Introduction
Hepatocellular carcinoma (HCC) is one of the most
frequently diagnosed cancers and is also one of the major causes of
tumor-related deaths worldwide (1). With the improvement of the surgical
techniques and the application of transarterial chemoembolization
(TACE), the radiofrequency ablation (RFA), and liver
transplantation, the overall survival and the prognosis have made
great progress and the 5-year survival rate of the early diagnosed
HCC with curative treatments ranges from 50 to 70%, but the
recurrence of early-stage HCC after resection occur in ~20, 50 and
75% of patients at 1, 3 and 5 years, respectively (2,3).
Therefore, the recurrence and the metastasis after resections are
intractable problems for achieving total control in HCC treatment,
while there are few drugs to resolve the crucial problem. At
present, the only approved systematic therapy for the
advanced-stage HCC patients is the multikinase tyrosine kinase
inhibitor sorafenib which used alone improves the median overall
survival by ~3 months (4,5). Thus, it is urgent to pursue new
agents to improve the prognosis of HCC.
Cancer targeting gene-viro-therapy (CTGVT) is a
promising approach to conquer the malignant tumor as it is endowed
with the ability to selectively infect and damage the tumor cells
with minimum harm to the normal tissue (6). The new strategy takes advantage of
gene therapy and virotherapy by utilizing the oncolytic adenovirus
containing the anticancer gene, which produces more effective
antitumor effects than either gene therapy or the viral therapy
alone (7,8). The adenovirus vectors are the most
common used gene delivery system, especially the adenovirus
serotype 5 (Wad5) due to its excellent characteristics. Adenovirus
can replicate in both dividing and non-dividing cells and it hardly
integrates into the host genome posing low risk of mutagenesis. The
easy manipulation and the capability to produce high tilters of
virus are favorable. Moreover, the adenovirus activates the humoral
and cellular immune response which might activate immune system for
the recognition of tumor antigens. The side effects of adenovirus
therapy are mild and rare. So adenovirus is an ideal candidate
vector for the CTGVT (9). The
oncolytic adenovirus, also called conditionally replicating
adenovirus, could theoretically selectively propagate in and lyse
the tumor cells and the released progeny virus will evade the
neighboring tumor cells and the virus replication will stop in the
normal cells (9,10). To achieve the targeting therapy of
HCC and safety for the normal cells, a dual-regulated oncolytic
vector by using liver cancer-specified promoters was then
constructed.
Human telomerase reverse transcriptase (hTERT) is
highly active in >80% of human tumor cells but not in most
normal cells and the hTERT promoter has been cloned by several
scientists and was utilized to drive the exogenous gene expression
(11,12). The adenovirus early region 1a (E1A)
plays a central role in the virus replication and cell cycle and
the E1A promoter can be replaced by the hTERT promoter (13). However, hTERT is also expressed in
the hematopoietic stem cells and generative cells, so the system
has a potential detriment to the normal cells and will produce side
effects in humans. To minimize the potentially adverse effects to
normal cells, more delicate regulatory systems should be
engineered. Human α-fetoprotein (AFP) is re-expressed in ~70% of
the HCC, so the AFP promoter is widely used to drive the adenovirus
E1B gene as an excellent tool for HCC targeting therapy (14,15).
The system can be used to target to the AFP-positive HCC specially,
but does not work in the low-AFP-generating cells or AFP-negative
cells so we created a fused AFP promoter, HREAF, by connecting the
hypoxia reactive element (HRE) enhancer with the AFP and the system
is able to enhance the replication capability of oncolytic
adenoviruses in both AFP-positive and AFP-negative liver cancer
cells (16,17). However, there exists a disadvantage
in this system that it can also kill the hepatic stem cells which
also express AFP, thus weakening the hepatic reserve and worsening
the prognosis of the HCC patients. It has been reported that the
HREAF promoter enhanced the translational strength and selectivity
of the oncolytic virus to achieve HCC-specific and tumor
environment-selective viral replication and tumor killing (17,18).
In order to achieve the hepatoma-restricted cytotoxicity and
enhanced replication, we integrated the hTERT promoter and HREAF
promoter into our oncolytic adenovirus where the hTERT promoter
drove the E1A expression and the HREAF drove the E1B expression,
then the Ad-hTERT-HREAF was constructed and we named it SG505.
Previous studies revealed that the focal adhesion
kinase (FAK) expression was upregulated in HCC and the high
expression was associated with a poor prognosis (19,20).
FAK besides contributing to the invasion and metastasis of the HCC,
also proved to be an important mediator of cell adhesion, growth,
proliferation, survival, angiogenesis and migration which were
often disturbed in the cancer cells, so it was a promising
therapeutic target for the HCC (21). Herein, we created the short-hairpin
RNA against FAK and inserted the gene in the recombined oncolytic
adenovirus to confirm the antitumor effect and the new system was
Ad-hTERT-HREAF-FAK-shRNA, or SG505-siFAK for short. We also created
other recombined adenoviruses, namely, Ad-hTERT-HREAF-EGFP-shRNA
(SG505-EGFP), Ad-PPE3-siFAK, and Ad-DC311-siFAK. In this study, we
explored the selective and efficient antitumor effect of the
recombined oncolytic adenovirus to offer better treatments for
HCC.
Materials and methods
Cell lines and culture conditions
HEK293 cells (embryonic kidney cell line containing
the E1A region of serotype adenovirus 5) were purchased from
Canadian Microbix Biosystem Inc. Hep3B and HepG2 (AFP-positive
human liver cancer cell), SMMC-7721 (AFP-negative human liver
cancer cell), L02 (human normal liver cell line), BJ (skin
fibroblasts), PANC-1 (poorly differentiated pancreatic carcinoma
cell line) and H460 (human large cell lung carcinoma cell line)
were obtained from Cell Bank of Shanghai Institutes for Biological
Sciences of the Chinese Academy of Sciences (Shanghai, China).
Cells were cultured in DMEM or RPMI-1640 supplemented with 10%
fetal bovine serum, 4 mmol/l glutamine, 50 U/ml penicillin and 50
μg/ml streptomycin under an atmosphere of 95% air and 5%
CO2.
Recombinant adenoviruses
Short hairpin RNA targeting FAK was constructed in
our lab. The hybrid HREAP promoter and hTERT were generated
referring to the previous studies (16,22).
Therefore, the shuttle plasmids Ad-DC311-siFAK, Ad-PPE3-siFAK and
the dual-specific antitumor oncolytic adenovirus SG505, SG505-EGFP,
SG505-siFAK were constructed. Briefly, shuttle plasmids were
constructed to generate the recombinant. Packing and production of
the recombinant adenoviruses were performed in the HEK-293 cells
using Lipofectamine 2000 (Gibco BRL, Grand Island, NY, USA)
according to the manufacturer’s protocol. Viral plaques appeared
9–14 days after cotransfection and were sublimated three times. The
recombinant adenoviruses were identified separately by PCR. The
viral titers were determined by the tissue culture infectious dose
50 (TCID50) assay in HEK293 cells.
Western blot analysis
Cells were infected with the recombinant
adenoviruses at a MOI of 3 pfu/cell. The cells were trypsinized,
harvested and resuspended in RIPA lysis buffer 3 days later. The
protein concentration was confirmed by the avidin-biotin complex
(ABC) technique as described by the manufacturer. Then, protein
samples were separated by 10–15% SDS-polyacrylamide gel
electrophoresis and transferred to polyvinylidene fluoride (PVDF)
membranes. Membranes were blocked in 5% bovine serum albumin (BSA)
and incubated with primary antibodies at 4°C overnight and then
were detected by the appropriate secondary antibodies marked with
horseradish peroxidase at 37°C for 2 h. Signals were visualized via
the enhanced chemiluminescence (ECL) Western blotting substrate
kit. Extracts of uninfected cells were used as the negative control
and β-actin was used as the internal control.
