Introduction
A series of naturally-occurring substances isolated
from the genus Curcuma have been widely used in cooking
worldwide, and at the same time, they have been traditionally
prescribed against not only general diseases such as wounds,
inflammation and fever but also cardiovascular and malignant
diseases mostly in Asian countries (1). One of the most common ones is
turmeric, which was isolated for the first time in the dried ground
rhizome of Curcuma longa (2), and the active constitute identified
as the curcumin (3) has been
determined to possess a wide variety of effects on inhibitions
against proliferation, growth, metastasis, invasion and
angiogenesis of tumor cells (4,5).
Whereas, the genus Curcuma contains large numbers of
species, and various constituents isolated as turmerin, essential
oils and curcuminoids including curcumin were reported to produce
the same efficacies (6,7). They have been investigated for
antioxidant, anti-inflammatory and antitumor activities (8–16),
and some were applied in clinical trials (17,18).
Curcuma zedoaria, otherwise known as zedoary
or white turmeric, was demonstrated to contain strong cytotoxic
activity against tumor cells, and several constituents were
identified (14). The extracts
from C. zedoaria have also been reported to possess
anti-mutagenic (19,20), antitumor (13,21,22),
antioxidant (23), antimicrobial
(24,25), anti-inflammation (26) and anti-angiogenesis (27,28)
activities. However, detailed mechanisms of their effectiveness
still remain to be investigated, and it has been expected that the
curcumin possesses various activities to regulate expression of
multiple cell signaling proteins (4).
Recently, we synthesized a series of glycans, and
demonstrated their potential as antitumor agents against various
tumor cells through the induction of apoptosis and autophagy in the
cells (29–31). As described previously (29), prognosis of patients suffering from
esophageal cancer is still poor irrespective of recently developed
treatments with surgery and chemotherapies. Recent studies have
also showed that the prevalence of esophageal cancer is expected to
increase gradually even though many other types of cancer are
expected to decrease in incidence by 2025 (32).
In this study, the C. zedoaria extract
prepared from the rhizome with ethanol was investigated both in
vitro and in vivo, and its antitumor effects on
esophageal cancer cells were elucidated together with determination
of molecular mechanisms involved.
Materials and methods
Materials
The rhizome powder of Curcuma zedoaria
(Christm.) Roscoe was obtained from Uchida Wakanyaku Ltd. (Tokyo,
Japan). TE-8 cells were purchased from RIKEN BioResource Center
(Ibaraki, Japan) and HET-1A cells were from American Type Culture
Collection (Manassas, VA, USA). Balb/c nude mice (6-week-old,
female) were purchased from Nihon CLEA (Tokyo, Japan). Antibodies
against caspase-9, caspase-3, PARP, Bcl-2, PTEN, phospho-Akt,
phospho-mTOR, phospho-STAT3, FGFR1 and MMP-2 were obtained from
Cell Signaling Technology Inc. (Danvers, MA, USA). Anti-β-actin and
DMSO were purchased from Sigma (St. Louis, MO, USA).
Preparation of C. zedoaria
extraction
Eight hundred grams of the rhizome powder from C.
zedoaria rhizome was extracted three times with 800 ml of
ethanol. The combined extracts were then concentrated using a
rotary evaporator, and the dried material (14.02 g) was
obtained.
Cell lines
Cell lines used in this study were human esophageal
squamous carcinoma (TE-8) (33)
and non-cancerous immortalized esophageal cells (HET-1A) (34). The cells were cultured at 37°C in
RPMI-1640 medium (Sigma) supplemented with 10% fetal bovine serum
and antibiotics (100 U/ml penicillin and 100 μg/ml
streptomycin).
Cell proliferation assay
Fifty μl of a cell suspension (1×104
cells) was seeded into each well of a 96-well plate (Falcon,
Franklin Lakes, NJ, USA) and incubated at 37°C overnight. Fifty μl
of medium containing various amounts of the crude extract of C.
zedoaria (7.8–1,000 μg/ml), which was dissolved with 10% DMSO,
was added to each well and the plate was incubated at 37°C for 24
h. Cell viability was determined with the aid of a cell counting
kit (Dojindo, Kumamoto, Japan) according to the manufacturer's
instructions. The absorbance at 450 nm (OD450) with the
reference wavelength at 620 nm was read and the results were
derived from triplicate experiments. The cell proliferation
inhibition (CPI) rate (%) was calculated by the following formula:
CPI rate (%) = (1 − OD450 of the treated
cells/OD450 of the untreated cells) × 100.
