied anti-CCL5
antibody for 24 h to the co-culture system to evaluate the
hypothesis, the data showed the activation of p-Akt (Ser473) was
reduced by adding anti-CCL5 neutralizing antibody to Huh7 cells
co-cultured with HS-5 cells (Fig.
7A), however, there was no significant change in p-ERK1/2
(Fig. 7B). We next focused on CCL5
expression in response to p-Akt (Ser473) and p-ERK1/2 signaling
pathways. Before we investigated the roles of p-Akt (Ser473) and
p-ERK1/2 signaling pathways, western blot analysis demonstrated
PI3K and ERK1/2 specific inhibitors which, respectively, were
LY294002 (20 μM) and PD98059 (10 μM) could decrease
the expression of p-Akt (Ser473) and p-ERK1/2 (Fig. 7A and B). Transwell assay
illustrated that the migration and invasion of Huh7 cells induced
by HS-5-CM were partly reversed by treatment with LY294002 but not
by PD98059 (Fig. 7C and D). In
addition, ELISA assay demonstrated that LY294002 reduced the
secretion of CCL5 in co-culture system (Fig. 7E). Thus, our results illustrated
that CCL5 derived from HS-5 cells had a pivotal role in migration
and invasion of Huh7 cells via PI3K-Akt signaling pathway.
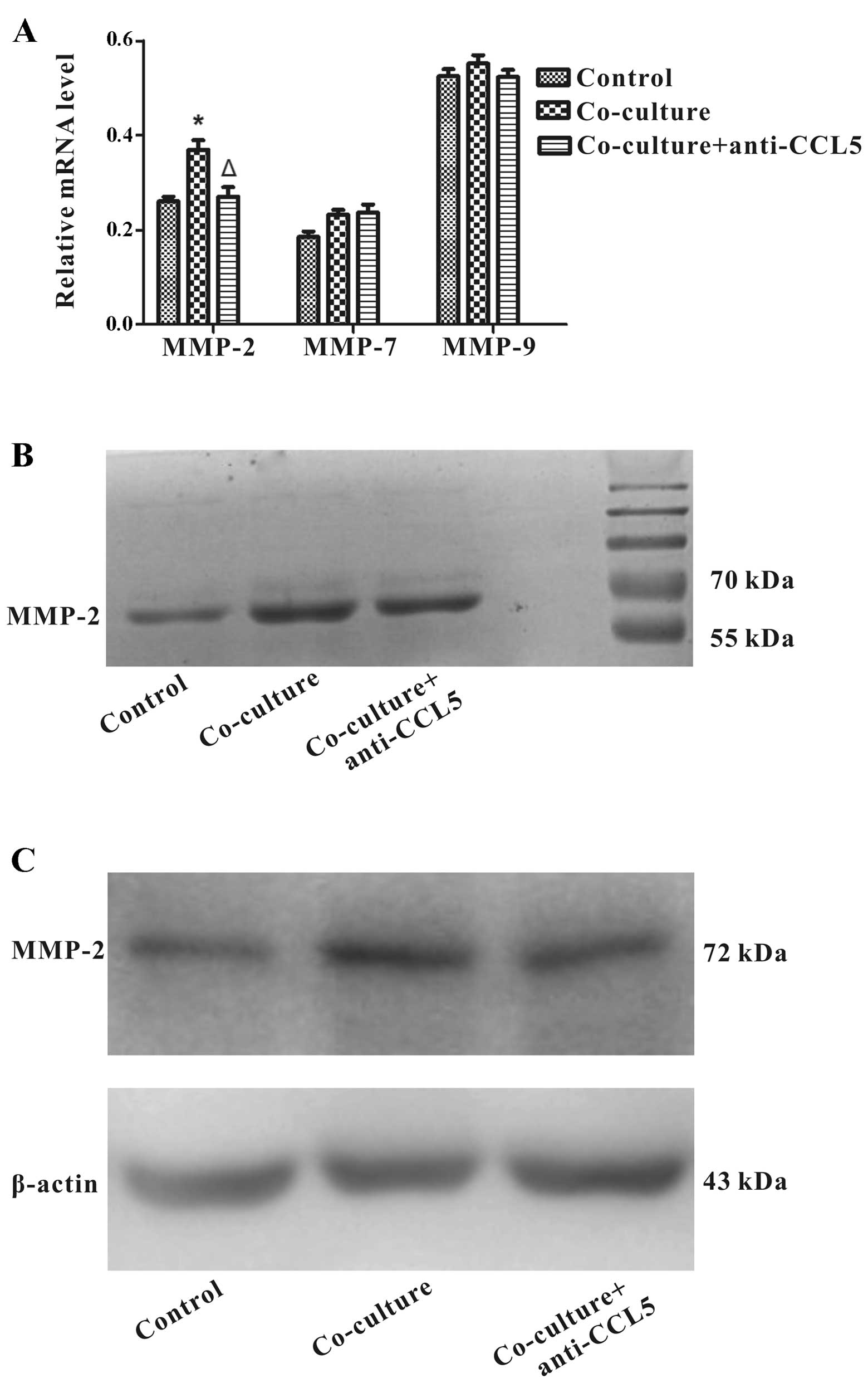
CCL5 downregulation from HS-5 cells
decreases MMP-2 expression in Huh7 cells
Cell movement is involved in the proteolytic
activity of MMPs, which regulate the dynamic ECM (extracellular
matrix)-cell and cell-cell interaction during migration (33). High expression of MMPs is related
to HCC progression (34,35). We investigated whether CCL5
regulated the levels of MMP-2, MMP-7 and MMP-9 in Huh7 cells
co-cultured with HS-5 cells. RT-PCR analysis showed that MMP-2
expression was up-regulated in Huh7 cells co-cultured with HS-5
cells at day 3, extraordinary, MMP-2 mRNA was downregulated through
the addition of anti-CCL5 neutralizing antibody. Nonetheless, the
expression of MMP-7 and MMP-9 mRNAs remained significantly
unchanged (Fig. 8A). To further
characterize the effect of CCL5 in MMP expression of Huh7 cells, we
applied gelatin zymography analysis to determine expression and
enzymatic activities of MMP-2 and MMP-9. The results revealed that
treatment of anti-CCL5 neutralizing antibody pointed to a decrease
in MMP-2 upregulation of Huh7 cells co-cultured with HS-5 cells,
whereas MMP-9 was not detected (Fig.
8B). Consistently, western blot assay confirmed that anti-CCL5
neutralizing antibody could reduce MMP-2 protein upregulation
