Introduction
For a number of decades the incidence of esophageal
adenocarcinoma has been steadily rising in several countries
(1–4). In the USA, for instance, the overall
EAC incidence increased from 0.4 cases per 100,000 in 1975 to 2.6
cases per 100,000 in 2009 (5). The
5-year survival has improved in this same period from ~5%, but
still remains rather poor at 15–20% (3,5). The
exact reasons for this rise in EAC incidence is unclear. With
respect to risk factors some studies have shown a relationship
between increased body mass index (BMI) with an increased risk for
EAC (3,6). Other important risk factors for EAC
are gastro-esophageal reflux disease (GERD) and the herewith
related Barrett’s esophagus (BE) (7–9). BE
is a metaplastic pre-malignant transformation of the esophageal
epithelium associated with GERD (7–9).
Although the outcomes of EAC have improved through the bettering of
conventional treatments, for instance through combining
chemoradiotherapy with surgery, outcomes of EAC are still poor
(10). There is, thus, a need for
a deeper molecular insight into EAC to develop new approaches and
treatments to improve patient outcomes.
A potential route to this deeper molecular insight
are kinases. This group of enzymes, through addition of a phosphate
group to specific targets, activate large and far-reaching
signaling cascades, which are pivotal in maintaining homeostasis in
living organisms. Their pivotal role also means that the precarious
biological balance they help sustain can be easily broken, through
the mutation or aberrant expression of these bio-molecules.
Elucidating phosphorylation activity with respect to specific
processes, such as cell proliferation, has been shown to be an
attractive avenue of research as molecular strategies developed
around inhibitors of the involved kinases might serve as anticancer
therapeutics. For instance, the targeting of the HER2 receptor
tyrosine kinase has been shown to be of benefit in breast cancer
treatment (11,12). Also in EAC, amplification of the
HER2 gene has been noticed in up to 21% of samples (13). A recent trial has shown improvement
in survival of patients with gastric and gastroesophageal junction
cancer upon combining chemotherapy with the HER2 targeting
immunoglobulin trastuzumab (14).
Therefore, identifying novel aberrancies of kinases involved in
cell proliferation could be useful in EAC. A potential source for
these kinases is the p16-Rb pathway, which is frequently affected
in cancer (15). The P16/INK4A
protein inhibits the cyclin D-CDK4/6 complexes that modulate
progression through the G1/S-phase checkpoint of the cell cycle,
through hyperphosphorylation of the Rb-E2F complex. Release of the
E2F transcription factors consequently leads to DNA replication
(15). Aberrancy of p16, such as
mutations, methylations and deletions, are some of the earliest
events in cancer (16). P16 is
also involved in the pathogenesis of EAC with a number of
P16 alterations such as gene locus loss, LOH (loss of
heterozygosity), mutations and methylation being present in BE, the
pre-malignant stage of EAC (17–20)
and consequently increasing with increasing tissue dysplasia
(17,18,20).
In a number of cancers it has been shown that aberration of p16
affect phosphorylation status of Rb (21,22).
It would be of interest to investigate whether a similar
biomolecular effect can be seen in EAC.
To elucidate phosphorylation mechanisms involved in
the pathogenesis of EAC we first employed a high-throughput
tyrosine kinase peptide chip, to investigate broad-scale kinase
activity in EAC. We additionally examined the p16-Rb pathway in EAC
and normal squamous esophageal biopsy specimens to investigate
whether phosphorylation of Rb might play a role in EAC
pathogenesis.
Materials and methods
Patient material
The present study received Institutional Review
Board approval from the Academic Medical Center (AMC, Amsterdam,
The Netherlands) and written informed consent was obtained from all
participating subjects. Biopsies were obtained from EAC patients
referred for endoscopy for disease staging. The study included
biopsies of EAC and squamous mucosa as confirmed in matched samples
taken for routine histological purposes. For the kinase chip,
immunoblotting and immunohistochemistry experiments normal squamous
EAC tissue samples were isolated from a total of 51 patients.
Samples were snap frozen and stored at −80°C until processing for
experiments. Of the patients, 43 were male and 8 female. Average
age was 66±12 years.
Esophageal cell lines
The human hTERT immortalized esophageal cell line
EPC2-hTERT was a kind gift from Professor A. Rustgi (University of
Pennsylvania, Philadelphia, PA, USA) (23) and was cultured in KSFM medium (Life
Technologies, Bleijswijk, The Netherlands) supplemented with human
recombinant epidermal growth factor (EGF) and bovine pituitary
extract (Life Technologies).
The OE19 and OE33 esophageal adenocarcinoma cell
line were obtained from the American Type Culture Collection (ATCC;
Manassas, VA, USA) and cultured in RPMI-1640 medium (Life
Technologies) supplemented with L-glutamine,
penicillin/streptomycin and 10% fetal bovine serum (FBS; Lonza
Group AG, Basel, Switzerland).
Tyrosine kinase peptide array
Fifteen EAC and coupled squamous biopsies were
analysed with the PAMgene tyrosine kinase peptide-microarray system
(PAMgene, Hertogenbosch, The Netherlands) for two replicate
experiments. This peptide-microarray contains 143 short peptides,
with sequences derived from literature, with known phosphorylation
sites representing 100 proteins. Biopsies were lysed in M-PER lysis
buffer (Thermo Fisher Scientific, Etten-Leur, The Netherlands) and
samples were tested in 2–3 replicates. The peptide-microarray
system measures kinase kinetics by detecting phosphorylation with
anti-phospho tyrosine fluorescently labelled immunoglobulins. End
level average signals and initial rates of the samples were
determined and corrected for signal saturation. These measurements
indicated phosphorylation activity. Kinase activity heat maps were
created with the CIMminer software (Genomics and Bioinformatics
group, LMP, CCR, National Cancer Institute, http://discover.nci.nih.gov/cimminer/).
