Introduction
The majority of identified tumors, including
prostate cancer (PCa) were proven to be originated from epithelial
cells (1). For many years,
tumorigenesis has been classically regarded as a largely
cell-autonomous process involving genetically transformed cancer
cells. Consequently, the focus of cancer research has been on
genetic changes in cancer cells, with the progression from normal
to malignant state. Recently, increasing number of studies suggest
that the progression of tumors might not only rely on the
cell-autonomous process of cancer cells themselves but tumors could
be also influenced by the surrounding cells (2). The tumor microenvironment (TME)
participation in tumor is now well accepted. Most of the solid
tumors are surrounded by complicated microenvironment, including
the extracellular matrix (ECM), different stromal cells (smooth
muscle cells and fibroblasts), other resident cells and recruited
inflammatory cells or bone marrow mesenchymal stem cells (BM-MSCs)
(2–4).
The peritumoral fibroblasts are considered as active
fibroblasts, which are also known as cancer associated fibroblasts
(CAFs) or myofibroblasts. In the normal tissue, fibroblasts can be
activated for wound healing or fibrosis (5). The activated myofibroblasts can gain
contractile stress fibers by expressing α-smooth muscle actin
(α-SMA) (6) that allows them to
gain better mobility via stronger contractile and secretory
capabilities. Therefore, more and more data suggest that
myofibroblasts or CAFs might have the capacity to promote tumor
growth and metastasis, either via direct interaction with tumor
epithelial cells or via the recruitment of inflammatory cells
(7,8).
Recent studies found PCa cells, but not normal
prostate cells, could better recruit BM-MSCs and the consequences
of such increased recruitment might then lead to enhanced PCa
metastasis (9). The involvement of
stromal cells in such enhanced PCa metastasis, however, remains
unclear. In this study, we focused on the roles of stromal cells in
the BM-MSCs capacity to enhance PCa growth and invasion and results
revealed that recruited BM-MSCs converted normal fibroblasts (NFs)
to more CAF-like cells to further promote PCa cell growth and
invasion.
Materials and methods
Cell culture
TRAMP-C1, CWR22Rv1, LNCaP, and C4-2 cell lines were
obtained from ATCC (Manassas, VA, USA). TRAMP-C1 cells were
maintained in Dulbecco’s modified Eagle’s medium with 10% FBS.
CWR22Rv1, LNCaP, and C4-2 cells were cultured in RPMI-1640 medium
with 10% FBS.
Mouse primary BM-MSCs were isolated from wild-type
C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME, USA) as previouly
described (10). Tibias and femurs
were dissected from 6-week-old mice. After bones were cut, the
marrows were flushed out with 5 ml DMEM by using a needle and
syringe, and re-suspended in DMEM plus 15% FBS. After filtration,
mono-nucleated cells were maintained in DMEM (Gibco) with 15% FBS,
2 mM L-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin, and
10 mM HEPES. All of the cells were incubated at 37°C with 5%
humidified CO2 for 24 h, then the non-adherent cells
were removed by replacing the medium every 3 days for ~1 week. When
the cells grew to confluence, they were harvested with 0.25%
trypsin and 1 mM EDTA (Hyclone) for experiments.
Human BM-MSCs were purchased from Stem Cell
Technologies (Vancouver, BC, Canada), and maintained in Human
MesenCult® Proliferation kit (Stem cell Technologies
Inc.). BM-MSCs were already verified by adipogenesis and
osteogenesis differentiations in our lab (10). As described previously (11), mouse CAFs was isolated from the
prostate stromal region of transgenic adenocarcinoma of the
prostate mice in our lab. Mouse NFs were isolated from the prostate
stromal region of benign prostate mice in the same way. pshTERT is
an immortalized stromal cell line, stably expressing the human
telomerase catalytic subunit-hTert (a kind gift from the New York
University, named pshTERT). NFs and CAFs were cultured in RPMI-1640
medium. All cells were maintained in a humidified 5% CO2
environment at 37°C.
Cell viability assay (MTT)
TRAMP-C1 cells (104) with different
treatment were seeded in 24-well plates, and cultured in 10% FBS
media for 0, 2, 4 and 6 days. Cells from different time-points were
harvested, then we added
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT,
0.5 mg/ml in normal media), incubated for 2 h and removed the
media. The cellular staining products were dissolved in 0.5 ml
DMSO. The absorbance at OD570 was measured by spectrophotometry
(Beckman Du640B). The data represent means ± SD from three
independent wells and confirmed by three independent
experiments.
BrdU labeling
Cells (103) were seeded on the 6-well
plates, given certain treatment (such as NFs or CAFs) and cultured
to 50% confluence. BrdU labeling reagent (Invitrogen) was added to
the cultured cells for 1:100 dilution in the culture media for 24
h. After labeling, staining was performed according to the manual
instructions (Invitrogen, BrdU staining kit). The data represent
means ± SD from three independent wells and confirmed by three
independent experiments.
Invasion assay
The 6-well transwells (Corning, #3422) were coated
with growth factor reduced matrix gel diluted with serum-free
RPMI-1640 media (1:5 diluted), and dried overnight at 37°C. Cells
(105) after different treatments were re-suspended with
serum-free media and seeded in the upper transwells. Media with 10%
FBS was put in the lower wells. After incubation for 48 h, the
cells on the upper surface of the transwells were removed with
cotton swabs. Cells on lower filter surfaces were fixed with 75%
ethanol, stained with 0.1% toluidine blue in PBS. Invaded cells
were counted under a microscope. The data represent means ± SD from
three independent wells and confirmed by three independent
experiments.
Western blot analysis
Harvested cells were washed with PBS twice and lysed
in RIPA buffer (50 mM Tris-HCl/pH 7.4, 1% NP-40, 150 mM NaCl, 1 mM
EDTA, 1 mM Na3VO4, 1 mM NaF, 1 mM okadaic
acid and 1 mg/ml aprotinin, leupeptin, and pepstatin) with
proteinase inhibitor. Samples (30 μg protein) were loaded on 10%
SDS-PAGE gel to be separated and transferred to PVDF membranes at
4°C (100 V, 90 min). Membranes were blocked in 5% fat-free milk
with 3% BSA in PBST for 1 h at room temperature, and incubated with
appropriate dilutions of primary antibodies (α-SMA, Abcam #ab7817,
1:300; GAPDH, Santa Cruz #sc-32233, 1:3,000) overnight at 4°C. Then
the membranes were washed, and incubated with HRP conjugated
anti-mouse IgG for 1 h at room temperature. The blots were
developed in ECL mixture and visualized by an imager.
