Introduction
Hemangiomas (HAs) are one of the most common benign
tumors, but the prevalence and the pathogenesis of HAs are not well
understood. HAs are characterized by three phases: proliferating,
involuting and involuted phases, and is defined by a period of
rapid proliferation of blood vessels in the first year of life,
followed by gradual regression of the vascular component with
eventual replacement by fibro-fatty tissue (1).
Insulin-like growth factor-II (IGF-II) plays an
important role in tumor development and progression through
activation of the receptor IGF2R. IGF-II is expressed in
cholangiocarcinoma (CC) cell lines, and inhibition of IGF-II by the
growth factor inhibitor influences the oncogene K-RAS genotype in
CC (2). Integrative genomic
analysis shows IGF-II/IGF2R signaling is activated in the
proliferation subclass of hepatocellular carcinoma (HCC), and
effective blockage of IGF1R signaling also provides the rationale
for testing HCC therapy in clinical trials (3). Also, the polymorphic variants of
IGF-axis genes act alone or jointly with other risk factors to
affect susceptibility to pancreatic cancer, and are used to
identify population subgroups that benefit from IGF2R-targeted
agents (4,5).
Thus, abnormalities in IGF-II/IGF2R signaling are
associated with the development of a wide variety of tumors
(6). Increased IGF-II expression
stimulates proliferation and results in a rapid conversion to
malignancy during tumor formation in primary mouse embryonic
fibroblasts (7). The 3′ UTR
IGF2R-A2/B2 variant is associated with increased tumor growth and
advanced stages in non-small cell lung cancer (8), and genetic variation of IGF2R may
advance distant metastasis, and influence the risk of developing
some cancers (9–11). Therefore, although there is
insufficient evidence supporting the associations between IGF1R
single-nucleotide polymorphisms (rs2272037) and brain tumor risk
(12), interaction of IGF-II with
IGF2R increases the risk of malignant transformation and enhances
the aggressive breast cancer phenotype, suggesting that IGF2R may
serve as a potential therapeutic target for HAs (13).
However, some studies have shown that mannose
6-phosphate/insulin-like growth factor II receptor (M6P/IGF2R) gene
can function as the tumor suppressor in cancer. M6P/IGF2R is found
inactivated in prostate cancer and the mutation of this gene is an
early event in the development of prostate cancer (14). M6P/IGF2R also functions as a growth
suppressor, but the loss or mutation of this gene contributes to
development and progression of some cancers (15,16)
and is involved in HBV-associated hepatocarcinogenesis (17). Moreover, M6P/IGF2R controls cell
adhesion and invasion by regulating αv integrin expression and
accelerating uPAR cleavage (18),
and restricts the metastatic propensity of squamous cell carcinomas
(19). These studies thereby
support that M6P/IGF2R may act as the tumor suppressor in
carcinogenesis.
In terms of the multi-functionality of IGF2R in
cancer, exploration of the expression and function of IGF-II/IGF2R
signaling in HAs is indispensable. In the present study, tissues
from 27 cases of HA tissues of different phases were collected, and
the expression of IGF-II and IGF2R was examined. Using
lentivirus-mediated IGF2R knockdown in HA-derived endothelial cells
(HDEC), we investigated the effects of IGF2R knockdown on the
biological behavior of human HA cells. We found that the expression
of IGF-II and IGF2R was increased in proliferating phase HAs, and
knockdown of IGF2R repressed proliferation and induced apoptosis in
HA cells in vitro and in vivo, suggesting that IGF2R
might represent a novel therapeutic target for the treatment of
human HAs.
Materials and methods
Materials
The primary HDEC and CRL-2586 EOMA cells used in
these experiments were from the Institute of Biochemistry and Cell
Biology (Shanghai, China). The lentivirus vector Lv-siIGF2R,
negative control vector (scramble-siRNA) and virion-packaging
elements were from Genechem (Shanghai, China). The IGF2R primer was
synthesized by ABI (Framingham, MA, USA) and all antibodies were
from Santa Cruz Biotechnology (Dallas, TX, USA).
Drugs and reagents
Dulbecco’s modified Eagle’s medium (DMEM) and fetal
bovine serum (FBS) were from Thermo Fisher Scientific Inc.
(Waltham, MA, USA); TRIzol reagent and Lipofectamine 2000 were from
Invitrogen (Carlsbad, CA, USA); M-MLV reverse transcriptase was
from Promega (Madison, WI, USA); SYBR Green Master Mixture was from
Takara (Otsu, Japan). ECL-Plus/kit was from GE Healthcare
(Piscataway, NJ, USA). Cell cycle analysis kit and apoptosis kit
[propidium iodide (PI), RNase A, Annexin V-FITC] were from KeyGen
Biology (Nanjing, China).
Tissue samples
Twenty-seven freshly resected human HAs and 18 cases
of adjacent normal skin tissue samples were collected at the
Department of General Surgery, and were classified according to
Mulliken criteria. Tissues and clinical information were obtained
as part of an approved study at Shanghai Jiao Tong University
School of Medicine. There were 15 cases of proliferating phase HAs
and 12 cases of involuting phase HAs. A portion of each tissue
sample was fixed with 10% formalin for histopathological and IHC
examination. All HA tissues were diagnosed by two independent
pathologists.
IHC staining
IHC staining for IGF-II (rabbit polyclonal, ab9572,
Abcam, MA, USA), IGF2R (rabbit polyclonal A0545, Sigma, USA) and
PCNA (mouse monoclonal PC10, Sigma) using polyclonal and monoclonal
antibodies was performed. Unstained sections were deparaffinized
and incubated overnight at 4°C with primary antibodies against the
proteins, then with biotinylated secondary antibody (1:200) at room
temperature for 1 h, followed by incubation with ABC peroxidase and
3,3′ diaminobenzidine (DAB; 30 mg dissolved in 100 ml Tris-buffer
containing 0.03% H2O2). Sections were
counterstained with hematoxylin. Digital images were acquired and
the integrated optical density (IOD) and positive expression rate
(cell positive area/cell total area) of semi-quantitative analysis
was performed.
Cell culture and transfection
The proliferating phase HDEC and CRL-2586 EOMA cells
were cultured in DMEM medium supplemented with 10% heat-inactivated
FBS, 100 U/ml of penicillin and 100 μg/ml of streptomycin. They
were all placed in a humidified atmosphere containing 5%
CO2 at 37°C. Lv-siIGF2R and negative control viruses
were transfected into proliferating phase HDEC and CRL-2586 EOMA
cells. Cells were subcultured at a 1:5 dilution in 300 μg/ml
G418-containing medium. On the day of transduction, HA cells were
replated at 5×104 cells/well in 24-well plates
containing serum-free growth medium with polybrene (5 mg/ml). When
the cells reached 50% confluence, they were transfected with
recombinant experimental virus or control virus at the optimal MOI
(multiplicity of infection) of 50, and cultured at 37°C and 5%
CO2 for 4 h. Then supernatant was discarded and serum
containing growth medium was added. At 4 days of post-transduction,
transduction efficiency was measured by the frequency of green
fluorescent protein (GFP)-positive cells. Positive stable
transfectants were selected and expanded for further study. The
clones transfected with the Lv-siIGF2R vectors were named as the
Lv-siIGF2R group, and the clones transfected with the negative
control vectors was named as the NC group.
