Contents
Introduction
Normal B cells
Chronic lymphocytic leukaemia
Hairy cell leukaemia
Diffuse large B-cell lymphoma
Follicular lymphoma
Marginal zone lymphoma
Mantle-cell lymphoma
Burkitt’s lymphoma
Hodgkin’s lymphoma
Conclusion
Introduction
Lymphocytes are by nature motile cells owing to
their pivotal role in immune surveillance. They traffic through the
immune tissues in search of the specific antigen that binds to
their unique antigen receptor. Binding of antigen to receptor
initiates an immune response designed to eliminate the pathogen
bearing the antigen. In the case of B cells, when antigen-naïve
lymphocytes encounter specific antigens they remain in the
lymphoreticular tissues for 2–3 days in order to differentiate into
mature effector cells. However, if they do not encounter antigen,
they exit the tissues within a matter of hours and continue their
search (1,2). The lymphocytes of B-cell lymphomas
mostly reside within the tissues and therefore differ from normal B
cells in their ability to traffic. Understanding the mechanisms by
which these cells are retained in the tissues may therefore open up
new avenues for novel therapy aimed at releasing the malignant
lymphocytes from their tissue micro-environment and, in doing so
deprive them of stimuli required for their growth and survival.
Normal B cells
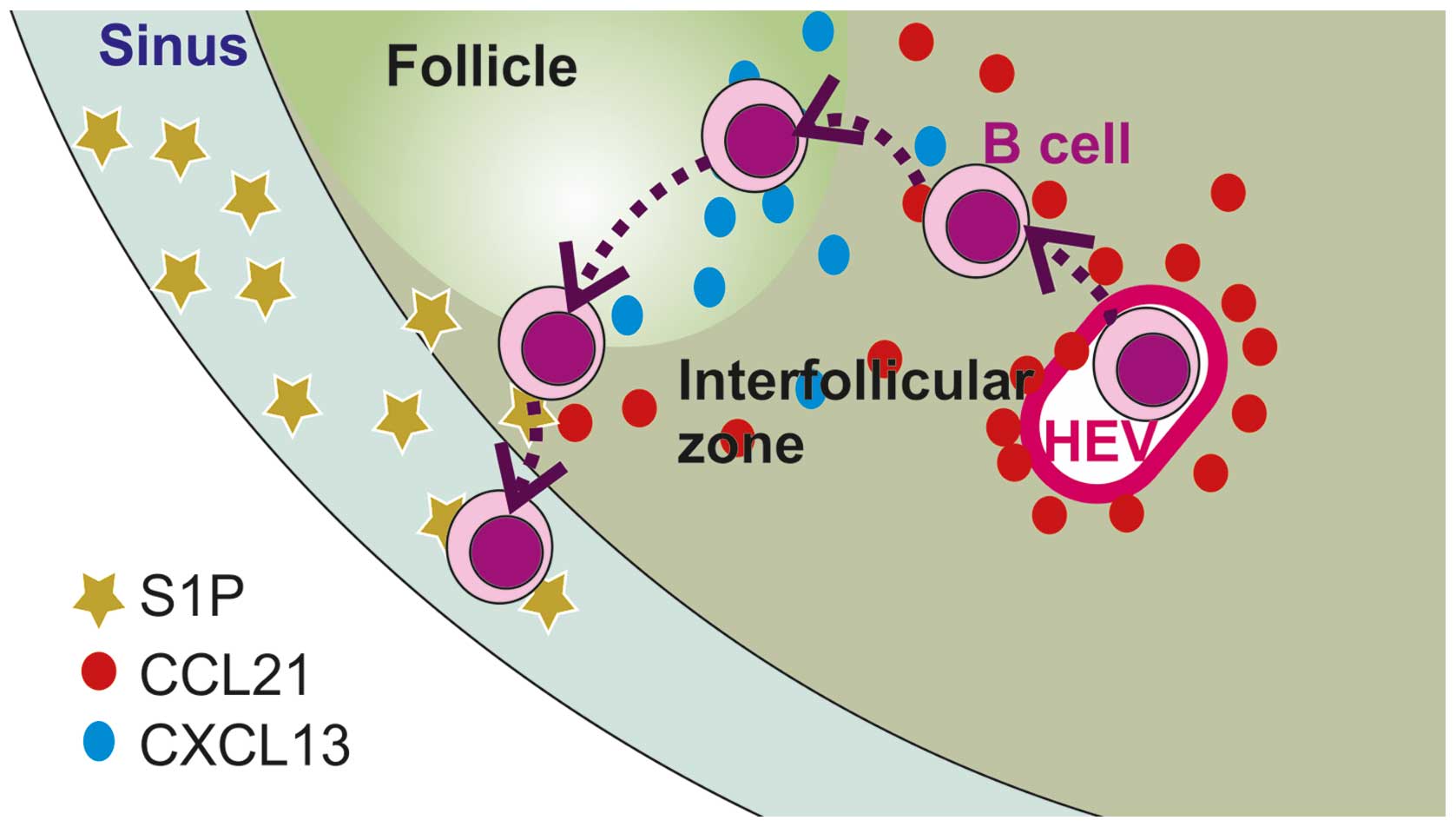
The factors controlling the trafficking of normal B
lymphocytes into, within and out of tissues have been extensively
studied (Fig. 1; Table I). Entry of lymphocytes into the
tissues is under the control of chemokines (3,4) and
adhesion molecules, whereas exit (egress) is dependent on the
sphingolipid, sphingosine-1-phosphate and its receptors S1PR1 and
S1PR3 and is independent of adhesion molecules (5,6).
