Introduction
Acute lymphoblastic leukemia (ALL), a malignant
disorder of lymphoid progenitor cells, arises mainly in children
and adolescents, and is the most common malignancy in these
generations (1). Despite recent
advances in the treatment of ALL, 20% of the patients cannot be
cured even for those types of ALL with the best prognosis (2). Older age, higher leukocyte count,
hypodiloidy, t(9;22), t(4;11), and extramedullary involvement (EMI)
are the factors that relate to poor prognosis (3,4). EMI
not only associates with poor prognosis but also induces painful
symptoms. At initial diagnosis, approximately 30–50, 8, 2.5–5 and
0.6% of patients show infiltration of leukemic cells into the
liver, mediastinum, central nervous system (CNS), and testis,
respectively (4–6). Moreover, solitary CNS or testicular
relapse is experienced in 20.9 or 5.3% of relapsed patients,
respectively (7). The T-cell
immunophenotype, hyperleucocytosis, the Philadelphia reciprocal
translocation between chromosome 9 and 22, and the presence of
leukemic cells in the cerebrospinal fluid are factors that predict
extramedullary relapse (3,5,7).
ALL is a clonal disorder that is characterized by
heterogeneous subpopulations of cells with different malignant
behavior. The dissemination of leukemic cells (also referred to as
metastasis in the context of solid tumors) is not a random event.
Instead, this is a process destined by characteristic molecular
events, with certain tumor cells having a specific affinity for the
microenvironment (8,9). In this context, significant effort
has been devoted to finding molecules that direct leukemic cells to
extramedullary organs. Clinically, overexpression of CXCR4,
interleukin-15, CCR9, CD56, CD103, matrix metalloproteinase-2
(MMP-2), and MMP-9, or underexpression of intracellular adhesion
molecule 1 (ICAM1) have been correlated with EMI (8,10–15).
Experimental evidence suggested that Notch1 controls CCR7
expression and guides leukemic cells to CNS in vivo
(16). Mass spectrometry revealed
that leukemic cell lines with a higher invasiveness expressed RAC2
(17). Recently, Castro et
al demonstrated that 5T4 oncofetal antigen enhances
invasiveness of leukemic cell lines in vitro and in
vivo (18).
Although an increasing number of studies are
reporting on searches for molecules that associate with EMI of ALL,
no study has used global gene expression analysis to compare
leukemic clones with high and low invasiveness derived from the
same cell line. In this study, we used the B-ALL cell line Tanoue
to obtain a cell line with high invasiveness by in vivo
selection and compared the gene signature with the parental
cells.
Materials and methods
Cell line
The human B lymphoblastic leukemia cell line Tanoue
was obtained from the Riken BioResource Center (Tsukuba, Japan).
Tanoue was cultured in RPMI-1640 (Lonza, Basel, Switzerland)
supplemented with 10% heat-inactivated fetal bovine serum (FBS;
Hyclone, Logan, UT, USA) at 37°C in a humidified atmosphere of 5%
CO.
Retroviral transfection of luciferase
gene
Expression of the firefly luciferase gene was
carried out as described, previously (19). Briefly, the retrovirus vector
pBABE-Luc-Hygro was transfected into the packaging cell line
Platinum-A (Cell Biolabs Inc., San Diego, CA, USA) using
Lipofectamine 2000 (Life Technologies Inc., Gaithersburg, MD, USA)
according to the manufacturer’s instructions. The supernatant was
collected, and Tanoue was infected with the virus in the presence
of 8 μg/ml hexadimethrine bromide (Sigma-Aldrich, St. Louis, MO,
USA). Luciferase-expressing Tanoue (Luc-Tanoue) cells were selected
by exposure to 250 μg/ml of hygromycin B (Wako Pure Chemical
Industries, Tokyo, Japan) for 1 week.
Non-invasive in vivo imaging and
selection of highly infiltrative leukemic cell line
The study protocol was approved by the Animal Ethics
Committee of Sapporo Medical University (no. 09-003). The tail vein
of NOD/SCID mice (female, 5-week old) were injected with
1×106 Luc-Tanoue in 0.1 ml RPMI. Bioluminescence signals
were monitored weekly using the IVIS Lumina II (Caliper Life
Sciences, Hopkinton, MA, USA) after isoflurane anesthesia was
administered to the animals. Before imaging, each mouse was
injected with 0.15 ml luciferin (20 mg/ml potassium salt; Promega,
Madison, WI, USA), intraperitoneally. To establish a highly
infiltrative leukemic cell line, the mice were monitored until
leukemic cells infiltrated the CNS. Thereafter, leukemic cells in
the brain was harvested, their culture in vitro generated
the line the Luc-Tanoue-F1. Repetition of this selection cycle 4
times generated Luc-Tanoue-F4 line.
In vitro cell invasion and migration
assay
Cells were seeded in the upper chamber of BD BioCoat
Matrigel Invasion Chamber (8-μm pore size; BD Bioscience, San Jose,
CA, USA) at a density of 1×106 cells per well in 0.5 ml
RPMI-1640 with 0.1% FBS and 0.5 ml of RPMI-1640 containing 1% FBS
was added to the lower chamber. Plates were incubated for 48 h at
37°C in a humidified atmosphere (5% CO) and cells that migrated
into the lower chamber were enumerated with an XE-5000
hemocytometer (Sysmex, Kobe, Japan). The percentage of invasive
cells was expressed as a percentage of total cells.
Cell migration assay was performed as described
above, except that the Transwell chamber (8-μm pore size; Corning,
Corning, NY, USA) was used as the assay plate, and the plates were
incubated for 24 h.
Cell proliferation assay
Cells were seeded in 24-well plates at a density of
1×105 cells per well in triplicate. Plates were
incubated at 37°C in a humidified atmosphere (5% CO) and cell
numbers were determined using an XE-5000 hemocytometer (Sysmex) at
the time-points indicated in each experiment.
In vitro cell adhesion assay
Flat-bottomed 96-well plates (Corning) were coated
with Matrigel (BD Bioscience) diluted with RPMI-1640 to 100 ng/μl,
seeded with 2×105 cells, incubated for 2 h at 37°C,
washed twice before measuring the number of attached cells using
the Cell Titer-Glo™ Luminescent Cell Viability assay (Promega),
according to the manufacturer’s instructions. The level of
ATP-derived luminescent signal was measured using a Veritas™
Microplate Luminometer (Promega). Adhered cells are described as a
percentage of total cells seeded.
Zymography
MMP-2 and MMP-9 activity of cultured media was
assessed by gelatin zymogram (Life Technologies Inc.) according to
the manufacturer’s instructions. Briefly, cells were cultured in
RPMI-1640 without FBS for 24 h, after which 10 μl of the media was
diluted 1:1 with Tris-glycine SDS sample buffer and separated by
electrophoresis on a 10%-Novex Zymogram (gelatin) gel. The gels
were incubated in Zymogram Renaturing buffer for 30 min at room
temperature, equilibrated with Zymogram Developing buffer and
incubated overnight at 37°C. The gels were stained with Coomassie
brilliant blue and MMP activity was detected as clear bands against
a dark background.
Quantitative reverse
transcriptase-polymerase chain reaction
Expression of CD7 mRNA was determined by
quantitative reverse transcriptase-polymerase chain reaction
(RT-PCR). Total RNA was isolated using the RNeasy Mini Kit
(Qiagen), according to the protocol provided by the manufacturer.
