Introduction
Gestational choriocarcinoma usually arises from a
prior molar pregnancy and occurs in 1 in 40,000 pregnancies
(1). Improvement in treatment of
gestational choriocarcinoma is required.
Dickkopf-1 is a 35-kDa secreted protein that was
originally characterized as a Wnt inhibitor (2). DKK1 plays a critical role in cell
patterning, proliferation, and fate determination during
embryogenesis (3). DKK1 was shown
downregulated in many human cancers, such as gastric cancer, lung
cancer, and breast cancer, indicating that it might act as a tumor
suppressor (4–6). However, the role of DKK1 in tumor
biology remains controversial. High expression of DKK1 is related
to lymphatic metastasis and indicates poor prognosis in
intrahepatic cholangiocarcinoma patients (7). The Wnt/β-catenin signaling pathway is
commonly dysregulated in various cancers (8–10).
The signaling pathway can directly alter gene expression and is a
key regulator of cell proliferation, apoptosis, differentiation,
polarity, and migration (11,12).
DKK1 can directly bind to the Wnt ligands (secreted
frizzled-related proteins, sFRPs) or by interacting with the low
density lipoprotein receptor-related protein 5/6 (LRP) coreceptors,
preventing binding of the Wnt proteins to the FRZ/LRP receptor
complex (13). However, Kawano and
Kypta (14) demonstrated that DKK1
suppressed the tumorigenicity of two human breast cancer cell lines
that lack activated Wnt signaling.
In this study, the effects of treating trophoblast
cell lines, JEG3 and BeWo, with human recombinant DKK1 protein were
determined in vitro and in vivo. As expected, DKK1
reduced the proliferation of JEG3 and BeWo cells. Moreover, the
cellular biological mechanisms of DKK1 were determined.
Materials and methods
Cell lines and culture conditions
Human choriocarcinoma cells, JEG-3 and BeWo (ATCC,
Manassas, VA, USA) were maintained in Dulbecco’s modified Eagle’s
medium (DMEM, Hyclone, Logan, UT, USA) supplemented with 10%
heat-inactivated FBS, 2 mM glutamine, penicillin (100 U/ml) and
streptomycin (100 μg/ml) at 37°C with 5% CO2.
Methylation analysis
Genomic DNA was extracted and purified from cells
using DNAzol (Invitrogen Life Technologies; Carlsbad, CA, USA)
according to the manufacturer’s protocol. The extracted DNA was
modified by treatment with sodium bisulfite using the EpiTect
Bisulfite kit (Qiagen, Hilden, Germany). The methylation status of
the DKK1 gene was assessed using both methylation specific PCR
(MSP) and bisulfate sequencing. The modified DNA was used as a
template both for MSP and unmethylated-specific PCR (USP). The
primer sequences for the methylated DKK1 gene were
5′-TTAAGGGGTCGGAATGTTTC-3′ (sense) and 5′-CACGAAACCGTACCGATTC-3′
(antisense) and for the unmethylated allele were
5′-TTTTAAGGGGTTGGAATGTTTT-3′ (sense) and
5′-CCACAAAACCATACCAATTCAAC-3′ (antisense). The PCR products were
separated in a 2% agarose gel with ethidium bromide and visualized
under UV illumination. To examine the effect of demethylation,
cells were incubated with medium containing 10 μM
5-aza-2′-deoxycytidine (5-aza-dC, Sigma-Aldrich, Carlsbad, CA, USA)
for 36 h. Then DNA was isolated and MSP carried out as described
above.
Immunofluorescence
Cells were washed with PBS, fixed in 4%
paraformaldehyde, permeabilized in 1% Triton X-100 for 15 min at
room temperature and blocked with 5% bovine serum albumin in PBS
for 1 h. DKK1 was detected using anti-DKK1 (Santa Cruz, sc-25516)
antibody for 1 h at room temperature. Cells were washed with PBS
and incubated with the appropriate fluorophore-conjugated secondary
antibody, Alexa Fluor® 594 Donkey anti-rabbit IgG (H+L),
for 1 h at room temperature, washed with PBS, and mounted using
Prolong anti-fade (Sigma).
Cell growth assay
Cells were seeded at 200 cells per well in 24-well
tissue culture plates and incubated under normal culture
conditions. After 24 h, cells were treated with various
concentrations of rhDKK1 protein (e.g., 0, 10, 50, 100 and 200
ng/ml for each). Plates were incubated for 3 weeks in a humidified
incubator at 37°C. Two weeks after seeding, colonies were stained
with 0.05% crystal violet containing 50% methanol and counted. The
colonies were counted in 4–5 random fields for each of the
duplicate samples by using a microscope at ×100 magnification.
Apoptosis detection
Cells were trypsinized, washed twice with cold PBS,
and resuspended in 200 μl binding buffer. Annexin V-FITC was added
to a final concentration of 0.5 μg/ml, according to the
manufacturer’s instructions (KeyGen, Nanjing, China). After 20-min
incubation at room temperature in the dark, 400 μl of binding
buffer containing propidium iodide (PI, 50 μg/μl) was added, and
samples were immediately analyzed on a FACSCalibur flow cytometer
(Becton-Dickinson Medical Devices, Shanghai, China).
Detection of mitochondrial transmembrane
potential (MMP)
The changes in the mitochondrial potential were
detected by using 5,5′,6,6′-tetrachloro-1,1′,3,3′
tetraethylbenzimidazolyl-carbocyanine iodide/chloride (JC-1,
KeyGen), a cationic dye that exhibits potential-dependent
accumulation in mitochondria. These changes were indicated by
fluorescence emission shift from red (590 nm) to green (525 nm) and
analyzed on a flow cytometer (BD).