Whether or not a lymphocyte remains in or exits the lymphoreticular
system, as well as its localisation within lymphoreticular tissues,
is determined by the balance of chemokines, S1P receptors and
adhesion molecules. With regard to chemokines involved in the
trafficking of normal B cells, CCR7 and its ligand CCL21 control
entry into lymph nodes (7),
whereas CXCR5 and its ligand CXCL13 direct B lymphocytes into the
follicular area of LNs (7,8) and the white pulp of the spleen
(8) and contributes to the
retention of B cells at these sites. finally, CXCR4 and its ligand
CXCL12 are involved in the homing and retention of B lymphocytes in
the bone marrow (BM) (9,10). With regard to adhesion molecules,
binding of α4β1 (VLA-4) on lymphocytes to VCAM-1 on HEV contributes
to the initial interaction of lymphocytes with HEV (11,12),
whereas binding of αLβ2 (LFA-1) on lymphocytes to its ligand ICAM-1
on the surface of HEV is essential for entry of lymphocytes into
LNs (13,14). Both α4β1 and αLβ2 are required for
entry of lymphocytes into the splenic white pulp (15), whereas α4β1 is also involved in the
motility and retention of B lymphocytes within the spleen (16) and BM (10,17).
α4 can also form heterodimers with β7; β7 integrins are responsible
for efficient trafficking and retention of lymphocytes in the gut
(18). When complexed with α4, β7
binds to MadCAM-1 on the HEVs of the mucosa-associated lymphoid
tissue (MALT) of the gut (19)
where it allows the entry of α4β7-expressing lymphocytes. Whereas
αEβ7 binds to E-cadherin and facilitates the retention
of effector and memory lymphocytes in the gut epithelium (18). Exit of lymphocytes from the LNs is
regulated by S1PR1, whereas S1PR3 regulates egress from the spleen
(20) and BM (21).
Compared to normal B lymphocytes, much less is known
about the trafficking of lymphoma cells. This review outlines the
chemokine receptors and integrins that are known to be expressed on
lymphoma cells, summarises the evidence supporting their role in
lymphoma biology and speculates on how this understanding might
translate into novel therapy.
Chronic lymphocytic leukaemia
Chronic lymphocytic leukaemia (CLL) is the most
extensively studied of the B-cell lymphomas with regard to the
factors involved in cell trafficking (Table II). This probably reflects its high
prevalence and almost universal blood involvement. CLL cells
migrate into and infiltrate all organs of the lymphoreticular
system including the BM, LNs, white pulp of the spleen and liver.
Invasion of LNs by CLL results in the complete destruction of the
normal architecture, with the malignant lymphocytes occupying both
the follicular and interfollicular areas (22).
 | Table II.The expression of chemokine receptors
and adhesion molecules involved in lymphocyte homing by B cell
lymphomas. |
Table II.
The expression of chemokine receptors
and adhesion molecules involved in lymphocyte homing by B cell
lymphomas.
| Disease | CCR7 | CXCR4 | CXCR5 | α4β1 | α4β7 | αLβ2 | S1P |
|---|
| CLL | +/+++ | +/+++ | +++ | −/++ | − | +/+++ | NT |
| HCL | −/+ | +++ | −/+ | ++ | − | −/+ | NT |
| DLBCL | NT | Y | Y | −/+++ | ND | −/+ |
S1P2 |
| FL | Y/Na | Y | Y | −/+++ | NT | NT | NT |
| MZL | NT | −/+ | −/+ | −/+ | − | NT | NT |
| MALT | NT | NT | NT | −/+ | + | NT | NT |
| MCL | Y | Y | Y | Y | +b | Y | NT |
| BL | +++ | NT | Y | Y | NT | + | NT |
| HL | | | | | | | |
| Classical | +++ | +++ | −/+ | NT | NT | Y | NT |
| Nodular | − | Y | −/+ | | | | |
CLL cell entry into lymph nodes resembles that of
normal B cells in that it requires CCL21 and αLβ2 (23). However, CLL cells differ from
normal B cells in that they also require α4β1 for transendothelial
cell migration (TEM). In keeping with this observation,
lymphadenopathy in CLL is associated with high levels of α4β1 and
CCR7 (23). Furthermore, high
expression of α4 (CD49d) has been observed to be an independent
adverse prognostic factor (24,25).
The dependence of CLL cells on α4β1 for TEM can be explained by a
defect in the polar clustering of αLβ2 that is overcome by α4β1
expression (24,25).
The role of CXCR4 and its potential involvement in
the accumulation of CLL cells in the BM has also been investigated.
CXCR4 mediates the migration of CLL cells through BM stromal cells,
which secrete its ligand CXCL12 (26), and the retention of CLL cells
within the BM (27). Furthermore,
high CXCR4 expression correlates with extensive tissue invasion and
adverse outcome (28). It is
unclear whether other chemokine receptors play a role in regulating
the migration of CLL cells into and within tissues. For example,
although CXCR5 has been reported to be overexpressed by CLL cells
(29), this chemokine receptor is
predominantly expressed in the follicles and is associated with
homing to this site (7,8). Since CLL cells do not accumulate in
the follicles, the role of CXCR5 in CLL-cell homing is unclear.
There is emerging evidence that novel therapeutic
agents that target components of the B-cell receptor signalling
pathway may act at least in part by interfering with CLL-cell
trafficking. For example, the phosphotidylinositol 3-kinase (PI3K)
δ inhibitor, idelalisib (CAL-101), has been reported to
down-regulate the expression of CXCL13 and reduce chemotaxis
towards CXCL12 and CXCL13 without affecting the expression of
chemokine receptors (30). These
observations are in keeping with the established role of PI3K in
the directional movement of lymphocytes (31,32).
