Introduction
Colorectal cancer (CRC) is the second most common
cause of death among cancer patients in the developed world and the
third most common malignancy in the United States (1). Other than surgery, treatment for CRC
patients relies primarily on chemotherapy, especially the patients
with advanced CRC. In clinic, application of 5-fluorouracil (5-FU)
makes a great contribution to the improvement of the life quality
and overall survival of CRC patients (2). A group of patients also suffer cancer
recurrence or metastasis when the standard treatment has been
completed or even during the course of post-surgical chemotherapy,
suggesting a development of 5-FU resistance. Drug resistance,
whether intrinsic or acquired, is believed to cause treatment
failure in >90% of patients with metastatic cancer (3). Clearly, if drug resistance could be
overcome, the impact on survival would be highly significant.
Altered regulation of nucleotide metabolism, amino acid metabolism,
cytoskeleton organization, transport, and oxygen metabolism have
been reported to confer 5-FU resistance (4). For example, high-level expression of
hENT1 correlated with poor clinical response to 5-FU among CRC
patients (5). BAX downregulation
could contribute as an important factor during cell resistance to
5-FU in colon cancer (6). Although
some progress has been achieved in the past 50 decades, much more
efforts are still needed to resolve 5-FU resistance in CRC.
Glycosylation is one of the most abundant
post-translational modifications found on more than half of all
secreted and cellular proteins. Most protein glycosylation is
either Asn-linked or initiated by O-linked GalNAc added to Ser or
Thr (7). Glycans on glycoproteins
mediate a dynamic protein state, involving folding, quality
control, secretion and catabolism. N-glycans are also related to
tumor progression and metastasis as well as to immune system
activity, and their potential relationship to chemoresistance has
recently been examined (8). Zhang
et al showed that N-glycomic alterations were associated
with adriamycin resistance in human leukemia (9). Increased levels and defective
N-glycosylation of multidrug resistance-associated proteins (MRPs)
in ovarian carcinoma cells resistant to oxaliplatin have also been
reported (10). In addition,
N-glycans bearing a β-1–6-linked GlcNAc branch are consistently
elevated in concert with increased expression of Mgat5, and direct
correlation has been made between Mgat5 overexpression and enhanced
drug resistance (11).
Swainsonine, an inhibitor of N-glycan biosynthesis, could reduce
5-FU tolerance of CRC cells (12).
Taken together, these studies indicate the existence of differences
between sensitive and resistant cells in the content and
composition of N-glycans.
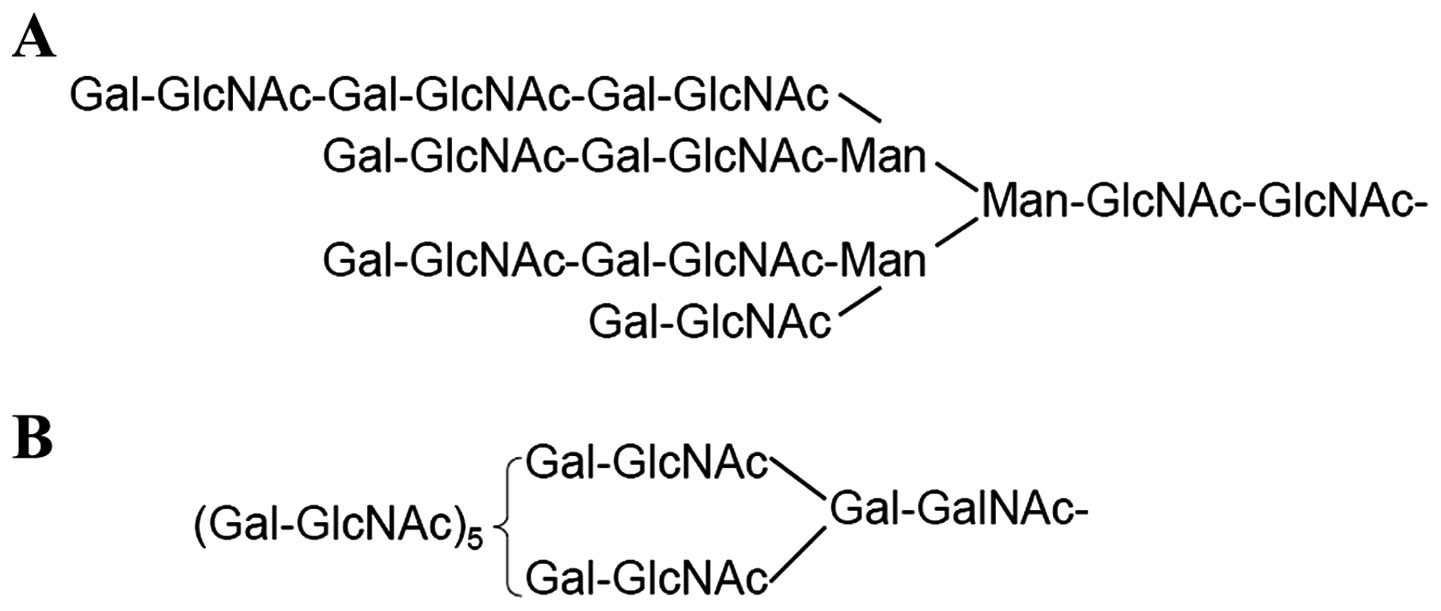
Polylactosamine is a linear carbohydrate polymer
composed of alternating GlcNAc and Gal residues involved in
cellular functions ranging from differentiation to metastasis
(13). It can be incorporated into
either N-linked or mucin-type O-linked glycans (Fig. 1). It is well known that
β-1–6-branched N-glycans serve as most preferred sites for addition
of polylactosamine (14).
Polylactosamine is synthesized by the alternative action of a
β-1,4-galactosyltransferase (β4GalT) and a
β-1,3-N-acetylglucosaminyltransferase (β3GnT) (15). β3GnT8, which was the most recently
identified enzyme among the β3GnTs, is involved in the biosynthesis
of polylactosamine on tetraantennary (β1,6-branched) N-glycans.
Ishida et al showed that most of the cell lines established
from CRC expressed higher levels of the β3GnT8 transcript (16). Recently, β3GnT8 has been reported
to be associated with cancer chemoresistance (9). Therefore, it is of interest to
clarify the relationship between β3GnT8 expression and drug
resistance in CRC.
In this study, a 5-FU-resistant CRC cell line was
established from the parental cell line SW620, and the role of
β3GnT8 in alteration of 5-FU resistance and the possible pathways
involved were investigated by RNA interference-based
approaches.
Materials and methods
Cell line generation
Human SW620 CRC cells (ATCC, Manassas, VA, USA) were
cultured in RPMI-1640 medium (Gibco-BRL, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (Gibco-BRL) in a
humidified atmosphere with 5% CO2 at 37°C. To establish
the drug-resistant cell subline SW620/5-FU, SW620 cells were
exposed to stepwise increasing 5-FU (Sigma, St. Louis, MO, USA)
concentrations from 10 to 100 μg/ml. SW620/5-FU cells were
incubated for 1 week in drug-free medium prior to their use in each
experiment.
MTT assay
Cells were seeded in 96-well plates at a density of
5.0×103 cells/ml. After treatment with the indicated
methods, medium was removed and 50 μl of MTT (Sigma) was added to
each well. Then the cells were incubated in the dark at 37°C for an
additional 4 h. The reaction was stopped by the addition of 150 μl
DMSO (Sigma) and the absorbance of samples at 570 nm was measured
with a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
The chemosensitivity of 5-FU was expressed as 50% inhibition
concentration (IC50). Cell viability was assessed after 48 h of
exposure to 10–100 μg/ml of 5-FU. The IC50 value calculation was
performed using GraphPad Prism 5.0 software.