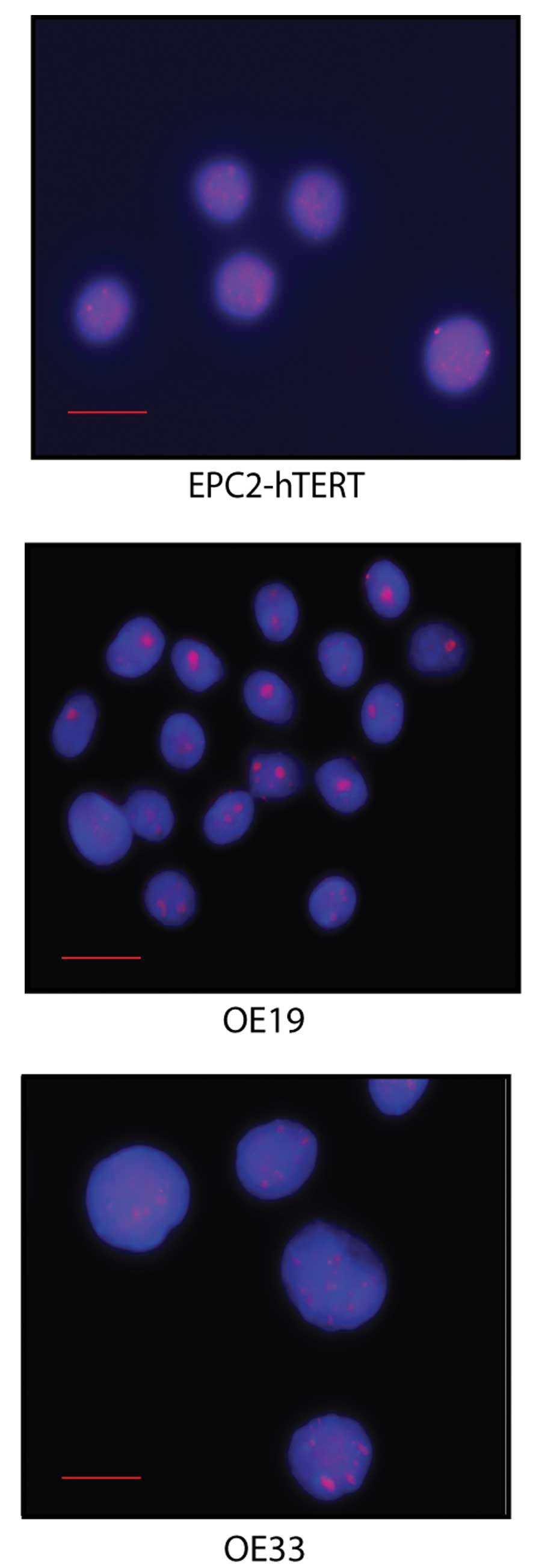
DNA fluorescence in situ hybridization
(FISH)
To detect the loss of the p16 gene locus in the cell
lines, DNA FISH was performed as previously described (24). For this experiment the specimens
were hybridized with a directly labelled probe mix which contained
the SpectrumRed-LSI p16 (9p21) locus specific probe for the 9p21
(P16) region on chromosome 9, obtained from Abbott Molecular
(Chicago, IL, USA).
Scoring was done with the 60× objective of the
Olympus BX51 microscope (Olympus Nederland BV, Zoeterwoude, The
Netherlands). Pictures were taken with the Olympus XM10 camera and
brightness and contrast adjustments were done with Olympus Cell^F
software. To define per sample P16 DNA-FISH status we used
previously determined frequency cut-off values for P16 gains
and losses (24). These values
were obtained from counts of 20 normal squamous epithelium
cytological patient specimens (100 nuclei evaluated per case) and
calculated as the mean percentage of squamous nuclei with either
p16 gain or loss plus 3× times standard deviation. The
P16 cut-off frequency was 10% for losses and 4% for
gains.
Western blotting
Biopsies and cell lines were lysed in M-PER lysis
buffer (Thermo Fisher Scientific) supplemented with protease and
phosphatase inhibitors (Halt protease and phosphatise inhibitor
cocktail; Thermo Fisher Scientific). For the cell line experiments
two independent lysate samples were made from each line and a
number of replicate blots were made from these samples.
Lysates combined with sample buffer (125 mM
Tris-HCl, pH 6.8; 4% SDS; 2% β-mercaptoethanol; 20% glycerol; 1 mg
bromophenol blue) were loaded onto SDS-protein gels and
subsequently transferred onto PVDF membranes (Millipore, Amsterdam,
The Netherlands). The blots were incubated overnight at 4°C with
the primary antibody of interest. After incubation with the primary
antibodies, blots were incubated for 1 h at room temperature with
the secondary antibody, the anti-rabbit HRP conjugated
immunoglobulin (Dako, Heverlee, Belgium). Consequently blots were
incubated in Lumilite plus (Boehringer-Mannheim, Mannheim, Germany)
and chemiluminescence was detected using a Fuji LAS 4000
illuminator (Fuji Film Medical Systems, Stamford, CT, USA).
Quantification of the blots was performed with ImageJ software
(version 1.44). Primary antibodies used were the rabbit
anti-phospho-Rb (T356), anti-phospho-Rb (S795) and anti-phospho-Rb
(S780) immunoglobulins (Abcam, Cambridge, UK). Also used were the
rabbit monoclonal anti-P16/INK4A (Epitomics/Bio-Connect BV, TE
Huissen, The Netherlands) and rabbit polyclonal anti-Rb (ab6075;
Abcam) antibodies. The rabbit polyclonal anti-actin (Santa Cruz
Biotechnology, Heidelberg, Germany) antibody was used for
quantification of the P16 and (phospho-)Rb levels.
The relative phospho-Rb levels were corrected for
the total level of the Rb protein to determine the phosphorylation
activity on the specific amino acid. Total Rb levels were
determined for each cell line and EAC and squamous patient
biopsies. From these levels an average Rb level was determined for
each of the aforementioned esophageal cell lines and the BE tissue
types. The average phospho-Rb levels were then corrected for these
total Rb levels for the respective cell line and tissue type.
For the tissue samples total Rb expression was
obtained for 24 squamous and 20 EAC samples. Phospho-Rb levels were
measured for 38 normal squamous and 32 EAC samples. Phospho-Rb
(S795), was investigated in protein lysates from 12 EAC and
squamous samples. Phospho-Rb (S780) was investigated in 4 EAC and
squamous samples. Phospho-Rb (T356) was investigated in 16 EAC and
22 squamous samples. P16 protein levels were measured for 30 normal
squamous and 20 EAC samples.
Immunohistochemistry
Eight squamous esophageal and 19 EAC samples were
used for the immunohistochemistry experiments. These samples were
formalin fixated after isolation and paraffin embedded. Subsequent
5-μm tissue sections of each biopsy were cut and used for the
stainings. For staining, tissue slides were de-paraffinized and
antigen retrieval was performed by boiling slides for 20 min in
pre-warmed citrate buffer, pH 6.0. Endogenous peroxidase was
blocked by incubation with 0.3% H2O2. Next,
slides were blocked with 10% normal goat serum (Bio-Connect) and an
avidin/biotin blocking kit (Vector Laboratories Inc., Burlingame,
CA, USA). Slides were washed in PBS and incubated with the primary
antibody for 90 min at room temperature. Primary antibodies used
were monoclonal rabbit anti phospho-Rb (T356) and polyclonal rabbit
anti phospho-Rb (S795) (Abcam). Slides were then incubated with the
respective biotin linked secondary reagents from the LSAB™2 Kits
(Dako) following the manufacturer’s instructions. The peroxidase
activity was visualized using DAB+ (Dako). Finally,
sections were counter-stained with Mayer’s haematoxylin, dehydrated
and mounted. Slides were photographed with a microscope equipped
with a digital camera (Leica CTR500; Leica Microsystems, Rijswijk,
The Netherlands) using the 10× objective.