Furthermore, administration of idelalisib to patients with CLL
results in a rapid reduction in LN size and a simultaneous increase
in blood involvement which subsequently declines over a period of
several months, suggesting an effect on the trafficking of CLL
cells into or out of lymph nodes (30). Administration of the SYK inhibitor
fostamatinib to patients with CLL also results in an transient
lymphocytosis (33), likely
reflecting the established role of SYK in signalling mediated
through integrins and chemokines (34). Furthermore, the BTK inhibitor
ibrutinib which also induces lymphocytosis inhibits chemokine and
BCR-mediated adhesion of the malignant lymphocytes via α4β1
(35). In summary, disruption of
CLL trafficking appears to be a consistent effect of these kinase
inhibitors, and it is intriguing to speculate that this might
account for at least some of their therapeutic activity. In theory,
CLL-cell trafficking could be targeted more directly by targeting
molecules such as α4β1 and CXCR4 (36,37)
which are known to play a crucial role in the migration and
survival of CLL cells. Indeed, inhibitors of both molecules have
already shown activity in other diseases including multiple
sclerosis, Crohn’s disease and chronic myeloid leukaemia (38,39).
Hairy cell leukaemia
Similar to CLL, the neoplastic lymphocytes of hairy
cell leukaemia (HCL) are usually found in the circulation as well
as in the tissues. Thus, despite its rarity (<1% of all
leukaemias), the factors involved in the trafficking and organ
involvement in HCL have been more extensively studied than in
other, more common, lymphoid malignancies (Table II) (40). The pattern of tissue involvement in
HCL differs from that of CLL. In particular, HCs do not infiltrate
the lymph nodes but are instead localised to the red pulp of the
spleen (41). The malignant
lymphocytes also infiltrate the BM where they secrete fibronectin
resulting in BM fibrosis (42). In
keeping with the absence of lymphadenopathy in HCL, HCs express
CCR7 at extremely low levels (43). In addition, the low expression of
CCR7 and CXCR5 (43), which are
required for entry into the splenic white pulp (44) and lymphoid follicles, respectively,
likely explains why HCs are not founds at these sites. The homing
of HCs to the BM can be attributed to their high expression of
CXCR4 (45).
With regard to adhesion molecules, HCs express α4β1,
whereas αLβ2 is either absent or expressed at very low levels
(46). Since αLβ2 is required for
TEM into LN, the low expression of this integrin, together with the
low expression of CCR7, provides an explanation for the absence of
LN involvement in HCL. In contrast, high expression of α4β1
(together with CXCR4 and CXCR5) would explain the homing of HCL
cells to, and their retention in, the spleen and BM. In addition,
the expression of αEβ7 characterises HCL, although as
HCs do not home to the gastointestinal tract, it is unclear why the
neoplastic B-cells express this integrin (47). In summary, the chemokine and
integrin receptor profile of HCs (with the exception of
αEβ7) fits perfectly with the unique tissue distribution
of HCL.
Diffuse large B-cell lymphoma
Despite being the most common type of NHL, very
little is known regarding the factors involved in the migration and
trafficking of the malignant cells in this disease (Table II) (48,49).
This is surprising given the effacement of LN architecture and
frequent dissemination both within and outside of the
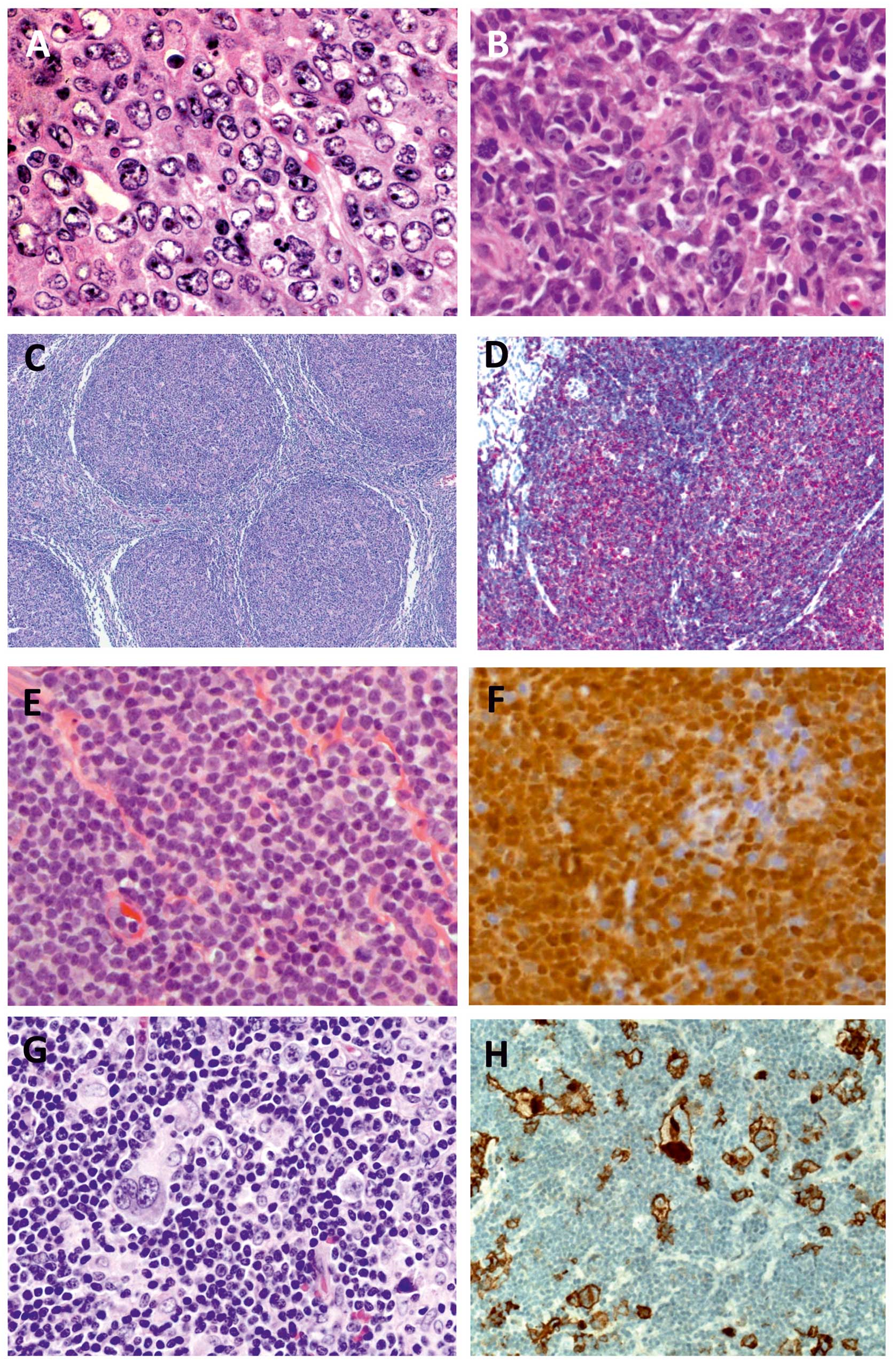
lymphoreticular system (Fig. 2A and
B). Diffuse large B-cell lymphoma (DLBCL) cells have been shown
to express the chemokine receptors CXCR4 and CXCR5 (48). Primary CNS lymphomas, a rare
subtype of DLBCL express these chemokines together with CCR7
(50); however, the expression of
the receptor is confined to the cytoplasm. Whether or not the
expression on other DLBCLs is on the surface, or in the cytoplasm
has not been explored. With regard to integrins, a proportion of
cases express α4β1 and αLβ2 (49),
with high expression of α4β1 being associated with advanced stage
disease (49).
