Introduction
Currently, complementary and alternative medicine
(CAM) is prevalent throughout the world, so the integration of
western and oriental medicine, utilizing both properties, is
increasingly required. Japanese traditional herbal medicine
(Kampo), for example, Juzentaihoto (JTT), which is also called
Shi-Quan-Da-Bu-Tang in Chinese herbal medicine, is prescribed for
various diseases, and one of its properties is strong
immunepotentiating activity, such as increasing antibody production
(1–7). In addition, pharmacokinetics analysis
of major active compound in JTT has been reported (8–11).
Vaccines are one of the major accomplishments in the
history of medicine. It is now also imperative to develop novel
vaccine adjuvants with high safety and efficacy, while novel
vaccines are increasingly undergoing advanced development and
contributing to unmet clinical needs (12).
Thus, we investigated whether the activity of Kampo
was suitable as immune adjuvant for treatment with vaccines.
Recently, we performed broad-ranging analysis of the adjuvant
effect of JTT on influenza vaccination in elderly people, who are a
high-risk group for influenza infection, in a multicenter
randomized controlled trial (9).
Consequently, JTT increased and prolonged antibody production after
influenza vaccination. Therefore, we clinically first achieved the
establishment of a new integrative vaccine therapy using Kampo as
an immune adjuvant (13).
On the other hand, a wide range of tumor vaccine
preparations, which have high immunoreaction specific to a
tumor-related antigen, have been tested throughout the last two
decades. In fact, more than twenty clinical studies that
investigated therapeutic potential of tumor-associated antigens
(TAAs) in tumor patients were reported in 2013 (14).
Given the success of JTT in influenza vaccination
(13), we extended the use of JTT
to other types of vaccine as immune adjuvant in the present
study.
Here, we report that JTT is an attractive immune
adjuvant candidate for tumor vaccines because of its marked
inhibitory effect on tumor growth in combination with a tumor
vaccine in a mouse model experiment.
Materials and methods
Reagents
Juzentaihoto (JTT) was obtained from Tsumura &
Co. (Tokyo, Japan). JTT was prepared as a spray-dried powder of a
hot water extract obtained from ten medical herbals in the
following ratio: JP astragalus root (3.0g), JP cinnamon bark (3.0
g), JP rehmannia root (3.0g), JP peony root (3.0 g), JP cnidium
rhizome (3.0 g), Japanese angelica root (3.0 g), JP ginseng (3.0
g), JP poria sclerotium (3.0 g), JP glycyrrhiza (1.5 g) and JP
Atractylodes lancea rhizome (3.0 g). Japanese pharmacopoeia
(JP) is established and published to regulate the properties and
quality of drugs by the Minister of Health, Labour and Welfare
after hearing the opinion of the Pharmaceutical Affairs and Food
Sanitation Council (The Japanese Pharmacopoeia 16th edition). JTT
has been approved by the Japanese Ministry of Health, Labor and
Welfare since 1986. Chemical characterization of JTT was ensured by
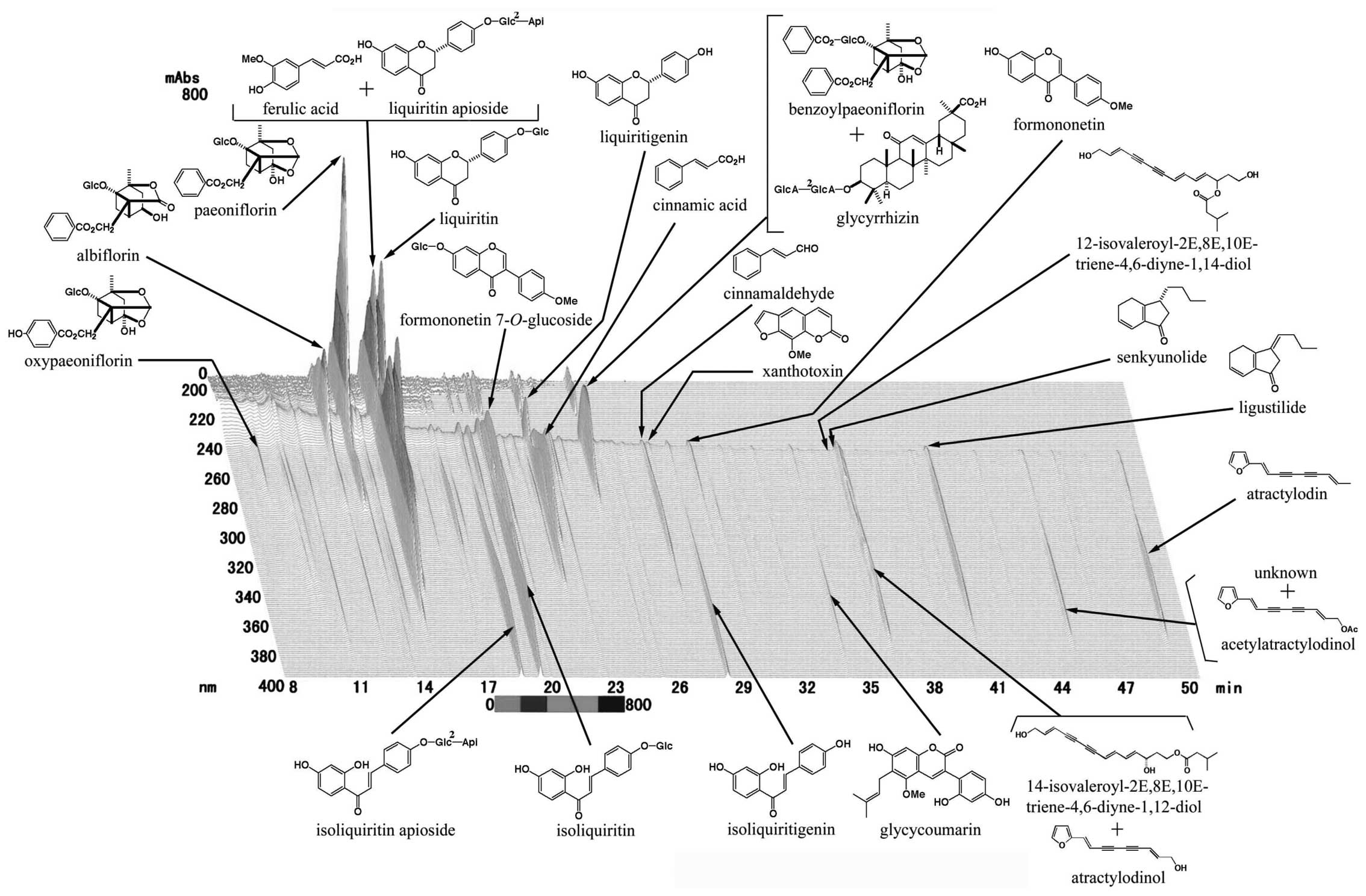
using three-dimensional (3D) HPLC analysis (Fig. 1). JTT was dissolved in water
immediately before administration to mice.
