Introduction
Despite recent advances in therapeutic treatments
using multimodal therapies, including the excision of malignant
tissue combined with radio- and chemotherapy, high oral squamous
cell carcinoma (OSCC) mortality rates of ~50% have not improved
over time (1). The main reason for
this is that early lymph node metastasis of cancer cells is
difficult to detect (2,3). When induction chemotherapy with
cis-diamminedichloroplatinum (II) (CDDP) and 5-fluorouracil
was introduced for treatment of squamous cell carcinoma of the head
and neck (SCCHN) in the early 1980s, response rates of 30–40% were
initially reported (4,5). A problem that emerged during OSCC
treatment was increased resistance against many of the approved
chemotherapeutic agents, such as paclitaxel, docetaxel, cetuximab
and CDDP (6).
CDDP is used to treat a range of malignancies,
including SCCHN. CDDP resistance studied in cultured cancer cells
has shown that cellular defense mechanisms in a highly complex
pleiotropic phenotype confers resistance by reducing apoptosis,
upregulating DNA damage repair mechanisms, altering cell-cycle
checkpoints, and disrupting assembly of the cytoskeleton (7). Alterations to the cytoskeleton
disrupt cellular protein trafficking, and redirect transporters
away from the cell surface. This results in cells that are
permanently resistant to CDDP, and also resistant to other
compounds that usually enter into cells via uptake transporters.
The pleiotropic mechanisms underlying CDDP resistance are well
described but poorly understood in their entirety and in terms of
clinical significance (7,8).
The Hippo pathway was initially identified for
sensing and regulating organ size in Drosophila and mammals
(9). In the Hippo pathway, YAP is
a transcriptional co-activator that participates in several
context-dependent transcriptional programs that regulate organ size
and promote cell proliferation (10). YAP was proposed as a candidate
oncogene and its dysregulation associated with hepatocellular
carcinoma, non-small cell lung carcinoma, esophageal squamous cell
carcinoma, ovarian cancer and gastric cancer (9,11–14).
YAP is also reported to cause epithelial-mesenchymal transition
(EMT), stimulate proliferation, inhibit apoptosis, and to promote
tumor progression in a tissue-specific manner (9,12).
Ge et al reported that YAP expression in primary SCCHN was
associated with nodal metastasis due to EMT (15). Zhao et al identified YAP as
an important player in response to apoptosis of cancer cells
induced by the anti-tubulin drug paclitaxel (16). Whether YAP overexpression confers
CDDP resistance to cancer cells is unclear, therefore we sought to
determine whether this was the case.
Materials and methods
Cell culture and establishment of
CDDP-resistant cell lines
OSCC cell lines were maintained on 100-mm plates at
37°C/5% CO2, and grown as monolayers in Dulbecco’s
modified Eagle’s medium (DMEM), supplemented with L-glutamine,
penicillin, streptomycin and 10% (v/v) fetal bovine serum (FBS).
The OSC-19 cell culture was provided by the Kanazawa University
Graduate School of Medical Science (Kanazawa, Japan). CDDP was
purchased from Nichi-Iko Pharmaceutical Co. Ltd. and stored as a
0.5 mg/ml stock solution in 0.9% (w/v) NaCl at room temperature and
shielded from light.
CDDP-resistant variants of the OSCC cell line were
isolated using stepwise selection by increasing the concentration
of CDDP. To generate the resistant cells the starting CDDP
concentration was 0.5 μg/ml; once cultures became confluent in
medium containing CDDP, its concentration was increased to 1.0,
1.5, 2.0, 2.5 or 3.0 μg/ml. Cells were continuously exposed to CDDP
for 4–8 weeks at each concentration, with culture medium refreshed
every 3 days. Cultures were passage once a week over a 40-week
period. Established CDDP-resistant cell cultures were designated
OSC-19-R, SCCKN-R, and HSC-3-R, and maintained in the presence of
CDDP.
Growth assays and determination of CDDP
sensitivity
The sensitivity of cells to CDDP was determined by
also using the Cell Counting Kit-8 (Dojindo, Kumamoto, Japan). On
day 1, cells (5.0x103 cells/well in 100 μl of DMEM) were
seeded in 96-well plates and cultured at 37°C. On day 2, the medium
was replaced with 100 μl of fresh DMEM containing 0.33–167 μM CDDP
and cells were allowed to incubate for 3 days. On day 5, 10 μl of
Cell Counting Kit-8 solution was added; after 3 h, the absorbance
in each well was measured at 450 nm using a microplate
spectrophotometer (Bio-Rad, Hercules, CA, USA). The fold increase
or decrease in resistance was determined by dividing the
IC50 value of CDDP for resistant cells with that for
parental cells.
Western blotting
Cell lysates were subjected to western blotting as
described previously (17). The
primary antibodies used were rabbit polyclonal antibodies against
YAP, phospho-YAP (serine-127), TEAD, LATS1, LATS2, AKT, and
phospho-AKT (serine-473) (Cell Signaling Technology, Boston, MA,
USA), glutathione S-transferase (GST)-π (Calbiochem, USA), ATP7B,
ERCC1, P73 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). We used
a goat polyclonal antibody against actin (Santa Cruz Biotechnology)
as an internal control. The secondary antibodies we used were
anti-goat and anti-rabbit immunoglobulin Gs (IgGs) conjugated with
alkaline phosphatase (Santa Cruz Biotechnology).