Plasmid transfection and RNA
interference
The pSilen-Circle-Si-β3GnT8 plasmid was constructed
by our Lab and identified by digestion with restriction enzymes
XhoI and EcoRI (MBI Fermentas, Vilnius, Lithuania)
(17). Plasmid DNA was purified as
described in the EndoFree plasmid purification handbook (Qiagen,
Ltd., Crawley, UK). For transfection studies, SW620/5-FU cells were
plated at a density of 2xl05 cells/well in 6-well plates
and incubated for 24 h. The cells were then transfected with 2–4 μg
of plasmid DNA using Lipofectamine 2000 according to the
manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). As the
negative control, the same amount of empty vector, pEGFP-c1, was
also transfected. Gene silencing effect was confirmed by western
blot analysis and qPCR at 24 h post-transfection.
Quantitative RT-PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen). Then the isolated RNA was quantified by
spectrophotometry (optical density 260/280 nm). Reverse
transcription to cDNA was conducted using the Superscript First
Strand synthesis system (Invitrogen). All PCR reactions were
carried out on an ABI PRISM® 7500 Sequence Detection
System (Applied Biosystems, Foster City, CA, USA) using the
SYBR-Green Real-Time PCR Master Mix kit (Toyobo, Osaka, Japan)
according to the manufacturer’s instruction. PCR conditions used
were: denaturation at 95°C for 30 sec, followed by 40 cycles of
denaturation at 95°C for 5 sec, annealing at 60°C for 30 sec and
elongation at 72°C for 30 sec. Primers of all genes are listed in
Table I. The data were collected
and analyzed using the comparative Ct (threshold cycle) method
using GADPH as the reference gene.
 | Table ISequences of the primers used for
quantitative RT-PCR. |
Table I
Sequences of the primers used for
quantitative RT-PCR.
| Primer name | Sequences
(5′→3′) |
|---|
| β3GnT1 | F:
AACACTGGACTTGGATATGG
R: TCACATATAGCATCTCATCTG |
| β3GnT2 | F:
ATACTGGAACCGAGAGCAAG
R: TCAGGTTCGCAGTAGTTCAG |
| β3GnT3 | F:
TATGTGCCAGAGGTGGTGAC
R: ACATACCCAGGAAGACATCAT |
| β3GnT4 | F:
TCAAGTCACAGCCTGGTCAC
R: TCATCAAACTCCCTACTCTCAT |
| β3GnT7 | F:
CTACTGCTATGGAATGAGAC
R: AGCTATTTATCTTACTTCTGTT |
| β3GnT8 | F:
GTCGCTACAGTGACCTGCTG
R: GTCTTTGAGCGTCTGGTTGA |
| P-gp | F:
TTGCTGCTTACATTCAGGTTTCA
R: AGCCTATCTCCTGTCGCATTA |
| GADPH | F:
CCAACCGCGAGAAGATGA
R: CCAGAG GCGTACAGGGATAG |
Western blot analysis
Harvested cells were lysed in lysis buffer
containing 50 mM Tris-HCl (pH 7.5), 1% NP-40, 2 mM EDTA, 10 mM
NaCl, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM DTT, 0.1% SDS, 1
mM PMSF and placed in ice for 30 min. After centrifugation for 15
min at 4°C, the supernatant was collected. Then proteins were
separated by 10% SDS-PAGE and transferred to PVDF membranes. After
blocking with 5% fat-free milk for 1 h at room temperature, the
membranes were incubated with the primary antibody overnight at 4°C
followed by incubation with horseradish peroxidase (HRP)-conjugated
secondary antibody. The proteins were visualized using an ECL
detection kit purchased from Beyotime Institute of Biotechnology
(Jiangsu, China). Rabbit anti-human β3GnT8 affinity pAb was
purified in our laboratory (18).
GADPH antibody was purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA).
Lectin blot analysis
SDS-PAGE and electrophoretic transferring were
performed in the same manner as described for western blot
analysis. After blocking with Carbo-Free Blocking Solution (Vector
Labs, Burlingame, CA, USA), the membranes were incubated with 2
μg/ml of biotinylated Lycopersicon esculentum agglutinin
(LEA) (Sigma) for 1 h. Reactive bands were detected with a diluted
HRP-conjugated streptavidin (Sigma), and then visualized using ECL
system (GE Healthcare, Pittsburgh, PA, USA).
Analysis of lectin labeling by flow
cytometry
Cells were collected and washed three times with
PBS. The cell density was adjusted to 2×106/ml, stained
with 10 μg/ml FITC-LEA (Sigma) in PBS (contain 0.5% BSA and 0.05%
sodium azide) at 4°C for 1 h, then washed three times with PBS. The
fluorescence intensity of the stained cells was measured with a
FACScan flow cytometer (Becton-Dickinson, Mountain View, CA, USA)
and analyzed with CellQuest.
Apoptosis analysis
Cells were harvested and fixed in cold 80% ethanol
overnight at 4°C and double stained with Annexin V-FITC and PI
(both from Sigma) for 30 min at room temperature in dark. Stained
cells were passed through a nylon-mesh sieve to remove cell clumps.
Apoptotic cells were detected using flow cytometry within 1 h
(Becton-Dickinson). Cells in the lower right quadrant represented
early apoptosis and in the upper right quadrant represented late
apoptotic cells.
Cell cycle analysis
A certain number of cells were trypsinized and fixed
with 80% ethanol at 4°C overnight prior to being stained with PI
using freshly prepared staining solution. The distribution of cells
in the different phases of the cell cycle was measured by flow
cytomety. The percentage of cells in G1 phase, S phase, and G2/M
phase was analyzed using standard ModiFit and CellQuest software
programs.
Statistical analysis
All values are expressed as mean ± SD from
triplicate experiments. Independent t-test was performed for
comparison of data from independent samples. P<0.05 was
considered significant.