The cDNA was synthesized using TaqMan Reverse Transcription
reagents (Applied Biosystems, Foster City, CA, USA). The
gene-specific primers and fluorescent hybridization probes used in
the quantitative PCR were as follows: CD7 forward primer,
5′-TCGGACACTGGCACCTACAC-3′; reverse primer,
5′-TGCCATCCTTGGGACTGTTC-3′; and probe, 5′-TGCCAGGCCATCACGGAGGTCAAT
(TAMRA)-3′. Expression level of CD7 was compared to the
level of GAPDH, which was determined using the GAPDH control
reagents (Applied Biosystems). For ITGA3 and ITGB2,
the primers and probes were purchased from Applied Biosystems.
Flow cytometric analysis and cell
sorting
Surface immunophenotyping and cell sorting was
performed using the EPICS XL-MCL flow cytometer (Beckman Coulter,
Fullerton, CA, USA) and FACS Aria II cell sorter
(Becton-Dickinson). Fluorescein isothiocyanate (FITC)-conjugated
anti-CD7 antibody (6603824) was purchased from Beckman Coulter.
Microarray analysis
Global gene expression profiling was carried out by
Hokkaido System Science (Sapporo, Japan) using Agilent RNA Spike-In
Kit for One color and Agilent SurePrint G3 Human GE 8x60K
Microarrays following the Agilent one-color microarray-based gene
expression analysis protocol (Agilent Technologies, Santa Clara,
CA, USA). The slides were scanned with an Agilent Technologies
Microarray Scanner and the image data was processed using Agilent
Feature Extraction software, version 10.7.3.1. The gene expression
levels were compared after global normalization.
Construction of CD7 expressing vectors
and the transfection procedure
Full length CD7 was amplified using
5′-GCTAGCAACATGGCCGGGCCTCCGAGGCTCC-3′ and
5′-ACCGGTTGGTACTGGTTGGGGGAGGACAGC-3′ as the forward and reverse
primer, respectively. Extracellular domain of CD7 was
amplified using 5′-GCTAGCAACATGGCCGGGCCTCCGAGGCTCC-3′ and
5′-ACCGGTCTCGCCAGCACACACGCCACCCC-3′ as the forward and reverse
primer, respectively. Intracellular domain of CD7 was
amplified using 5′-GCTAGCACCATGGCGAGGACACAGATAAAGAAAC-3′ and
5′-ACCGGTTGGTACTGGTTGGGGGAGGACAGC-3′ as the forward and reverse
primer, respectively. The PCR products were cloned into PCR4-TOPO
vector (Life Technologies), sequenced, and the cDNA then excised by
restriction digestion prior to cloning into the NheI and
AgeI sites of pTurbo-GFP-N vector (Evrogen, Moscow, Russia),
resulting in pTurboCD7-GFP, pTurboCD7-EC-GFP and pTurboCD7-IC-GFP.
The plasmids were transfected into cells using Lipofectamine 2000
(Invitrogen) according to the manufacturer’s protocol. GFP-positive
cells were selected using FACS Aria II cell sorter
(Becton-Dickinson).
Silencing of CD7 and ITGB2 by short
interfering RNA (siRNA
Stealth siRNAs against CD7 (HSS101524,
HSS190108) and ITGB2 (HSS105563, HSS105564) were purchased
from Life Technologies. Non-silencing siRNA control was purchased
from Applied Biosystems. All siRNAs were transfected into cells by
electroporation, using Amaxa cell line Nucleofector Kit T (Lonza,
Gaithersburg, MD, USA) Nucleofector II (Lonza) and the program
C-005, according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis of the data was conducted using
Microsoft Excel®. Statistical significance was evaluated
with the Student’s t-test.
Results
Selection of a highly invasive leukemic
cell line
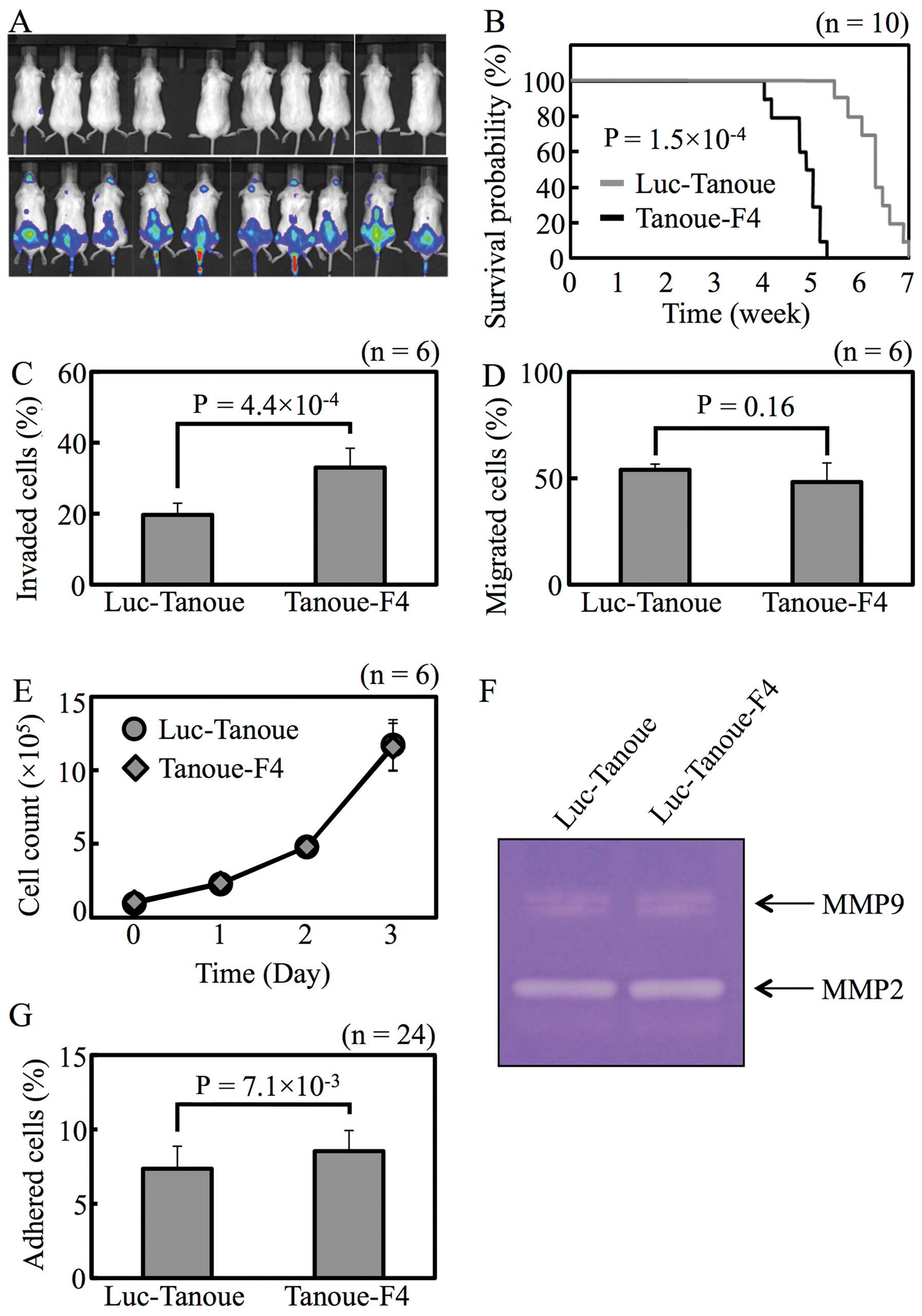
The highly invasive cell line Luc-Tanoue-F4 was
obtained by 4 rounds of in vivo selection. To confirm that
Luc-Tanoue-F4 is more invasive than Luc-Tanoue, the cells were
injected into the tail vein of non-obese diabetic/severe combined
immunodeficient (NOD/SCID) mice, and EMI was monitored
non-invasively in vivo. After 2 weeks, none of NOD/SCID
mouse injected with Luc-Tanoue showed apparent CNS involvement;
however, 9 of 10 mice injected with Luc-Tanoue-F4 revealed
involvement of the brain or olfactory bulb (Fig. 1A). Mean survival was shorter in
mice injected with Luc-Tanoue-F4 than Luc-Tanoue (Fig. 1B; 34.0±3.0 vs. 44.0±3.4 days,
P=1.5×10−4).