Western blot analyses
TE-8 and HET-1A cells (1×106 cell/ ml)
were treated with the C. zedoaria extract (100 μg/ml) for 24
h. Both cell types incubated for 12 and 24 h were obtained together
with those without treatment as controls. Cells were washed twice
in an ice-cold medium and the cell lysate was prepared after
solubilizing cells with PRO-PREP Protein Extraction Solution
(iNtRON Biotechnology, Gyeonggi-do, Korea). Protein concentrations
of the lysate were determined with a Pierce BCA protein assay kit
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) using bovine
serum albumin as a standard. The extract (40 μg) was subjected to
electrophoresis on a Mini-PROTEAN TGX Precast Gels 4–20% (Bio-Rad,
Tokyo, Japan) and the gel was electro-transferred to a Hybond ECL
(7×8 cm) membrane (GE Life Science Healthcare, Amersham Place,
Buckinghamshire, UK). Changes of expression levels of various
proteins after treatment with the extract were analyzed using
western blotting of each protein. β-actin was used as a loading
control. MagicMark™ XP Western Protein Standard (Invitrogen Life
Science Technology, Waltham, MA, USA), which consists of nine
recombinant proteins in the range of 20–220 kDa was used as a
loading marker. Bands on the membrane were detected using an Image
Quant LAS4000 with the aid of an enhanced chemiluminescence
detection system (GE Life Science).
Colony assay
TE-8 cells were seeded at 250 cells in a
25-cm2 cell culture flask (Corning Life Science,
Rochester, NY, USA) and incubated for 24 h and then, after washing
with the RPMI medium, the cells were treated with the medium
containing the C. zedoaria extract with different
concentrations for 48 h. Each test was performed in triplicate.
After incubation for 10 days with change of medium every 2 days,
the number of colonies formed was counted under the microscope
(35). The survival rate of
colonies (%) was calculated from the following formula: survival
rate (%) = (numbers of colonies formed after treatment/numbers of
colonies formed without treatment) × 100.
Cell invasion assay
The cell invasion assay was examined in the BD
BioCoat™ Matrigel invasion chamber (8.0 μm, BD Bioscience, San
Jose, CA, USA) according to the manufacturer's instructions. TE-8
cells were incubated at 37°C for 48 h with the medium containing
the C. zedoaria extract with different concentrations. Five
hundred μl of treated cells suspension containing
2.5×104 cells and 750 μl of the medium were added to
each invasion chamber. After incubation for 12 h, the cells were
stained with a Diff-Quick kit (Sysmex Corp., Kobe, Japan) and
observed under the microscope. The five visual fields in every
membrane were photographed and all samples were tested in
triplicate.
Animal experiments
TE-8 cells were suspended in 100 μl of the medium
containing 1×106 cells and mixed with 100 μl of the
Matrigel (BD Biosciences). The mixture was then injected
subcutaneously into the flank of the Balb/c nude mice (6-week-old,
female) according to the method described previously (36). Two hundred μl of 10% DMSO
containing 5 mg of the C. zedoaria extract was administered
orally everyday with the aid of a disposable feeding needle
(Fuchigami Kikai Co., Ltd., Kyoto, Japan). The same treatment with
only 10% DMSO solution was also conducted as controls (n=10 each).
Tumor volume (V) formed was estimated according to the following
formula: V = 1/2 × (a × b2), where V, a and b indicate
the tumor volume in mm3, the smallest and largest tumor
size (mm), respectively (37). The
mice were sacrificed by euthanasia using Somnopentyl (Kyoritsu
Seiyaku Co. Ltd., Tokyo, Japan) on the 29th day after injection of
the cells. The experiment was approved by the Animal Care and
Experimentation Committee of Gunma University.
Statistical analysis
Statistical analysis was performed using JMP 5.0
software (SAS Institute Inc., NC, USA). The differences were
considered statistically significant when the P-value was
<0.05.
