Introduction
Worldwide, lung cancer is the leading cause of
cancer mortality. Non-small cell lung cancer (NSCLC) accounts for
~85% of all lung cancers. Standard first-line treatment options for
NSCLC depend on disease and patient characteristics and may include
surgery, platinum-based doublet chemotherapy and radiotherapy
(1). Although surgical resection
is curative if diagnosis occurs at early stage I or stage II
disease, ~70% of all newly diagnosed patients present with local
advanced or metastatic disease and require systemic chemotherapy
(1,2). Chemotherapy combinations for more
advanced diseases have shown to convey no benefit on overall
survival or quality of life beyond 4–6 cycles (3,4).
NSCLC patients with epidermal growth factor receptor (EGFR)
mutations initially respond to EGFR tyrosine kinase inhibitors,
however, most patients experience a relapse within 1 year (5,6).
Despite the development of novel molecular therapies, the prognosis
of lung cancer is still poor and the 5-year survival remains
<20% (7–9). Hence, novel and more effective
approaches are needed for the treatment of advanced lung
cancer.
Oncolytic viruses represent an emerging therapeutic
platform for the treatment of human cancer with unique attributes
compared with conventional therapeutic modalities, which is able to
selectively infect and lyse tumor cells where after the released
progeny virions reinfect neighboring tumor cells and also enter the
blood stream to infect metastasized tumor cells, while ideally
leaving normal cells unharmed (10). Among them, adenoviruses were widely
used as oncolytic viral agents in cancer therapy, as they possess
an inherent potential to kill the cells that sustain their
replication, and they are considered to be safe and have been used
in several clinical settings (11–13).
To restrict cytocidal effect to tumor cells, the conditionally
replicative adenoviruses (CRAds) have been developed to restrict
viral replication to target cancerous tissues and inhibit
replication in normal healthy cells. This has been attempted by
exploiting loss-of-function mutations in viral sequences, or
linking genes E1A/E1B to cancer-specific promoters, such as the
telomerase or prostate-specific rat probasin promoters or the human
prostate-specific enhancer/promoter (14,15).
H101, a conditionally replicative adenovirus, was
generated by both E1B and E3 gene deletion, which
selectively infects and kills tumor cells through viral oncolysis
(16). Without E1B to inactivate
p53, H101 adenovirus cannot replicate and lyse normal cells where
p53 is active, which does not have significant cytopathic effects
on normal cells (17). In
addition, the deletion of a 78.3–85.8-nm gene segment in the E3
region, which encodes the adenovirus death protein, may improve the
safety of the product (5). In
China, H101 has been clinically approved for the treatment of
several malignancies (5).
In the present study, we describe the CAR expression
of lung cancer cells and cytopathologic effects of H101 infection
on cancer cells in vitro, and we further demonstrate that
H101 virus suppressed lung cancer xenografts growth in
vivo.
Materials and methods
Cell culture and recombinant adenovirus
H101
XWLC-05, human lung adenocarcinoma cell line, was
kindly provided as a gift by Kunming College University, which was
established from the primary tumor of a Xuanwei woman with lung
adenocarcinoma (18). The human
embryonic kidney cell line HEK293A and lung squamous cell carcinoma
SK-MES-1 were purchased from the Cell Bank of Kunming Institute of
Zoology, Chinese Academy of Sciences. All cell lines were cultured
in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10%
fetal bovine serum (FBS), 100 U/ml−1 penicillin and 100
μg/ml−1 streptomycin in a humidified atmosphere with 5%
CO2 at 37°C. Recombinant adenovirus H101 was obtained
from Shanghai Sunway Biotech Co., Ltd. (Shanghai, China).
Measurement of coxsackievirus adenovirus
receptor (CAR) expression
CAR expression of lung cancer was analyzed as
previously described (19). In
brief, cells growing on cover glass were incubated with the mouse
monoclonal anti-CAR antibody (1:50; Santa Cruz Biotechnology, Santa
Cruz, CA, USA) in binding buffer at 4°C overnight. Afterwards, the
immune reaction was visualized using the EnVision™ detection system
(Dako, Glostrup, Denmark). Analysis of stained cells was performed
under a microscope.
For RT-PCR detection of CAR mRNA expression,
total RNA was extracted from cells by using RNAiso Plus kit
(Takara, Dalian, China). The cDNA was synthesized by using First
Strand cDNA Synthesis kit (Invitrogen, Carlsbad, CA, USA).
Oligonucleotide primers with the following sequences were used:
CAR, forward primer, TTCAGGTGCGAGATG TTA and reverse primer,
GAATGATTACTGCCGATG; GAPDH, forward primer, AGAAGGCTGGGGCTCATTTG and
reverse primer, AGGGGCCATCCACAGTCTTC. The PCR was performed by
using the following parameters: 94°C 5 min; 94°C 30 sec, 56°C 30
sec, 72°C 30 sec × 35 cycles; 72°C 7 min. PCR product was
visualized by 1% agarose gel electrophoresis using 0.5 μg/ml
ethidium bromide.
Quantification of infection
efficiency
To assess the susceptibility to H101 infection of
the XWLC-05 cell lines, cells were plated at a density of
106 cells/well in 6-well plates. Six hours after
seeding, the cells were infected with adenoviruses harboring the
green fluorescent protein reporter gene (Ad-GFP) at multiplicity of
infection (MOI) of 1, 10, 100 and 1000 for 2 h at 37°C. At the end
of the incubation period, the virus was removed and the cells were
maintained in their standard medium. Infected cells expressing GFP
were identified 48 h after infection using a fluorescent microscope
(Nikon, Tokyo, Japan) and BD Accuri C6 flow cytometry (BD
Biosciences, San Jose, CA, USA). The mean ratio of infected cancer
cells was calculated. Results are presented as the means ± SD of at
least three independent experiments.