Immunocytochemistry
The primary antibodies we used in our
immunocytochemistry analysis included rabbit anti-YAP (Cell
Signaling Technology) and a mouse polyclonal antibody against human
actin (Santa Cruz). Cultured cells were fixed in 3.7%
paraformaldehyde for 20 min at room temperature. After blocking
with 2% (w/v) bovine serum albumin (BSA), cells were treated with a
primary antibody at 4°C overnight. Cells were washed, and then
incubated with an anti-rabbit IgG conjugated to fluorescein
isothiocyanate (FITC) or an anti-mouse IgG conjugated to rhodamine
phalloidin (Cytoskeleton, Denver, CO, USA). Cells were then stained
with 4,6-diamidino-2-phenylindole (DAPI). Fluorescence images were
obtained using a confocal laser-scanning microscope (LSM 510
version 3.2; Carl Zeiss Co. Ltd., Oberkochen, Germany).
Transfection of siRNAs
Transfection of siRNAs was conducted as described
previously (17). Cells were
cultured in DMEM supplemented with 10% FBS for 24 h and transfected
with 5 μM siRNA using Thermo Scientific DharmaFECT Transfection
reagents (Roche, Indianapolis, IN, USA) according to the
manufacturer’s instructions. SMART pool siRNAs targeting YAP
(L-012200-00-0020) and control siRNA, and On-Target plus GAPDH
(D-001830-01-20) were purchased from Dharmacon Inc. (Lafayette, CO,
USA).
Statistical analysis
Data are expressed as means ± SD. Results were
analyzed, and individual group means were compared using Student’s
t-test. A p-value of <0.05 was considered statistically
significant.
Results
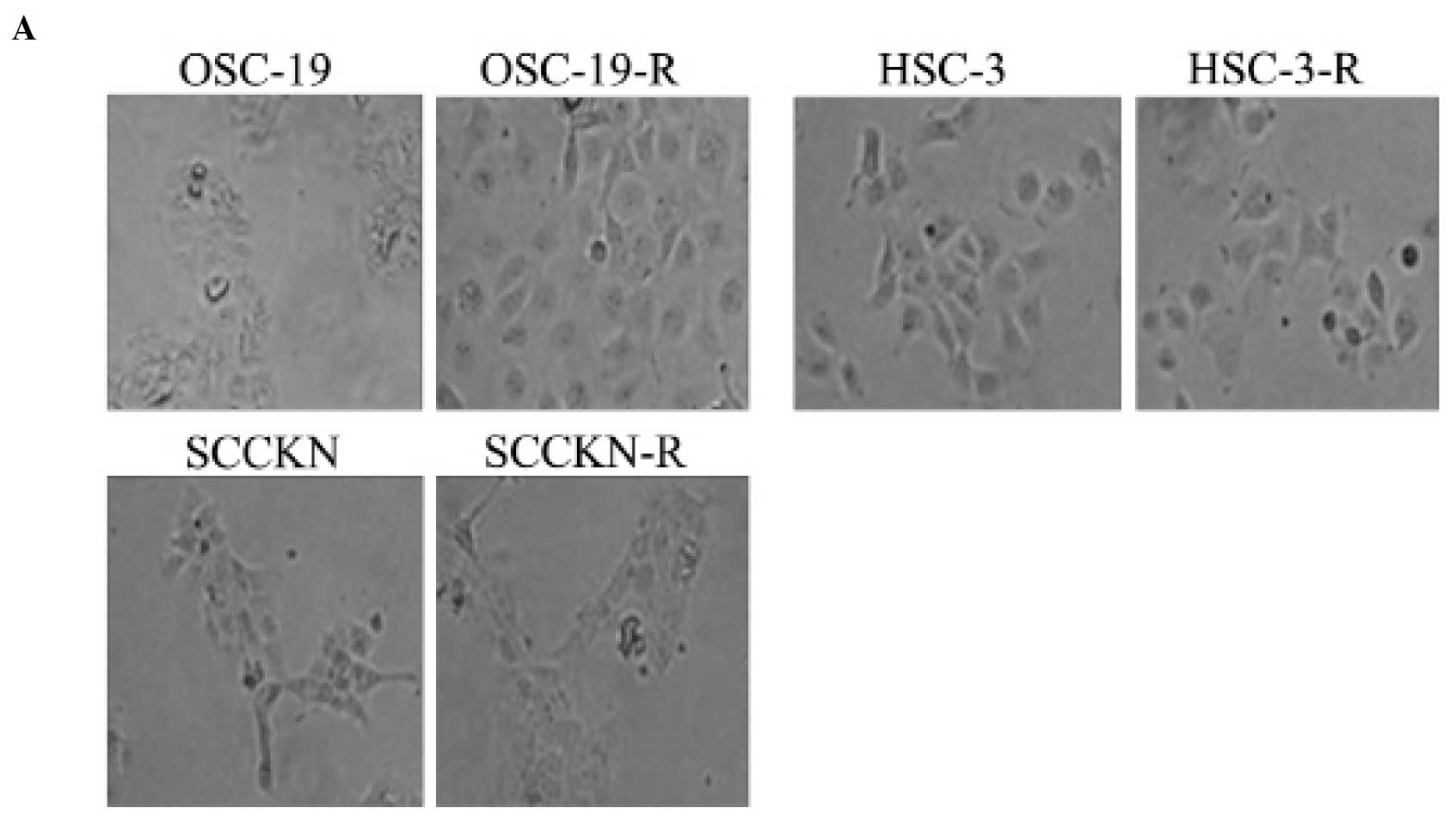
CDDP-resistant cell lines
When the CDDP concentration was raised after a step,
~30% cell death occurred. After ~1 month, OSCC cells acquired
resistance to CDDP step-by-step. The CDDP-resistant cell lines we
generated from SCCKN, OSC-19, and HSC-3 were designated SCCKN-R,
OSC-19-R, and HSC-3-R (Fig. 1A).