Results
Effects of the C. zedoaria extract on
viability of esophageal cells
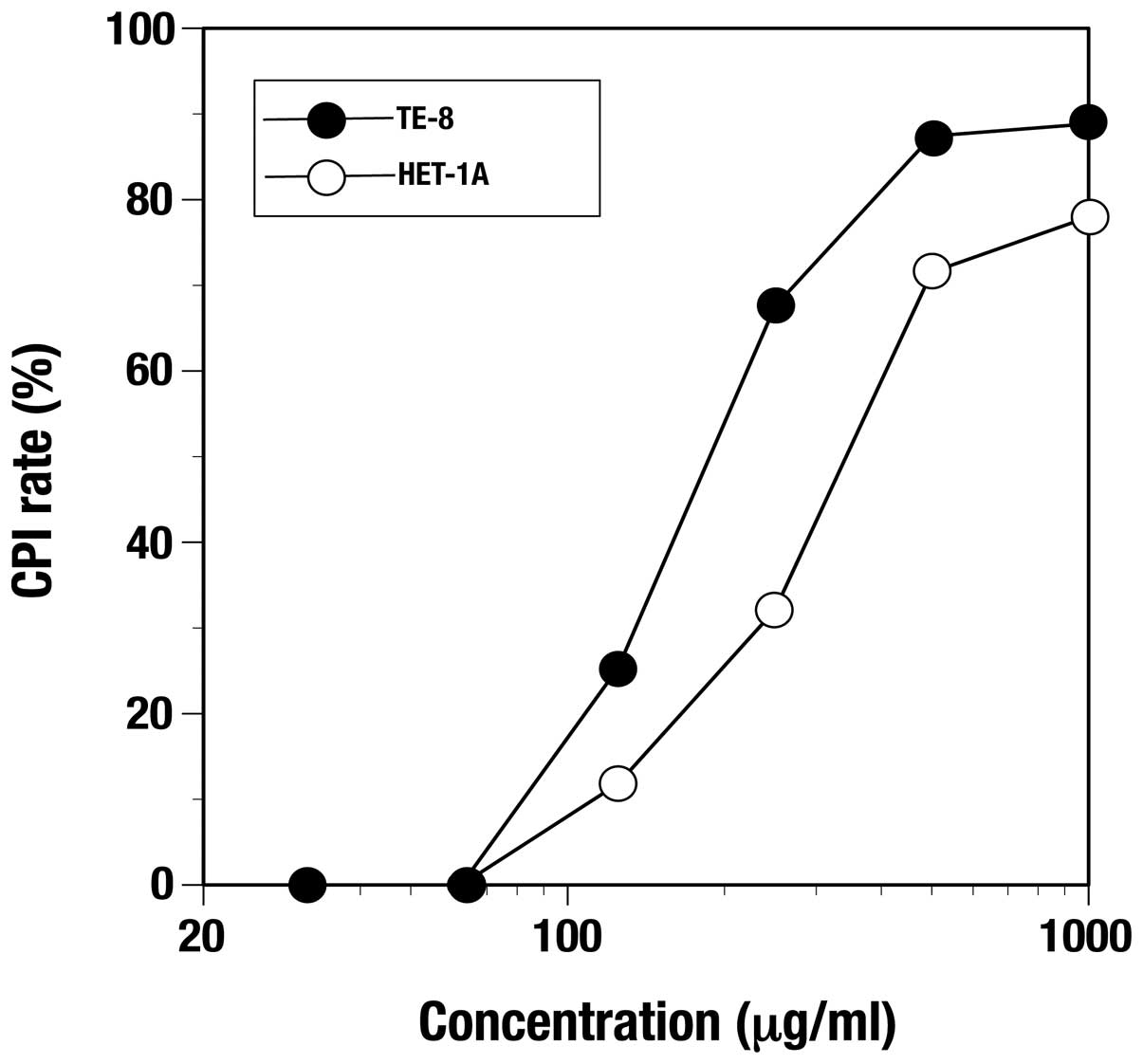
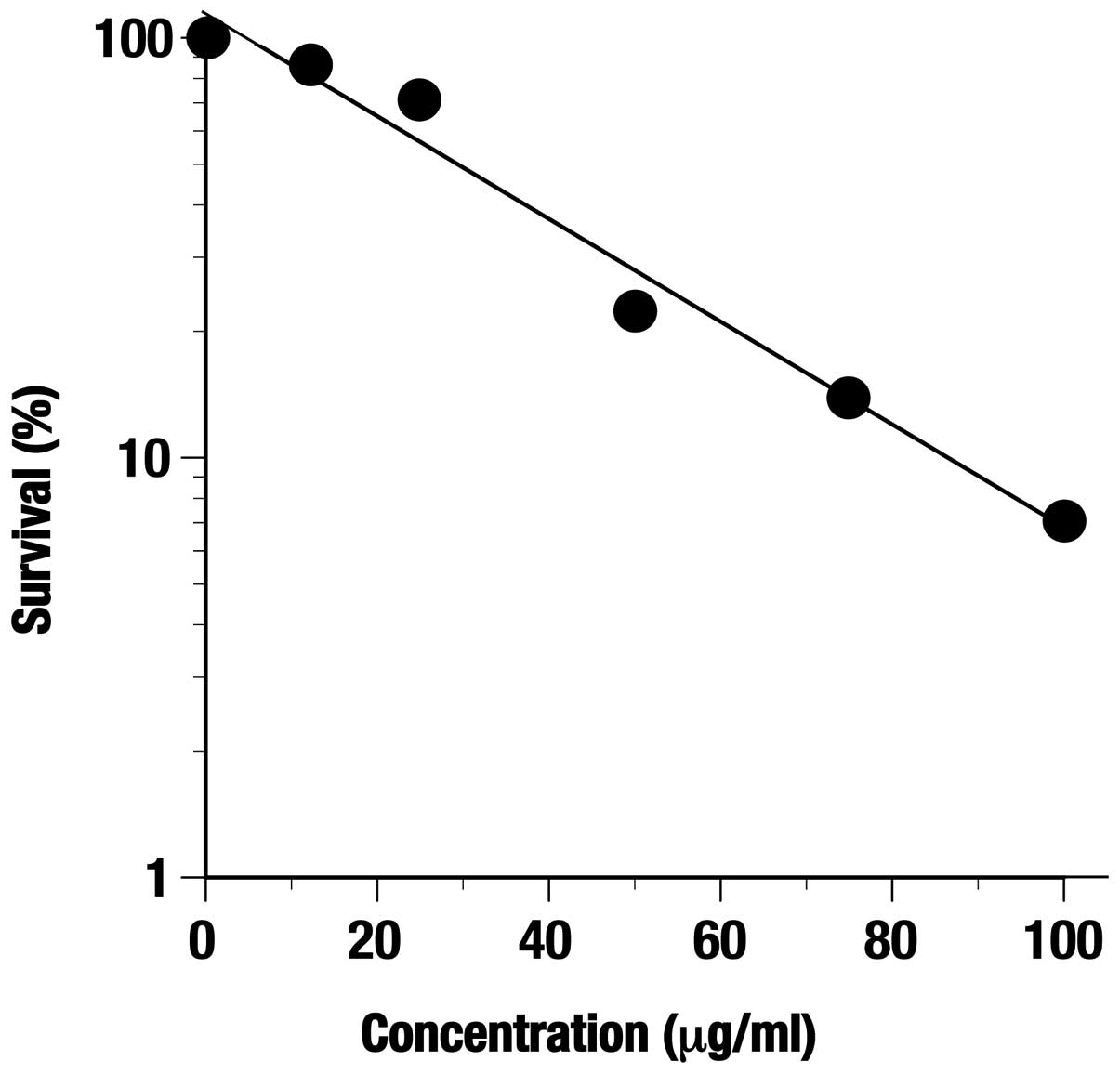
Inhibiting activity of the C. zedoaria
extract against proliferation was observed at 24 h in both TE-8 and