Luc-Tanoue-F4 was more invasive than Luc-Tanoue
in vitro (Fig. 1C). Cell
migration, proliferation, adhesion, and protease activity, all of
which are the factors that affect cell invasiveness, were
characterized in vitro. There were no differences between
the 2 lines in terms of cell migration (Fig. 1D), proliferation (Fig. 1E), or protease activity (Fig. 1F). Cell adhesion was the only
factor that differed between Luc-Tanoue and Luc-Tanoue-F4 (Fig. 1G).
CD7 expression is higher in Luc-Tanoue-F4
than Luc-Tanoue
Next, Luc-Tanoue and Luc-Tanoue-F4 were subjected to
microarray analysis to compare gene-expression signatures. When the
cut-off value of gene expression level was set at 2-fold, 286 and
236 genes were expressed at higher and lower levels in
Luc-Tanoue-F4 than Luc-Tanoue, respectively (GEO accession nos.
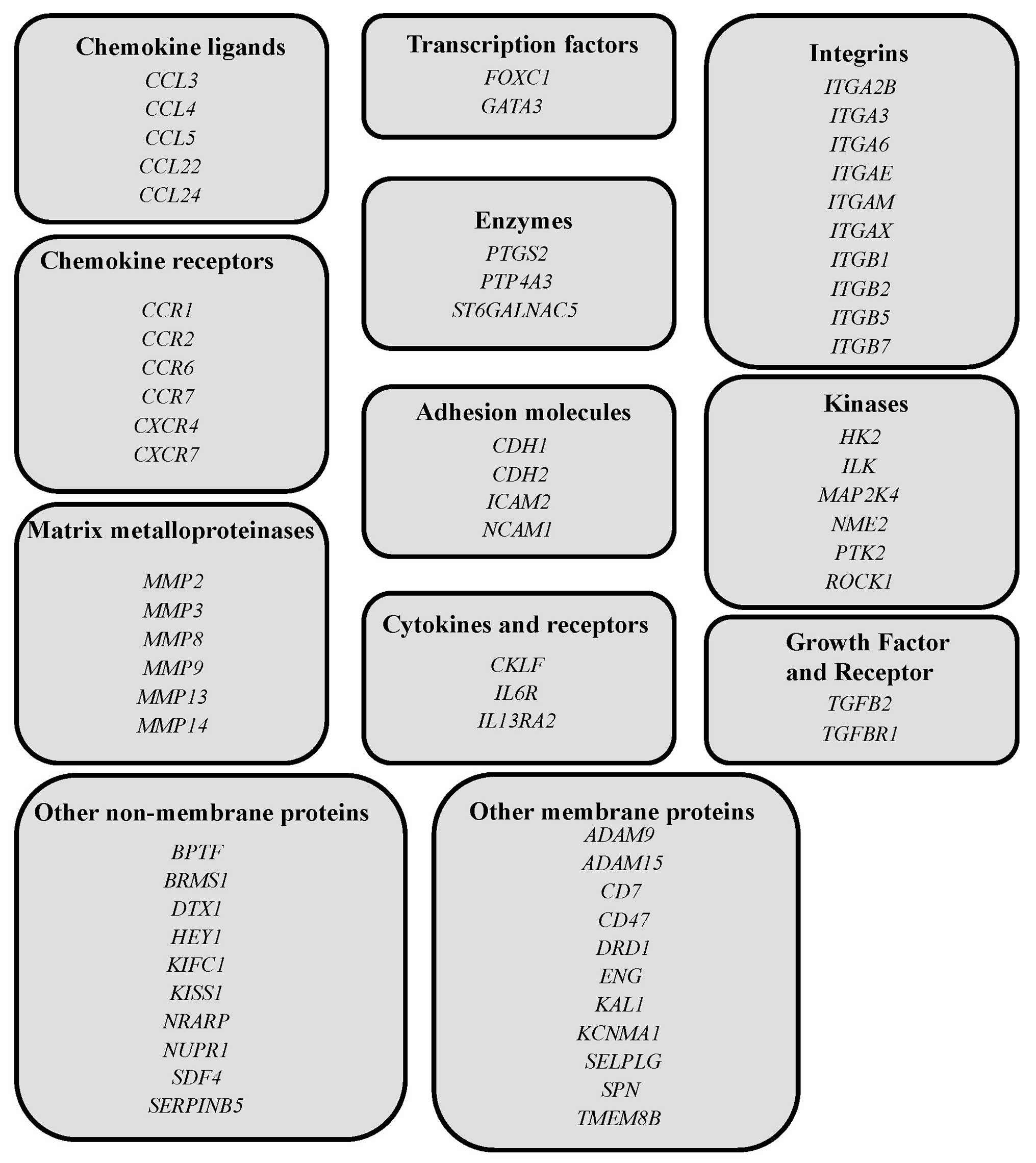
GSE53651). Analysis of the expression of genes that relate to EMI
or brain metastasis (listed in Fig.
2) indicated that CD7 showed the largest difference
(4.59-fold) in transcript levels between the 2 cell lines. Levels
of ITGB2 (2.17-fold) and ITGA3 (2.13-fold)
transcripts were also higher in Luc-Tanoue-F4 than Luc-Tanoue.