A semi-quantitative approach was used to score the
stained tissue samples taking into account the strength of staining
and the nuclei stained. Only nuclei of epithelial cells were
considered for scoring. For the anti-phospho-Rb (T356, S795)
antibodies, staining was scored from 0 to 4. Scores 0, 1, no or
very light staining in few nuclei; score 2, staining in few nuclei;
score 3, staining in 33–50% of nuclei; score 4, clear staining in
almost all nuclei. Slides were scored by one researcher (D.S.).
Statistical analysis
The Paired t-test and the Wilcoxon paired-rank test
to determine peptides significantly differently phosphorylated in
tumor compared to normal tissues were performed in Microsoft Excel
and with SPSS 20 (IBM, Amsterdam, The Netherlands). Statistical
significance was set at P<0.05. The T-test, the Wilcoxon paired
rank and the Mann-Whitney U test to look for significant
differences in quantified protein expression were performed with
GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA).
Statistical significance was set at P<0.05. SEM of the
quantified protein expressions was also determined with
GraphPad.
Results
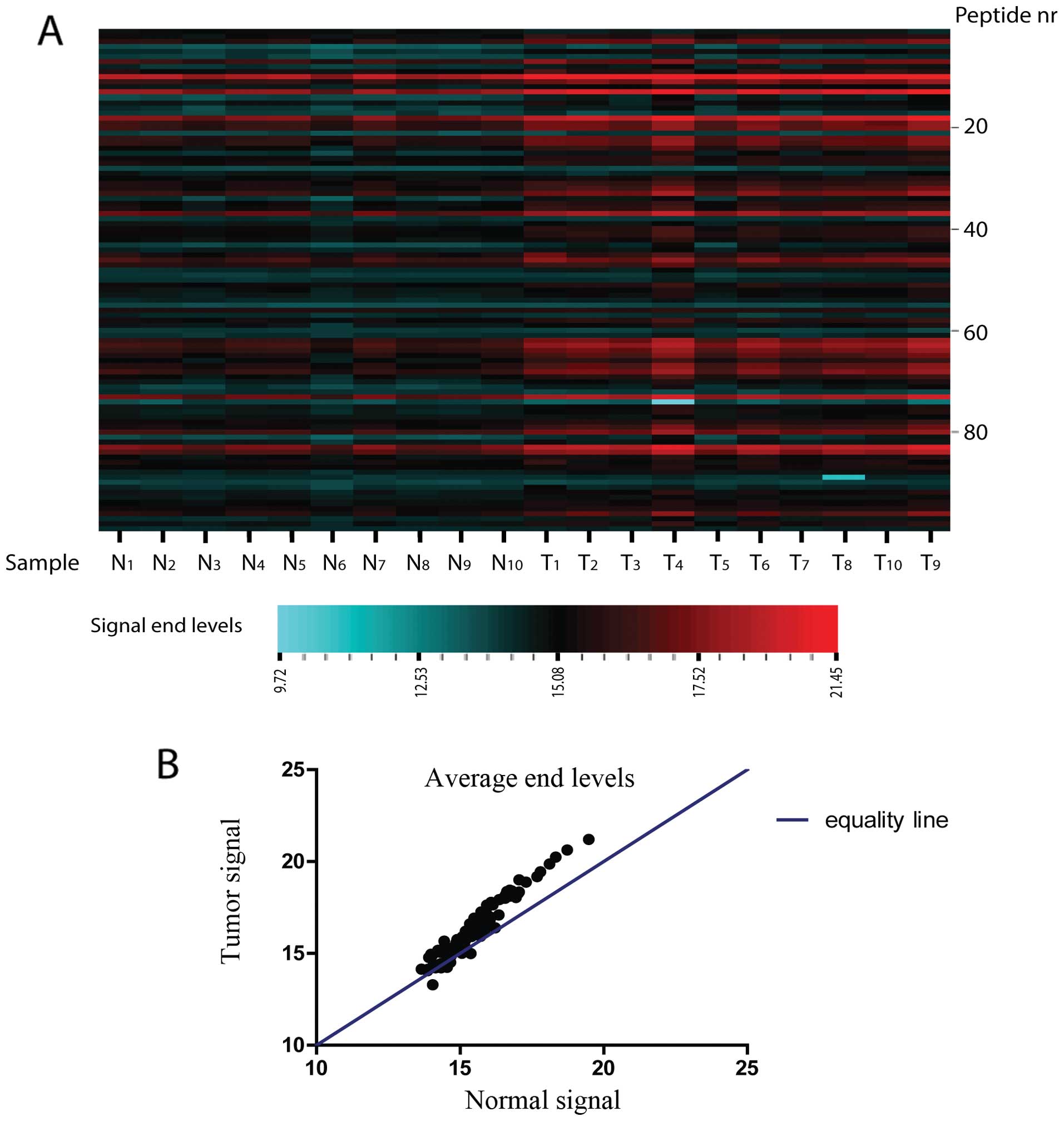
Tyrosine kinase activity and
phosphorylation of the Rb protein in esophageal adenocarcinoma
Phosphorylation activity in EAC biopsies was
compared to the matching normal squamous samples for 15 patients
with the PAMgene tyrosine kinase peptide-microarray system in two
independent experiments. In both experiments phosphorylation
activity showed an overall increase in tumor samples compared to
the matching normal squamous biopsies (Fig. 1). For one experiment,
phosphorylation levels were too low to be analysed for all the
peptides. In this case there were 100 analysable peptides. For the
other experiment 142 peptides were analysable. When averaged over
both experiments, of the 142 analysable peptides, 122 (86%) were
more phosphorylated and 11 (8%) were less phosphorylated in EAC.
Nine (6%) peptides had equal phosphorylation levels in tumor
compared to normal squamous tissues. In both experiments we found a
number of peptides for which the difference in phosphorylation
between tumor and coupled normal squamous tissues was significant
(P<0.05; paired t-test and Wilcoxon paired rank test). We listed
all peptides that were either significantly differently
phosphorylated for tumor vs. normal tissues in both experiments or
in one experiment if it was only analysable in a single experiment.
