Introduction
Endometrial cancer is the most common gynecological
malignancy in developed countries and represents the eighth leading
cause of cancer related death in women (1). Apart from surgery, irradiation,
hormonal-therapy and chemotherapy are used to cure this malignancy
(2). The majority of patients with
advanced endometrial cancers relapse because a proportion of
primary tumours are intrinsically refractory to treatment.
Therefore, a definite need for new treatment strategies exists.
Cordyceps Sinensis (Cordy), Ganoderma lucidum
(Reishi) and Agaricus Blazi Murill (ABM) are fungi
widely used in traditional Chinese medicine. The biological
activities and numerous pharmacological effects such as antitumour,
immunomodulatory, anti-inflammatory, anti-diabetes, anti-hepatitis,
anti-hypercholesterolemia, anti-heart disease and anti-oxidant
properties of Cordy, Reishi and ABM have been well
documented (3–5). Of course, every fungi contains a
variety of bioactive compounds, including triterpenes,
polysaccharides, sterols, nucleoside and nucleotides, as well as
their degradation products and derivatives (6–8).
Multiple compound-based drugs may provide important combination
therapies that simultaneously influence multiple pharmacological
targets and provide clinical efficacy beyond that of single
compound-based drugs (9).
However, up to now the biological pathways involved
in pharmacological activities of Cordy, Reishi and
ABM are still not clear. Furthermore, no data exist on the
effects of these fungi on endometrial carcinomas. Therefore, we
studied the effects of a hot-water-extract derived from Cordy,
Reishi and ABM on endometrial cancer cells in
vitro. We used endometrial cancer cell lines derived from
different stages; e.g. Ishikawa cells (stage I tumour); Hec-1A
cells (stage II tumour) and AN3-CA cells (stage III tumour). Our
purpose was to examine the efficiency of crude extracts from
Cordy alone and a mixture composed of Reishi and
ABM for treatment of endometrial cancer in vitro
systems. After demonstrating growth-inhibition in a dose- and
time-dependent manner, we started to enlighten the molecular basis
for the observed inhibition of proliferation caused by the
different fungi extracts.
Materials and methods
Cell culture
AN3-CA, Hec-1A and Ishikawa cells were obtained from
American Type Culture Collection (Manassas, VA, USA). Hec-1A and
Ishikawa cells were grown in RPMI-1640 medium (PAA, Cölbe, Germany)
supplemented with 10% fetal calf serum, 2 mM glutamine, 1%
penicillin/streptomycin and 0.5% sodium pyruvate solution. AN3-CA
cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)
(PAA) containing 10% fetal calf serum, 2 mM glutamine, 1%
penicillin/streptomycin and 0.5% sodium pyruvate solution.
Fungi extracts
The Cordyceps Sinensis as well as the mixture
composed of 50% (w/w) Reishi and 50% (w/w) ABM was
supplied by MycoVital (Limeshain, Germany). Whole mushrooms were
dried (below 35 °C) and homogenized to a powder by the
manufacturer. The mushrooms were not treated with any compound or
chemical. The mushroom extracts were directly ordered by the
manufacturer and used within the indicated best-before date. A
stock solution of 50 mg/ml was prepared as previously described
(10). Briefly, the fungi powder
was suspended by adding water and then boiled for 5 min. After
brief centrifugation, the supernatant was collected and sterile
filtrated. The stock solution was stored at 4 °C in the dark. Such
a hot water extraction is used in most studies with mushrooms
because some compounds such as polysaccharides are found inside
indigestible cell walls and only hot water extraction can release
and thereby maintaining the structural integrity of these compounds
(11). Two different lot numbers
were tested from both mushroom preparations. We observed no
significant differences between the two different lot numbers.
Cytotoxicity MTT assay
To quantify the cytotoxicity of the fungi extracts
the viability of cells was measured with a non-radioactive cell
viability assay. Therefore, cells were cultured in 96-well
flat-bottom plates, in humidified 37 °C and 5% CO2
atmosphere. The cell density was initially adjusted to
2×105 cells/ml in a final volume of 50 μl/well.
Cells were treated with different concentrations of fungi extracts
as indicated for 24, 48 and 72 h, respectively. During the last 4 h
of incubation, cells were pulsed with 10 μl of tetrazolium
salt [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide,
MTT] labelling reagent (Roth, Karlsruhe, Germany) at a final
concentration of 0.5 mg/ml. The colorimetric assay is based on the
reduction of yellow MTT to pure violet formazan crystals by
metabolic active cells (12). The
crystals were solubilised by addition of 100 μl 10% SDS in
0.01 M HCl to each well. Absorbance was measured
spectrophotometrically using a 540-nm wavelength ELISA reader
(Tecan, Männedorf, Switzerland) and Magellan software. The
experiments were performed 6-fold. At least 3 independent
experiments were performed for each cell line.
Preparation of cell lysates and western
blotting
Preparation of cell lysates was performed as
previously described (13).
Membranes were probed overnight with anti-Phospho-AKT antibody and
anti-AKT-antibody from Epitomics (Burlingame, CA, USA), anti-LC3B
antibody from Cell Signaling (Frankfurt, Germany) or anti-β-actin
antibody from Abcam (Cambridge, UK), respectively. Secondary
horseradish peroxidase (HRP)-conjugated antibodies were obtained
from Cell Signaling. The chemiluminescent HRP substrate solution
(Millipore, Schwalbach, Germany) was used for detection.
NK-cell preparation and lysis assay
PBMC were isolated from healthy volunteers by
density gradient centrifugation (Biocoll; Biochrom AG, Berlin,
Germany). Monocytes were depleted by adherence and the remaining
non-adherent PBL were further cultured on irradiated (30 Gy)
RPMI-8866 feeder cells to obtain polyclonal NK-cell populations
(19). After 6 days of co-culture
500 units of IL-2 (Peprotech, Hamburg, Germany) were added per ml
and after 48 h the polyclonal NK-cell population (effector cells)
was used in different killing assays. Therefore the NK-cells were
labeled with eFluor 670 (eBioscience, Frankfurt, Germany). The
lytic activity against CFSE-stained (eBioscience) tumour cells
(targets; 105 cells/well) was assessed in a modified 5 h
FATAL assay using various effector:target ratios (20). Cells were detached by
trypsinisation. The target cell lysis was determined by flow
cytometric analysis of 30,000 target cells in a FACScan flow
cytometer (Calibur, BD Biosciences, Heidelberg, Germany). eFluor
670-negative target cells were selected by gating and the
percentage of CFSE cells within this population was determined.
Spontaneous leakage of CFSE was determined by incubating the target
cells with medium alone.
Flow cytometry
For cell cycle analysis, cells were treated with
fungi extracts as indicated, harvested, fixed and permeabilized
overnight in ice-cold 70% ethanol (Merck, Darmstadt, Germany). The
cells were washed twice with PBS. RNA was digested with RNase A
(Gibco Life Technologies, Paisley, UK). The DNA was stained with
propidium iodide (50 μg/ml). Fluorescence was recorded in a
FACSCalibur (Becton Dickinson, Heidelberg, Germany). Instrument
settings were adjusted to move the G0/G1 peak to 200 relative
fluorescence units. Cells to the left of this peak appeared to have
DNA content below 2n, indicative of cell death. Aggregated cells
were gated out. A total of 2×104 cells per condition
were recorded.
Apoptosis assay
Cellular apoptosis was measured by Annexin V and
propidium iodide staining using Annexin V Apoptosis Detection kit
FITC (eBioscience) according to the manufacturer’s protocol.