Mice
Five-week-old female C57BL/6 mice were purchased
from Sankyo Labo Service (Hamamatsu, Japan) for use in experiments
from 6 to 8 weeks of age. This study was carried out in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of University of Toyama. The protocol was
approved by the Committee on the Ethics of Animal Experiments of
University of Toyama (permission no. S-2009 INM-3). All surgery was
performed under sodium pentobarbital anesthesia, and all efforts
were made to minimize suffering.
Cell culture
CD8-OVA1.3 cells, a murine T-T hybridoma that is
specific for OVA257-264H-2Kb29, were maintained in
Dulbecco's modified Eagle's medium (DMEM) (Life Technologies Japan
Ltd., Tokyo, Japan) supplemented with 10% fetal bovine serum (FBS)
(Invitrogen), and 2-ME (50 μM, Invitrogen), E.G7-OVA tumor cells
(H-2b; OVA-transfectant of EL4 murine thymoma cells) were
maintained in RPMI-1640 medium supplemented with 10% FBS, 2-ME (50
μM) and G418 (400 μg/ml). Murine dendritic DC2.4 cells (H-2b) (T-T
hybridoma against OVA + H-2Kb) were grown in complete RPMI-1640
medium supplemented with non-essential amino acid (100 μM) and 2-ME
(50 μM). Primary DC cells were positively selected from spleen
(C57BL/6 mice, H-2b) using an anti-mouse CD11c-magnetic activated
cell sorting (MACS) kit, according to the protocol provided by the
manufacturer (Miltenyi Biotec K.K., Tokyo, Japan). The purity of
primary DC cells was analyzed by CD11c antibodies conjugated to PE
(BD Biosciences, CA, USA) with a FACSCanto flow cytometer (BD
Pharmingen).
Phagocytosis in DC2.4 cells
Fluorescence isothiocyanate (FITC) conjugated OVA
(OVA-FITC; Life Technologies Japan Ltd.) was dissolved to a
concentration of 10 μg/ml in balanced salt solution (BSS).
Phagocytosis in DC2.4 cells was performed by modification of a
previously reported method (30).
In this assay, AIM-V media (Life Technologies) was used instead of
growth media. Briefly, 5×105 cells/well were seeded in a
3.5-cm dish (Corning International K.K., Tokyo, Japan) and
incubated with JTT (200 μg/ml), OVA-FITC (50 μg/ml) and Lipofectine
(20 μg/ml; Life Technologies) for 20 h at 37°C. Vectashield
mounting medium for DAPI staining (Vector Laboratories, Inc., CA,
USA) was added to the cells. Florescence images were captured using
a Leica TCS SP5 microscope (Leica Microsystems, Wetzlar,
Germany).
Antigen presentation
The in vitro assay for antigen processing and
presentation was performed as described previously (31). In brief, DC2.4 cells
(2×104) cultured on 96-well plates were incubated with
JTT (200 μg/ml) or the ten herbal ingredients of JTT (100 μg/ml) at
37°C for 1 h. OVA (50 μg/ml) with Lipofectine (20 μg/ml) was added
and incubated at 37°C for 20 h. The cells were fixed with 0.05%
glutaraldehyde and washed three times, CD8-OVA1.3 cells
(1×105) were added to each well. After 24-h cultivation,
the response of stimulated CD8-OVA1.3 cells was determined by their
IL-2 secretion levels using ELISA (R&D Systems, MN, USA).
Tumor protection assay
C57BL/6 mice were subcutaneously immunized with
OVA-peptide formulatied antigens at 100 μg/100 μl incomplete Freund
adjuvant/mouse. Seven days after immunization, these mice were
intradermally inoculated with E.G7-OVA cells (5×105).
Ten mice were used for each experimental group. Tumor volume was
calculated as follows: (tumor volume; mm3) = (major
axis; mm) × (minor axis; mm)2 × 1/2. Tumor measurements
were determined until the volume exceeded 1,000 mm3.
Tumor-bearing mice continued treatment as indicated above after 37
days. Mice were allowed to live up to their natural death or were
sacrificed when their tumor volume was >1,000 mm3.
Sacrifice was performed under sodium pentobarbital anesthesia, and
all efforts were made to minimize suffering. Kaplan-Meier survival
curves were plotted and statistically analyzed. Animal body weight
was measured and recorded every 4 d during the treatment for the
monitoring of the condition of the animals. JTT or water was orally
administered to 10 mice (2 g/kg/day) from day 7 to day 37
consecutively.
ELISPOT
The ELISPOT assay (R&D Systems) was performed
according to the manufacturer's instructions. Briefly, 96-well PVDF
plates (Millipore, MA, USA) were coated overnight at 4°C with 0.1
mg/well of purified AN-18 (anti-IFN-γ). The membrane on the plates
was pre-wetted by adding 50 μl 70% ethanol to each well, then
washed and blocked for 30 min with complete RPMI. C57BL/6
splenocytes (1×105 cells/well) were seeded on the
96-well PVDF plates with OVA (50 mg/ml; Sigma-Aldrich, MO, USA) at
37°C for 24 h. After staining with streptavidin-alkaline
phosphatase (BD Biosciences, CA, USA), IFN-γ-secreting T cells in
the spleens were calculated under a microscope.