Quantitative real-time PCR
To quantitatively determine the mRNA expression
level of IGFII and IGF2R in proliferating phase HA tissues and
cells, Real-time PCR was used. Total RNA of each clone was
extracted with TRIzol according to the manufacturer’s protocol.
Reverse-transcription was carried out using M-MLV and cDNA
amplification was carried out using SYBR Green Master Mix kit
according to the manufacturer’s protocol. The IGF2R gene was
amplified using specific oligonucleotide primer and human
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as
an endogenous control. The PCR primer sequences were listed as
follows: IGFII, 5′-AGAGAGGCCAAACGTCATCGT-3′ and 5′-TCACC
CCACCTTGCAGAATTA-3′; IGF2R, 5′-GAAAACACAAG AATGAAGGTCAA-3′ and
5′-CCACCAGCTCTCCTCCAC ATA-3′; GAPDH, 5′-CAACGAATTTGGCTACAGCA-3′ and
5′-AGGGGTCTACATGGCAACTG-3′. Data were analyzed using the
comparative Ct method (2−ΔΔCt). Three separate
experiments were performed for each clone.
Western blot assay
The proliferating phase HDEC and CRL-2586 EOMA cells
were harvested and extracted using lysis buffer (Tris-HCl, SDS,
mercaptoethanol, glycerol). Cell extracts were boiled for 5 min in
loading buffer and then an equal amount of cell extracts from each
sample was separated on 15% SDS-PAGE gels. Separated protein bands
were transferred onto polyvinylidene fluoride (PVDF) membranes and
the membranes were blocked in 5% skim-milk powder dissolved in
PBST. The primary antibodies against IGF-II (rabbit polyclonal
ab9572, Abcam), IGF2R (rabbit polyclonal A0545, Sigma), PCNA (mouse
monoclonal PC10, Sigma), Ki-67 (rabbit polyclonal ab888, Abcam),
Bcl-2 (rabbit monoclonal SP66, Sigma), Bax (rabbit monoclonal
ab32503, Abcam) and Cyclin D1 (rabbit monoclonal sc-718, Santa
Cruz, USA) were diluted according to the manufacturer’s
instructions of antibodies and incubated overnight at 4°C. Then,
horseradish peroxidase-linked secondary antibodies were added at a
dilution ratio of 1:1,000, and incubated at room temperature for 2
h. The membranes were washed three times with PBS for three times
and the immunoreactive bands were visualized using ECL-Plus/kit
according to the manufacturer’s instructions. The relative protein
level in different cell lines was normalized to the endogenous
level of β-actin protein. Three separate experiments were performed
for each clone.
Cell proliferation assay
Cell proliferation was analyzed with the MTT assay.
Briefly, cells infected with Lv-siIGF2R were incubated in
96-well-plates (8 wells each group) at a density of
1×105 cells per well with DEME medium supplemented with
10% FBS. Cells were treated with 20 μl MTT dye at 0, 24, 48, and 72
h and then incubated with 150 μl of DMSO for 5 min. The color
reaction was measured at 570 nm with enzyme immunoassay analyzer
(Bio-Rad, USA). The proliferation activity was calculated for each
clone.
Cell apoptosis analysis
To detect cell apoptosis, the proliferating phase
HDEC and CRL-2586 EOMA cells were trypsinized, washed with cold PBS
and resuspended in binding buffer according to the instruction of
the apoptosis kit. FITC-Annexin V and PI were added to the fixed
cells for 20 min in darkness at room temperature. Then, Annexin V
binding buffer was added to the mixture before the fluorescence was
measured on FACsort flow cytometer (Becton-Dickinson, Mountain
View, CA, USA). The cell apoptosis was analyzed using CellQuest
software (Becton-Dickinson). Cell apoptotic rate = the number of
cell apoptosis/the number of apoptotic cells and the normal cells.
Three separate experiments were performed for each clone.
Cell cycle analysis
To detect cell cycle variation, the proliferating
phase HDEC and CRL-2586 EOMA cells were trypsinized, washed with
PBS and fixed with 80% cold ethanol overnight at −20°C. After
another wash with PBS, the fixed cells were stained with PI in the
presence of RNase A for 30 min at room temperature in darkness.
Each sample was filtered through a 50-μm nylon filter to obtain
single-cell suspension. The samples were then analyzed on FACsort
flow cytometer (Becton-Dickinson). ModFit3.0 software (Verity
Software House, Topsham, ME, USA) was used for cell cycle analysis.
Three separate experiments were performed for each clone.
Subcutaneous tumor model and gene
therapy
Six-week-old female immune-deficient nude mice
(BALB/c-nu) were bred at the laboratory animal facility (Institute
of Chinese Academy of Sciences, Shanghai, China), and were housed
individually in microisolator ventilated cages with free access to
water and food. All experimental procedures were performed
according to the regulations and internal biosafety and bioethics
guidelines of Shanghai Jiaotong University and the Shanghai
Municipal Science and Technology Commission.
Four mice were injected subcutaneously with
1×107 HDEC cells in 50 μl of PBS pre-mixed with an equal
volume of Matrigel matrix (Becton-Dickinson). Mice were monitored
daily, and three out of four mice developed a subcutaneous tumor.
When the tumor size reached ~5 mm in length, it was surgically
removed, cut into 1–2 mm3 pieces, and re-seeded
individually into other mice on the right flanks. When tumor size
reached ~5 mm in length, the mice were randomly assigned as PBS
group, negative control (NC) group and Lv-siIGF2R-treated group. In
treatment group, 15 μl of Lv-siIGF2R was injected into subcutaneous
tumors using a multi-site injection format (at least three sites).
Mice in the PBS (HDEC) group and the NC group received 15 μl of PBS
with or without negative control lentivirus, respectively.
Injections were repeated every other day after initial treatment.
The tumor volume was measured every three days with a caliper,
using the formula volume = (length × width)2/2.
Statistical analysis
SPSS 20.0 was used for the statistical analysis.
One-way analysis of variance (ANOVA) was used to analyze the
differences between groups. The LSD method of multiple comparisons
was used when the probability for ANOVA was statistically
significant. Statistical significance was P<0.05. In the
figures, the error bars depict standard deviation.