RNA isolation and quantitative
analysis
Total cellular RNAs were extracted by TRIzol reagent
(Invitrogen) according to the manufacturer’s instructions. Total
RNA (1 ng) was used to synthesize first-strand complementary DNA by
reverse transcriptase Superscript III (Invitrogen). The amount of
certain cDNA was measured in real-time PCR assays using a SYBR
green Bio-Rad CFX96 system. Primers were: mouse α-SMA: sense
5′-CCCAGACATCAGGGAGTAATGG-3′, antisense
5′-TCTATCGGATACTTCAGCGTCA-3′; mouse CD90: sense
5′-TGCTCTCAGTCTTGCAGGTG-3′, antisense
5′-TGGATGGAGTTATCCTTGGTGTT-3′; mouse IGF-1: sense
5′-CACATCATGTCGTCTTCACACC-3′, antisense
5′-GGAAGCAACACTCATCCACAATG-3′; mouse IGF-2: sense
5′-GTGCTGCATCGCTGCTTAC-3′, antisense 5′-CGGTCCGAACAGACAAACT-3′;
mouse PDGFA: sense 5′-TGGCTCGAAGTCAGATCCACA-3′, antisense
5′-TTCTCGGGCACATGGTTAATG-3′; mouse TGFβ-1: sense
5′-CCACCTGCAAGACCATGGAC-3′, antisense 5′-CTGGCGAGCCTTAGTTTGGAC-3′;
mouse GM-CSF: sense 5′-GGCCTTGGAAGCATGTAGAGG-3′, antisense
5′-GGAGAACTCGTTAGAGACGACTT-3′; human IGF-1: sense
5′-ATGCTCTTCAGTTCGTGTGTG-3′, antisense 5′-GCACTCCCTCTACTTGCGTTC-3′;
human IGF-2: sense 5′-GTGGCATCGTTGAGGAGTG-3′, antisense
5′-CACGTCCCTCTCGGACTTG-3′; human TGFβ-1: sense
5′-GGCCAGATCCTGTCCAAGC-3′, antisense 5′-GTGGGTTTCCACCATTAGCAC-3′;
human GM-CSF: sense 5′-GGGAGCATGTGAATGCCATC-3′, antisense
5′-GCAGTGTCTCTACTCAGGTTCAG-3′. The expression level of the other
genes was normalized to the expression of mouse GAPDH (sense
5′-AGGTCGGTGTGAACGGATTTG-3′, antisense
5′-TGTAGACCATGTAGTTGAGGTCA-3′) or human GAPDH (sense
5′-TGGCTTCATAGGTGACTTCCA-3′, antisense
5′-AAGGACCTGTCTAGGTTTGATGC-3′).
Immunofluorescence (IF) staining
NFs with different treatments were seeded on 4-well
chamber slides and fixed with 4% paraformaldehyde in PBS for 1 h at
15–25°C, washed with PBS. Then slides were incubated in
permeabilisation solution (0.5% Triton X-100 in PBS) for 5 min.
Following blockage with 5% BSA for 1 h samples were incubated with
primary antibody (anti-mouse α-SMA antibody, Abcam #ab7817, 1:100)
at 4°C overnight. After washing in 1× PBS, incubated with 1:200
diluted fluorescent secondary antibody for IF (Alexa 488 tagged).
Signals were observed under fluorescence microscope.
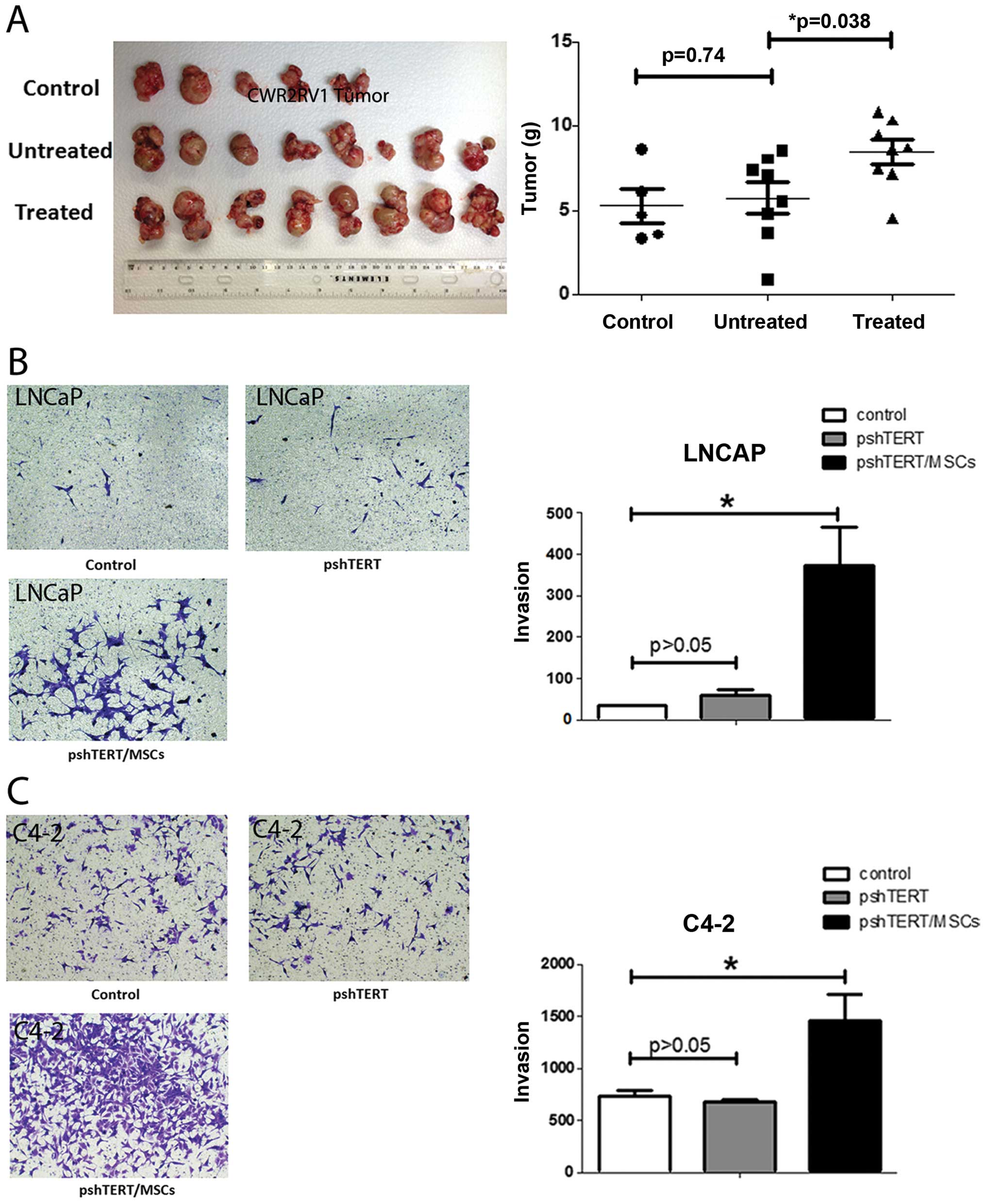
In vivo tumor studies
Male 6- to 8-week-old nude mice were used.