mediated by HS-5 cells in Huh7 cells (Fig. 8C). The data indicated that CCL5
downregulation from HS-5 cells decreased MMP-2 expression in Huh7
cells.
Discussion
HCC invasion and metastasis are very strongly
related to cancer patient survival, but the detailed mechanisms are
still unknown (36). Advanced HCC
patients presenting with bone metastases are different from breast
and prostate cancer patients, particularly in liver function
impairment, prognosis and available effective treatments (37). Cirrhosis is an independent
prognostic factor for osteoporosis (38). Bone marrow is the most common
source of MSCs, which are found to take part in tumor
microenvironment that mediates tumor cell progress. However,
crosstalk of the tumor-MSCs is complicated, the controversy on the
effects of MSCs on the tumor cell progression remains to be
debated, especially in HCC. Herein, we adopted HS-5-CM to eliminate
the interaction with HCC cells, and it demonstrated that HS-5 cells
could promote proliferation, migration and invasion of Huh7 cells
in vitro, and the increase of proliferation was verified by
the in vivo results. Our results are consistent with a large
proportion of research (16,17,20).
However, the tumor metastasis in liver and lung regions were not
found, demonstrating that Huh7 cell invasion ability is limited
in vivo. Despite the above, some reports stated that MSCs
could inhibit HCC cell growth (22,39).
It is proposed that the time of MSCs introduction into tumors might
be a critical factor for the contradicting results (9). In this study, we consider that MSCs
are helpful for HCC progression.
MSC secretions include a complex mixture of
cytokines, growth factors and chemokines, which have an influence
on cancer cell migration and invasion. In this study, the data
determined that in co-culture with Huh7 cells, HS-5 cells expressed
an increased level of CCL5. To circumvent variability in cell-cell
interaction subsequent experiments were done using conditioned
medium from the ‘co-culture’ cell lines, and it was shown to
consistently alter the CCL5 level. To our knowledge, chemokines and
receptors play a number of non-redundant roles in tumor
progression. Besides, some reports indicate that CCL5 is an
effective inducer of tumor cell migration and invasion, such as in
breast, colorectal, osteosarcoma and prostate tumor cells, acting
through paracrine and autocrine manners (40–44).