Briefly, cells were treated with fungi extracts as indicated,
harvested, washed once with binding buffer [10 mM HEPES/NaOH (pH
7.4), 140 mM NaCl, 2.5 mM CaCl2] and resuspended in
binding buffer at a cell density of 1×106 cells/ml.
FITC-conjugated Annexin V (5 μl) was added to 100 μl
of the cell suspension and incubated 15 min at room temperature.
Then the cells were washed with binding buffer and finally
resuspended in 200 μl binding buffer. After addition of 5
μl propidium iodide (2 μg/ml) the cells were analyzed
by flow cytometry on a FACSCalibur (Becton Dickinson).
Autophagy assay
The degree of autophagic death was measured by
acridine orange (Sigma-Aldrich, St. Louis, MO, USA) staining as
previously described (14).
Briefly, cells were treated with fungi extracts as indicated and
acridine orange was added at a final concentration of 1 mg/ml 20
min before the cells were harvested. Cells were removed from the
plate with trypsin-EDTA, and collected in phenol red-free growth
medium. A total of 1×104 cells per condition were
analyzed by flow cytometry on a FACSCalibur (Becton Dickinson).
Results
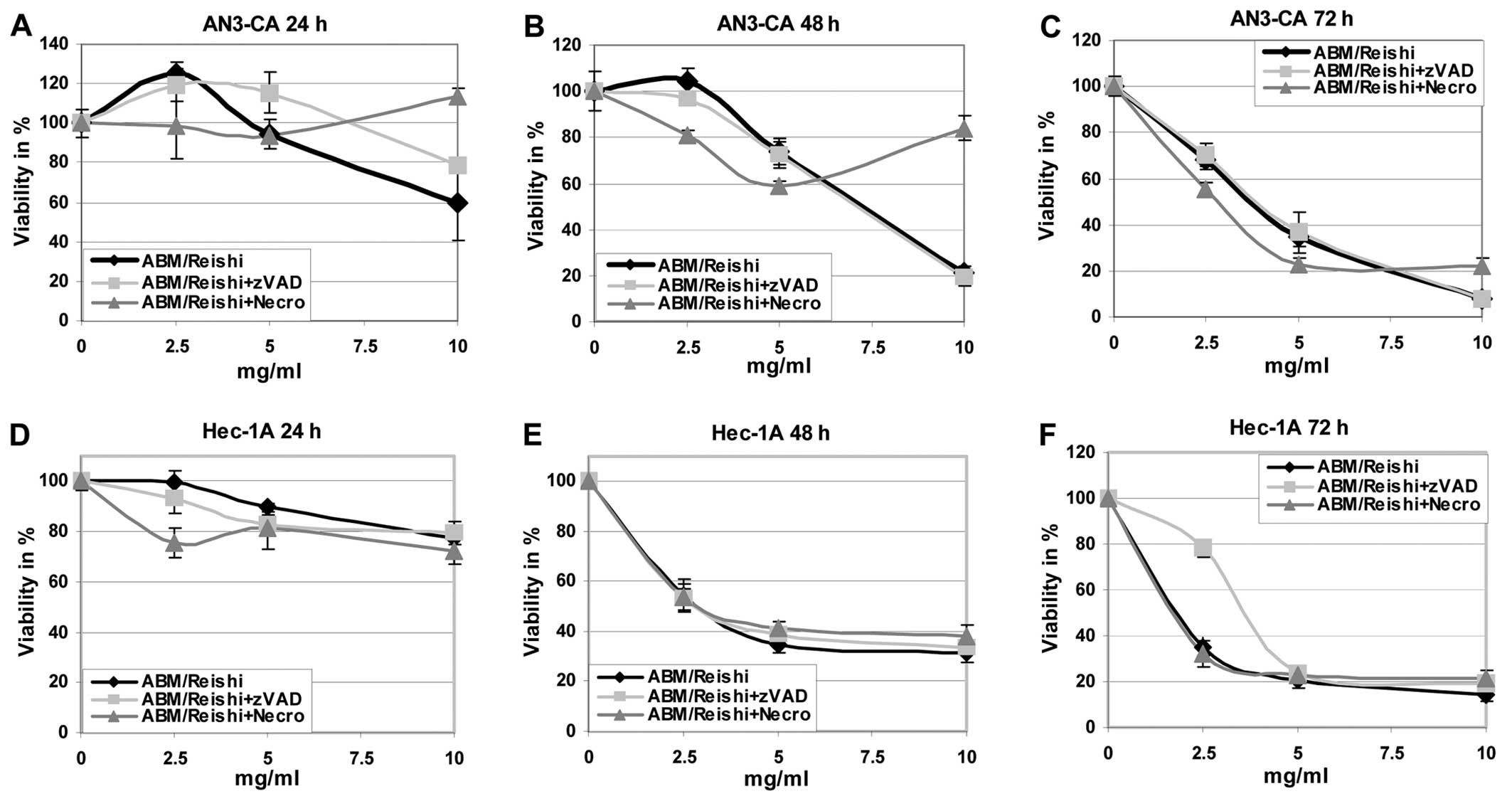
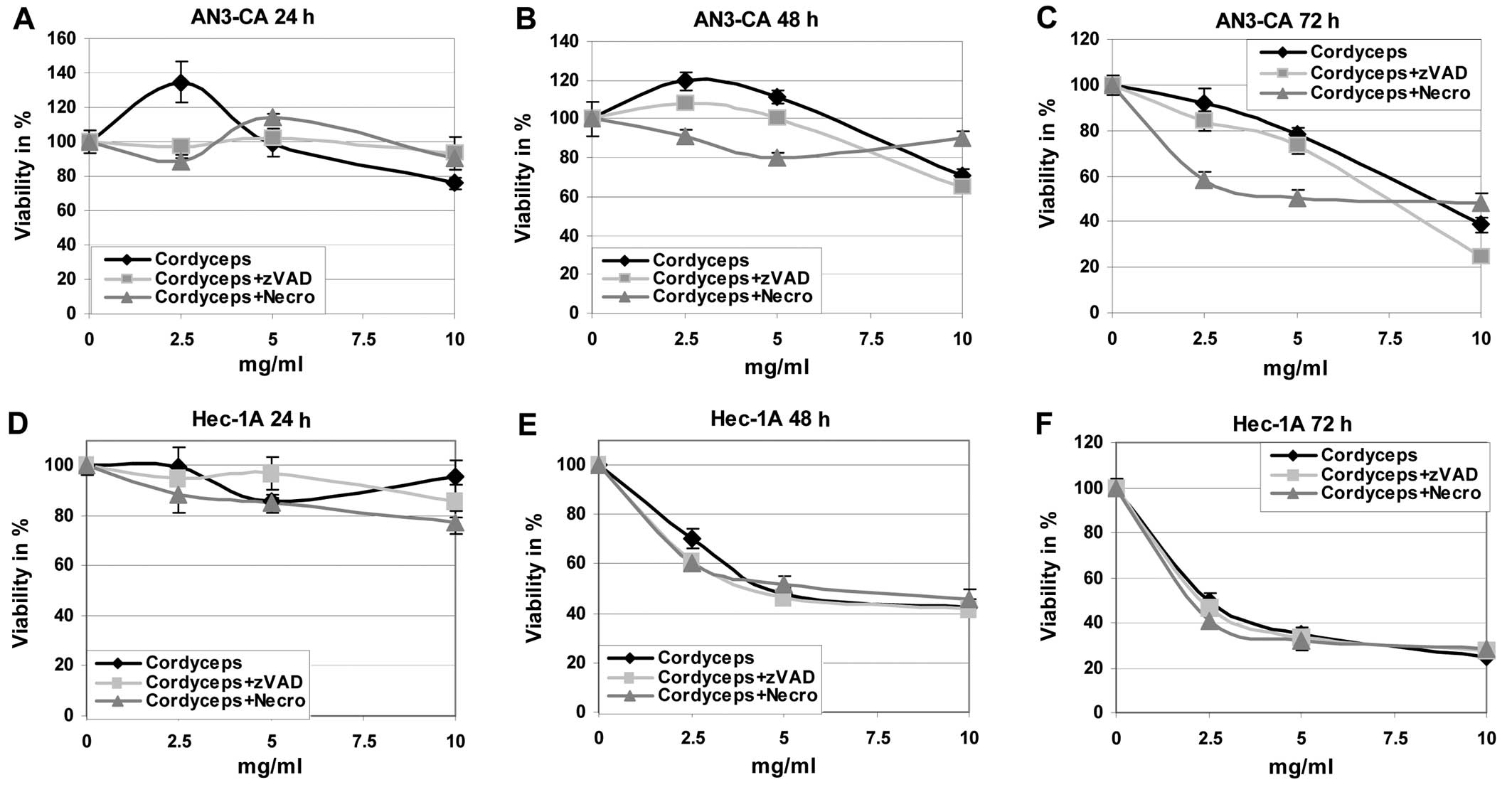
First of all the effects of the different fungi
extracts on the viability and growing of endometrial cancer cell
lines were evaluated (Figs. 1 and
2). It is obvious that the tumour
cells are killed by the ABM/Reishi (Fig. 1) and Cordyceps (Fig. 2) extracts in a time- and
dose-dependent manner. The viability of all endometrial cancer
cells are <20% compared to untreated control cells after
incubation with 10 mg/ml of ABM/Reishi extracts for 72 h
(Fig. 1). Of all endometrial cell
lines used in this study, AN3-CA seems to be the most sensitive
cells for treatment with ABM/Reishi extracts. Incubation for
48 h of AN3-CA cells with 10 mg/ml of ABM/Reishi extracts
resulted in killing of 80% of the cells (Fig. 1). Moreover, the incubation of
endometrial cancer cells with Cordyceps extract resulted in
a clearly reduced viability (Fig.
2). Only in Ishikawa cells the killing potency of
Cordyceps extract is comparable to the effects observed with
ABM/Reishi extracts. In both other cell lines, the killing
efficiency of Cordyceps extract is slightly diminished
compared to the effects exerted by ABM/Reishi extracts.
To further analyze the basis of the observed
cytotoxic effects, cells were co-incubated with the fungi extracts