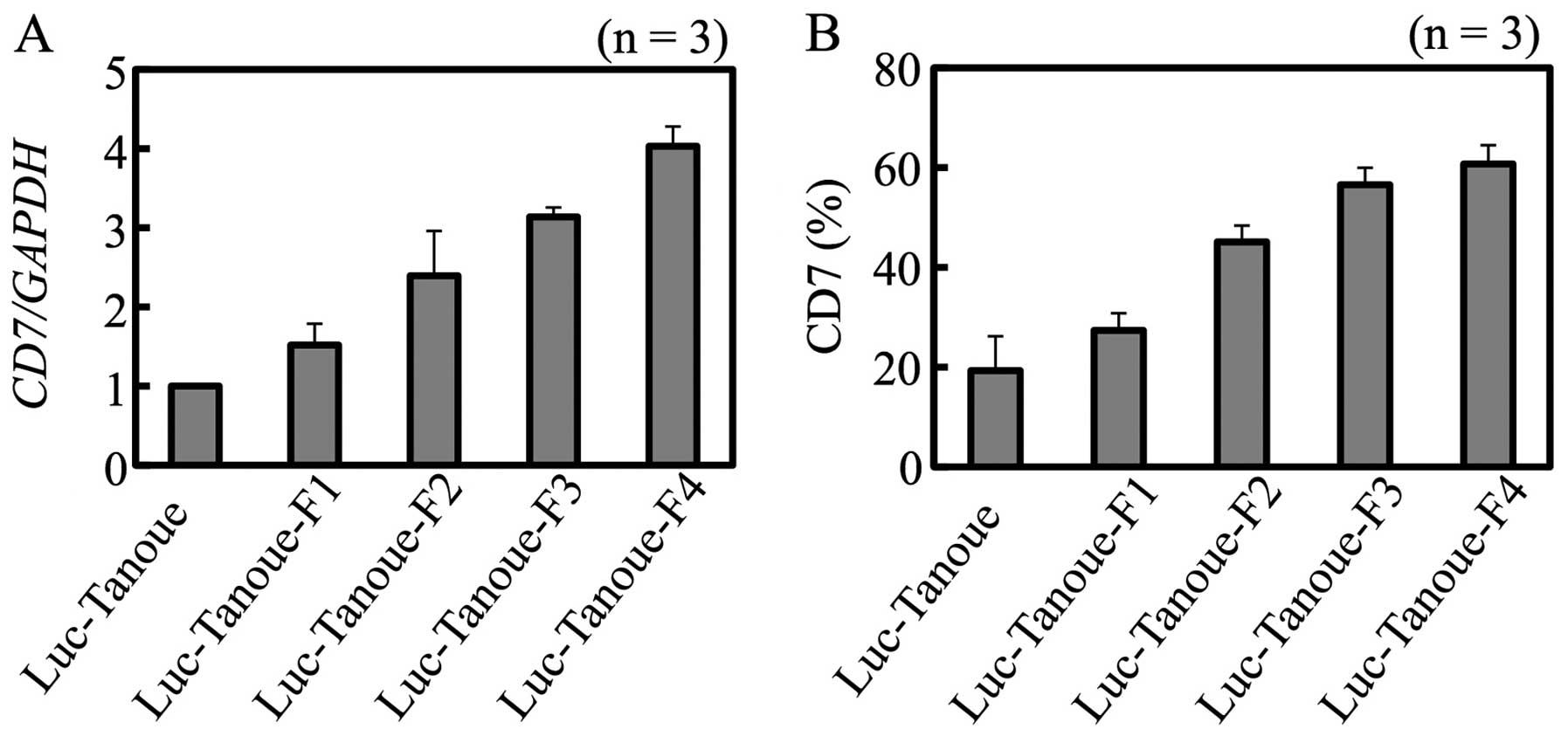
Levels of CD7 transcripts in Luc-Tanoue and
Luc-Tanoue-F1-F4 increased as the cells underwent in vivo
selections (Fig. 3A). Analysis of
CD7 protein abundance by fluorescence-activated cell sorting (FACS)
was consistent with the result of gene expression analysis
(Fig. 3B).
CD7 promotes cell invasiveness and
adhesion
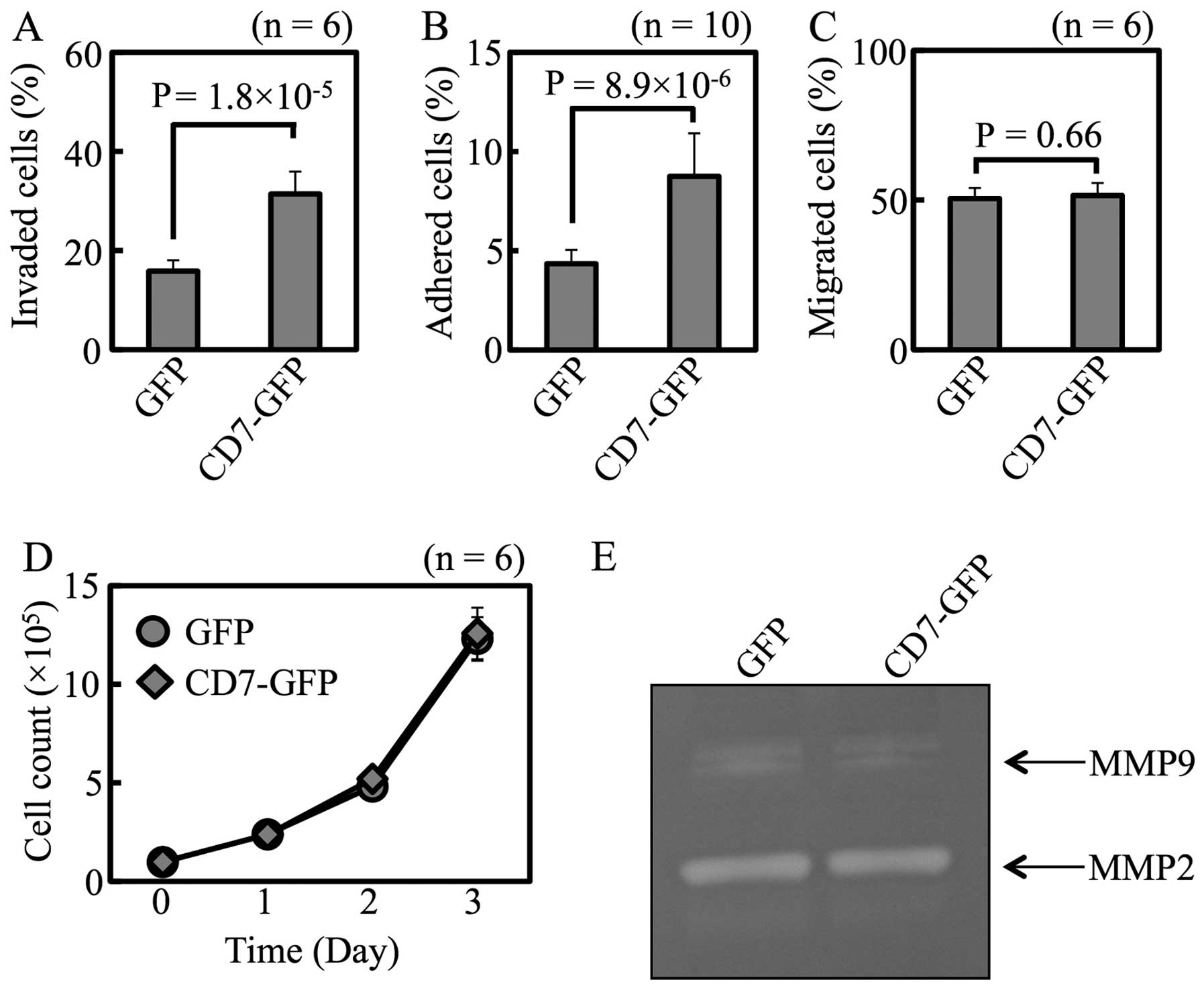
The CD7 protein was overexpressed as a fusion to
green fluorescent protein (GFP) in Luc-Tanoue, a line that
expresses CD7 at a low level. The CD7 mRNA expression level
was 51.0-fold higher in CD7-GFP transfectant than in the
mock-transfected cells.