HET-1A cells in a dose-dependent manner (Fig. 1). The minimum concentration of the
extract giving a 50% inhibition of cell proliferation inhibition
(CPI50) in TE-8 cells and HET-1A was 200 and 362.5
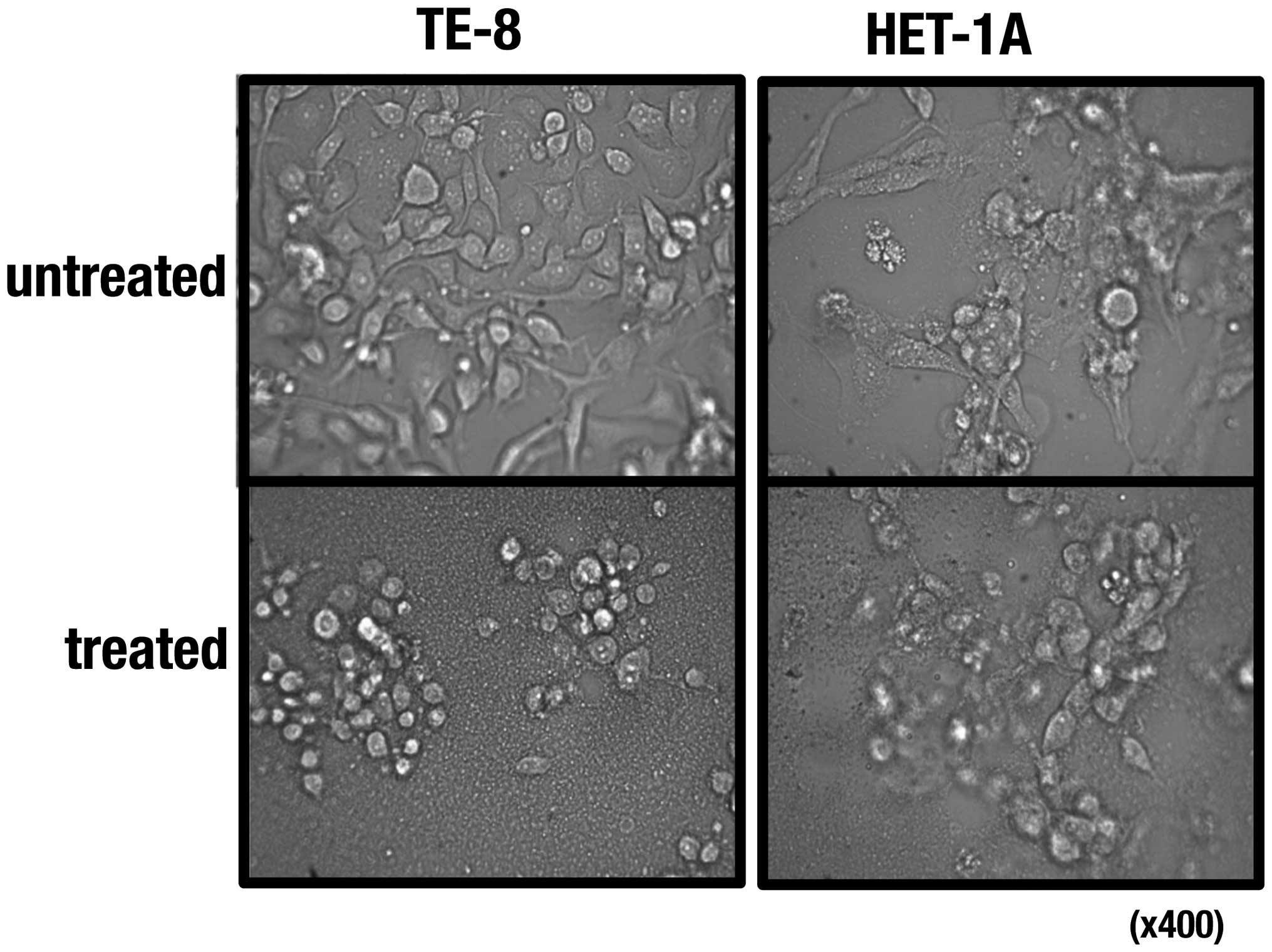
μg/ml, respectively. Morphological changes of the cells treated
with the extract were observed under a microscope (Fig. 2), and cell death seemed to occur
faster in TE-8 cells than in HET-1A cells (data not shown).