The CDDP IC50 for each cell line was determined using an
MTT assay (Table I); the SCCKN-R,
OSC-19-R, and HSC-3-R cell lines were 5.7-, 6.6- and 5.5-fold more
resistant to CDDP than their parental cells, respectively. There
was a significant association for each IC50 value
between parental and CDDP-resistant cell lines.
 | Table ISensitivity of CDDP for each of the
cell lines we used and generated.a |
Table I
Sensitivity of CDDP for each of the
cell lines we used and generated.a
| Cell line | IC50 in μM
(mean ± SD) | Fold resistance to
CDDP compared with parental cell line | p-value |
|---|
| OSC-19 | 17.1±12.7 | - | |
| OSC-19-R | 113.3±17.6 | 6.6 | <0.01 |
| SCCKN | 1.7±0.3 | - | |
| SCCKN-R | 9.8±0.8 | 5.7 | <0.01 |
| HSC-3 | 9.9±0.9 | - | |
| HSC-3-R | 54.6±4.5 | 5.5 | <0.01 |
Western blotting of ATP7B protein
levels
The CDDP-resistant cell lines, SCCKN-R and HSC-3-R,
showed overexpression of the ATP-7B protein (Fig. 1B). There were no obvious difference
in expression levels of GST-π and ERCC1 between parental and
