Introduction
Breast cancer is the most common female cancer in
the world and bone metastasis occurs in up to 70–80% of patients
with advanced breast cancer (1,2).
Bone metastasis of breast cancer typically leads to osteolysis and
results in bone destruction and skeletal-related events, including
severe bone pain, pathologic fractures, spinal cord and nerve
compression syndromes, and life-threatening hypercalcemia, which
are a common cause of morbidity and mortality (3). Current treatments for bone metastasis
have limited efficacy and are only palliative (4). Side-effects like renal toxicity and
osteonecrosis of the jaw are considered clinically significant,
potentially painful, and debilitating enough to decrease life
quality of breast cancer patients (2,5,6).
Thus, it is important that therapeutic strategies are developed for
the prevention and treatment of bone metastasis in breast
cancer.
The spread of breast cancer to the bone begins with
the initiation of bone demolition, the entry of malignant cells in
the bone marrow, and subsequently, an increase in osteolysis
(7). Bone metastasis is
characterized by the complicated interaction between cancer cells
and bone micro-environment (i.e., osteoclasts, osteoblasts and
stroma cells) (7). Osteolytic bone
metastasis is driven by the ‘vicious cycle’ of cancer-mediated
upregulation of osteoclasts and bone stroma-induced cancer
progression (8). Osteoclasts are
large, multi-nucleated cells derived from hematopoietic precursor
cells of the monocyte/ macrophage lineage (9). Their differentiation is mediated by
two cytokines, including macrophage colony stimulating factor
(M-CSF) and receptor activator of nuclear factor κB ligand (RANKL)
(10). RANKL is crucial for
osteoclast function by binding to its receptor RANK, while M-CSF
maintains the proliferation and survival of osteoclasts, and
upregulates RANK receptor expression in osteoclast precursor cells
(11).
In addition to osteoclasts, breast cancer cells can
also interact with osteoblasts to support osteoclast formation
(12). Breast cancer cells
increase the expressions of osteoclast differentiation factors,
M-CSF and RANKL, and decrease osteoprotegerin (OPG) level in
osteoblasts, leading to osteoclastogenesis (13–15).
Therefore, molecular targeting of both cancer-mediated
osteoclastogenesis or mending the imbalance of
osteoblast-osteoclast interaction can reduce bone destruction
(osteolytic bone metastasis).
Wedelolactone
(7-methoxy-5,11,12-trihydroxy-coumestan, Fig. 1A) (Sigma-Aldrich, St. Louis, MO,
USA) is a natural coumarin isolated from Wedelia chinensis
and Eclipta prostrata, which are traditionally used for hair
and skin health and for preventing liver damage due to alcohol
overdose (16–19). Previous studies show that WDL has
diverse pharmacologic effects such as anti-hepatotoxic,
anti-androgenic, and anti-human immunodeficiency activities
(19–21). Recent research has highlighted the
antitumor effects of WDL. Combinations of active compounds in W.
chinensis, including WDL, apigenin, and luteloin, have been
found to suppress the growth of prostate cancer cells in
vitro and in vivo (20,22).
Treatment with WDL inhibits the growth of pituitary adenoma cells
and mammary carcinosarcomas in vitro (23,24).
Moreover, WDL can induce caspase-dependent apoptosis in prostate
cancer cells via downregulation of PKCɛ (25).
This study demonstrates that WDL inhibits
MDA-MB-231-mediated osteoclastogenesis through the downregulation
of the Akt/mTOR signaling pathway. Moreover, WDL downregulates
M-CSF expression in MDA-CM-stimulated osteoblasts and inhibits
osteoblast-mediated osteoclastogenesis.
Materials and methods
Cell culture
Human breast cancer MDA-MB-231 cells were purchased
from the American Type Culture Collection (ATCC) (Manassas, VA,
USA). MDA-MB-231 cells were cultured in standard modified essential
medium (α-MEM) [α-MEM supplemented with 10% fetal calf serum (FCS),
penicillin, and streptomycin]. Human primary osteoblasts were
obtained from Lonza (Walkersville, MD, USA) and cultured in
osteoblast growth medium.
For conditioned medium (CM) collection, the
MDA-MB-231 cells were treated with vehicle (0.1% DMSO) or various
concentrations of WDL for 12 h. After washing, fresh α-MEM was
added and cultured for another 24 h. The supernatants were
collected, filtered (0.22 mm), and defined as MDA-CM or WDL-MDA-CM.
Osteoblasts were cultured with MDA-CM or WDL-MDA-CM (20%) for
another 24 h and the supernatants were then collected and filtered
(0.22 mm). The collected supernatants were grouped as osteoblast-CM
(OB-CM), MDA-OB-CM and WDL-MDA-OB-CM.
Osteoclast differentiation
Human peripheral blood, obtained from healthy adult
volunteers, was collected in syringes containing 1000 U/ml of
preservative-free heparin. The PBMCs were isolated by density
centrifugation using Ficoll-Hypaque and were re-suspended in RPMI
supplemented with 10% heat-inactivated fetal bovine serum. The
PBMCs were then plated and incubated overnight at 37°C.
CD14+ monocytes were isolated using CD14+
mAb-conjugated magnetic beads (MACS MicroBeads; Miltenyi Biotec),
according to the manufacturer’s protocol. Monocytes were grown in
culture medium containing vehicle or 200 ng/ml human M-CSF and 100
ng/ml human RANKL for 14–21 days. The medium was replaced every
five days. The Institutional Review Board of the Kaohsiung Medical
University Chung-Ho Memorial Hospital approved the study protocol
and all patients provided written informed consent in accordance
with the Declaration of Helsinki.
