Introduction
Solid tumors characteristically contain areas of
hypoxia (low oxygen tension), which is a powerful stimulus for the
expression of genes involved in proliferation, glycolysis, invasion
and angiogenesis. Adaptation of tumor cells to hypoxic environment
results in an aggressive and metastatic cancer phenotype that is
associated with resistance to radiation therapy, chemotherapy, and
a poor treatment outcome (1).
The transcription factor hypoxia-inducible factor-1
(HIF-1) is central to the regulation of a number of
hypoxia-activated genes and consists of HIF-1α and HIF-1β subunits.
The expression and activity of HIF-1α subunit are uniquely
regulated by the intracellular oxygen concentration (2). Under normoxic conditions, HIF-1α
protein is degraded rapidly and continuously by ubiquitination and
proteasome degradation pathway. Under hypoxic conditions, HIF-1α
protein accumulates and translocates to the nucleus where it forms
an active complex with HIF-1β, which activates transcription of its
target genes important for the adaptation and survival under
hypoxia (3). Overexpression of
HIF-1α protein has been demonstrated in many human cancers and
their metastases, and it is associated with increased vascularity,
invasion and metastasis, tumor progression and treatment resistance
(4). Therefore, HIF-1α has been
identified as an important molecular target for tumor therapy.
Oligomer procyanidins (F2) (degree of
polymerization: 2–15) is a natural fraction extracted from grape
seeds, the characterization and composition of which have been
reported previously (5–7). We previously found that F2 could
cross the blood-brain barrier, enhance the •OH scavenging ability
in rat brain and protect mouse brain against ethanol-induced
oxidative DNA damage (8). Since
both the overproduction of •OH and oxidative DNA damage occur in
tumors, we further investigated the antitumor effect of F2 in
vitro, and found that F2 could inhibit the growth of multiple
cancer cells and induce U87 cells paraptosis (9). We also found that F2 could
significantly inhibit the migration and invasion of fMLF (a FPR1
agonist)-induced U87 cells due to its partial agonist action on
formyl peptide receptor 1 (FPR1) (10). FPR1 is a membrane receptor
overexpressed in U87 cells, regulates the cell growth, survival,
migration and invasion (11,12).
Moreover, an in vivo study showed that repeated
administration of F2 to mice had no adverse effects (13). These data suggest that F2 may serve
as a safe and efficient antitumor agent. However, the underlying
antitumor mechanism of F2 is not clear and needs considerably more
research.
Based on the evidence that HIF-1α was highly
expressed in human astrocytoma U251 and human hepatoma Hep3B cell
lines and mediated their multiple tumor biology (14,15),
and our findings that cell viability of both cell lines could be
significantly inhibited by F2 under normoxic conditions (9), we selected the U251 and Hep3B cell
lines to investigate the antitumor effect of F2 targeting HIF-1α to
further explain its antitumor mechanism, which will help define the
characteristics of F2 and may contribute to the discovery of novel
antitumor or prevention agents.
Materials and methods
Preparation of F2
The grape seed (Vitis vinifera, cv. Fernão
Pires) powder (200 g) was firstly extracted using 3 l of
methanol-water (80:20, v/v), followed by 3 l acetone-water (75:25,
v/v) to obtain crude phenolic extract as described previously
(5). After removing the organic
solvents, the crude phenolic extract was chromatographed on a
Lichroprep RP-18 (200×25 mm i.d.; 25–40 m particle size; Merck,
Darmstadt, Germany) column to isolate F2 with the procedures
similar to those already described (6). Briefly, F2 was extracted from the
grape seed methanolic extract and evaporated at <30°C to
dryness, and dissolved in double distilled water prior to
lyophilization. The chemical and structural characterization of F2
was determined by normal-phase and reverse-phase HPLC,
thioacidolysis-HPLC, ESI-MS analyses, formaldehyde-HCl
precipitation and elemental analysis (7). The powder of F2 obtained was
endotoxin free and stored at −20°C until used. For cells culture
work in vitro, a 10 mg/ml aqueous stock solution was
prepared and sterilized using the 0.22-μm filter.
Cell lines and reagents
Human astrocytoma U251 cell line (ATCC, Rockville,
MD, USA), human hepatoma Hep3B cell line (ATCC) and human umbilical
vein endothelial cells (HUVECs) (National Center for Medical
Culture Collection, China) were routinely cultured in DMEM and/or
RPMI-1640 supplemented with 10% FBS (Gibco-BRL, Rockville, MD, USA)
and maintained at 37°C in a humidified incubator with 5%
CO2 (referred to as normoxic conditions). U251-HRE
(cells stably transfected with pGL2-TK-HRE plasmid) and U251-pGL3
(cells stably transfected with pGL3-control plasmid) cells were
cultured in RPMI-1640 with 5% FBS and 100 μg/ml G418 (Sigma, CA,
USA). For cell culture under hypoxic conditions, cells were grown
in a modular incubator chamber (CA, USA) containing 1%
O2, 5% CO2 and 94% N2 at 37°C.
Hypoxia can also be induced using the hypoxia mimetic agent
desferoxamine (Sigma).
Rapamycin, LY294002, cycloheximide (CHX), MG132 and
dimethyloxalylglycine (DMOG) were purchased from Sigma. PD98059 was
purchased from Beyotime (S1805, China). Recombinant human VEGF and
EGF were purchased from Peprotech (Rocky Hill, NJ, USA). HIF-1α
siRNA and control siRNA were purchased from Santa Cruz (CA, USA).
Primary antibodies against HIF-1α and HIF-1β were purchased from
BD-Transduction Laboratories (MA, USA). Antibodies specific for
phosphorylated (Ser-308) or total AKT, phosphorylated (Ser-2448) or
total mTOR, phosphorylated (Thr-389) or total p70S6K,
phosphorylated (Ser-65) or total 4E-BP1, phosphorylated p44/42
ERK1/2 (T202/Y204) or p44/42ERK1/2 and phosphorylated (Tyr-992) or
total EGFR and β-actin were purchased from Cell Signaling
Technology (CA, USA).
Transient transfection and luciferase
reporter assays
pGL2-TK-HRE plasmid, containing three copies of the
hypoxia-responsive element from the inducible nitric oxide synthase
promoter upstream of the firefly luciferase reporter gene, was
kindly provided by Giovanni Melillo (National Tumor Institute,
Frederick, MD, USA). Hep3B cells were transiently transfected with
0.5 μg pGL2-TK-HRE plasmid. Afterward, cells were treated with
different concentrations of F2 (μg/ml) followed by exposure to
hypoxia for 16 h. Cell lysates were collected and luciferase levels
were subsequently assayed using the Bright-Glo™ luciferase assay
system (Promega, WI, USA).
Western blot analysis
Western blot analysis was performed as described
previously (10). In brief, equal
amounts of total protein extracts from cultured cells were
fractionated by 4–15% SDS-PAGE and electrically transferred onto
polyvinylidene difluoride (PVDF) membranes (Millipore, Darmstadt,
Germany). Mouse or rabbit primary antibodies and appropriate
horseradish peroxidase (HRP)-conjugated secondary antibodies (CST,
CA, USA) were used to detect the designated proteins.
Membrane-bound secondary antibodies were detected with ECL reagents
(Invitrogen, CA, USA) and exposed to X-ray film. Results were
normalized to the internal control (β-actin).
Real-time PCR and RT-PCR
Total RNA was isolated using RNeasy Mini kit
(Qiagen, Germany) following the manufacturer’s instructions. RNA (1
μg) was reverse transcribed with a RevertAid First Strand cDNA
Sythesis kit (Toyobo, Japan). For quantitative PCR, analysis was
carried out using iQ SYBR Green Supermix (Bio-Rad, CA, USA) and a
CFX96 Real-Time PCR Detection System (Bio-Rad) as instructed by the
manufacturer. RT-PCR reactions were performed as described
previously (8).
Quantitation of VEGF production
Cells were incubated in the 6-well culture plates
(Corning Costar, NY, USA) and treated with F2 (μg/ml) under
normoxic and hypoxic conditions for 16 h. Cells culture medium were
collected and centrifuged at 800 rpm for 5 min at 4°C to remove
cellular debris. VEGF in the medium was measured by using the
Quantikine human VEGF ELISA kit (R&D Systems, MN, USA)
according to the manufacturer’s instructions.
Cell invasion assay
Cells (10–20×104) were plated in cell
basal medium in the upper chamber of a Transwell (8 μm, Corning
Costar), pre-coated with matrigel (BD, MA, USA). Serum-free
RPMI-1640 medium with or without of F2 (μg/ml) was added to the
upper and lower chamber of the Transwell. After 12 or 16 h,
non-migrated cells were removed with cotton swab and migrated cells
were stained and examined using ImageXpress Micro XLS Widefield
High Content Screening System (Molecular Devices, CA, USA), the
number of migrated cells was quantified by counting the cell
number.