MTT assay and BrdU incorporation
assay
The MTT colorimetric assay was carried out to detect
cell viability (23,24). To assess the cytotoxic effect of
the virus, cells on logarithmic phase were seeded onto 96-well
plates at 1×103–2×103 cells per well and
infected with oncolytic adenovirus. The viruses were diluted by
serum-free culture solution. The cells were then infected with
various concentrations (at MOI of 0.1, 0.2, 0.5, 1, 2, 5, 10 and
100 pfu/cell) of recombinant adenovirus 24 h later. The cells
without virus infection were used as controls and 4 duplications
were set corresponding to a certain MOI value. The media was
replaced by 100 μl phosphate-buffered saline (PBS) per well. The
cells were treated with 5 μl MTT (5 mg/ml) and incubating for 4 h
at 37°C to allow MTT metabolization. Then the culture medium was
removed and the crystals formed were dissolved in 150 μl MTT lysate
solution (70% dimethylsulfoxide and 0.02 mol/l hydrochloric acid)
for 4 h at 37°C. Cell viability was determined by measuring
absorbance at 595 nm, using a microplate reader. The controls at
each day were set as 100% viability. The percentage of cell
survival rate treated with the recombinant adenovirus was expressed
using the following formula: [100% × (absorbance value of
experimental cells) / (absorbance value of control cells)]. All
measurements were performed in triplicate. Bromodeoxyuridine (BrdU)
incorporation was measured using BrdU Cell Proliferation Assay
Chemicon cat. no. 2750. Transfected cells were incubated with BrdU
for the last 12 h of the 72-h treatment with FAK siRNA and BrdU
incorporation was assessed as described in the manufacturer’s
protocol. The concentration resulting in 50% of cell growth
inhibition (IC50) was calculated using SPSS version
17.0.
Real-time RT-PCR analysis
Real-time PCR was carried out on the Real-Time PCR
system (Applied Biosystems) with the following conditions: 95°C, 15
sec for 1 cycle; then 95°C, 5 sec; and 60°C, 30 sec for 45 cycles.
The mRNA expression of FAK was measured on ABI 7,500 Real-Time PCR
system with β-actin used as a control. Each experiment was repeated
three times. RNA levels were calculated from the mean relative
quantity (RQ) (RQ=2−ΔΔCt).
Tumor xenograft experiment
Eighty female BALB/c nude mice (4-week-old) were
purchased from the Shanghai Experimental Animal Center (Shanghai,
China). To establish xenograft tumors, 5×106 Hep3B cells
in 500 μl normal saline were subcutaneously injected into the right
flank of each mouse. When the largest diameter of tumors reached 5
mm, 40 mice were divided randomly into four groups (10 mice per
group). PBS or 2×108 pfu of Wad5, SG505, SG505-siFAK
were administered to each mouse by intravenous injection every
other day into the tumors five times. All injections were given
every 2 days during the first week (days 3, 5 and 7 after grouping)
and once weekly for 2 more weeks (days 14 and 21 and so on after
grouping). The remaining 40 nude mice received the treatments and
measurements as described above by intratumoral injection. Tumor
size was measured using calipers twice a week and calculated with a
formula of [0.52 × (smallest diameter)2 × (largest
diameter)] (25), tumor inhibition
rates was calculated using the formula (1 - tumor weight of
experimental group/average tumor weight of control group) × 100%.
During the animal experiment, all animals were monitored daily and
sacrificed at the end of the experiment. Ethics approval was
granted by the Ethics Committee of the Affiliated Sixth People’s
Hospital of Shanghai Jiao Tong University.
Immunohistochemical analysis
Tumors were harvested and fixed in 4%
paraformaldehyde, embedded in paraffin and cut into 4-μm sections
for immunohistochemical analysis after sacrifice. These sections
were stained with monoclonal anti-FAK antibodies at a 1:500
dilution. The slides were then washed with PBS and incubated with
the avidin-biotin-peroxidase complex reagent. Then the differential
expression of FAK in various tissues were studied. The criteria
applied to evaluate the FAK staining intensity were described
previously (19).
Statistical analysis
Statistical analysis was carried out by SPSS 17.0.