Luciferase expressing CWR22Rv1 cells (1×106), stably
transfected by pCDNA3.0-luciferase plasmid, were mixed with
Matrigel (1:1 in volume) and injected to the anterior lobes of 5
nude mice as control group. Eight mice were injected with CWR22Rv1
cells together with parental pshTERT cells (1:1). And another 8
mice were injected with CWR22Rv1 cells together with BM-MSCs
pre-treated pshTERT cells. Six weeks after injection, mice were
sacrificed and tumors were weighed. The research was approved and
conducted following the rules and regulations of the University
Committee of Animal Research (UCAR), No. 2002-296), which was fully
credited by Association for Assessment and Accreditation of
Laboratory Animal Care (AAALAC, No. A-3292-01), at the University
of Rochester Medical Center.
Statistical analysis
Values were expressed as mean ± standard deviation
(SD). The Student’s t- and ANOVA tests were used to calculate
p-values. p-values were two-sided, and considered statistically
significant when <0.05.
Results
Identification of NFs and CAFs isolated
from the PCa bearing mice
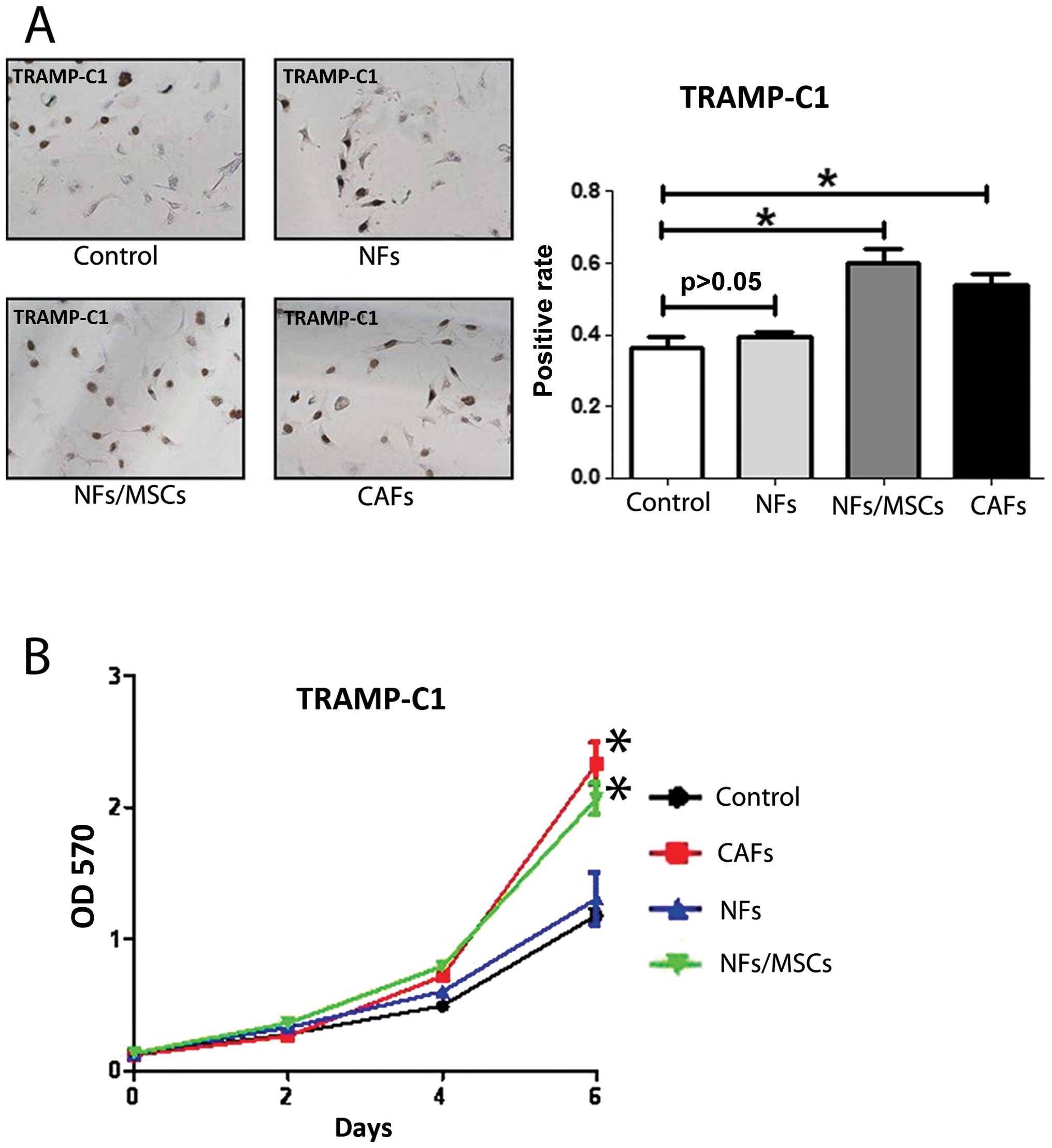
We first confirmed NFs and CAFs that were isolated