It was demonstrated that stroma-derived CCL5 was particularly
important in inducing pro-malignancy impact in CCR5-expressing
breast cancer cells (12).
However, in tumor microenvironment, whether HS-5 cells mediated
CCL5 plays a pivotal role in HCC migratory and invasion, has not
been extensively studied. We found that Huh7 cells revealed a
higher level of CCR5 mRNA than CCR1 and CCR3, and an obvious
increase in CCR5 mRNA after co-culture. Furthermore, a recent
report determined that CCR5 was involved to HCC inflammation
(45). CCR5 is more important than
CCR1 and CCR3 in HCC progress in our study. Anti-CCL5
neutralization antibody decreased the migration and invasion of
Huh7 cells mediated by HS-5-CM. The results indicated that the
CCL5/CCR5 axis was associated with migration and invasion of Huh7
cells in tumor micro-environment. It cannot be denied that other
cytokines play a pivotal role of malignant progression like CXCL12
promoting tumor cell proliferation and survival via paracrine and
auto-crine mechanisms (46,47).
It was proposed that CXCL12 could promote HCC cells migration
(48). Nevertheless, in mimicking
HCC microenvironment, it cannot be ignored that CCL5-secreted from
MSCs is a critical factor in Huh7 cell migration and invasion.
Activation of PI3K contributes to invasion and
metastasis of HCC (49). ERK1/2
pathway is connected with the migratory or invasive behavior of a
variety of malignancies (50).
Thus, whether the progression of HCC induced by MSCs is involved in
the PI3K/Akt and ERK1/2 signaling pathways has not been yet
established. In particular, we demonstrated that the PI3K inhibitor
LY294002 antagonized enhancement in migration and invasion of Huh7
cells, and also reduced CCL5 secretion. However, co-culture did not
activate the p-ERK1/2 signaling pathway and ERK1/2 specific
inhibitor PD98059 did not reduce the mobility and invasiveness of
Huh7 cells. It is possible that ERK1/2 signaling pathway regulates
another biological function in HCC microenvironment. It is
conceivable that other signaling pathways may synergistically or
antagonistically regulate the HCC progress. Our data indicated that
PI3K/Akt signaling pathway could play an important part in
CCL5-mediated migration and invasion of Huh7 cells.
MMPs play a key role in cancer cell invasion. Some
data showed that the expression of MMP-2 increased in hepatic
fibrosis (51), others reported
that certain cytokines could increase MMP-2 and MMP-9 secretion in
human sarcoma cells (52). Xiang
et al showed that MMP-2 was an independent prognostic factor
for lymph node metastasis in HCC (53). Previous studies showed that CCL5
promoted the expression of MMP-9 in tumor cells (44,54,55).
In this study, we demonstrated that HS-5 cells did not elevate
MMP-9 expression but MMP-2 expression in Huh7 cells. The results
suggest that anti-CCL5 neutralization antibody could depress the
secretion of MMP-2 in Huh7 cells. Therefore, MMP-2 may be a
CCL5-responsive mediator, and it causes the degradation of ECM,
which may lead to subsequent HCC migration and metastasis.
In conclusion, the current observations indicate
that HS-5 cells can promote the proliferation, migration and
invasion, and inhibit apoptosis of Huh7 cells. Exocrine CCL5
secreted from MSCs promotes migration and invasion of Huh7 cells
via PI3K/Akt signaling pathway, and accompanies the MMP-2
upregulation. Hence, CCL5 may be an important factor in HCC with
bone metastases.
Acknowledgements
We would like to thank Dr Tongchuan He
(University of Chicago) for the gift of human hepatocellular
carcinoma cell lines and normal human liver cell lines. This study
was supported by the National Natural Science Foundation of China
(NSFC 31200971), National Ministry of Education Foundation of China
(20115503110009) and the 973 Program of the Ministry of Science and
Technology of China (2011CB707906).