Tube formation assay
Matrigel (50 μl) mixed with serum-free RPMI-1640
medium (1:1) were placed into each well of the 96-well-plate
(Corning Costar) and allowed to polymerize by incubation at 37°C
for 30 min. A mixture of HUVECs (5×104/well) (100 μl)
was seeded on the gel with or without culture medium (CM) (100%),
then incubated at 37°C for 12 h. Cells were stained and examined,
and tube formation (branches/field) was examined and
quantified.
Statistical analysis
Results are expressed as means ± SE of three
independent experiments done in triplicate. One-way ANOVA followed
by Dunnett’s t- and T3-test were used for statistical analysis
(SPSS 16.0 software, SPSS, USA).
Results
F2 inhibits HIF-1α expression in U251 and
Hep3B cells
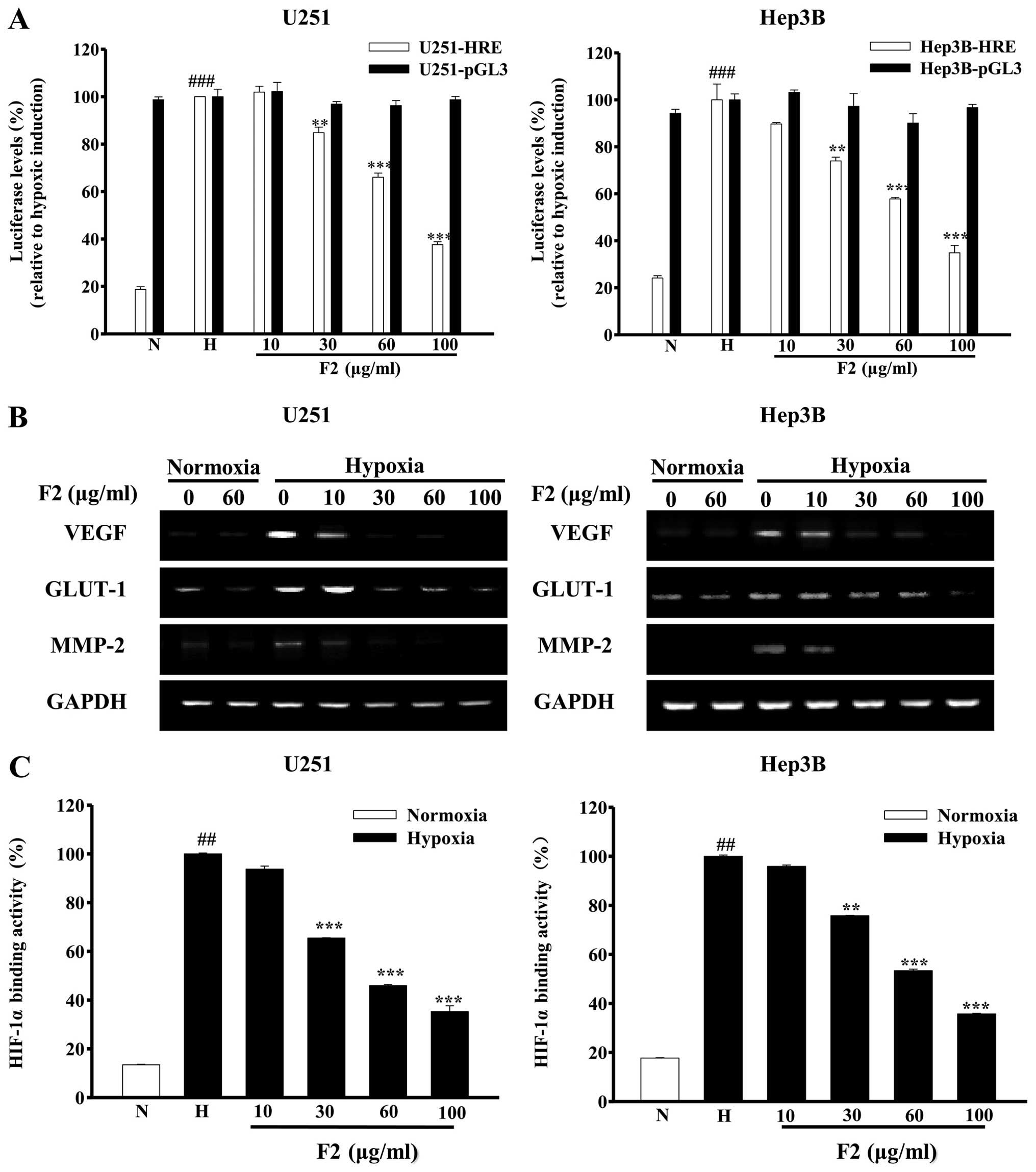
We used U251-HRE and U251-pGL3 cells (HIF-1α
inhibitor screening model) to detect whether F2 inhibited the
HIF-1α activity (16). As shown in
Fig. 1A, the hypoxia-induced
luciferase expression in U251-HRE cells, but not the constitutive
luciferase in U251-pGL3 control cells, was significantly decreased
by F2 in a concentration-dependent manner (P<0.01). Similar
results were obtained in Hep3B-HRE and Hep3B-pGL3 transiently
transfected cells (P<0.01). The effects of F2 on the expression
of HIF-1 target genes mRNA were examined. As shown in Fig. 1B, hypoxic induction of VEGF, GLUT-1
and MMP-2 mRNA were markedly blocked by F2 in a
concentration-dependent manner. In addition, HIF-1α DNA binding
activity in both cell lines was also significantly decreased in the
presence of F2 (P<0.01, Fig.
1C).
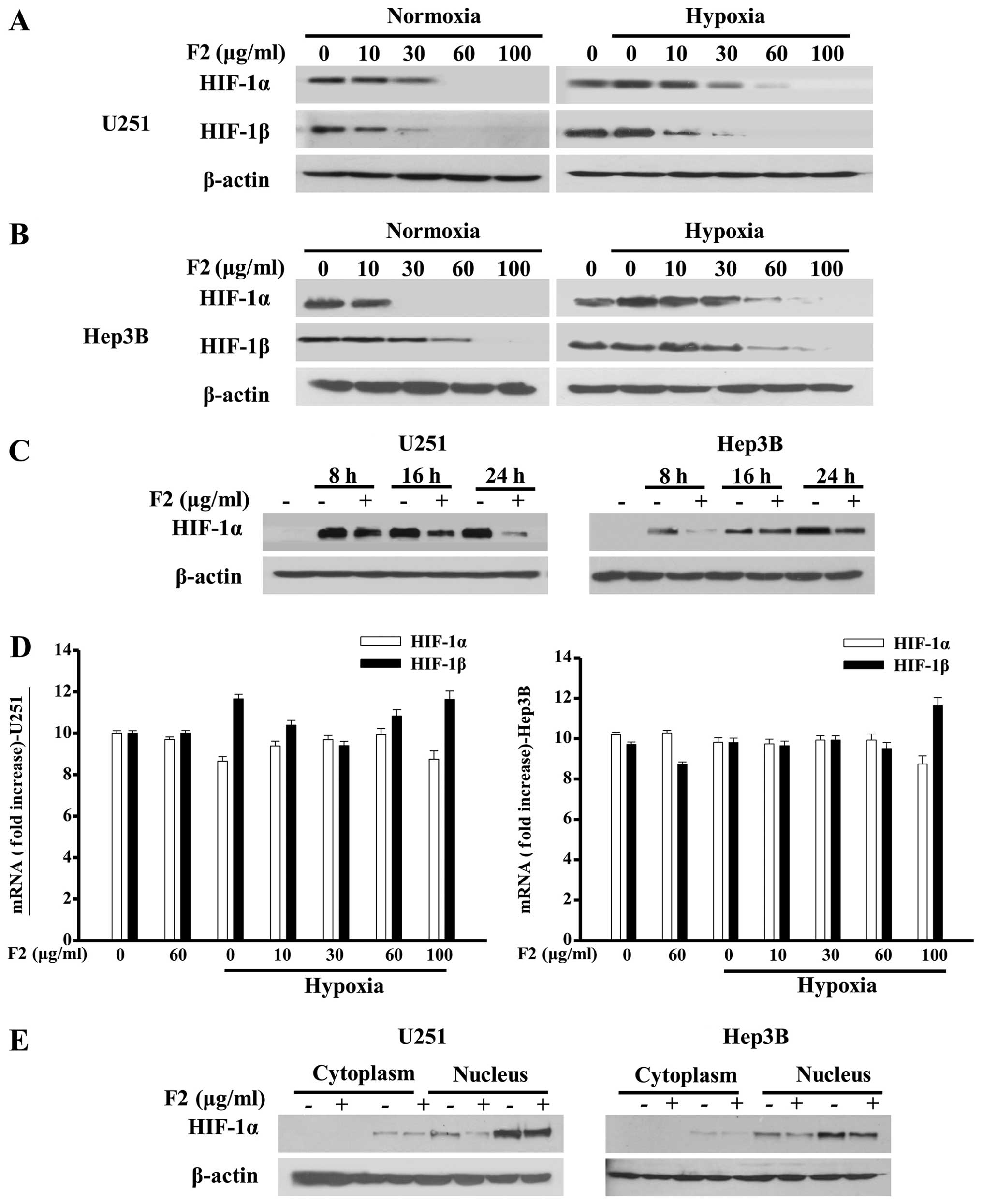
In order to evaluate how F2 inhibits HIF-1α
activity, HIF-1α and HIF-1β in both cells were investigated at
protein and mRNA levels by western blot analysis and real-time PCR,
respectively. As shown in Fig.