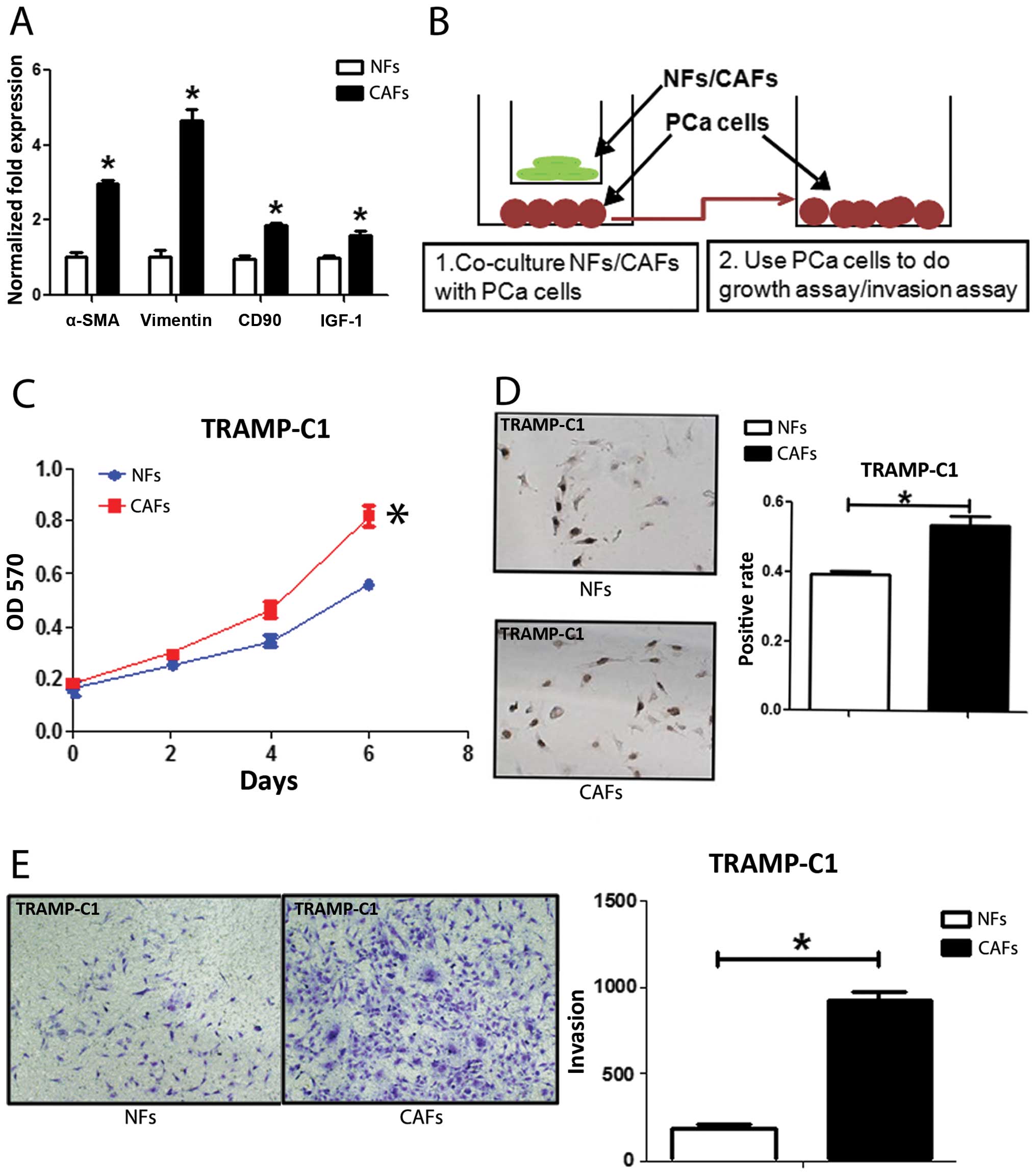
from TRAMP mice with several markers for CAFs. The co-expression of
α-SMA and vimentin is commonly used to define CAFs (12). CD90 (8) and IGF-1 (13) were also reported highly expressed
in CAFs. We found the expression of α-SMA, vimentin, CD90 and IGF-1
in CAFs were higher in CAFs than NFs with qPCR (Fig. 1A), western blot analysis (Fig. 5D) and IF assays (Fig. 5E). We further compared their
ability to enhance PCa cell growth and invasion as early reports
documented that CAFs have much better capacity than NFs to enhance
PCa cell growth and invasion (14). We co-cultured PCa epithelial cells
with NFs vs CAFs in the co-culture systems (Fig. 1B), and results from MTT (Fig. 1C) and BrdU staining (Fig. 1D) assays revealed that the CAFs
have better capacity than NFs to enhance PCa cells growth and
proliferation. Furthermore, the results from the transwell invasion
assay also showed that the CAFs had much better capacity than NFs
to enhance PCa cell invasion (Fig.
1E).
Together, results from Fig. 1 confirmed the CAFs and NFs used in
these studies are correct by showing CAFs have better capacity than
NFs to enhance PCa cell growth and invasion.