Results
The expression of IGF-II, IGF2R and PCNA
in human HAs
The protein expression of IGF-II, IGF2R and PCNA was
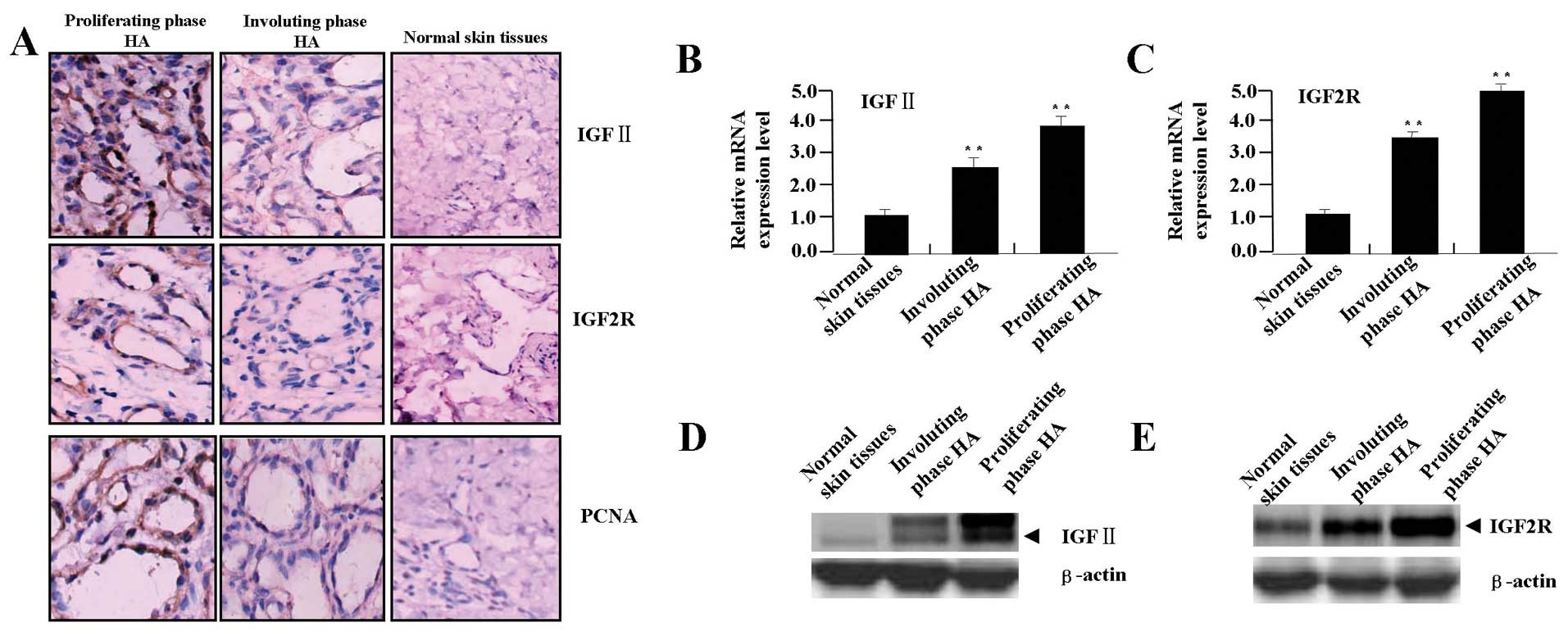
evaluated using IHC analysis. As shown in Fig. 1A, the positive expression of
IGF-II, IGF2R and PCNA was found increased in proliferating phase
HAs, but decreased in involuting phase HAs. From the cell
localization, the positive staining of IGF-II was mainly in the
cytoplasm, that of IGF2R was in the membrane and cytoplasm, but
PCNA was in the cellular nucleus in HA and normal tissue cells. The
average IOD and positive rates of IGF-II, IGF2R and PCNA were
significantly higher in proliferating phase HAs than those in
involuting phase HAs or in normal tissues (Table I, each P<0.01). Spearman
correlation analysis showed the positive correlation between the
IGF-II, IGF2R and PCNA expression in HA tissues of different phases
(r=0.625, P=0.015). Then, we examined the mRNA and protein
expression levels of IGF-II and IGF2R in HA tissues by real-time
PCR (Fig. 1B and C) and western
blot assays (Fig. 1D and E), and
further found that the expression of IGF-II and IGF2R was increased
in proliferating phase HAs, but decreased in involuting phase
HA.
 | Table IExpression of IGFII, IGF2R and PCNA
in different phase HAs. |
Table I
Expression of IGFII, IGF2R and PCNA
in different phase HAs.
| | IGFII | IGF2R | PCNA |
|---|
| |
|
|
|
|---|
| Group | Case | IOD | Positive rate | IOD | Positive rate | IOD | Positive rate |
|---|
| Normal skin
tissues | 18 | 2.53±0.67 | 0.04±0.01 | 3.65±0.71 | 0.02±0.01 | 2.15±0.82 | 0.02±0.01 |
| Involuting phase
HAs | 12 | 7.29±1.17a | 0.12±0.03a | 10.78±2.05a | 0.09±0.03a | 8.16±2.19a | 0.10±0.03a |
| Proliferating phase
HAs | 15 | 13.05±1.21ab | 0.18±0.04ab | 12.98±1.45ab | 0.13±0.03ab | 13.01±2.12ab | 0.15±0.04ab |
The siRNA knockdown efficiency for IGF2R
expression
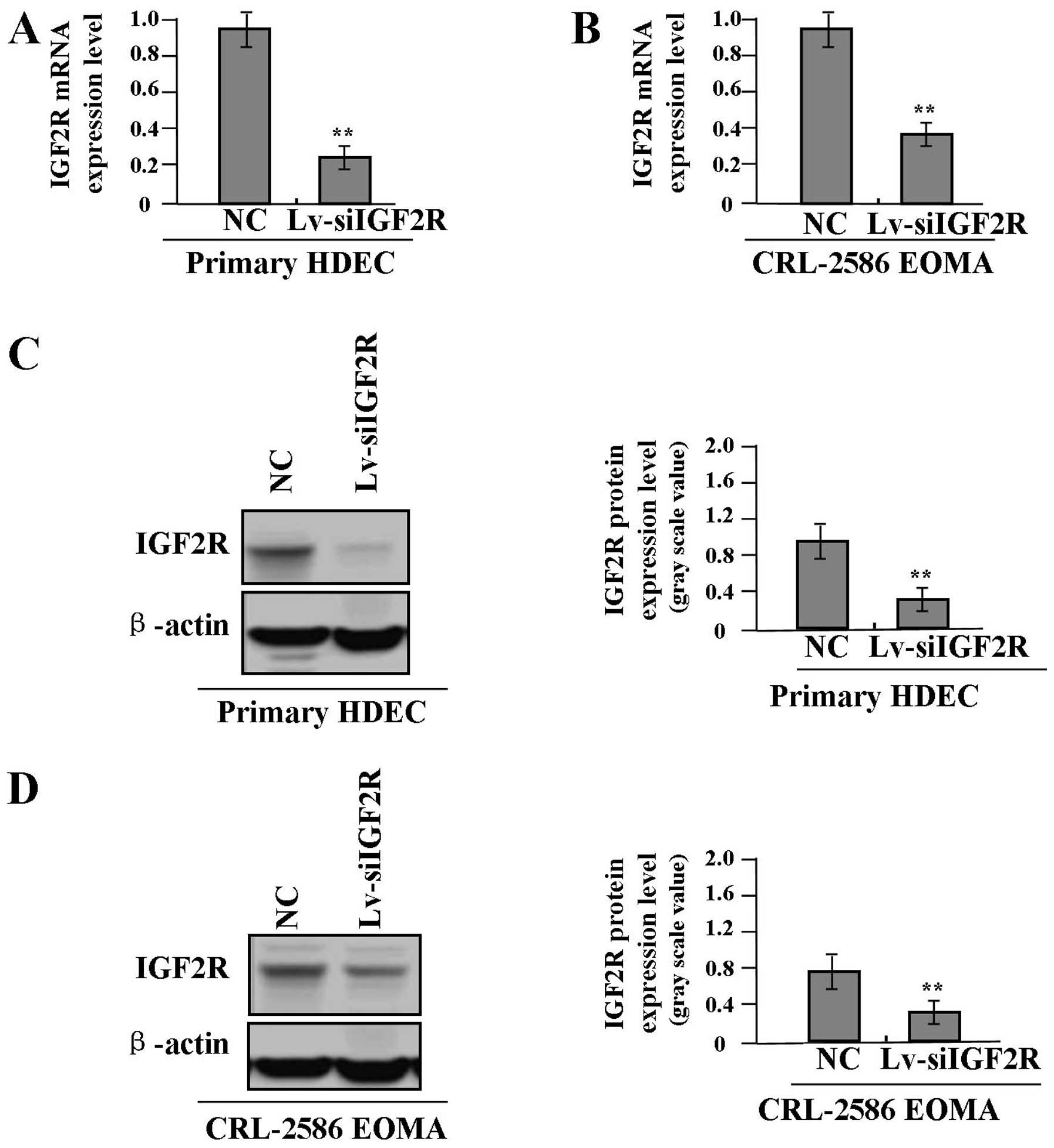
To examine the Lv-siIGF2R knockdown efficiency for
IGF2R expression in proliferative phase HDEC and CRL-2586 EOMA
cells, we investigated the expression levels of IGF2R in
Lv-siIGF2R-transfected HA cells using real-time PCR and western
blot assays. As shown in Fig. 2,
the knockdown efficiency for IGF2R expression was found to be
significantly increased in Lv-siIGF2R-transfected HDEC (Fig. 2A and C) and CRL-2586 EOMA cells
(Fig. 2B and D), respectively
reaching >80 and 70% (each **P<0.01).