2A–C, F2 inhibited the expressions of HIF-1α and HIF-1β protein
in both cells under normoxic and hypoxic conditions in a
concentration- and/or time-dependent manner without obvious changes
in the cell viability (P>0.05, data not shown). For the
concentration-dependent inhibition of HIF-1α protein levels, no
significant difference between U251 and Hep3B cells was observed.
In addition, F2 also exerted a concentration-dependent inhibition
of hypoxic-induced accumulation of HIF-1α protein in other cell
lines, such as A549 and MDA-MB-435S cell lines (data not
shown).
For the HIF-1α and HIF-1β mRNA, no appreciable
effects of F2 were found in either cell line, leaving a random
distribution and an inconsistent pattern (P>0.05, Fig. 2D). Since HIF-1 activity is
primarily determined by HIF-1α subunit in the nucleus, we also
determined the presence of HIF-1α in the nucleus and cytoplasm, and
found that the nuclear translocation of HIF-1α in both cell lines
were not changed in the presence of F2 (Fig. 2E) under normoxic or hypoxic
conditions.
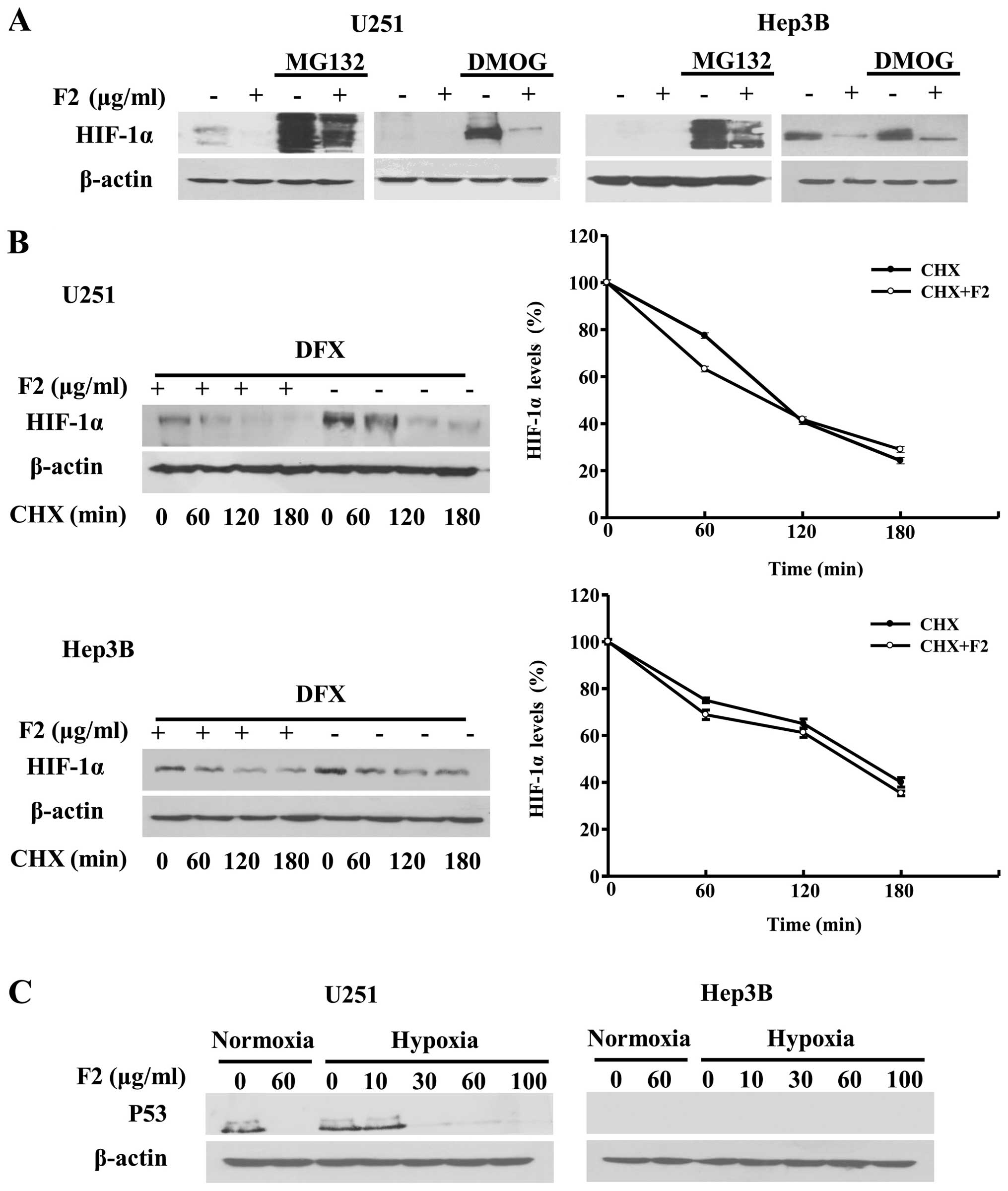
F2 does not affect the degradation or
stability of HIF-1α
In order to test whether the inhibitory effect of F2
on HIF-1α protein expression was associated with the degradation
induction of HIF-1α via the prolyl-hydroxyltion-proteasome pathway,
we assessed the impact of F2 on HIF-1α protein degradation in the
presence of MG132 (a proteasome inhibitor) and DMOG (a prolyl
hydroxylase inhibitor) (17). As
shown in Fig. 3A, the MG132 (20
μM) and DMOG (100 μM)-induced increase in expression of HIF-1α was
decreased by F2 (60 μg/ml) under normoxic condition.
Immuno-fluorescence assay showed that F2 also downregulated the
expression level of hydroxylation-HIF-1α (hy-HIF-1α) induced by
MG132 in Hep3B cells (data not shown). In this study, HIF-1α
protein was expressed as two bands and slightly dispersed in cells
treated with MG132 due to its poly-ubiquitylation modification,
which was consistent with previous results (18). These results indicate that F2
suppresses the accumulation of HIF-1α through a pathway independent
of hydroxylation-proteasome degradation.
These results were compatible with the possibility
that F2 affected the half-life of HIF-1α protein and promoted its
degradation through a different pathway (17). To investigate this hypothesis,
experiments were performed using cycloheximide (CHX) which could
inhibit de novo protein synthesis. HIF-1α protein was
measured before (time 0) and at different times after CHX was
added, however, as shown in Fig.
3B, there was no change in HIF-1α half-life in U251 or Hep3B
cells (P>0.05) after treatment with F2 (60 μg/ml).
A study also exists revealing that p53 might
negatively regulate HIF-1α stability by interacting directly or
indirectly by targeting it for HDM2-mediated degradation (19). Therefore, the effect of F2 on p53
expression was tested to see whether F2 affected the expression of
HIF-1α with p53 involved. It is reported that U251 and Hep3B cells
express mutant-p53 and deleted-p53, respectively (20,21).
As shown in Fig. 3C, U251 cells
expressed p53, which was accordance with a previous report that
U251 cells expressed mutant-p53 (20), while the level of p53 was decreased
in the presence of F2; p53 was not detected in Hep3B cells, which
might be due to the Hep3B cells expressing the deleted p53
(21). F2 also inhibited HIF-1α
expression in A549 cells which expressed wild-p53. It therefore
seems that p53 is uninvolved in the regulation of HIF-1α expression
by F2, because HIF-1α expressions were inhibited by F2 regardless
of the type of p53 in cells or whether p53 existed or not.
Taken together, these data indicate that F2 does not
influence the degradation or stability of HIF-1α, raising the
possibility that it may affect its translation.