With regard to S1P receptors, DLBCL cells
preferentially express S1PR2, and mutations which inactivate the
receptor were found in 27% of cases (51). S1PR2 inactivation is thought to
play a critical role in the development of DLBCL as following the
conditional knockout of S1PR2 in B cells 50% of mice developed the
disease (51). S1PR2 differs from
S1PR1 and S1PR3 in that it is coupled to G12/13 rather
than Gi. Hence, whereas S1PR1 and S1PR3 signal through
Rac and promote motility, S1PR2 activates Rho and thereby inhibits
motility (52).
Follicular lymphoma
Most patients with follicular lymphoma (FL) present
with advanced-stage disease involving multiple LNs and BM (53). However, very little is known about
how the malignant lymphocytes become disseminated (Table II). As would be predicted for
lymphocytes that home to the lymphoid follicles, FL cells express
CXCR5 (48,54). They also produce CXCL13 (the ligand
for CXCR5), which may therefore play a role in attracting
additional FL cells to existing sites of involvement (48). FL cells also express CXCR4
(29,48,54),
which likely explains the high frequency of BM involvement.
Although one might expect the surface expression of CXCR5 and CXCR4
to be downregulated in the LN and BM, respectively, due to
ligand-induced receptor internalisation, expression of these two
receptors is similar in different tissue compartments (29). There are conflicting reports
concerning whether or not follicular lymphoma cells express CCR7
(29,54). With regard to adhesion molecules,
the expression of α4β1 on FL cells varies not only in the number of
positive cells but also in the intensity of expression on the
positive cells (49). However, it
is unclear whether α4β1 expression is associated with stage or
prognosis. It is clear that the susceptibility of FL cells to
anti-lymphoma therapy is strongly influenced by interaction with
non-malignant cells in the tumour microenvironment (55) (Fig. 2C
and D). In addition, it has been recently shown that there is
bidirectional migration of lymphoma cells from the LN to the BM,
and the cells that reside in the BM are responsible for relapse
following chemotherapy (56).
Marginal zone lymphoma
Marginal zone lymphomas (MZL) are classified
according to their tissue localisation into extranodal MZL of MALT,
nodal MZL and splenic MZL. Extranodal MALT lymphomas comprises
50–70% of MZL and occur at mucosal sites including stomach,
salivary glands, lacrimal glands, parotid glands, as well as skin,
thyroid, lung and other organs. These lymphomas typically present
as an isolated lesion, and the disease follows an indolent clinical
course. More than 80% of MZLs arising in the stomach achieve
10-year survival when the Helicobacter pylori infection
which drives the disease is treated (57). In contrast, nodal MZL, which
typically affects peripheral and intra-abdominal LNs and bone
marrow, tends to be less responsive to treatment (58). Splenic MZL is a distinctive disease
with splenic, BM and blood involvement (58). Given the unique tissue distribution
of the different MZL subtypes, remarkably little is know about the
factors that determine disease localisation (Table II). As might be predicted, MALT
lymphomas express α4β7 (59), with
some cases also expressing α4β1 (60). With regard to chemokines, MZL are
reported to express low levels of CXCR4 and CXCR5 and migrate
poorly in response to these chemokines (61). There is no information as to
whether or not the expression of these chemokine receptors
corresponds to infiltration at particular sites. Further
investigation of the molecules involved in the tissue localisation
of MZL is therefore needed with the potential to inform of novel
therapeutic strategies to dislodge the lymphoma cells from their
protective microenvironmental niches.
Mantle-cell lymphoma
Mantle-cell lymphoma (MCL) is a challenging disease
to treat as it is neither low-grade nor curable with conventional
therapy. In addition to LNs (Fig. 2E
and F), the disease is usually present in the bone marrow and
sometimes the blood. One very striking feature of MCL is its
propensity to form colonic polyps which occur in a high proportion
of patients (62). Although the
treatment of MCL has improved in recent years owing to the use of
high-dose cytarabine, stem-cell transplantation and rituximab
(63,64), developing more effective therapy
for this disease is a priority. MCL cells express high levels of
all three chemokine receptors associated with lymphocyte homing,
namely CXCR4, CXCR5 and CCR7 (61)
(Table II). They also express both
αLβ2 and α4β1 (49) (Table II). Inhibition of the latter
integrin on MCL cells has been shown to inhibit motility beneath
(65), and adhesion to, marrow
stromal cells in vitro (66). Furthermore, adhesion of MCL cells
to a stromal cell line has been shown to protect them from
drug-induced apoptosis in vitro. However, inhibition of
α4β1-mediated adhesion with the monoclonal antibody natalizumab had
a limited effect in overcoming protection in this system (66), suggesting that cytoprotection is
likely mediated by soluble factors secreted by the stromal cells.