Invasion assays
Transwell chamber (8-μM pore size polycarbonate
membrane, Cell Biolabs, San Diego, CA, USA) Matrigel-invasion
assays were performed to detect the invasion of cells. Cells
(30,000) were placed in the upper chamber and allowed to invade for
24 h. Ten fields of cells were acquired at ×10 magnification and
quantified. Relative invasion was expressed as a ratio of control
cells.
In vivo antitumor effect
All animal studies were conducted by the Animal Care
and Use Committee of China Medical University. Fifty NOD SCID mice
(Institute of Animal Center, Chinese Academy of Sciences, Shanghai,
China), male, aged 4–6 weeks, were used in the in vivo
experiment for observing antitumor efficacy of DKK1. Tumor
xenografts were established by subcutaneous inoculation of
3×107 JEG-3 cells into the back of mice. When tumors
reached 5–7 mm in diameter, the mice were randomly divided into
five groups (untreated, PBS, DKK1, DKK1+si-LGR6 #1 and DKK1+si-LGR6
#2). Mice were then intratumorally injected with 100 μl of PBS,
DKK1, DKK1+si-LGR6 #1 or DKK1+si-LGR6 #2. Synthetic siRNA (100 nM)
(si-LGR6) specifically targeting LGR6 (Invitrogen) were used to
transfect cells using Oligofectamine (Invitrogen). The injections
were repeated every two days. Tumor growth was monitored by
periodic measurements with calipers. All animals were sacrificed
five weeks post-treatment and tumors were dissected for
pathological examination. Another 80 NOD SCID nude mice treated in
the same way as described above and used to observe the survival
time. The survival status of the mice was observed until the
experiments were terminated for unacceptable tumor burden.
Enzyme-linked immunosorbent assay
(ELISA)
To measure the serum DKK1 concentration from mouse,
a sandwich enzyme-linked immunosorbent assay (ELISA) was performed
using DKK1 ELISA kit (R&D Systems) according to its
protocol.
Western blot analysis
Cells and tissues were harvested, washed twice with
PBS, lysed on ice for 30 min in 100 μl lysis buffer [120 mM NaCl,
40 mM Tris (pH 8.0), 0.1% NP-40] and then centrifuged at 13,000 g
for 15 min. The supernatants were collected from the lysates and
the protein concentration was determined. Aliquots of the lysates
(50 μg of protein) were boiled for 5 min and electrophoresed using
a 10% sodium dodecysulfate-polyacrylamide gel. The blots in the
gels were transferred onto nitrocellulose membranes (Bio-Rad,
Hercules, CA, USA), which were then incubated with primary
antibodies. Axin2 (2151, 1:500) was purchased from Cell Signaling
Technology (Beverly, MA, USA). LRP6 (sc-25317, 1:500), glycogen
synthase kinase (GSK-3β, sc-377213, 1:200), β-catenin (sc-7963,
1:200), Bax (sc-7480, 1:500), Bcl-xL (sc-8392, 1:200), Bcl-2
(sc-783, 1:500), phospho-Bcl-2 (Ser 87) (sc-16323, 1:200),
β-tubulin (sc-5274, 1:500) and β-actin (sc-103656, 1:1,000)
antibodies were purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). The nitrocellulose membranes were further incubated
with secondary immunoglobulin-G-horseradish peroxidase conjugates.
Immunostaining was detected using an enhanced chemiluminescence
(ECL) system (Amersham Biosciences, Westborough, MA, USA).
In silico experiment
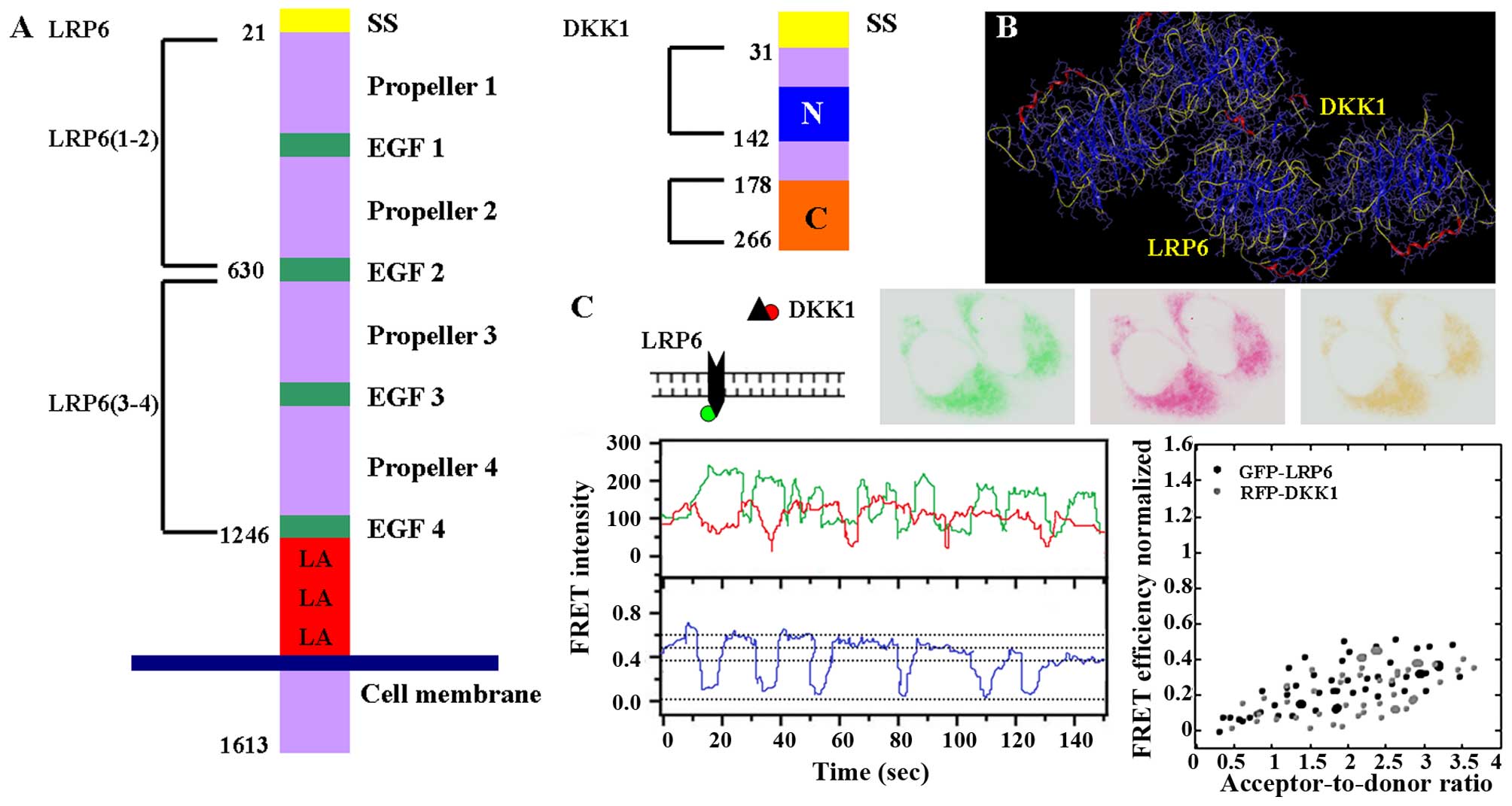
The interaction between DKK1 and LRP6 protein was
analyzed by SYBYL-X 1.3 (Tripos International, St. Louis, MO, USA).