and z-VAD-fmk, a broad caspase-inhibitor, or necrostatin-1, a
necroptosis inhibitor, respectively (Figs. 1 and 2). Addition of z-VAD-fmk resulted in
increased cell viability in AN3-CA cells after 24 h and in Hec-1A
cells after 72 h of co-incubation with ABM/Reishi extracts
(Fig. 1A and F). Co-incubation of
necrostatin with ABM/Reishi extracts resulted in a
significant protective effect only in AN3-CA cells at the highest
concentration of ABM/Reishi extracts used in this study at
all time points as well as in Ishikawa cells after 72 h (Fig. 1A–C and I). In contrast, the
co-incubation of Cordyceps extract with z-VAD-fmk or
necrostatin-1 had only slight effects on the cell viability
(Fig. 2).
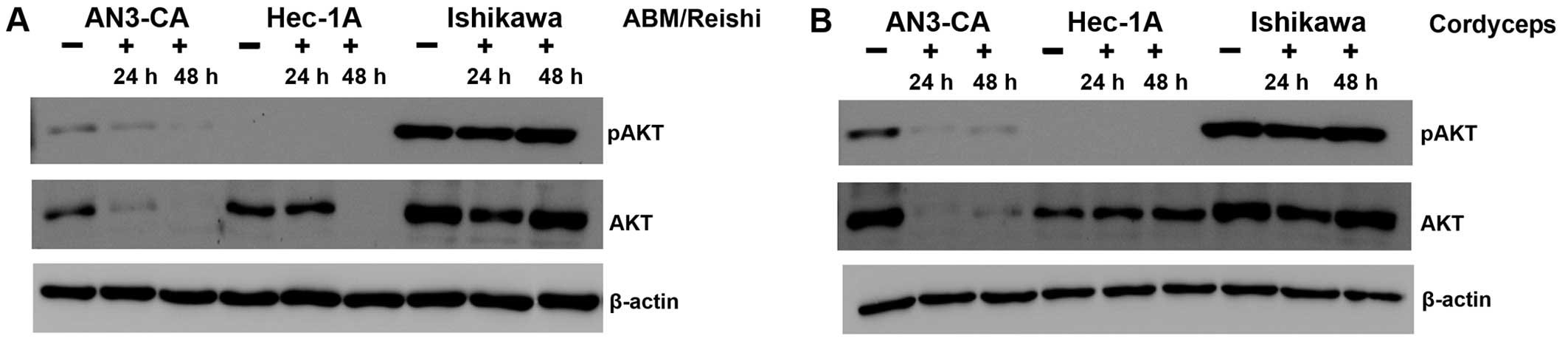
In the next step, we addressed the question if the
PI3K/AKT pathway known to be highly active in a broad variety of
tumours is influenced by the different fungi extracts. Because of
the fact that phosphorylated AKT (pAKT) is indicative for the
activity of the PI3K/AKT pathway, the expression level of pAKT as
well as AKT was analyzed with specific antibodies in a western blot
(Fig. 3). After incubation of
AN3-CA cells with ABM/Reishi (Fig. 3A) or Cordyceps extracts
(Fig. 3B) the pAKT expression was
reduced. Prolonged incubation with fungi extracts resulted in a
stronger inhibition of pAKT expression. In the same range, the
total AKT expression is strongly reduced in AN3-CA cells by
incubation with ABM/Reishi (Fig. 3A) and Cordyceps extracts
(Fig. 3B). As expected in Hec-1A
cells the pAKT expression is below the detection level, because of
the wild-type PTEN expression in this cell line (15,16).
But nevertheless, the AKT expression is strongly decreased by
incubation with ABM/Reishi extract for 48 h (Fig. 3A). In contrast, the incubation of
Hec-1A cells with Cordyceps extract did not significantly
alter the AKT expression (Fig.
3B). In Ishikawa cells activated AKT (pAKT) was highly
expressed due to an inactivating mutation of PTEN (15,16)
and only a slight decrease in the amount of pAKT was observed after
incubation of these cells with the fungi extracts for 24 h
(Fig. 3). Even in Ishikawa cells,
the expression of AKT was reduced by both fungi extracts after
incubation for 24 h (Fig. 3).
Therefore, it seems that both fungi extracts exert at least to a
certain degree their effect on the molecular level by decreasing
AKT expression.