Effect of Lv-siIGF2R on cell
proliferation
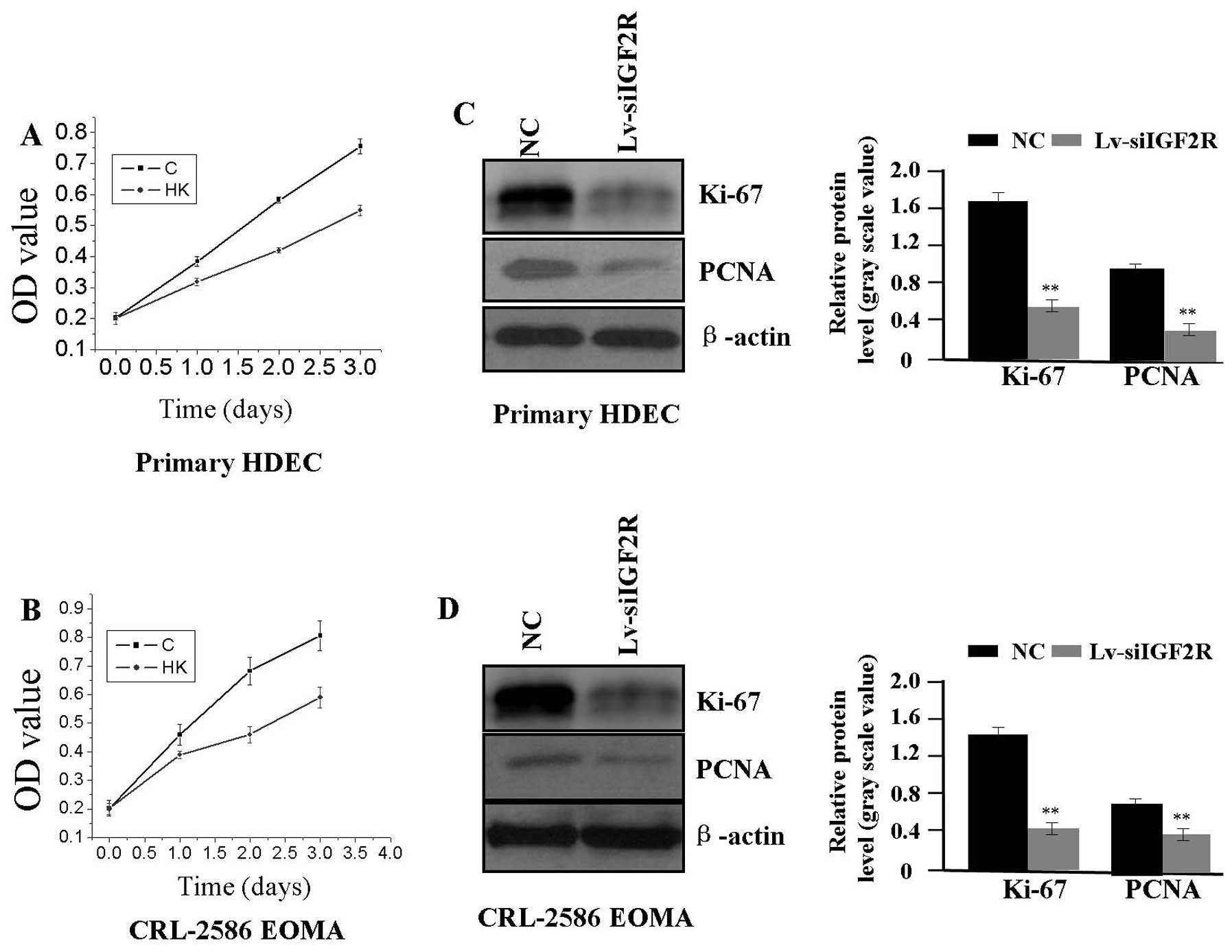
To investigate the effect of Lv-siIGF2R on cell
proliferation in HA cells (HDEC and CRL-2586 EOMA), we examined
cell proliferative activities using MTT assay. We found that
Lv-siIGF2R could significantly diminish the proliferative activity
of HA cells in a time-dependent manner compared with the NC group
(Fig. 3A and B). In addition, to
determine whether IGF2R knockdown suppressed the endogenous
expression of Ki-67 and PCNA, we examined the protein expression of
Ki-67 and PCNA by western blot assay. The amount of Ki-67 and PCNA
proteins was significantly decreased in the Lv-siIGF2R group
compared with the NC group (each **P<0.01) (Fig. 3C and D), suggesting that knockdown
of IGF2R might inhibit HA cell proliferation through downregulation
of Ki-67 and PCNA expression.