All continuous data were presented as mean ± standard deviation
(SD). Categorical variables were compared by the χ2 test
or Fisher’s exact test. The independent sample t-test or ANOVA was
used to compare the mean values of different groups. P-value
<0.05 was considered statistically significant.
Results
Construction and characterization of the
recombinant oncolytic adenovirus
SG505-siFAK was a dual-regulated oncolytic
adenovirus with E1A driven by the hTERT promoter and E1B driven by
the HREAF promoter and it was armed with the therapeutic gene
siFAK. The SG505 was blank vector and SG505-EGFP was armed by the
reporter gene enhanced green fluorescent protein (EGFP).
Replicative Ad-PDC311-siFAK virus and replication-defective
Ad-PPE3-siFAK virus were initially introduced by the siFAK to
confirm the function of RNA interference targeting to the FAK
(Fig. 1A). The sequences of the
inserted parts in all of the recombinant vectors were analyzed and
proved to be correct.
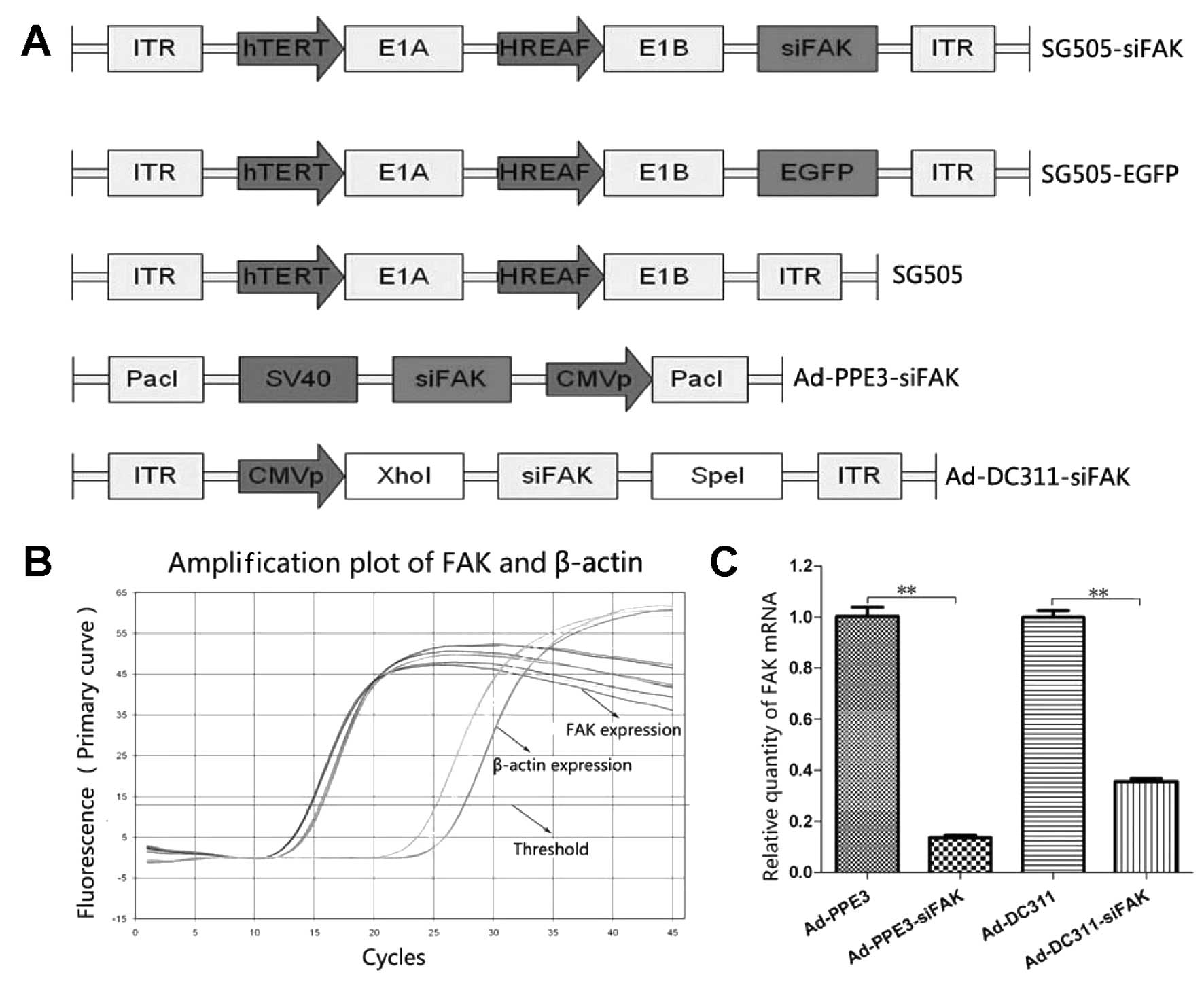 | Figure 1The structure of the recombined
oncolytic adenovirus and the function of the FAK-shRNA. (A)
Schematic diagram of recombinant adenoviruses. Schematic diagram of
organization depict the elements in the recombinant adenovirus
(SG505-siFAK, SG505-EGFP and SG505); the hTERT promoter drove the
E1A expression and the hybrid HREAF promoter drove the E1B
expression. The Ad-PPE3-siFAK and Ad-DC311-siFAK contained the
siFAK elements. (B) Amplification plot based on quantitative
real-time PCR of HepG2 FAK expression and β-actin expression. The
siFAK was transfected to Ad-PPE3 and Ad-DC311 separately. (C)
Quantification of the FAK mRNA transcript level in the Hep3B by
four different viruses. The relative levels of the FAK mRNA were
examined by the RT-PCR and reflected the relative expression
compared with control sample, the results indicated that
short-hairpin RNA against FAK could efficiently inhibit the FAK
expression at the transcriptional level. All results were
normalized and were representatives of triplicate readings from
each treatment (**P<0.01). (D) The siFAK specially
inhibited the growth of tumor cells. Silencing of FAK impaired the
cell viability of Hep3B and SMMC-7721 cells. After transfection
with Ad-PPE3, Ad-PPE3-siFAK, Ad-DC311, Ad-DC311-siFAK for 48 h, BJ,
L02, Hep3B and SMMC-7721 cells were seeded into 96-well plates
(2,000/well) and incubated for the indicated times. Measurement of
cell viability of normal cells (BJ and L02) and liver cancer cells
(Hep3B and SMMC-7721) every day after injection with different
recombined adenovirus at a MOI of 1 pfu/cell by MTT assay. Points
indicated the mean value (n=3); bars indicated SD,
**P<0.01. (E) BrdU incorporation assay at 72 h. The
siFAK had no obvious effect on the growth rate of normal BJ cells
and L02 cells. However, the growth rates of liver cancer line Hep3B
infected with Ad-PPE3-siFAK and Ad-DC311-siFAK were significantly
inhibited compared to the ones infected with the Ad-PPE3 and
Ad-DC311 (0.457±0.051 vs. 0.880±0.032, P<0.01 and 0.695±0.045
vs.1.114±0.001, P<0.01, respectively). Ad-PPE3-siFAK and
Ad-DC311-siFAK could also decrease the SMMC-7721 growth rate
compared to the Ad-PPE3 and Ad-DC311 (0.575±0.011 vs. 1.117±0.007,
P<0.01 and 0.542±0.020 vs. 1.073±0.042, P<0.01,
respectively). The points indicated the mean value (n=3); bars
indicated SD. **P<0.01. |
It has been reported that the FAK was overexpressed
in the HCC tumor but not in the normal tissue, so we constructed a
targeting siFAK which will inhibit the FAK expression by the RAN
interference technology (19,20,26).
The synthetic gene was then inserted into the replicative
adenovirus Ad-PPE3 and replication-defective adenovirus AD-DC311.
To evaluate FAK expression in tumor cells, we employed the
real-time PCR to analyze the levels of the FAK mRNA in
vitro. All of the samples were analyzed and the expression of
β-actin mRNA transcripts were used as internal control. We found
that the relative expression level of FAK mRNA in Hep3B cells
infected by the Ad-PPE3-siFAK was significantly lower than that
infected by the Ad-PPE3 (0.137±0.015 vs. 1.003±0.06; P<0.01) and