Real-time fluorecent quantitative PCR for
viral Hexon mRNA
XWLC-05 was infected with oncolytic virus H101 at a
MOI of 100 as described above. At 24, 48 and 72 h post-infection,
virus replication was evaluated by studying relative changes of
viral Hexon mRNA expression. Total RNA was extracted from cells by
using RNAiso Plus kit (Takara). cDNA was synthesized by using First
Strand cDNA Synthesis kit (Invitrogen) according to the
manufacturer’s instructions. Gene expression was quantified by
real-time quantitative PCR using SYBR® Premix Ex Taq™ II
(Takara) and primers recognizing Hexon as previously described
(20).
Virus-mediated cytotoxicity assays
Cells were plated in 96-well plates at
2×104 cells/well. After 6 h, cells were infected with
H101 at MOI of 1, 10, 100 and 1000 or Ad-GFP at a MOI of 100, or
vehicle treatment (phosphate-buffer saline, PBS). Virus-induced
cytotoxicity was analyzed daily for 5 days by measuring the release
of lactate dehydrogenase (LDH) in conditioned media using the
CytoTox 96® Non-Radioactive Cytotoxicity assay kit
(Promega, Madison, WI, USA) and by spectrophotometry (Bio-Rad
Laboratories, Hercules, CA, USA) at 490 nm. Percent cytotoxicity =
1 – 100% × [(experimental LDH release - spontaneous LDH
release)/(maximum LDH release - spontaneous LDH release)]. Samples
were measured in triplicate.
Cell cycle and cell apoptosis
detection
For cell cycle detection, cells were seeded at
105 cells/well in 6-well plates. Cells were harvested at
24, 48 and 72 h post-infection with H101 (MOI=100) or Ad-GFP
(MOI=100) or vehicle treatment (PBS). Cells were washed twice with
PBS and stained with 10 μg/ml propidium iodile (PI). The samples
were analyzed with BD Accuri C6 flow cytometry to determine cell
cycle distribution. Apoptosis was quantified by detecting surface
exposure of phosphatidylserine in apoptotic cells using the Annexin
V-FITC/PI apoptosis detection kit (BD Biosciences Clontech, CA,
USA). Cancer cells were infected for 24 h with H101 (MOI=100),
apoptotic cells were detected according to the manufacturer’s
instruction, using BD Accuri C6 flow cytometry.
Mouse procedures
Animal experiments were approved by and performed in
accordance with institutional guidelines of the Yunnan Animal Care
and Use Committee. Immunodeficient homozygous SCID Beige male mice,
7–8 weeks of age, were obtained from Vital River Laboratories