Osteoclast formation was measured by quantifying
cells positively stained by tartrate-resistant acid phosphatase
(TRAP) (Sigma-Aldrich). Briefly, cells were fixed by formaldehyde
for 30 min and then stained with naphthol AS-BI phosphate and a
tartrate solution for 1 h at 37°C, followed by counter-staining
with a hematoxylin solution. Osteoclasts were determined to be
TRAP-positive staining multi-nuclear (>3 nuclei) cells by light
microscopy. The total number of TRAP-positive cells and number of
nuclei per TRAP-positive cell in each well were counted.
Bone resorption assay
CD14+ monocytes were plated into a
calcium phosphate apatite-coated 48-well plate bone resorption
assay (Cosmo Bio Co., Ltd., Tokyo, Japan) in the same culture
conditions as described above. After a 14-day culture, each well
was washed with saline. A solution of 5% sodium hypochlorite was
left in the well for 5 min to detach the cells. The number of pits
in each well was counted under a microscope.
Immunoblotting
Cells were lysed on ice for 15 min by M-PER lysis
reagent (Thermo Fisher Scientific, Rockford, IL, USA). Cell lysate
was centrifuged at 14,000 × g for 15 min and the supernatant
fraction was collected for immunoblotting. Equivalent amounts of
protein were resolved by SDS-PAGE (6–8%) and transferred to
polyvinylidene difluoride membranes. After blocking for 1 h in 5%
non-fat dry milk in TBS, the membrane was incubated with the
desired primary Ab for 1–16 h. The membrane was then treated with
appropriate peroxidase-conjugated secondary Ab and the
immuno-reactive proteins were detected using an ECL kit
(Millipore), according to the manufacturer’s instructions.
Enzyme-linked immuno-sorbent assay
(ELISA)
The levels of M-CSF were assessed by M-CSF ELISA kit
(R&D Systems, Minneapolis, MN, USA). The RANKL and OPG levels
of osteoblast were quantified using the DuoSet ELISA kits (R&D
Systems).
Statistical analysis
Data were expressed as means ± SD. Statistical
comparisons were made using analysis of variance. Significant
differences (p<0.05) between two test groups were analyzed by
Student’s t-test.
Results
WDL exhibits direct inhibitory effect on
osteoclast differentiation and bone resorption activity
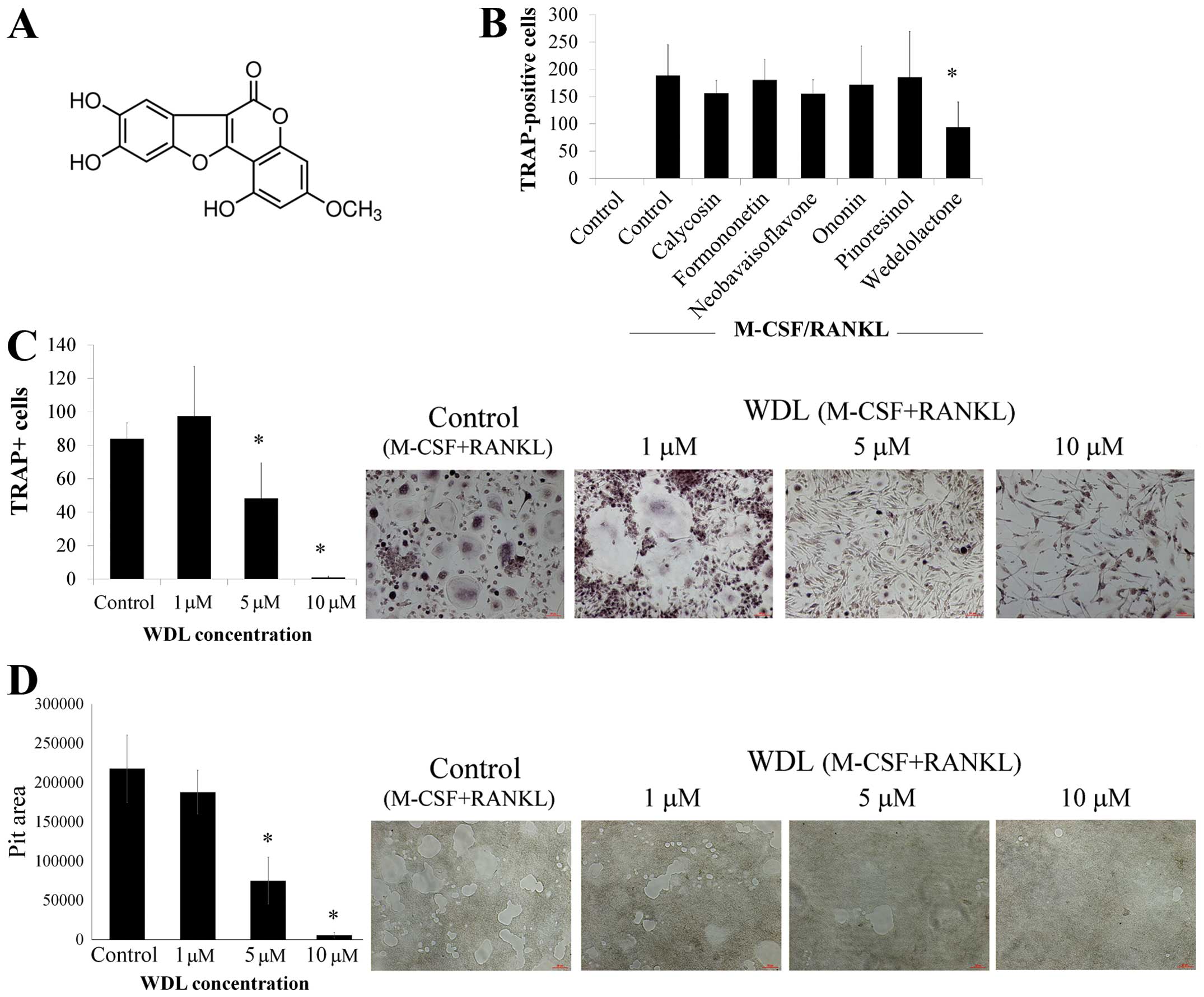
To investigate if novel agents can inhibit
osteoclasts, the effects of various phytoestrogens, including
calycosin, formononetin, neobavaisoflavone, ononin, pinoresinol,
and wedelolactone (WDL), on osteoclast differentiation were
assessed. The addition of RANKL and M-CSF caused the formation of
numerous TRAP-positive multi-nucleated osteoclasts (Fig. 1B). The differentiation of
osteoclasts was significantly inhibited by WDL but other agents
failed to affect osteoclastogenesis. Treatment of WDL decreased
osteoclastogenesis in a dose-dependent manner (Fig. 1C). Moreover, WDL treatment
substantially reduced osteoclastic bone resorption in a
dose-dependent manner (Fig. 1D).