Results
Generation of a 5-FU-resistant cell
line
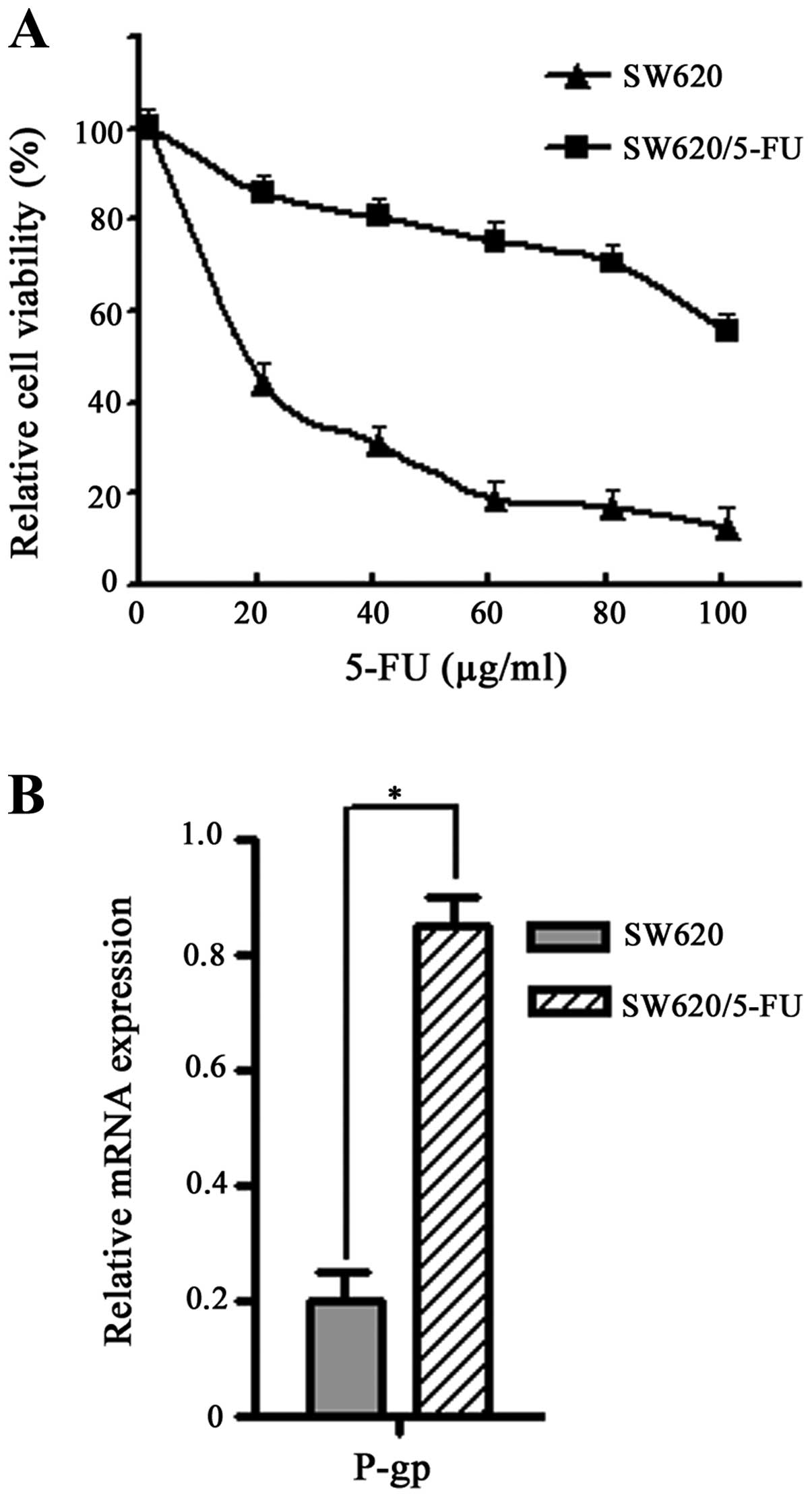
The 5-FU-resistant cell line SW620/5-FU was
established from its parent cell line (SW620) by exposure to 5-FU
in stepwise increase concentrations from 10 to 100μg/ml over a
period of 3 months. Then cells were exposed to various
concentrations of 5-FU for 48 h and cell viability was measured by
MTT assay. We found SW620/5-FU cells were ~10-fold more resistant
to 5-FU as compared with the parental cells (IC50, 128 vs. 13
μg/ml) (Fig. 2A).
P-glycoprotein (P-gp) is a plasma membrane
glycoprotein often involved in the resistance of cancer cells
towards multiple anticancer agents (19). To further confirm the
5-FU-resistant phenotype, mRNA expression of P-gp in SW620/5-FU
cells was examined by quantitative RT-PCR. As shown in Fig. 2B, high level of P-gp mRNA was
detected in the SW620/5-FU cells. It confirmed that a
5-FU-resistant cell line was successfully constructed, and can be
used for the successive experiments.
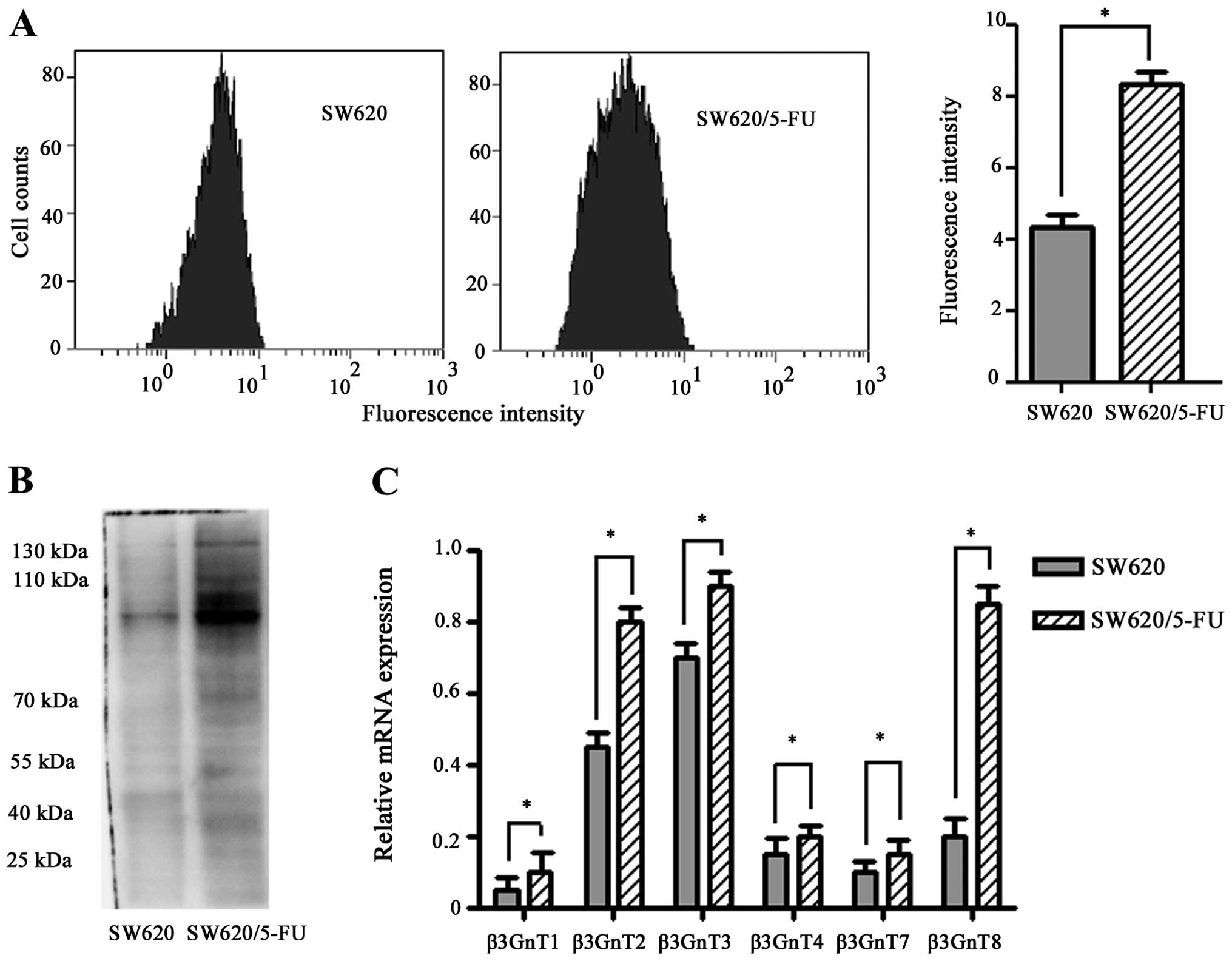
Increased expression of polylactosamine
chains and β3GnT8 in SW620/5-FU cells
Polylactosamine chains can be specifically
identified by LEA (20). To
determine the alteration of polylactosamine in SW620/5-FU cells,
each cell group was bound with LEA. As shown in Fig. 3A, the mean fluorescence intensities
of LEA-labeling cells of SW620/5-FU and SW620 were 8.38±0.15 and
4.25±0.19, respectively. Significant differences were seen between
the drug-sensitive and -resistance cells (P<0.05). Next, the
separated glycoproteins were transferred onto PVDF membranes.