CDDP-resistant cell lines. We did not observe any difference in
expression levels of the apoptotic cascade protein caspase-3/7 in
OSC-19 and OSC-19-R cells (data not shown).
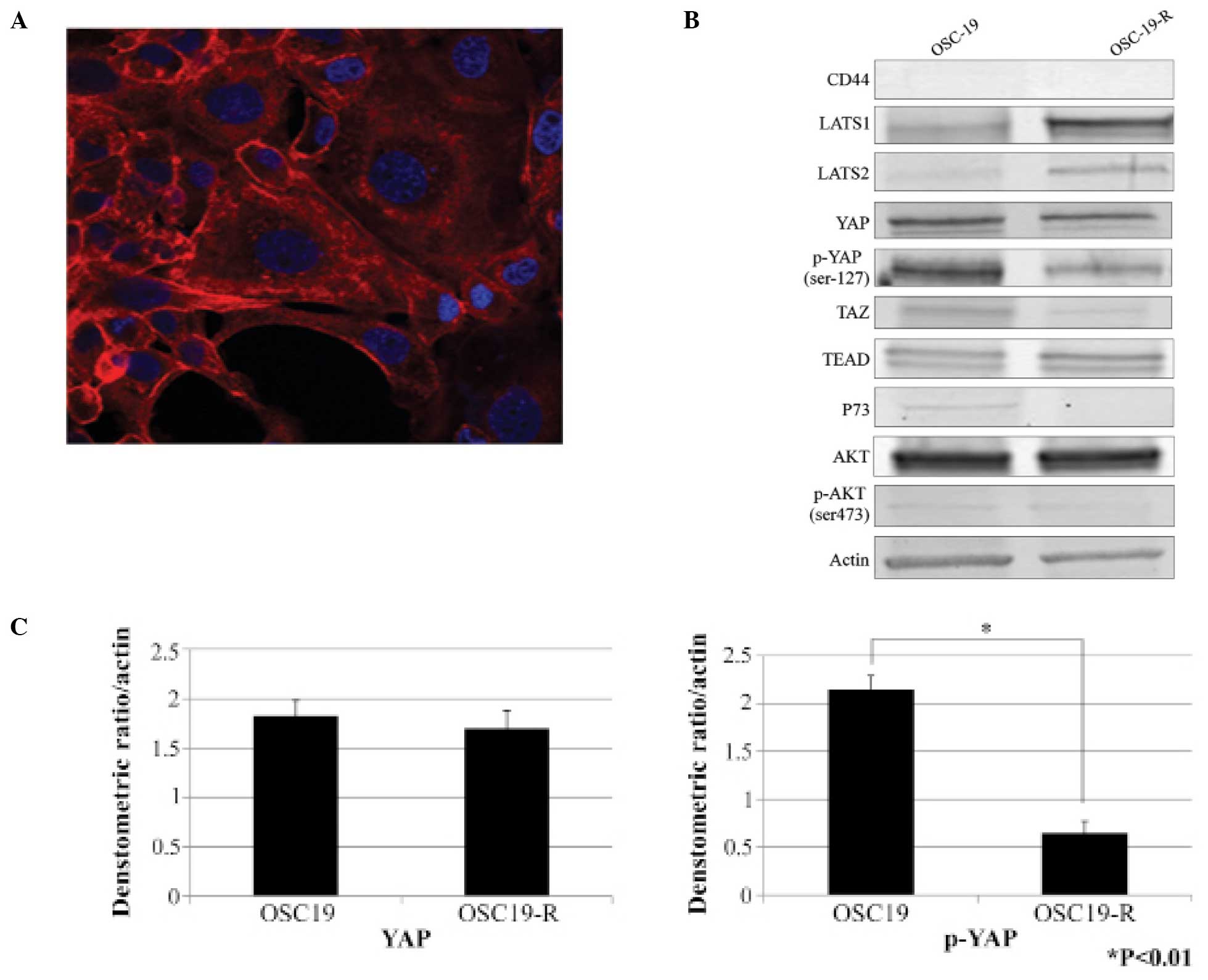
A new mechanism involving YAP induced
CDDP resistance
Compared with parental OSC-19 cells, OSC-19-R cells
were obviously larger (Fig. 2A).
We investigated the Hippo pathway as it is known to regulate organ
size. Expression levels of YAP were not significantly different in
the OSC-19 and OSC-19-R cell lines, however, expression levels of
phosphorylated YAP in OSC-19-R cells were decreased (Fig. 2B and C). In addition, there was no
difference in CD44, TEAD, P73, AKT, or phosphorylated AKT
expression levels. While high expression levels of LATS1/2 were
revealed, our results indicated that expression levels of
non-phosphorylated YAP, a substrate of LATS1/2, were decreased. Our
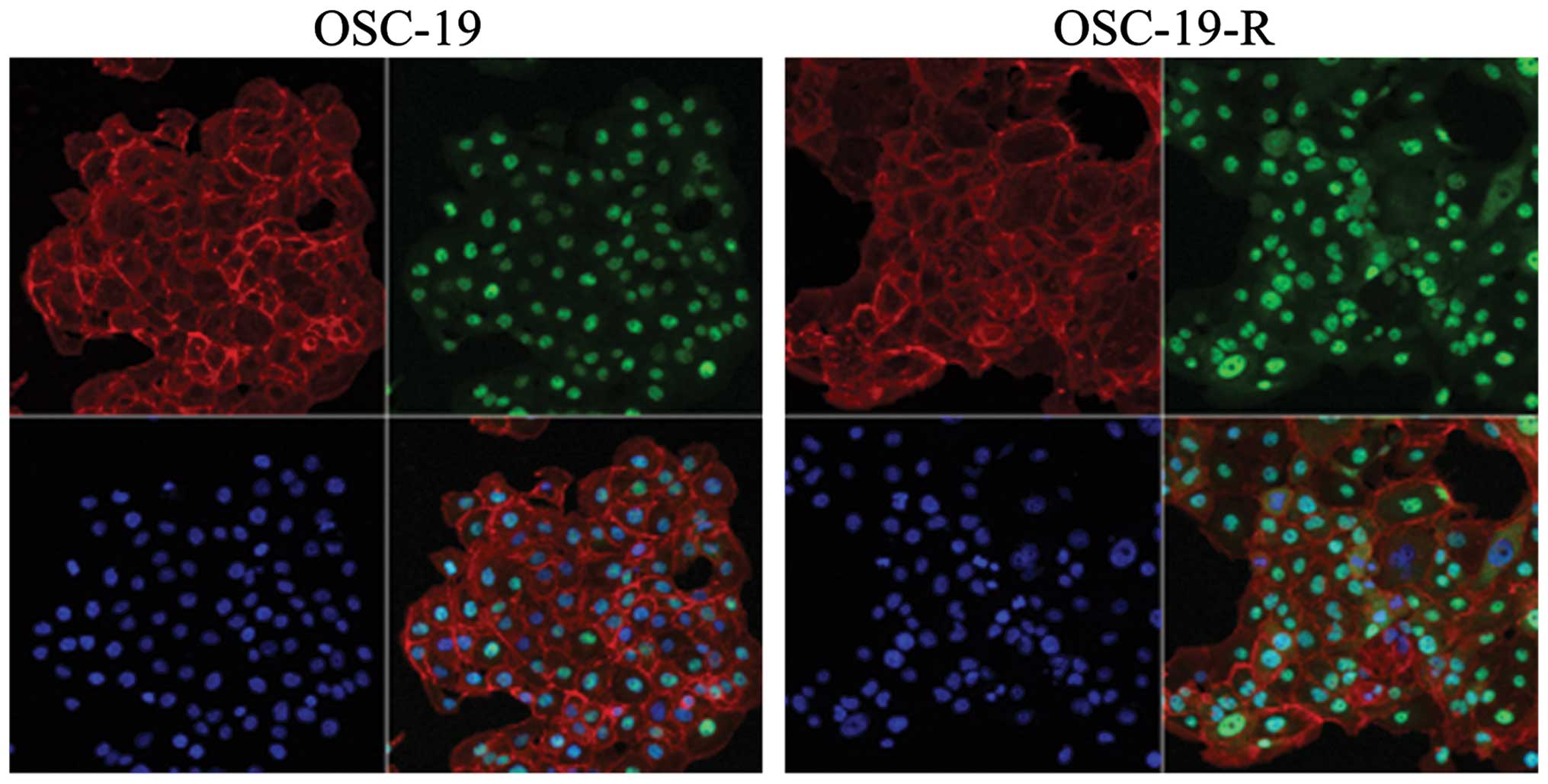
immunocytochemistry results revealed that YAP translocated from the
cytoplasm to the nucleus in OSC-19-R cells (Fig. 3).