the relative expression level of FAK mRNA in Hep3B cells infected
by the Ad-DC311-siFAK was also significantly lower than that
infected by the Ad-DC311 (0.357±0.021 vs. 1.000±0.044; P<0.01),
so we can confirm that the FAK-siRNA has potential to be an
effective therapeutic gene for HCC (Fig. 1C).
As FAK is a potential therapy targeting the tumor
and knockdown of the FAK suppressed the growth of the HCC, as
established herein, to evaluate the antitumor ability of the
oncolytic adenovirus expressing the FAK shRNA in vitro, we
measured the cell viability by MTT assay. We found that the liver
cancer cell lines (Hep3B and SMCC7721) were sensitive to the
Ad-PPE3 and Ad-DC311 infection, whereas the normal cell lines (BJ
and L02) showed much lower sensitivity, demonstrating safety of the
vectors expressing siFAK to the normal cells (Fig. 1D). Similar results were also
confirmed by BrdU incorporation assay (Fig. 1E). Collectively, these data
indicated that RNA interference against FAK functioned as a
suppressive factor of FAK. The primary studies paved the way for
further investigations for HCC treatment.
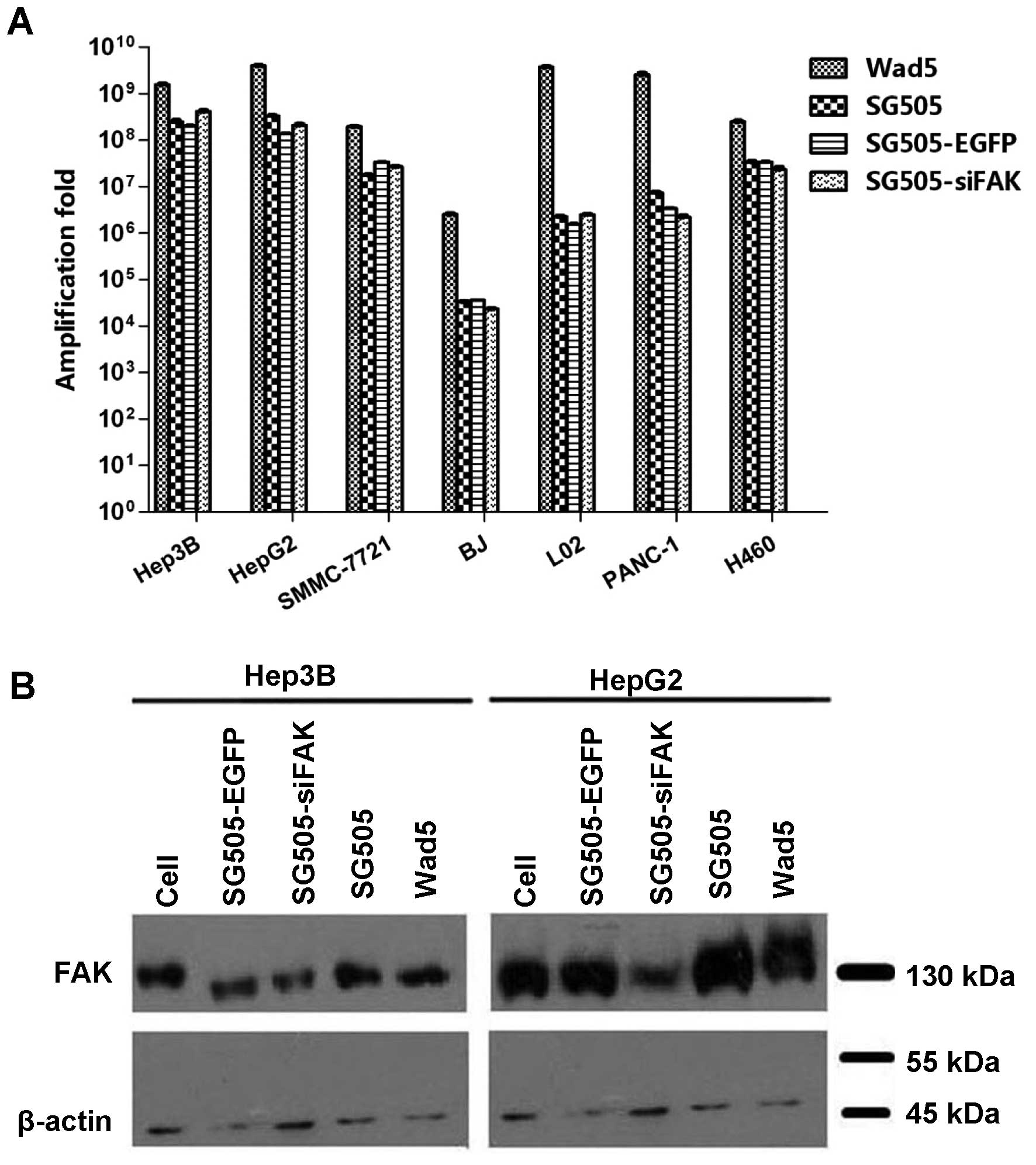
Selective replicative ability of the
recombined oncolytic adenovirus to AFP-positive liver cancer
cells
To confirm the virus infective ability, the virus
yields of different viruses were assayed using the standard tissue
culture infectious dose 50 (TCID50) assay. Virus
replication rates were determined at 96 h after the cells were
infected with Wad5, SG505, SG505-EGFP and SG505-siFAK separately at
a MOI of 5 pfu/cell. Previous studies have already assayed the AFP
secretion ability of various liver cell line, which indicated that
Hep3B and HepG2 cell lines secreted AFP at level of 1,174±17.33 and
38.20±1.29 ng/ml and the secretion of AFP by L02 and SMMC-7721 was
undetectable (7). The recombined
oncolytic adenoviruses showed more potent replicative abilities in
the Hep3B and HepG2 cell lines with the replication increasing by
~108-fold, compared to the AFP-negative cell lines,
including the SMMC-7721, L02, BJ, PANC-1 and H460 cell lines
(P<0.05) (Fig. 2A). Notably,
Wad5 displayed the strongest replicative ability among various
kinds of viruses in the cell lines. Although the recombined
adenoviruses were slightly low in virus yield compared to Wad5, it
was potent enough to drive the expression of the exogenous gene.
The FAK expression was significantly downregulated in the Hep3B and
HepG2 cell lines infected by the SG505-siFAK (Fig. 2B). Therefore, it also confirmed
that SG505 was a promising vector to deliver the siFAK which could
effectively suppress the FAK expression. In addition, the virus
tilters of SG505, SG505-EGFP and SG505-siFAK in the normal BJ and
L02 cell were remarkably weaker than that of Wad5, which indicated
less damage to the normal cells. The results demonstrated that
recombined adenoviruses had significantly higher replication rates
in the AFP-positive liver cancer Hep3B, and HepG2 cells and the
safety concerning the recombined adenoviruses was remarkably
balanced.
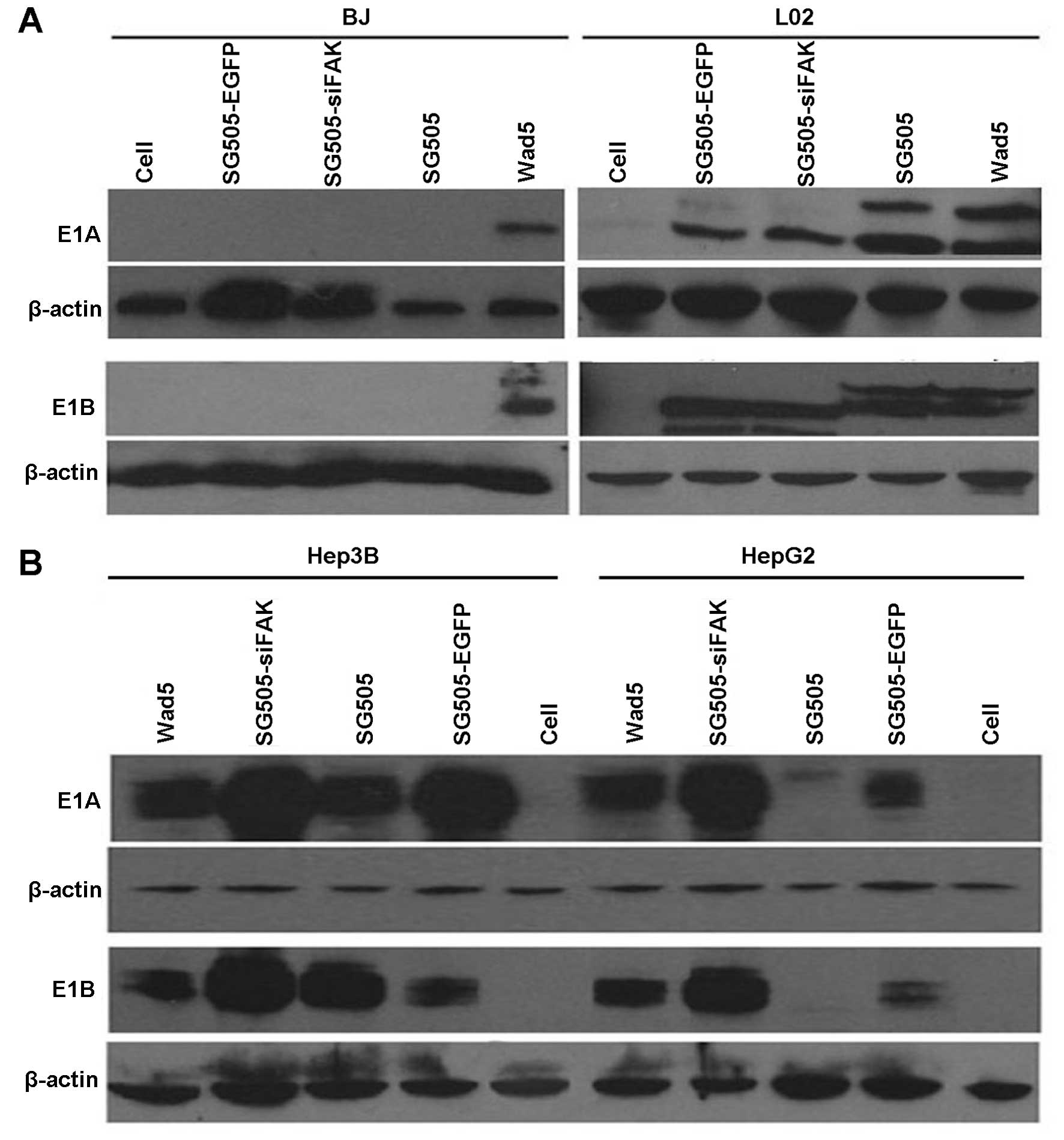
To further investigate the selective invasion of the
dual-regulated oncolytic adenovirus, we applied western blot
analysis to analyze the E1A and E1B expression in different cell
lines infected by various viruses. E1A and E1B gene expression in
the infected cells provided an independent verification of virus
replication because they were essential for the virus replication
(27–29). In BJ cells, it was clear that
detectable levels of E1A and E1B were only produced by the
Wad5-infected cells while they were not detected in the other four
oncolytic adenoviruses (Fig. 3A).
Besides, in the normal liver cell line (L02), the E1A and E1B were
weakly expressed in the cells infected by SG505-siFAK, SG505 and
SG505-EGFP compared to Wad5, which confirmed the safety to the
normal viable cells (Fig 3A). The
expression of E1A and E1B in HepG2 and Hep3B infected by the
oncolytic adenovirus was comparable to the ones by Wad5, which
implied that the oncolytic adenoviruses produced large amounts of
progeny viruses (Fig. 4B).