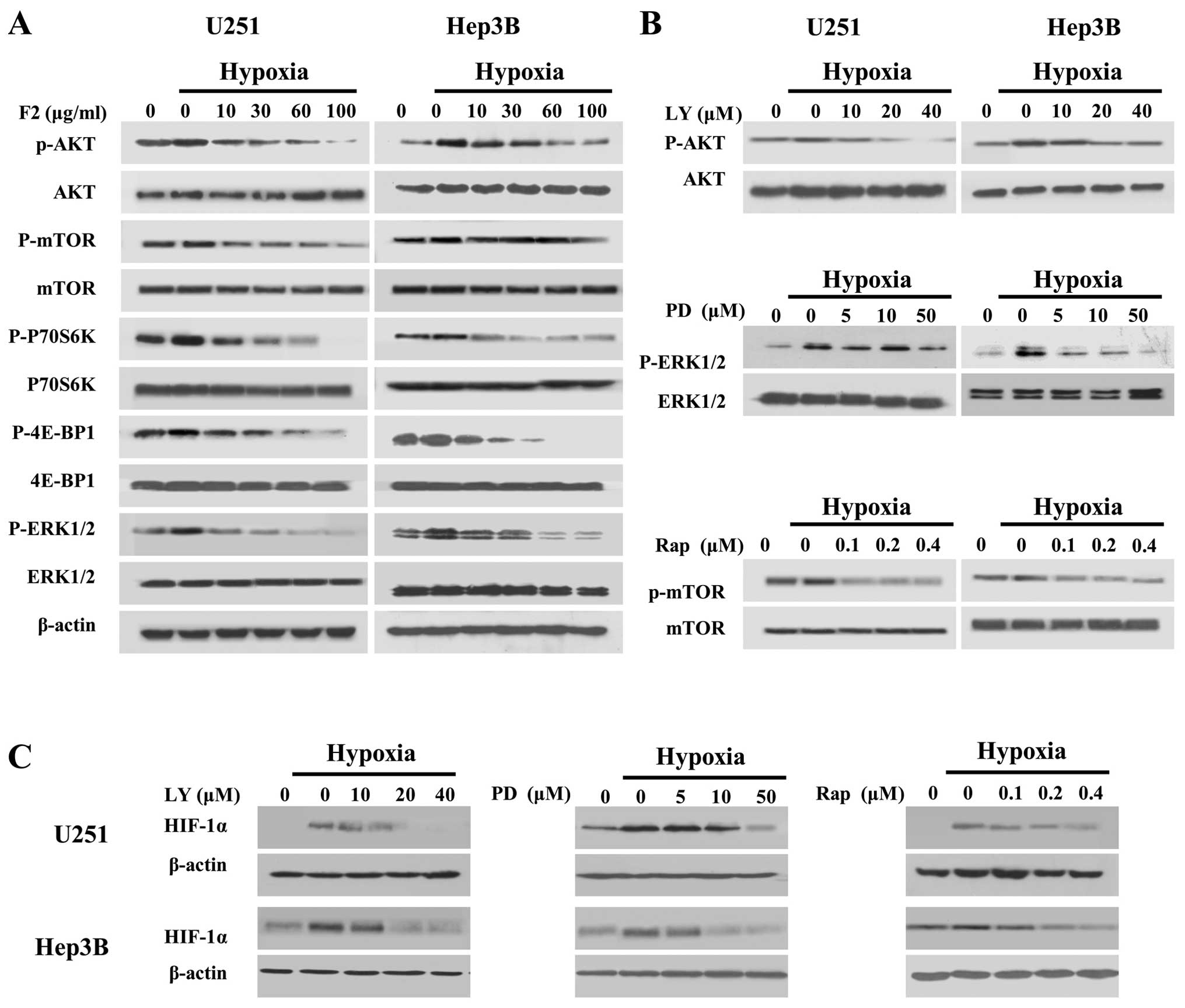
F2 inactivates hypoxia-induced
PI3K-AKT-mTOR and MAPK-ERK1/2 pathways
We next investigated whether F2 affected the
expression of HIF-1α through the PI3K-AKT-mTOR and ERK1/2 pathways,
by inhibiting the HIF-1α translational regulators P70S6K and 4E-BP1
(22–24). As shown in Fig. 4A, hypoxic induction of the p-AKT,
p-mTOR, p-P70S6K, p-4E-BP1 and p-ERK1/2 in both cell lines were
decreased by F2 in a concentration-dependent manner. In addition,
LY294002 (PI3K inhibitor), rapamycin (mTOR inhibitor) and PD98059
(MEK1 inhibitor) could inhibited the p-AKT, p-mTOR and p-ERK1/2
induced by hypoxia followed by downregulation of the level of
HIF-1α (Fig. 4B and C). These
results indicate that the inhibitory effect of F2 on HIF-1α protein
expression is associated with the inactivation of PI3K-AKT-mTOR and
MAPK-ERK1/2 pathways.
F2 inhibits the activation of EGFR
signaling pathway
Epidermal growth factor receptor (EGFR) is
overexpressed and activated in a variety of tumors, and has been
reported to regulate the translation and expression of HIF-1α
through the PI3K-AKT-mTOR and MAPK-ERK1/2 pathways (25,26).
In this study, we found that hypoxia promoted the phosphorylation
of EGFR (p-EGFR) in both U251 and Hep3B cells, and this could be
abrogated in the presence of F2 (Fig.
5A). As a ligand of EGFR and one target gene of HIF-1, EGF (10
ng/ml) also could dramatically induce the expression of HIF-1α and
VEGF, both of which were decreased by F2 in a
concentration-dependent manner (Fig.
5B). In addition, EGF induction of HIF-1α protein and
luciferase levels (P<0.05) were both inhibited by LY294002,
rapamycin and PD98059 (P<0.05, Fig.
5C and D). These results are consistent with the previous
report and confirm that EGFR acts as an upstream mediator of HIF-1α
in both cell lines, and probably is a potential target of F2.
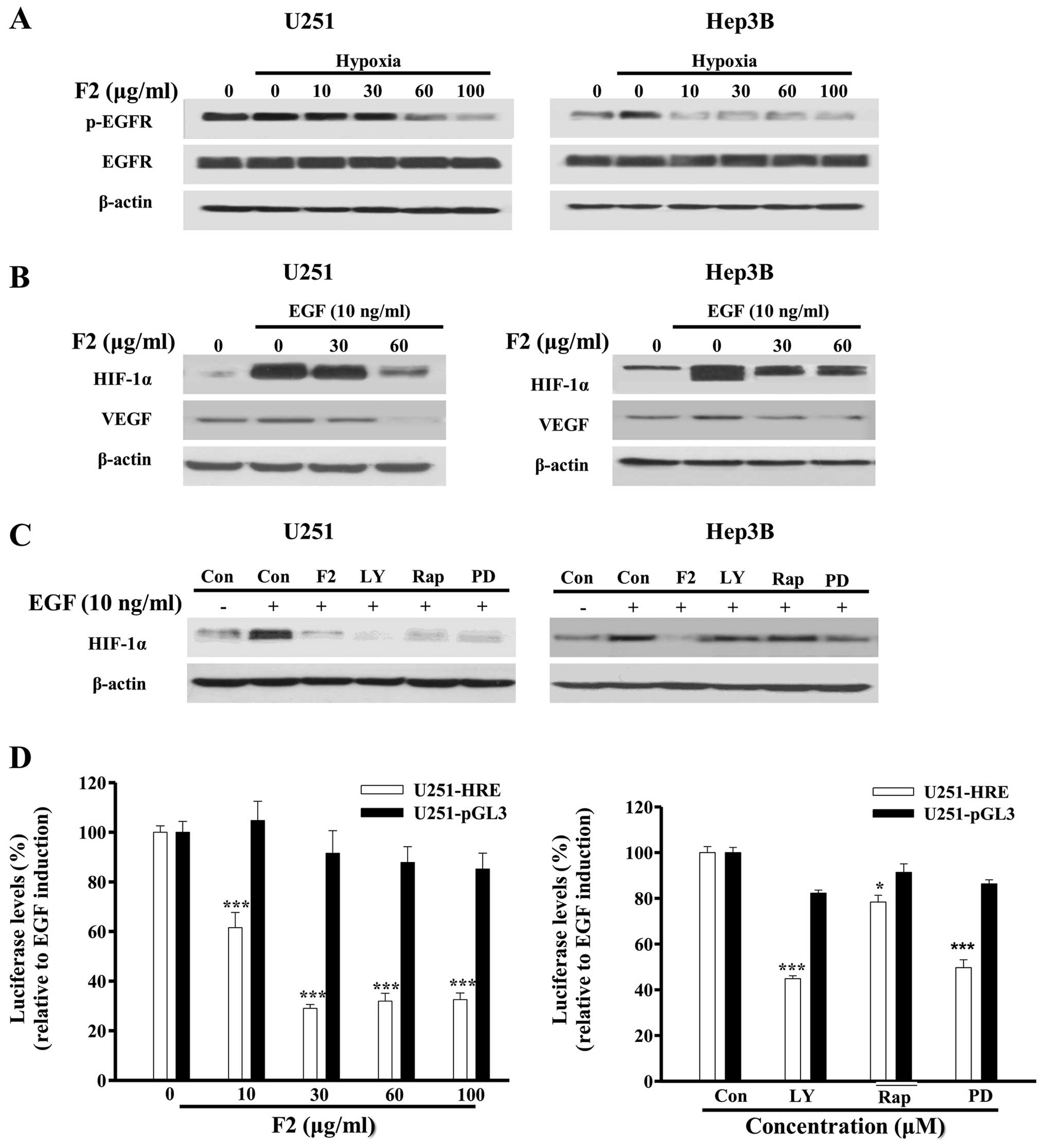 | Figure 5F2 inhibits the activation of EGFR
pathway in U251 and Hep3B cells. (A) Western blot analysis of total
proteins of cells treated with different concentrations of F2