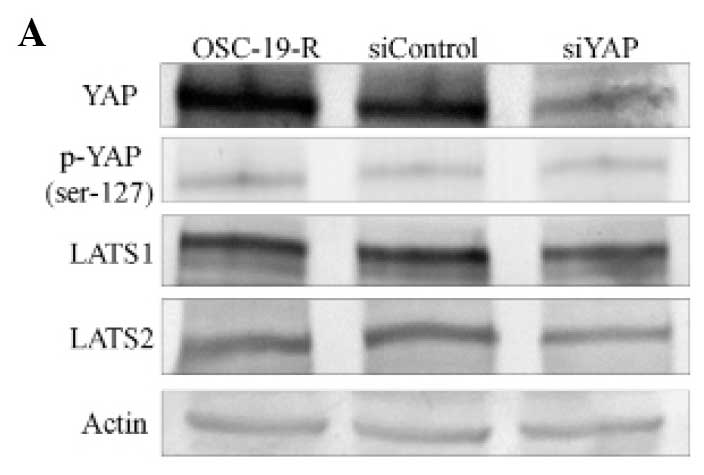
YAP nuclear translocation inhibited by
siRNAs
To clarify the translocation of YAP in
CDDP-resistant cells, knockdown experiments using siRNAs were
conducted. We observed a dramatic reduction of YAP expression in
YAP-specific siRNA-treated cells compared with cells treated with
control siRNAs using western blotting. Expression levels of
phospho-YAP and LATS1/2 were not inhibited by siRNA treatments
(Fig. 4A and B). Our
immunocytochemistry results confirmed that expression levels of YAP
were reduced in YAP-specific siRNA-treated cells (Fig. 4C). The OSC-19-R cells treated with
YAP-specific siRNAs showed a reduction in YAP nuclear
translocation.
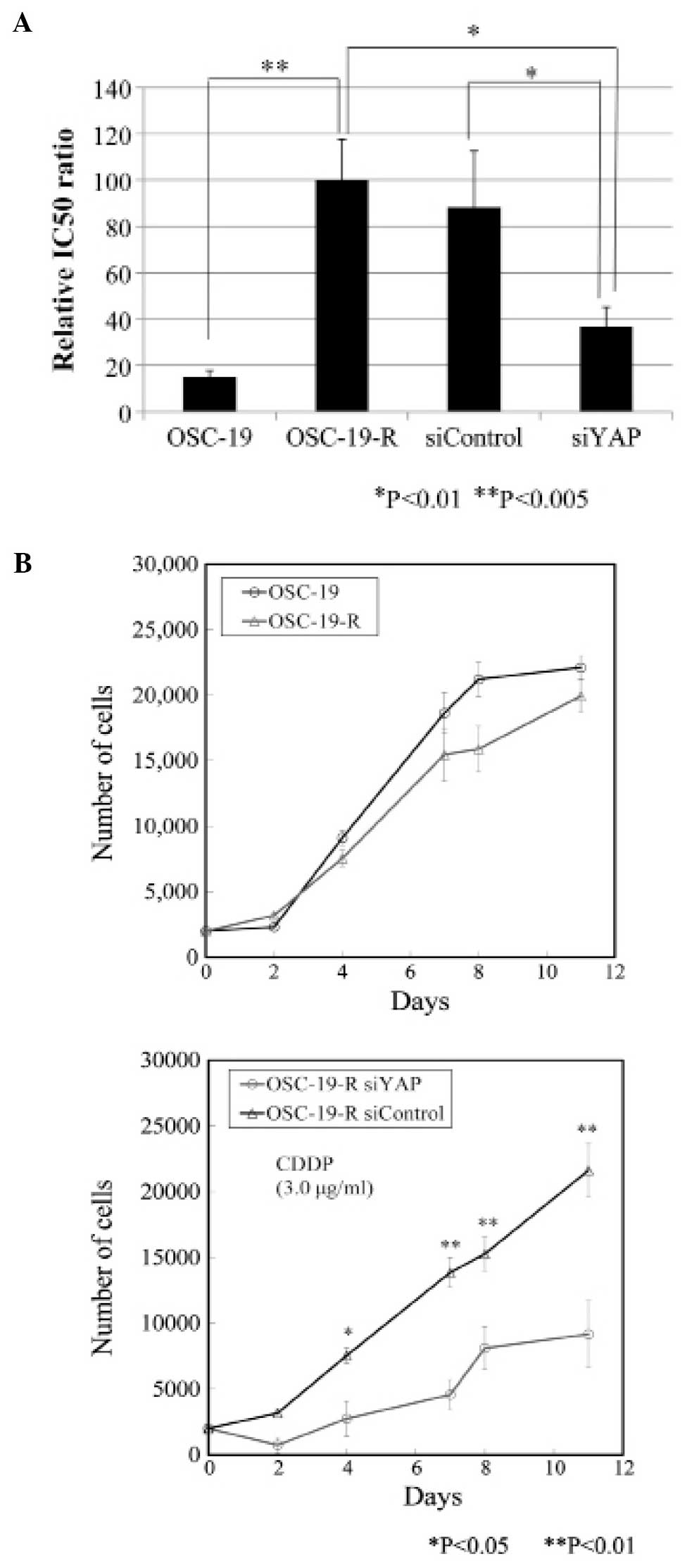
Overcoming acquired CDDP resistance with
YAP siRNAs
Nuclear translocation of YAP appeared to induce
acquired CDDP resistance in the OSC-19 cell line. Treatment with
YAP-specific siRNAs ameliorated acquired CDDP resistance, with
sensitivity to CDDP increased in OSC-19-R cells transfected with
siYAP, and an IC50 reduction rate of 38.0% calculated
(Fig. 5A). Statistically, there
was no difference between the OSC-19 and OSC-19-R cells as shown by
growth curves, but there was obviously difference between
OSC-19-R-siControl and OSC-19-R-siYAP cells treated with CDDP
(Fig. 5B). Proliferation of
OSC-19-R-siYAP cells was significantly inhibited by CDDP from day 2
onwards.
Discussion
Many studies have been conducted to investigate
mechanisms of acquired resistance in tumor cells to CDDP (18). The molecular mechanisms that
underlie CDDP resistance are not well understood. From our results
in the present study, we propose a new mechanism of acquired
resistance to CDDP that is related to the Hippo pathway. The Hippo
pathway controls organ size in diverse species, and dysregulation
of this pathway can induce tumors (19). To the best of our knowledge, we are
the first to report that translocation of YAP induced acquired
resistance to CDDP in OSCC, and that repression of YAP expression
with siRNAs increased sensitivity to CDDP in cells that have
acquired CDDP resistance.
We established three CDDP-resistant cell lines, with
two of these cell lines exhibiting higher expression levels of