Upregulation of polylactosamine chains was also observed in
SW620/5-FU cells as detected by lectin blot analysis (Fig. 3B).
β3Gn-T1, -T2, -T3, -T4, -T7, and -T8 have been shown
to possess the ability to synthesize polylactosamine chains
(20). To identify and evaluate
candidate genes involved in polylactosamine synthesis in SW620/5-FU
cells, quantitative RT-PCR analysis was performed. We found
β3Gn-T1, -T2, -T3, -T4, -T7, and -T8 were both highly expressed in
SW620/5-FU cells(P<0.05) (Fig.
3C). However, the change of β3GnT8 mRNA expression was more
obvious than other β3GnTs (i.e., >4-fold higher). These data
indicated that overexpression of β3GnT8 may be responsible for the
increased levels of polylactosamine chains in SW620/5-FU cells.
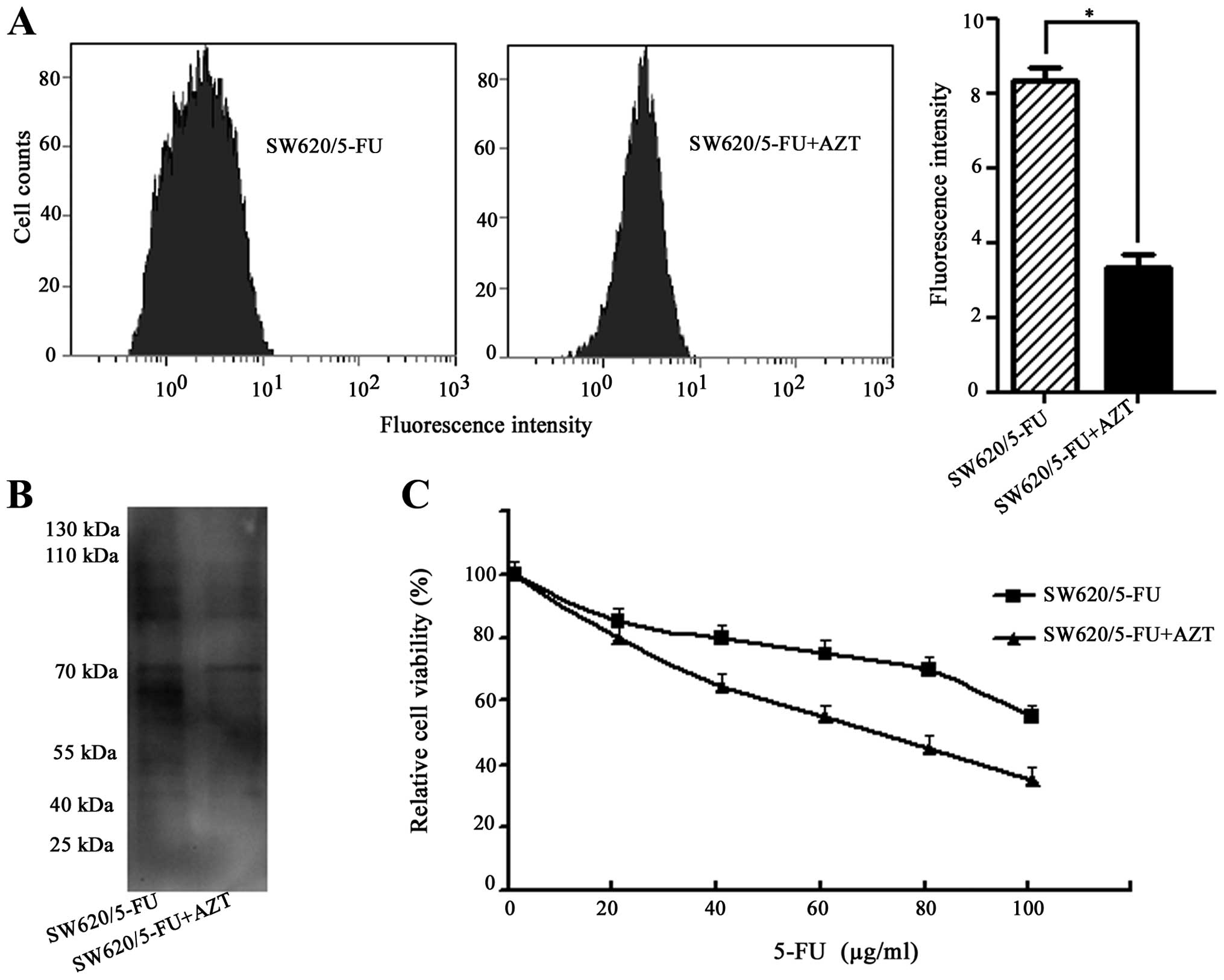
Effects of 3′-azidothymidine (AZT) on
chemosensitivity of SW620/5-FU cells
To explore whether polylactosamine was associated
with 5-FU resistance, AZT was used. AZT is a thymidine analogue
that is able to inhibit the synthesis of polylactosamine (21). SW620/5-FU and its parent cells
showed similar sensitivity to AZT (Sigma). From both cell lines
>90% of cells were killed by treatment with 120 μM of AZT,
whereas 80% of cells survived at 20 μM AZT (data not shown).
Therefore, we applied 5 μM AZT, at which concentration no
cytotoxicity was observed. As shown in Fig. 4A and B, AZT treatment resulted in
decreased polylactosamine in SW620/5-FU cells as determined by flow
cytometry and lectin blot analysis. Then SW620/5-FU cells were
pre-treated with AZT for 24 h before exposed to various
concentrations of 5-FU for 48 h. MTT assay showed that
pre-treatment with AZT reduced the IC50 value against 5-FU of the
resistant cells (72 vs. 128 μg/ml) (Fig. 4C). These results further confirmed
that the inhibition of polylactosamine was able to reverse 5-FU
resistance.