(μg/ml) for 1 h. (B and C) Cells were starved for 24 h, then
treated with F2 (μg/ml), LY (20 μM), Rap (400 nM), PD (50 μM) in
the presence or absence of EGF (10 ng/ml) for 16 h, cell total
proteins were collected for western blot analysis. (D) U251-HRE
cells were starved for 24 h, then treated with different
concentrations of F2 (μg/ml), LY (20 μM), Rap (400 nM), PD (50 μM)
in the presence or absence of EGF (10 ng/ml) for 16 h. Luciferase
levels were tested using Bio-Glo bright luciferase reagent. The
diagram shows relative amounts of luciferase levels in the treated
group vs. EGF group. Values are means ± SE of three independent
experiments performed in triplicate, *P<0.05;
***P<0.001. |
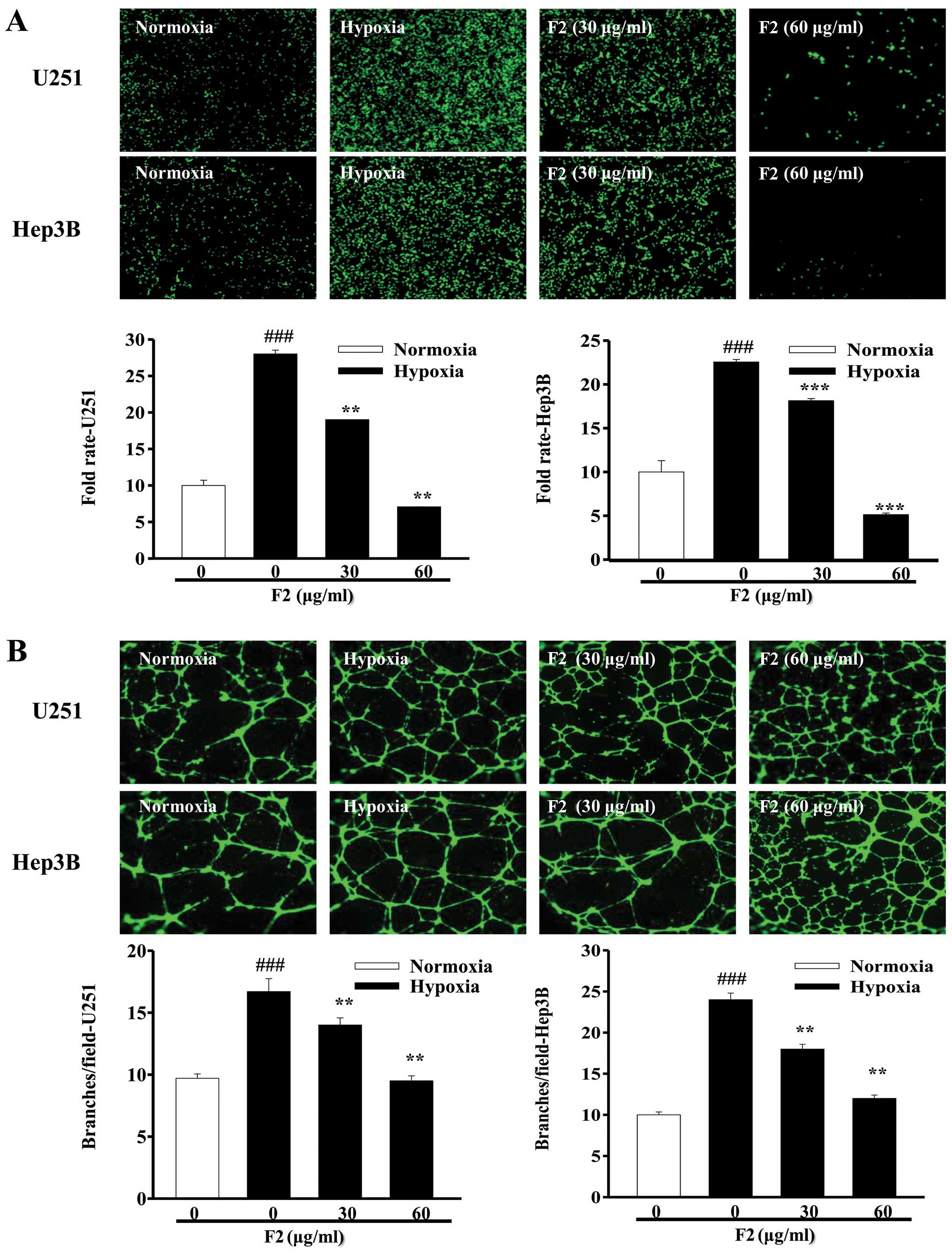
F2 inhibits tumor angiogenesis in
vitro
The results above showed that F2 could inhibit the
expression levels of HIF-1α and its target gene-VEGF mRNA. Given
the key roles of VEGF and HIF-1α in the regulation of tumor
angiogenesis (27,28), we investigated the effect of F2 on
tumor angiogenesis using the tube formation assay and HUVECs
invasion assay. As shown in Fig.
6A, conditioned media (CM) collected from both cell types
exposed to hypoxia were all able to promote the invasion of HUVECs
(P<0.001), whereas CM collected from cells exposed to hypoxia in
the presence of F2 decreased the invasion ability of HUVECs
(P<0.01). A similar result was obtained in the tube formation
assay (Fig. 6B). Moreover, the
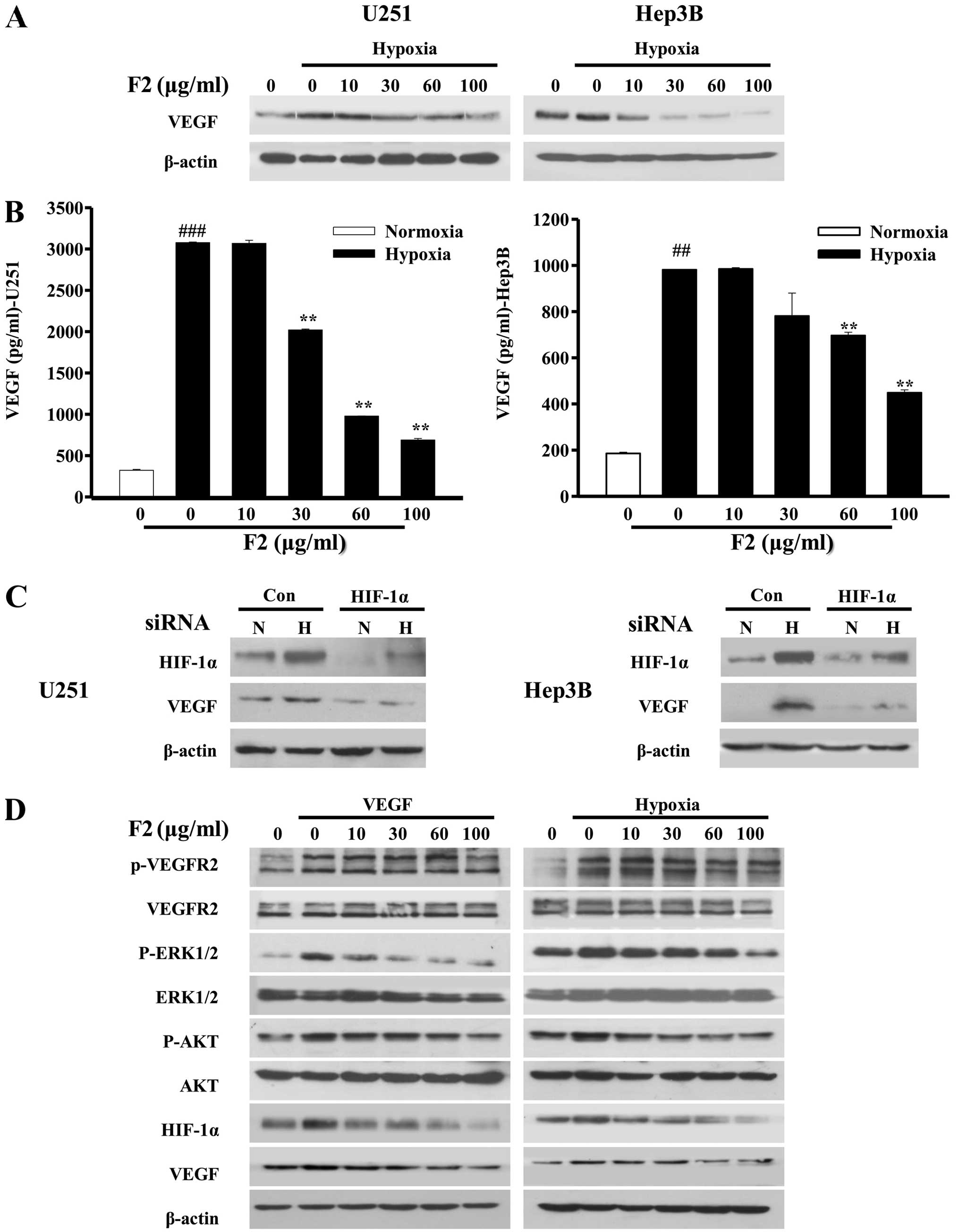
levels of cellular and secreted VEGF proteins induced by hypoxia in
both cell types were significantly inhibited by F2 in a
concentration-dependent manner (P<0.01, Fig. 7A and B). To further confirm that
VEGF level is controlled by HIF-1α in both U251 and Hep3B cells, we
examined whether RNA interference of HIF-1α was able to knock down
the protein level of VEGF. As shown in Fig. 7C, when HIF-1α expression was
knocked down, the levels of HIF-1α and VEGF were lower under
normoxic conditions and unable to be upregulated by hypoxia,
confirming that HIF-1α is an upstream mediator of VEGF in U251 and
Hep3B cells.