These findings suggested that WDL inhibited osteoclast
differentiation and bone resorption activity in vitro.
WDL inhibited breast cancer-mediated
osteoclastogenesis
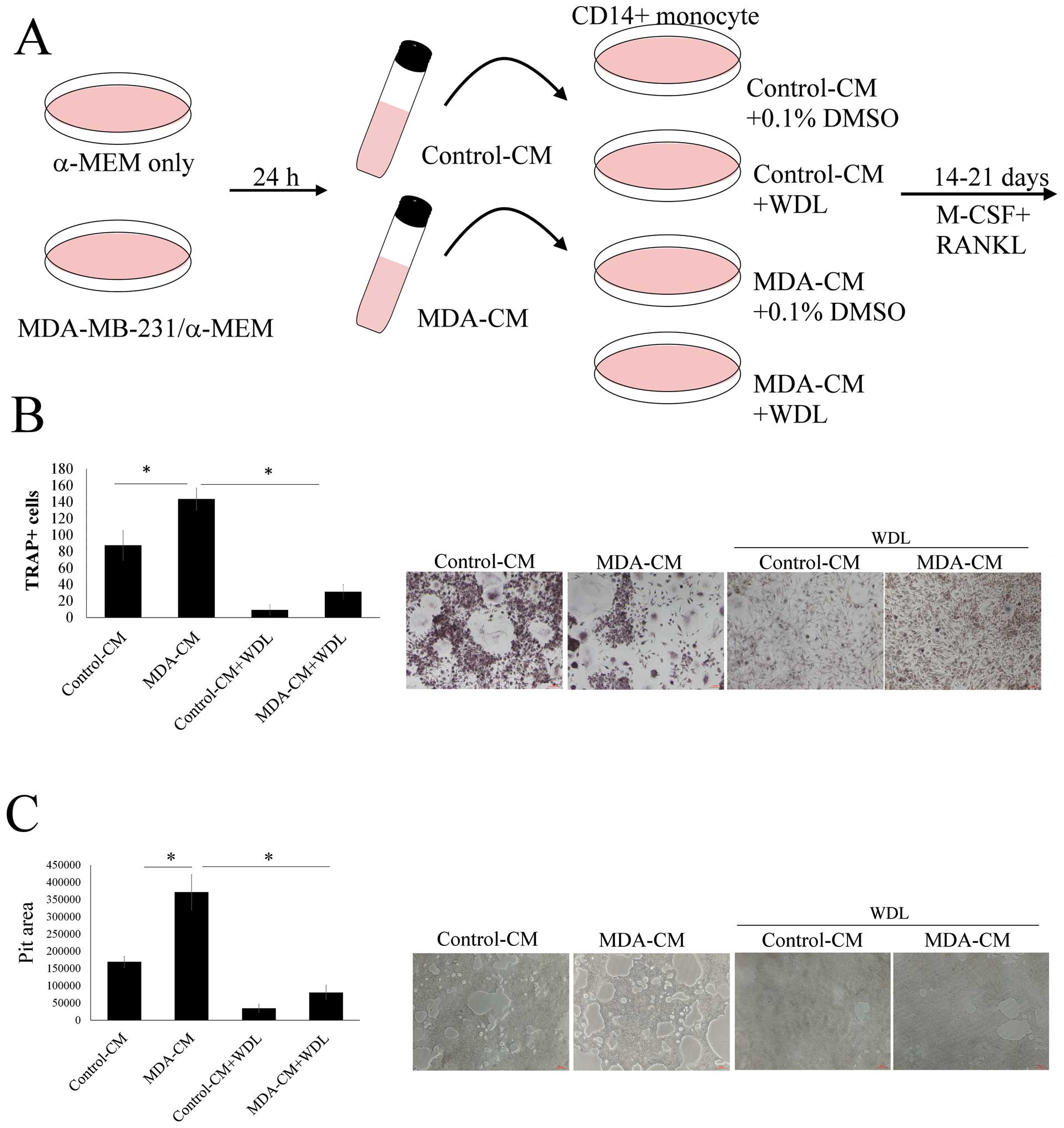
The effect of WDL on breast cancer-mediated
osteoclasto genesis was evaluated (Fig. 2A). MDA-MB-231-CM markedly increased
osteoclastogenesis (TRAP-positive multi-nucleated osteoclasts)
(Fig. 2B). This stimulatory effect
of breast cancer MDA-MB-231 cells was significantly decreased by
WDL. MDA-MB-231-CM also enhanced the bone resorption activity of
osteoclasts and this effect of breast cancer was also reduced by
WDL (Fig. 2C). These results
indicated that WDL inhibited MDA-MB-231 cell-mediated
osteoclastogenesis and osteoclast activity.