These peptides can be assigned to proteins which have a function in
a variety of processes including development, immunity and also
cell structure and motility (Table
I).
 | Table IProteins with significantly different
(P<0.05) phosphorylation signals in tumor (T) compared to normal
squamous (N) esophageal tissues as measured by the kinase
array. |
Table I
Proteins with significantly different
(P<0.05) phosphorylation signals in tumor (T) compared to normal
squamous (N) esophageal tissues as measured by the kinase
array.
| Pathway | Protein (gene) -
[p-Tyr group] | Fold-change
(T/N) |
|---|
| Cytoskeletal
structure/cell motility | Annexin A2
(ANXA2) - [Y24] | 4.9 |
| Adapter molecule
Crk (CRK) - [Y221] | 3.0 |
|
Macrophage-stimulating protein receptor
(RON) - [Y1353] | 2.4 |
| β-catenin
(CTNB1) - [Y86] | 1.9 |
|
Development/homeostasis | Tyrosine-protein
kinase JAK2 (JAK-2) - [Y570] | 1.6 |
| Myelin basic
protein (MBP) - [Y203] | 1.5 |
| Immunity | T-cell surface
glycoprotein CD3 ζ chain (CD3Z) - [Y123,153] | 4.0, 5.5 |
| Signal transducer
and activator of transcription 4 (STAT4) - [Y725] | 4.0 |
| B-cell antigen
receptor complex-associated protein α-chain (CD79A)
[Y182/188] | 1.6 |
| Lymphocyte
cell-specific protein-tyrosine kinase (LCK) - [Y394] | 1.4 |
| Linker for
activation of T-cells family member 1 (LAT) - [Y255] | 1.2 |
| Phosphoinositide
phospholipase C-γ-1 (PLCG1) - [Y1253] | 0.63 |
We found that also peptides representing proteins of
the G1/S checkpoint, specifically CDK2 and Rb, were differently
phosphorylated in EAC vs. normal esophageal tissues. Both peptides
showed higher levels of phosphorylation in tumor vs. normal
tissues, with ratios of 1.7 and 1.5, respectively for CDK2 and Rb.
Notably, phosphorylation of the CDK2-peptide was on a presumed
inhibitory residue at the Y15 position (25), which should lead to a lower level
of CDK2 activity. Nonetheless, the peptide derived from the Rb
protein showed higher phosphorylation in EAC compared to the normal
tissues. This is most likely due to a larger contribution of CDK4
activity, resulting in a net effect of increased Rb phosphorylation
in EAC.
P16 gene status and protein expression in
normal squamous and malignant esophageal cell lines
To investigate in cell lines if increased levels of
Rb phosphorylation were associated with p16 abnormalities in EAC,
we first validated gene status and the relative protein expression
of P16 in cell lines representing normal squamous epithelium
(EPC2-hTERT) and EAC (OE19 and OE33). The P16 (9p21) gene
locus status of the cells was investigated through DNA FISH
(Fig. 2) and protein expression of
P16 by immunoblotting. EPC2-hTERT was considered normal for
P16 gene status as assessed by DNA-FISH (Table II). Both EAC cell lines had large
frequencies of P16 FISH abnormalities. OE19 showed cells
with either losses or gains of the P16 allele, while OE33
exhibited exclusively gains of the gene (Table II). When considering the protein
levels, EPC2-hTERT had the highest relative p16 protein expression.
In comparison, the cancer cell line OE33 which actually showed
gains of P16, had a lower P16 protein expression, while OE19
had the lowest expression level (Table II and Fig. 3A). The low protein levels of P16 in
OE33 is likely due to P16 gene promoter hypermethylation
which is a frequent event in EAC and also has been observed in OE33
(26). Thus, P16 protein levels
correlated with P16 gene status as assessed by DNA-FISH in
EPC2-hTERT and OE19. This correlation was not directly seen for
OE33. Nevertheless, both techniques indicated aberrancies of p16 in
cancer cells compared to normal squamous cells. However, the
assessed P16 protein levels in EAC seemed to correlate better with
the reported functionality of the protein.
 | Table IIFrequencies for p16 locus loss and
gain in esophageal cell lines by DNA FISH. |
Table II
Frequencies for p16 locus loss and
gain in esophageal cell lines by DNA FISH.
| Cell lines | EPC2-hTERT | OE19 | OE33 |
|---|
| P16 FISH
frequencies (%) |
| Loss | 0 | 13 | 0 |
| Gain | 1 | 24 | 68 |
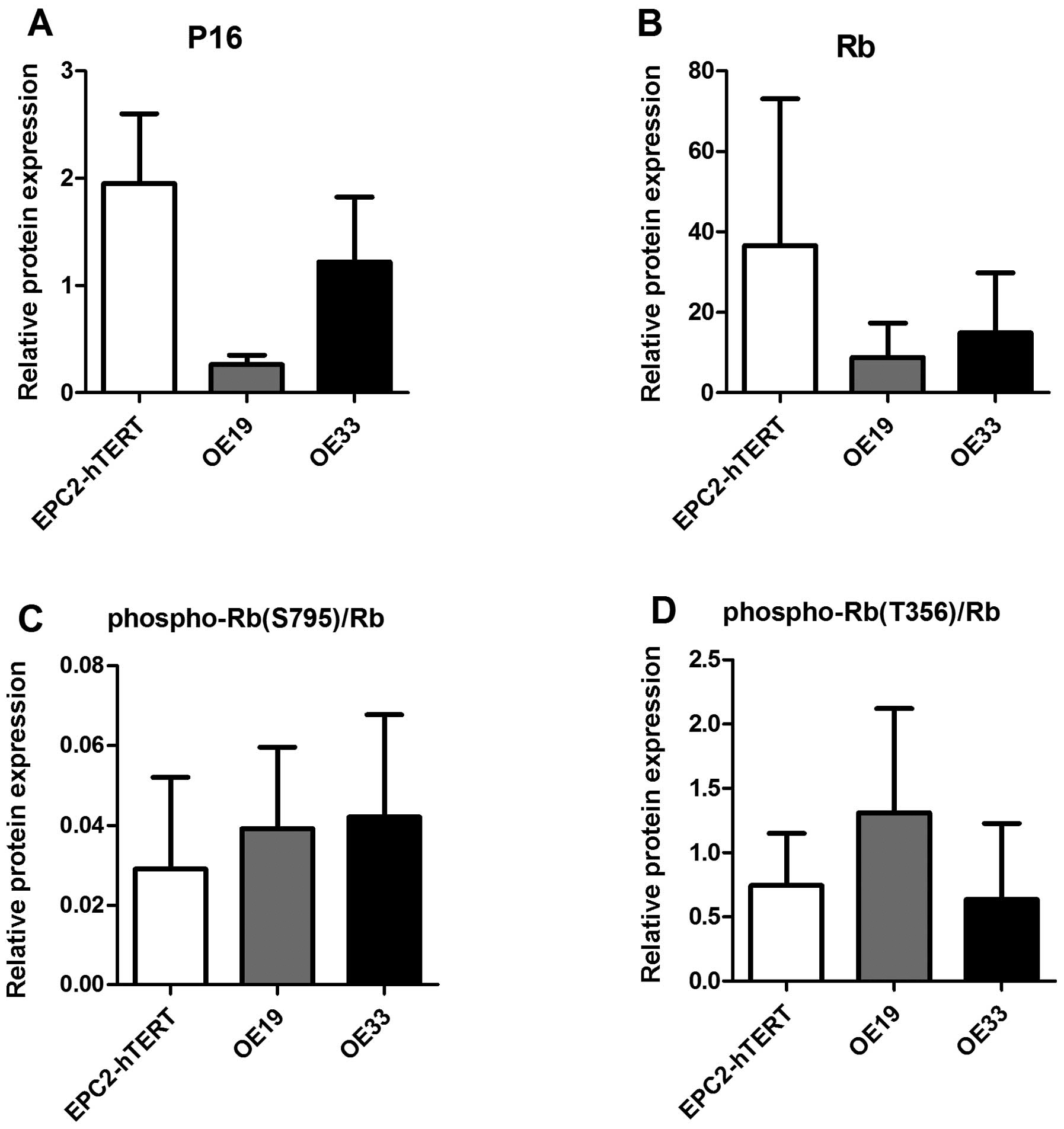
Phospho-Rb expression in normal and EAC
cell lines
Consequently, we compared the P16 protein expression
levels to the relative Rb protein phosphorylation in the
EPC2-hTERT, OE19 and OE33 cell lines. Rb has several
phosphorylation sites. We investigated phosphorylation on the T356
and S795 amino acids of Rb in the cell lines. We found that on
average Rb phosphorylation on these two residues inversely
correlated with the P16 protein levels (Fig. 3). P16 protein levels were lower for
both EAC cell lines, OE19 and OE33, compared to the normal squamous
cell line, EPC2-hTERT (Fig. 4A).