References
|
1.
|
Yang JD and Roberts LR: Hepatocellular
carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 7:448–458.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Ma W, Wong CC, Tung EK, Wong CM and Ng IO:
RhoE is frequently down-regulated in hepatocellular carcinoma (HCC)
and suppresses HCC invasion through antagonizing the
Rho/Rho-kinase/myosin phosphatase target pathway. Hepatology.
57:152–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ho CL, Chen S, Yeung DW and Cheng TK:
Dual-tracer PET/CT imaging in evaluation of metastatic
hepatocellular carcinoma. J Nucl Med. 48:902–909. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Natsuizaka M, Omura T, Akaike T, et al:
Clinical features of hepatocellular carcinoma with extrahepatic
metastases. J Gastroenterol Hepatol. 20:1781–1787. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Xiang ZL, Zeng ZC, Tang ZY, et al:
Chemokine receptor CXCR4 expression in hepatocellular carcinoma
patients increases the risk of bone metastases and poor survival.
BMC Cancer. 9:1762009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
7.
|
Chung LW, Baseman A, Assikis V and Zhau
HE: Molecular insights into prostate cancer progression: the
missing link of tumor microenvironment. J Urol. 173:10–20. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Chackal-Roy M, Niemeyer C, Moore M and
Zetter BR: Stimulation of human prostatic carcinoma cell growth by
factors present in human bone marrow. J Clin Invest. 84:43–50.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Klopp AH, Gupta A, Spaeth E, Andreeff M
and Marini F III: Concise review: Dissecting a discrepancy in the
literature: do mesenchymal stem cells support or suppress tumor
growth? Stem Cells. 29:11–19. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Maestroni GJ, Hertens E and Galli P:
Factor(s) from nonmacrophage bone marrow stromal cells inhibit
Lewis lung carcinoma and B16 melanoma growth in mice. Cell Mol Life
Sci. 55:663–667. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kucerova L, Matuskova M, Hlubinova K,
Altanerova V and Altaner C: Tumor cell behaviour modulation by
mesenchymal stromal cells. Mol Cancer. 9:1292010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Karnoub AE, Dash AB, Vo AP, et al:
Mesenchymal stem cells within tumour stroma promote breast cancer
metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lin JT, Wang JY, Chen MK, et al: Colon
cancer mesenchymal stem cells modulate the tumorigenicity of colon
cancer through interleukin 6. Exp Cell Res. 319:2216–2229. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Khakoo AY, Pati S, Anderson SA, et al:
Human mesenchymal stem cells exert potent antitumorigenic effects
in a model of Kaposi’s sarcoma. J Exp Med. 203:1235–1247.
2006.PubMed/NCBI
|
|
15.
|
Zhang C, Soori M, Miles FL, et al:
Paracrine factors produced by bone marrow stromal cells induce
apoptosis and neuroendocrine differentiation in prostate cancer
cells. Prostate. 71:157–167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Hernanda PY, Pedroza-Gonzalez A, van der
Laan LJ, et al: Tumor promotion through the mesenchymal stem cell
compartment in human hepatocellular carcinoma. Carcinogenesis.
34:2330–2340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Mizuguchi T, Hui T, Palm K, et al:
Enhanced proliferation and differentiation of rat hepatocytes
cultured with bone marrow stromal cells. J Cell Physiol.
189:106–119. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Jing Y, Han Z, Liu Y, et al: Mesenchymal
stem cells in inflammation microenvironment accelerates
hepatocellular carcinoma metastasis by inducing
epithelial-mesenchymal transition. PLoS One. 7:e432722012.
View Article : Google Scholar
|
|
19.
|
Li GC, Ye QH, Dong QZ, Ren N, Jia HL and
Qin LX: Mesenchymal stem cells seldomly fuse with hepatocellular
carcinoma cells and are mainly distributed in the tumor stroma in
mouse models. Oncol Rep. 29:713–719. 2013.PubMed/NCBI
|
|
20.
|
Li GC, Ye QH, Xue YH, et al: Human
mesenchymal stem cells inhibit metastasis of a hepatocellular
carcinoma model using the MHCC97-H cell line. Cancer Sci.
101:2546–2553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Niess H, Bao Q, Conrad C, et al: Selective
targeting of genetically engineered mesenchymal stem cells to tumor
stroma microenvironments using tissue-specific suicide gene
expression suppresses growth of hepatocellular carcinoma. Ann Surg.