The structure of DKK1 (PDB code: 3S8V) and LRP6 (PDB code: 3S8Z)
protein were retrieved from the Protein Data Bank (http://www.rcsb.org).
Determination of the FRET efficiency in a
confocal image
FRET measurements were performed on the confocal
laser scanning microscope (Bio-Rad) as previously described
(15). Donor signal I1 (excitation
= 488 nm, emission = 505–530 nm), FRET signal I2 (excitation = 488
nm, emission > 585 nm), and acceptor signal I3 (excitation = 543
nm, emission > 585 nm) were collected.
Statistical analysis
Statistical analyses were carried out using GraphPad
Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA). Data were
expressed as mean ± standard deviation of three independent
experiments performed in triplicate. Data comparisons in relation
to control were performed by one-way ANOVA. The Kaplan-Meier method
was used to estimate the probability of mouse survival. Differences
were considered statistically significant at P-value <0.05.
Results
DKK1 promoter methylation analysis
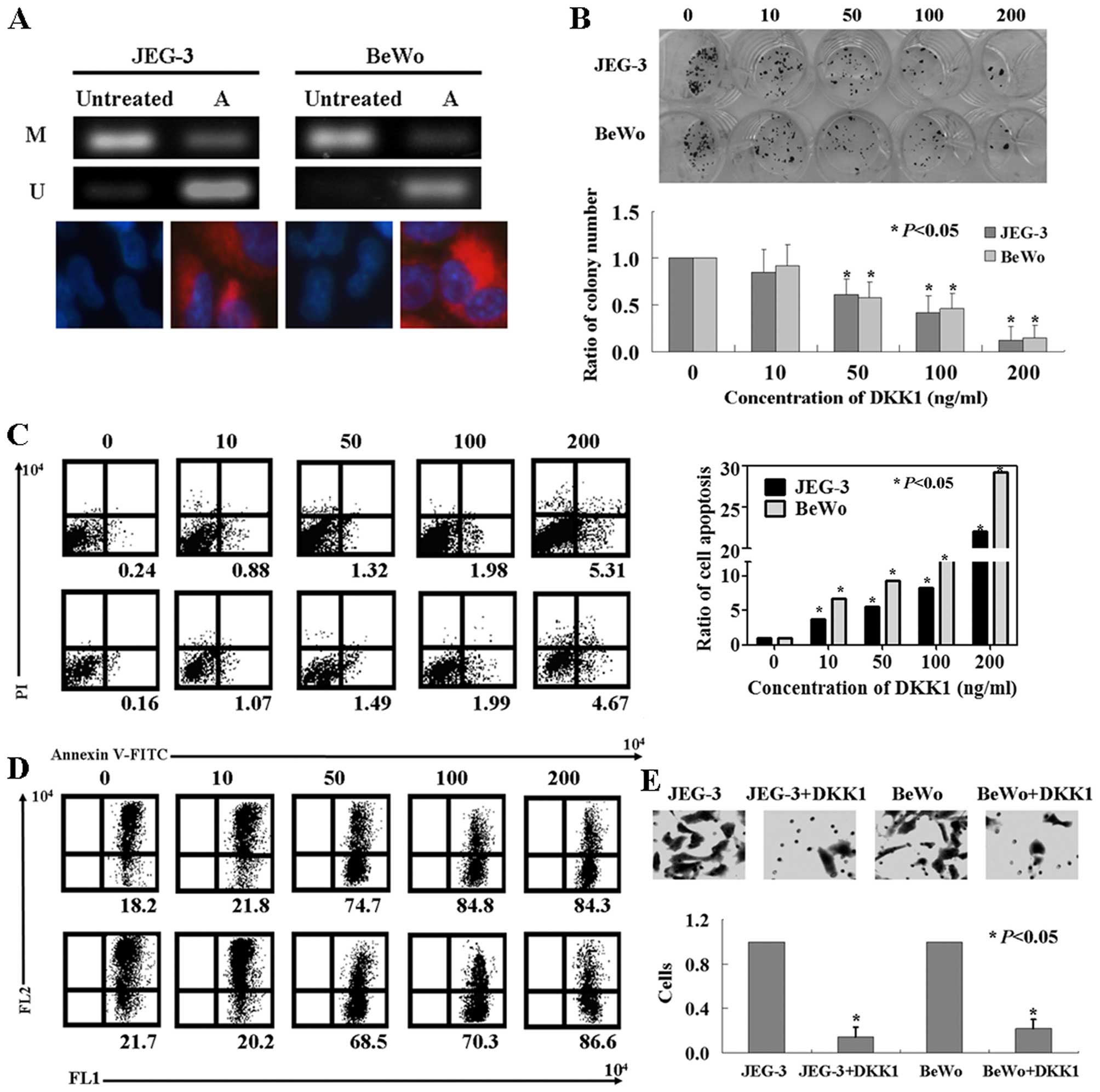
A correlation was observed between DKK1
promoter methylation and decreased DKK1 mRNA levels in both
JEG3 and BeWo cells (Fig. 1A).