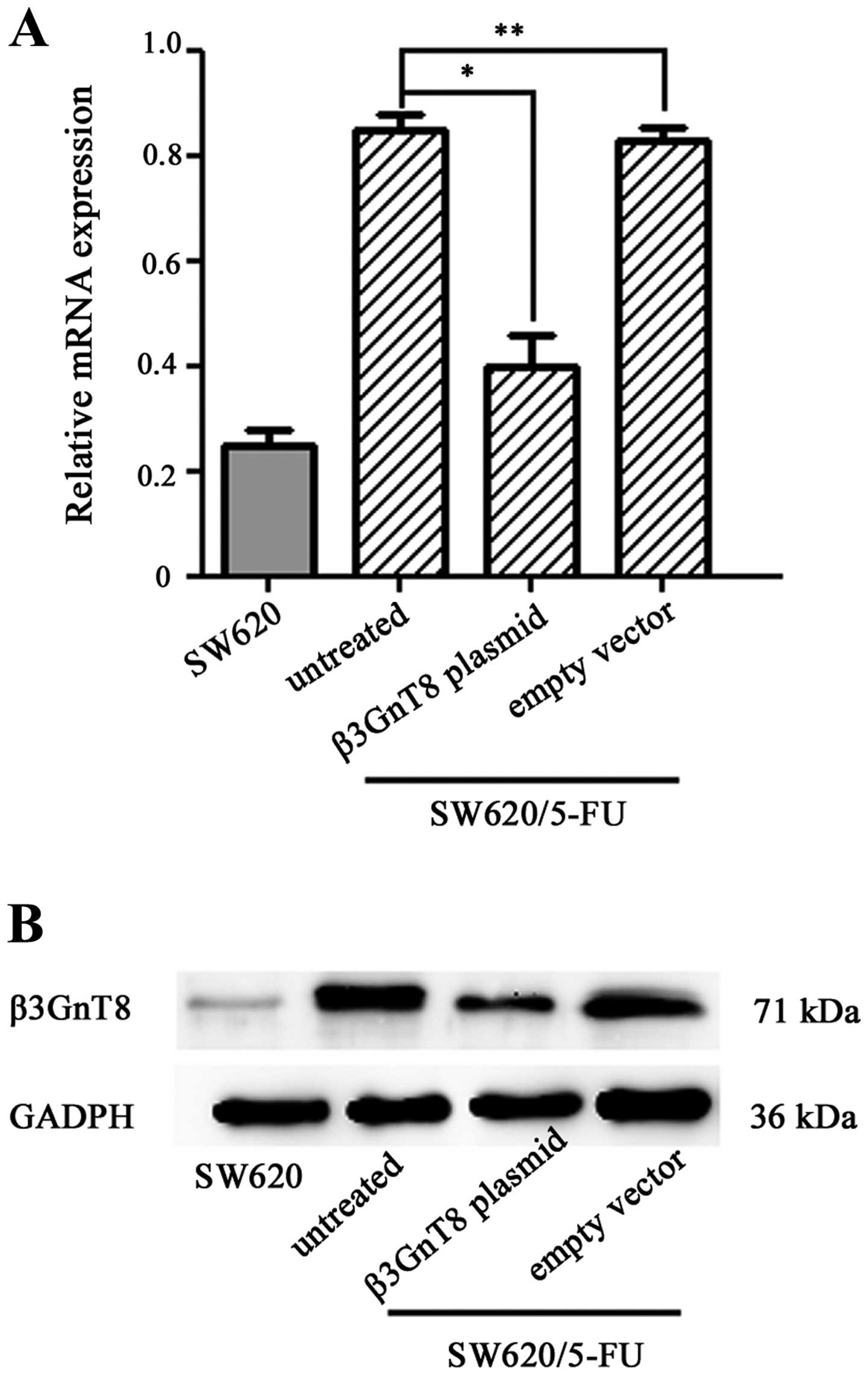
Knockdown of β3GnT8 inhibits the
formation of polylactosamine in SW620/5-FU cells
To investigate the potential activity of elevated
expression of β3GnT8 in SW620/5-FU cells, pSilenCircle-Si-β3GnT8
plasmid targeting β3GnT8 was used to transfect SW620/5-FU cells.
Then β3GnT8 mRNA and protein expression was detected, respectively,
by quantitative RT-PCR and western blot analysis. As shown in
Fig. 5A and B, β3GnT8 expression
was significantly inhibited (P<0.05) in the SW620/5-FU cells
transfected with pSilenCircle-Si-β3GnT8 plasmid, whereas no
significant inhibitory effect was observed in the untreated cells
and cells treated with empty vector (P>0.05).
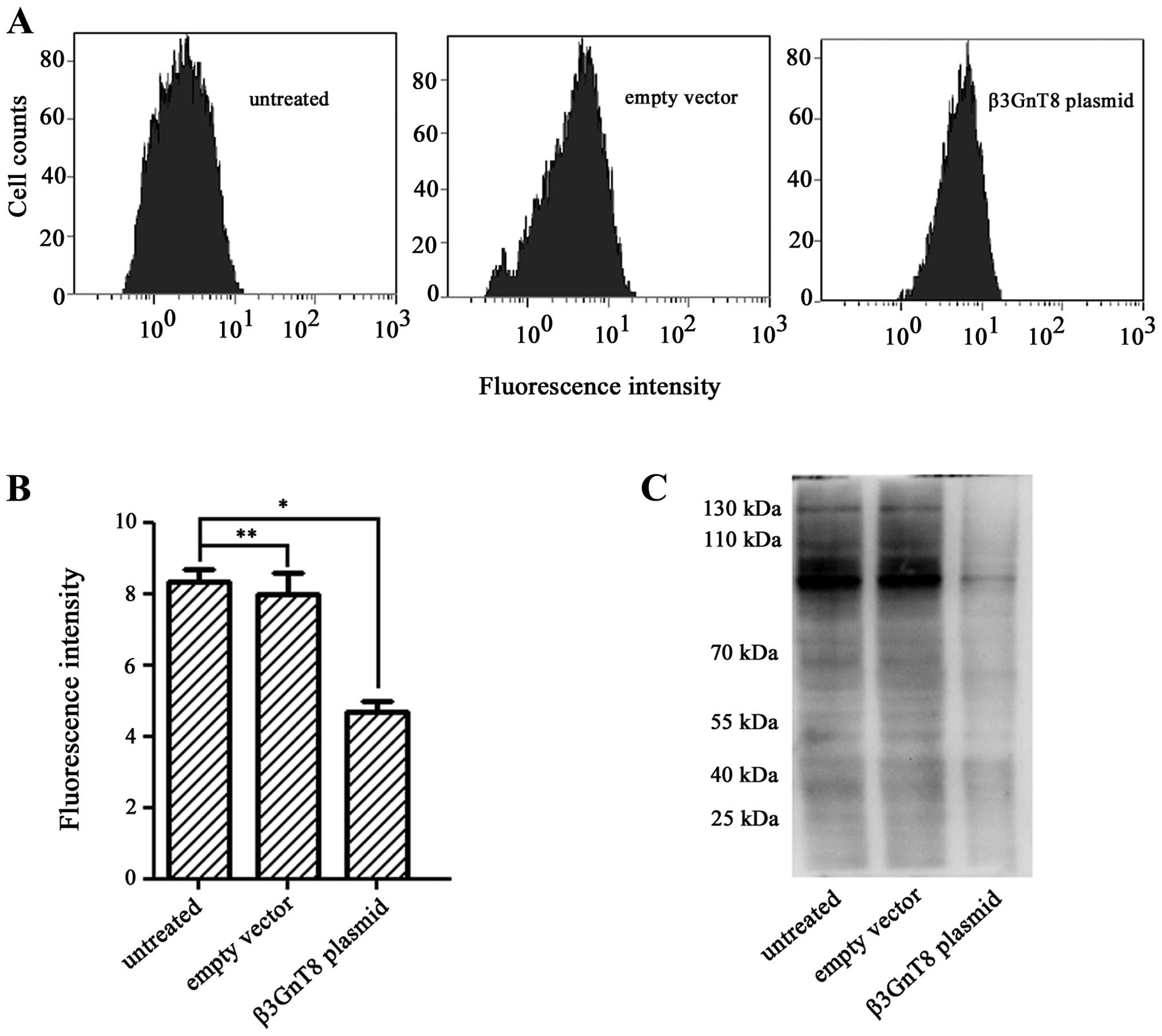
To evaluate whether β3GnT8 knockdown could modify
polylactosamine-type N-glycans, each cell group was also bound to
FITC-LEA. Fig. 6A and B showed
that β3GnT8 knockdown resulted in a decrease of fluorescence
intensity compared with untreated cells and cells treated with
empty vector (P<0.05). In addition, the result of lectin blot
analysis revealed that knockdown of β3GnT8 by
pSilenCircle-Si-β3GnT8 plasmid led to the downregulation of
polylactosamine levels in SW620/5-FU cells (Fig. 6C). These results clearly proved
that β3GnT8 contributes to development of 5-FU resistance in CRC
cells via regulating the N-glycosylation profile in terms of
polylactosamine chains.