ATP7B than those of other molecules when they acquired CDDP
resistance. With respect to CDDP transport, CTR1 and ATP7B
influence its uptake and efflux, respectively. ATP7B is a
Cu2+-ATPase, with these enzymes known to be homologous
in structure and function, and able to mediate the efflux of
Cu2+ (20). The major
Cu2+ uptake transporter is copper transporter 1 (CTR1).
Recent results have shown that the Cu2+ homeostasis
system also regulates the uptake, intracellular
compartmentalization and efflux of CDDP (20–22).
It was reported that cell lines acquired resistance to CDDP due to
overexpression of ATP7B, resulting in greater efflux of CDDP
(23). Based on the finding that
overexpression of ATP7B induced acquired resistance to CDDP in
OSCC, we ensured that ATP7B was overexpressed in the SCCKN-R and
HSC-3-R cell lines we established. The previous study (23) also reported overexpression of ATP7B
in CDDP-resistant OSC-19 cells they generated, however, we did not
observe this in our OSC-19-R cells. Moreover, the IC50
of OSC-19-R was 30.6 μM (23),
which is about one-third the level of that determined in our
present study. We postulate that this discrepancy is a result of
the difference in how the resistance was acquired. Dose escalation
of CDDP was more harsh in the previous report compared with the
method we used (23). Under
clinical situations, high dose CDDP chemotherapy, which is an
example of intra-arterial super-selective chemotherapy, is widly
and safely used on a daily basis. Therefore, we sought to establish
CDDP-resistant cell lines that were dependent upon higher fixed
concentrations of CDDP exposure.
Although much progress has been made towards our
understanding of the role of the Hippo pathway with regards to
tumorigenesis, organ size control, and stem cell renewal and
differentiation (24–27), its function(s) with respect to
chemotherapeutic drug response is largely unknown (28). In human HNSCC and OSCC lines,
amplification of the chromosomal region that encodes YAP, 11q21-22,
is frequently seen (29,30). Reports also exist showing that core
components of the Hippo pathway might be involved in the response
of cancer cells to chemotherapeutic drugs such as paclitaxel
(16,31–34).