In addition, it has been shown that expression of α4β7 by MCL cells
in peripheral LNs was associated with gastrointestinal tract
involvement in 5/7 cases studied; all cases were also positive for
α4β1 (67). Whether or not MCL
cells express αEβ7 is unclear, one report suggests that
mRNA levels were higher in non-nodal MCL (68), whereas another that the protein is
not expressed (47). Further
investigation of the factors involved in the spread of the tumour
to different organs is clearly warranted.
Since BCR signalling is also thought to play a role
in MCL, as with CLL, the BTK inhibitor ibrutinib has also been
tested in the treatment of MCL where it also induces lymphocytosis
and a decrease in LN size, suggesting that treatment displaces the
MCL cells from their microenvironmental niches (69,70).
Burkitt’s lymphoma
Burkitt’s lymphoma (BL) is a highly aggressive
disease that typically present with large abdominal masses plus
bone marrow or CNS involvement in 70 and 40% of patients,
respectively. A significant proportion of patients are not cured by
intensive chemotherapy, and relapsed or refractory disease is
associated a dismal outcome (71).
Given that the CXCL13/CXCR5 axis was first identified in BL
(72), and that CCR7 was
identified as one of the most upregulated genes in BL (73) it is surprising that there have been
no further studies regarding the role of chemokines and their
receptors in primary BL cells (Table
II). Aberrant expression of CXCL13 in the CNS has been
associated with involvement of the brain as in DLBCL (74), and in the recruitment of B cells to
the brain in paediatric Opsoclonus-myoclonus syndrome (75). It therefore seems likely that
expression of CXCR5 by BL cells could control their homing to the
CNS. With regard to integrins, BL cells express α4β1 and αLβ2
(76) (Table II). However, levels of αLβ2 are
lower than in normal B cells owing to the overexpression of
c-myc (77).
Hodgkin’s lymphoma
Hodgkin’s lymphoma (HL) is usually confined to the
LNs. Lymphadenopathy is usually localised with involvement of
contiguous nodes. Occasionally, the disease involves extra-nodal
sites, the BM and lung being most commonly affected. The majority
of patients with HL are curable. However, patients who fail
frontline therapy have an uncertain prognosis (78). The expression of chemokine
receptors by the malignant Reed-Sternberg (R-S) cells varies
according to the subtype of HL (Table
II). In classical HL, the R-S express high levels of CCR7 and
CXCR4 and are located in the interfollicular areas. In contrast, in
nodular lymphocyte predominant HL, the malignant cells expresses
CXCR4, but not CCR7 (54).
Expression of CXCR5 is typically low or absent and not linked to
any particular subtype (54). The
malignant R-S cells are surrounded by a dense infiltrate of
non-malignant leukocytes which provide a protective and stimulatory
microenvironment (Fig. 2G and H)
(55,79,80).
Consequently, in addition to the chemokines responsible for the
localisation of R-S cells within the lymphoid tissues, it is also
important to consider the chemokines responsible for attracting
non-malignant cells to the R-S cells (80). In fact, R-S cells have been shown
to produce CCL17, CCL22, CXCL9, CXCL10 and CX3CL1 which are thought
to attract T cells and monocytes into the tumour (79,80).
In contrast, little is known about which adhesion molecules are
important in HL, although early reports indicate that R-S cells
express αLβ2 and αXβ2 (81)
(Table II). The importance of
interaction between R-S cells and other leukocytes in HL offers the
hope of a novel therapy that disrupts these interactions.
Conclusion
B-cell lymphomas are a diverse group of diseases
that share many of the homing characteristics of normal B
lymphocytes. The anatomical distribution of different B-cell
malignancies can be partially explained by the profile of adhesion
molecules, chemokine receptors and S1P receptors expressed,
although there are still many unanswered questions. Drugs that
target B-cell receptor signalling pathways clearly have an effect
on cell trafficking, and it is intriguing to speculate that the
therapeutic effects of these drugs might be mediated at least in
part by dislodging malignant B cells from their protective
micro-environment. Improving our understanding of the molecular
mechanisms responsible for guiding malignant B cells to, and
retaining them in, particular tissue sites is important as it
provides an opportunity to develop new approaches to therapy based
on the disruption of these processes.
Acknowledgements
We thank Dr Geetha K. Menon for proof
reading the manuscript and for providing the images for Fig. 2A, G and H.
References
|
1.
|
Cyster JG: Chemokines,
sphingosine-1-phosphate, and cell migration in secondary lymphoid
organs. Ann Rev Immunol. 23:127–159. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
von Andrian U and Mackay CR: T-cell
function and migration: two sides of the same coin. N Engl J Med.
343:1020–1034. 2000.
|
|
3.
|
Campbell JJ and Butcher EC: Chemokines in
tissue-specific and microenvironment-specific lymphocyte homing.
Curr Opin Immunol. 12:336–341. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Okada T, Ngo VN, Ekland EH, et al:
Chemokine requirements for B cell entry into lymph nodes and peyers
patches. J Exp Med. 196:65–75. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Matloubain M, Lo CG, Cinamon G, et al:
Lymphocyte egress from thymus and peripheral lymphoid organs is
dependent on S1P receptor 1. Nature. 427:355–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Lo CG, Xu Y, Proia RL and Cyster JG:
Cyclical modulation of sphingosine-1-phosphate receptor 1 surface
expression during lymphocyte recirculation and relationship to
lymphoid organ transit. J Exp Med. 201:291–301. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Muller G, Hopken UE and Lipp M: The impact
of CCR7 and CXCR5 on lymphoid organ development and systemic
immunity. Immunol Rev. 195:117–135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Cyster JG, Ansel KM, Reif K, et al:
Follicular stromal cells and lymphocyte homing to follicles.