When measured in vitro, both cell
invasiveness (Fig. 4A) and
adhesion (Fig. 4B) were higher in
CD7-GFP transfectants than mock-transfectants. There were no
differences in cell migration, proliferation, or protease activity
between these 2 transfectants (Fig.
4C–E).
These transfectants failed to express the GFP-fusion
protein in vivo. Thus, Luc-Tanoue was separated into CD7-low
expressing cells (Tanoue-CD7) and CD7-high expressing cells
(Tanoue-CD7) by FACS. Tanoue-CD7 showed higher levels of
invasiveness and cell adhesion than Tanoue-CD7 in vitro
(data not shown). Whereas all mice injected with Tanoue-CD7 showed
olfactory bulb involvement, mice injected with Tanoue-CD7 showed no
apparent EMI (data not shown). These results show that CD7 enhances
the invasiveness of Tanoue cells in vitro and in
vivo.
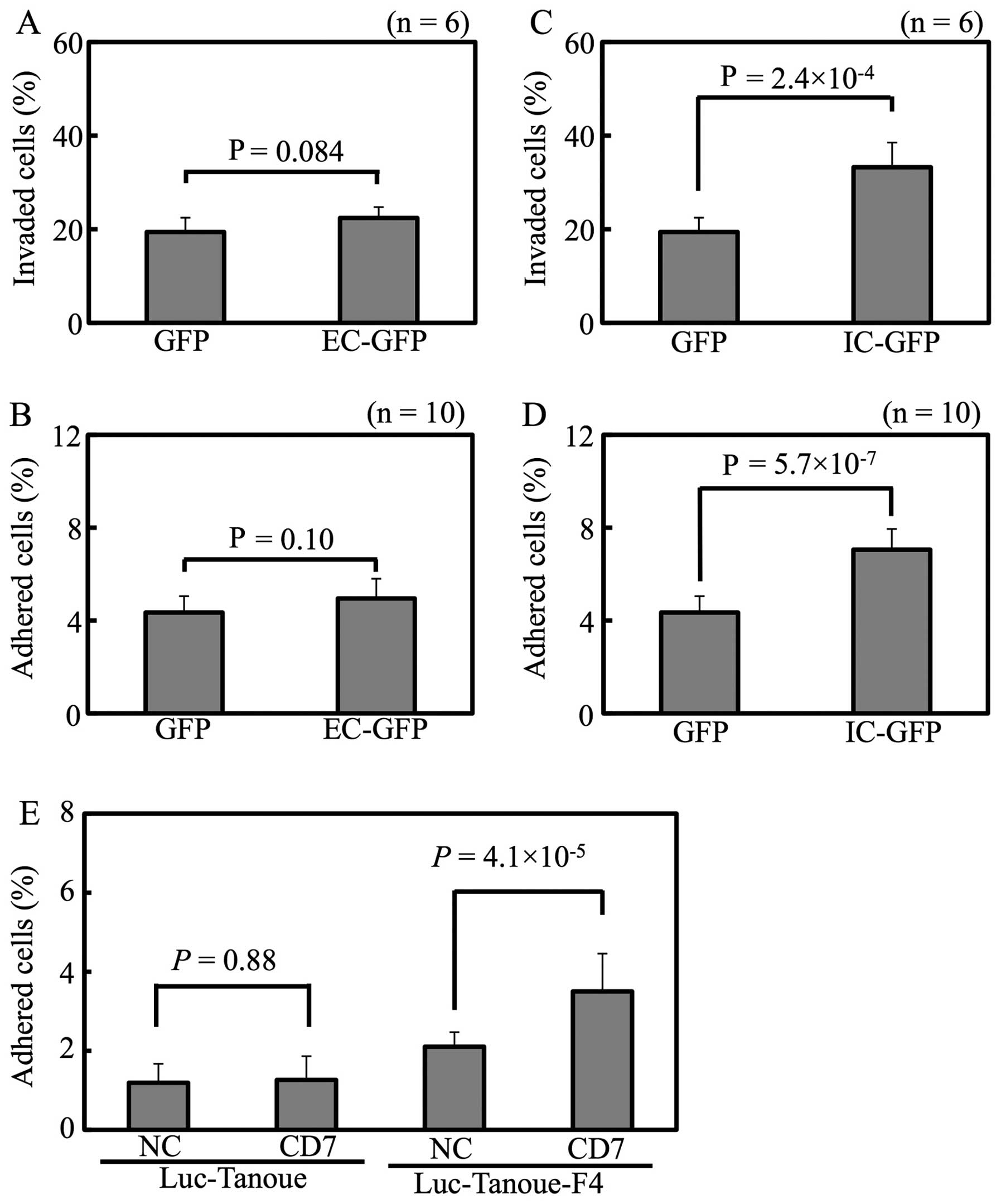
CD7 intracellular domain promotes cell
invasiveness and adhesion
To gain insight into the mechanism by which CD7
enhances the adhesion of leukemic cells, the intracellular and
extracellular domains of CD7 were overexpressed separately
in Luc-Tanoue. Whereas expression of the extracellular domain
neither enhanced cell invasiveness (Fig. 5A) nor adhesiveness (Fig. 5B), the intracellular domain
enhanced both cell invasiveness (Fig.
5C) and adhesiveness (Fig.
5D). Given the lack of availability of an anti-CD7 blocking
antibody, Luc-Tanoue and Luc-Tanoue-F4 were stimulated with
CD7-agonistic antibody; whereas the CD7-agonistic antibody enhanced
adhesion of the Luc-Tanoue-F4 (which expresses a high level of
CD7), it had little effect on the Luc-Tanoue (which expresses a low
level of CD7) (Fig. 5E). These
results show that signal transduction evoked by the intracellular
domain of CD7 enhanced the cell invasiveness and adhesive capacity
of Tanoue cells.