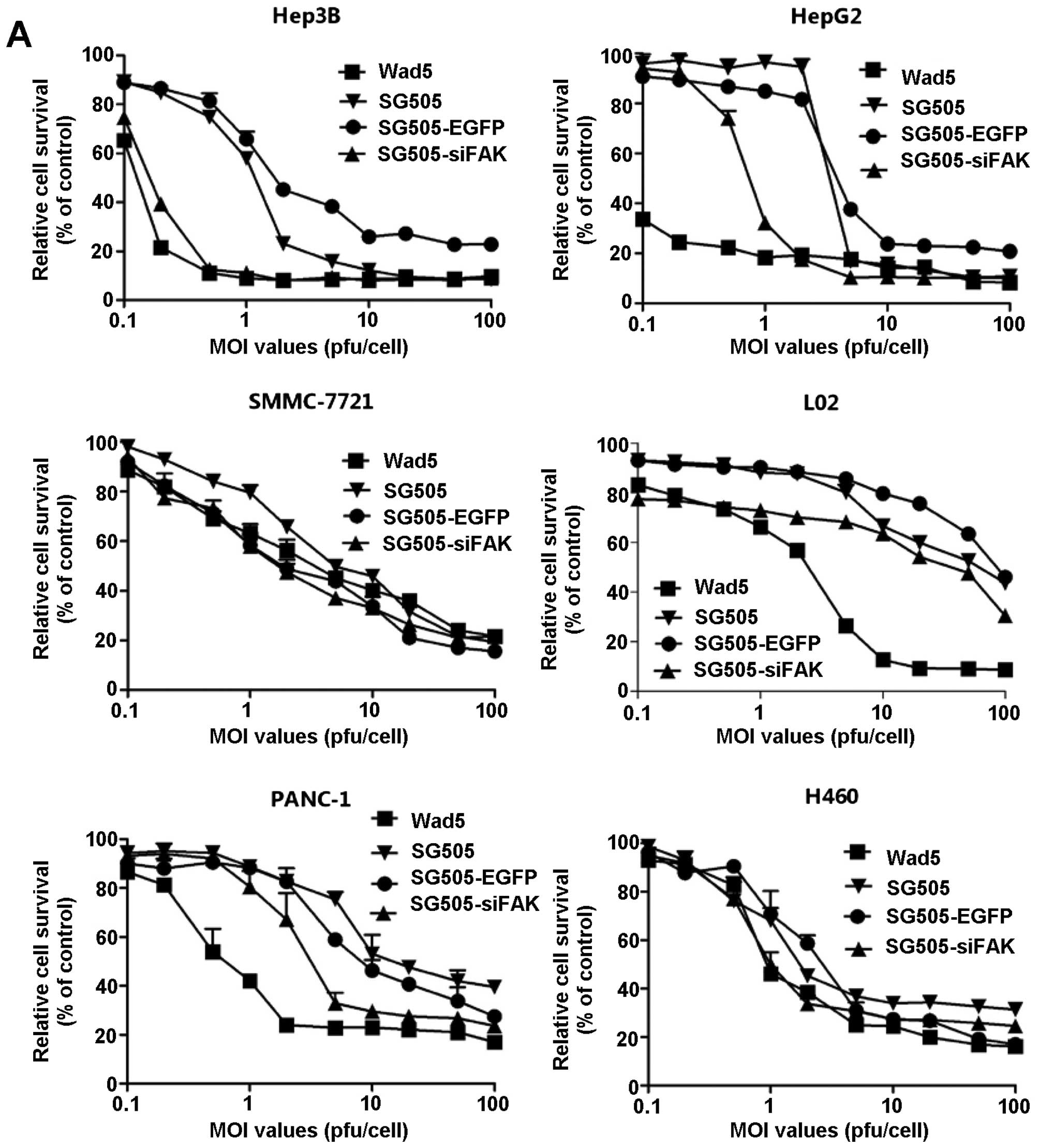
The cytotoxic effects of dual-regulated
oncolytic adenovirus in AFP-positive liver cancer cell lines
Next, we investigated the antitumor effect of the
viruses on cell viability. The AFP-positive liver cancer cell lines
(Hep3B and HepG2), AFP-negative liver cancer cell line (SMMC-7721),
human normal liver cell line (L02), PANC-1 and H460 were infected
with Wad5, SG505, SG505-siFAK and SG505-EGFP at consistent MOIs
ranging from 0.1 to 100 pfu/cell. Overall, the cell viability
declined gradually with the increasing MOI values in various cell
lines. SG505-siFAK and Wad5 showed most evident cytotoxic effects
in the Hep3B and HepG2 cell lines. To further study the cytotoxic
effects of the viruses, we compared the IC50 values in
the cell lines infected with Wad5, SG505, SG505-EGFP and
SG505-siFAK. The IC50 values of SG505-siFAK were
0.092±0.009, 0.424±0.414, 14.796±2.520, 48.709±0.927, 5.970±0.945
and 2.710±0.244 pfu/cell in Hep3B, HepG2, SMMC-7721, L02, PANC-1
and H460, respectively. The IC50 values further
confirmed that the SG505-siFAK showed strong cytotoxic effects to
the AFP-positive tumor cell line, and was weakly cytotoxic in the
pancreatic cancer line PANC-1 and large cell lung cancer cell line
H460. Although Wad5 showed similar cytotoxic effects in Hep3B and
HepG2 comparing to the SG505-siFAK, it had more potent cytotoxic
effect to the normal liver cell L02 (P<0.01), therefore
SG505-siFAK was mild to the normal liver cell comparing to
Wad5.
Antitumor effect of dual-regulated
oncolytic adenovirus in the nude mouse xenografts
To evaluate and compare the antitumor activity of
the recombined oncolytic adenovirus in vivo, a liver cancer
cell tumor xengraft model was established by inoculating
AFP-positive Hep3B cells into the right back of the nude mice. When
the long diameter reached 5 mm, PBS, SG505-EGFP and SG505-siFAK
(2×108 pfu/mice per time) were administered to the mice
by either intravenous injection or intratumoral injection in order
to determine which kind of administration would cause more potent
antitumor effect. Each nude mouse in the experimental group
received a total of 1×109 pfu of virus and all of the
mice were sacrificed after 8 weeks. The volumes of tumors in the
mice treated with PBS in both group grew rapidly and reached ~1,300
mm3 within 4 weeks, suggesting the malignancy of the
Hep3B in the study. SG505 and SG505-siFAK began to show remarkable
inhibition effect by day 14 compared to the blank control group.
Nude mice treated with SG505-siFAK at a total dosage of
1×109 pfu per mouse showed similar antitumor ability
when compared to the Wad5 in both groups (P>0.05) and stronger
antitumor efficiency than SG505-EGFP in each group (P<0.01). It
is worth noting that SG505-siFAK resulted in potent tumor growth
inhibition without tumor regression, which resembled survival with
tumor in the clinical scenario (Fig.
5A and B). The discrepancy of antitumor efficiency between two
groups was accomplished by comparing the tumor inhibition rates,
which demonstrated that SG505-siFAK and Wad5 had more robust
antitumor capability in the intratumoral injection group than that
in the intravenous injection (Fig.
5C). The main reason for the phenomenon was that the oncolytic
adenovirus would be feasibly trapped and eliminated in the liver
and the intratumoral injection could directly produce faster
antitumor effects.