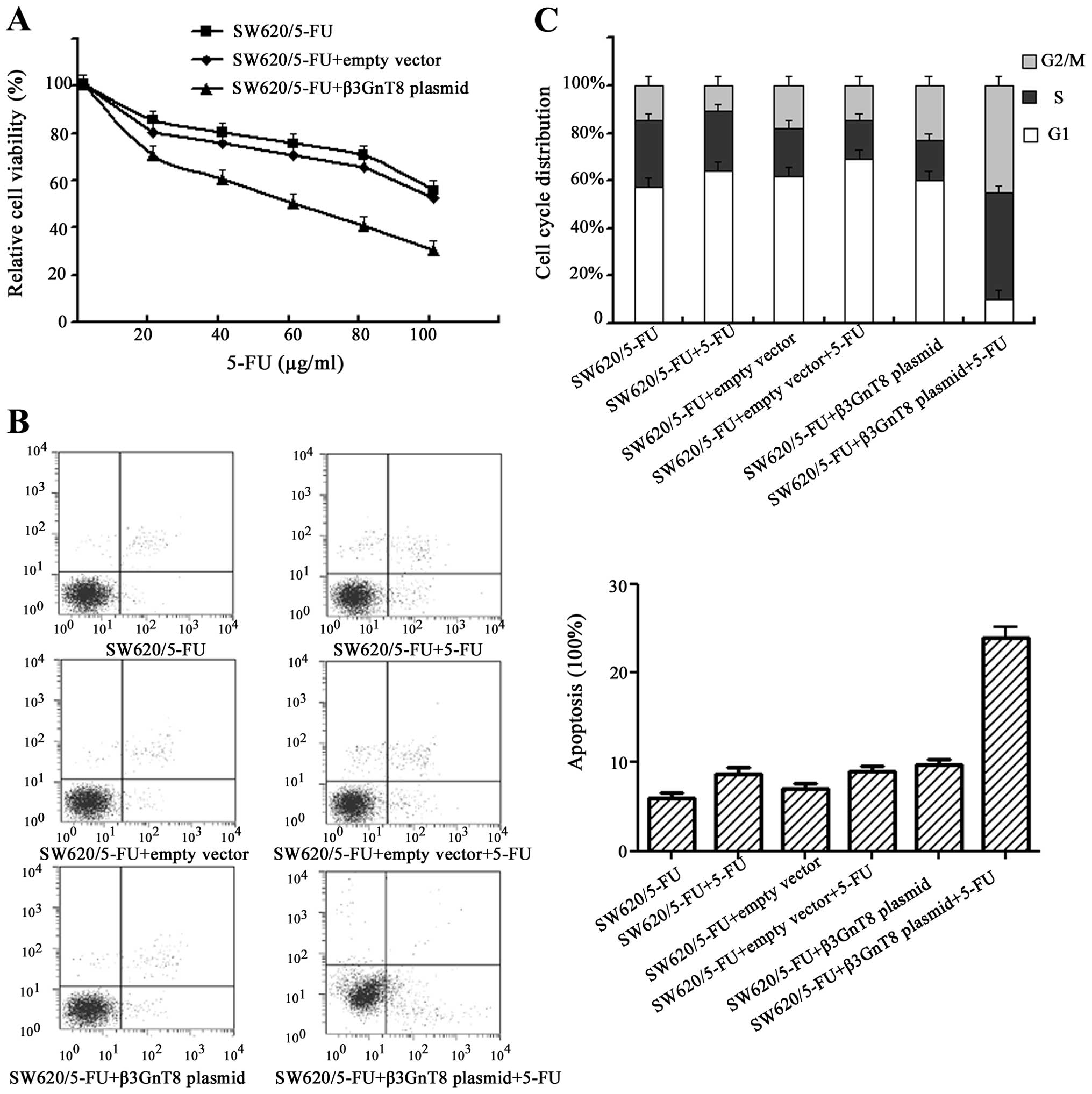
Reversal of 5-FU resistance by knockdown
of β3GnT8
Having demonstrated that β3GnT8 overexpression may
participate in the resistance to 5-FU, we sought to determine
whether decreased β3GnT8 expression would render CRC cells more
sensitive to 5-FU. Then SW620/5-FU cells were transiently
transfected with pSilenCircle-Si-β3GnT8 plasmid or empty vector,
followed 24 h later with the 5-FU treatment for a further 48 h,
before cell viability was measured using the MTT assay. As shown in
Fig. 7A, IC50 value of 5-FU in
SW620/5-FU cells was significantly (P<0.05) reduced while β3GnT8
expression was inhibited, suggesting that inhibition of β3GnT8 may
increase drug sensitivity.
Flow cytometry assays were further performed to
evaluate potential effects of β3GnT8 knockdown on the apoptosis and
cell cycle arrest. Cells were exposed to 128 μg/ml 5-FU for 48 h.
As shown in Fig. 7B, no
significant difference in the proportion of apoptotic cell was
observed between the untreated cells and cells treated with empty
vector when exposed to 5-FU (P>0.05). However, notable apoptosis
was found in SW620/5-FU cells exposed to 5-FU, when β3GnT8 was
knocked down (P<0.05). In addition, knockdown of β3GnT8 alone
could also induce a slight increase in apoptosis in SW620/5-FU
cells. Furthermore, no significant difference in the cycle
distribution was observed between the untreated cells and cells
treated with empty vector when exposed to 5-FU (P>0.05)
(Fig. 7C). On the other hand, when
β3GnT8 expression was inhibited, significant reduction in cells in
G1 phase and the accumulation of cells in S and G2/M phases was
observed in SW620/5-FU cells exposed to 5-FU (P<0.05).
Discussion
Long-term chemotherapy unavoidably leads to drug
resistance and this has become a major challenge to the triumph of
chemotherapy. Over the past 50 years, despite its many advantages,
clinical applications of 5-FU have been greatly limited due to drug
resistance. The overall response rate for advanced CRC of 5-FU
alone is still only 10–15% and the combination of 5-FU with other
anti-tumor drugs has merely improved the response rates to 40–50%
(4). Although previous studies
have reported various mechanisms of 5-FU resistance, there are many
‘unknowns’ that need further clarification. In the present study,
SW620/5-FU cells were generated by exposure to a gradually
increasing 5-FU concentration. Higher IC50 value against 5-FU of
the resistant cells were found compared to its parental cells.
Drug-resistant biomarker such as P-gp was also highly expressed.
Such resistance was stable upon the removal of 5-FU and was
maintained for a considerable period of time. Thus, it can be
considered that SW620/5-FU cells are a useful model for the
investigation of 5-FU resistance in CRC.
Cell surface glycans are a class of sophisticated
biomolecules related to cancer development and progression, and
their analysis is of great significance for early cancer diagnosis
and treatment. Colon cancer cells frequently express glycans at
different levels or with fundamentally different structures than
those observed on normal cells. For example, aberrant O-glycans,
such as Galβ1, 3GalNAc (T antigen), were commonly found in colon
cancer (22). Structures
containing a bisecting GlcNAc were found to be decreased in the
colorectal tumor, whereas sulfated glycans, paucimannosidic
glycans, and glycans containing a sialylated Lewis type epitope
were shown to be increased in tumor tissues (23). Rencently, aberrant changes in
N-glycans have been shown to be associated with drug resistance
(9,24). Swainsonine, an inhibitor of
N-glycan biosynthesis, could reduce 5-FU tolerance in the
multistage resistance of CRC cells (12). Therefore, monitoring of the
N-glycan profile in CRC would be an important step in the
prevention of side-effects and would increase our understanding of
5-FU resistance mechanisms. It is well known that lectins are
carbohydrate-binding proteins or glycoproteins of non-immune origin
that recognize and reversibly bind to glycans without altering
their covalent structure. LEA lectin, obtained from Lycopersicum
esculentum, has specific affinity for polylactosamine sugar
residues (25). In this study, the
alteration of polylactosamine in SW620/5-FU cells was detected by
flow cytometry and lectin blot analysis assays. The flow cytometry
analysis is an effective approach to measure the linkage of
FITC-lectin to cell surface carbohydrate not only qualitatively but
also quantitatively (26). This
study clearly showed that LEA signal was significantly upregulated
in SW620/5-FU cells. It suggested that polylactosamine chains were
associated with 5-FU resistance in cancer cells.