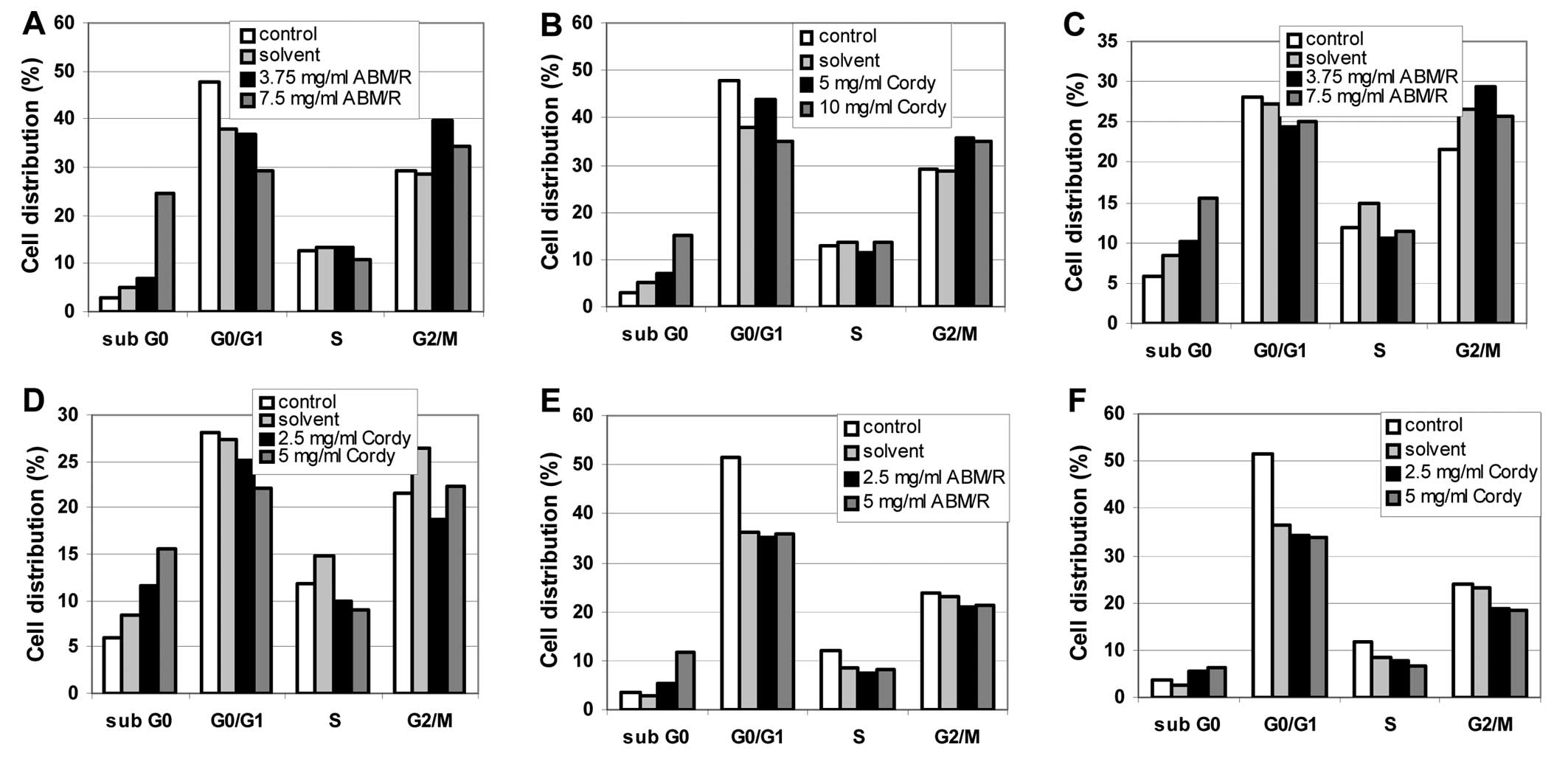
To enlighten further the effects of the fungi
extracts on the endometrial cell lines, FACS analyses were
performed. In cell cycle FACS analyses, an increase in the sub-G0
phase was observed after incubation of the cells (AN3-CA in
Fig. 4A and B; Hec-1A in Fig. 4C and D; Ishikawa in Fig. 4E and F) for 24 h with the indicated
concentrations of fungi extracts [ABM/Reishi extract in
Fig. 4A, C and E; Cordyceps
(Cordy) extract in Fig. 4B, D and
F] in all cases.
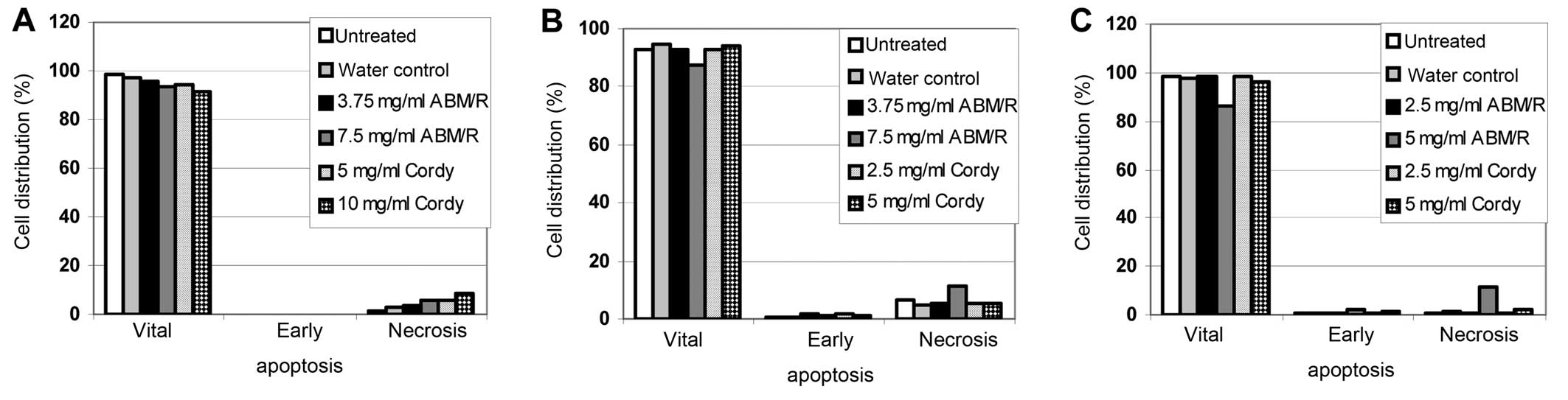
In FACS-based apoptosis assays, only slight effects
were found after incubation of the cells (AN3-CA in Fig. 5A; Hec-1A in Fig. 5B; Ishikawa in Fig. 5C) for 24 h with the indicated
concentrations of fungi extracts (Fig.
5). In agreement with the observation from the MTT assay with
and without z-VAD-fmk (Figs. 1 and
2) no apoptotic cells were
detected (Fig. 5). Furthermore,
only a minor increase in necrosis was observed (Fig. 5). Therefore, it must be concluded
that neither necrosis nor the programmed cell death (apoptosis)
played a predominant role in the fungi extracts mediated cell death
of endometrial cancer cells.