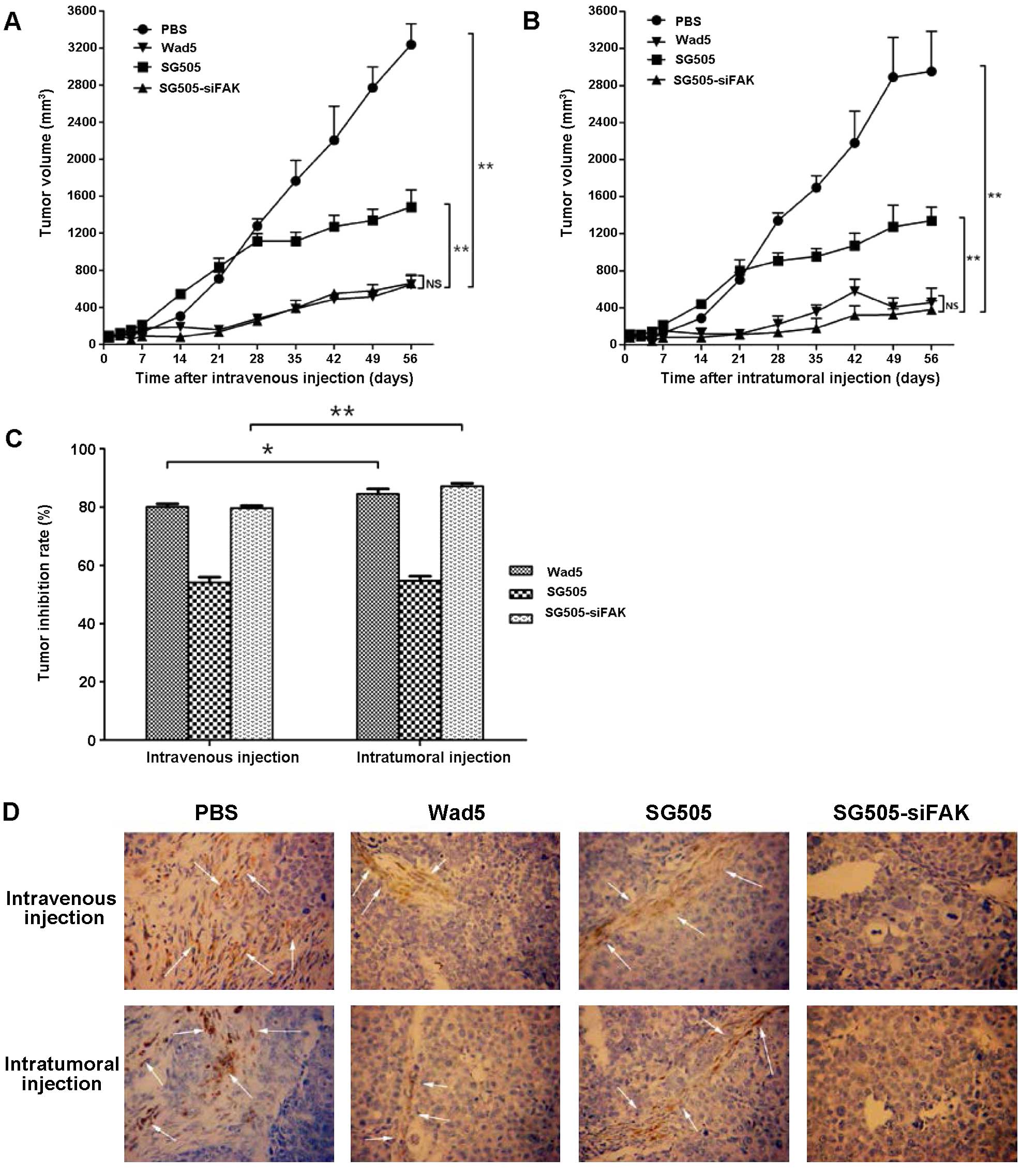 | Figure 5SG505-siFAK inhibited the growth of
xeongraft tumors. (A) The tumor volumes of different groups treated
with PBS, Wad5, SG505 and SG505-siFAK by intravenous or
intratumoral injection. The tumor volumes treated intravenously
with PBS, Wad5, SG505 and SG505-siFAK at day 56 were 3,237.5±223.2,
645.6±107.6, 1,168.3±38.0 and 659.2±82.4 mm3,
respectively. The tumor volumes treated intratumorally with PBS,
Wad5, SG505 and SG505-siFAK at day 56 were 2952.1±435.3,
456.5±155.7, 1339.2±147.6 and 379.6±95.6 mm3. Tumor
volumes were recorded as mm3 ± SD, points indicated the
mean value (n=10) and bars indicated SD; **P<0.01;
*P<0.05; NS, no significant difference. (C) Data
collected 56 days after the initial virus treatment were analyzed
to compare the tumor inhibition rates between the intravenous group
and intratumoral injection. In the intravenous injection group,
tumor inhibition rates of Wad5, SG505 and SG505-siFAK were 80.06,
54.14 and 79.63%, respectively; in the intratumoral injection
group, tumor inhibition rates of Wad5, SG505 and SG505-siFAK were
84.54, 56.64 and 87.14%, respectively. (D) Representative
micrographs of immonohistochemical staining for FAK in the groups.
The specimens from various tumors injected with different
adenoviruses were assayed by IHC and they revealed evidence of
antitumor effects of SG505-siFAK in vivo. The FAK in
cytoplasm of tumor cell was yellow or brown. FAK in the cells
treated by the SG505-siFAK was stained negatively and FAK in the
cells treated by the PBS, Wad5 and SG505 was stained positively,
implying the successful gene knockdown by the shRNA. Arrow
indicated cells that stained positively for FAK. |
To confirm that the antitumor efficiency was
mediated by the FAK siRNA engineered in the dual-regulated
adenovirus, expression of FAK was assayed by immunohistochemistry
in the tumor specimen when the mice were sacrificed. The FAK
expression levels in the tumors treated with SG505-siFAK
intratumorally or intravenously were markedly lower than those
treated with PBS or SG505. Although SG505-siFAK and Wad5 showed
similar antitumor capability, the FAK expression in the tumor
tissue infected by the former was positive, and weakly positively
infected by the latter (Fig. 5D).
We thus deduced that SG505-siFAK might exert antitumor efficiency
in vivo by combining the selective suppression effects of
double-regulated oncolytic adenovirus to the liver cancer cells and
the downregulation of FAK expression.
Discussion
In this study, we constructed double-regulated
oncolytic adenovirus which proved to be a safe and efficient
approach to suppress the development of AFP-positive liver cancer
both in vitro and in vivo. Several promoters have
been applied to achieve the selectivity for the tumor cells in the
oncolytic systems, such as HRE, the prostate-specific antigen,
osteo-calcin, MUC1, midkine, Lplastin, E2F-1, UPII and Survivin
genes (30–32). The promoters of these reconstructed
oncolytic adenoviruses can be only activated in certain tumor cells
which produced the special transcription factor and finally
resulted in massive virus replication. We were inspired by the
previous meaningful studies and decided to utilize the hTERT
promoter to drive the E1A expression and hybrid HREAF promoter to
drive the E1B expression, thus, SG505 was engineered based on the
framework of Wad5 and the targeting therapeutic gene was introduced
into the vector. The reason why AFP-promoter was chosen was that
AFP is a widely-used clinical marker and it was feasible to choose
suitable objects to receive the treatments. The recombined virus
replication was potent in the AFP-positive liver cell lines (Hep3B
and HepG2), exceeding that in the AFP-negative liver cell line
(SMMC-7721), large cell lung cancer line (H460) and pancreatic
cancer line (PANC-1) in spite of the enhanced HREAP promoter.
Therefore, the recombined adenovirus showed specific infection
capability in the AFP-positive liver cancer cells. SG505-siFAK was
designed to selectively invade the AFP-positive liver cancer cell
lines (Hep3B and HepG2) and produced more progeny viruses compared
with the normal cell lines (BJ and L02). SG505-siFAK and SG505-EGFP
derived from the SG505 shown similar selective replication
(Fig. 2A). The western blotting
also demonstrated the replication abilities of SG505, SG505-EGFP
and SG505-siFAK were attenuated in the normal cells but were weaker
than the wild adenovirus (Fig. 3).
Thus, our data demonstrated the SG505 was a safe and efficient
vector in the CTGVT.