After the cells were treated with 5-aza-dC, we found that the
DKK1 mRNA and protein expression was restored (Fig. 1A).
The roles of rhDKK1 protein in JEG3 and
BeWo cells
Colony formation assay was used to detect cell
viability. As shown in Fig. 1B,
the proliferation rate of both JEG3 and BeWo cells were inhibited
by rhDKK1 protein in a dose-dependent manner (P<0.05). The
IC50 values for JEG3 and BeWo cells were 83.4 and 97.3
ng/ml, respectively. The results of Annexin V-FITC and PI double
staining showed that the apoptotic ratio of the cells after DKK1
treatment was increased significantly in these cells (Fig. 1C, P<0.05). Mitochondrial damage
was indicated by the fluorescence emission shift from red to green.
JEG3 and BeWo cells showed mitochondrial damage following DKK1
treatment (Fig. 1D). In addition,
we found that significantly less cells migrated to the lower
membrane compared with untreated ones (Fig. 1E, P<0.05).
DKK1 inhibits the Wnt signaling pathway
and induced the mitochondrial apoptosis pathway
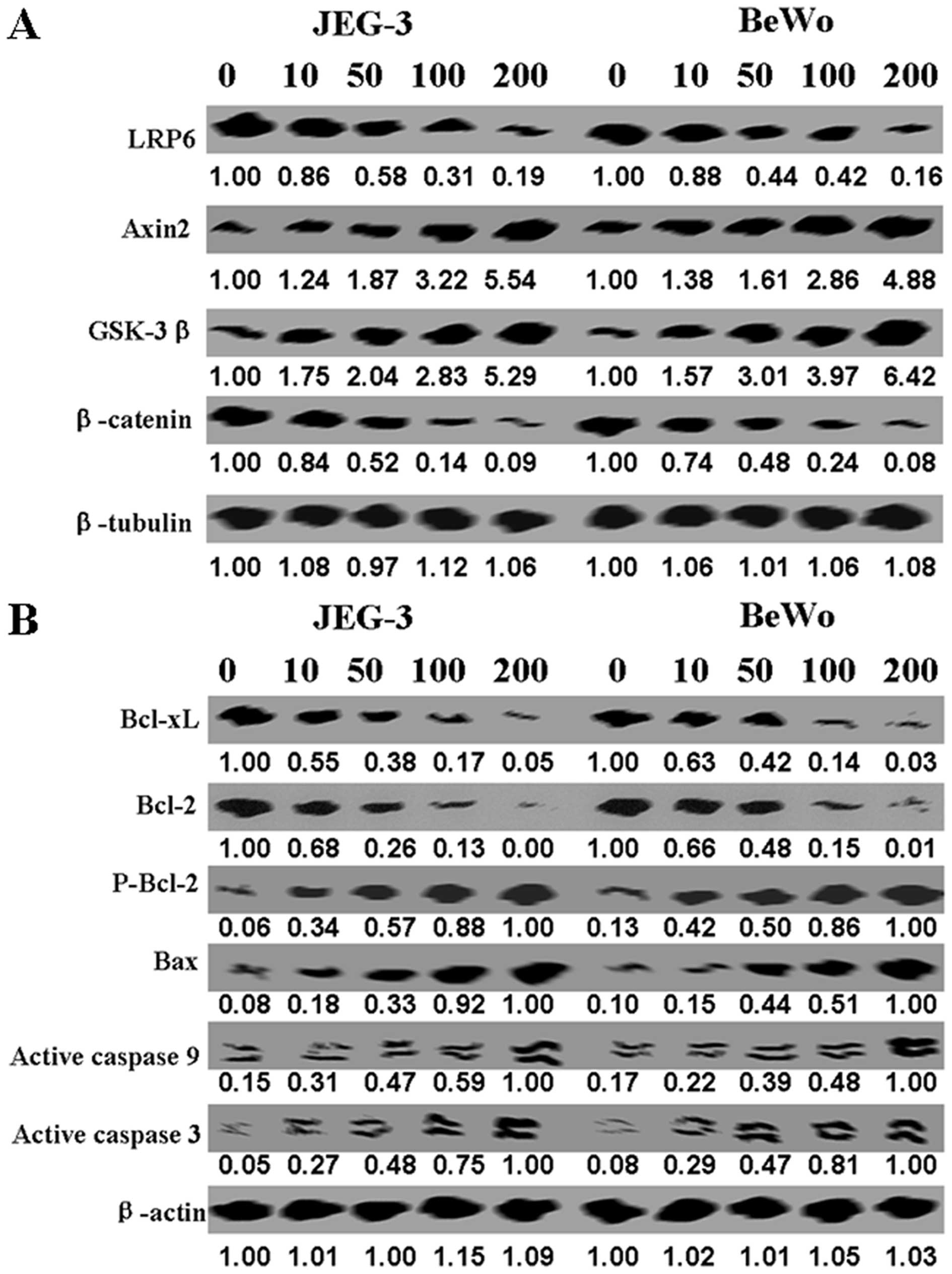
Furthermore, we carried out western blot analysis to
identify the mechanism of apoptosis induced by DKK1. We found that
LRP6 and β-catenin protein levels were lower in treated cells than
untreated ones, while Axin2 and GSK-3 protein levels were increased
in the treated ones (Fig. 2A).