Polylactosamine is a fundamental structure of
glycans carried on N- and O-glycans (27). Polylactosamine preferentially adds
to β1–6GlcNAc linked antennae attached to the trimannosyl core of
complex-type N-glycans. There are a number of reports regarding the
functions and distributions of polylactosamine-type N-glycans. For
example, some cancer cells such as U937 (human T-lymphoma) and
MKN45 (human gastric cancer) cells specifically express
polylactosamine-type N-glycans and such glycans were often modified
with fucose and sulfate residues (28). Togayachi et al have reported
that polylactosamine on N-glycans was a putative immune regulatory
factor presumably suppressing excessive responses during immune
reactions (27). Common
glycoproteins expressing polylactosamine-type N-glycans on matched
patient primary and metastatic melanoma cells always showed
different glycan profiles (29).
In a study on CRC cell lines, highly metastatic cell lines were
found to synthesize more N-glycans that contain polylactosamine
than poorly metastatic cell lines (30). A highly fucosylated polylactosamine
type N-glycan was also expressed on CRC SW1116 cells (31). Therefore, it is interested to
clarify the relationship between polylactosamine-type N-glycans and
5-FU resistance in SW620/5-FU cells. AZT was the first approved
antiviral for the treatment of human immunodeficiency virus
(32). It has been reported that
AZT could inhibit the biosynthesis of highly branched N-glycans and
polylactosamine chains in melanoma cells (21). Synergistic antitumor effect of AZT
in combination with 5-FU in human CRC cell lines was also observed
(33). Here, we found AZT
pre-treatment resulted in a reduction in the amount of
polylactosamine chains, although the mechanism by which this occurs
is not yet clear. We also found that the inhibition of
polylactosamine by AZT was able to reverse 5-FU resistance in
SW620/5-FU cells. To the best of our knowledge, this study is the
first revealing the expression patterns of polylactosamine-type
N-glycans in 5-FU-resistant cancer cells and the correlation with
reversal of resistance. Furthermore, we made some effort to explore
the possible mechanisms, our preliminary results are promising.
Aberrant glycosylation is associated with
differential expression of enzymes such as glycosyltransferase and
glycosidases (34). The aberrant
expressions of the enzymes in turn cause cancer cells to produce
glycoproteins with specific cancer-associated aberrations in glycan
structures. Eight members in the β3GnT family (β3GnT1-T8) have been
identified thus far, and their activities have been characterized.
Several of the enzymes, β3Gn-T1, -T2, -T3, -T4, -T7 and -T8, have
been shown to mediate polylactosamine synthesis (20). It is worth noting that β3GnT2
showed the strongest activity for polylactosamine synthesis in
initial in vitro experiments (35). By contrast, β3GnT3 and β3GnT4 were
found to have very weak polylactosamine synthase activity (15). β3GnT8, which has been cloned by our
and another groups, is the most recently identified enzyme among
the β3GnTs (16,36). It has been reported that β3GnT2 and
β3GnT8 can form a complex with enhanced enzymatic activity
(37). However, the presence of
β3GnT8 can stimulate the activity of β3GnT2. Overexpression of
β3GnT8, but not β3GnT2, may induce an increase in
polylactosamine-type N-glycans in malignant tumor cells (20). Herein, as an alternative strategy,
we hypothesized that β3GnT8 is responsible for the synthesis of
polylactosamine-type N-glycans in SW620/5-FU cells. We found
β3Gn-T1, -T2, -T3, -T4, -T7, and -T8 were both highly expressed in
SW620/5-FU cells. As expected, our results demonstrated the change
of β3GnT8 mRNA expression was more obvious than other β3GnTs. Based
on the findings of our study and other reports, we thought
overexpression of β3GnT8 should contribute to development of drug
resistance in cancer cells, and knockdown of β3GnT8 may restore the
sensitivity to anticancer agents.
To investigate the correlation between β3GnT8 and
5-FU resistance in SW620/5-FU cells, the expression of β3GnT8 was
downregulated by pSilenCircle-Si-β3GnT8 plasmid. We found β3GnT8
knockdown led to the downregulation of polylactosamine levels in
SW620/5-FU cells. In addition, IC50 value of 5-FU in SW620/5-FU
cells was significantly reduced while β3GnT8 expression was
inhibited. When β3GnT8 was knocked down by RNA interference, we
also observed inhibition of cell proliferation and increase in
apoptosis in cells with exposure to 5-FU, indicating a reversal of
5-FU resistance by β3GnT8 knockdown. Although the reports focusing
on β3GnT8 and drug resistance remain limited and preliminary, some
correlations between overexpression of β3GnT8 and drug resistance
(9,11) encouraged us to presume that β3GnT8
should be involved in the development of drug resistance.
In conclusion, we confirmed that β3GnT8 expression
was upregulated in 5-FU-resistant cancer cells and that the
knockdown of β3GnT8 reversed the 5-FU resistance through, at least
partly, suppression the biosynthesis of polylactosamine-type
N-glycans. Thus, β3GnT8 is a potential molecular target to overcome
anticancer drug resistance in CRC. Whether or not there are other
signal transduction pathways involved, and the elucidation of the
underlying mechanisms are warranted.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (31170772), the Research and
Innovation Project for College Graduates of Jiangsu Province
(CXZZ13_0827), and Health Department of Hubei Province
(JX3A20).
References
|
1
|
Sarfaty M, Doroshenk M, Hotz J, et al:
Strategies for expanding colorectal cancer screening at community
health centers. CA Cancer J Clin. 63:221–231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang B, Zhang B, Chen X, Bae S, Singh K,
Washington MK and Datta PK: Loss of Smad4 in colorectal cancer
induces resistance to 5-fluorouracil through activating Akt
pathway. Br J Cancer. 110:946–957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He Z, Gao J, Wang Q, et al: S100P
contributes to chemosensitivity of human ovarian cancer cell line
OVCAR3. Oncol Rep. 20:325–332. 2008.PubMed/NCBI
|
|
4
|
Zhang N, Yin Y, Xu SJ and Chen WS:
5-Fluorouracil: mechanisms of resistance and reversal strategies.
Molecules. 13:1551–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Phua LC, Mal M, Koh PK, Cheah PY, Chan EC
and Ho HK: Investigating the role of nucleoside transporters in the
resistance of colorectal cancer to 5-fluorouracil therapy. Cancer
Chemother Pharmacol. 71:817–823. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Manoochehri M, Karbasi A, Bandehpour M and
Kazemi B: Down-regulation of BAX gene during carcinogenesis and
acquisition of resistance to 5-FU in colorectal cancer. Pathol
Oncol Res. Oct 14–2013.(Epub ahead of print).
|
|
7
|
Anugraham M, Jacob F, Nixdorf S,
Everest-Dass AV, Heinzelmann-Schwarz V and Packer NH: Specific
glycosylation of membrane proteins in epithelial ovarian cancer
cell lines: glycan structures reflect gene expression and DNA
methylation status. Mol Cell Proteomics. May 22–2014.(Epub ahead of
print).
|
|
8
|
Lau KS and Dennis JW: N-Glycans in cancer
progression. Glycobiology. 18:750–760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Z, Zhao Y, Jiang L, Miao X, Zhou H
and Jia L: Glycomic alterations are associated with multidrug
resistance in human leukemia. Int J Biochem Cell Biol.