In the present study, we established YAP as an important protein
for CDDP drug resistance, indicating a strong link between EMT and
drug resistance (35). It is
possible that phosphorylation and inactivation of YAP might
compromise its ability to trans-activate another transcription
factor that suppresses E-cadherin and induces EMT. Moreover,
because EMT plays important roles in stem cell renewal, cell
migration, anoikis, invasion, and metastasis (16,36,37),
it would also be interesting to further explore how phosphorylation
of YAP under physiological condition regulates these processes.
In conclusion, our results revealed a new mechanism
for YAP, which could be used as a molecular target to overcome
acquired resistance of CDDP. Further examination of the
relationship between YAP levels, translocation, and phosphorylation
status of YAP, and the survival of clinical cancer patients before
and after treatment with CDDP, are required to sufficiently
determine if YAP and phospho-YAP can be used as prognostic
biomarkers for predicting sensitivity to CDDP. It is possible YAP
and phospho-YAP could be potential therapeutic targets for the
treatment of cancer patients that are drug-resistant.
Acknowledgements
The authors thank Dr Keiko Wakai for preparation of
the experiments and Ms. Takako Nanba for management of grants. This
study was supported by Grants-in-Aid for JSPS KAKENHI (grant nos.
25463123 to K.N. and 26861760 to K.Y.).
Abbreviations:
|
OSCC
|
oral squamous cell carcinoma
|
|
CDDP
|
cisplatin
|
|
SCCHN
|
squamous cell carcinoma of the head
and neck
|
|
EMT
|
epithelial-mesenchymal transition
|
|
DMEM
|
Dulbecco’s modified Eagle’s medium
|
References
|
1
|
Haddad RI and Shin DM: Recent advances in
head and neck cancer. N Engl J Med. 359:1143–1154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Overgaard J, Mohanti BK, Begum N, Ali R,
Agarwal JP, Kuddu M, Bhasker S, Tatsuzaki H and Grau C: Five versus
six fractions of radiotherapy per week for squamous-cell carcinoma
of the head and neck (IAEA-ACC study): A randomised, multi-centre
trial. Lancet Oncol. 11:553–560. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roepman P, Wessels LF, Kettelarij N,
Kemmeren P, Miles AJ, Lijnzaad P, Tilanus MG, Koole R, Hordijk GJ,
van der Vliet PC, et al: An expression profile for diagnosis of
lymph node metastases from primary head and neck squamous cell
carcinomas. Nat Genet. 37:182–186. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weaver A, Fleming S, Ensley J, Kish JA,
Jacobs J, Kinzie J, Crissman J and Al-Sarraf M: Superior clinical
response and survival rates with initial bolus of cisplatin and 120
hour infusion of 5-fluorouracil before definitive therapy for
locally advanced head and neck cancer. Am J Surg. 148:525–529.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rooney M, Kish J, Jacobs J, Kinzie J,
Weaver A, Crissman J and Al-Sarraf M: Improved complete response
rate and survival in advanced head and neck cancer after
three-course induction therapy with 120-hour 5-FU infusion and
cisplatin. Cancer. 55:1123–1128. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang D and Lippard SJ: Cellular processing
of platinum anti-cancer drugs. Nat Rev Drug Discov. 4:307–320.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hall MD, Okabe M, Shen DW, Liang XJ and
Gottesman MM: The role of cellular accumulation in determining
sensitivity to platinum-based chemotherapy. Annu Rev Pharmacol
Toxicol. 48:495–535. 2008. View Article : Google Scholar
|
|
8
|
Shen DW, Pouliot LM, Hall MD and Gottesman
MM: Cisplatin resistance: A cellular self-defense mechanism
resulting from multiple epigenetic and genetic changes. Pharmacol
Rev. 64:706–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Avruch J, Zhou D, Fitamant J and Bardeesy
N: Mst1/2 signalling to Yap: Gatekeeper for liver size and tumour
development. Br J Cancer. 104:24–32. 2011. View Article : Google Scholar :
|
|
10
|
Wang K, Degerny C, Xu M and Yang XJ: YAP,
TAZ, and Yorkie: A conserved family of signal-responsive
transcriptional coregulators in animal development and human
disease. Biochem Cell Biol. 87:77–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuan M, Tomlinson V, Lara R, Holliday D,
Chelala C, Harada T, Gangeswaran R, Manson-Bishop C, Smith P,
Danovi SA, et al: Yes-associated protein (YAP) functions as a tumor
suppressor in breast. Cell Death Differ. 15:1752–1759. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vidal M and Cagan RL: Drosophila models
for cancer research. Curr Opin Genet Dev. 16:10–16. 2006.
View Article : Google Scholar
|
|
13
|
Nylander K, Coates PJ and Hall PA:
Characterization of the expression pattern of p63 alpha and delta
Np63 alpha in benign and malignant oral epithelial lesions. Int J
Cancer. 87:368–372. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lo Muzio L, Santarelli A, Caltabiano R,
Rubini C, Pieramici T, Trevisiol L, Carinci F, Leonardi R, De Lillo
A, Lanzafame S, et al: p63 overexpression associates with poor
prognosis in head and neck squamous cell carcinoma. Hum Pathol.