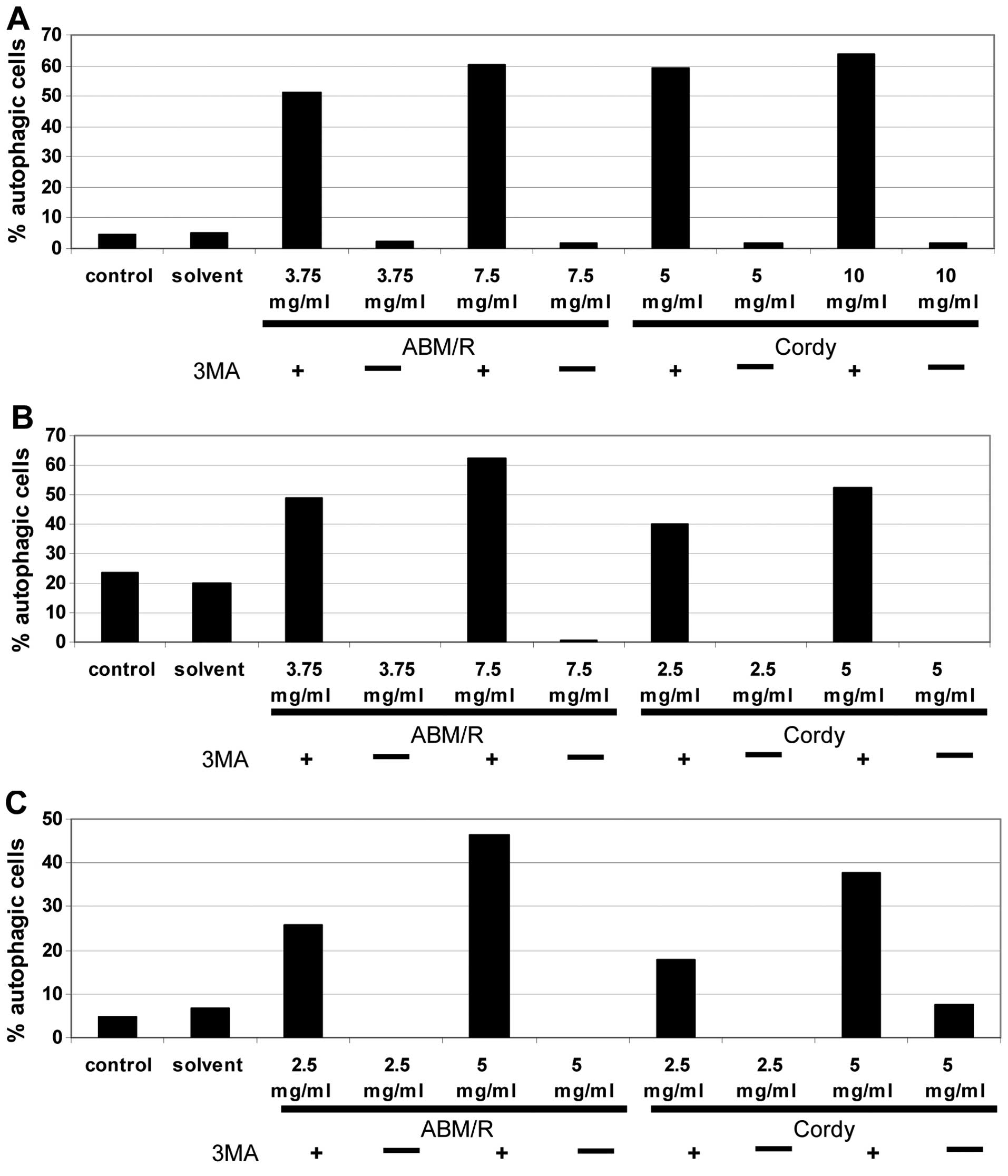
Another mechanism of programmed cell death often
observed in tumour cells is autophagic death (17–19).
To test the degree of autophagic death caused by both fungi
extracts, the endometrial cells were stained with acridine orange
after pre-incubation of the cells with Cordy and
ABM/Reishi extracts and thereafter analysed by FACS (AN3-CA
in Fig. 6A; Hec-1A in Fig. 6B; Ishikawa in Fig. 6C). In all probes, incubation with
fungi extracts for 24 h resulted in an increase in autophagy in
comparison to the untreated control cells and the solvent controls.
To show the specificity the cells were co-incubated with the fungi
extracts and the well established autophagy inhibitor
3’-methyladenine (3MA) (20)
(Fig. 6). Surprisingly Hec-1A
cells contained a high-level of autophagic cells even in the
untreated control culture (Fig.
6B). In comparison to the control cultures of both other
endometrial cancer cell lines in Hec-1A cells, nearly four times
more autophagic cells were detected. By addition of
3’-methyladenine, the effects of the Cordy and
ABM/Reishi extracts were completely abolished. Furthermore,
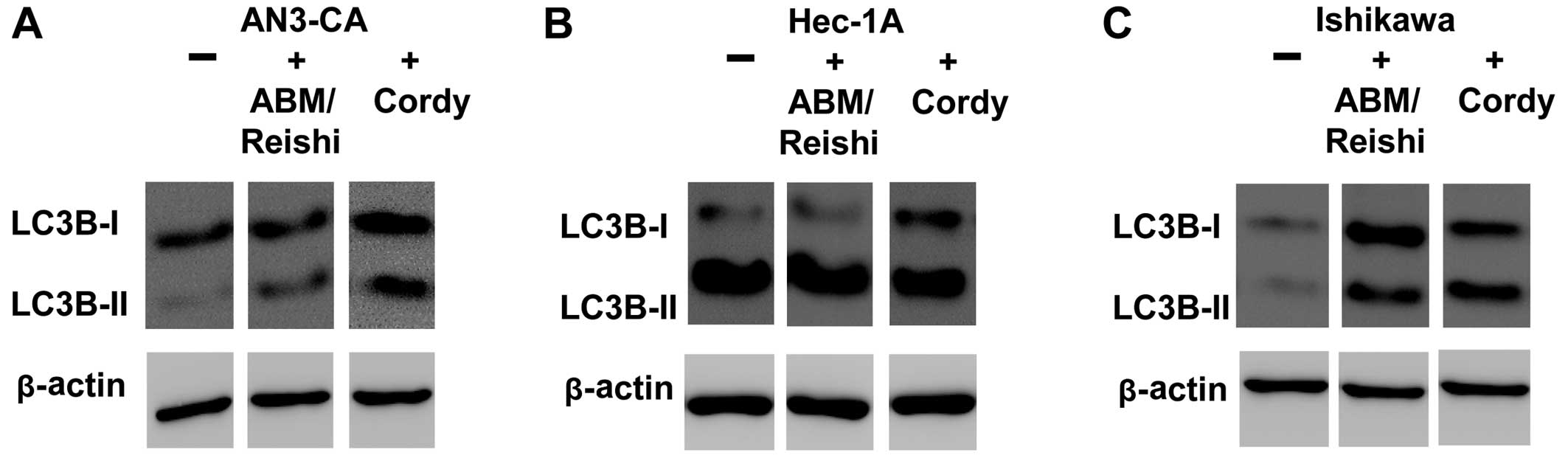
the induction of autophagic death in endometrial cancer cells by
Cordy and ABM/Reishi extracts were verified on the
protein level by western blotting using a specific antibody against
LC3 protein (Fig. 7). The
conversion of LC3B-I to the faster migrating form LC3B-II is a well
established indicator of autophagy. As shown for AN3-CA cells
(Fig. 7A) and Ishikawa cells
(Fig. 7C) incubation with fungi
extract resulted in an increased amount of LC3B-II. In Hec-1A cells
(Fig. 7B) it is difficult to
detect an increase in LC3B-II after incubation with fungi extracts
due to the already increased amount of autophagic cells in the
untreated culture that was already observed in the FACS based
detection of autophagy (Fig.
6B).