In addition, hypoxia also regulates the function of
endothelial cells through the VEGFR2/HIF-1 pathway, which is also a
classical pathway for endothelial angiogenesis (29,30).
As shown in Fig. 7D, p-VEGFR2,
p-AKT and p-ERK1/2 induced by hypoxia or VEGF (10 ng/ml) were all
decreased in the presence of F2 in HUVECs. Surprisingly, HIF-1α and
VEGF were also inhibited by F2 at the same time (Fig. 7D), suggesting that F2 disturbs the
HIF-1/VEGF pathway not only in tumor cells, but also in endothelial
cells. These results suggest that F2 may be a potential
anti-angiogenesis candidate which could inhibit tumor angiogenesis
by targeting the HIF-1/VEGF pathway.
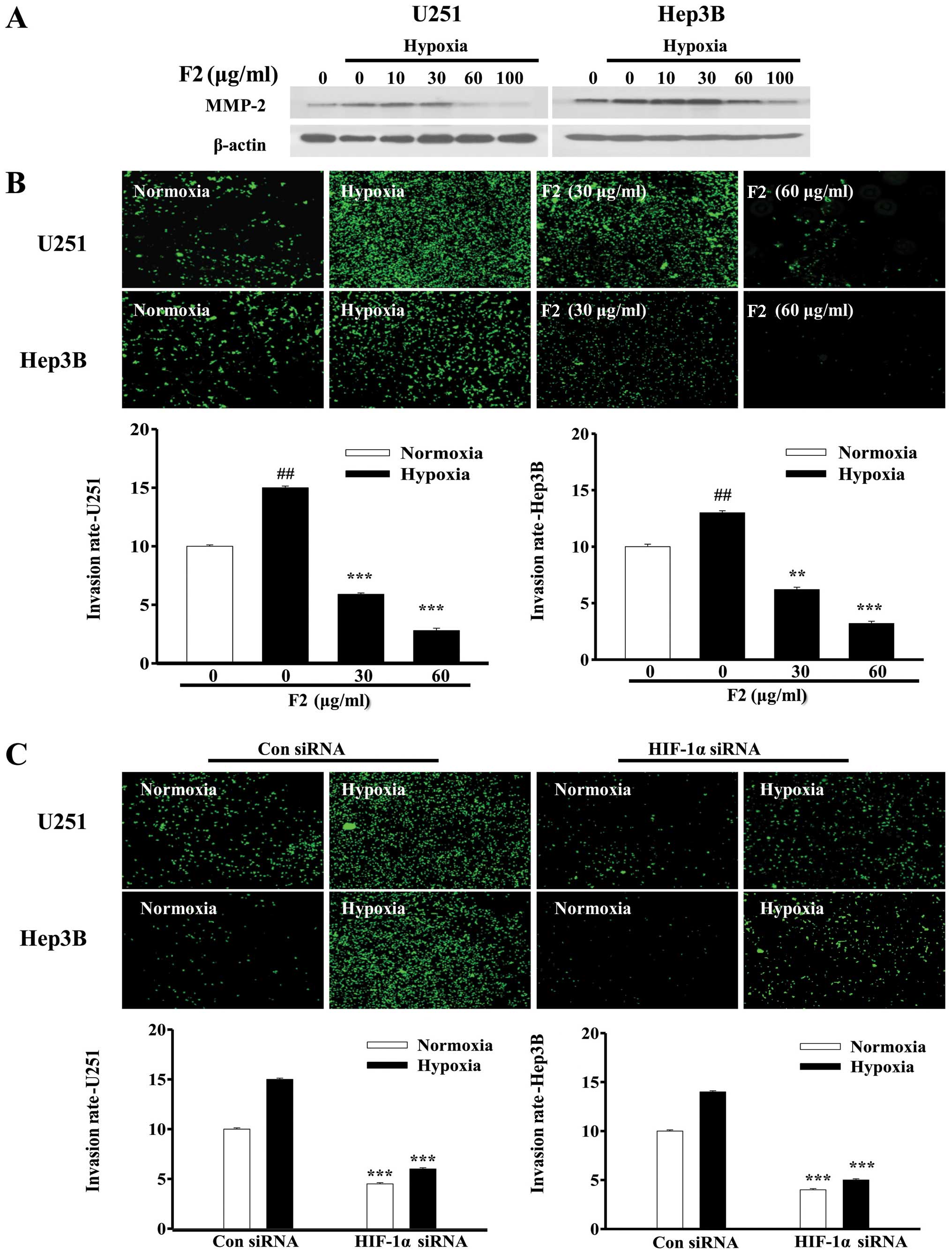
F2 inhibits tumor cell invasion in
vitro
Hypoxia also stimulates the invasion and migration
of tumor cell through activating the HIF-1 target genes, such as
MMP-2 (31). In this study, both
MMP-2 mRNA and protein induced by hypoxia were decreased in the
presence of F2 (Figs. 1B and
8A), indicating that F2 may
influence the tumor cell invasion by regulating HIF-1α expression.
As shown in Fig. 8B, Transwell
assay results showed that hypoxia significantly induced the
invasion of U251 and Hep3B cells (P<0.01), which could be
reversed by F2 (P<0.01). To further confirm that hypoxia-induced
cell invasion was mediated by HIF-1α, HIF-1α siRNA was used to
knock down the HIF-1α expression in both cell types. Invasion of
both types of tumor cells were impaired under normoxic and hypoxic
conditions when HIF-1α expression was knocked down (P<0.001,
Fig. 8C), suggesting that HIF-1α
mediated both U251 and Hep3B cell invasion. These results
demonstrate that F2 has strong effect upon inhibiting cell invasion
through downregulation of HIF-1α expression.