Compared with untreated control, we found a decrease in Bcl-2 and
Bcl-xL protein, and an increase in p-Bcl-2 and Bax protein levels
in DKK1-treated cells (Fig. 2B).
Active caspase-3 and -9 were also increased in DKK1-treated cells
(Fig. 2B).
DKK1 inhibits tumor growth in mouse
xenograft models
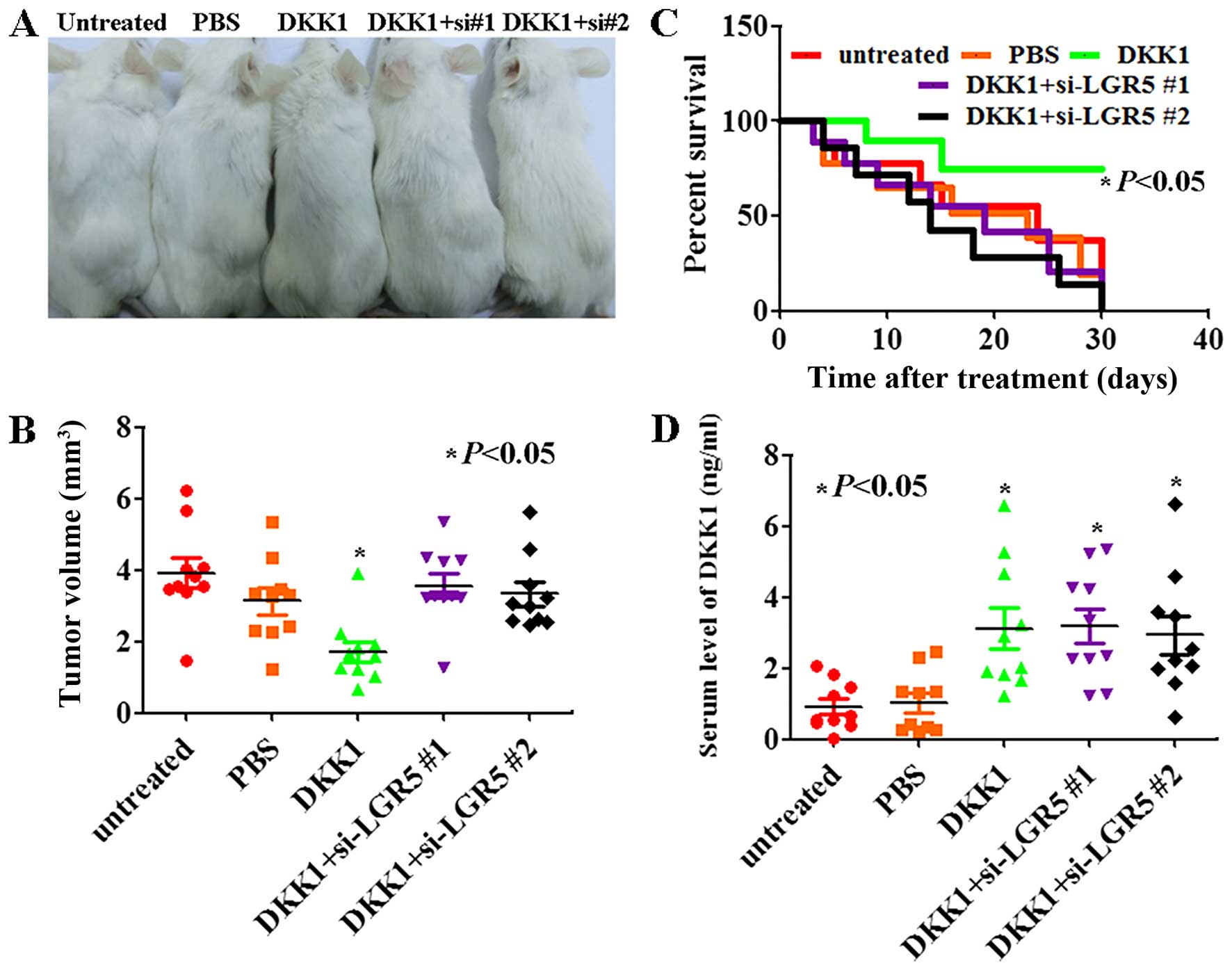
We next determined the antitumor properties of DKK1
in established xenograft tumor models. As shown in Fig. 3B, the tumor volume of DKK1-treated
JEG-3 mice was less than untreated or PBS treated JEG-3 mice
(P<0.05). In addition, the survival rate of mice with tumors
treated with DKK1 was significantly improved (Fig. 3C, P<0.05). The serum levels of
DKK1 in the mice treated with DKK1 were higher than that in
untreated or PBS treated ones (Fig.