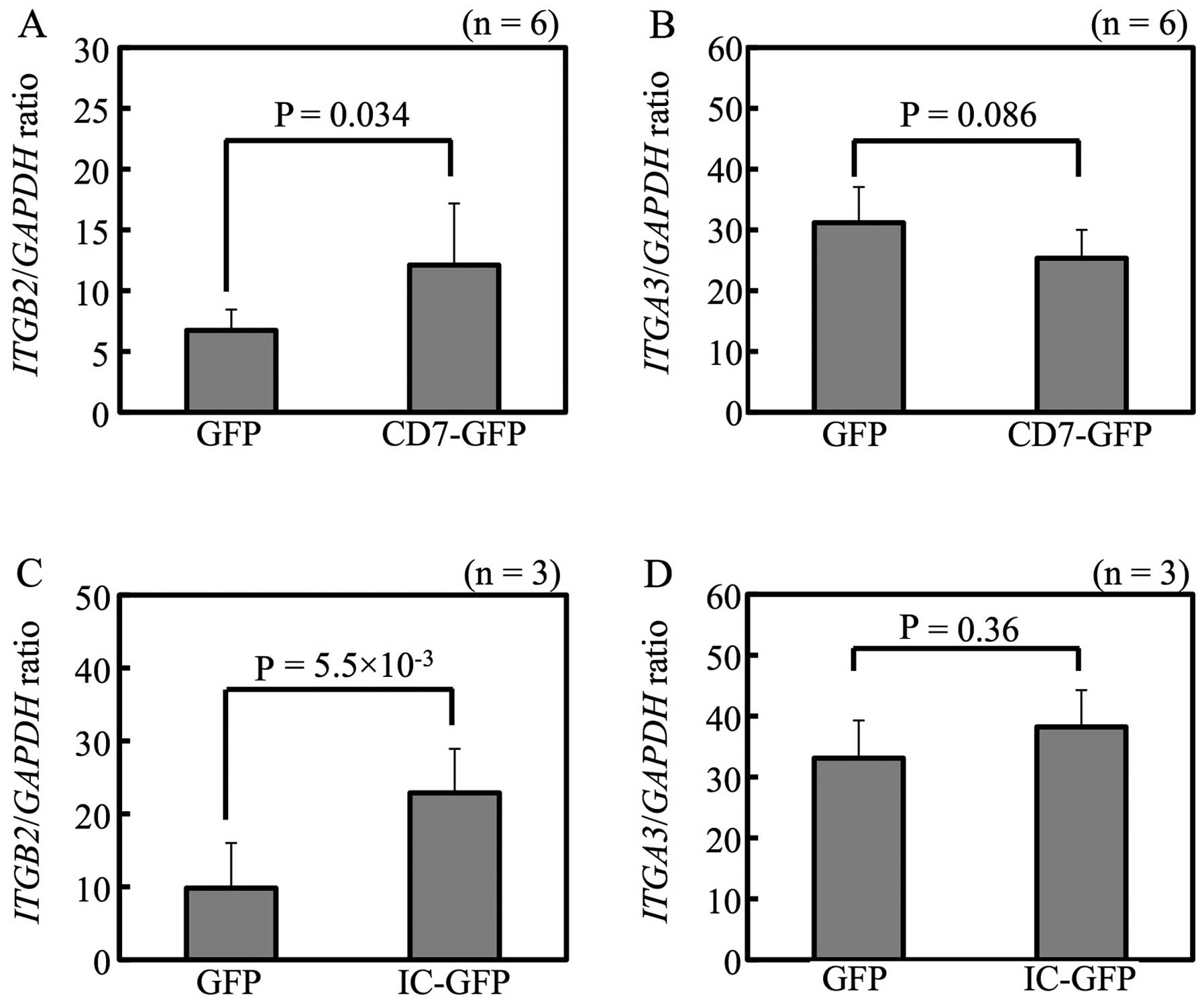
Integrin β2 mediates cell adhesion of
Tanoue
To check whether CD7 regulates expression levels of
ITGB2 and ITGA3, pTurbo-GFP-N, pTurboCD7-GFP and
pTurboCD7-IC-GFP transfectants were analyzed. As shown in Fig. 6A and C, pTurboCD7-GFP and
pTurboCD7-IC-GFP transfectants showed higher levels of ITGB2
expression than pTurboGFP-N transfectants. Transfection of
pTurboCD7-GFP and pTurboCD7-IC-GFP did not increase ITGA3
expression (Fig. 6B and D).
Expression level of integrin β2 was higher in Luc-Tanoue-F4 than
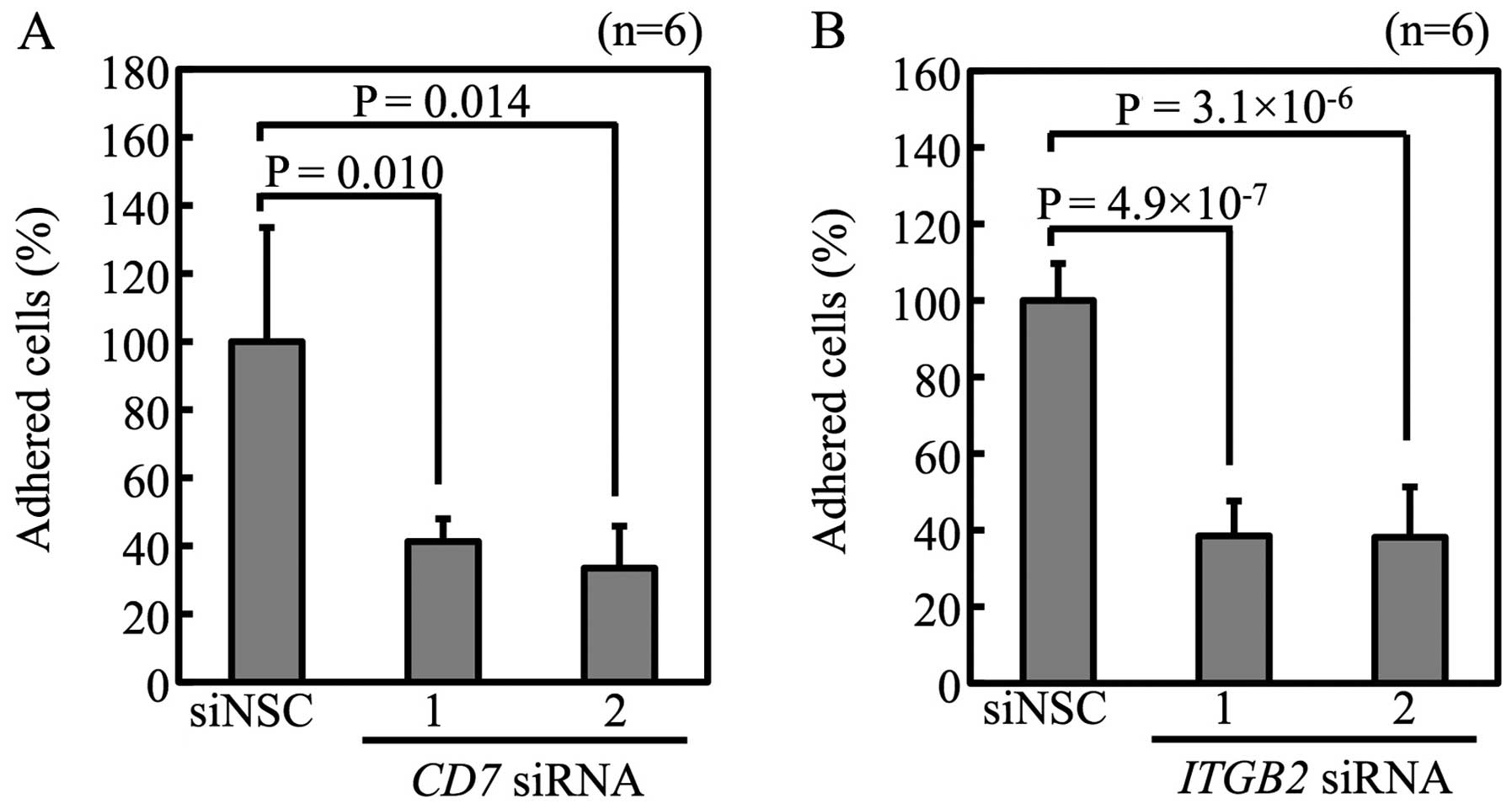
Luc-Tanoue, confirmed by FACS (data not shown). Suppression of
CD7 and ITGB2 by siRNA reduced adhesion of
Luc-Tanoue-F4 (Fig. 7). These
results show that CD7 and integrin β2 regulate adhesiveness of the
Tanoue leukemic cell line.