254:767–775. 2011. View Article : Google Scholar
|
|
22.
|
Qiao L, Xu Z, Zhao T, et al: Suppression
of tumorigenesis by human mesenchymal stem cells in a hepatoma
model. Cell Res. 18:500–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Ho IA, Toh HC, Ng WH, et al: Human bone
marrow-derived mesenchymal stem cells suppress human glioma growth
through inhibition of angiogenesis. Stem Cells. 31:146–155. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Sahin H, Trautwein C and Wasmuth HE:
Functional role of chemokines in liver disease models. Nat Rev
Gastroenterol Hepatol. 7:682–690. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Makinoshima H and Dezawa M: Pancreatic
cancer cells activate CCL5 expression in mesenchymal stromal cells
through the insulin-like growth factor-I pathway. FEBS Lett.
583:3697–3703. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Luther SA and Cyster JG: Chemokines as
regulators of T cell differentiation. Nat Immunol. 2:102–107. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Charni F, Friand V, Haddad O, et al:
Syndecan-1 and syndecan-4 are involved in RANTES/CCL5-induced
migration and invasion of human hepatoma cells. Biochim Biophys
Acta. 1790:1314–1326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Sutton A, Friand V, Papy-Garcia D, et al:
Glycosaminoglycans and their synthetic mimetics inhibit
RANTES-induced migration and invasion of human hepatoma cells. Mol
Cancer Ther. 6:2948–2958. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Liou JM, Lin JT, Huang SP, et al:
RANTES-403 polymorphism is associated with reduced risk of gastric
cancer in women. J Gastroenterol. 43:115–123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Kondo T, Ito F, Nakazawa H, Horita S,
Osaka Y and Toma H: High expression of chemokine gene as a
favorable prognostic factor in renal cell carcinoma. J Urol.
171:2171–2175. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Honczarenko M, Le Y, Swierkowski M, Ghiran
I, Glodek AM and Silberstein LE: Human bone marrow stromal cells
express a distinct set of biologically functional chemokine
receptors. Stem Cells. 24:1030–1041. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Tyner JW, Uchida O, Kajiwara N, et al:
CCL5-CCR5 interaction provides antiapoptotic signals for macrophage
survival during viral infection. Nat Med. 11:1180–1187. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Bao M, Chen Z, Xu Y, et al: Sphingosine
kinase 1 promotes tumour cell migration and invasion via the
S1P/EDG1 axis in hepatocellular carcinoma. Liver Int. 32:331–338.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Palagyi A, Neveling K, Plinninger U, et
al: Genetic inactivation of the Fanconi anemia gene FANCC
identified in the hepatocellular carcinoma cell line HuH-7 confers
sensitivity towards DNA-interstrand crosslinking agents. Mol
Cancer. 9:1272010. View Article : Google Scholar
|
|
35.
|
Shi F, Shi M, Zeng Z, et al: PD-1 and
PD-L1 upregulation promotes CD8(+) T-cell apoptosis and
postoperative recurrence in hepatocellular carcinoma patients. Int
J Cancer. 128:887–896. 2011.
|
|
36.
|
Chen J, Liu WB, Jia WD, et al:
Overexpression of mortalin in hepatocellular carcinoma and its
relationship with angiogenesis and epithelial to mesenchymal
transition. Int J Oncol. 44:247–255. 2014.PubMed/NCBI
|
|
37.
|
Montella L, Addeo R, Palmieri G, et al:
Zoledronic acid in the treatment of bone metastases by
hepatocellular carcinoma: a case series. Cancer Chemother
Pharmacol. 65:1137–1143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Crawford BA, Kam C, Pavlovic J, et al:
Zoledronic acid prevents bone loss after liver transplantation: a
randomized, double-blind, placebo-controlled trial. Ann Intern Med.
144:239–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Lu YR, Yuan Y, Wang XJ, et al: The growth
inhibitory effect of mesenchymal stem cells on tumor cells in vitro
and in vivo. Cancer Biol Ther. 7:245–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Wang SW, Wu HH, Liu SC, et al: CCL5 and
CCR5 interaction promotes cell motility in human osteosarcoma. PLoS
One. 7:e351012012. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Cambien B, Richard-Fiardo P, Karimdjee BF,
et al: CCL5 neutralization restricts cancer growth and potentiates
the targeting of PDGFRβ in colorectal carcinoma. PLoS One.