3D, P<0.05). Interestingly, DKK1 showed no effects on LRP6
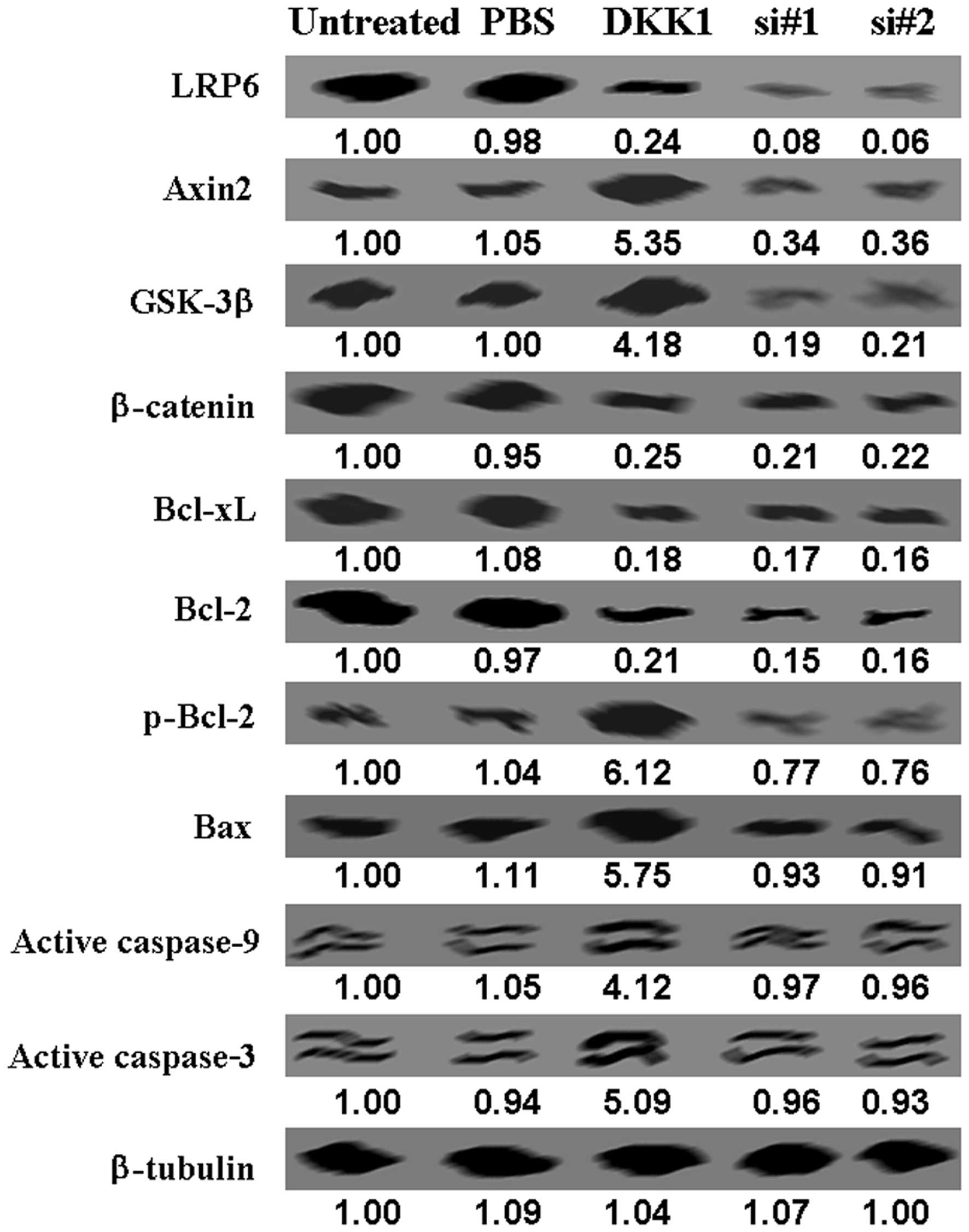
knockdown JEG-3 cells (Fig. 3,
P<0.05). We knocked down LRP6 expression in JEG-3 cells by using
synthetic siRNA (si-LGR6) and noticed that the effect of DKK1 on
Wnt signaling pathway and mitochondrial apoptosis pathway was
offset by si-LGR6 (Fig. 4). These
results indicated that DKK1 may cause its antitumor effect by LGR6
in JEG-3 cells.
DKK1 binds to LGR6 and inhibits the Wnt
signaling pathway
We found that DKK1 was ab;e to bind to LGR6 by using
the modeling software SYBYL-X 1.3 (Fig. 5B). Furthermore, we carried out FRET
to identify the interaction between DKK1 and LGR6. To permit
spectroscopic analyses of receptor assembly and function in the
living cell, we tagged DKK1 protein with RFP and labeled LGR6 with
GFP. The results of FRET confirmed an almost linear increased donor
(DKK1) and acceptor (LGR6) dimer (Fig.
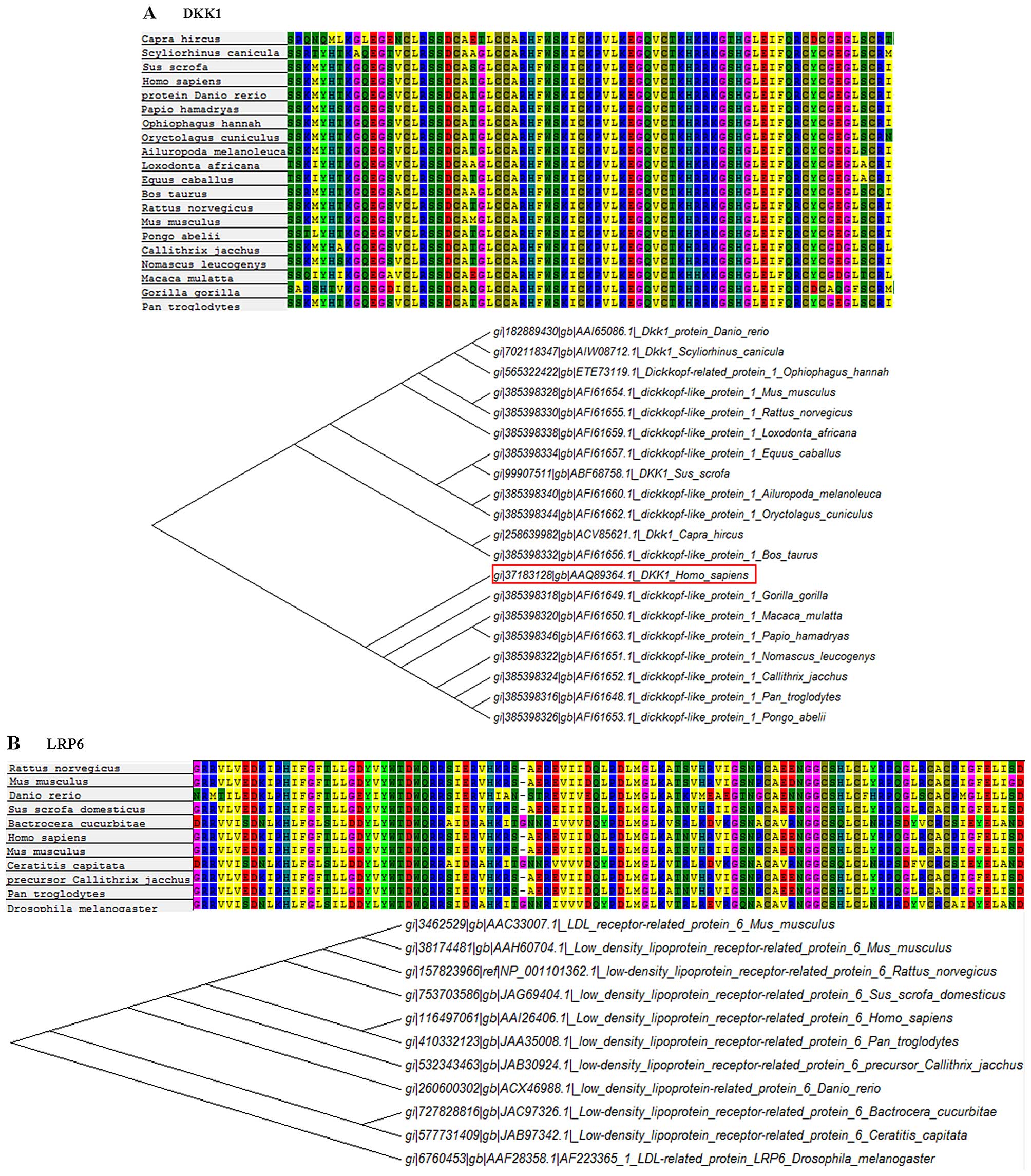
5C). Furthermore, we carried out multiple sequence alignment
and phylogenetic analysis. Additionally, we confirmed both the DKK1
and LGR6 as highly conserved proteins (Fig. 6), indicating that our results can
be applied to other species.
Discussion
DKK-1 has been previously reported to be
downregulated in human colon cancer for promoter hypermethylation
(16). In our study, we also
confirmed that decreased DKK1 in trophoblast tumor cell lines was
related to promoter hypermethylation. The Wnt/β-catenin signaling
pathway plays a pivotal role in regulating cellular processes
involved in development and differentiation (17). Aberrant Wnt/β-catenin signaling is
widely implicated in cancer, such as lung cancer (18), gastric cancer (19) and colorectal cancer (20). This signaling pathway is activated
by the binding of a Wnt agonist to low density lipoprotein receptor
(LDLR)-related protein 6 (LRP6) that are present at the cell
surface (21). In the absence of
Wnt signaling, GSK-3 is active and phosphorylates β-catenin in the
scaffolding protein complex of adenomatous polyposis coli (APC) and
Axin (22). Antagonists that
interfere with Wnt ligand/receptor interactions may therefore be