WDL decreased osteoclastogenesis by
inhibiting Akt/mTOR
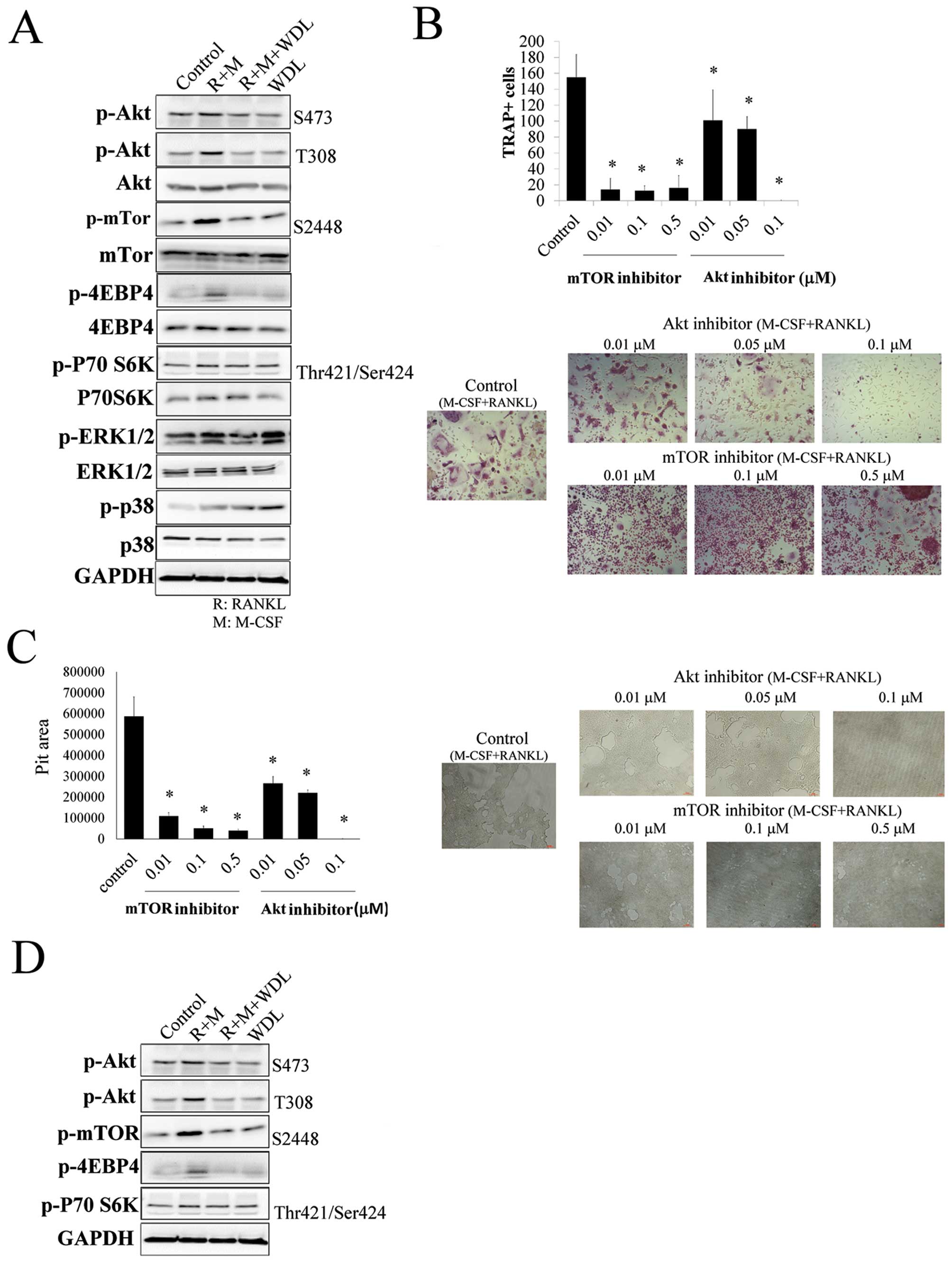
The molecular mechanism of WDL on osteoclastogenesis
was investigated. The addition of M-CSF/RANKL increased the
phosphorylation of Akt, mTOR, ERK1/2 and p38. The targets of mTOR,
p70S6K, and 4EBP4 were also activated by M-CSF/RANKL, showing that
Akt/mTOR signaling cascade was triggered in osteoclastogenesis. The
activation of osteoclast inducers (M-CSF and RANKL) on Akt/mTOR
signaling was inhibited by WDL (Fig.
3A).