(Beijing, China). Back subcutaneous tumors were established by
injection of 5×106 XWLC-05 cells in 100 μl of PBS. When
tumor volume averaged ~100 mm3, 1×108 PFU of
H101 in 100 μl was injected in tumor daily for 4 days. Tumor
dimensions were measured by caliper every 4 days for 28 days, and
tumor volume (V) was estimated by the formula (long diameter ×
short diameter2)/2. The tumor growth inhibition rate was
calculated according to the previously described method (21). Mice were sacrificed at 32 days from
the first H101 injection, tumors and other important organism were
harvested, fixed in formalin and embedded in paraffin. Sections
were deparaffinized with xylene, hydrated in ethanol and distilled
water, and stained with hematoxylin and eosin (H&E). Tumor
sections were also evaluated for Hexon protein expression of H101
viruses using FITC conjugated mouse anti-Hexon monoclonal antibody
(GeneTex, Inc., Irvine, CA, USA), nuclei were counterstained with
DAPI and the sections visualized by fluorescent microscope
(Olympus, Tokyo, Japan).
Results
Lung cancer cells express CAR-specific
mRNA and protein
Ad enters a cell via receptor-mediated endocytosis
involving the binding of the fiber knob of viral capsid proteins to
the primary receptor CAR. We investigated the CAR mRNA expression
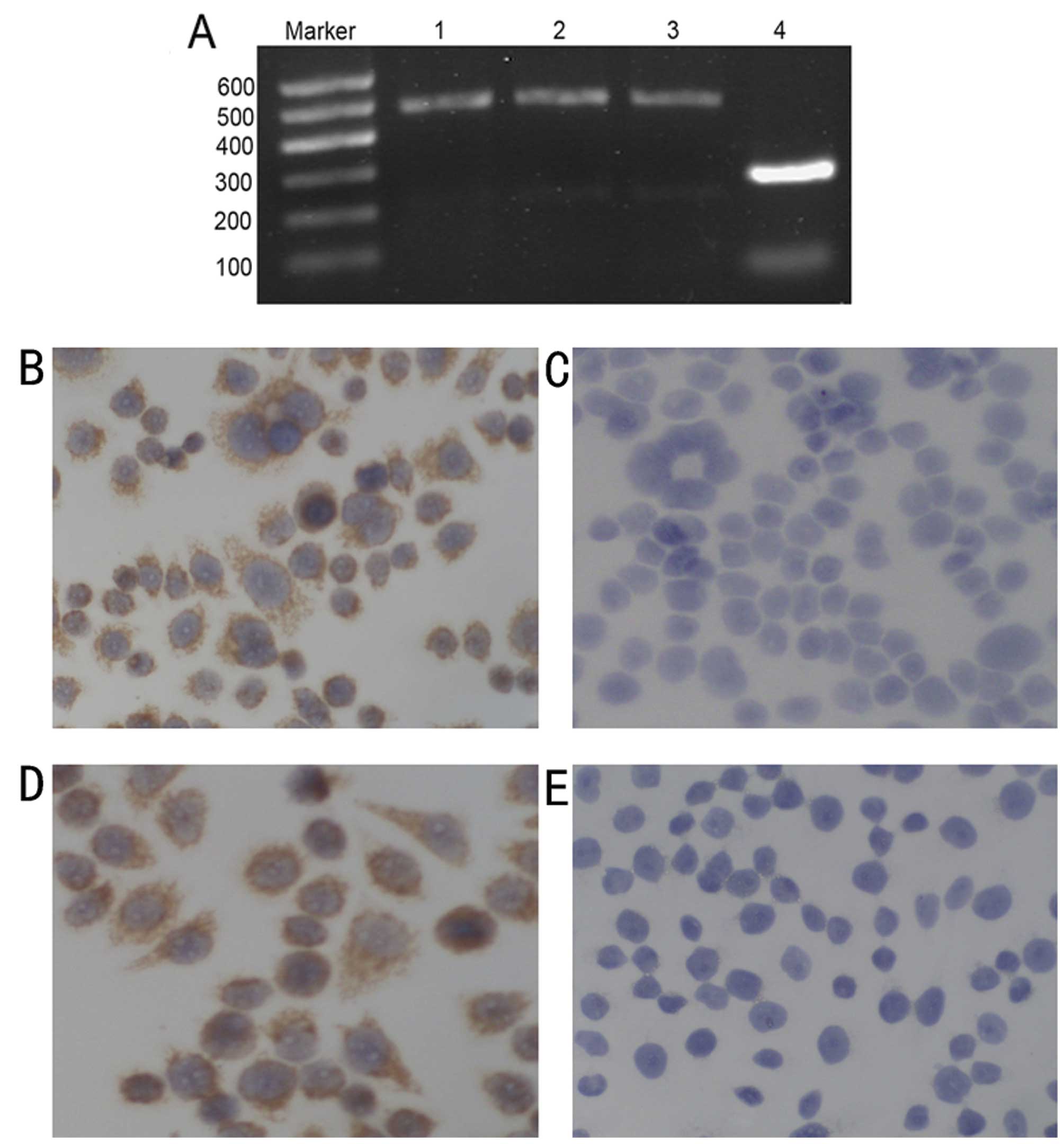
on lung cancer cell lines by RT-PCR assays. The results showed that
both XWLC-05 and SK-MES-1 expressed CAR mRNA similarly to 293 cells
(Fig. 1A). Using
immunocytochemistry, we also analyzed the presence of CAR protein
in XWLC-05 (Fig. 1B) and SK-MES-1
cells (Fig. 1D). RT-PCR and
immunochemistry analysis confirmed that lung cancer cells expressed
CAR, which provided a molecular base for oncolytic adenovirus H101
infection.
The susceptibility of XWLC-05 cells to
H101 infection
As the adenovirus type 5 infected cells use common
mechanism, the susceptibility of lung cancer cells to infection
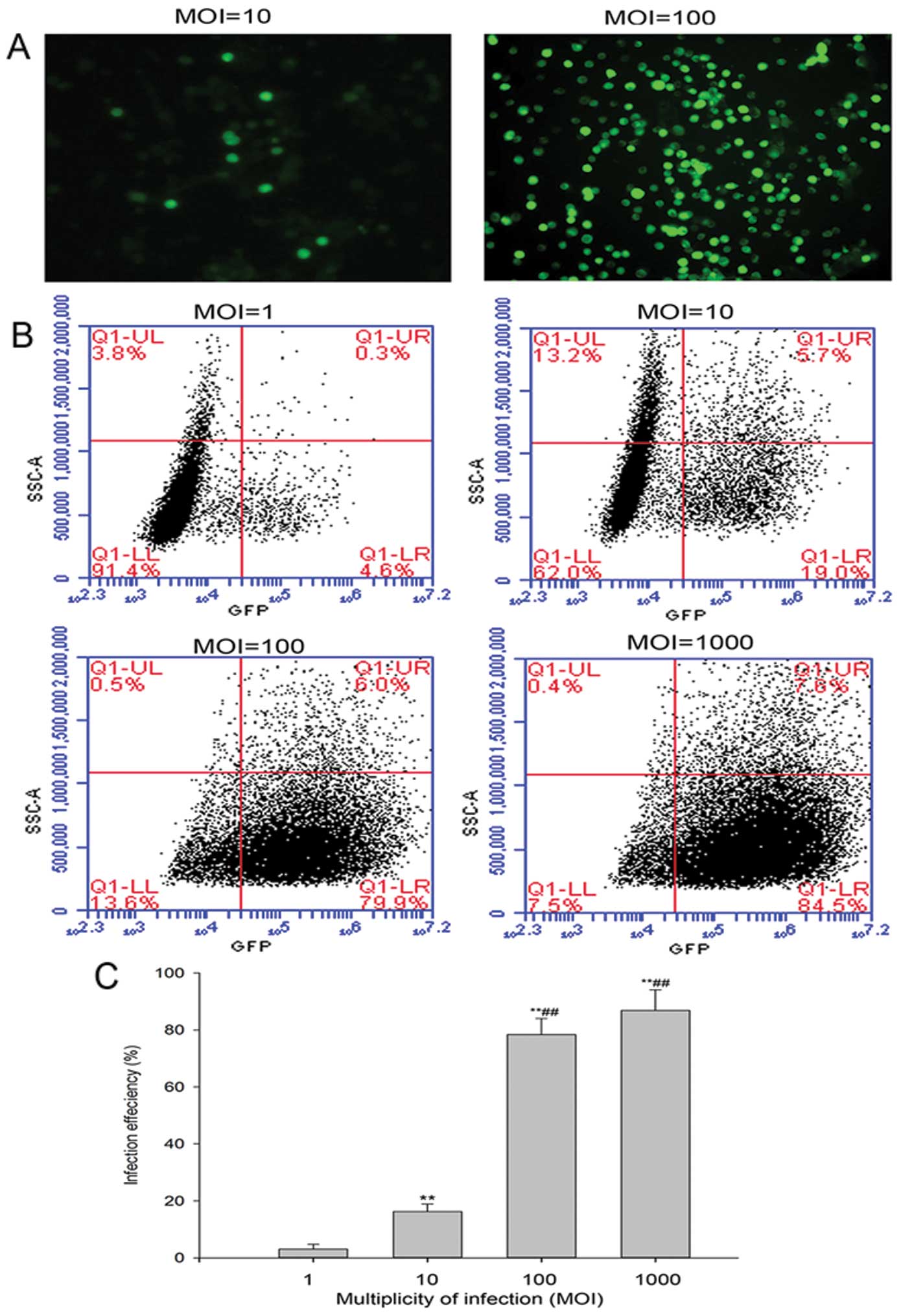
with Ad5 was determined by using Ad-GFP. As illustrated in Fig. 2, infected cancer cells expressing
GFP were visualized using a fluorescent microscope (Fig. 2A), and flow cytometric analysis
revealed that the percentage of Ad-GFP positive cells was increased
at a dose-dependent manner (Fig. 2B
and C). These results indicated that Ad-GFP transduced XWLC-05
efficiently.