ELISA
OVA solutions (10 mg/ml) were added to 96-well ELISA
plates and incubated at 4°C overnight. Unbound OVA on the plate was
removed by washing, and the plate was further incubated with
blocking reagent (Morinaga Institute of Biological Science, Inc.,
Yokohama, Japan) at 37°C for 1 h. Dilutions of mouse serum
immunized with OVA with or without the administration of JTT were
added to the wells (100 μl/well) and incubated at 37°C for 1 h.
After washing, anti-mouse IgG1-HRP (15H6, G1 chain specific;
SouthernBiotech, AL, USA) or IgG2a-HRP (HOPC-1, G2a chain specific;
SouthernBiotech) was added to the wells and incubated at 37°C for 1
h. These IgG1 and IgG2 levels were determined using TMBZ
(3,3′,5,5′-tetramethylbenzidine; Dojindo, Kumamoto, Japan)
according to the manufacturer's instructions.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD) and were analyzed statistically by the two-tailed Student's
t-test. Survival was analyzed by the Kaplan-Meier method and
compared between groups by use of the log-rank test. Analyses were
performed by means of JMP (Version 11, SAS Institute Inc., NC,
USA). Results with P<0.05 were considered significant and
similar results were obtained from three independent
experiments.
Results
Increasing effects of JTT and its herbal
ingredients on antigen presentation to MHC class I pathway in DC2.4
cells and primary DC cells
Presently, JTT is manufactured according to the
Ethical Kampo Medicine Drug GMP regulations and the self-imposed
regulations of the Japan Kampo-Medicine Manufacturers Association.
The quality of its main component was guaranteed through
three-dimensional (3D) HPLC analysis (Fig. 1).
To determine JTT ability in the uptake of antigen
phagocytosis, murine dendritic DC2.4 cells were incubated with
soluble OVA-FITC. DC2.4 cells were fixed and labeled with DAPI, a
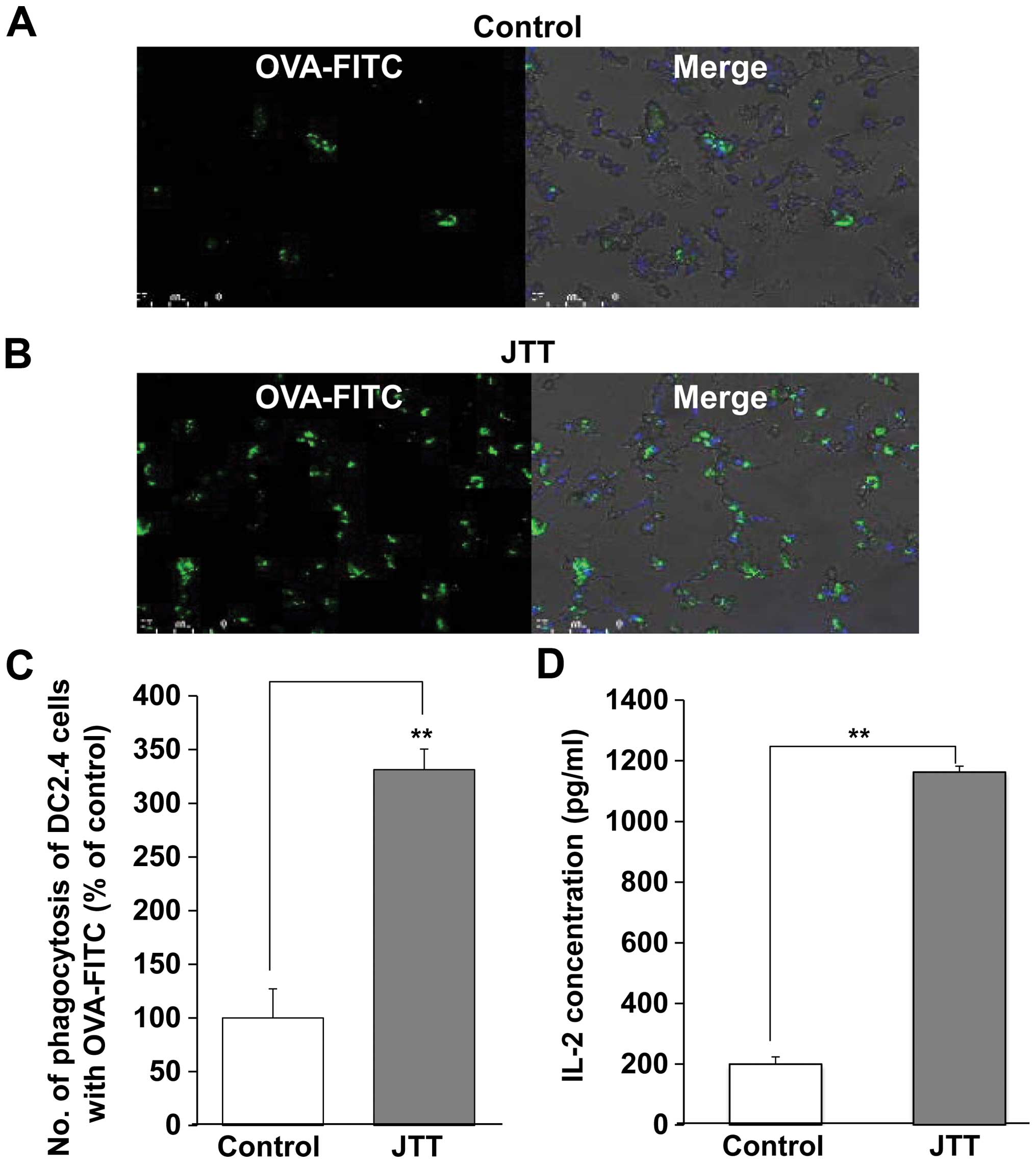
nucleotide marker. DC2.4 cells slightly phagocytosed OVA-FITC in
the absence of JTT (Fig. 2A). On
the other hand, the numbers of phagocytosis of DC2.4 cells with
OVA-FITC significantly increased 3.3-fold in the presence of JTT
(Fig. 2B and C). However, no
change of the fluorescence intensity per single DC was observed by
using of image analyzer (data not shown). Therefore, JTT may not
improve the efficacy of phagocytosis by single DC.