The role of Akt/mTOR on osteoclastogenesis and bone
resorption was further supported by using both Akt (Akt inhibitor
IV) and mTOR inhibitors (rapamycin), which decreased
osteoclastogenesis and its activity (Fig. 3B and C). In addition, WDL not only
decreased M-CSF/RANKL-mediated Akt/mTOR activation, but also
reduced the reinforcing effect of breast cancer on this signaling
pathway (Fig. 3D). These results
revealed that WDL inhibited the Akt/mTOR signaling pathway, which
was critical in osteoclastogenesis induced by M-CSF/RANKL and
MDA-MB-231 cells.
WDL decreased the stimulatory effect of
breast cancer on M-CSF expression in osteoblasts
Osteoblasts are known to play a critical role on the
deterioration of bone metastasis by increasing M-CSF and RANKL, and
decreasing OPG expression (13–15).
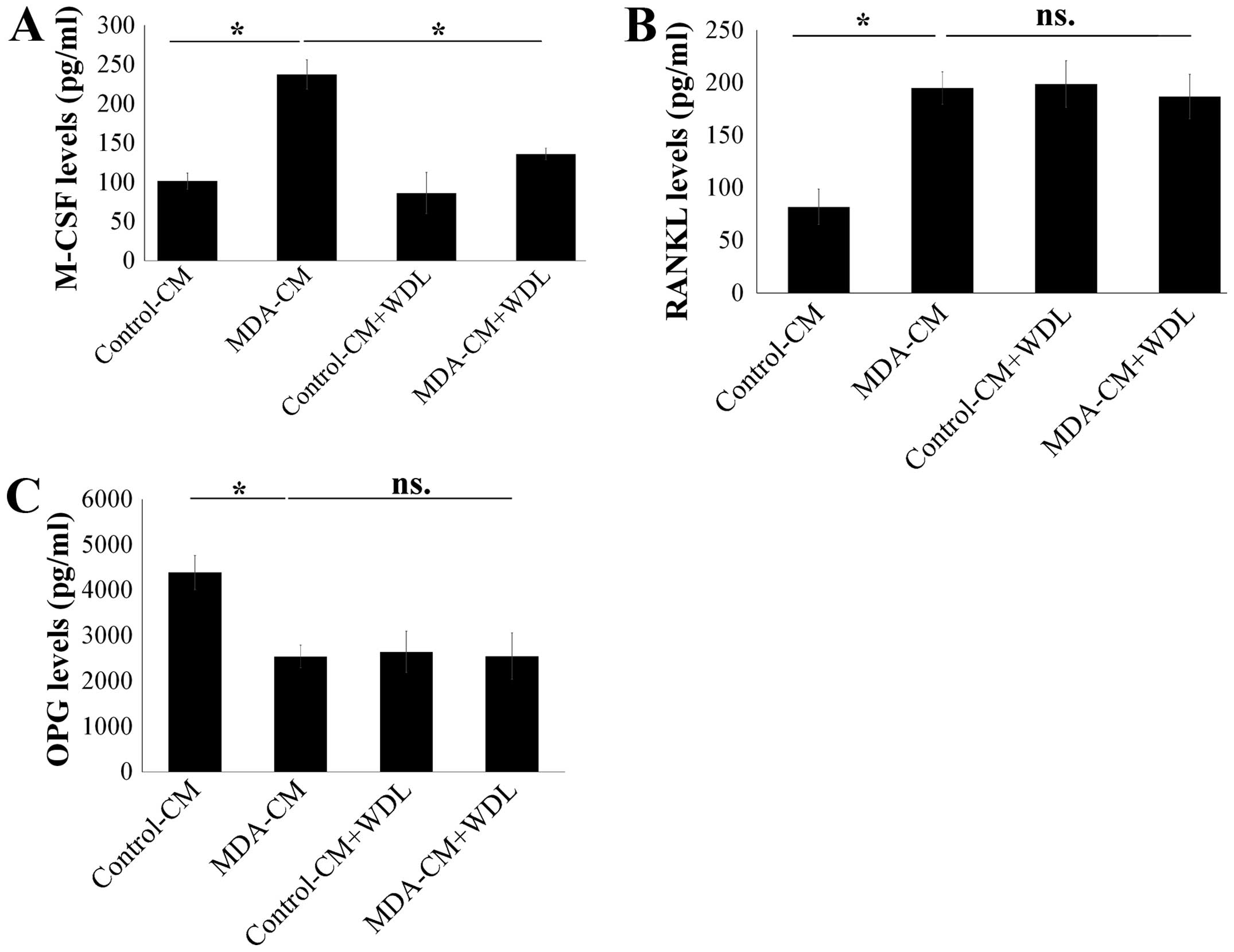
As such, the effect of WDL on the secretion of M-CSF, RANKL, and
OPG in osteoblasts after stimulation of breast cancer was assessed.
MDA-MB-231-CM markedly increased the expressions of M-CSF and RANKL
in osteoblasts (Fig. 4A and B).
Furthermore, MDA-MB-231-CM also decreased OPG expression in
osteoblasts (Fig. 4C). WDL
decreased the stimulatory effect of breast cancer on the production
of M-CSF, although it did not affect the expressions of RANKL and
OPG in osteoblasts (Fig. 4).