Effect of Lv-siIGF2R on cell
apoptosis
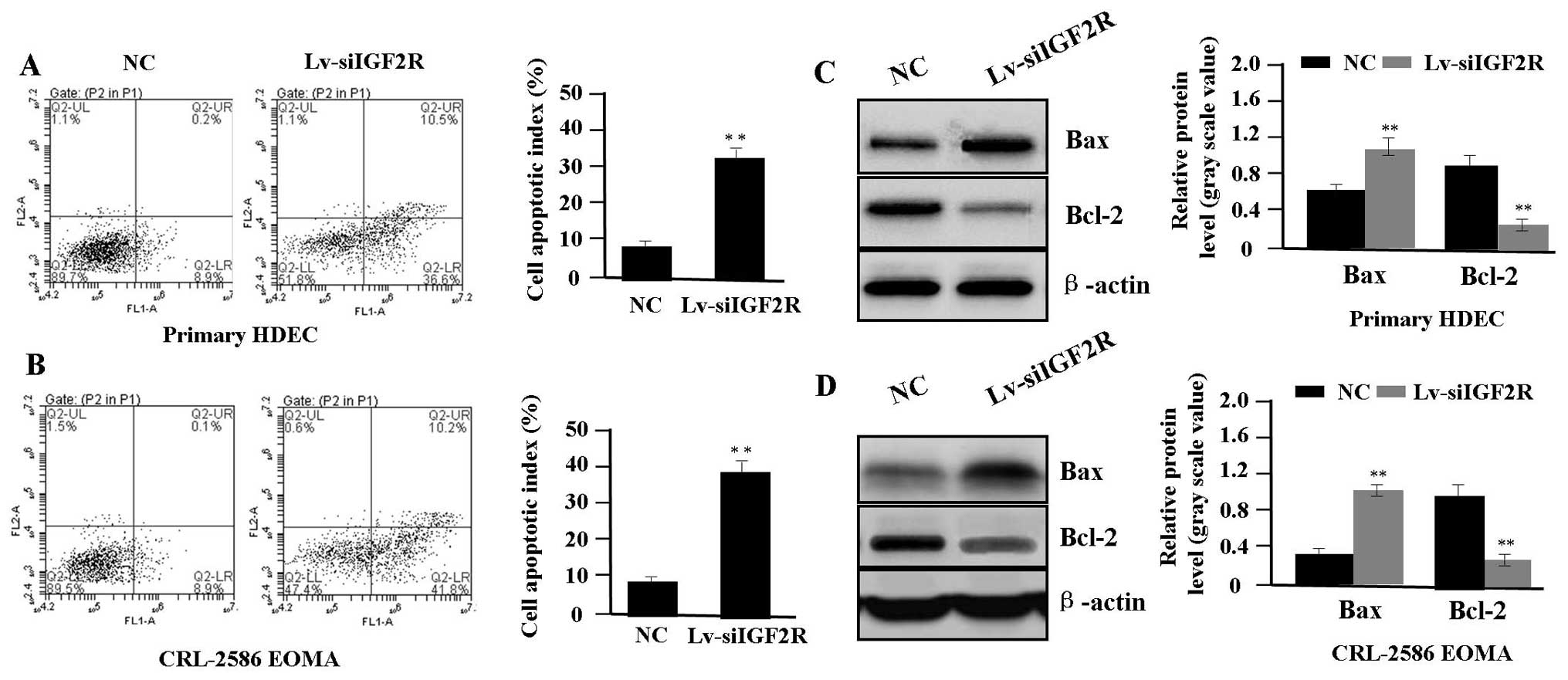
To determine whether IGF2R knockdown affected the
apoptosis in HA cells (HDEC and CRL-2586 EOMA), flow cytometric
analysis was performed. Cell apoptotic rates were markedly
increased in Lv-siIGF2R group compared with the NC group (each
**P<0.01) (Fig. 4A and
B). To determine whether IGF2R knockdown influences the
expression of Bax and Bcl-2, we examined the expression of Bax and
Bcl-2 in HA cells by western blot assay. It was found that the
amount of Bax was markedly increased, while that of Bcl-2 protein
was decreased in the Lv-siIGF2R group compared with the NC group
(each **P<0.01) (Fig. 4C
and D), suggesting that knockdown of IGF2R might induce HA cell
apoptosis through regulation of the expression of Bax and
Bcl-2.
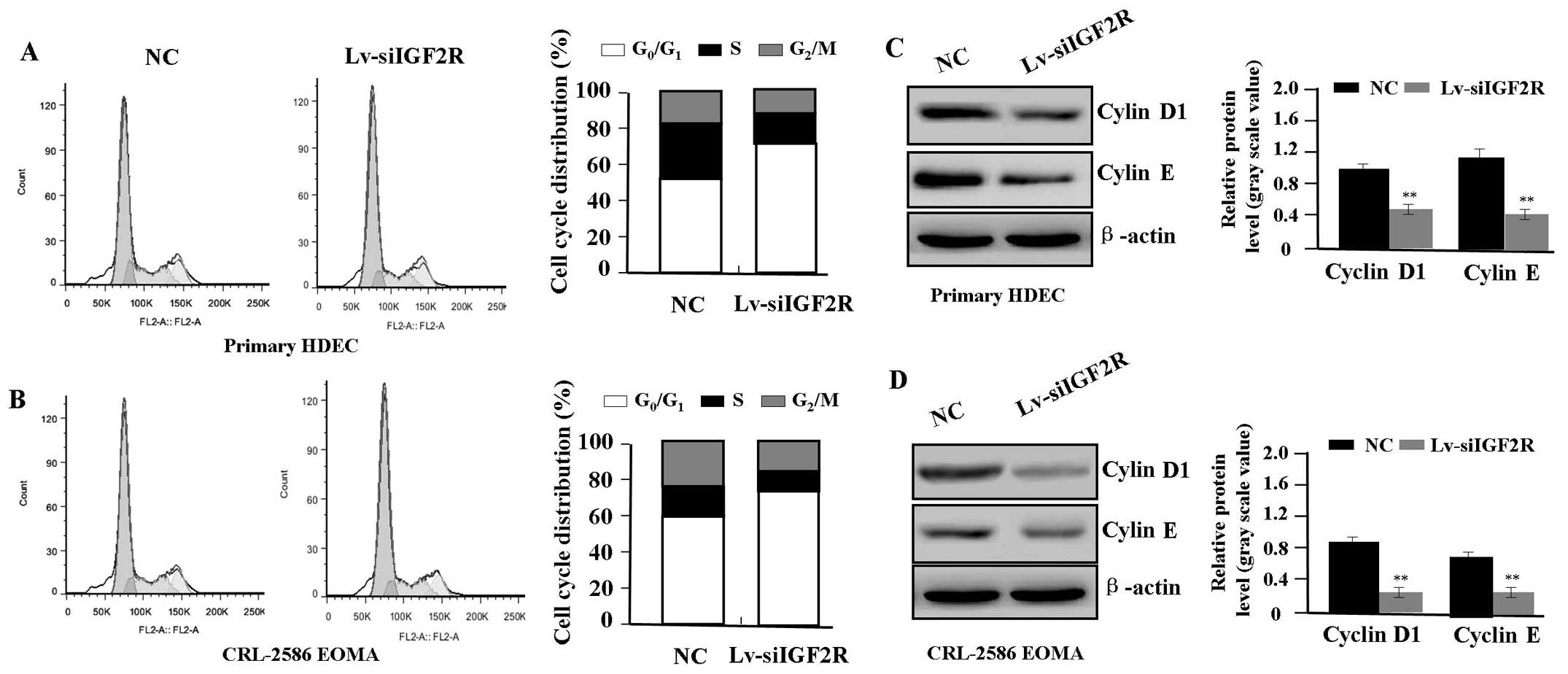
Effect of Lv-siIGF2R on cell cycle
distribution
To determine whether IGF2R knockdown influenced
cycle distribution in HA cells (HDEC and CRL-2586 EOMA), flow
cytometry analysis was performed. We showed that the
G0/G1 phase fraction was increased, while the
S and G2/M phase fractions were decreased, and more HA
cells were arrested in the G0/G1 phase in the
Lv-siIGF2R group compared with the NC group (Fig. 5A and B). In order to determine
whether IGF2R knockdown suppressed the expression of Cyclin D1 and
E, we examined the expression of Cyclin D1 and E by western blot
assay. The amount of Cyclin D1 and E protein was markedly decreased
in the Lv-siIGF2R group compared with the NC group (each
**P<0.01) (Fig. 5C and
D), suggesting that knockdown of IGF2R might induce cycle
arrest in HA cells through downregulation of Cyclin D1 and E
expression.