Replication of oncolytic adenovirus H101
in lung cancer cells
Since adenovirus transduced XWLC-05 cells
efficiently, we investigated the replicative potential of H101 in
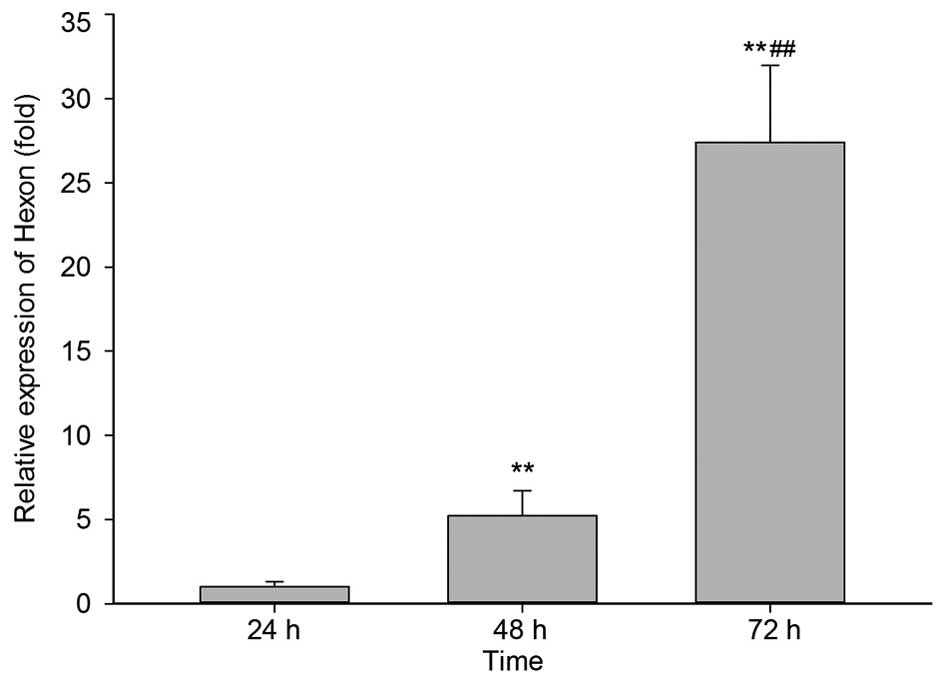
the cancer cells. XWLC-05 cells were infected with H101 virus and
analyzed for the amount of Hexon mRNA by real-time fluorescent
quantitative PCR. Results showed that H101 replicated efficiently
in XWLC-05 cells with comparative Hexon mRNA copy number increased
in the cells time-dependently (Fig.
3). Compared with 24 h, Hexon mRNA was significantly increased
by 5.2-fold at 48 h and 27.4-fold at 72 h. Increases in Hexon mRNA
between 24 and 72 h post-infection were indicative of viral
replication. These results indicated that the H101 virus was able
to replicate efficiently in lung cancer cells.
Cytopathic effects of H101 on XWLC-05
cells
XWLC-05 cells were used to further investigate the
cytopathic changes triggered by H101 infection. The cytopathic
effect was evaluated by viral cytotoxicity, cell cycle progression
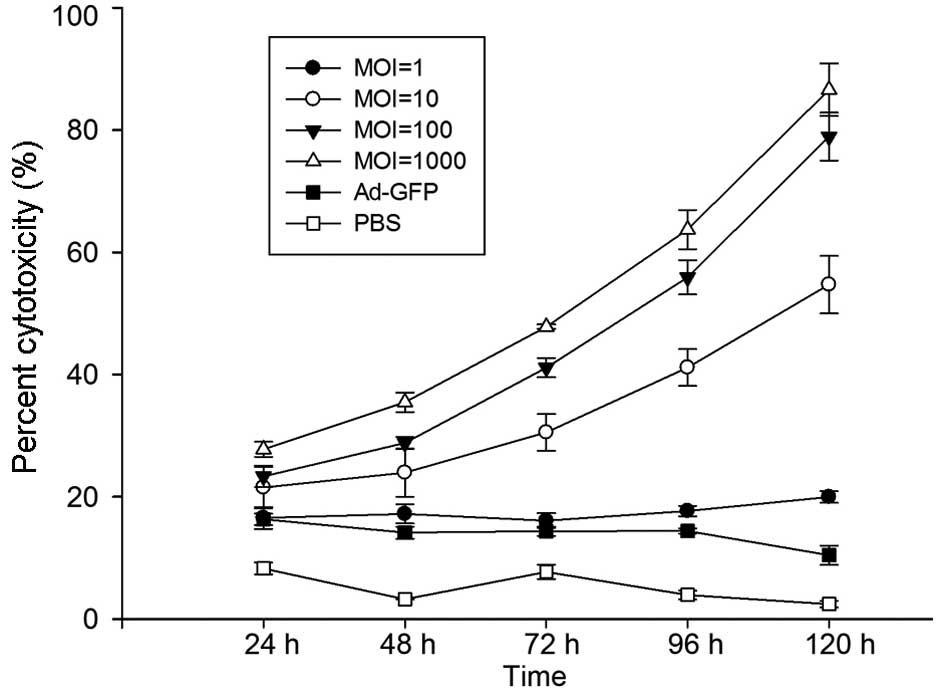
and apoptosis analysis. In agreement with the above results, LDH
assays indicated that H101 killed XWLC-05 cells efficiently with