potent cancer treatments (23). In
this study, we also confirmed that DKK1 inhibited the proliferation
of trophoblast tumor cells by suppressing the Wnt signaling
pathway.
Furthermore, DKK1 was able to activate the
mitochondrial apoptosis pathway in trophoblast tumor cells. DKK1
showed no effects on LRP6 knockdown trophoblast tumor cells. Our
results indicated that the effects of DKK1 on mitochondria were
through the Wnt signaling pathway. Activation of Wnt/β-catenin
signaling activates caspase-3 and -9 and reduces the mitochondrial
membrane potential (24). The
anti-apoptotic Bcl-2 protein is a target gene of the canonical
Wnt/β-catenin signaling pathway (25). Our results are consistent with the
above previous studies.
In conclusion, the principal findings of our study
are that: i) the antitumor roles of DKK1 in trophoblast tumor
cells, ii) DKK1 induces apoptosis in trophoblast tumor cells by
activating mitochondrial apoptosis pathway and suppressing the
Wnt/β-catenin signaling pathway, and iii) DKK1-mediated apoptosis
is regulated by the expression of LRP6 in vivo and in
vitro.
Acknowledgements
We thank Dr Yu Jian for his valuable comments and
excellent technical assistance.
References
|
1
|
Milenković V, Lazović B, Mačvanski M,
Jeremić K and Hrgović Z: Clinical outcome of a FIGO sage IV
gestational choriocarcinoma. Case Rep Oncol. 6:504–507. 2013.
View Article : Google Scholar
|
|
2
|
Bafico A, Liu G, Yaniv A, Gazit A and
Aaronson SA: Novel mechanism of Wnt signalling inhibition mediated
by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol.
3:683–686. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ogoshi K, Kasamatsu A, Iyoda M, Sakuma K,
Yamatoji M, Sakamoto Y, Ogawara K, Shiiba M, Tanzawa H and Uzawa K:
Dickkopf-1 in human oral cancer. Int J Oncol. 39:329–336.
2011.PubMed/NCBI
|
|
4
|
Gao C, Xie R, Ren C and Yang X: Dickkopf-1
expression is a novel prognostic marker for gastric cancer. J
Biomed Biotechnol. 2012:8045922012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li S, Qin X, Guo X, Cui A, He Y, Wei S,
Wang X and Shan B: Dickkopf-1 is oncogenic and involved in invasive
growth in non small cell lung cancer. PLoS One. 8:e849442013.