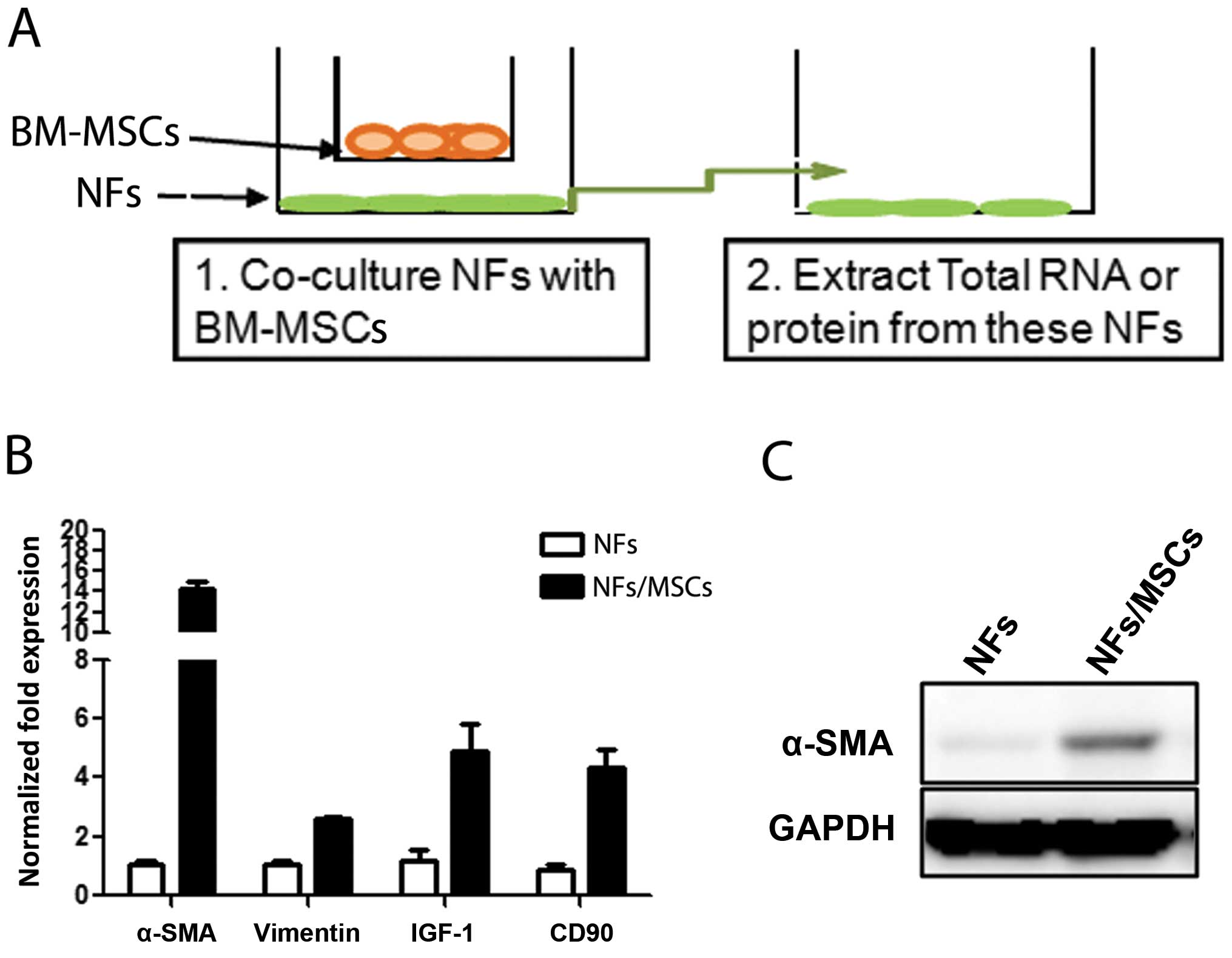
Molecular characteristics of NFs are
changed upon co-culture with BM-MSCs
To investigate whether BM-MSCs have any influence on
the conversion of prostate NFs to CAF cells, we co-cultured BM-MSCs
with NFs (Fig. 2A) at a ratio of
1:10 for 7 days, and results from qPCR and western blot analyses
revealed increased CAF markers, including α-SMA, CD90, and IGF-1 in
NFs upon co-culture with BM-MSCs (Fig.
2B and C). Similar results were obtained when we replaced the
ratio of BM-MSCs with NFs from 1:1 to 1:2 or 1:4 (data not shown).
These results suggest that BM-MSCs can influence the conversion of
NFs to cells with characteristics more close to CAFs.
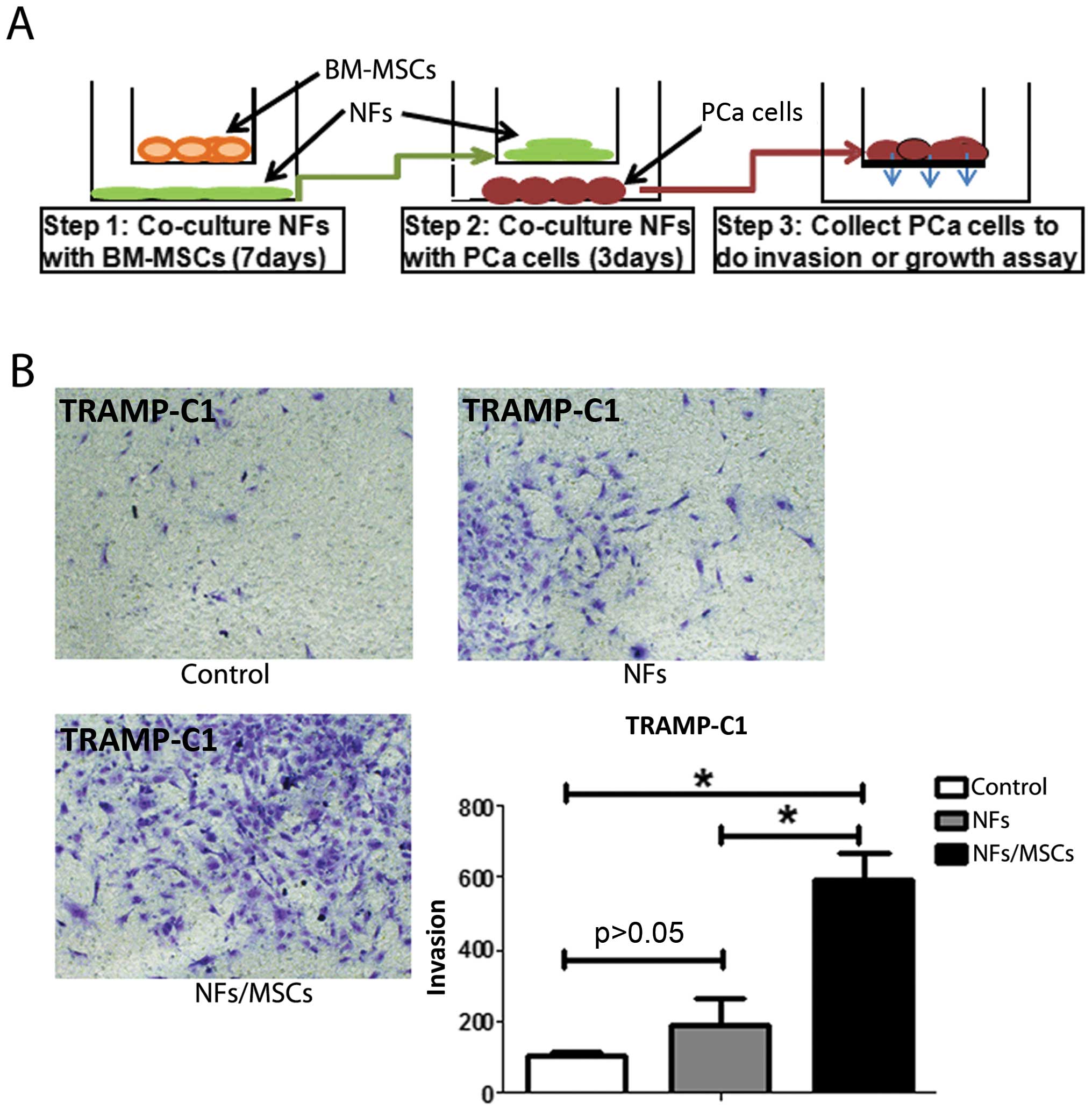
NFs promote PCa cell invasion when
pre-treated with BM-MSCs
One of the key phenotypes of CAFs is their
capability to promote tumor metastasis (Fig. 1E) (2). Our next experiment was to test
whether BM-MSCs-treated NFs also would have a similar capability.