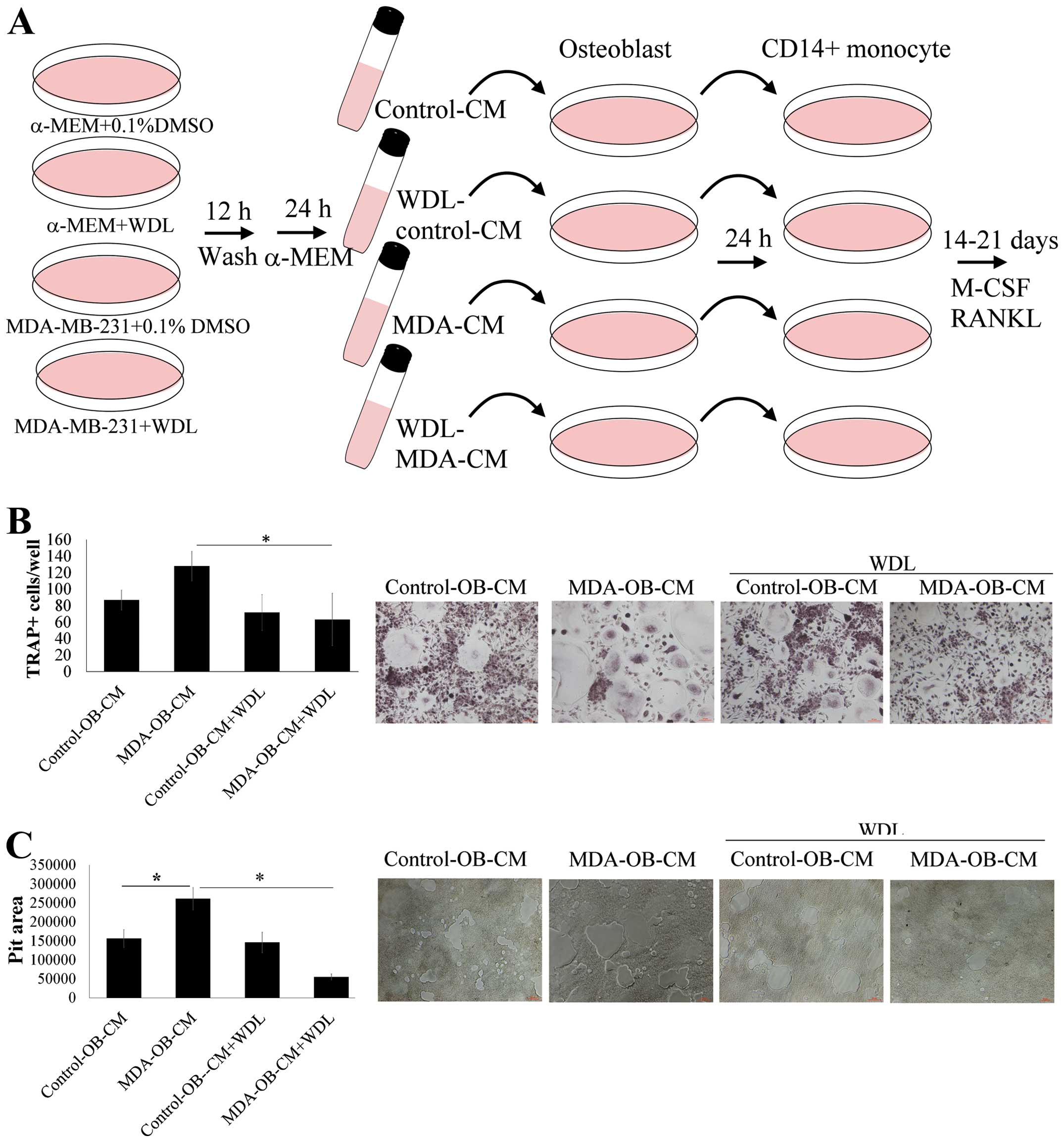
WDL reduces the enhancement effect of
breast cancer on osteoblast-mediated osteoclastogenesis
The activity of WDL on breast cancer-stimulated
interaction of osteoblasts and osteoclasts was further investigated
(Fig. 5A). Breast cancer
MDA-MB-231 stimulated osteoblast-induced osteoclastogenesis,
compared to results of the osteoclastogenesis cultured in
unstimulated osteoblast supernatants (Fig. 5B). The stimulatory effect of
MDA-MB-231 cells was inhibited by WDL treatment. Similarly, breast
cancer MDA-MB-231 cells increased the promotion of osteoblasts on
osteoclast bone resorption and this effect was prevented by WDL
(Fig. 5C). These findings suggest
that WDL inhibited the deterioration of osteoblasts on breast
cancer-mediated osteolytic bone metastasis.
Discussion
Bone metastasis is a devastating development in
patients with breast cancer because once this occurs, morbidity and
mortality rates are markedly increased (2,4).
Drugs that target osteoclastogenesis, such as bisphosphonates or
anti-RANKL antibody (denosumab), decrease the incidence of skeletal
complications and have been prescribed for the inhibition of
cancer-mediated bone destruction (6). However, 30–50% of patients on such
therapies still develop new bone metastasis, skeletal
complications, and disease progression, emphasizing the need for
new therapies (2,4,6). The
present study is the first to demonstrate that WDL can inhibit
breast cancer-induced osteolytic bone metastasis by decreasing the
stimulatory effect of breast cancer on osteoclasts and breast
cancer-mediated interaction of osteoblasts and osteoclasts.
Evaluation of osteoclast differentiation is
characterized in breast cancer bone metastasis (4,12).
An increase in osteoclastic bone resorption leads to the release of
bone-derived growth factors that promote the growth and progression
of breast cancer cells (4,12). Inhibiting osteoclast function
provides a potential approach to preventing bone metastasis.
Isolated from Eclipta prostrata and Wedelia
chinensis, WDL belongs to the coumarin category of
phytoestrogens. This is the first study that investigated and
determined that WDL exhibits a direct inhibitory effect on
osteoclast differentiation and bone resorption activity.