Immunol Rev. 176:181–193. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Nie Y, Waite J, Brewer F, Sunshine MJ,
Littman DR and Zou YR: The role of CXCR4 in maintaining peripheral
B cell compartments and humoral immunity. J Exp Med. 200:1145–1156.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Glodek AM, Honczarenko M, Le Y, Campbell
JJ and Silberstein LE: Sustained activation of cell adhesion is a
differentially regulated process in B lymphopoiesis. J Exp Med.
197:461–473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Harnett MH: B cells spread and gather.
Science. 312:709–710. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Laudanna C, Kim JY, Constantin G and
Butcher EC: Rapid leukocyte activation by chemokines. Immunol Rev.
186:37–46. 2002. View Article : Google Scholar
|
|
13.
|
Ostermann G, Weber K, Zernecke A, Schroder
A and Weber C: JAM-1 is a ligand of the β2 integrin
LFA-1 involved in transendothelial migration of leukocytes. Nat
Immunol. 3:151–158. 2002.
|
|
14.
|
Shulman Z, Shinder V, Klein E, et al:
Lymphocyte crawling and transendothelial migration require
chemokine triggering of high-affinity LFA-1 integrin. Immunity.
30:384–396. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Lo CG, Lu TT and Cyster JG:
Integrin-dependence of lymphocyte entry into the white pulp. J Exp
Med. 197:353–361. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Cinamon G, Matloubain M, Lesneski M, et
al: Sphingosine 1-phosphate receptor promotes B cell localization
in the splenic marginal zone. Nat Immunol. 5:713–720. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ryan DH, Nuccie BL, Abboud CN and Winslow
JM: Vascular cell adhesion molecule-1 and the integrin VLA-4
mediate adhesion of human B cell precursors to cultured bone marrow
adherent cells. J Clin Invest. 88:995–1004. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Gorfu G, Rivera-Nieves J and Ley K: Role
of β7 integrins in intestinal lymphocyte homing and retention. Curr
Mol Med. 9:836–850. 2009.
|
|
19.
|
Hamann A, Andrew DP, Jablonski-Westrich D,
Holzmann B and Butcher EC: Role of α4-integrins in
lymphocytes homing to mucosal tissues in vivo. J Immunol.
152:3282–3293. 1994.
|
|
20.
|
Rosen H and Goetzl EJ: Sphingosine
1-phosphate and its receptors: an autocrine and paracrine network.
Nat Rev Immunol. 5:560–570. 2005. View
Article : Google Scholar
|
|
21.
|
Donovan EE, Pelanda R and Torres R: S1P3
confers differential S1P-induced migration by autoreactive and
non-autoreactive immature B cells and is required for normal B-cell
development. Eur J Immunol. 40:688–698. 2010. View Article : Google Scholar
|
|
22.
|
Foucar K: B cell chronic lymphocytic and
prolymphocytic leukemia. Williams and Wilkins; Baltimore: 1992
|
|
23.
|
Till KJ, Lin K, Zuzel M and Cawley JC: The
chemokine receptor CCR7 and α4 integrin are important for migration
of chronic lymphocytic leukemia cells into lymph nodes. Blood.
99:2977–2984. 2002.
|
|
24.
|
Shanafelt TD, Bone ND, Geyer SM, et al:
Prognostic importance of CD49d expression in chronic lymphocytic
leukemia. Blood. 108:786a2006.
|
|
25.
|
Gattei V, Bulian P, Del Principe MI, et
al: High CD49d protein expression predict short overall survival
and early progression in chronic lymphocytic leukemia patients.
Leuk Lymph. 48(Suppl 1): S602007.
|
|
26.
|
Burger JA, Burger M and Kipps TJ: Chronic
lymphocytic leukemia B cells express functional CXCR4 chemokine
receptors that mediate spontaneous migration beneath bone marrow
stromal cells. Blood. 94:3658–3667. 1999.
|
|
27.
|
Burger JA and Peled A: CXCR4 antagonists:
targeting the micro-environment in leukemia and other cancers.
Leukemia. 23:43–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Calissano C, Damle RJ, Hayes G, et al:
In vivo intra- and interclonal kinetic heterogeneity in
B-cell chronic lymphocytic leukaemia. Blood. 114:4832–4842. 2009.
View Article : Google Scholar
|
|
29.
|
Lopez-Giral S, Quintana NE, Caberixo M, et
al: Chemokine receptors that mediated B cell homing to secondary
lymphoid tissues are highly expressed in B cell chronic lymphocytic
leukemia and non-Hodgkin lymphomas with widespread nodular
dissemination. J Leukoc Biol. 76:462–471. 2004. View Article : Google Scholar
|
|
30.
|
Hoellenriegel J, Meadows SA, Sivina M, et
al: The phosphoinositide 3′ kinase delta inhibitor, CAL-101,
inhibits B-cell receptor signaling and chemokine networks in
chronic lymphocytic leukemia. Blood. 118:3603–3612. 2011.
|
|
31.
|
Kinashi T: Intracellular signaling
controlling integrin activation in lymphocytes. Nat Rev Immunol.