After co-culturing mouse BM-MSCs and mouse NFs for 7 days, we
removed the mouse BM-MSCs (to eliminate the BM-MSCs effect on PCa
cells) and collected mouse NFs. These pre-treated NFs were
subsequently incubated with the mouse PCa epithelial TRAMP-C1 cells
(at the ratio of 1:1) for 3 days (see detailed procedure in
Fig. 3A). The results showed that
NFs pre-treated/incubated with BM-MSCs have the capacity to enhance
the PCa cell invasion (Fig.
3B).
Similar results were obtained when we replaced mouse
BM-MSCs and mouse NFs with human BM-MSCs and human stromal pshTERT
cells showing pshTERT cells (after co-cultured with human BM-MSCs)
were able to promote PCa C4-2 (Fig.
1) and LNCaP cells invasion (Fig.
6B).
The above, together with the results in Fig. 3 suggest that the prostate NFs may
acquire CAFs characteristics via co-culture with BM-MSCs to enhance
PCa cell invasion.
NFs promote PCa cell growth when
pre-treated with BM-MSCs
Another key phenotype of CAFs is their capability to
promote tumor growth (2). Using
BrdU staining assay and MTT assay to detect PCa cells proliferation
(Fig. 4A) and growth (Fig. 4B), we found that the NF pre-treated
BM-MSCs increased the PCa cells growth (Fig. 4A) and proliferation (Fig. 4B). In vivo mouse studies
using mice with orthotopically xenografted CWR22Rv1 cells
co-implanted with NF cells pre-treated with either BM-MSCs or
control media for 7 days also showed that NFs pre-treated with
BM-MSCs have larger tumor size as compared to the media control
(Fig. 6A).
The in vitro and in vivo results from
Figs. 4 and 6A suggest that the prostate NFs may
acquire the CAF characteristics via co-culture with BM-MSCs to
enhance PCa cell growth and proliferation.
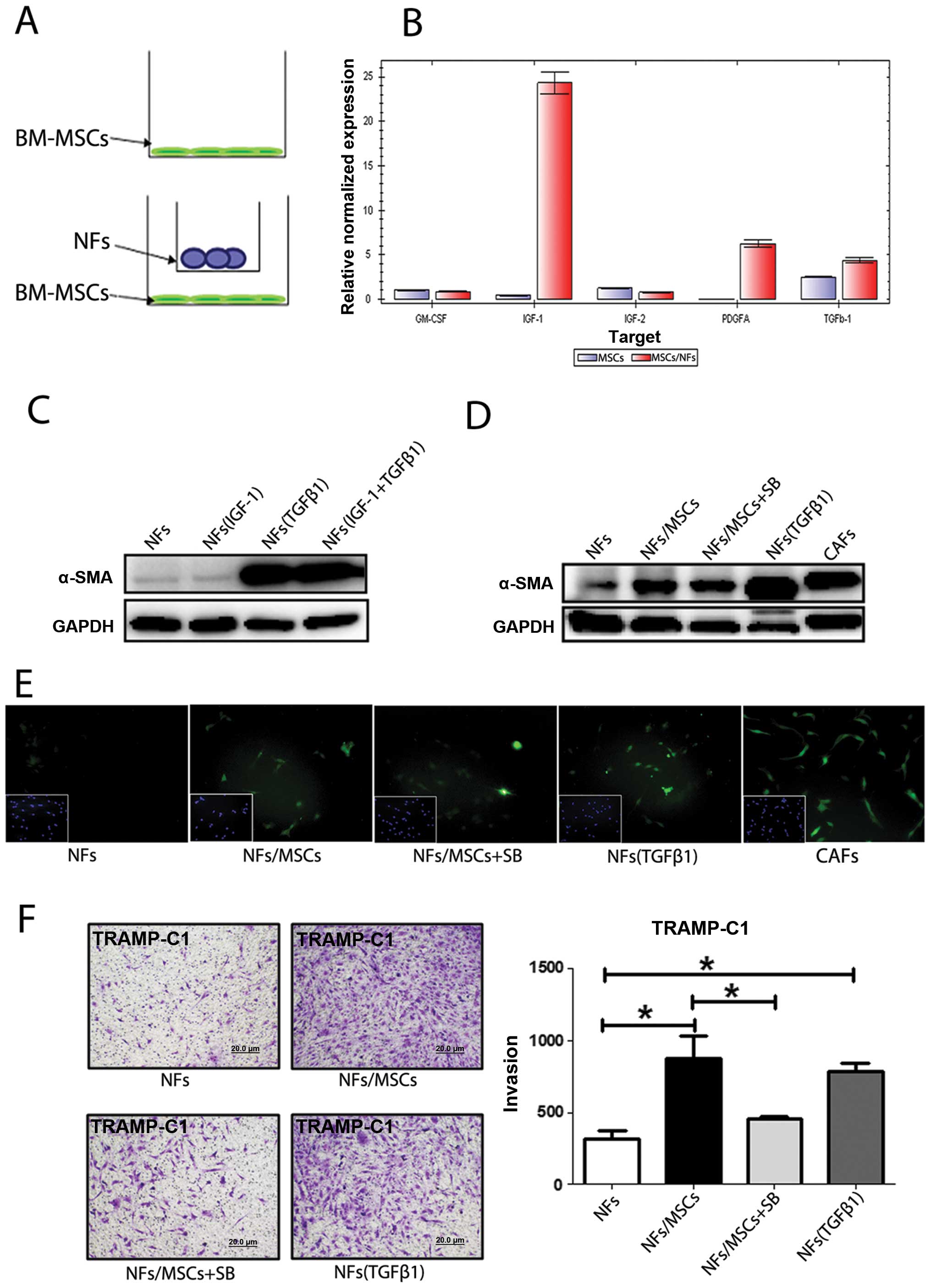
BM-MSCs secrete TFGβ-1 to promote the
conversion from NFs to CAFs
Recent studies demonstrated that some cytokines or
growth factors including GM-CSF, IGF-1, IGF-2, PDGFA and TFGβ-1
induced the conversion of NFs to CAFs (15–17).