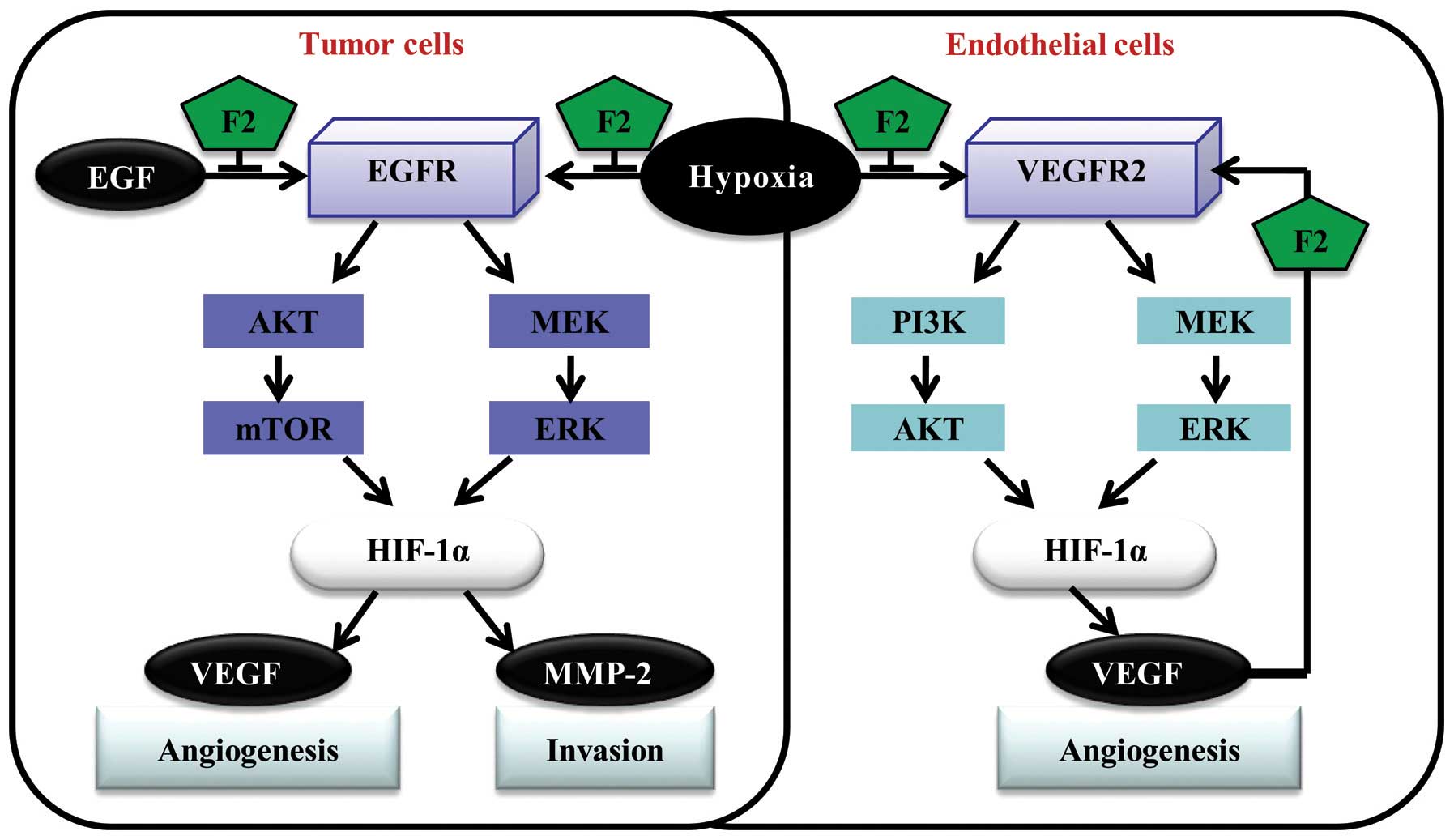
Discussion
Regarding the novel F2 natural antitumor product we
extracted, studies to date have shown that it may be a potential
antitumor agent, which have multiple antitumor effects through
various signaling pathways involved in cell survival, death,
migration and invasion (8,10). In the present study, F2 decreased
HIF-1α expression in U251 and Hep3B cells mainly through disturbing
the PI3K-AKT-mTOR and MAPK-ERK1/2 pathways, and finally inhibited
tumor angiogenesis and cell invasion in vitro (Fig. 9). These findings provide new
insights into our previous studies and potentially explain that
antitumor effect of F2 may be linked to HIF-1α.
As the only oxygen-regulated subunit, HIF-1α is
highly expressed in solid tumors and regulates expression of
multiple genes involved in tumorigenesis. Many studies have shown
that knocking down the expression of HIF-1α could significantly
control the tumor growth, inhibit angiogenesis and invasion
(29,32,33),
so we focused our study on HIF-1α. To date it has been shown that
multiple signaling pathways are involved in the regulation of
hypoxia-induced HIF-1α protein stabilization and expression. To
define the signaling mechanisms by which F2 inhibited
hypoxia-induced HIF-1α expression, we examined the effects of F2 on
the activation of PI3K-AKT-mTOR and ERK1/2 pathways in U251 and
Hep3B cells in response to hypoxia. Our results indicated that F2
significantly inhibited hypoxia-mediated activation of AKT, mTOR
and ERK1/2 in both cell types, which were consistent with its
inhibitory effects on hypoxia-induced HIF-1α protein accumulation
and HIF-1α target gene expression. mTOR is known to regulate the
HIF-1α protein translation by controlling the phosphorylation of
the downstream effectors P70S6K and 4E-BP1 (34). We found that F2 could inhibit the
hypoxia-induced P70S6K and 4E-BP1 in U251 and Hep3B cell lines.
Collectively, these findings suggest that F2 probably inhibits
HIF-1α translation by blocking the activation of PI3K/AKT-mTOR and
ERK1/2 pathways.
EGFR might increase the cellular response to hypoxia
by increasing HIF-1α expression (35). The anti-EGFR antibody, cetuximab,
can sensitize human head and neck squamous cell carcinoma cells to
radiation in part through inhibiting radiation-induced HIF-1α
(36). In our study, we
demonstrated for the first time that both hypoxia and EGF could
induce the expression of HIF-1α and VEGF in both cells by
activation of EGFR. F2 was able to inhibit the expression of HIF-1α
in both cell lines induced by hypoxia or EGF. These results
indicate that EGFR is a regulator of HIF-1α in both U251 and Hep3B
cells, and may also be a potential target of F2, but the latter
speculation needs further study.
Overexpression of HIF-1α has been found to be
closely related with tumor angiogenesis and invasion (26,37).
F2 could inhibit the hypoxia-simulated tumor angiogenesis and cell
invasion in vitro in a HIF-1α-dependent manner, and MMP-2
may be the key protein participating in the inhibitory effect of F2
on cell invasion because it could be involved in the breakdown of
the extracellular matrix (31).
However, there are other proteins participating in the
hypoxia-induced tumor invasion, such as CXC chemokine receptor 4
(CXCR4) and interleukin-8 (IL-8) (38,39).
Accordingly, additional studies are underway to identify the
associated genes that are directly or indirectly involved in the
inhibitory effect of F2 on tumor cell invasion in response to
hypoxia and/or HIF-1α overexpression.
Under hypoxic conditions, HIF-1α is stabilized and
forms heterodimers with constantly-expressed HIF-1β. It is the
current opinion that hypoxia has no effect on the levels of HIF-1β
(40,41). So we and most other researchers
only focus our study on the oxygen-regulated HIF-1α subunit.
However, in our study, hypoxia (16 h) also slightly induced the
expression of HIF-1β in both U251 and Hep3B cells. F2 also
inhibited the expression of HIF-1β protein under normoxic and
hypoxic conditions in accordance with HIF-1α. A study published
recently reported that hypoxia might induce the expression of
HIF-1β in Hep3B cell lines by inducing the expression of HIF-1α,
but this relation is cell-type specific and not widely existing in
tumor cells (42). Hence, it is
speculated that F2 may decrease the level of HIF-1β by inhibiting
HIF-1α expression, and HIF-1β should be further investigated,
especially regarding tumorigenesis, because HIF-1β regulation is
far more complex than appreciated today.
In conclusion, we have shown for the first time that
F2 decreased HIF-1α, VEGF and MMP-2 expression in U251 and Hep3B
cells mainly through interfering with PI3K-AKTmTOR and ERK1/2
pathways, leading to inhibition of tumor angiogenesis and cell
invasion in vitro.
Acknowledgements
We are grateful to Dr Giovanni Melillo for his
generous gift of the U251-HRE, U251-pGL3 cell lines and the
pGL2-TK-HRE plasmid. This study was partially supported by the
National Natural Science Foundation of China (no. 30973560) and
National High Technology Research and Development Program of China
(863 Program) (no. 2012AA020305).
References
|
1
|
Melillo G: Targeting hypoxia cell
signaling for tumor therapy. Cancer Metastasis Rev. 26:341–352.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Semenza GL: HIF-1 and tumor progression:
pathophysiology and therapeutics. Trends Mol Med. 8:62–67. 2002.
View Article : Google Scholar
|
|
3
|
Semenza GL: Targeting HIF-1 for tumor
therapy. Nat Rev Cancer. 3:1–11. 2003. View
Article : Google Scholar
|
|
4
|
Semenza GL: HIF-1: upstream and downstream
of tumor metabolism. Curr Opin Genet Dev. 20:51–56. 2010.
View Article : Google Scholar
|
|
5
|
Sun BS, Belchior GP, Ricardo da Silva JM
and Spranger MI: Isolation and purification of dimeric and trimeric
procyanidins from grape seeds. J Chromatogr A. 841:115–121. 1999.