LDH increases in a time- and dose-dependent manner (Fig. 4). Microscopy examination also
revealed nuclear swelling and DNA release from the nucleus into the
cytosol at later time-points after infection, indicating that most
cells died of necrosis (data not shown). In order to understand the
underlying mechanisms of cytopathic effect by H101 treatment, we
analyzed its effects on cell cycle phase distribution and apoptosis
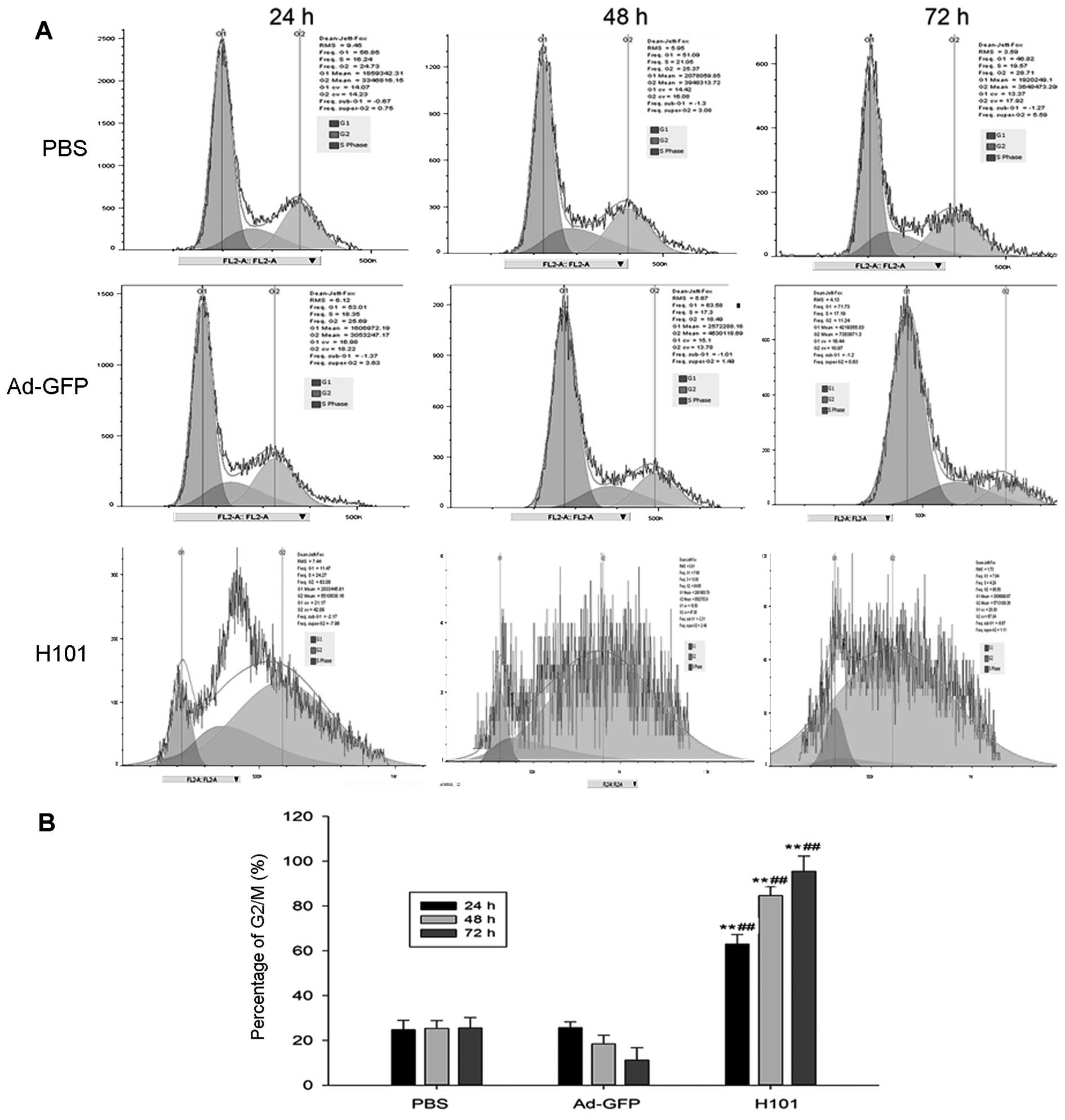
in XWLC-05 cells. As illustrated in Fig. 5, H101 induced strong accumulation
of cells in the G2/M phase in XWLC-05 cells at a MOI of 100 after
24-, 48- or 72-h treatments, which was associated with parallel
depletion of cells in the G0/G1 phase (Fig. 5A). Compared to vehicle or Ad-GFP,
the percentage of G2/M phase cells in H101 infected cells was
significantly different at all time-points (Fig. 5B). However, the percentage of
apoptotic cells was very low and not significantly different at 24
h post-infection by propidium iodide/Annexin V staining analysis
(data not shown). The results suggest that H101 infection induced
cytotoxic effects on XWLC-05 cells by cell cycle arrest and cell
necrosis.