5:546–559. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Sasaki AT, Chun C, Takeda K and Firtel RA:
Localised Ras signaling at the leading edge regulates PI3K, cell
polarity, and directional cell movement. J Cell Biol. 167:505–518.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Friedberg JW, Sharman J, Sweetenham J, et
al: Inhibition of Syk with fostamatinib disodium has significant
clinical activity in non-Hodgkin lymphoma and chronic lymphocytic
leukemia. Blood. 115:2578–2585. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Mocsai A, Ruland J and Tybulewicz VLJ: The
SYK tyrosine kinase: a crucial player in diverse biological
functions. Nat Rev Immunol. 10:387–402. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
de Rooij MFM, Kuil A, Geest CR, et al: The
clinically active BTK inhibitor PCI-32765 targets B-cell receptor-
and chemokine-controlled adhesion and migration in chronic
lymphocytic leukemia. Blood. 119:2590–2594. 2012.PubMed/NCBI
|
|
36.
|
Burger M, Hartmann T, Fujii N, Kipps TJ
and Burger JA: CXCR4 chemokine receptor antagonists inhibit
activation, migration, and survival of chronic lymphocytic leukemia
cells in response to stromal cell-derived factor 1 (SDF-1/CXCL12).
Blood. 102:15852003.
|
|
37.
|
Zuccchetto A, Viasitti T, Benedetti D, et
al: The CD49d/CD29 complex is physically and functionally
associated with CD38 in B-cell chronic lymphocytic leukemia cells.
Leukemia. 26:1293–1300. 2012.
|
|
38.
|
Mackay CR: Moving targets: cell migration
inhibitors as new anti-inflammatory therapies. Nat Immunol.
9:988–998. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Weisberg E, Azab AK, Manley PW, et al:
Inhibition of CXCR4 in CML cells disrupts their interaction with
the bone marrow microenvironment and sensitizes them to nilotinib.
Leukemia. 26:985–990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Cawley JC: Hairy cell leukemia and the
microenvironment. Leuk Lymph. 52(Suppl 2): S93–S95. 2011.
View Article : Google Scholar
|
|
41.
|
Cawley JC, Zuzel M and Caligaris-Cappio F:
Biology of the hairy cell. Hairy Cell Leukemia. Tallman M and
Polliack A: Harwood Academic Publishers; Newark: pp. 9–18. 1999
|
|
42.
|
Aziz KA, Till KJ, Zuzel M and Cawley JC:
Involvement of CD44-hyaluronan interaction in malignant cell homing
and fibronectin synthesis in hairy cell leukemia. Blood.
96:3161–3167. 2000.PubMed/NCBI
|
|
43.
|
Basso K, Liso A, Tiacci E, et al: Gene
expression profiling of hairy cell leukemia reveals a phenotype
related to memory B cells with altered expression of chemokine and
adhesion receptors. J Exp Med. 199:59–68. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Potsch C, Vohringer D and Pircher H:
Distinct migration patterns of naive and effector CD8 T cells in
the spleen: correlation with CCR7 receptor expression and chemokine
reactivity. Eur J Immunol. 29:3652–3570. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Burger JA, Sivina M and Ravandi F: The
microenvironment in hairy cell leukemia: pathways and potential
therapeutic targets. Leuk Lymph. 52(Suppl 2): S94–S98. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Burthem J, Vincent A and Cawley JC:
Integrin receptors and hairy cell leukaemia. Leuk Lymph.
21:211–215. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Venkataraman G, Aguhar C, Kreitman RJ,
Yuan CM and Stetler-Stevenson M: Characteristic CD103 and CD123
expression pattern defines hairy cell leukemia: usefulness of CD123
and CD103 in the diagnosis of mature B-cell lymphoproliferative
disorders. Am J Clin Pathol. 136:625–630. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Pals ST, de Gorter DJ and Spaargaren M:
Lymphoma dissemination: the other face of lymphocyte homing. Blood.
110:3102–3111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Terol M-J, Lopez-Giuillermo A, Bosch F, et
al: Expression of beta-integrin adhesion molecules in non-Hodgkin’s
lymphoma: correlation with clinical and evolutive features. J Clin
Oncol. 17:1869–1875. 1999.PubMed/NCBI
|
|
50.
|
Janke K, Coupland S, Na I-K, et al:
Expression of the chemokine receptors CXCR4, CXCR5 and CCR7 in
primary central nervous system lymphoma. Blood. 106:384–385. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Cattoretti G, Mandelbaum J, Lee N, et al:
Targeted disruption of the S1P2 sphingosine
1-phosphate receptor gene leads to diffuse large B-cell lymphoma
formation. Cancer Res. 69:8686–8692. 2009.PubMed/NCBI
|
|
52.
|
Lepley D, Paik J-H, Hla T and Ferrer F:
The G protein-coupled receptor S1P2 regulates Rho/Rho
kinase pathway to inhibit tumor cell migration. Cancer Res.
65:3788–3795. 2005.PubMed/NCBI
|
|
53.
|
Klapper W: Pathobiology and diagnosis of
follicular lymphoma. Semin Diagn Pathol. 28:146–160. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Hopken UE, Foss HD, Meyer D, et al:
Up-regulation of the chemokine receptor CCR7 in classical but not
in lymphocyte-predominant Hodgkin disease correlates with distinct
dissemination of neoplastic cells in lymphoid organs. Blood.
99:1109–1116. 2002. View Article : Google Scholar
|
|
55.
|
Coupland SE: The challenge of the
microenvironmnent in B-cell lymphomas. Histopathology. 58:69–80.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Wartenberg M, Vasil P, Meyer C, et al:
Somatic hypermutation analysis in follicular lymphoma provides
evidence suggesting bidirectional cell migration between lymph node
and bone marrow during disease progression and relapse.
Haematologica. 98:1433–1441. 2013. View Article : Google Scholar
|
|
57.
|
Gisbert J and Calvet X: Review article:
common misconceptions in the management of Helicobacter
pylori-associated gastric MALT-lymphoma. Aliment Pharmacol
Ther. 34:1047–1062. 2011.PubMed/NCBI
|
|
58.
|
Piris MA, Arribas A and Mollejo M:
Marginal zone lymphoma. Semin Diagn Pathol. 28:135–145. 2011.