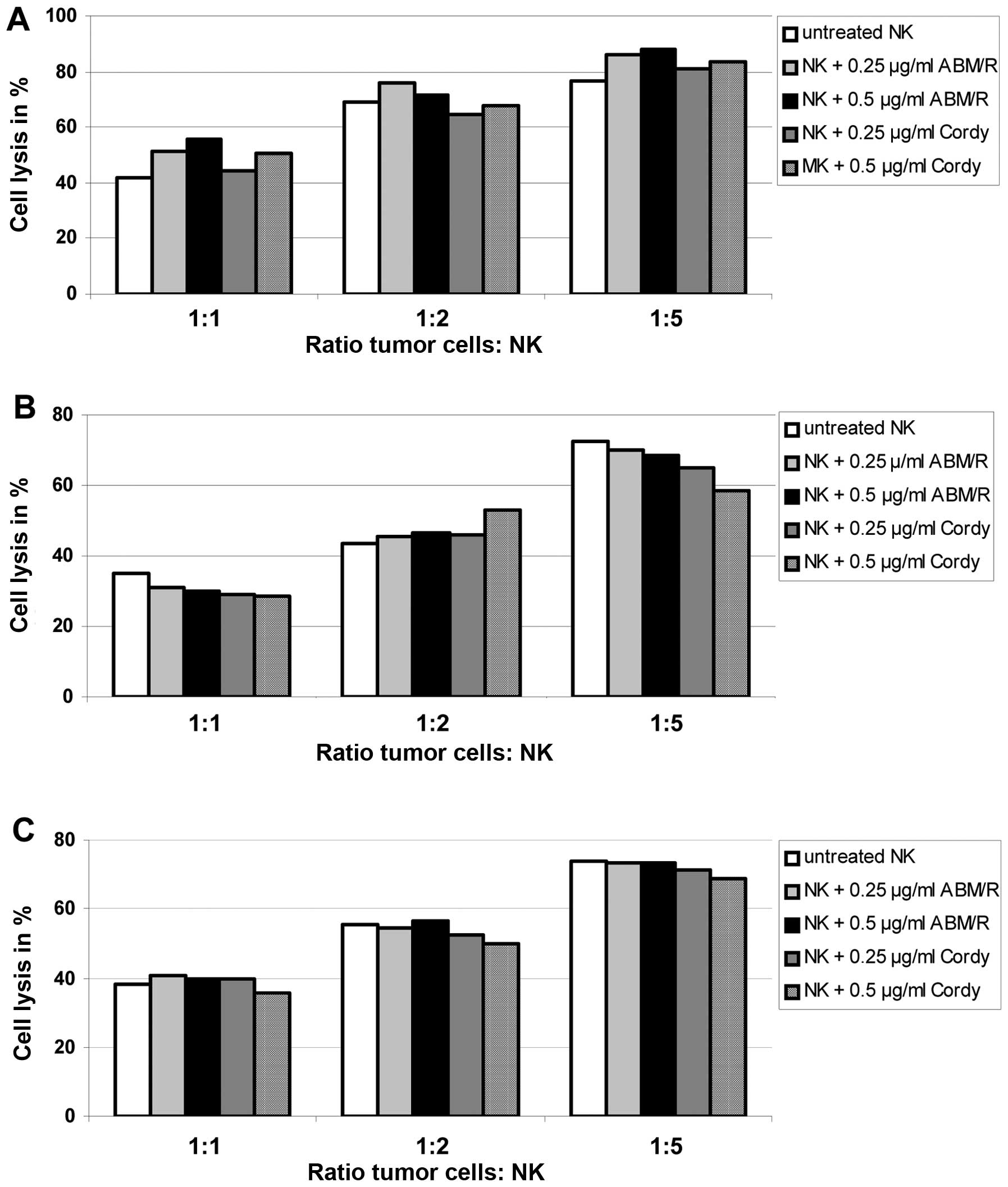
Since different fungi extracts are able to stimulate
the non-specific immune system and to exert antitumour activity
through the stimulation of the host’s defence mechanism, we
analyzed the effect on killing of endometrial cancer cells (AN3-CA
in Fig. 8A; Hec-1A in Fig. 8B; Ishikawa in Fig. 8C) by NK-cells matured with and
without addition of Cordy and ABM/Reishi extracts in
the indicated concentrations (Fig.
8). The killing capacity of NK-cells was not significantly
influenced by the maturation in the presence of either Cordy
or ABM/Reishi extracts compared to NK-cells matured without
addition of fungi extracts (Fig.
8). Furthermore, the incubation of tumour cells with the fungi
extracts did not alter the killing efficiency of NK-cells (data not
shown). Therefore, it seems that the used extracts from
Cordy and ABM/Reishi do not influence the
immunological interactions between tumour cells and NK-cells.
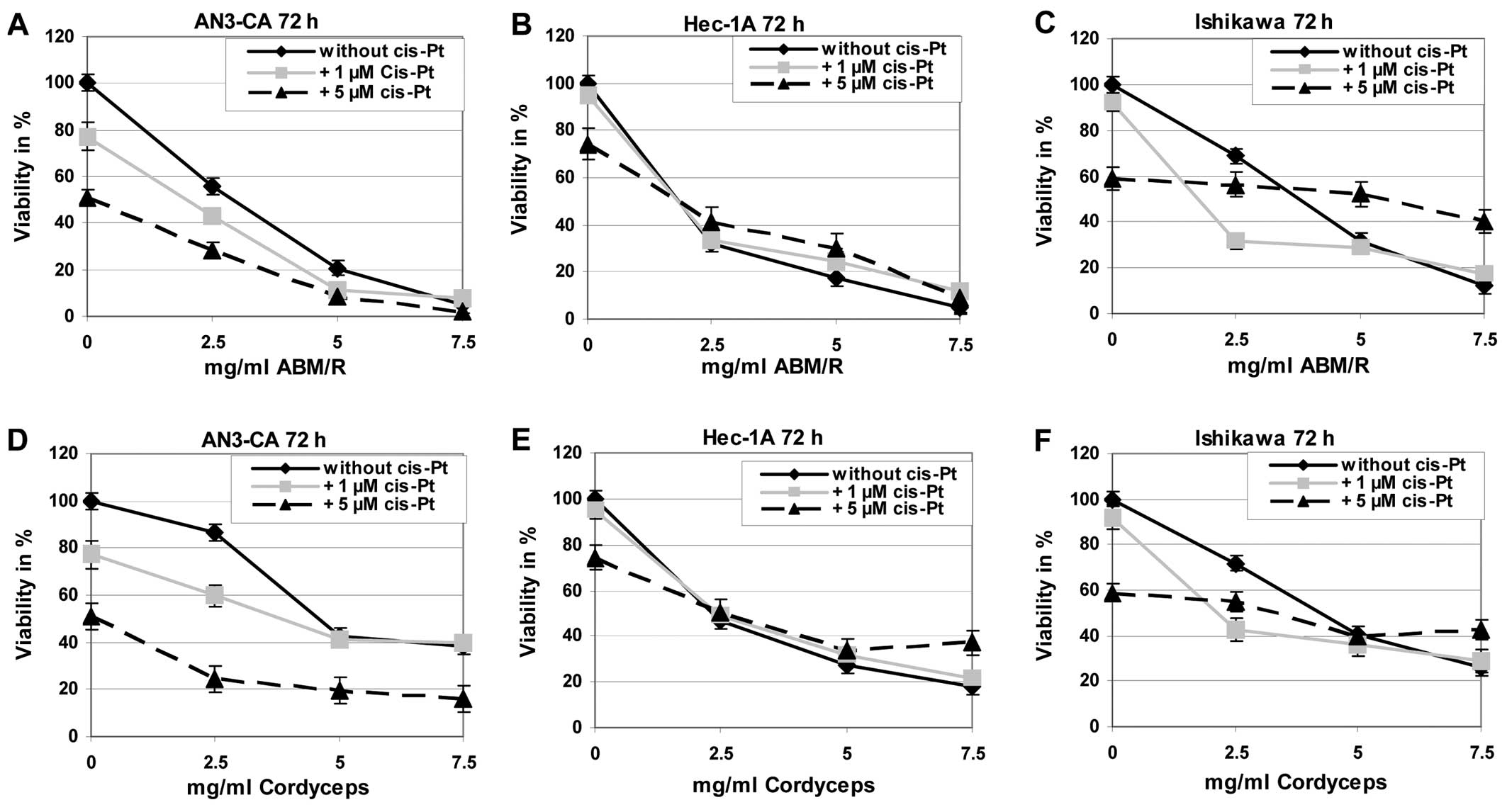
For treatment of endometrial cancer
chemotherapeutics are often used, among those cisplatin is known to
be one of the most effective in this disease. Therefore, we tested
if combined effects can be observed by incubation of endometrial
cancer cells with cisplatin and fungi extracts in comparison to
treatment of the tumour cells with one of these substances alone.