For the T356 residue, EPC2-hTERT, showed a lower phospho-Rb (T356)
level compared to the EAC cell line OE19 and comparable levels to
OE33. For phospho-Rb (S795) a higher level of phosphorylation was
seen for both EAC cell lines as compared to the normal squamous
cell line.
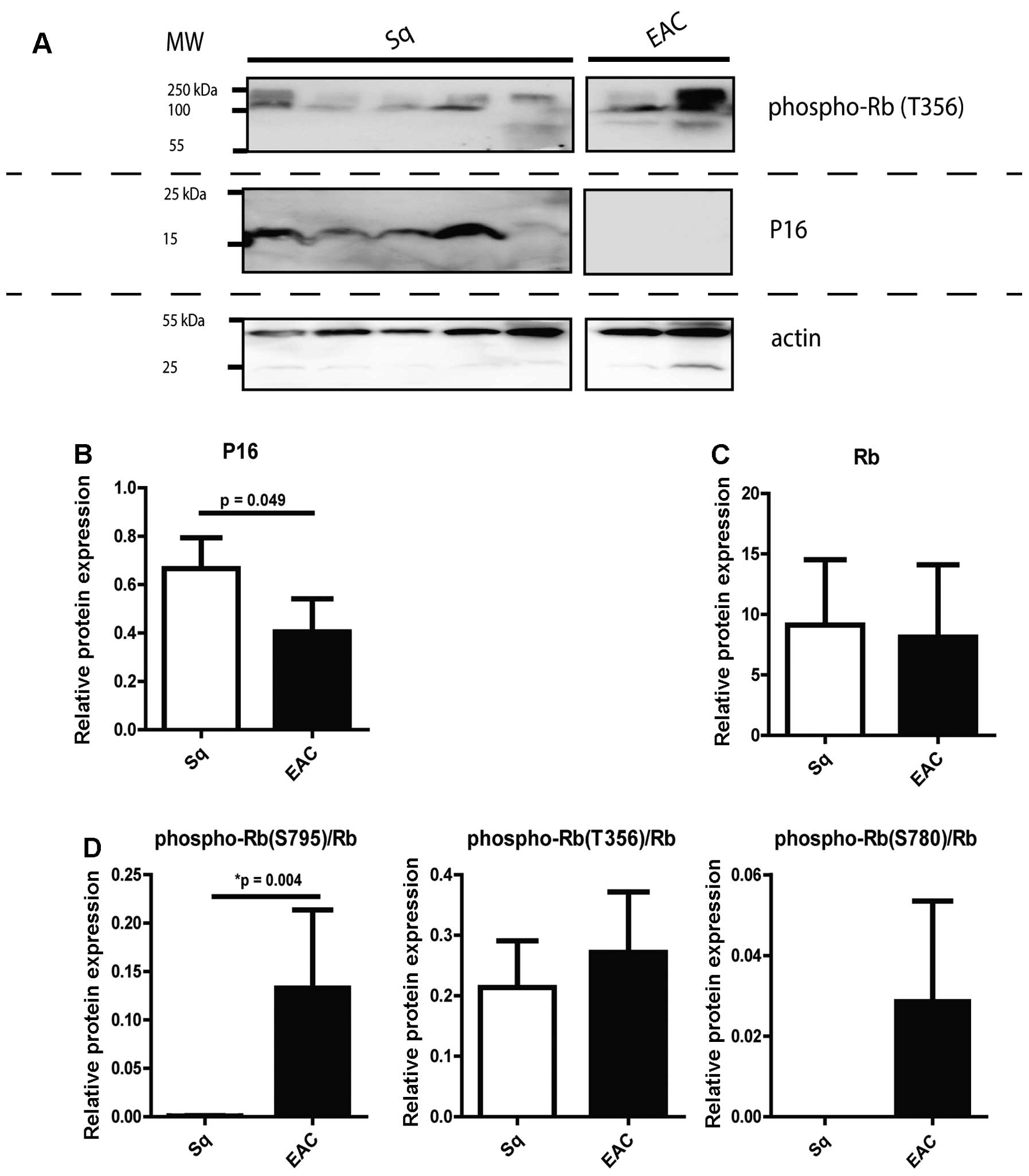
Rb phosphorylation and P16 expression in
esophageal tissues
Rb protein phosphorylation was also investigated in
patient biopsies with EAC and normal squamous epithelial tissues
and compared with P16 protein expression (Fig. 4). For these samples phosphorylation
on the S795, S780 and T356 amino acids of Rb was investigated. In
all cases, the tissue phospho-Rb levels were corrected for total Rb
expression. The protein expression levels within the specific
tissue types were rather variable (Fig. 4B–D). On average, expression of P16
was decreased in EAC biopsies when compared to the normal squamous
esophageal tissues (Fig. 4B,
t-test; P=0.049). Inversely, we found increased phosphorylation for
all three investigated Rb sites in EAC as compared to normal
squamous tissues (Fig. 4D). On
average, Rb phosphorylation at the S795 site was significantly
higher in EAC compared to coupled squamous tissues (Wilcoxon paired
rank test, P=0.004). Considering the specific phosphorylation
sites, for phospho-Rb (S795), 9 of 12 (75%) of EAC cases showed
increased phosphorylation as compared to matching squamous
controls. For phospho-Rb (S780), 4 of 4 (100%) and for phospho-Rb
(T356), 8 of 16 (50%) had increased phosphorylation in EAC. The
inverse correlation between a higher Rb phosphorylation ratio
compared to P16 protein expression in EAC falls in line with the
functional relation of P16 and Rb phosphorylation.