Owing to the increase of phagocytosis, we next
examined whether JTT introduced OVA antigen into the MHC class I
presentation pathway in dendritic cells. No antigen presentation
was detected by T cells alone or by T cells cocultured with DC 2.4
cells not loaded with OVA and preincubated with JTT. DC2.4 cells
preincubated with JTT efficiently presented OVA antigen on their
MHC class I molecules (Fig. 2C),
suggesting that the antigen introduced into the cytoplasm directly
by JTT was processed into OVA peptides (OVA257-264 (SL8) SIINFEKL)
(15). In this case, JTT had an
EC50 of 133.7 μg/ml against antigen presentation to the
MHC class I pathway in DC2.4 cells. We next investigated whether
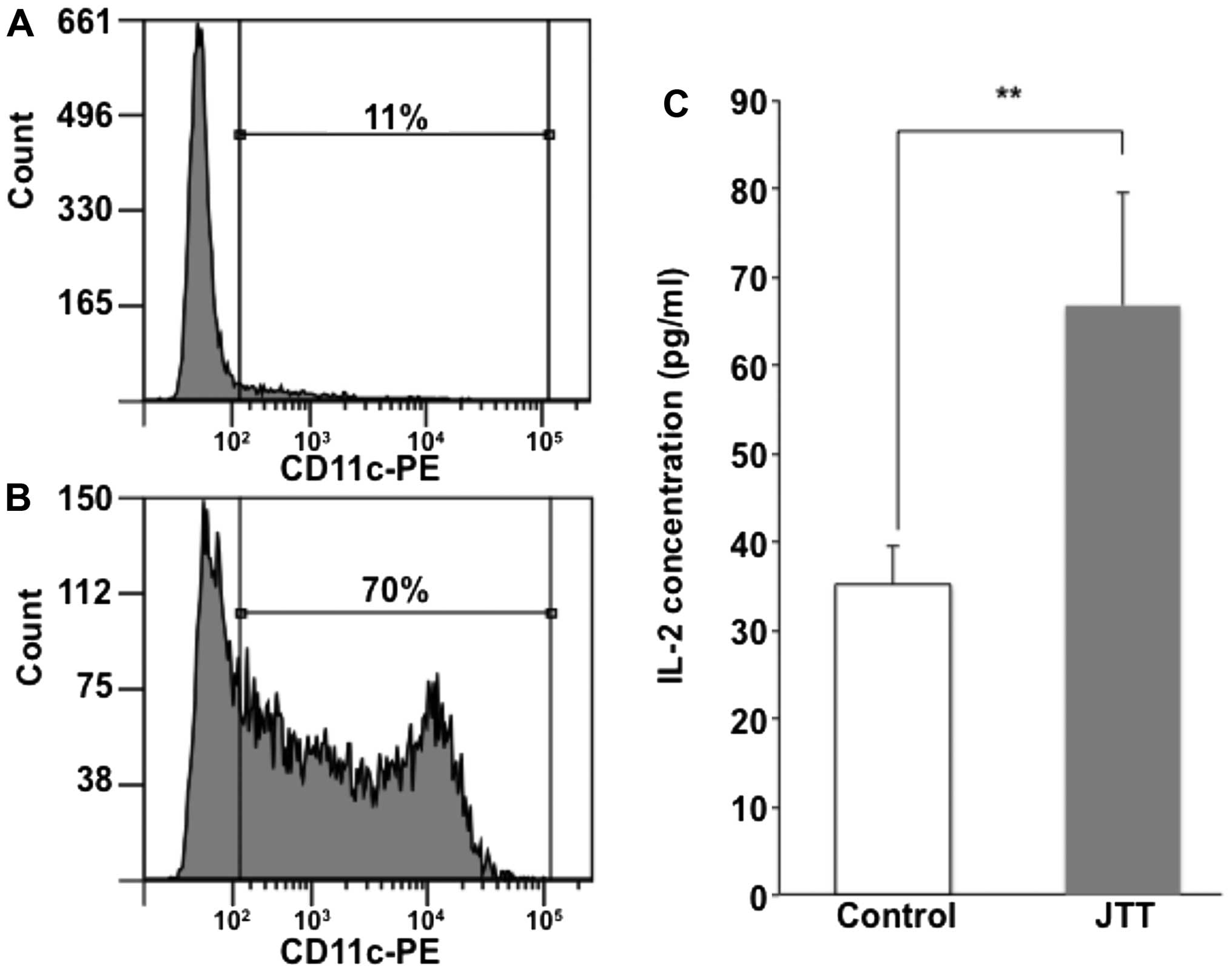
JTT induced antigen presentation in primary dendritic cells as well
as in dendritic cell lines. The antigen presentation of primary
dendritic cells was also enhanced by JTT (Fig. 3).
On the other hand, JTT was composed of ten medical
herbals. Of the 10 medical herbal ingredients, peony root and poria
sclerotium have the strongest effects on increasing antigen
presenting ability (Table I).
 | Table IEffects of ten medical herbals on
antigen presentation to MHC class I pathway in DC2.4 cells in
vitro. |
Table I
Effects of ten medical herbals on
antigen presentation to MHC class I pathway in DC2.4 cells in
vitro.
| Herbal | Percentage of control
(%) |
|---|
| Astragalus root | 98.0 |
| Cinnamon bark | 25.1 |
| Rehmannia root | 123.5 |
| Peony root | 282.3 |
| Cnidium
rhizome | 175.5 |
| Japanese angelica
root | 236.9 |
| Ginseng | 131.6 |
| Poria
sclerotium | 313.8 |
| Glycyrrhiza | 183.4 |
| Atractylodes
lancea rhizome | 176.6 |
No cytotoxicity in DC2.4 cells was observed by
treatment with JTT and the 10 medical herbals, except for cinnamon
bark at 200 μg/ml (data not shown).