H101 inhibited the growth of subcutaneous
XWLC-05 xenografts
In vitro, we investigated the oncolytic
effect of H101 on XWLC-05 cells, which significantly induced
cytotoxicity in XWLC-05 cells as compared to Ad-GFP or vehicle
control. We next evaluated the susceptibility of these cells to
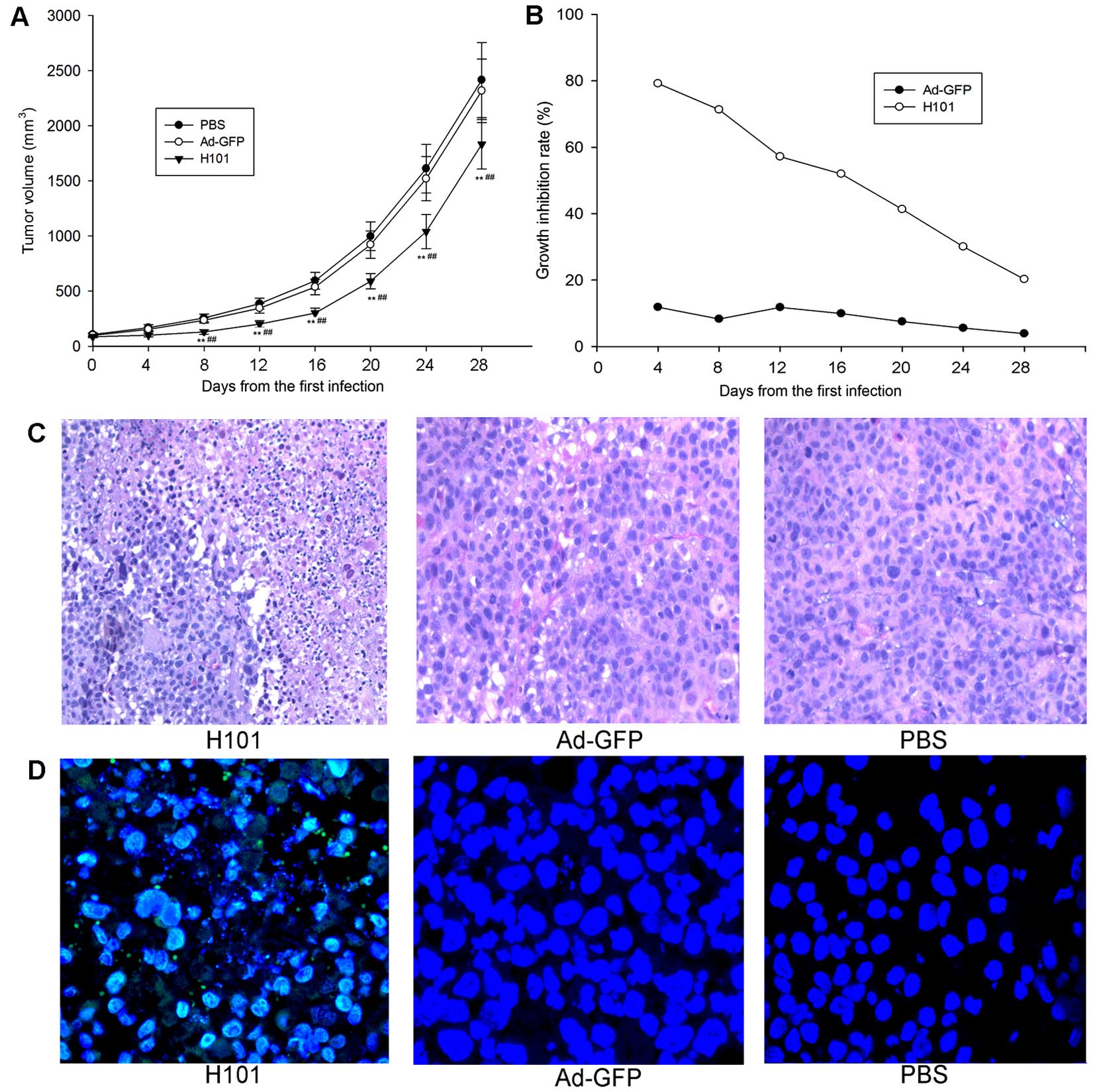
H101 treatment in vivo. H101 significantly suppressed the
growth of XWLC-05 tumor xenografts as compared to Ad-GFP or PBS
from 8 day to 28 day end-point of the study (Fig. 6A). The growth inhibition rate of
the H101-treated group was markedly increased as compared with
Ad-GFP group, and it gradually decreased due to limited viral
replication and propagation of the entire tumor mass (Fig. 6B). Histological examination of
Ad-GFP or PBS-treated tumors revealed healthy cancer cells arranged
in tumor architecture. However, in H101 virus-treated group, H101
induced cytocidal effects on XWLC-05 cells, which were
characterized by scattered necrotic patches surrounded by healthy
tumor cells, indicative of incomplete oncolysis due to limited
viral replication and propagation of cancer cells (Fig. 6C). To associate cell death and
active viral infection, immunofluorescence (IF) staining with FITC
anti-Hexon was performed on paraffin-embedded sections collected 28
days after virus injection, and IF staining showed the presence of
viral proteins in the tumor cells of H101 virus-treated group
(Fig. 6D). In general, H101
inhibited the growth of subcutaneous XWLC-05 xenografts by virus
replication and virus-induced cytotoxicity in vivo.
Discussion
Contrary to the improved survival outcomes for many
other types of cancers, the prognosis for people diagnosed with
lung cancer remains poor despite conventional therapy with surgery,
chemotherapy and new molecular target therapies, with 5-year
relative survival being ~6–14% among males and 7–18% among females
(2). This poor survival means that
new treatment options with completely novel mechanisms of
therapeutic activity are needed for lung cancer patients to improve
outcome.
Oncolytic virotherapy have proposed as a promising
therapeutic approach to fight cancer. A variety of viruses have
shown oncolytic properties including adenovirus, herpes simplex
virus, Newcastle disease virus, vesicular stomatitis virus and
reovirus (22). Among a variety of
oncolytic viral agents, CRAds specifically aimed at killing tumor
cells while sparing normal cells have been developed as new agents
for cancer therapy (23,24), and demonstrated efficacy and safety
in preclinical (25,26) and clinical trials (27–29).
Various methods have been used to achieve a selective expression.
These approaches included the use of tumor-specific promoters to
drive E1A gene expression (30) or
the E1B deletion, which restricts the oncolytic activity to
p53-defective tumor cells. ONYX-015 was the first to be tested in
clinical trials, revealing itself as a well-tolerated and safe tool
and a promising therapeutic agent in cancer (31).
In the present study, we describe a novel
application of adenovirus H101 in lung cancer, which was very
similar to ONYX-015. H101 was generated by deleting both E1B
and E3 genes of adenovirus type 5 (Ad5), which selectively
infects and kills tumor cells through viral oncolysis while leaving
normal cells unharmed without E1B to inactivate P53, and improves
the safety of the product with the E3 deletion. Previous reports
have shown the therapeutic potentials in other tumors. In clinic, a
multicenter randomized phase 3 clinical trial showed that the
combination therapy with H101 for head and neck squamous cell
carcinoma and esophageal cancer yielded a 27% increase in overall
response rates compared with fluorouracil plus cisplatin-based
chemotherapy alone (22). This
virus was approved by the Chinese State Food and Drug
Administration for the treatment of late-stage refractory
nasopharyngeal cancer.