In addition, WDL also inhibits breast cancer
MDA-MB-231-mediated osteoclastogenesis and bone degradation. This
finding suggests that WDL possesses therapeutic potential in the
treatment of breast cancer bone metastasis. The impact of
metastasized cancer cells on the bone interrupts the interaction
between the activities of osteoclasts and osteoblasts (26). Osteoblasts perform vital roles in
the regulation of skeleton physiology because they not only act as
precursors of osteocytes but are also dual regulators of osteoclast
differentiation (27,28). Osteoblasts stimulate osteoclast
formation from osteoclast precursors by producing M-CSF and RANKL
(29). In contrast, osteoblasts
inhibit osteoclastogenesis via secreting OPG, a decoy receptor for
RANKL to block the binding of RANKL to its receptors on
pre-osteoclasts, RANK (13).
Cancer can alter the expressions of M-CSF, RANKL,
and OPG in osteoblasts, facilitating cancer-associated osteolytic
bone metastasis (14,30). In this study, breast cancer
MDA-MB-231 cells increased the expressions of M-CSF and RANKL but
decreased OPG expression, thereby increasing osteoclastogenesis and
the bone resorption activity. This upregulation of M-CSF by
MDA-MB-231 is prevented by WDL, resulting in a decrease in breast
cancer-associated osteocleogenesis and bone resorption. This study
revealed that WDL can mend the imbalanced interaction of
osteoblasts and osteoclasts in breast cancer-mediated skeletal
micro-environment.
Akt is a critical mediator of cell proliferation,
survival and differentiation in a variety of cell types (31). Akt has been identified as being
involved in the regulation of osteoclast survival and
differentiation, whereas its deficiency in osteoclasts results in
impaired bone resorption (32,33).
mTOR, a downstream molecule of Akt, has also been shown to be
involved in the regulation of osteoclast survival (34). Inhibition of the mTOR pathway by
rapamycin, an mTOR inhibitor, decreases the number of TRAP-positive
multi-nucleated osteoclasts in the chondro-osseous junction in rats
(35).
In this study, osteoclast differentiation induced by
M-CSF/RANKL increased the activation of Akt and mTOR signaling
cascade. Inhibitors of both Akt and mTOR markedly suppressed
osteoclast differentiation and bone resorption activity, suggesting
that the Akt/mTOR pathway plays a crucial role in
osteoclastogenesis and osteoclast functions. Moreover, breast
cancer MDA-MB-231 cells augment the activation of Akt/mTOR
signaling, resulting in enhanced osteoclastogenesis. WDL not only
blocks M-CSF/RANKL-induced Akt/ mTOR activation, but also prevents
the reinforcing effect of breast cancer on this signaling pathway.
These data suggest that WDL is an Akt/mTOR inhibitor, targeting
both the induction of osteoclast differentiation and the cancer
inducible activation of Akt/mTOR.
In conclusion, WDL has protective potential against
breast cancer-induced bone destruction by directly decreasing
cancer cell mediated osteoclast differentiation and bone resorption
and by restoring the balance of osteoblast-osteoclast interaction.
Taken together, WDL possesses potential and dual ameliorating
effects on breast cancer-associated osteolytic bone metastasis.
Acknowledgements
This study was supported by grants from the National
Science Council of Taiwan (NSC 102-2628-B-037-002-MY3; NSC
102-2632-B-037-001-MY3; NSC 102-2314-B-037-035-MY3), the Excellence
for Cancer Research Center Grant, the Ministry of Health and
Welfare, Executive Yuan, Taipei, Taiwan (MOHW 103-TD-B-111-05), and
the Kaohsiung Medical University Hospital (KMUH102-2M61). The
authors thank the Center for Research Resources and Development of
Kaohsiung Medical University for their support with the
instrumentation.
References
|
1
|
Akhtari M, Mansuri J, Newman KA, Guise TM
and Seth P: Biology of breast cancer bone metastasis. Cancer Biol
Ther. 7:3–9. 2008. View Article : Google Scholar
|
|
2
|
Rordorf T, Hassan AA, Azim H, et al: Bone
health in breast cancer patients: a comprehensive statement by
CECOG/SAKK intergroup. Breast. 23:511–525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rove KO and Crawford ED: Metastatic cancer
in solid tumors and clinical outcome: skeletal-related events.
Oncology. 23:21–27. 2009.
|
|
4
|
Weilbaecher KN, Guise TA and McCauley LK:
Cancer to bone: a fatal attraction. Nat Rev Cancer. 11:411–425.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gupta S, Gupta H, Mandhyan D and
Srivastava S: Bisphophonates related osteonecrosis of the jaw. Natl
J Maxillofac Surg. 4:151–158. 2013. View Article : Google Scholar
|
|
6
|
Verron E, Schmid-Antomarchi H,
Pascal-Mousselard H, Schmid-Alliana A, Scimeca JC and Bouler JM:
Therapeutic strategies for treating osteolytic bone metastases.
Drug Discov Today. 19:1419–1426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roodman GD: Mechanisms of bone metastasis.
N Engl J Med. 350:1655–1664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mundy GR: Metastasis to bone: causes,
consequences and therapeutic opportunities. Nat Rev Cancer.
2:584–593. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–1514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suda T, Takahashi N, Udagawa N, Jimi E,
Gillespie MT and Martin TJ: Modulation of osteoclast
differentiation and function by the new members of the tumor
necrosis factor receptor and ligand families. Endocr Rev.