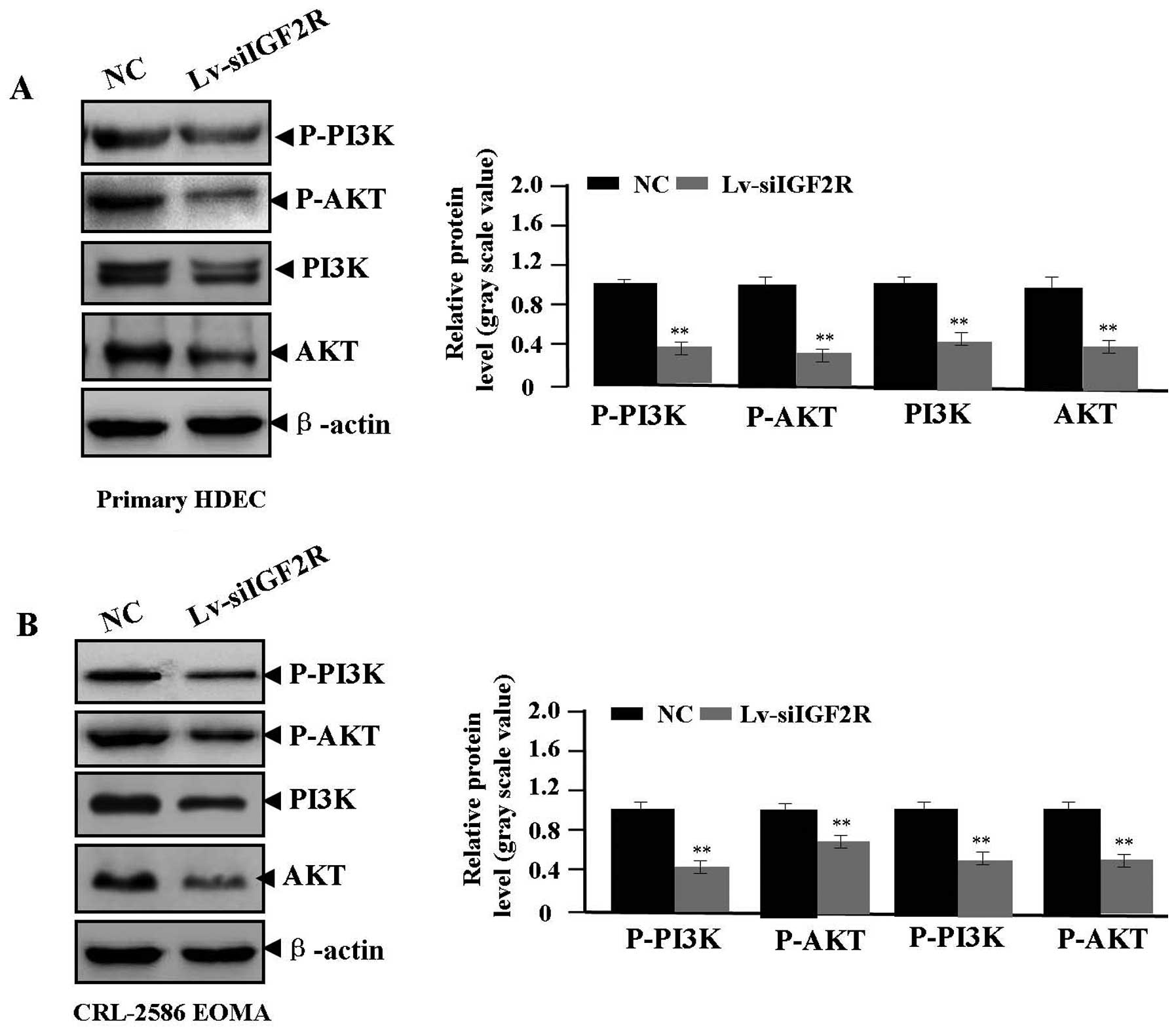
Effect of Lv-siIGF2R on signaling
transduction of PI3K/AKT
Many studies have demonstrated that the PI3K/AKT
pathway is involved in regulating HA formation (20,21).
Whether Lv-siIGF2R affects PI3K/AKT signaling transduction of
PI3K/AKT needed to be explored. Western blot assays were performed
to assess the expression of P-PI3K, P-AKT, PI3K and AKT in
Lv-siIGF2R-transfected HA cells. We found that the activity of
P-PI3K, P-AKT, PI3K and AKT proteins was reduced in the Lv-siIGF2R
group compared with the NC group (each **P<0.01)
(Fig. 6), suggesting that
knockdown of IGF2R might block the PI3K/AKT signaling transduction
in HA cells.
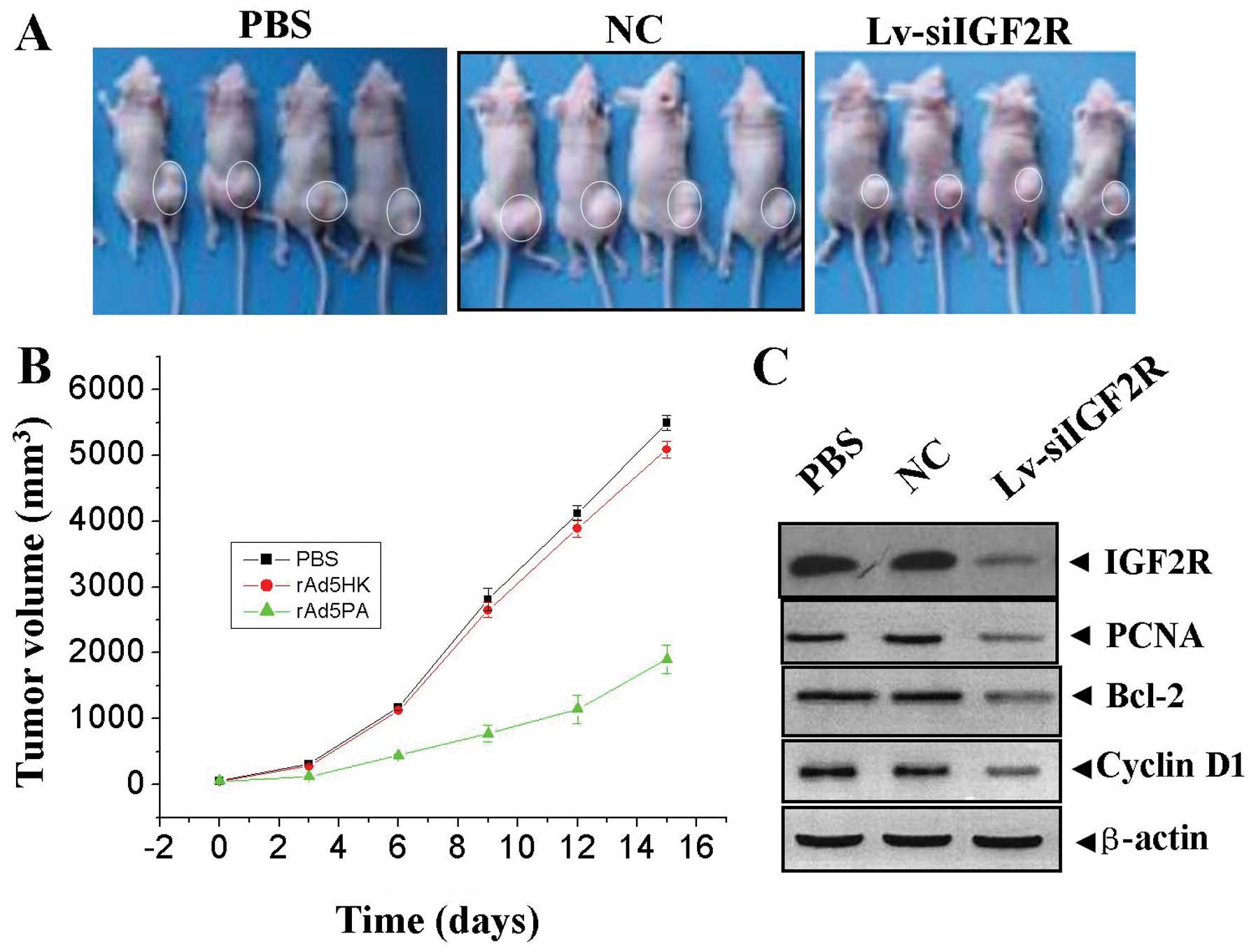
Antitumor effect of Lv-siIGF2R on the
HDEC xenograft model
Our in vitro experiments demonstrated that
knockdown of IGF2R could efficiently inhibit proliferation and
induce apoptosis in HA cells. Therefore, we further investigated
the antitumor effect of Lv-siIGF2R in vivo using the HDEC
xenograft model and lentivirus-mediated gene therapy. The mean
volume of tumors in all experimental mice before treatment was
78.30±8.20 mm3. During the first 3 days of recovery, the
tumors in each group grew slowly, with no obvious difference in
tumor size. However, from the 3rd day after the second treatment
and beyond, the tumors in PBS and NC groups grew rapidly until the
end of the observation period on the 15th day. Conversely, the
tumors treated with Lv-siIGF2R maintained a slower growth rate
(Fig. 7A and B), and there was a
significant difference in tumor volume between Lv-siIGF2R group and
PBS and NC groups over the two-week observation period (Fig. 6, P<0.01). After recovery of two
weeks, the tumor samples were removed for western blot analysis of
the protein expression of IGF2R, PCNA, Bcl-2 and Cyclin D1. It was
found that the protein expression levels of IGF2R, PCNA, Bcl-2 and
Cyclin D1 were remarkably downregulated in Lv-siIGF2R group
compared with those in PBS and NC groups (Fig. 7C).
Discussion
The interaction between IGF2R and IGF-II plays an
important role in cellular physiology and pathological progression
including tumor growth, invasion and metastasis (22). However, some studies show that the
expression of IGF-II and IGF2R is strongly decreased in carcinomas
and metastatic breast cancer, but increased in normal gland and
adenomas (23). M6P/IGF2R reduces
tumorigenicity and invasive potential of HCC, but knockdown of
M6P/IGF2R enhances cell motility and invasiveness (24). To define the role of IGF-II/IGF2R
signaling in HAs, we examined the expression of IGF-II and IGF2R in
human HA tissues by IHC, real-time PCR and western blot assays. It
was found that the expression of IGF-II and IGF2R was significantly
increased in proliferating phase HAs, but decreased in involuting
phase HAs, suggesting IGF-II and IGF2R may be implicated in the
development of human HA.
Survival factors including IGF2R play critical roles
in regulating cell growth in normal and cancer cells. Genetic
screen has identifies IGF2R as a cell surface marker in tumor
cells, suggesting that IGF2R is required for tumor growth in a
mouse model (25). However, few
studies exist on the function of IGF-II/IGF2R signaling in HAs. In
the present study, our findings indicated that knockdown of IGF2R
gene significantly inhibited cell proliferation, and induced cell
apoptosis and cycle arrest in HA cells in vitro and in
vivo. Tovar et al (3)