36:187–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ge L, Smail M, Meng W, Shyr Y, Ye F, Fan
KH, Li X, Zhou HM and Bhowmick NA: Yes-associated protein
expression in head and neck squamous cell carcinoma nodal
metastasis. PLoS One. 6:e275292011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Y, Khanal P, Savage P, She YM, Cyr TD
and Yang X: YAP-induced resistance of cancer cells to antitubulin
drugs is modulated by a Hippo-independent pathway. Cancer Res.
74:4493–4503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamamura M, Noguchi K, Nakano Y, Segawa E,
Zushi Y, Takaoka K, Kishimoto H, Hashimoto-Tamaoki T and Urade M:
Functional analysis of Zyxin in cell migration and invasive
potential of oral squamous cell carcinoma cells. Int J Oncol.
42:873–880. 2013.PubMed/NCBI
|
|
18
|
Köberle B, Tomicic MT, Usanova S and Kaina
B: Cisplatin resistance: Preclinical findings and clinical
implications. Biochim Biophys Acta. 1806:172–182. 2010.PubMed/NCBI
|
|
19
|
Liu AM, Wong KF, Jiang X, Qiao Y and Luk
JM: Regulators of mammalian Hippo pathway in cancer. Biochim
Biophys Acta. 1826:357–364. 2012.PubMed/NCBI
|
|
20
|
Solioz M and Vulpe C: CPx-type ATPases: A
class of P-type ATPases that pump heavy metals. Trends Biochem Sci.
21:237–241. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Culotta VC, Lin SJ, Schmidt P, Klomp LW,
Casareno RL and Gitlin J: Intracellular pathways of copper
trafficking in yeast and humans. Adv Exp Med Biol. 448:247–254.
1999.PubMed/NCBI
|
|
22
|
Katano K, Safaei R, Samimi G, Holzer A,
Rochdi M and Howell SB: The copper export pump ATP7B modulates the
cellular pharmacology of carboplatin in ovarian carcinoma cells.
Mol Pharmacol. 64:466–473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Samimi G, Katano K, Holzer AK, Safaei R
and Howell SB: Modulation of the cellular pharmacology of cisplatin
and its analogs by the copper exporters ATP7A and ATP7B. Mol
Pharmacol. 66:25–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Safaei R and Howell SB: Copper
transporters regulate the cellular pharmacology and sensitivity to
Pt drugs. Crit Rev Oncol Hematol. 53:13–23. 2005. View Article : Google Scholar
|
|
25
|
Yoshizawa K, Nozaki S, Kitahara H, Ohara
T, Kato K, Kawashiri S and Yamamoto E: Copper efflux transporter
(ATP7B) contributes to the acquisition of cisplatin-resistance in
human oral squamous cell lines. Oncol Rep. 18:987–991.
2007.PubMed/NCBI
|
|
26
|
Hong W and Guan KL: The YAP and TAZ
transcription co-activators: Key downstream effectors of the
mammalian Hippo pathway. Semin Cell Dev Biol. 23:785–793. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sudol M and Harvey KF: Modularity in the
Hippo signaling pathway. Trends Biochem Sci. 35:627–633. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu FX and Guan KL: The Hippo pathway:
Regulators and regulations. Genes Dev. 27:355–371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo X and Zhao B: Integration of
mechanical and chemical signals by YAP and TAZ transcription
coactivators. Cell Biosci. 3:332013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lai D, Visser-Grieve S and Yang X: Tumour
suppressor genes in chemotherapeutic drug response. Biosci Rep.
32:361–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carey TE, Van Dyke DL and Worsham MJ:
Nonrandom chromosome aberrations and clonal populations in head and
neck cancer. Anticancer Res. 13(6B): 2561–2567. 1993.PubMed/NCBI
|
|
32
|
Snijders AM, Schmidt BL, Fridlyand J,
Dekker N, Pinkel D, Jordan RC and Albertson DG: Rare amplicons
implicate frequent deregulation of cell fate specification pathways
in oral squamous cell carcinoma. Oncogene. 24:4232–4242. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lai D, Ho KC, Hao Y and Yang X: Taxol
resistance in breast cancer cells is mediated by the hippo pathway
component TAZ and its downstream transcriptional targets Cyr61 and
CTGF. Cancer Res. 71:2728–2738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Visser S and Yang X: Identification of
LATS transcriptional targets in HeLa cells using whole human genome
oligonucleotide microarray. Gene. 449:22–29. 2010. View Article : Google Scholar
|
|
35
|
Kawahara M, Hori T, Chonabayashi K, Oka T,
Sudol M and Uchiyama T: Kpm/Lats2 is linked to chemosensitivity of
leukemic cells through the stabilization of p73. Blood.
112:3856–3866. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cordenonsi M, Zanconato F, Azzolin L,
Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR,
Poletti A, et al: The Hippo transducer TAZ confers cancer stem
cell-related traits on breast cancer cells. Cell. 147:759–772.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Frisch SM, Schaller M and Cieply B:
Mechanisms that link the oncogenic epithelial-mesenchymal
transition to suppression of anoikis. J Cell Sci. 126:21–29. 2013.
View Article : Google Scholar : PubMed/NCBI
|