To investigate which growth factors or cytokines secreted by
BM-MSCs might induce this transformation, we extracted RNAs from
BM-MSCs, with or without co-culture with NFs (Fig. 5A), to assay their differential
expression of these growth factors/cytokines. The qPCR analyses
revealed that expression of PDGFa, IGF-1 and TGFβ-1 in BM-MSCs was
increased after co-culture with NFs (Fig. 5B). Similar results with increased
IGF-1 and TGFβ-1 also occurred when we replaced mouse BM-MSCs/NFs
cells with human BM-MSCs/NFs cells PCa (data not shown).
We then applied two different approaches to further
confirm these results: first we added IGF-1 and TGFβ-1 recombinant
proteins to NFs to mimic the BM-MSCs effect and found only TGFβ-1
induced expression of α-SMA (Fig.
5C). We then used an interruption approach via addition of
TGFβ-1 inhibitor SB431542 to the co-cultured NFs and BM-MSCs and
found slightly suppressed α-SMA expression (Fig. 5D and E). Importantly, addition of
the TGFβ-1 inhibitor SB431542 suppressed the BM-MSCs/NFs induced
PCa cells invasion (Fig. 5F).
Results in Fig. 5
suggest that BM-MSCs may be able to trigger the conversion of NFs
to CAFs via altering the secretion of TGFβ-1.
Discussion
PCa is the most common malignant tumor of men in the
USA with the second highest death rate (1). Even though the current standard
therapy of androgen deprivation therapy (ADT) for the later stage
PCa is effective in the beginning, eventually the PCa still
progresses into the castration resistant stage with metastasis
(18). Several hypotheses were
used to explain the mechanisms by which PCa could escape from ADT
(19), yet none of them was
applied successfully to cure PCa. It is now widely recognized that
PCa, like other tumors, is regulated by multiple signaling from
multiple cells existing in the TME, including luminal epithelial
cells (19), epithelial basal
cells, stromal cells, stem/progenital cells (20), BM-MSCs (9), endothelial cells (21), and macrophages (22).
The stromal CAFs in TME, were also reported to be
able to influence the PCa progression (2). However, the origin of CAFs remained
unclear. Early studies suggested that the most important source of
CAFs could come from the resident NFs (17), especially the spindle-like
fibroblasts surrounding tumors (so-called peritumor fibroblasts),
and not the fibroblasts far away from tumor sites, with distinct
characteristics of highly expressed α-SMA, CD90, IGF-1 (8,23,24)
that can promote tumor growth and invasion (7,14).
The second source of CAFs could come from the
endothelial-mesenchymal transition (EndMT) (25) with increased mesenchymal markers of
fibroblast-specific protein-1 (FSP1) and decreased CD31/PECAM
(25). Importantly, BM-MSCs were
reported to be the potential third source of CAFs (26) that might contribute ~25% of the CAF
population (27).
The bone marrow is a complex tissue containing
hematopoietic stem/progenitor cells and their connective tissue
mesenchymal cells constituting the bone marrow microenvironment.
BM-MSCs comprise multifunctional non-hematopoietic stem cells that
have differentiating capabilities (28). In mouse models, BM-MSCs were
demonstrated to be able to migrate and incorporate into other
tissues where BM-MSCs were able to elicit tissue-specific
differentiation. For example, BM-MSCs contributed to tissue repair
by differentiation into tissue-specific cell types or by production
of trophic factors at the site of injury to stimulate tissue repair
and/or to reduce self-inflicted damage mediated by the immune
system (29). In PCa, BM-MSCs were
also proven to be able to migrate to PCa and influence PCa cell
growth (30), and invasion with
increased PCa stem cell population (9).
We found, for the first time, that BM-MSCs, in
addition to differentiating to CAFs or their direct function on PCa
epithelial cells, they might also be able to promote the conversion
of NFs to CAFs via secretion of TGFβ-1. These results suggest that
the recruited BM-MSCs may be able to transform into CAFs and
further promote the conversion of normal fibroblasts to CAFs. The
consequences of such increased CAFs may then enhance the PCa cell
growth and invasion.
The significance of the above findings is not only
adding new evidence of cross-talk between different cell types
within the TME, it also provides a new potential target to suppress
PCa progression. Targeting the infiltrating BM-MSCs via either
interruption of interaction between PCa and BM-MSCs or preventing
the conversion of NFs to CAFs via inhibition of TGFβ-1 signal may
result in suppression of the PCa progression.
Acknowledgements
We thank Karen Wolf for help with manuscript
preparation. This study was supported by NIH grant CA156700 and
George Whipple Professorship Endowment, Taiwan Department of Health
Clinical Trial and Research Center of Excellence grant
DOH99-TD-B-111-004 and China 973 grant CB518304.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liao D, Luo Y, Markowitz D, Xiang R and
Reisfeld RA: Cancer associated fibroblasts promote tumor growth and
metastasis by modulating the tumor immune microenvironment in a 4T1
murine breast cancer model. PLoS One. 4:e79652009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu P, Baek SH, Bourk EM, Ohgi KA,
Garcia-Bassets I, Sanjo H, Akira S, Kotol PF, Glass CK, Rosenfeld
MG, et al: Macrophage/cancer cell interactions mediate hormone
resistance by a nuclear receptor derepression pathway. Cell.
124:615–629. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zeng Y, Opeskin K, Goad J and Williams ED:
Tumor-induced activation of lymphatic endothelial cells via
vascular endothelial growth factor receptor-2 is critical for
prostate cancer lymphatic metastasis. Cancer Res. 66:9566–9575.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gabbiani G, Ryan GB and Majne G: Presence
of modified fibroblasts in granulation tissue and their possible
role in wound contraction. Experientia. 27:549–550. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier
C and Brown RA: Myofibroblasts and mechano-regulation of connective
tissue remodelling. Nat Rev Mol Cell Biol. 3:349–363. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Franco OE, Shaw AK, Strand DW and Hayward
SW: Cancer associated fibroblasts in cancer pathogenesis. Semin
Cell Dev Biol. 21:33–39. 2010. View Article : Google Scholar :
|
|
8
|
Zhao H and Peehl DM: Tumor-promoting
phenotype of CD90hi prostate cancer-associated fibroblasts.