have previously found that IGF2R is activated and associated with
the mTOR signaling in HCC, and a selective inhibition of IGF2R
significantly decreases cell viability and proliferation,
suggesting that IGF2R may be a promising target that promotes tumor
progression in human HAs.
As non-histone nuclear proteins, PCNA and Ki-67 are
associated with cell growth and plays a critical role in the
initiation of cell proliferation. They have proved to be quite
useful as the markers for proliferating cells (26). Apoptotic cell death is regulated by
complex interactions between pro-survival members and two subgroups
of pro-apoptotic members of Bcl-2 protein family. Bax/Bcl-2 as a
primary or secondary oncogenic event is critical in maintaining
tumor development, and inducing therapeutic resistance (27). Cyclin D1 and E are cell cycle
regulators that are also frequently altered in cancers, as they are
found to be overexpressed and involved in cancers (28). Some studies have shown the
association between these indexes and HAs. PCNA and Ki-67 are
expressed by the majority of endothelial cells in the proliferating
phase HAs, but their expression is negligible in the involuting
phase (29–31). Bcl-2 contributes to the low
apoptosis effect seen in HAs (32). However, the effects of IGF2R on the
expression of PCNA, Ki-67, Bax, Bcl-2, Cyclin D1 and E have not
been examined in HA cells. In the present study, we found that
knockdown of IGF2R could significantly downregulate the expression
of PCNA, Ki-67, Bcl-2, Cyclin D1 and E and upregulated the
expression of Bax in HA cells and xenograft HA samples, suggesting
that IGF2R may promote the development and progression of human HA
via upregulation of PCNA, Ki-67, Bcl-2, Cyclin D1 and E expression
and downregulation of Bax expression. More importantly, PI3K/AKT
has been found to play a role in the development of HAs (20,21).
Activation of the PI3K/AKT pathway promotes tumor growth and
invasion through upregulation of the PCNA and Ki-67 expression
(33), blocks cell apoptosis
through decrease of the Bax/Bcl-2 ratio (34), and prompts cell cycle progression
through upregulation of Cyclin D/E expression (35). We further found knockdown of IGF2R
decreased the expression of p-PI3K and p-AKT in HA cells,
suggesting that the PI3K/AKT pathway might mediate the regulation
of IGF2R on the expression of PCNA, Ki-67, Bcl-2, Cyclin D1 and E
implicated in HA proliferation and apoptosis.
In conclusion, our findings indicate that the
expression of IGF-II and IGF2R is increased in proliferating phase
HAs, and knockdown of IGF2R suppresses proliferation and induces
apoptosis in HA cells in vitro and in vivo,
suggesting that IGF2R may represent a novel therapeutic target for
the treatment of human HAs.
Acknowledgements
This study was supported by Shanghai Science and
Technology Committee scientific and technological innovation
project (no. 12140901102), and Shanghai City Board of education
research and innovation project (no. 12YZ042).
References
|
1
|
Mulliken JB, Fishman SJ and Burrows PE:
Vascular anomalies. Curr Probl Surg. 37:517–584. 2000. View Article : Google Scholar
|
|
2
|
Xu L, Hausmann M, Dietmaier W, et al:
Expression of growth factor receptors and targeting of EGFR in
cholangiocarcinoma cell lines. BMC Cancer. 10:3022010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tovar V, Alsinet C, Villanueva A, et al:
IGF activation in a molecular subclass of hepatocellular carcinoma
and pre-clinical efficacy of IGF-1R blockage. J Hepatol.