We investigated the CAR mRNA and protein expression
in a lung cancer cell line, as CAR is the entry gateway for
adenovirus and needed for efficient adenovirus-mediated oncolysis.
Our finding clearly indicated the high CAR mRNA and protein
expression in lung cancer cells including adenocarcinoma XWLC-05
and squamous cell carcinoma SK-MES-1. Therefore, it is speculated
that lung cancer may have susceptibility to adenovirus H101
infection. In a previous study, high CAR mRNA and protein
expression was observed in other tumors such as breast cancer and
early-stage oesophageal adenocarcinoma (19,32).
Functionally, CAR may promote early carcinogenesis as suggested for
cervix and ovarian cancers, with CAR-expressing cell lines
displaying less sensitivity towards apoptotic stimuli (33). As for lung cancer cells, it was
suggested that CAR might be required for effective xenograft
formation (34,35).
We next demonstrated that adenovirus H101 was able
to efficiently infect and replicate in XWLC-05 cell lines in
vitro, leading to cell growth stress and oncolysis. H101
infection induced the accumulation of G2/M phase cells, and nuclear
swelling and DNA release from the nucleus into cytosol at late
time-points, which indicated that the underlying mechanism for
cytopathic effects of H101 involved cell cycle stress and necrosis,
which is in agreement with a previous study (20).
In vivo, we showed that the tumor volume of
mice bearing XWLC-05 xenograft was significantly reduced compared
with mock controls when treated with H101. For safe and effective
virotherapy, target tissue-restricted virus expression is
desirable. In the present study, the intratumoral injection of H101
in XWLC-05 xenografts exhibited localized intratumoral Hexon
protein expression. Moreover, there was no evidence of viral spread
to any other organs including brain, liver, heart and gut, based on
Hexon protein expression under a fluorescence microscope (data not
shown), indicating tumor-specific viral infection and activity.
Although oncolytic adenovirus H101 are highly efficient at killing
tumor cells in vitro, the activity in vivo was
moderate, and the tumor growth inhibition gradually decreased due
to limited viral proliferation and rapid propagation of cancer
cells. In other studies, the results exhibited relative activities
of oncolytic virus in animals and the clinic, thus, combination
therapy is often suggested for therapeautic potential in
vivo. For example, others have reported previously that
radiation can induce greater vector replication, causing increased
oncolytic activity (36,37). Based on our results, it would be of
significant clinical importance to evaluate the effect of
combination therapy of H101 and chemotherapy or radiation.
In conclusion, the present study demonstrates
therapeutic potential of adenovirus H101 in lung cancer, which
provides novel insights into the treatment of lung cancer using the
oncolytic adenovirus.
Acknowledgements
The present study was supported by the Fundamental
Research Funds (2010ZC186) and the Reserve Talent Funds (2008Y003)
of Yunnan Province.
References
|
1
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ramalingam SS, Owonikoko TK and Khuri FR:
Lung cancer: New biological insights and recent therapeutic
advances. CA Cancer J Clin. 61:91–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roy M, Luo YH, Ye M and Liu J: Nonsmall
cell lung cancer therapy: Insight into multitargeted small-molecule
growth factor receptor inhibitors. BioMed Res Int. 2013:9647432013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lorigan P, Verweij J, Papai Z, Rodenhuis
S, Le Cesne A, Leahy MG, Radford JA, Van Glabbeke MM, Kirkpatrick
A, Hogendoorn PC, et al; European Organisation for Research and
Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. Phase
III trial of two investigational schedules of ifosfamide compared
with standard-dose doxorubicin in advanced or metastatic soft
tissue sarcoma: A European Organisation for Research and Treatment
of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol.
25:3144–3150. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carrera S, Buque A, Azkona E, Aresti U,
Calvo B, Sancho A, Arruti M, Nuño M, Rubio I, de Lobera AR, et al:
Epidermal growth factor receptor tyrosine-kinase inhibitor
treatment resistance in non-small cell lung cancer: Biological
basis and therapeutic strategies. Clin Transl Oncol. 16:339–350.
2014. View Article : Google Scholar
|
|
7
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pao W and Girard N: New driver mutations
in non-small-cell lung cancer. Lancet Oncol. 12:175–180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo ZS, Thorne SH and Bartlett DL:
Oncolytic virotherapy: Molecular targets in tumor-selective
replication and carrier cell-mediated delivery of oncolytic
viruses. Biochim Biophys Acta. 1785:217–231. 2008.PubMed/NCBI
|
|
11
|
Jiang G, Yang CS, Xu D, Sun C, Zheng JN,
Lei TC and Liu YQ: Potent antitumour activity of a novel
conditionally replicating adenovirus for melanoma via inhibition of
migration and invasion. Br J Cancer. 110:2496–2505. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Immonen A, Vapalahti M, Tyynelä K,
Hurskainen H, Sandmair A, Vanninen R, Langford G, Murray N and
Ylä-Herttuala S: AdvHSV-tk gene therapy with intravenous
ganciclovir improves survival in human malignant glioma: A
randomised, controlled study. Mol Ther. 10:967–972. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khuri FR, Nemunaitis J, Ganly I, Arseneau
J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C,
et al: A controlled trial of intratumoral ONYX-015, a
selectively-replicating adenovirus, in combination with cisplatin
and 5-fluorouracil in patients with recurrent head and neck cancer.
Nat Med. 6:879–885. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Doloff JC and Waxman DJ: Adenoviral
vectors for prodrug activation-based gene therapy for cancer.