Discussion
The present study revealed that CD7 promotes EMI of
the B-ALL line Tanoue. To the best of our knowledge, this is the
first study to show that CD7 promotes EMI by inducing integrin β2
in hematological malignancy. Comparison of the global gene
expression profile of cells with different EMI potential that were
all obtained from the same B-ALL cell line and found no significant
increases in any of the genes previously associated with EMI,
including CXCR4, IL-15, CCR9, CD103, CD56, MMP2 or
MMP9; nor did it suggest that a decrease in ICAM1
expression was associated with EMI.
CD7 is a 40-kDa type-I transmembrane single-chain
glycoprotein that belongs to immunoglobulin (Ig) superfamily. It is
expressed on thymocytes, T cells, and natural killer (NK) cells, as
well as in subpopulation of early immature B and myeloid cells
(20). The extracellular domain of
CD7 shares homology with the variable region of Igκ-chains and the
γ-chains of T-cell receptors (21). It is thought that CD7 exists as a
homodimer, and that cross-linking antibodies that recognize CD7
stimulates its downstream signaling (22). There is a YEDM motif in the
intracellular domain of CD7, and phosphatidylinositol 3-kinase and
tyrosine kinase play critical roles in its activation (22–25).
Physiologically, activation of CD7 augments IL-2
production by T cells (26),
induces the production of granulocyte macrophage colony-stimulating
factor by myeloid progenitor cells (27), stimulates IFN-γ production in NK
cells (28), and regulates the
capacity for adhesion in T cells (29) and NK cells (28).
Clinically, the expression of CD7 correlates with a
poor prognosis for acute myeloid leukemia (30–35),
non-Hodgkin’s lymphoma (36) and
B-lymphoblastic leukemia (37).
Moreover, CD7+, CD4−, CD8− acute
lymphoblastic leukemia is reported to show poor clinical
characteristics that involves extramedullary organs, including the
mediastenum, skin, and CNS (38).
This study showed that CD7 induces integrin β2 (also
referred to as CD18; Fig. 6A) and
enhanced the capacities of leukemic cells for adhesion (Fig. 4B). Notwithstanding, there are
observations that CD7 regulates the capacities of T and NK cells to
adhere to extracellular matrix, there is no evidence that CD7 plays
an adhesion molecule role. Together with the results shown in
Fig. 5A–D that CD7-induced
signaling (but not extracellular domain of CD7) enhanced the
adhesive capacities of Tanoue cells, it seems possible that the
induction of integrin β2 enables CD7 to promote cell adhesiveness
in the Tanoue B-ALL line. These observations are consistent with
previous reports that CD7-induced cell adhesion is mediated by
integrin β2 (29,39).
Integrin β2 is an important adhesion molecule in
leucocytes. Genetic mutations in ITGB2 results in the
immunodeficiency caused by a decreased capacity of leucocytes to
adhere (leukocyte adhesion deficiency) (40,41).
Furthermore, integrin β2 is critically required for leucocyte
extravasation (42,43). Together with our results, these
observations indicate that the CD7/integrin β2 axis may contribute
to extravasation during the EMI of leukemic cells.
Results of this study imply that CD7 and integrin β2
are potential molecular targets in leukemia therapy. Indeed,
antibodies against integrin β2 inhibit leukemia and lymphoma
dissemination in experimental models (44–46).
It remains to be established whether a blocking antibody against
CD7 can inhibit dissemination of leukemia.
In conclusion, CD7 promotes EMI of the B-ALL line
Tanoue in an integrin β2-dependent manner. CD7 and integrin β2 are
potential molecular targets in leukemia therapy.
Acknowledgements
We thank Dr S.H. Kim (National Cancer Center, Korea)
for providing us with firefly luciferase expressing vector
(pBABE-Luc-Hygro).
References
|
1
|
Pui CH and Evans WE: Acute lymphoblastic
leukemia. N Engl J Med. 339:605–615. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schrappe M, Nachman J, Hunger S, et al:
Educational symposium on long-term results of large prospective
clinical trials for childhood acute lymphoblastic leukemia
(1985–2000). Leukemia. 24:253–254. 2010.PubMed/NCBI
|
|
3
|
Pui CH, Robison LL and Look AT: Acute
lymphoblastic leukaemia. Lancet. 371:1030–1043. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schrappe M, Reiter A, Ludwig WD, et al:
Improved outcome in childhood acute lymphoblastic leukemia despite
reduced use of anthracyclines and cranial radiotherapy: results of
trial ALL-BFM 90. German-Austrian-Swiss ALL-BFM Study Group. Blood.
95:3310–3322. 2000.
|
|
5
|
Lazarus HM, Richards SM, Chopra R, et al:
Central nervous system involvement in adult acute lymphoblastic
leukemia at diagnosis: results from the international ALL trial MRC
UKALL XII/ECOG E2993. Blood. 108:465–472. 2006. View Article : Google Scholar
|
|
6
|
Reiter A, Schrappe M, Ludwig WD, et al:
Chemotherapy in 998 unselected childhood acute lymphoblastic
leukemia patients. Results and conclusions of the multicenter trial
ALL-BFM 86. Blood. 84:3122–3133. 1994.
|
|
7
|
Jacobs JE and Hastings C: Isolated
extramedullary relapse in childhood acute lymphocytic leukemia.
Curr Hematol Malig Rep. 5:185–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Annels NE, Willemze AJ, van der Velden VH,
et al: Possible link between unique chemokine and homing receptor
expression at diagnosis and relapse location in a patient with
childhood T-ALL. Blood. 103:2806–2808. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Langley RR and Fidler IJ: Tumor cell-organ
microenvironment interactions in the pathogenesis of cancer
metastasis. Endocr Rev. 28:297–321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Crazzolara R, Kreczy A, Mann G, et al:
High expression of the chemokine receptor CXCR4 predicts
extramedullary organ infiltration in childhood acute lymphoblastic
leukaemia. Br J Haematol. 115:545–553. 2001. View Article : Google Scholar
|
|
11
|
Kuittinen O, Savolainen ER, Koistinen P,
Mottonen M and Turpeenniemi-Hujanen T: MMP-2 and MMP-9 expression
in adult and childhood acute lymphatic leukemia (ALL). Leuk Res.
25:125–131. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mielcarek M, Sperling C, Schrappe M, Meyer
U, Riehm H and Ludwig WD: Expression of intercellular adhesion
molecule 1 (ICAM-1) in childhood acute lymphoblastic leukaemia:
correlation with clinical features and outcome. Br J Haematol.
96:301–307. 1997. View Article : Google Scholar
|
|
13
|
Ravandi F, Cortes J, Estrov Z, et al: CD56
expression predicts occurrence of CNS disease in acute
lymphoblastic leukemia. Leuk Res. 26:643–649. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schneider P, Costa O, Legrand E, et al: In
vitro secretion of matrix metalloproteinase-9 is a prognostic
marker in childhood acute lymphoblastic leukemia. Leuk Res.
34:24–31. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu S, Fischer L, Gokbuget N, et al:
Expression of interleukin 15 in primary adult acute lymphoblastic
leukemia. Cancer. 116:387–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Buonamici S, Trimarchi T, Ruocco MG, et
al: CCR7 signalling as an essential regulator of CNS infiltration
in T-cell leukaemia. Nature. 459:1000–1004. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Holland M, Castro FV, Alexander S, et al:
RAC2, AEP, and ICAM1 expression are associated with CNS disease in
a mouse model of pre-B childhood acute lymphoblastic leukemia.