View Article : Google Scholar
|
|
59.
|
Drillenburg P, van der Voort R, Koopman G,
et al: Preferential expression of the mucosal homing receptor
integrin α4β7 in gastrointestinal non-Hodgkin’s lymphomas. Am J
Pathol. 150:919–927. 1997.
|
|
60.
|
Liu YX, Yoshino T, Ohara N, et al: Loss of
expression of α4β7 integrin and L-selectin is associated with
high-grade progression of low-grade MALT lymphoma. Mod Pathol.
14:798–805. 2001.
|
|
61.
|
Trentin L, Cabrelle A, Facco M, et al:
Homeostatic chemokines drive migration of malignant B cells in
patients with non-Hodgkin lymphomas. Blood. 104:502–508. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Sander B: Mantle cell lymphoma: recent
insights into pathogenesis, clinical variability, and new
diagnostic markers. Semin Diagn Pathol. 28:245–255. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
63.
|
Kluin-Nelemans JC, Hoster E and Walewski
J: R-CHOP Versus R-FC followed by maintenance with rituximab versus
interferon-α: outcome of the first randomized Trial for elderly
patients with mantle cell lymphoma. Blood. 118:4932011.
|
|
64.
|
Sjöberg J, Halthur C, Kristinsson SY, et
al: Progress in Hodgkin lymphoma: a population-based study on
patients diagnosed in Sweden from 1973–2009. Blood. 119:990–996.
2012.PubMed/NCBI
|
|
65.
|
Kurtova AV, Tamayo AT, Ford RJ and Burger
JA: Mantle cell lymphoma cells express high levels of CXCR4, CXCR5,
and VLA-4 (CD49d): importance for interactions with the stromal
microenvironment and specific targeting. Blood. 113:4604–4613.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
66.
|
Mraz M, Zent CS, Church AK, et al: Bone
marrow stromal cells protect lymphoma B-cells from
rituximab-induced apoptosis and targeting integrin α-4-β-1 (VLA-4)
with natalizumab can overcome this resistance. Br J Haematol.
155:53–64. 2011.PubMed/NCBI
|
|
67.
|
Geissmann F, Ruskoné-Fourmestraux A,
Hermine O, et al: Homing receptor α4β7 integrin expression predicts
digestive tract involvement in mantle cell lymphoma. Am J Pathol.
153:1701–1705. 1998.
|
|
68.
|
Del Giudice I, Messina M, Chiaretti S, et
al: Behind the scenes of non-nodal MCL: downmodulation of genes
involved in actin cytoskeleton organization, cell projection, cell
adhesion, tumour invasion, TP53 pathway and mutate status of
immunoglobulin heavy chain genes. Br J Haematol. 156:601–611.
2012.
|
|
69.
|
Advani RH, Buggy JJ, Sharman JP, et al:
Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has
significant activity in patients with relapsed/refractory B-cell
malignancies. J Clin Oncol. 31:88–94. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70.
|
Chang BY, Francesco M, De Rooij J, et al:
Egress of CD19+CD5+ cells into peripheral
blood following treatment with the BTK inhibitor ibrutinib in
mantle cell lymphoma patients. Blood. 122:2414–2422. 2013.
|
|
71.
|
Ferry JA: Burkitt’s lymphoma:
clinicopathologic features and differential diagnosis. Oncologist.
11:375–383. 2006.
|
|
72.
|
Gunn MD, Ngo VN, Ansel KM, Ekland EH,
Cyster JG and Williams LT: A B-cell-homing chemokine made in
lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature.
391:799–803. 1998.PubMed/NCBI
|
|
73.
|
Birkenbach M, Josefsen K, Yalamanchili R,
Lenoir G and Kieff E: Epstein-Barr virus-induced genes: first
lymphocyte-specific G protein-coupled peptide receptors. J Virol.
67:2209–2220. 1993.PubMed/NCBI
|
|
74.
|
Lalor S and Segal BM: Lymphoid chemokines
in the CNS. J Neuroimmunol. 224:56–61. 2010. View Article : Google Scholar
|
|
75.
|
Pranzatelli MR, Tata ED, McGee NR, et al:
Key role of CXCL13/CXCR5 axis for cerebrospinal fluid B cell
recruitment in pediatric OMS. J Neuroimmunol. 243:81–88. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
76.
|
Rincon J, Prieto J and Patarroyo M:
Expression of integrins and other adhesion molecules in
Epstein-Barr virus-transformed B lymphoblastoid cells and Burkitt’s
lymphoma cells. Int J Cancer. 51:452–458. 1992.PubMed/NCBI
|
|
77.
|
Inghirami G, Grignani F, Sternas L,
Lombardi L, Knowles DM and Dalla-Favera R: Down-regulation of LFA-1
adhesion receptors by c-myc oncogenie in human B
lymphoblastoid cells. Science. 250:682–686. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
78.
|
Kadin M and Rathore B: Hodgkin’s lymphoma
therapy: past, present, and future. Exp Opin Pharmacother.
11:2891–2906. 2010.
|
|
79.
|
Steidl C, Connors JM and Gascoyne RD:
Molecular pathogenesis of Hodgkin’s lymphoma: increasing evidence
of the importance of the microenvironment. J Clin Oncol.
29:1812–1826. 2011.
|
|
80.
|
Maggio E, van den Berg A, Diepstra A,
Kluiver J, Visser L and Poppema S: Chemokines, cytokines and their
receptors in Hodgkin’s lymphoma cell lines and tissues. Ann Oncol.
13:52–56. 2002.
|
|
81.
|
Ellis PA, Hart DN, Colls BM, Nimmo JC,
MacDonald JE and Angus HB: Hodgkin’s cells express a novel pattern
of adhesion molecules. Clin Exp Immunol. 90:117–123. 1992.
|