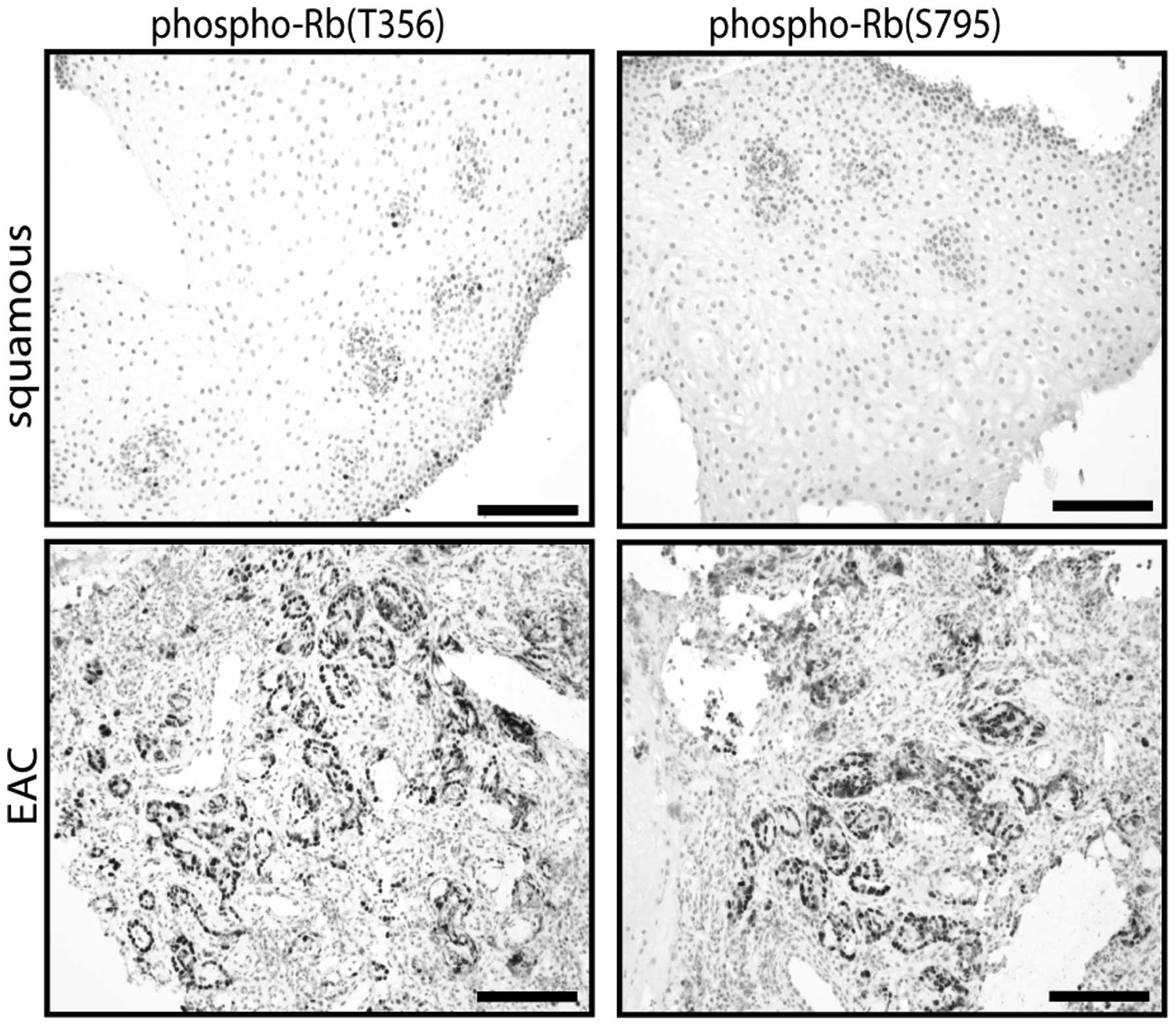
Phospho-Rb immunohistochemistry staining
in esophageal tissues
To validate and visualize the Rb phosphorylation as
detected in the biopsy protein lysates we performed
immunohistochemistry staining for Rb phosphorylated on the S795 and
T356 groups on BE patient tissue samples. To this end 8 squamous
esophageal and 19 EAC samples were stained. Overall, the stainings
showed that the EAC tissues had a stronger phosphorylation on both
the S795 and T356 groups than the squamous tissues (Fig. 5). To better (semi-)quantify these
results we designed a scoring system for the staining performed,
based on strength of staining and number of stained nuclei. The
scoring confirmed that Rb phosphorylation on S795 and T356 was much
more pronounced in EAC compared to squamous esophageal tissues
(Table III). If we considered
the two highest scoring categories as indicative of high Rb
phosphorylation, than 84.2% of the EAC tissues showed a high
phosphorylation of S795 and T356 compared to 0% of the squamous
tissues. Another interesting result of the staining was that
sequential EAC tissue sections stained for the two Rb groups did
not show a complete overlap with respect to stained nuclei. This
indicates that certain nuclei might have increased phosphorylation
on one amino acid while lacking this increased phosphorylation on
the other amino acid.
 | Table IIIScoring percentages for phospho-Rb
immunohistochemistry staining on BE tissue samples. |
Table III
Scoring percentages for phospho-Rb
immunohistochemistry staining on BE tissue samples.
| Score |
|---|
|
|
|---|
| 0/1 | 2 | 3 | 4 |
|---|
| Phospho-Rb
(T356) |
| Sq | 100 | 0 | 0 | 0 |
| EAC | 10.5 | 5.26 | 47.4 | 36.8 |
| Phospho-Rb
(S795) |
| Sq | 100 | 0 | 0 | 0 |
| EAC | 0 | 15.8 | 63.1 | 21.1 |
Discussion
Our explorative search into phosphorylation activity
as a method to gain more insight into the pathogenesis of EAC
confirmed that kinase activity is largely upregulated in this
cancer. High phosphorylation levels have been described earlier in
EAC as such our findings are in accordance with earlier studies
(27,28). To the best of our knowledge,
however, this is the first report that describes the
phosphorylation of specific Rb sites in EAC.
A list of the proteins for which phosphorylation was
significantly different in EAC compared to normal esophageal
tissues shows a broad range of molecules involved in varied
processes (Table I). A large part
of this list represents proteins involved in cytoskeletal structure
organization and cell adhesion, but also proteins involved in
immunity are represented. EAC is associated with chronic
inflammation due to active reflux esophagitis (27–29).
Aberrant phosphorylation related to the inflammatory process in EAC
compared to normal squamous tissues falls in line with the
literature (29–31). Tissue structure and inflammation
related processes might be interesting candidates for molecular
targets in EAC.
A pathway of interest that we investigated in the
present study was the phosphorylation of the Rb protein in EAC. A
1.5-fold higher phosphorylation of Rb was observed by the kinase
chip in EAC compared to normal esophageal tissues. This aberrant
phosphorylation of Rb is likely due to aberrations of its upstream
regulator, the p16. Aberrant p16 as detected through methylation,
loss of heterozygosity and immunohistochemistry has been frequently
observed in EAC pathogenesis (17–20).
P16 has thus been a molecule of interest in EAC. In the present
study we first investigated p16 status in EAC. There was a lower
P16 protein expression in EAC tissues and cell lines compared to
the normal squamous tissues (Figs.