Tumor regression by the combination of
OVA vaccination and JTT
To clarify the efficacy of JTT as an adjuvant for
tumor vaccines, mice immunized with OVA were intradermally
challenged with E.G7-OVA tumor expressing the SL8 epitope as the
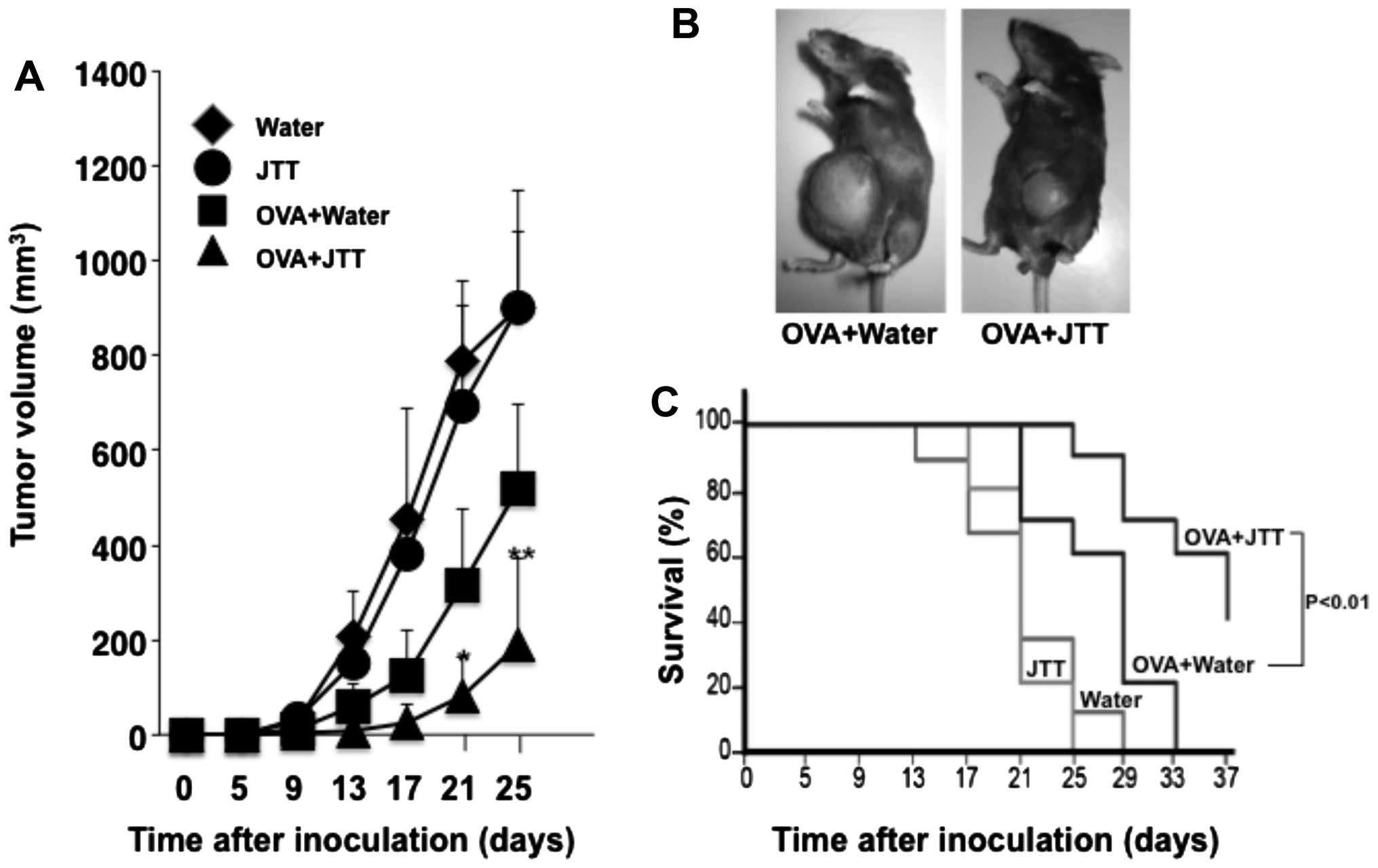
model tumor antigen. Oral administration of JTT alone did not
generate protective immunity against tumor growth, the same as the
control, whereas tumor growth was inhibited by OVA vaccine
(Fig. 4A and B). Together with
JTT, OVA vaccine markedly inhibited the growth of E.G7-OVA
tumor.
Consequently, tumor volume significantly decreased
to <40%, comparing the combination of OVA vaccine and JTT to OVA
vaccine alone. While no mouse immunized with OVA vaccine alone
completely rejected E.G7-OVA tumor formation, the combination of
OVA vaccine and JTT resulted in 2 of 10 mice exhibiting complete
rejection, and prolonged survival was observed in mice treated with
the combination compared to OVA vaccine alone (Fig. 4C).