The current strategy of CTGVT is to insert the
targeting gene in the oncolytic adenovirus (33), therefore an appropriate antitumor
gene is crucial for the success of CTGVT. FAK has been reported in
the liver cancer tissue and proved to be a potential therapeutic
target for HCC. The present reports clarified that FAK was required
for c-Met/β-catenin-driven hepatocarcinogenesis and activation of
the FAK-Src signaling pathways contributes to HCC growth and
metastasis, so gene knockdown of FAK provided a promising strategy
to treat HCC (34,35). Therefore, we decided to introduce a
robust tool to knock down the FAK expression by RNA interference
(36,37). RNA interference has rapidly become
a powerful tool for target validation and the most widely used
gene-silencing technique in functional genomics due to its
extremely high inhibitory activity and the fact that the inhibition
is very specific (38). Our
results demonstrated that a significant suppression of SG505-siFAK
was achieved in AFP-positive Hep3B and HepG2 cell lines without
causing too much damage to the normal liver cells (Fig. 4), which rendered the
double-regulated oncolytic adenovirus remarkable safety. Regarding
to the antitumor efficiency, The IC50 value of SG505 in
Hep3B and HepG2 was 1.199±0.073 and 15.026±0.926 pfu/cell,
respectively, which was larger than that of SG505-siFAK
(P<0.05). The results meant that the CTGVT therapy combined the
benefits of virotherapy and gene therapy, producing comprehensive
and synergetic effect (39).
We further confirmed the strong antitumor ability of
SG505-siFAK in nude mouse xenograft model (Fig. 5). The SG505-siFAK induced more
significant tumor regression than the SG505-EGFP in both
intratumoral injection group and intravenous group. Besides, the
SG505 was able to produce a considerable tumor growth inhibitory
effect compared with the blank control group. It indicated that
oncolytic adenovirus could cause tumor inhibition by itself and
with the oncolytic adenovirus armed with antitumor gene. The
potential side effects of the oncolytic adenovirus is hardly under
the manipulation in nude mice because mice are known to be
non-permissive to the human adenovirus, therefore the antitumor
efficiency discrepancy among the Wad5 and SG505-siFAK was not
remarkable.
Although RNAi was more potent than other gene
silencing methods, several inherent limitations in RNAi technology
need to be overcame before carrying out clinical trials, including
transfection, incomplete silencing of tumor cells, low specificity,
immunological reactions, undesired gene insertions (40). Above all, the RNA interference
technology only knocked down gene expression but generally didn’t
eliminate it (41). Consistent
with the previous studies, our data also indicated that the tumors
were not eradicated totally (Fig.
5) (42). To accomplish the
total tumor inhibition, we postulated that two therapeutic genes
could be used in the dual-regulated adenovious vector, which will
completely eliminate the hepatoma xenograft (39). The liver cancer suppressor gene
included tumor necrosis factor-related apoptosis inducing ligand
(TRAIL), hepatocellular carcinoma suppressor 1 (HCCS1), and
suppressor of cytokine signaling 3 (SOCS3) (43–45).
The previous studies on the HCC and colorectal tumor xenografts
have proved the viability and feasibility (7). Besides, the CTGVT could also be
combined with chemotherapeutic drugs, thus reducing the side
effects and drug resistance (46).
In conclusion, the present data demonstrated that
SG505-siFAK, a novel recombined dual-regulated oncolytic
adenovirus, could infect and kill liver cancer cells specifically
and effectively. In addition, our study provided evidence that
siRNA directly delivered against FAK by the SG505 possessed the
ability to downregulate the targeting gene and suppressed the
progression of human liver cancer cells in the xenografts in the
mouse model. Hence, the CTGVT approach provides a promising method
for the effective treatment of HCC and more studies are needed to
verify its potential therapeutic effects in clinical trials.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China, no. 30872512.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tabrizian P, Roayaie S and Schwartz ME:
Current management of hepatocellular carcinoma. World J
Gastroenterol. 20:10223–10237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Fuster J and Bruix J:
Intention-to-treat analysis of surgical treatment for early
hepatocellular carcinoma: Resection versus transplantation.
Hepatology. 30:1434–1440. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bai W, Wang YJ, Zhao Y, Qi XS, Yin ZX, He
CY, Li RJ, Wu KC, Xia JL, Fan DM, et al: Sorafenib in combination
with transarterial chemoembolization improves the survival of
patients with unresectable hepatocellular carcinoma: A propensity
score matching study. J Dig Dis. 14:181–190. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Graf D, Vallböhmer D, Knoefel WT, Kröpil
P, Antoch G, Sagir A and Häussinger D: Multimodal treatment of
hepatocellular carcinoma. Eur J Intern Med. 25:430–437. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Russell SJ, Peng KW and Bell JC: Oncolytic
virotherapy. Nat Biotechnol. 30:658–670. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao X, Yang M, Wei RC, Zeng Y, Gu JF,
Huang WD, Yang DQ, Li HL, Ding M, Wei N, et al: Cancer targeting
gene-viro-therapy of liver carcinoma by dual-regulated oncolytic
adenovirus armed with TRAIL gene. Gene Ther. 18:765–777. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei RC, Cao X, Gui JH, Zhou XM, Zhong D,
Yan QL, Huang WD, Qian QJ, Zhao FL and Liu XY: Augmenting the
antitumor effect of TRAIL by SOCS3 with double-regulated
replicating oncolytic adenovirus in hepatocellular carcinoma. Hum
Gene Ther. 22:1109–1119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu ZB, Wu CT, Wang H, Zhang QW, Wang L,
Wang RL, Lu ZZ and Wang LS: A simplified system for generating
oncolytic adenovirus vector carrying one or two transgenes. Cancer
Gene Ther. 15:173–182. 2008. View Article : Google Scholar
|
|
10
|
Kasala D, Choi JW, Kim SW and Yun CO:
Utilizing adenovirus vectors for gene delivery in cancer. Expert
Opin Drug Deliv. 11:379–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu XY, Gu JF and Shi WF: Targeting
gene-virotherapy for cancer. Acta Biochim Biophys Sin. 37:581–587.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang Q, Zhang X, Wang H, et al: A novel
conditionally replicative adenovirus vector targeting
telomerase-positive tumor cells. Clin Cancer Res. 10:1439–1445.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hahn WC: Targeting cancer with telomerase:
commentary re Q. Huang et al:, a novel conditionally replicative
adenovirus vector targeting telomerase-positive tumor cells. Clin
Cancer Res, 10: 1439–1445, 2004. Clin Cancer Res. 10:1203–1205.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tangkijvanich P, Anukulkarnkusol N,
Suwangool P, Lertmaharit S, Hanvivatvong O, Kullavanijaya P and
Poovorawan Y: Clinical characteristics and prognosis of
hepatocellular carcinoma: Analysis based on serum alpha-fetoprotein
levels. J Clin Gastroenterol. 31:302–308. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Yu DC, Chen Y, Amin P, Zhang H,
Nguyen N and Henderson DR: A hepatocellular carcinoma-specific
adenovirus variant, CV890, eliminates distant human liver tumors in
combination with doxorubicin. Cancer Res. 61:6428–6436.
2001.PubMed/NCBI
|
|
16
|
Ido A, Uto H, Moriuchi A, Nagata K, Onaga
Y, Onaga M, Hori T, Hirono S, Hayashi K, Tamaoki T, et al: Gene
therapy targeting for hepatocellular carcinoma: Selective and
enhanced suicide gene expression regulated by a hypoxia-inducible
enhancer linked to a human alpha-fetoprotein promoter. Cancer Res.
61:3016–3021. 2001.PubMed/NCBI
|
|
17
|
Kwon OJ, Kim PH, Huyn S, Wu L, Kim M and
Yun CO: A hypoxia- and {alpha}-fetoprotein-dependent oncolytic
adenovirus exhibits specific killing of hepatocellular carcinomas.