Blood. 118:638–649. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Castro FV, McGinn OJ, Krishnan S, et al:
5T4 oncofetal antigen is expressed in high risk of relapse
childhood pre-B acute lymphoblastic leukemia and is associated with
a more invasive and chemotactic phenotype. Leukemia. 26:1487–1498.
2012. View Article : Google Scholar
|
|
19
|
Kuribayashi K, Finnberg N and El-Deiry WS:
Studying p53-dependent cell death in vitro and in vivo. Methods
Enzymol. 446:159–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sempowski GD, Lee DM, Kaufman RE and
Haynes BF: Structure and function of the CD7 molecule. Crit Rev
Immunol. 19:331–348. 1999.PubMed/NCBI
|
|
21
|
Aruffo A and Seed B: Molecular cloning of
two CD7 (T-cell leukemia antigen) cDNAs by a COS cell expression
system. EMBO J. 6:3313–3316. 1987.PubMed/NCBI
|
|
22
|
Lazarovits AI, Osman N, Le Feuvre CE, Ley
SC and Crumpton MJ: CD7 is associated with CD3 and CD45 on human T
cells. J Immunol. 153:3956–3966. 1994.
|
|
23
|
Chan AS, Mobley JL, Fields GB and Shimizu
Y: CD7-mediated regulation of integrin adhesiveness on human T
cells involves tyrosine phosphorylation-dependent activation of
phosphatidylinositol 3-kinase. J Immunol. 159:934–942. 1997.
|
|
24
|
Lee DM, Patel DD, Pendergast AM and Haynes
BF: Functional association of CD7 with phosphatidylinositol
3-kinase: interaction via a YEDM motif. Int Immunol. 8:1195–1203.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rabinowich H, Lin WC, Herberman RB and
Whiteside TL: Signaling via CD7 molecules on human NK cells.
Induction of tyrosine phosphorylation and beta 1 integrin-mediated
adhesion to fibronectin. J Immunol. 153:3504–3513. 1994.PubMed/NCBI
|
|
26
|
Jung LK, Roy AK and Chakkalath HR: CD7
augments T cell proliferation via the interleukin-2 autocrine
pathway. Cell Immunol. 141:189–199. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hou Z, Leta E and Jung LK: Cross-linking
CD7 on myeloblasts results in granulocyte-macrophage
colony-stimulating factor production. Blood. 88:124–129.
1996.PubMed/NCBI
|
|
28
|
Rabinowich H, Pricop L, Herberman RB and
Whiteside TL: Expression and function of CD7 molecule on human
natural killer cells. J Immunol. 152:517–526. 1994.PubMed/NCBI
|
|
29
|
Shimizu Y, van Seventer GA, Ennis E,
Newman W, Horgan KJ and Shaw S: Crosslinking of the T cell-specific
accessory molecules CD7 and CD28 modulates T cell adhesion. J Exp
Med. 175:577–582. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Del Poeta G, Stasi R, Venditti A, et al:
Prognostic value of cell marker analysis in de novo acute myeloid
leukemia. Leukemia. 8:388–394. 1994.PubMed/NCBI
|
|
31
|
Jensen AW, Hokland M, Jorgensen H,
Justesen J, Ellegaard J and Hokland P: Solitary expression of CD7
among T-cell antigens in acute myeloid leukemia: identification of
a group of patients with similar T-cell receptor beta and delta
rearrangements and course of disease suggestive of poor prognosis.
Blood. 78:1292–1300. 1991.
|
|
32
|
Kita K, Miwa H, Nakase K, et al: Clinical
importance of CD7 expression in acute myelocytic leukemia. The
Japan Cooperative Group of Leukemia/Lymphoma. Blood. 81:2399–2405.
1993.PubMed/NCBI
|
|
33
|
Saxena A, Sheridan DP, Card RT, McPeek AM,
Mewdell CC and Skinnider LF: Biologic and clinical significance of
CD7 expression in acute myeloid leukemia. Am J Hematol. 58:278–284.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yumura-Yagi K, Hara J, Kurahashi H, et al:
Clinical significance of CD7-positive stem cell leukemia. A
distinct subtype of mixed lineage leukemia. Cancer. 68:2273–2280.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chang H, Yeung J, Brandwein J and Yi QL:
CD7 expression predicts poor disease free survival and
post-remission survival in patients with acute myeloid leukemia and
normal karyotype. Leuk Res. 31:157–162. 2007. View Article : Google Scholar
|
|
36
|
Yumura-Yagi K, Ishihara S, Hara J, et al:
Poor prognosis of mediastinal non-Hodgkin’s lymphoma with an
immature phenotype of CD2+, CD7 (or CD5)+,
CD3−, CD4−, and CD8. Cancer. 63:671–674.
1989.
|
|
37
|
Hussein S, Gill KZ, Sireci AN, et al:
Aberrant T-cell antigen expression in B lymphoblastic leukaemia. Br
J Haematol. 155:449–456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kurtzberg J, Waldmann TA, Davey MP, et al:
CD7+, CD4−, CD8− acute leukemia: a
syndrome of malignant pluripotent lymphohematopoietic cells. Blood.
73:381–390. 1989.
|
|
39
|
Chan AS, Reynolds PJ and Shimizu Y:
Tyrosine kinase activity associated with the CD7 antigen:
correlation with regulation of T cell integrin function. Eur J
Immunol. 24:2602–2608. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kishimoto TK, Hollander N, Roberts TM,
Anderson DC and Springer TA: Heterogeneous mutations in the beta
subunit common to the LFA-1, Mac-1, and p150,95 glycoproteins cause
leukocyte adhesion deficiency. Cell. 50:193–202. 1987. View Article : Google Scholar
|
|
41
|
Mathew EC, Shaw JM, Bonilla FA, Law SK and
Wright DA: A novel point mutation in CD18 causing the expression of
dysfunctional CD11/CD18 leucocyte integrins in a patient with
leucocyte adhesion deficiency (LAD). Clin Exp Immunol. 121:133–138.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kling D, Fingerle J and Harlan JM:
Inhibition of leukocyte extravasation with a monoclonal antibody to
CD18 during formation of experimental intimal thickening in rabbit
carotid arteries. Arterioscler Thromb. 12:997–1007. 1992.
View Article : Google Scholar
|
|
43
|
Walzog B, Scharffetter-Kochanek K and
Gaehtgens P: Impairment of neutrophil emigration in CD18-null mice.
Am J Physiol. 276:G1125–G11130. 1999.PubMed/NCBI
|
|
44
|
Cohen S, Haimovich J and Hollander N:
Anti-idiotype x anti-LFA-1 bispecific antibodies inhibit metastasis
of B cell lymphoma. J Immunol. 170:2695–2701. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Harning R, Myers C and Merluzzi VJ:
Monoclonal antibodies to lymphocyte function-associated antigen-1
inhibit invasion of human lymphoma and metastasis of murine
lymphoma. Clin Exp Metastasis. 11:337–342. 1993. View Article : Google Scholar
|
|
46
|
Zahalka MA, Okon E and Naor D: Blocking
lymphoma invasiveness with a monoclonal antibody directed against
the beta-chain of the leukocyte adhesion molecule (CD18). J
Immunol. 150:4466–4477. 1993.PubMed/NCBI
|