52:550–559. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dong X, Javle M, Hess KR, et al:
Insulin-like growth factor axis gene polymorphisms and clinical
outcomes in pancreatic cancer. Gastroenterology. 139:464–473. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dong X, Li Y, Tang H, et al: Insulin-like
growth factor axis gene polymorphisms modify risk of pancreatic
cancer. Cancer Epidemiol. 36:206–211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harris LK and Westwood M: Biology and
significance of signalling pathways activated by IGF-II. Growth
Factors. 30:1–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hernandez L, Kozlov S, Piras G, et al:
Paternal and maternal genomes confer opposite effects on
proliferation, cell-cycle length, senescence, and tumor formation.
Proc Natl Acad Sci USA. 100:13344–13349. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kotsinas A, Evangelou K, Sideridou M, et
al: The 3′ UTR IGF2R-A2/B2 variant is associated with increased
tumor growth and advanced stages in non-small cell lung cancer.
Cancer Lett. 259:177–185. 2008.
|
|
9
|
Zavras AI, Pitiphat W, Wu T, et al:
Insulin-like growth factor II receptor gene-167 genotype increases
the risk of oral squamous cell carcinoma in humans. Cancer Res.
63:296–297. 2003.PubMed/NCBI
|
|
10
|
Yoon AJ, Zavras AI, Chen MK, et al:
Association between Gly1619ARG polymorphism of IGF2R domain 11
(rs629849) and advanced stage of oral cancer. Med Oncol.
29:682–685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoyo C, Schildkraut JM, Murphy SK, et al:
IGF2R polymorphisms and risk of esophageal and gastric
adenocarcinomas. Int J Cancer. 125:2673–2678. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lönn S, Rothman N, Shapiro WR, et al:
Genetic variation in insulin-like growth factors and brain tumor
risk. Neuro Oncol. 10:553–559. 2008.PubMed/NCBI
|
|
13
|
Kalla Singh S, Tan QW, Brito C, et al:
Insulin-like growth factors I and II receptors in the breast cancer
survival disparity among African-American women. Growth Horm IGF
Res. 20:245–254. 2010.PubMed/NCBI
|
|
14
|
Hu CK, McCall S, Madden J, et al: Loss of
heterozygosity of M6P/IGF2R gene is an early event in the
development of prostate cancer. Prostate Cancer Prostatic Dis.
9:62–67. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Z, Ge Y, Landman N, et al: Decreased
expression of the mannose 6-phosphate/insulin-like growth factor-II
receptor promotes growth of human breast cancer cells. BMC Cancer.
2:182002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oka Y, Waterland RA, Killian JK, et al:
M6P/IGF2R tumor suppressor gene mutated in hepatocellular
carcinomas in Japan. Hepatology. 35:1153–1163. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang EB, Qin LL, Zhao YN, et al: Genetic
changes and expression of the mannose 6-phosphate/insulin-like
growth factor II receptor gene in human hepatitis B
virus-associated hepatocellular carcinoma. Int J Mol Med.
11:773–778. 2003.
|
|
18
|
Schiller HB, Szekeres A, Binder BR, et al:
Mannose 6-phosphate/insulin-like growth factor 2 receptor limits
cell invasion by controlling alphaVbeta3 integrin expression and
proteolytic processing of urokinase-type plasminogen activator
receptor. Mol Biol Cell. 20:745–756. 2009. View Article : Google Scholar
|
|
19
|
Probst OC, Puxbaum V, Svoboda B, et al:
The mannose 6-phosphate/insulin-like growth factor II receptor
restricts the tumourigenicity and invasiveness of squamous cell
carcinoma cells. Int J Cancer. 124:2559–2567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji Y, Chen S, Li K, et al: Signaling
pathways in the development of infantile hemangioma. J Hematol
Oncol. 7:132014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Amin RM, Hiroshima K, Miyagi Y, et al:
Role of the PI3K/Akt, mTOR, and STK11/LKB1 pathways in the
tumorigenesis of sclerosing hemangioma of the lung. Pathol Int.
58:38–44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brown J, Jones EY and Forbes BE:
Interactions of IGF-II with the IGF2R/cation-independent
mannose-6-phosphate receptor mechanism and biological outcomes.
Vitam Horm. 80:699–719. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Klopfleisch R, Hvid H, Klose P, et al:
Insulin receptor is expressed in normal canine mammary gland and
benign adenomas but decreased in metastatic canine mammary
carcinomas similar to human breast cancer. Vet Comp Oncol.
8:293–301. 2010. View Article : Google Scholar
|
|
24
|
Puxbaum V, Nimmerfall E, Bäuerl C, et al:
M6P/IGF2R modulates the invasiveness of liver cells via its
capacity to bind mannose 6-phosphate residues. J Hepatol.
57:337–343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gelman MS, Ye XK, Stull R, et al:
Identification of cell surface and secreted proteins essential for
tumor cell survival using a genetic suppressor element screen.
Oncogene. 23:8158–8170. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shin DM, Hittelman WN and Hong WK:
Biomarkers in upper aerodigestive tract tumorigenesis: a review.
Cancer Epidemiol Biomarkers Prev. 3:697–709. 1994.PubMed/NCBI
|
|
27
|
Kelly PN and Strasser A: The role of Bcl-2
and its pro-survival relatives in tumourigenesis and cancer
therapy. Cell Death Differ. 18:1414–1424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim JK and Diehl JA: Nuclear cyclin D1: an
oncogenic driver in human cancer. J Cell Physiol. 220:292–296.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tan ST, Velickovic M, Ruger BM, et al:
Cellular and extracellular markers of hemangioma. Plast Reconstr
Surg. 106:529–538. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Murakami M, Sakai H, Kodama A, et al:
Expression of the anti-apoptotic factors Bcl-2 and survivin in
canine vascular tumours. J Comp Pathol. 139:1–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mohamed AM, Elwakil TF, Taher IM, et al:
Cyclin D1 gene amplification in proliferating haemangioma. Cell
Tissue Res. 338:107–115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakamura T: Apoptosis and expression of
Bax/Bcl-2 proteins in pyogenic granuloma: a comparative study with
granulation tissue and capillary hemangioma. J Cutan Pathol.
27:400–405. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fu Y, Zhang Q, Kang C, et al: Inhibitory
effects of adenovirus mediated Akt1 and PIK3R1 shRNA on the growth
of malignant tumor cells in vitro and in vivo. Cancer Biol Ther.
8:1002–1009. 2009. View Article : Google Scholar
|
|
34
|
Raja Singh P, Arunkumar R, Sivakamasundari
V, et al: Anti-proliferative and apoptosis inducing effect of
nimbolide by altering molecules involved in apoptosis and IGF
signalling via PI3K/AKT in prostate cancer (PC-3) cell line. Cell
Biochem Funct. 32:217–228. 2014.PubMed/NCBI
|
|
35
|
Chen C, Chang YC, Lan MS, et al: Leptin
stimulates ovarian cancer cell growth and inhibits apoptosis by
increasing cyclin D1 and Mcl-1 expression via the activation of the
MEK/ERK1/2 and PI3K/Akt signaling pathways. Int J Oncol.
42:1113–1119. 2013.PubMed/NCBI
|