Clin Cancer Res. 16:6071–6082. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang TG, Savontaus MJ, Shinozaki K,
Sauter BV and Woo SL: Telomerase-dependent oncolytic adenovirus for
cancer treatment. Gene Ther. 10:1241–1247. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuan Z, Zheng Q, Fan J, Ai KX, Chen J and
Huang XY: Expression and prognostic significance of focal adhesion
kinase in hepatocellular carcinoma. J Cancer Res Clin Oncol.
136:1489–1496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujii T, Koshikawa K, Nomoto S, Okochi O,
Kaneko T, Inoue S, Yatabe Y, Takeda S and Nakao A: Focal adhesion
kinase is over-expressed in hepatocellular carcinoma and can be
served as an independent prognostic factor. J Hepatol. 41:104–111.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Golubovskaya VM: Focal adhesion kinase as
a cancer therapy target. Anticancer Agents Med Chem. 10:735–741.
2010. View Article : Google Scholar
|
|
22
|
He D, Sun L, Li C, Hu N, Sheng Y, Chen Z,
Li X, Chi B and Jin N: Anti-tumor effects of an oncolytic
adenovirus expressing hemagglutinin-neuraminidase of Newcastle
disease virus in vitro and in vivo. Viruses. 6:856–874. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boncler M, Różalski M, Krajewska U,
Podsędek A and Watala C: Comparison of PrestoBlue and MTT assays of
cellular viability in the assessment of anti-proliferative effects
of plant extracts on human endothelial cells. J Pharmacol Toxicol
Methods. 69:9–16. 2014. View Article : Google Scholar
|
|
24
|
Ford AL, An H, Kong L, Zhu H, Vo KD,
Powers WJ, Lin W and Lee JM: Clinically relevant reperfusion in
acute ischemic stroke: MTT performs better than Tmax and TTP.
Transl Stroke Res. 5:415–421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu L, Wu W, Zhu G, Liu L, Guan G, Li X,
Jin N and Chi B: Therapeutic efficacy of an hTERT promoter-driven
oncolytic adenovirus that expresses apoptin in gastric carcinoma.
Int J Mol Med. 30:747–754. 2012.PubMed/NCBI
|
|
26
|
Itoh S, Maeda T, Shimada M, Aishima S,
Shirabe K, Tanaka S and Maehara Y: Role of expression of focal
adhesion kinase in progression of hepatocellular carcinoma. Clin
Cancer Res. 10:2812–2817. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu DC, Chen Y, Seng M, Dilley J and
Henderson DR: The addition of adenovirus type 5 region E3 enables
calydon virus 787 to eliminate distant prostate tumor xenografts.
Cancer Res. 59:4200–4203. 1999.PubMed/NCBI
|
|
28
|
Nettelbeck DM, Rivera AA, Balagué C,
Alemany R and Curiel DT: Novel oncolytic adenoviruses targeted to
melanoma: Specific viral replication and cytolysis by expression of
E1A mutants from the tyrosinase enhancer/promoter. Cancer Res.
62:4663–4670. 2002.PubMed/NCBI
|
|
29
|
Leitner S, Sweeney K, Oberg D, Davies D,
Miranda E, Lemoine NR and Halldén G: Oncolytic adenoviral mutants
with E1B19K gene deletions enhance gemcitabine-induced apoptosis in
pancreatic carcinoma cells and anti-tumor efficacy in vivo. Clin
Cancer Res. 15:1730–1740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dong X, Qu W, Ma S, Zhu Z, Zheng C, He A,
Karlsson A, Xu K and Zheng X: Potent antitumoral effects of
targeted promoter-driven oncolytic adenovirus armed with Dm-dNK for
breast cancer in vitro and in vivo. Cancer Lett. 328:95–103. 2013.
View Article : Google Scholar
|
|
31
|
Wang L, Zhang Y, Zhao J, Xiao E, Lu J, Fu
S and Wang Z: Combination of bladder cancer-specific oncolytic
adenovirus gene therapy with cisplatin on bladder cancer in vitro.
Tumour Biol. 35:10879–10890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu XR, Cai Y, Cao X, Wei RC, Li HL, Zhou
XM, Zhang KJ, Wu S, Qian QJ, Cheng B, et al: A new oncolytic
adenoviral vector carrying dual tumour suppressor genes shows
potent anti-tumour effect. J Cell Mol Med. 16:1298–1309. 2012.
View Article : Google Scholar
|
|
33
|
He B, Huang X, Liu X and Xu B: Cancer
targeting gene-viro-therapy for pancreatic cancer using oncolytic
adenovirus ZD55-IL-24 in immune-competent mice. Mol Biol Rep.
40:5397–5405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shang N, Arteaga M, Zaidi A, Stauffer J,
Cotler SJ, Zeleznik-Le NJ, Zhang J and Qiu W: FAK is required for
c-Met/beta-catenin-driven hepatocarcinogenesis. Hepatology.
61:214–226. 2014. View Article : Google Scholar
|
|
35
|
Dai Z, Zhou SL, Zhou ZJ, Bai DS, Xu XY, Fu
XT, Chen Q, Zhao YM, Zhu K, Yu L, et al: Capn4 contributes to
tumour growth and metastasis of hepatocellular carcinoma by
activation of the FAK-Src signalling pathways. J Pathol.
234:316–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ko BS, Jan YJ, Chang TC, Liang SM, Chen
SC, Liu TA, Wu YM, Wang J and Liou JY: Upregulation of focal
adhesion kinase by 14-3-3ɛ via NFκB activation in hepatocellular
carcinoma. Anticancer Agents Med Chem. 13:555–562. 2013. View Article : Google Scholar
|
|
37
|
Cai L, Han J, Zhuo X, Xiong Y, Dong J and
Li X: Overexpression and significance of focal adhesion kinase in
hepatocellular carcinoma and its relationship with HBV infection.
Med Oncol. 26:409–414. 2009. View Article : Google Scholar
|
|
38
|
Takeshita F and Ochiya T: Therapeutic
potential of RNA interference against cancer. Cancer Sci.
97:689–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu XY: Targeting gene-virotherapy of
cancer and its prosperity. Cell Res. 16:879–886. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Grünweller A, Wyszko E, Bieber B, Jahnel
R, Erdmann VA and Kurreck J: Comparison of different antisense
strategies in mammalian cells using locked nucleic acids,
2′-O-methyl RNA, phosphorothioates and small interfering RNA.
Nucleic Acids Res. 31:3185–3193. 2003. View Article : Google Scholar
|
|
41
|
Prados J, Melguizo C, Roldan H, Alvarez
PJ, Ortiz R, Arias JL and Aranega A: RNA interference in the
treatment of colon cancer. BioDrugs. 27:317–327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu W, Zhu F, Jiang Y, Sun D, Yang B and
Yan H: siRNA targeting survivin inhibits the growth and enhances
the chemosensitivity of hepatocellular carcinoma cells. Oncol Rep.
29:1183–1188. 2013.
|
|
43
|
Pei Z, Chu L, Zou W, Zhang Z, Qiu S, Qi R,
Gu J, Qian C and Liu X: An oncolytic adenoviral vector of Smac
increases antitumor activity of TRAIL against HCC in human cells
and in mice. Hepatology. 39:1371–1381. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang J, Gan Y, Gu J, Hu J, Liu X and Zhao
X: Potent anti-hepatoma efficacy of HCCS1 via dual tumor-targeting
gene-virotherapy strategy. Oncol Rep. 20:1035–1040. 2008.PubMed/NCBI
|
|
45
|
Wu WY, Kim H, Zhang CL, Meng XL and Wu ZS:
Loss of suppressors of cytokine signaling 3 promotes aggressiveness
in hepatocellular carcinoma. J Invest Surg. 27:197–204. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Guo X, Wang W, Zhou F, Lu Z, Fang R, Jia
F, Bu X, Li R, Zhang B, Wu M, et al: siRNA-mediated inhibition of
hTERT enhances chemosensitivity of hepatocellular carcinoma. Cancer
Biol Ther. 7:1555–1560. 2008. View Article : Google Scholar : PubMed/NCBI
|