Antitumor effect by immunological
modulation by JTT
To verify the immune adjuvant effects of JTT,
humoral and cellular immune responses to the OVA vaccine were
measured. The humoral immune responses specific to the OVA vaccine
were investigated by OVA antigen-specific IgG in mouse serum by
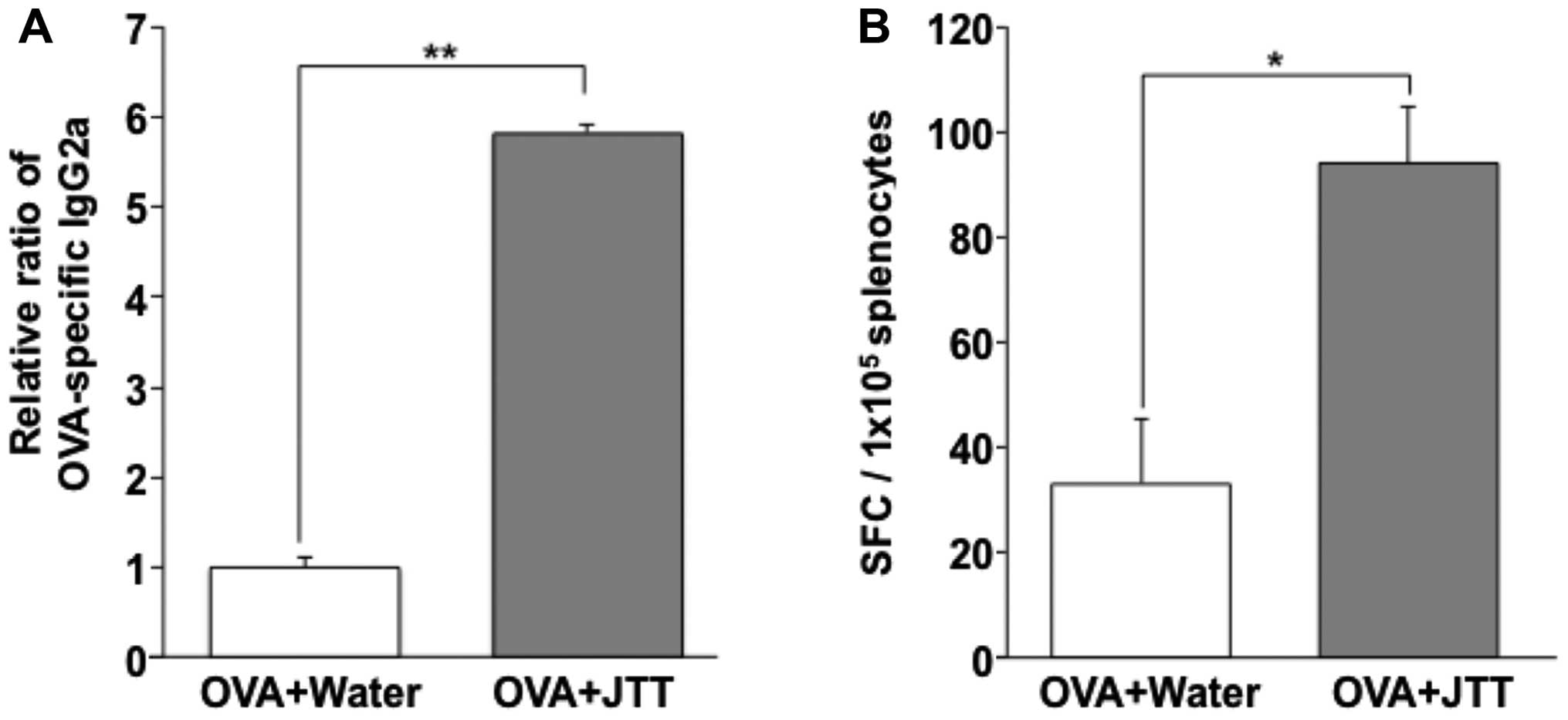
ELISA. OVA antigen-specific IgG2b in the OVA and JTT mouse group
was markedly increased ~6-fold compared to the OVA alone group
(Fig. 5A), and compared to OVA
antigen-specific IgG1 (data not shown). T-cell responses to the
vaccine were measured by the IFN-γ ELISPOT assay with splenocytes
isolated from mice immunized with OVA with oral administration of
JTT. OVA antigen-specific IFN-γ-secreting CD8 T cells significantly
increased ~3-fold compared to the OVA vaccine alone mouse group
with OVA and with oral administration of JTT (Fig. 5B).
Discussion
There are a number of reports that some Kampo
medicine activates innate immunity. JTT is often used clinically in
tumor patients who have received chemotherapy or radiation therapy
to improve anorexia or remission of leucopenia (Fig. 1).
It has been reported that JTT can enhance natural
killer (NKT) cell-inducing activity and the ability to produce
antibodies in the liver (1).
Ohnishi et al reported that liver metastasis was suppressed
by oral administration of JTT to mice and its efficacy was induced
by macrophages and T cells (5,6). In
addition, as reported by Chino et al, JTT enhanced
LPS-induced IL-12 and IFN-γ production via toll-like receptor (TLR)
in macrophages (7). Thus, JTT is
considered to be a drug with potential as a biological response
modifier (BRM), boosting its efficacy and activating innate
immunity.
However, while its role as a BRM is expected, the
effect of Kampo on the acquired immune system, such as
antigen-specific immune induction, i.e., vaccines, remains unclear.
In order to induce tumor antigen-specific immune responses using
tumor-associated antigens inoculated as vaccines, first of all, the
antigen needs to be taken up by an antigen-presenting cells (APC),
such as dendritic cells. Then, in the cytoplasm of APC, an epitope
peptide fragmented by vaccine antigens needs to be presented to MHC
class I, which are cell membrane surface molecules in APC (16).
In this study, we examined whether JTT can induce a
vaccine antigen-specific immune response, and whether it can be
applied as a tumor vaccine adjuvant.
Firstly, the effect of JTT on the phagocytic ability
of a vaccine model antigen (OVA) in dendritic cells was
investigated (Fig. 2). We also
examined the impact of JTT on the antigen-presenting ability of
dendritic cell lines (DC2.4 cells) and primary dendritic cells to T
cells using IL-2 production from MHC class I and OVA
complex-restricted T cell clones as an indicator. As a result, JTT
strongly enhanced the antigen-presenting ability of both DC2.4
(Fig. 2C) and primary dendritic
cells (Fig. 3C). From these
findings, the possibility that JTT has an adjuvant effect on
vaccines was suggested. Therefore, we next examined the efficacy of
JTT as an adjuvant for tumor vaccines using inoculated EG7 mouse
tumors presented with tumor model antigen (OVA) (17,18).
As a result, despite the failure to observe
antitumor effects from the administration of JTT alone, we were
able to observe that inoculation of OVA vaccine allowed the
induction of antitumor specific immunity against EG7. We also
observed that the combined use of JTT inhibited tumor growth
significantly and prolonged the tumor engraftment period as well as
the survival period (Fig. 4).
These findings confirmed that JTT has adjuvant efficacy for tumor
vaccines. In order to demonstrate the efficacy of JTT as an
adjuvant, we analyzed the vaccine antigen-specific immune induction
mechanism (vaccine antigen-specific IgG, IFN-γ-secreting CD8 T
cells, and CTL).