Prostate. 69:991–1000. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo J, Ok Lee S, Liang L, Huang CK, Li L,
Wen S and Chang C: Infiltrating bone marrow mesenchymal stem cells
increase prostate cancer stem cell population and metastatic
ability via secreting cytokines to suppress androgen receptor
signaling. Oncogene. 33:2768–2778. 2014. View Article : Google Scholar
|
|
10
|
Huang CK, Tsai MY, Luo J, Kang HY, Lee SO
and Chang C: Suppression of androgen receptor enhances the
self-renewal of mesenchymal stem cells through elevated expression
of EGFR. Biochim Biophys Acta. 1833.1222–1234. 2013.
|
|
11
|
Slavin S, Yeh CR, Da J, Yu S, Miyamoto H,
Messing EM, Guancial E and Yeh S: Estrogen receptor α in
cancer-associated fibroblasts suppresses prostate cancer invasion
via modulation of thrombospondin 2 and matrix metalloproteinase 3.
Carcinogenesis. 35:1301–1309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tuxhorn JA, Ayala GE, Smith MJ, Smith VC,
Dang TD and Rowley DR: Reactive stroma in human prostate cancer:
induction of myofibroblast phenotype and extracellular matrix
remodeling. Clin Cancer Res. 8:2912–2923. 2002.PubMed/NCBI
|
|
13
|
Reinertsen T, Halgunset J, Viset T,
Flatberg A, Haugsmoen LL and Skogseth H: Gene expressional changes
in prostate fibroblasts from cancerous tissue. APMIS. 120:558–571.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cirri P and Chiarugi P: Cancer associated
fibroblasts: The dark side of the coin. Am J Cancer Res. 1:482–497.
2011.PubMed/NCBI
|
|
15
|
Haubeiss S, Schmid JO, Mürdter TE,
Sonnenberg M, Friedel G, van der Kuip H and Aulitzky WE: Dasatinib
reverses cancer-associated fibroblasts (CAFs) from primary lung
carcinomas to a phenotype comparable to that of normal fibroblasts.
Mol Cancer. 9:1682010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishii K, Mizokami A, Tsunoda T, Iguchi K,
Kato M, Hori Y, Arima K, Namiki M and Sugimura Y: Heterogenous
induction of carcinoma-associated fibroblast-like differentiation
in normal human prostatic fibroblasts by co-culturing with prostate
cancer cells. J Cell Biochem. 112:3604–3611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cirri P and Chiarugi P:
Cancer-associated-fibroblasts and tumour cells: A diabolic liaison
driving cancer progression. Cancer Metastasis Rev. 31:195–208.
2012. View Article : Google Scholar
|
|
18
|
Miyamoto H, Messing EM and Chang C:
Androgen deprivation therapy for prostate cancer: Current status
and future prospects. Prostate. 61:332–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Niu Y, Altuwaijri S, Lai KP, Wu CT, Ricke
WA, Messing EM, Yao J, Yeh S and Chang C: Androgen receptor is a
tumor suppressor and proliferator in prostate cancer. Proc Natl
Acad Sci USA. 105:12182–12187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee SO, Tian J, Huang CK, Ma Z, Lai KP,
Hsiao H, Jiang M, Yeh S and Chang C: Suppressor role of androgen
receptor in proliferation of prostate basal epithelial and
progenitor cells. J Endocrinol. 213:173–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Lee SO, Xia S, Jiang Q, Luo J, Li
L, Yeh S and Chang C: Endothelial cells enhance prostate cancer
metastasis via IL-6→androgen receptor→TGF-β→MMP-9 signals. Mol
Cancer Ther. 12:1026–1037. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Izumi K, Fang LY, Mizokami A, Namiki M, Li
L, Lin WJ and Chang C: Targeting the androgen receptor with siRNA
promotes prostate cancer metastasis through enhanced macrophage
recruitment via CCL2/CCR2-induced STAT3 activation. EMBO Mol Med.
5:1383–1401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gonda TA, Varro A, Wang TC and Tycko B:
Molecular biology of cancer-associated fibroblasts: Can these cells
be targeted in anti-cancer therapy? Semin Cell Dev Biol. 21:2–10.
2010. View Article : Google Scholar
|
|
24
|
Navab R, Strumpf D, Bandarchi B, Zhu CQ,
Pintilie M, Ramnarine VR, Ibrahimov E, Radulovich N, Leung L,
Barczyk M, et al: Prognostic gene-expression signature of
carcinoma-associated fibroblasts in non-small cell lung cancer.
Proc Natl Acad Sci USA. 108:7160–7165. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeisberg EM, Potenta S, Xie L, Zeisberg M
and Kalluri R: Discovery of endothelial to mesenchymal transition
as a source for carcinoma-associated fibroblasts. Cancer Res.
67:10123–10128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mishra PJ, Mishra PJ, Humeniuk R, Medina
DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW and Banerjee D:
Carcinoma-associated fibroblast-like differentiation of human
mesenchymal stem cells. Cancer Res. 68:4331–4339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Direkze NC, Hodivala-Dilke K, Jeffery R,
Hunt T, Poulsom R, Oukrif D, Alison MR and Wright NA: Bone marrow
contribution to tumor-associated myofibroblasts and fibroblasts.
Cancer Res. 64:8492–8495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Comite P, Cobianchi L, Avanzini MA, Zonta
S, Mantelli M, Achille V, De Martino M, Cansolino L, Ferrari C,
Alessiani M, et al: Isolation and ex vivo expansion of bone
marrow-derived porcine mesenchymal stromal cells: Potential for
application in an experimental model of solid organ transplantation
in large animals. Transplant Proc. 42:1341–1343. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang H, Chen Z and Bie P: Bone
marrow-derived mesenchymal stem cells as immunosuppressants in
liver transplantation: A review of current data. Transfus Med Rev.
26:129–141. 2012. View Article : Google Scholar
|
|
30
|
Placencio VR, Li X, Sherrill TP, Fritz G
and Bhowmick NA: Bone marrow derived mesenchymal stem cells
incorporate into the prostate during regrowth. PLoS One.
5:e129202010. View Article : Google Scholar : PubMed/NCBI
|