3 and 4). On the genetic level
P16 showed an aberrant status in EAC cell lines compared to a
normal squamous esophageal cell line (Table II). The discrepancy of gains of
the P16 locus seen in one EAC cell line combined with a lower P16
protein expression, is likely due to hypermethylation of the p16
gene promoter. A process frequently observed in EAC (17,18,20).
We further found an inverse correlation between P16 expression and
phosphorylation of Rb forms in EAC cell lines and biopsies
(Figs. 3 and 4). Overall, EAC had lower P16 protein
expression levels and increased phosphorylation levels of Rb.
The specific Rb phosphorylation sites of S795, S780
and T356, that we investigated all shared the same upstream cyclin
dependent kinase 4 (CDK4). Phosphorylation at these amino acids
leads to the detachment of Rb from its interacting proteins and
stimulation of progression through the G1/S checkpoint (32–34).
In the tissue lysates there was a higher rate of Rb phosphorylation
on all three positions in EAC compared to the normal tissues.
Overall, this effect seemed to be more pronounced for the S795 and
S780, than the T356 site, and only for phosphorylation on the S795
position did this appear to be significant. However, this might be
due to the relatively small number of samples investigated. Also in
the immunohistochemistry stained slides the EAC tissues showed a
higher expression of Rb phosphorylated on the S795 and T356 groups
compared to the normal tissues. It is not clear how phosphorylation
on the different sites would differ with respect to functioning.
The S795 group lies within the Rb C-terminal tail, believed to
regulate binding of Rb interactors, such as E2F, to the Rb pocket
domain (35,36). The T356 group lies in the
N-terminal interdomain linker region of Rb and is also believed to
be involved in the regulation of binding of the E2F transcription
factor (37,38). However, as Rb interacts with a
number of other different proteins through the different binding
domains (35), it is possible that
phosphorylation on the different Rb residues affect interactions
with varied proteins and pathways. Notably, the
immunohistochemistry staining patterns for phospho-Rb (S795) and
phospho-Rb (T356) when comparing sequential slides differed
indicating that specific nuclei might have an increased
phosphorylation on a specific residue but not on the other. Future
experiments should more closely examine whether specific
phosphorylation on the different Rb residues might lead to
differential results in EAC. Also it might be of interest to
investigate if there are specific Rb sites that are more
phosphorylated in the earlier stages of EAC development, i.e. in
BE.
Research has shown that inhibitors of kinases
involved in cell proliferation might serve as anticancer
therapeutics. With respect to Rb phosphorylation some research has
been done on specific molecules affecting this process in EAC. For
instance, Song et al (40)
found that in cell lines of EAC and its pre-malignant stage of
Barrett’s esophagus, the green tea extract mixture of polyphenon E
leads to an inhibition of proliferation and induction of apoptosis.
This anti-proliferative function of the compound seemed to occur
through the downregulation of the expression of cyclin D1, an
upstream phosphorylating regulator of Rb. Accordingly, this led to
a lower expression of phospho-Rb in EAC and Barrett’s cells
(40). In another experiment the
effects of flavopiridol, a pan-inhibitor of CDKs, on a mouse model
of Barrett’s and related EAC, were investigated (41). In this model esophagojejunostomy
induced reflux combined with carcinogen exposure in a p27 null
background, induced Barrett’s development. These researchers found
a significantly lower prevalence of BE and EAC in flavopiridol
treated animals. This was combined with a lower expression of
cyclin D1 in tumors of treated animals, with some tentative
evidence for lower expression of phospho-Rb in treated mice
(41). There are thus potential
therapeutics that could be tested in EAC to further investigate how
modulation of the specific phosphorylation of Rb could influence
the disease.
In conclusion, overall phosphorylation activity is
upregulated in EAC. This aberrant upregulation seems to be
especially pronounced in processes related to inflammation and
tissue structure organization. With respect to the p16-Rb pathway,
Rb is significantly higher phosphorylated on the S795 residue. This
residue might be of interest as a putative target for treatment and
further molecular insight in EAC and should be further
investigated.
References
|
1
|
Pera M: Trends in incidence and prevalence
of specialized intestinal metaplasia, Barrett’s esophagus, and
adenocarcinoma of the gastroesophageal junction. World J Surg.
27:999–1008. 2003. View Article : Google Scholar
|
|
2
|
Holmes RS and Vaughan TL: Epidemiology and
pathogenesis of esophageal cancer. Semin Radiat Oncol. 17:2–9.
2007. View Article : Google Scholar
|
|
3
|
Lagergren J and Lagergren P: Recent
developments in esophageal adenocarcinoma. CA Cancer J Clin.
63:232–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Edgren G, Adami HO, Weiderpass E and Nyrén
O: A global assessment of the oesophageal adenocarcinoma epidemic.
Gut. 62:1406–1414. 2013. View Article : Google Scholar
|
|
5
|
Hur C, Miller M, Kong CY, Dowling EC,
Nattinger KJ, Dunn M and Feuer EJ: Trends in esophageal
adenocarcinoma incidence and mortality. Cancer. 119:1149–1158.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoyo C, Cook MB, Kamangar F, Freedman ND,
Whiteman DC, Bernstein L, Brown LM, Risch HA, Ye W, Sharp L, et al:
Body mass index in relation to oesophageal and oesophagogastric
junction adenocarcinomas: a pooled analysis from the International
BEACON Consortium. Int J Epidemiol. 41:1706–1718. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cossentino MJ and Wong RK: Barrett’s
esophagus and risk of esophageal adenocarcinoma. Semin Gastrointest
Dis. 14:128–135. 2003.PubMed/NCBI
|
|
8
|
Spechler SJ, Sharma P, Souza RF, Inadomi
JM and Shaheen NJ; American Gastroenterological Association.
American Gastroenterological Association technical review on the
management of Barrett’s esophagus. Gastroenterology. 140:e18–e52.
2011. View Article : Google Scholar
|
|
9
|
Rubenstein JH and Taylor JB:
Meta-analysis: the association of oesophageal adenocarcinoma with
symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther.
32:1222–1227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Hagen P, Hulshof MC, van Lanschot JJ,
Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ,
Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, et al: Preoperative
chemoradiotherapy for esophageal or junctional cancer. N Engl J
Med. 366:2074–2084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baselga J, Carbonell X, Castañeda-Soto NJ,
Clemens M, Green M, Harvey V, Morales S, Barton C and Ghahramani P:
Phase II study of efficacy, safety, and pharmacokinetics of
trastuzumab monotherapy administered on a 3-weekly schedule. J Clin
Oncol. 23:2162–2171. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Piccart-Gebhart MJ, Procter M,
Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga
J, Bell R, Jackisch C, et al: Trastuzumab after adjuvant
chemotherapy in HER2-positive breast cancer. N Engl J Med.