It is known that IL-4 of Th2-type cytokines is
involved in class switching to IgG1 from IgM, while cytokine IFN-γ
of Th1 type is involved in class switching to IgG2a (19,20).
IFN-γ is a cytokine involved in the induction of tumor-specific
CTL, and IFN-γ produced from Th1 cells tilts the Th1/Th2 balance to
Th1 dominantly (21).
As a result, we observed the significant production
of OVA-antigen specific IgG2a and IFN-γ-secreting CD8 T cells by
the combination of OVA vaccine and oral administration of JTT
(Fig. 5). In addition, the
tendency of increasing of OVA antigen-specific CTL (cytotoxic T
lymphocytes) by JTT could be observed using MHC tetramer by FACS
(data not shown). Further study will focus on the CTL for clearing
of the antitumor mechanism of JTT.
For the future development of novel adjuvants from
Kampo formulas, it is important to identify the active compounds
derived from medical herbal components, which have remarkable
vaccine antigen-specific immune responses. As shown in Fig. 1, JTT consists of ten medical herbal
ingredients (astragalus root, cinnamon bark, rehmannia root, peony
root, cnidium rhizome, Japanese angelica root, ginseng, poria
sclerotium, glycyrrhiza and Atractylodes lancea rhizome). In
DC.2.4 cells, peony root and poria sclerotium were found to have
the strongest effect on increasing antigen presenting ability of
the ten medical herbs (Table I).
We will thus try to identify an active compound with this ability
in the two medical herbs.
In wake of the discovery of the tumor antigen
(22), tumor vaccine therapy has
drawn attention, with attempts to obtain an antitumor effect by
inducing tumor antigen-specific immune responses in the host.
However, despite the current progress, in which
useful tumor antigens have been identified and the whole aspect of
the immune system surrounding tumors is being elucidated, tumor
vaccine therapy is not satisfactory compared with other treatments
such as chemotherapy. To improve the therapeutic effect, various
approaches have been attempted, including the development of
vaccine materials (8,10).
Here we focused on Japanese traditional herbal
medicine (Kampo) as an immune adjuvant for tumor vaccine.
Currently, Kampo is used in a number of clinical situations such as
cancer therapy, and contributes to health care (23–28).
Therefore, it is extremely important to confirm the
enhancement of the antitumor effect by combining Kampo with
chemotherapy using existing antitumor agents or tumor vaccine
treatments which have been carried out in clinical practice.
On the other hand, OVA was used as a tumor model
antigen in this study. For this reason, in order to introduce the
combined use of Kampo for tumor vaccine treatment into clinical
practice, in our future key studies it will be necessary to examine
the potential efficacy of JTT as an adjuvant by using an actual
tumor-related antigen, and to explore better Kampo drugs than JTT
to serve as more potent adjuvants.
Acknowledgements
This study was supported by a Grant-in-Aid for
Health Labour Sciences Research Grant and a Grant-in-Aid for the
Cooperative Research Project from Institute of Natural Medicine,
University of Toyama. We thank Dr Tsuyoshi Nakanishi (Laboratory of
Hygienic Chemistry and Molecular Toxicology, Department of
Biopharmaceutical Sciences, Gifu Pharmaceutical University, Japan),
Dr Takeshi Yamamoto, Dr Makoto Kadowaki (Division of
Gastrointestinal Pathophysiology, Institute of Natural Medicine of
Toyama, Japan) and Dr. Kazuo Ogawa (Tsumura Research Laboratories)
for helpful advice and technical support.
References
|
1
|
Matsumoto T, Sakurai MH, Kiyohara H and
Yamada H: Orally administered decoction of Kampo (Japanese herbal)
medicine, ‘Juzen-Taiho-To’ modulates cytokine secretion and induces
NKT cells in mouse liver. Immunopharmacology. 46:149–161. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kiyohara H, Matsumoto T and Yamada H:
Combination effects of herbs in a multi-herbal formula: Expression
of Juzen-taiho-to's immuno-modulatory activity on the intestinal
immune system. Evid Based Complement Alternat Med. 1:83–91. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kiyohara H, Matsumoto T, Takemoto N,
Kawamura H, Komatsu Y and Yamada H: Effect of oral administration
of a pectic polysaccharide fraction from a Kampo (Japanese herbal)
medicine ‘juzen-taiho-to’ on antibody response of mice. Planta Med.
61:429–434. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iijima K, Sun S, Cyong JC and Jyonouchi H:
Juzen-taiho-to, a Japanese herbal medicine, modulates type 1 and
type 2 T cell responses in old BALB/c mice. Am J Chin Med.
27:191–203. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohnishi Y, Fujii H, Hayakawa Y, Sakukawa
R, Yamaura T, Sakamoto T, Tsukada K, Fujimaki M, Nunome S, Komatsu
Y, et al: Oral administration of a Kampo (Japanese herbal) medicine
Juzen-taiho-to inhibits liver metastasis of colon 26-L5 carcinoma
cells. Jpn J Cancer Res. 89:206–213. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Onishi Y, Yamaura T, Tauchi K, Sakamoto T,
Tsukada K, Nunome S, Komatsu Y and Saiki I: Expression of the
anti-metastatic effect induced by Juzen-taiho-to is based on the
content of Shimotsu-to constituents. Biol Pharm Bull. 21:761–765.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chino A, Sakurai H, Choo MK, Koizumi K,
Shimada Y, Terasawa K and Saiki I: Juzentaihoto, a Kampo medicine,
enhances IL-12 production by modulating Toll-like receptor 4
signaling pathways in murine peritoneal exudate macrophages. Int
Immunopharmacol. 5:871–882. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Munekage M, Ichikawa K, Kitagawa H,
Ishihara K, Uehara H, Watanabe J, Kono T and Hanazaki K: Population
pharmacokinetic analysis of daikenchuto, a traditional Japanese
medicine (Kampo) in Japanese and US health volunteers. Drug Metab
Dispos. 41:1256–1263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie Y, Jiang ZH, Zhou H, Ma WZ, Wong YF,
Liu ZQ and Liu L: The pharmacokinetic study of sinomenine,
paeoniflorin and paeonol in rats after oral administration of a
herbal product Qingfu Guanjiesu capsule by HPLC. Biomed Chromatogr.