View Article : Google Scholar
|
|
6
|
Sun BS, Leandro C, Ricardo da Silva JM and
Spranger MI: Separation of grape and wine proanthocyanidins
according to their degree of polymerization. J Agr Food Chem.
46:1390–1396. 1998. View Article : Google Scholar
|
|
7
|
Spranger MI, Sun BS, Mateus A, et al:
Chemical characterization and antioxidant activities of oligomeric
and polymeric procyanidin fractions from grape seeds. Food Chem.
108:519–532. 2008. View Article : Google Scholar
|
|
8
|
Huang M, Sun BS, Zhao YQ, et al: Effects
of catechin and its polymers on ethanol-induced ascorbic acid and
hydroxyl radical release in mouse striatum. J Chin Nat Med.
1:34–40. 2003.
|
|
9
|
Zhang FJ, Yang JY, Mou YH, et al:
Inhibition of U-87 human glioblastoma cell proliferation and formyl
peptide receptor function by oligomer procyanidins (F2) isolated
from grape seeds. Chem Biol Interact. 179:419–429. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang JY, Wang Q, Zhao RJ, et al:
Identification of oligomer proanthocyanidins (F2) isolated from
grape seeds as a formyl peptide receptor 1 partial agonist. Int
Immunopharmacol. 15:756–763. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang J, Chen K, Chen J, et al: The
G-protein-coupled formyl-peptide receptor FPR confers a more
invasive phenotype on human glioblastoma cells. Br J Cancer.
102:1052–1060. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Huang J, Zhou Y, et al: The role of
chemo-attractant receptors in the progression of glioma. INTECH.
21:286–305. 2011.
|
|
13
|
Guo L, Wang LH, Sun BS, et al: Direct in
vivo evidence of protective effects of grape seed procyanidin
fractions and other antioxidants against ethanol-induced oxidative
DNA damage in mouse brain cells. Food Chem. 55:5881–5891. 2007.
View Article : Google Scholar
|
|
14
|
Liu Y, Li YM, Tian RF, et al: The
expression and significance of HIF-1α and GLUT-3 in glioma. Brain
Res. 1304:149–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang HF, Qian DZ, Tan YS, et al: Digoxin
and other cardiac glycosides inhibit HIF-1α synthesis and block
tumor growth. Proc Natl Acad Sci USA. 105:19579–19586. 2008.
View Article : Google Scholar
|
|
16
|
Rapisarda A, Uranchimeg B, Scudiero DA, et
al: Identification of small molecule inhibitors of
hypoxia-inducible factor-1 transcriptional activation pathway.
Cancer Res. 62:4316–4324. 2002.PubMed/NCBI
|
|
17
|
Xia MH, Bi K, Huang RL, et al:
Identification of small molecule compounds that inhibit the HIF-1
signaling pathway. Mol Cancer. 8:1–13. 2009. View Article : Google Scholar
|
|
18
|
Chau NM, Rogers P, Aherne W, et al:
Identification of novel small molecule inhibitors of
hypoxia-inducible factor-1 that differentially block
hypoxia-inducible factor-1 activity and hypoxia-inducible factor-1α
induction in response to hypoxic stress and growth factors. Cancer
Res. 65:4918–4928. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao Y, Wu JH, Wu SP, et al: A recombinant
cell-permeable p53 fusion protein is selectively stabilized under
hypoxia and inhibits tumor cell growth. Cancer Lett. 279:101–107.
2009. View Article : Google Scholar
|
|
20
|
Kamat CD, Green DE, Warnke L, et al:
Mutant p53 facilitates pro-angiogenic, hyper-proliferative
phenotype in response to chronic relative hypoxia. Cancer Lett.
249:209–219. 2007. View Article : Google Scholar
|
|
21
|
Zeng M, Xiao F, Zhong X, et al: Reactive
oxygen species play a central role in hexavalent chromium-induced
apoptosis in Hep3B cells without the functional roles of p53 and
caspase-3. Cell Physiol Biochem. 32:279–290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kizaka-Kondoh S, Kuchimaru T and
Kadonosono T: Pathophysiological response to hypoxia-from the
molecular mechanisms of malady to drug discovery: hypoxia-inducible
factor-1 (HIF-1) active cells as a target for tumor therapy. J
Pharmacol Sci. 115:440–445. 2011. View Article : Google Scholar
|
|
23
|
Jung HJ, Park JW, Lee JS, et al: Silibinin
inhibits expression of HIF-1α through suppression of protein
translation in prostate tumor cells. Biochem Biophys Res Commun.
390:71–76. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jung HJ, Suh SI, Suh MH, et al:
Pentamidine reduces expression of hypoxia-inducible factor-1α in
DU145 and MDA-MB-231 tumor cells. Cancer Lett. 303:39–46. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karar J and Maity A: PI3K/AKT/mTOR pathway
in angiogenesis. Front Mol Neurosci. 4:1–8. 2011. View Article : Google Scholar
|
|
26
|
Zhong H, Chiles K, Feldser D, et al:
Modulation of hypoxia-inducible factor 1α expression by the
epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP
pathway in human prostate cancer cells: implications for tumor
angiogenesis and therapeutics. Cancer Res. 00:1541–1545. 2000.
|
|
27
|
Ban HS, Uno M and Nakamura H: Suppression
of hypoxia-induced HIF-1α accumulation by VEGFR inhibitors:
different profiles of AAL993 versus SU5416 and KRN633. Cancer Lett.
296:17–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang QZ, Tang XD, Lu QY, et al: Green tea
extract and (−)-epigallocatechin-3-gallate inhibit hypoxia- and
serum-induced HIF-1α protein accumulation and VEGF expression in
human cervical carcinoma and hepatoma cells. Mol Cancer Ther.
5:1227–1238. 2010. View Article : Google Scholar
|
|
29
|
Medici D and Olsen BR: Rapamycin inhibits
proliferation of hemangioma endothelial cells by reducing
HIF-1-dependent expression of VEGF. PLoS One. 7:e429132012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Namiki A, Brogi E, Kearney M, et al:
Hypoxia induces vascular endothelial growth factor in cultured
human endothelial cells. J Biol Chem. 270:31189–31195. 2010.
|
|
31
|
Fujiwara S, Nakagawa K, Harada H, et al:
Silencing hypoxia-inducible factor-1 inhibits cell migration and
invasion under hypoxic environment in malignant gliomas. Int J
Oncol. 30:793–802. 2010.
|
|
32
|
Lu JM, Zhang KQ, Chen S and Wen W: Grape
seed extract inhibits VEGF expression via reducing HIF-1α protein
expression. Carcinogenesis. 30:636–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Manolescu B, Oprea E, Busu C and Cercasov
C: Natural compounds and the hypoxia-inducible factor (HIF)
signaling pathway. Biochimie. 91:1347–1358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wullschleger S, Loewith R and Hall MN: TOR
signaling in growth and metabolism. Cell. 124:471–484. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Swinson DE and O’Byrne KJ: Interactions
between hypoxia and epidermal growth factor receptor in
non-small-cell lung tumor. Clin Lung Cancer. 7:250–256. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu HQ, Liang K, Lu Y and Fan Z: The
anti-EGFR antibody cetuximab sensitizes human head and neck
squamous cell carcinoma cells to radiation in part through
inhibiting radiation-induced upregulation of HIF-1α. Cancer Lett.
322:78–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheung W, Wellman TL and Lounsbury KM:
VEGF and HIF-1α expression are increased in advanced stages of
epithelial ovarian tumor. Gynecol Oncol. 91:513–517. 2003.
View Article : Google Scholar
|
|
38
|
Ahn JK, Koh EM, Cha HS, et al: Role of
hypoxia-inducible factor-1 in hypoxia-induced expressions of IL-8,
MMP-1 and MMP-3 in rheumatoid fibroblast like. Rheumatology.
47:834–839. 2003. View Article : Google Scholar
|
|
39
|
Wang XB, Li CX, Chen Y, et al: Hypoxia
enhances CXCR4 expression favoring microglia migration via HIF-1α
activation. Biochem Biophys Res Commun. 371:283–288. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Berchner Pfannschmidt U, Frede S, Wotzlaw
C and Fandrey J: Imaging of the hypoxia-inducible factor pathway:
insights into oxygen sensing. Eur Respir J. 32:210–217. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Semenza GL: Hypoxia-inducible factors in
physiology and medicine. Cell. 148:399–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wolff M, Jelkmann W, Dunst J and Depping
R: The aryl hydrocarbon receptor nuclear translocator (ARNT/HIF-1β)
is influenced by hypoxia and hypoxia-mimetics. Cell Physiol
Biochem. 32:849–858. 2013. View Article : Google Scholar
|