20:345–357. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee ZH and Kim HH: Signal transduction by
receptor activator of nuclear factor kappa B in osteoclasts.
Biochem Biophys Res Commun. 305:211–214. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen YC, Sosnoski DM and Mastro AM: Breast
cancer metastasis to the bone: mechanisms of bone loss. Breast
Cancer Res. 12:2152010. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mountzios G, Dimopoulos MA, Bamias A, et
al: Abnormal bone remodeling process is due to an imbalance in the
receptor activator of nuclear factor-kappaB ligand
(RANKL)/osteoprotegerin (OPG) axis in patients with solid tumors
metastatic to the skeleton. Acta Oncol. 46:221–229. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McGrath EE: OPG/RANKL/RANK pathway as a
therapeutic target in cancer. J Thorac Oncol. 6:1468–1473. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Azim HA, Kamal NS and Azim HA Jr: Bone
metastasis in breast cancer: the story of RANK-ligand. J Egypt Nat
Cancer Inst. 24:107–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patel M, Kadakia V and Mishra S:
Simultaneous estimation of andrographolide and wedelolactone in
herbal formulations. Indian J Pharm Sci. 70:6892008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roy RK, Thakur M and Dixit VK: Hair growth
promoting activity of Eclipta alba in male albino rats. Arch
Dermatol Res. 300:357–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh B, Saxena AK, Chandan BK, Agarwal SG
and Anand KK: In vivo hepatoprotective activity of active fraction
from ethanolic extract of Eclipta alba leaves. Indian J Physiol
Pharmacol. 45:435–441. 2001.
|
|
19
|
Wagner H, Geyer B, Kiso Y, Hikino H and
Rao GS: Coumestans as the main active principles of the liver drugs
Eclipta alba and Wedelia calendulacea. Planta Med. 370–374. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin FM, Chen LR, Lin EH, et al: Compounds
from Wedelia chinensis synergistically suppress androgen activity
and growth in prostate cancer cells. Carcinogenesis. 28:2521–2529.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tewtrakul S, Subhadhirasakul S,
Cheenpracha S and Karalai C: HIV-1 protease and HIV-1 integrase
inhibitory substances from Eclipta prostrata. Phytother Res.
21:1092–1095. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsai CH, Lin FM, Yang YC, et al: Herbal
extract of Wedelia chinensis attenuates androgen receptor activity
and orthotopic growth of prostate cancer in nude mice. Clin Cancer
Res. 15:5435–5444. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vender JR, Laird MD and Dhandapani KM:
Inhibition of NFkappaB reduces cellular viability in GH3 pituitary
adenoma cells. Neurosurgery. 62:1122–1128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Idris AI, Libouban H, Nyangoga H,
Landao-Bassonga E, Chappard D and Ralston SH: Pharmacologic
inhibitors of IkappaB kinase suppress growth and migration of
mammary carcinosarcoma cells in vitro and prevent osteolytic bone
metastasis in vivo. Mol Cancer Ther. 8:2339–2347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sarveswaran S, Gautam SC and Ghosh J:
Wedelolactone, a medicinal plant-derived coumestan, induces
caspase-dependent apoptosis in prostate cancer cells via
downregulation of PKCɛ without inhibiting Akt. Int J Oncol.
41:2191–2199. 2012.PubMed/NCBI
|
|
26
|
Sterling JA, Edwards JR, Martin TJ and
Mundy GR: Advances in the biology of bone metastasis: how the
skeleton affects tumor behavior. Bone. 48:6–15. 2011. View Article : Google Scholar
|
|
27
|
Furugaki K, Moriya Y, Iwai T, et al:
Erlotinib inhibits osteolytic bone invasion of human non-small-cell
lung cancer cell line NCI-H292. Clin Exp Metastasis. 28:649–659.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hsu YL, Huang MS, Yang CJ, Hung JY, Wu LY
and Kuo PL: Lung tumor-associated osteoblast-derived bone
morphogenetic protein-2 increased epithelial-to-mesenchymal
transition of cancer by Runx2/Snail signaling pathway. J Biol Chem.
286:37335–37346. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takayanagi H: New immune connections in
osteoclast formation. Ann NY Acad Sci. 192:117–123. 2010.
View Article : Google Scholar
|
|
30
|
Yoneda T, Tanaka S and Hata K: Role of
RANKL/RANK in primary and secondary breast cancer. World J Orthop.
4:178–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Skeen JE, Bhaskar PT, Chen CC, et al: Akt
deficiency impairs normal cell proliferation and suppresses
oncogenesis in a p53-independent and mTORC1-dependent manner.
Cancer Cell. 10:269–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moon JB, Kim JH, Kim K, et al: Akt induces
osteoclast differentiation through regulating the GSK3β/NFATc1
signaling cascade. J Immunol. 188:163–169. 2012. View Article : Google Scholar
|
|
33
|
Cao H, Zhu K, Qiu L, et al: Critical role
of AKT protein in myeloma-induced osteoclast formation and
osteolysis. J Biol Chem. 288:30399–30410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sugatani T and Hruska KA: Akt1/Akt2 and
mammalian target of rapamycin/Bim play critical roles in osteoclast
differentiation and survival, respectively, whereas Akt is
dispensable for cell survival in isolated osteoclast precursors. J
Biol Chem. 280:3583–3589. 2005. View Article : Google Scholar
|
|
35
|
Sanchez CP and He YZ: Bone growth during
rapamycin therapy in young rats. BMC Pediatr. 9:32009. View Article : Google Scholar : PubMed/NCBI
|