View Article : Google Scholar
|
|
6
|
Kyvernitakis I, Rachner TD, Urbschat A,
Hars O, Hofbauer LC and Hadji P: Effect of aromatase inhibition on
serum levels of sclerostin and dickkopf-1, bone turnover markers
and bone mineral density in women with breast cancer. J Cancer Res
Clin Oncol. 140:1671–1680. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi RY, Yang XR, Shen QJ, Yang LX, Xu Y,
Qiu SJ, Sun YF, Zhang X, Wang Z, Zhu K, et al: High expression of
Dickkopf-related protein 1 is related to lymphatic metastasis and
indicates poor prognosis in intrahepatic cholangiocarcinoma
patients after surgery. Cancer. 119:993–1003. 2013. View Article : Google Scholar
|
|
8
|
Xu W, Wang Z, Zhang W, Qian K, Li H, Kong
D, Li Y and Tang Y: Mutated K-ras activates CDK8 to stimulate the
epithelial-to-mesenchymal transition in pancreatic cancer in part
via the Wnt/β-catenin signaling pathway. Cancer Lett. 356B:613–627.
2015. View Article : Google Scholar
|
|
9
|
Tong X, Li L, Li X, Heng L, Zhong L, Su X,
Rong R, Hu S, Liu W, Jia B, et al: SOX10, a novel
HMG-box-containing tumor suppressor, inhibits growth and metastasis
of digestive cancers by suppressing the Wnt/β-catenin pathway.
Oncotarget. 5:10571–10583. 2014.PubMed/NCBI
|
|
10
|
Liu H, Yan ZQ, Li B, Yin SY, Sun Q, Kou
JJ, Ye D, Ferns K, Liu HY and Liu SL: Reduced expression of SOX7 in
ovarian cancer: A novel tumor suppressor through the Wnt/β-catenin
signaling pathway. J Ovarian Res. 7:872014. View Article : Google Scholar
|
|
11
|
Apte U, Zeng G, Thompson MD, Muller P,
Micsenyi A, Cieply B, Kaestner KH and Monga SP: beta-catenin is
critical for early postnatal liver growth. Am J Physiol
Gastrointest Liver Physiol. 292:G1578–G1585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nejak-Bowen K and Monga SP:
Wnt/beta-catenin signaling in hepatic organogenesis. Organogenesis.
4:92–99. 2008. View Article : Google Scholar
|
|
13
|
Mikheev AM, Mikheeva SA, Maxwell JP, Rivo
JV, Rostomily R, Swisshelm K and Zarbl H: Dickkopf-1 mediated tumor
suppression in human breast carcinoma cells. Breast Cancer Res
Treat. 112:263–273. 2008. View Article : Google Scholar
|
|
14
|
Kawano Y and Kypta R: Secreted antagonists
of the Wnt signalling pathway. J Cell Sci. 116:2627–2634. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vámosi G, Baudendistel N, von der Lieth
CW, Szalóki N, Mocsár G, Müller G, Brázda P, Waldeck W, Damjanovich
S, Langowski J, et al: Conformation of the c-Fos/c-Jun complex in
vivo: A combined FRET, FCCS, and MD-modeling study. Biophys J.
94:2859–2868. 2008. View Article : Google Scholar
|
|
16
|
González-Sancho JM, Aguilera O, García JM,
Pendás-Franco N, Peña C, Cal S, García de Herreros A, Bonilla F and
Muñoz A: The Wnt antagonist DICKKOPF-1 gene is a downstream target
of beta-catenin/TCF and is downregulated in human colon cancer.
Oncogene. 24:1098–1103. 2005. View Article : Google Scholar
|
|
17
|
Chien AJ, Conrad WH and Moon RT: A Wnt
survival guide: From flies to human disease. J Invest Dermatol.
129:1614–1627. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu X, Kim JE, Sun PL, Yoo SB, Kim H, Jin Y
and Chung JH: Immunohistochemical demonstration of alteration of
β-catenin during tumor metastasis by different mechanisms according
to histology in lung cancer. Exp Ther Med. 9:311–318.
2015.PubMed/NCBI
|
|
19
|
Cui J, Xi H, Cai A, Bian S, Wei B and Chen
L: Decreased expression of Sox7 correlates with the upregulation of
the Wnt/β-catenin signaling pathway and the poor survival of
gastric cancer patients. Int J Mol Med. 34:197–204. 2014.PubMed/NCBI
|
|
20
|
Silva AL, Dawson SN, Arends MJ, Guttula K,
Hall N, Cameron EA, Huang TH, Brenton JD, Tavaré S, Bienz M, et al:
Boosting Wnt activity during colorectal cancer progression through
selective hypermethylation of Wnt signaling antagonists. BMC
Cancer. 14:8912014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.PubMed/NCBI
|
|
22
|
Kitagawa M, Hatakeyama S, Shirane M,
Matsumoto M, Ishida N, Hattori K, Nakamichi I, Kikuchi A and
Nakayama K and Nakayama K: An F-box protein, FWD1, mediates
ubiquitin-dependent proteolysis of β-catenin. EMBO J. 18:2401–2410.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee JS, Hur MW, Lee SK, Choi WI, Kwon YG
and Yun CO: A novel sLRP6E1E2 inhibits canonical Wnt signaling,
epithelial-to-mesenchymal transition, and induces
mitochondria-dependent apoptosis in lung cancer. PLoS One.
7:e365202012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ming M, Wang S, Wu W, Senyuk V, Le Beau
MM, Nucifora G and Qian Z: Activation of Wnt/β-catenin protein
signaling induces mitochondria-mediated apoptosis in hematopoietic
progenitor cells. J Biol Chem. 287:22683–22690. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jaeger A, Baake J, Weiss DG and Kriehuber
R: Glycogen synthase kinase-3beta regulates differentiation-induced
apoptosis of human neural progenitor cells. Int J Dev Neurosci.
31:61–68. 2013. View Article : Google Scholar
|