Exemplarily the results after incubation of the cells for 72 h are
shown (Fig. 9). The incubation of
the different endometrial cell lines with cisplatin in combination
with ABM/Reishi (Fig. 9A–C)
or Cordyceps (Fig. 9D–F)
extracts resulted in a decreased viability compared to cisplatin
incubation alone.
Discussion
Our purpose was to prove the efficiency of crude
extracts from Cordy alone and a mixture composed of
Reishi and ABM for treatment of endometrial cancer in
model systems in vitro. We have demonstrated here that
hot-water-extract derived from Cordy and ABM/Reishi
is able to suppress the growth of different human endometrial
cancer cell lines in vitro. After this proof-of-concept the
molecular basis was enlightened; most probably the suppression of
pAKT is involved in the observed antitumour effects. This finding
in endometrial cancer cell lines is in agreement with previous
studies on the effect of Cordy and Reishi extracts in
leukemia cells as well as in ovarian, breast, prostate and gastric
cancer (10,21–25).
The effective concentrations of fungi extracts in endometrial
cancer cells are in the same range as previously described for
ovarian cancer cells (10).
Furthermore, our data argue for an interaction of the different
fungi extracts with the PI3K/AKT signalling pathway known to be
involved in a broad variety of cancers (26). We and others have examined the role
of PI3K/AKT pathway in human tumours and we have recently
demonstrated the role of pAKT level for different aspects of cancer
cells, e.g. cisplatin based resistance and NK-cell mediated killing
(13,27). Moreover, it has been demonstrated
that inhibition of the PI3K/AKT signalling pathway results in an
increased tumour cell death (28–30).
Another role of AKT has been published recently; it was
demonstrated that in a panel of various human tumour types pAKT is
necessary for phosphorylation of Beclin-1, an essential autophagy
and tumour suppressor protein (31). If Beclin-1 is not phosphorylated
increased autophagy, reduced anchorage-independent growth, and
inhibited AKT-driven tumourigenesis was observed (31). In light of this observation, the
correlation between increased autophagic death and decreased pAKT
level in the endometrial tumour cells is explainable. In addition,
the high amount of autophagic cells in the untreated cultures of
Hec-1A cells (Fig. 6B) and the
high expression level of LC3B-II (Fig.
7B) in these cells is most probably based on the low pAKT level
in Hec-1A cells (Fig. 3).
Surprisingly the Cordy and ABM/Reishi
extracts used in this study did not influence the interaction
between endometrial tumour cells and NK-cells in experiments in
vitro. Previously it has been reported that some
polysaccharides or polysaccharide-protein complexes from mushrooms
are able to stimulate the non-specific immune system and to exert
anti-tumour activity through the stimulation of the host’s defence
mechanism (11,32–34).
The fungi extracts activate effector cells such as macrophages, T
lymphocytes and NK-cells to secrete cytokines (TNF-α, IFN-γ and
IL-1β), which are anti-proliferative and induce apoptosis in tumour
cells (11,32–34).
All these effects of fungi extracts on the cells of the immune
system have been evaluated in animal models and in human clinical
practice (35) and clearly this
complex interacting system of different stimulating and each other
influencing cells that exist in vivo cannot be completely
simulated in vitro. Therefore, our results concerning the
tumour cell killing by NK-cells in an in vitro model must be
re-analysed in an immunocompetent in vitro mouse model. But
nevertheless our preliminary data show that no inhibitory effects
are exerted by the different fungi extracts onto the NK-cell
mediated tumour cell killing.
In any case, it should kept in mind that the origin
as well as preparation of the fungi extracts can influence the
effects to a very high degree as it was demonstrated exemplarily
for breast cancer cells by Xie et al (36). Therefore, it is of pivotal
importance in studies dealing with the effects of natural compound
extracts to use one and the same standardized extraction method for
the biological material from the same origin as we have done here
and in previous studies (37).
Because of the fact that every fungi contains
various bio active compounds, including triterpene,
polysaccharides, sterols, nucleoside and nucleotides as well as
their degradation products and derivatives (6–8) it
is necessary to separate, isolate and analyze the different
bioactive compounds in further studies. Nevertheless, multiple
compound-based drugs may provide important combination therapies
that simultaneously influence multiple pharmacological targets and
provide clinical efficacy beyond that of single compound-based
drugs (9).
In conclusion, to our knowledge this is the first
study showing that fungi widely used in traditional Chinese
medicine are able to suppress the growth of different human
endometrial cancer cell lines in vitro. It seems to be very
likely that the different fungi extracts act by suppression of AKT
phosphorylation. The decreased level of pAKT results in an
increased cell death. According to our data presented here an
autophagic cell death is triggered by the fungi extracts in
endometrial cancer cell lines. However, we must keep in mind that
autophagy could act in two ways. Autophagy can play either
pro-survival or pro-death roles (17,38).
Activation of autophagy may function as tumour suppressor mechanism
by degrading cells or this pathway may be exploited by cancer cells
to generate nutrients during periods of starvation and hypoxia
(17,19,39).
Therefore, the Cordy and ABM/Reishi extracts may be
used as adjuvants in endometrial tumour therapy in combination with
chemotherapeutics to prevent tumour cells using the induced
autophagy in a pro-survival manner.
Acknowledgements
We appreciate the permission to use
the INTAS ChemoStar Imager (Department of Microbiology, University
of Würzburg). Therefore, we thank especially Professor T. Rudel and
Dr B. Bergmann. This work was supported by IZKF Würzburg.
References
|
1.
|
Chaudhry P and Asselin E: Resistance to
chemotherapy and hormone therapy in endometrial cancer. Endocr
Relat Cancer. 16:363–380. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Marnitz S and Köhler C: Current therapy of
patients with endometrial carcinoma. A critical review.
Strahlenther Onkol. 188:12–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Patel S and Goyal A: Recent developments
in mushrooms as anti-cancer therapeutics: a review. 3 Biotech.
2:1–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Lindequist U, Niedermeyer TH and Jülich
WD: The pharmacological potential of mushrooms. Evid Based
Complement Alternat Med. 2:285–299. 2005. View Article : Google Scholar
|
|
5.
|
Lima CU, Cordova CO, Nóbrega Ode T,
Funghetto SE and Karnikowski MG: Does the Agaricus blazei
Murill mushroom have properties that affect the immune system?