6:e288422011.PubMed/NCBI
|
|
42.
|
Vaday GG, Peehl DM, Kadam PA and Lawrence
DM: Expression of CCL5 (RANTES) and CCR5 in prostate cancer.
Prostate. 66:124–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Gallo M, De Luca A, Lamura L and Normanno
N: Zoledronic acid blocks the interaction between mesenchymal stem
cells and breast cancer cells: implications for adjuvant therapy of
breast cancer. Ann Oncol. 23:597–604. 2012. View Article : Google Scholar
|
|
44.
|
Long H, Xie R, Xiang T, et al: Autocrine
CCL5 signaling promotes invasion and migration of CD133+
ovarian cancer stem-like cells via NF-κB-mediated MMP-9
upregulation. Stem Cells. 30:2309–2319. 2012.
|
|
45.
|
Barashi N, Weiss ID, Wald O, et al:
Inflammation-induced hepatocellular carcinoma is dependent on CCR5
in mice. Hepatology. 58:1021–1030. 2013. View Article : Google Scholar
|
|
46.
|
Orimo A, Gupta PB, Sgroi DC, et al:
Stromal fibroblasts present in invasive human breast carcinomas
promote tumor growth and angiogenesis through elevated SDF-1/CXCL12
secretion. Cell. 121:335–348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Mo W, Chen J, Patel A, et al: CXCR4/CXCL12
mediate autocrine cell-cycle progression in NF1-associated
malignant peripheral nerve sheath tumors. Cell. 152:1077–1090.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Liu H, Pan Z, Li A, et al: Roles of
chemokine receptor 4 (CXCR4) and chemokine ligand 12 (CXCL12) in
metastasis of hepatocellular carcinoma cells. Cell Mol Immunol.
5:373–378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Li X, Yang Z, Song W, et al:
Overexpression of Bmi-1 contributes to the invasion and metastasis
of hepatocellular carcinoma by increasing the expression of matrix
metalloproteinase (MMP)-2, MMP-9 and vascular endothelial growth
factor via the PTEM/PI3K/Akt pathway. Int J Oncol. 43:793–802.
2013.PubMed/NCBI
|
|
50.
|
Suthiphongchai T, Promyart P, Virochrut S,
Tohtong R and Wilairat P: Involvement of ERK1/2 in invasiveness and
metastatic development of rat prostatic adenocarcinoma. Oncol Res.
13:253–259. 2003.PubMed/NCBI
|
|
51.
|
Diaz-Gil JJ, Garcia-Monzon C, Rua C, et
al: The anti-fibrotic effect of liver growth factor is associated
with decreased intrahepatic levels of matrix metalloproteinases 2
and 9 and transforming growth factor beta 1 in bile ductligated
rats. Histol Histopathol. 23:583–591. 2008.PubMed/NCBI
|
|
52.
|
Roomi MW, Kalinovsky T, Rath M and
Niedzwiecki A: In vitro modulation of MMP-2 and MMP-9 in
pediatric human sarcoma cell lines by cytokines, inducers and
inhibitors. Int J Oncol. 44:27–34. 2014.
|
|
53.
|
Xiang ZL, Zeng ZC, Fan J, et al: Gene
expression profiling of fixed tissues identified hypoxia-inducible
factor-1α, VEGF, and matrix metalloproteinase-2 as biomarkers of
lymph node metastasis in hepatocellular carcinoma. Clin Cancer Res.
17:5463–5472. 2011.PubMed/NCBI
|
|
54.
|
Wang YH, Dong YY, Wang WM, et al: Vascular
endothelial cells facilitated HCC invasion and metastasis through
the Akt and NF-κB pathways induced by paracrine cytokines. J Exp
Clin Cancer Res. 32:512013.PubMed/NCBI
|
|
55.
|
Gao J, Ding F, Liu Q and Yao Y: Knockdown
of MACC1 expression suppressed hepatocellular carcinoma cell
migration and invasion and inhibited expression of MMP2 and MMP9.
Mol Cell Biochem. 376:21–32. 2013. View Article : Google Scholar : PubMed/NCBI
|