353:1659–1672. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gowryshankar A, Nagaraja V and Eslick GD:
HER2 status in Barrett’s esophagus & esophageal cancer: a
meta-analysis. J Gastrointest Oncol. 5:25–35. 2014.PubMed/NCBI
|
|
14
|
Bang YJ, van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): a phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sherr CJ and McCormick F: The RB and p53
pathways in cancer. Cancer Cell. 2:103–112. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rocco JW and Sidransky D:
p16(MTS-1/CDKN2/INK4a) in Cancer Progression. Exp Cell Res.
264:42–55. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vieth M, Schneider-Stock R, Rohrich K, May
A, Ell C, Markwarth A, Roessner A, Stolte M and Tannapfel A:
INK4a-ARF alterations in Barrett’s epithelium, intraepithelial
neoplasia and Barrett’s adenocarcinoma. Virchows Arch. 445:135–141.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hardiea LJ, Darnton SJ, Wallis YL, Chauhan
A, Hainaut P, Wilda CP and Cassond AG: p16 expression in Barrett’s
esophagus and esophageal adenocarcinoma: association with genetic
and epigenetic alterations. Cancer Lett. 217:221–230. 2005.
View Article : Google Scholar
|
|
19
|
Wong DJ, Paulson TG, Prevo LJ, Galipeau
PC, Longton G, Blount PL and Reid BJ: p16INK4a lesions
are common, early abnormalities that undergo clonal expansion in
Barrett’s metaplastic epithelium. Cancer Res. 61:8284–8289.
2001.PubMed/NCBI
|
|
20
|
Wang JS, Guo M, Montgomery EA, Thompson
RE, Cosby H, Hicks L, Wang S, Herman JG and Canto MI: DNA promoter
hypermethylation of p16 and APC predicts neoplastic progression in
Barrett’s esophagus. Am J Gastroenterol. 104:2153–2160. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaune KM, Neumann C, Hallermann C, Haller
F, Schön MP and Middel P: Simultaneous aberrations of single CDKN2A
network components and a high Rb phosphorylation status can
differentiate subgroups of primary cutaneous B-cell lymphomas. Exp
Dermatol. 20:331–335. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan J, Knorr J, Altmannsberger M,
Goeckenjan G, Ahr A, Scharl A and Strebhardt K: Expression of p16
and lack of pRB in primary small cell lung cancer. J Pathol.
189:358–362. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harada H, Nakagawa H, Oyama K, Takaoka M,
Andl CD, Jacobmeier B, von Werder A, Enders GH, Opitz OG and Rustgi
AK: Telomerase induces immortalization of human esophageal
keratinocytes without p16INK4a inactivation. Mol Cancer Res.
1:729–738. 2003.PubMed/NCBI
|
|
24
|
Rygiel AM, van Baal JW, Milano F, Wang KK,
Ten Kate FJ, Fockens P, Rosmolen WD, Bergman JJ, Peppelenbosch MP
and Krishnadath KK: Efficient automated assessment of genetic
abnormalities detected by fluorescence in situ hybridization on
brush cytology in a Barrett esophagus surveillance population.
Cancer. 109:1980–1988. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gu Y, Rosenblatt J and Morgan DO: Cell
cycle regulation of CDK2 activity by phosphorylation of Thr160 and
Tyr15. EMBO J. 11:3995–4005. 1992.PubMed/NCBI
|
|
26
|
Hong J, Resnick M, Behar J, Wang LJ, Wands
J, DeLellis RA, Souza RF, Spechler SJ and Cao W: Acid-induced p16
hypermethylation contributes to development of esophageal
adenocarcinoma via activation of NADPH oxidase NOX5-S. Am J Physiol
Gastrointest Liver Physiol. 299:G697–G706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kalinina T, Bockhorn M, Kaifi JT,
Thieltges S, Güngör C, Effenberger KE, Strelow A, Reichelt U,
Sauter G, Pantel K, et al: Insulin-like growth factor-1 receptor as
a novel prognostic marker and its implication as a cotarget in the
treatment of human adenocarcinoma of the esophagus. Int J Cancer.
127:1931–1940. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hector A, Montgomery EA, Karikari C, Canto
M, Dunbar KB, Wang JS, Feldmann G, Hong SM, Haffner MC, Meeker AK,
et al: The Axl receptor tyrosine kinase is an adverse prognostic
factor and a therapeutic target in esophageal adenocarcinoma.
Cancer Biol Ther. 10:1009–1018. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Poehlmann A, Kuester D, Malfertheiner P,
Guenther T and Roessner A: Inflammation and Barrett’s
carcinogenesis. Pathol Res Pract. 208:269–280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lao-Sirieix P and Fitzgerald RC: Role of
the micro-environment in Barrett’s carcinogenesis. Biochem Soc
Trans. 38:327–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abdel-Latif MM, Duggan S, Reynolds JV and
Kelleher D: Inflammation and esophageal carcinogenesis. Curr Opin
Pharmacol. 9:396–404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zarkowska T and Mittnacht S: Differential
phosphorylation of the retinoblastoma protein by G1/S
cyclin-dependent kinases. J Biol Chem. 272:12738–12746. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kitagawa M, Higashi H, Jung HK,
Suzuki-Takahashi I, Ikeda M, Tamai K, Kato J, Segawa K, Yoshida E,
Nishimura S, et al: The consensus motif for phosphorylation by
cyclin D1-Cdk4 is different from that for phosphorylation by cyclin
A/E-Cdk2. EMBO J. 15:7060–7069. 1996.PubMed/NCBI
|
|
34
|
Connell-Crowley L, Harper JW and Goodrich
DW: Cyclin D1/Cdk4 regulates retinoblastoma protein-mediated cell
cycle arrest by site-specific phosphorylation. Mol Biol Cell.
8:287–301. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dick FA: Structure-function analysis of
the retinoblastoma tumor suppressor protein - is the whole a sum of
its parts? Cell Div. 2:262007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rubin SM, Gall AL, Zheng N and Pavletich
NP: Structure of the Rb C-terminal domain bound to E2F1-DP1: a
mechanism for phosphorylation-induced E2F release. Cell.
123:1093–1106. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Burke JR, Deshong AJ, Pelton JG and Rubin
SM: Phosphorylation-induced conformational changes in the
retinoblastoma protein inhibit E2F transactivation domain binding.
J Biol Chem. 285:16286–16293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heilmann AM1 and Dyson NJ: Phosphorylation
puts the pRb tumor suppressor into shape. Genes Dev. 26:1128–1130.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Song S, Krishnan K, Liu K and Bresalier
RS: Polyphenon E inhibits the growth of human Barrett’s and
aerodigestive adenocarcinoma cells by suppressing cyclin D1
expression. Clin Cancer Res. 15:622–631. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lechpammer M, Xu X, Ellis FH,
Bhattacharaya N, Shapiro GI and Loda M: Flavopiridol reduces
malignant transformation of the esophageal mucosa in p27 knockout
mice. Oncogene. 24:1683–1688. 2005. View Article : Google Scholar : PubMed/NCBI
|