Anticancer Agents Med Chem. 14:115–126. 2014. View Article : Google Scholar :
|
|
15
|
Kawashima T, Kagawa S, Kobayashi N,
Shirakiya Y, Umeoka T, Teraishi F, Taki M, Kyo S, Tanaka N and
Fujiwara T: Telomerase-specific replication-selective virotherapy
for human cancer. Clin Cancer Res. 10:285–292. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kasuya H, Takeda S, Shimoyama S, Shikano
T, Nomura N, Kanazumi N, Nomoto S, Sugimoto H and Nakao A:
Oncolytic virus therapy - foreword. Curr Cancer Drug Targets.
7:123–125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang H, Wang H, Zhang J, Qian G, Niu B,
Fan X, Lu J, Hoffman AR, Hu JF and Ge S: Enhanced therapeutic
efficacy by simultaneously targeting two genetic defects in tumors.
Mol Ther. 17:57–64. 2009. View Article : Google Scholar
|
|
18
|
Yan FC, Wang QQ, Ruan YH, Ma LJ, Jia JT
and Jin KW: Establishment and biological characteristics of lung
cancer cell line XWLC-05. Ai Zheng. 26:21–25. 2007.(In Chinese).
PubMed/NCBI
|
|
19
|
Anders M, Christian C, McMahon M,
McCormick F and Korn WM: Inhibition of the Raf/MEK/ERK pathway
up-regulates expression of the coxsackievirus and adenovirus
receptor in cancer cells. Cancer Res. 63:2088–2095. 2003.PubMed/NCBI
|
|
20
|
Song X, Zhou Y, Jia R, Xu X, Wang H, Hu J,
Ge S and Fan X: Inhibition of retinoblastoma in vitro and in vivo
with conditionally replicating oncolytic adenovirus H101. Invest
Ophthalmol Vis Sci. 51:2626–2635. 2010. View Article : Google Scholar
|
|
21
|
Zhang C, Awasthi N, Schwarz MA, Hinz S and
Schwarz RE: Superior antitumor activity of nanoparticle
albumin-bound paclitaxel in experimental gastric cancer. PLoS One.
8:e580372013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu TC and Kirn D: Gene therapy progress
and prospects cancer: Oncolytic viruses. Gene Ther. 15:877–884.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bischoff JR, Kirn DH, Williams A, Heise C,
Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, et al:
An adenovirus mutant that replicates selectively in p53-deficient
human tumor cells. Science. 274:373–376. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Post DE, Khuri FR, Simons JW and Van Meir
EG: Replicative oncolytic adenoviruses in multimodal cancer
regimens. Hum Gene Ther. 14:933–946. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ranki T, Särkioja M, Hakkarainen T, von
Smitten K, Kanerva A and Hemminki A: Systemic efficacy of oncolytic
adenoviruses in imagable orthotopic models of hormone refractory
metastatic breast cancer. Int J Cancer. 121:165–174. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rajecki M, Kanerva A, Stenman UH, Tenhunen
M, Kangasniemi L, Särkioja M, Ala-Opas MY, Alfthan H, Sankila A,
Rintala E, et al: Treatment of prostate cancer with Ad5/3Delta24hCG
allows non-invasive detection of the magnitude and persistence of
virus replication in vivo. Mol Cancer Ther. 6:742–751. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nemunaitis J, Tong AW, Nemunaitis M,
Senzer N, Phadke AP, Bedell C, Adams N, Zhang YA, Maples PB, Chen
S, et al: A phase I study of telomerase-specific replication
competent oncolytic adenovirus (telomelysin) for various solid
tumors. Mol Ther. 18:429–434. 2010. View Article : Google Scholar :
|
|
28
|
Yu W and Fang H: Clinical trials with
oncolytic adenovirus in China. Curr Cancer Drug Targets. 7:141–148.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Freytag SO, Movsas B, Aref I, Stricker H,
Peabody J, Pegg J, Zhang Y, Barton KN, Brown SL, Lu M, et al: Phase
I trial of replication-competent adenovirus-mediated suicide gene
therapy combined with IMRT for prostate cancer. Mol Ther.
15:1016–1023. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamamoto M, Davydova J, Wang M, Siegal GP,
Krasnykh V, Vickers SM and Curiel DT: Infectivity enhanced,
cyclooxy-genase-2 promoter-based conditionally replicative
adenovirus for pancreatic cancer. Gastroenterology. 125:1203–1218.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ganly I, Kirn D, Eckhardt G, Rodriguez GI,
Soutar DS, Otto R, Robertson AG, Park O, Gulley ML, Heise C, et al:
A phase I study of Onyx-015, an E1B attenuated adenovirus,
administered intratumorally to patients with recurrent head and
neck cancer. Clin Cancer Res. 6:798–806. 2000.PubMed/NCBI
|
|
32
|
Anders M, Rösch T, Küster K, Becker I,
Höfler H, Stein HJ, Meining A, Wiedenmann B and Sarbia M:
Expression and function of the coxsackie and adenovirus receptor in
Barrett’s esophagus and associated neoplasia. Cancer Gene Ther.
16:508–515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brüning A, Stickeler E, Diederich D, Walz
L, Rohleder H, Friese K and Runnebaum IB: Coxsackie and adenovirus
receptor promotes adenocarcinoma cell survival and is
expressionally activated after transition from preneoplastic
precursor lesions to invasive adenocarcinomas. Clin Cancer Res.
11:4316–4320. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qin M, Escuadro B, Dohadwala M, Sharma S
and Batra RK: A novel role for the coxsackie adenovirus receptor in
mediating tumor formation by lung cancer cells. Cancer Res.
64:6377–6380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fuxe J, Liu L, Malin S, Philipson L,
Collins VP and Pettersson RF: Expression of the coxsackie and
adenovirus receptor in human astrocytic tumors and xenografts. Int
J Cancer. 103:723–729. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamamoto M and Curiel DT: Current issues
and future directions of oncolytic adenoviruses. Mol Ther.
18:243–250. 2010. View Article : Google Scholar :
|
|
37
|
Pesonen S, Kangasniemi L and Hemminki A:
Oncolytic adenoviruses for the treatment of human cancer: Focus on
translational and clinical data. Mol Pharm. 8:12–28. 2011.
View Article : Google Scholar
|