28:1294–1302. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Ma Y and Ma W: Pharmacokinetics
and bioavailability of cinnamic acid after oral administration of
Ramulus Cinnamomi in rats. Eur J Drug Metab Pharmacokinet.
34:51–56. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao W-J, Wang BJ, Wei CM, Yuan GY, Bu FL
and Guo RC: Determination of glycyrrhetic acid in human plasma by
HPLC-MS method and investigation of its pharmacokinetics. J Clin
Pharm Ther. 33:289–294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aranda F, Vacchelli E, Eggermont A, Galon
J, Sautès-Fridman C, Tartour E, Zitvogel L, Kroemer G and Galluzzi
L: Trial Watch: Peptide vaccines in cancer therapy. OncoImmunology.
2:e266212013. View Article : Google Scholar
|
|
13
|
Saiki I, Koizumi K, Goto H, Inujima A,
Namiki T, Raimura M, Kogure T, Tatsumi T, Inoue H, Sakai S, et al:
The long-term effects of a Kampo medicine, juzentaihoto, on
maintenance of antibody titer in elderly people after influenza
vaccination. Evid Based Complement Alternat Med. 2013:5680742013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reed SG, Orr MT and Fox CB: Key roles of
adjuvants in modern vaccines. Nat Med. 19:1597–1608. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bacik I, Cox JH, Anderson R, Yewdell JW
and Bennink JR: TAP (transporter associated with antigen
processing)-independent presentation of endogenously synthesized
peptides is enhanced by endoplasmic reticulum insertion sequences
located at the amino- but not carboxyl-terminus of the peptide. J
Immunol. 152:381–387. 1994.PubMed/NCBI
|
|
16
|
Harding CV: Class I MHC presentation of
exogenous antigens. J Clin Immunol. 16:90–96. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Babu JS, Nair S, Kanda P and Rouse BT:
Priming for virus-specific CD8+ but not CD4+
cytotoxic T lymphocytes with synthetic lipopeptide is influenced by
acylation units and liposome encapsulation. Vaccine. 13:1669–1676.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takahashi H, Takeshita T, Morein B, Putney
S, Germain RN and Berzofsky JA: Induction of CD8+
cytotoxic T cells by immunization with purified HIV-1 envelope
protein in ISCOMs. Nature. 344:873–875. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Snapper CM and Paul WE: Interferon-gamma
and B cell stimulatory factor-1 reciprocally regulate Ig isotype
production. Science. 236:944–947. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu Z, Zan H, Pone EJ, Mai T and Casali P:
Immunoglobulin class-switch DNA recombination: Induction, targeting
and beyond. Nat Rev Immunol. 12:517–531. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gajewski TF and Fitch FW:
Anti-proliferative effect of IFN-gamma in immune regulation. I
IFN-gamma inhibits the proliferation of Th2 but not Th1 murine
helper T lymphocyte clones. J Immunol. 140:4245–4252.
1988.PubMed/NCBI
|
|
22
|
van der Bruggen P, Traversari C, Chomez P,
Lurquin C, De Plaen E, Van den Eynde BJ, Knuth A and Boon T: A gene
encoding an antigen recognized by cytolytic T lymphocytes on a
human melanoma. J Immunol. 178:2617–2621. 2007.PubMed/NCBI
|
|
23
|
Uezono Y, Miyano K, Sudo Y, Suzuki M,
Shiraishi S and Terawaki K: A review of traditional Japanese
medicines and their potential mechanism of action. Curr Pharm Des.
18:4839–4853. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamakawa J, Motoo Y, Moriya J, Ogawa M,
Uenishi H, Akazawa S, Sasagawa T, Nishio M and Kobayashi J:
Significance of Kampo, traditional Japanese medicine, in supportive
care of cancer patients. Evid Based Complement Alternat Med.
2013:7464862013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Okumi H and Koyama A: Kampo medicine for
palliative care in Japan. Biopsychosoc Med. 8:62014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suzuki H, Asakawa A, Amitani H, Fujitsuka
N, Nakamura N and Inui A: Cancer cachexia pathophysiology and
translational aspect of herbal medicine. Jpn J Clin Oncol.
43:695–705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iwase S, Yamaguchi T, Miyaji T, Terawaki
K, Inui A and Uezono Y: The clinical use of Kampo medicines
(traditional Japanese herbal treatments) for controlling cancer
patients' symptoms in Japan: A national cross-sectional survey. BMC
Complement Altern Med. 12:2222012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mantani N, Kasahara Y, Kamata T, Sekiya N,
Shimada Y, Usuda K, Sakakibara I, Hattori N and Terasawa K: Effect
of Seihai-to, a Kampo medicine, in relapsing aspiration pneumonia -
an open-label pilot study. Phytomedicine. 9:195–201. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hayashi A, Wakita H, Yoshikawa T,
Nakanishi T, Tsutsumi Y, Mayumi T, Mukai Y, Yoshioka Y, Okada N and
Nakagawa S: A strategy for efficient cross-presentation of
CTL-epitope peptides leading to enhanced induction of in vivo tumor
immunity. J Control Release. 117:11–19. 2007. View Article : Google Scholar
|
|
30
|
Kato S, Koizumi K, Yamada M, Inujima A,
Takeno N, Nakanishi T, Sakurai H, Nakagawa S and Saiki I: A
phagocytotic inducer from herbal constituent, pentagalloylglucose
enhances lipoplex-mediated gene transfection in dendritic cells.
Biol Pharm Bull. 33:1878–1885. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakanishi T, Hayashi A, Kunisawa J,
Tsutsumi Y, Tanaka K, Yashiro-Ohtani Y, Nakanishi M, Fujiwara H,
Hamaoka T and Mayumi T: Fusogenic liposomes efficiently deliver
exogenous antigen through the cytoplasm into the MHC class I
processing pathway. Eur J Immunol. 30:1740–1747. 2000. View Article : Google Scholar : PubMed/NCBI
|