44:1244–1253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beretta GL, Benedetti V, Cossa G, et al:
Increased levels and defective glycosylation of MRPs in ovarian
carcinoma cells resistant to oxaliplatin. Biochem Pharmacol.
79:1108–1117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma H, Miao X, Ma Q, Zheng W, Zhou H and
Jia L: Functional roles of glycogene and N-glycan in multidrug
resistance of human breast cancer cells. IUBMB Life. 65:409–422.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hamaguchi J, Nakagawa H, Takahashi M, et
al: Swainsonine reduces 5-fluorouracil tolerance in the multistage
resistance of colorectal cancer cell lines. Mol Cancer. 6:582007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee PL, Kohler JJ and Pfeffer SR:
Association of beta-1,3-N-acetylglucosaminyltransferase 1 and
beta-1,4-galactosyltransferase 1, trans-Golgi enzymes involved in
coupled poly-N-acetyllactosamine synthesis. Glycobiology.
19:655–664. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Srinivasan N, Bane SM, Ahire SD, Ingle AD
and Kalraiya RD: Poly N-acetyllactosamine substitutions on N- and
not O-oligosaccharides or Thomsen-Friedenreich antigen facilitate
lung specific metastasis of melanoma cells via galectin-3.
Glycoconj J. 26:445–456. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Narimatsu H: Human glycogene cloning:
focus on beta 3-glycosyltransferase and beta 4-glycosyltransferase
families. Curr Opin Struct Biol. 16:567–575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishida H, Togayachi A, Sakai T, et al: A
novel beta1,3-N-acetylglucosaminyltransferase (beta3Gn-T8), which
synthesizes poly-N-acetyllactosamine, is dramatically upregulated
in colon cancer. FEBS Lett. 579:71–78. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hua D, Qin F, Shen L, et al: β3GnT8
regulates laryngeal carcinoma cell proliferation via targeting
MMPs/TIMPs and TGF-β1. Asian Pac J Cancer Prev. 13:2087–2093.
2012.
|
|
18
|
Jiang Z, Ge Y, Zhou J, Xu L and Wu SL:
Subcellular localization and tumor distribution of human
beta3-galactosyltransferase by beta3GalT7 antiserum. Hybridoma
(Larchmt). 29:141–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen K, Cui D, Sun L, Lu Y, Han M and Liu
J: Inhibition of IGF-IR increases chemosensitivity in human
colorectal cancer cells through MRP-2 promoter suppression. J Cell
Biochem. 113:2086–2097. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seko A and Yamashita K: Activation of
beta1,3-N-acetylglucosaminyltransferase-2 (beta3Gn-T2) by
beta3Gn-T8. Possible involvement of beta3Gn-T8 in increasing
poly-N-acetyllactosamine chains in differentiated HL-60 cells. J
Biol Chem. 283:33094–33100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Steet RA, Melancon P and Kuchta RD:
3′-Azidothymidine potently inhibits the biosynthesis of highly
branched N-linked oligosaccharides and poly-N-acetyllactosamine
chains in cells. J Biol Chem. 275:26812–26820. 2000.
|
|
22
|
Hung JS, Huang J, Lin YC, et al: C1GALT1
overexpression promotes the invasive behavior of colon cancer cells
through modifying O-glycosylation of FGFR2. Oncotarget.
5:2096–2106. 2014.PubMed/NCBI
|
|
23
|
Balog CI, Stavenhagen K, Fung WL, et al:
N-glycosylation of colorectal cancer tissues: a liquid
chromatography and mass spectrometry-based investigation. Mol Cell
Proteomics. 11:571–585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kudo T, Nakagawa H, Takahashi M, et al:
N-glycan alterations are associated with drug resistance in human
hepatocellular carcinoma. Mol Cancer. 6:322007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Villacampa N, Almolda B, González B and
Castellano B: Tomato lectin histochemistry for microglial
visualization. Methods Mol Biol. 1041:261–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Elloway EA, Armstrong RA, Bird RA, Kelly
SL and Smith SN: Analysis of Acanthamoeba polyphaga surface
carbohydrate exposure by FITC-lectin binding and fluorescence
evaluation. J Appl Microbiol. 97:1319–1325. 2004.
|
|
27
|
Togayachi A, Kozono Y, Ishida H, et al:
Polylactosamine on glycoproteins influences basal levels of
lymphocyte and macrophage activation. Proc Natl Acad Sci USA.
104:15829–15834. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mitsui Y, Yamada K, Hara S, Kinoshita M,
Hayakawa T and Kakehi K: Comparative studies on glycoproteins
expressing polylactosamine-type N-glycans in cancer cells. J Pharm
Biomed Anal. 70:718–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kinoshita M, Mitsui Y, Kakoi N, Yamada K,
Hayakawa T and Kakehi K: Common glycoproteins expressing
polylactosamine-type glycans on matched patient primary and
metastatic melanoma cells show different glycan profiles. J
Proteome Res. 13:1021–1033. 2014. View Article : Google Scholar
|
|
30
|
Ni J, Jiang Z, Shen L, et al: β3GnT8
regulates the metastatic potential of colorectal carcinoma cells by
altering the glycosylation of CD147. Oncol Rep. 31:1795–1801.
2014.
|
|
31
|
Terada M, Khoo KH, Inoue R, et al:
Characterization of oligosaccharide ligands expressed on SW1116
cells recognized by mannan-binding protein. A highly fucosylated
polylactosamine type N-glycan. J Biol Chem. 280:10897–10913. 2005.
View Article : Google Scholar
|
|
32
|
Sirivolu VR, Vernekar SK, Ilina T,
Myshakina NS, Parniak MA and Wang Z: Clicking 3′-azidothymidine
into novel potent inhibitors of human immunodeficiency virus. J Med
Chem. 56:8765–8780. 2013.
|
|
33
|
Andreuccetti M, Allegrini G, Antonuzzo A,
et al: Azidothymidine in combination with 5-fluorouracil in human
colorectal cell lines: in vitro synergistic cytotoxicity and
DNA-induced strand-breaks. Eur J Cancer. 32A:1219–1226. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meany DL and Chan DW: Aberrant
glycosylation associated with enzymes as cancer biomarkers. Clin
Proteomics. 8:72011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shiraishi N, Natsume A, Togayachi A, et
al: Identification and characterization of three novel
β1,3-N-acetylglucosaminyltransferases structurally related to the
β1,3-galactosyltransferase family. J Biol Chem. 276:3498–3507.
2001.
|
|
36
|
Huang C, Zhou J, Wu S, Shan Y, Teng S and
Yu L: Cloning and tissue distribution of the human B3GALT7 gene, a
member of the beta1,3-Glycosyltransferase family. Glycoconj J.
21:267–273. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Seko A and Yamashita K: Characterization
of a novel galactose beta1,3-N-acetylglucosaminyltransferase
(beta3Gn-T8): the complex formation of beta3Gn-T2 and beta3Gn-T8
enhances enzymatic activity. Glycobiology. 15:943–951. 2005.
View Article : Google Scholar : PubMed/NCBI
|