An integrative review. J Med Food. 14:2–8. 2011.
|
|
6.
|
Zhu JS, Halpern GM and Jones K: The
scientific rediscovery of a precious ancient Chinese herbal
regimen: Cordyceps sinensis: part II. J Altern Complement
Med. 4:429–457. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Li SP, Yang FQ and Tsim KW: Quality
control of Cordyceps sinensis, a valued traditional Chinese
medicine. J Pharm Biomed Anal. 41:1571–1584. 2006.
|
|
8.
|
Firenzuoli F, Gori L and Lombardo G: The
medicinal mushroom Agaricus blazei Murrill: review of
literature and pharmacotoxicological problems. Evid Based
Complement Alternat Med. 5:3–15. 2008.
|
|
9.
|
Schmidt BM, Ribnicky DM, Lipsky PE and
Raskin I: Revisiting the ancient concept of botanical therapeutics.
Nat Chem Biol. 3:360–366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Zhao S, Ye G, Fu G, et al: Ganoderma
lucidum exerts anti-tumor effects on ovarian cancer cells and
enhances their sensitivity to cisplatin. Int J Oncol. 38:1319–1327.
2011.
|
|
11.
|
Chihara G, Maeda Y, Hamuro J, Sasaki T and
Fukuoka F: Inhibition of mouse sarcoma 180 by polysaccharides from
Lentinus edodes (Berk.). Nature. 222:687–688. 1969.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Campling BG, Pym J, Galbraith PR and Cole
SP: Use of the MTT assay for rapid determination of
chemosensitivity of human leukemic blast cells. Leuk Res.
12:823–831. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hahne JC, Honig A, Meyer SR, et al:
Downregulation of AKT reverses platinum resistance of human ovarian
cancers in vitro. Oncol Rep. 28:2023–2028. 2012.PubMed/NCBI
|
|
14.
|
Traganos F and Darzynkiewicz Z: Lysosomal
proton pump activity: supravital cell staining with acridine orange
differentiates leukocyte subpopulations. Methods Cell Biol.
41:185–194. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Jin X, Gossett DR, Wang S, et al:
Inhibition of AKT survival pathway by a small molecule inhibitor in
human endometrial cancer cells. Br J Cancer. 91:1808–1812. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Block M, Fister S, Emons G, et al:
Antiproliferative effects of antiestrogens and inhibitors of growth
factor receptor signaling on endometrial cancer cells. Anticancer
Res. 30:2025–2031. 2010.PubMed/NCBI
|
|
17.
|
Tsujimoto Y and Shimizu S: Another way to
die: autophagic programmed cell death. Cell Death Differ. 12(Suppl
2): 1528–1534. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
González-Polo RA, Boya P, Pauleau AL, et
al: The apoptosis/autophagy paradox: autophagic vacuolization
before apoptotic death. J Cell Sci. 118:3091–3102. 2005.PubMed/NCBI
|
|
19.
|
Ouyang L, Shi Z, Zhao S, et al: Programmed
cell death pathways in cancer: a review of apoptosis, autophagy and
programmed necrosis. Cell Prolif. 45:487–488. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Amaral C, Borges M, Melo S, et al:
Apoptosis and autophagy in breast cancer cells following exemestane
treatment. PLoS One. 7:e423982012. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Jiang J, Slivova V, Harvey K,
Valachovicova T and Sliva D: Ganoderma lucidum suppresses
growth of breast cancer cells through the inhibition of Akt/NF-κB
signaling. Nutr Cancer. 49:209–216. 2004. View Article : Google Scholar
|
|
22.
|
Jin CY, Kim GJ and Choi YH: Induction of
apoptosis by aqueous extract of Cordyceps militaris through
activation of caspases and inactivation of Akt in human breast
cancer MDA-MB-231 cells. J Microbiol Biotechnol. 18:1997–2003.
2008.
|
|
23.
|
Jiang J and Sliva D: Novel medicinal
mushroom blend suppresses growth and invasiveness of human breast
cancer cells. Int J Oncol. 37:1529–1536. 2010.PubMed/NCBI
|
|
24.
|
Calviño E, Manjón JL, Sancho P, et al:
Ganoderma lucidum induced apoptosis in NB4 human leukemia
cells: involvement of Akt and Erk. J Ethnopharmacol. 128:71–78.
2010.
|
|
25.
|
Dotan N, Wasser SP and Mahajna J:
Inhibition of the androgen receptor activity by Coprinus
comatus substances. Nutr Cancer. 63:1316–1327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Coutte L, Dreyer C, Sablin MP, Faivre S
and Raymond E: PI3KAKT-mTOR pathway and cancer. Bull Cancer.
99:173–180. 2012.(In French).
|
|
27.
|
Hahne JC, Meyer SR, Gambaryan S, et al:
Immune escape of AKT overexpressing ovarian cancer cells. Int J
Oncol. 42:1630–1635. 2013.PubMed/NCBI
|
|
28.
|
Honig A, Hahne JC, Meyer S, et al: PI3K
inhibitor D-116883 is effective in in vitro models of
ovarian cancer. Anticancer Res. 32:2035–2041. 2012.PubMed/NCBI
|
|
29.
|
Pant A, Lee II, Lu Z, et al: Inhibition of
AKT with the orally active allosteric AKT inhibitor, MK-2206,
sensitizes endometrial cancer cells to progestin. PLoS One.
7:e415932012. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Prasad R, Vaid M and Katiyar SK: Grape
proanthocyanidin inhibit pancreatic cancer cell growth in
vitro and in vivo through induction of apoptosis and by
targeting the PI3K/Akt pathway. PLoS One. 7:e430642012. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Wang RC, Wei Y, An Z, et al: Akt-mediated
regulation of autophagy and tumorigenesis through Beclin 1
phosphorylation. Science. 338:956–959. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Mizuno T: The extraction and development
of antitumor-active polysaccharides from medicinal mushrooms in
Japan (review). Int J Med Mushr. 1:9–30. 1999. View Article : Google Scholar
|
|
33.
|
Wasser SP and Weis AL: Medicinal
properties of substances occurring in higher Basidiomycetes
mushrooms: current perspectives (review). Int J Med Mushr. 1:31–62.
1999. View Article : Google Scholar
|
|
34.
|
Reshetnikov SV, Wasser SP and Tan KK:
Higher basidiomycetes as a source of antitumor and
immunostimulating polysaccharides (review). Int J Med Mushr.
3:361–394. 2001.
|
|
35.
|
Wasser SP: Medicinal mushrooms as a source
of antitumor and immunomodulating polysaccharides. Appl Microbiol
Biotechnol. 60:258–274. 2002.PubMed/NCBI
|
|
36.
|
Xie YZ, Li SZ, Yee A, et al: Ganoderma
lucidum inhibits tumour cell proliferation and induces tumour
cell death. Enzyme Microb Technol. 40:177–185. 2006. View Article : Google Scholar
|
|
37.
|
Kiriakidis S, Högemeier O, Starcke S, et
al: Novel tempeh (fermented soyabean) isoflavones inhibit in vivo
angiogenesis in the chicken chorioallantoic membrane assay. Br J
Nutr. 93:317–323. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Wu WK, Coffelt SB, Cho CH, et al: The
autophagic paradox in cancer therapy. Oncogene. 31:939–953. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Carew JS, Kelly KR and Nawrocki ST:
Autophagy as a target for cancer therapy: new developments. Cancer
Manag Res. 4:357–365. 2012.
|