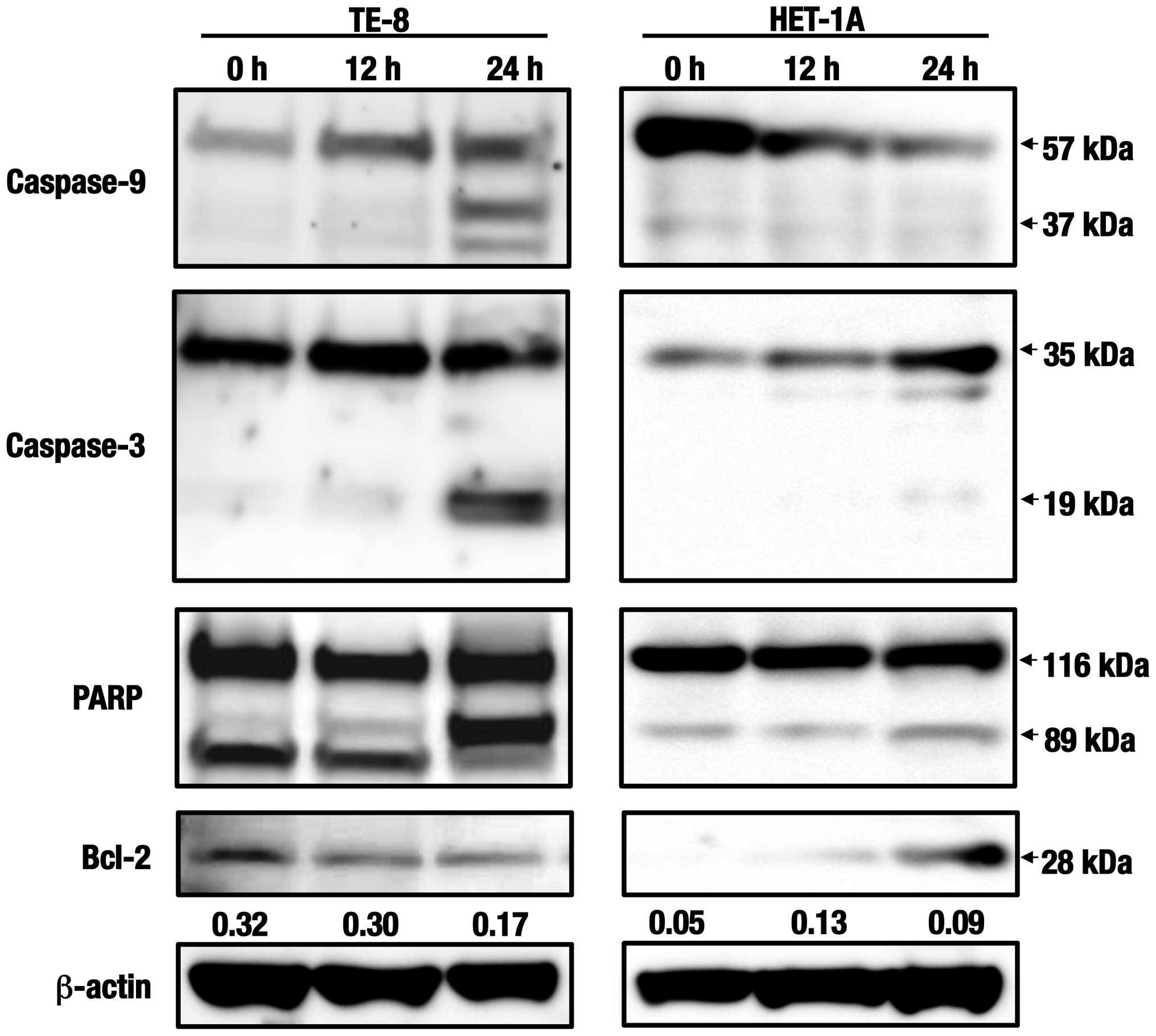
Western blot analyses of the caspase
cascade-related proteins
Since apoptotic cell death seemed to be induced in
esophageal cells that had been treated with the C. zedoaria
extract (Fig. 2), the expression
levels of the proteins related to apoptosis were analyzed in both
TE-8 and HET-1A cells (Fig. 3).
Expression levels of the initiator caspase, caspase-9 and the
effector caspase, caspase-3 were found to increase in TE-8 cells at
24 h indicating that active fragments with 37 kDa and 19 kDa,
increased, respectively by treatment with the extract. Furthermore,
the activation of poly(ADP-ribose) polymerase (PARP) seemed to be
induced simultaneously since increased levels of the active
fragment with 89 kDa were also determined in the same duration.
Reversely, the expression levels of one of the anti-apoptotic
molecules, Bcl-2, was shown to decrease in the treated TE-8 cells
in a time-dependent manner. No activated fragments were found in
caspase-9 or caspase-3 in HET-1A cells treated with the same
extract. Expression of PARP and Bcl-2 was slightly detected but no
significant change was observed in the cells.
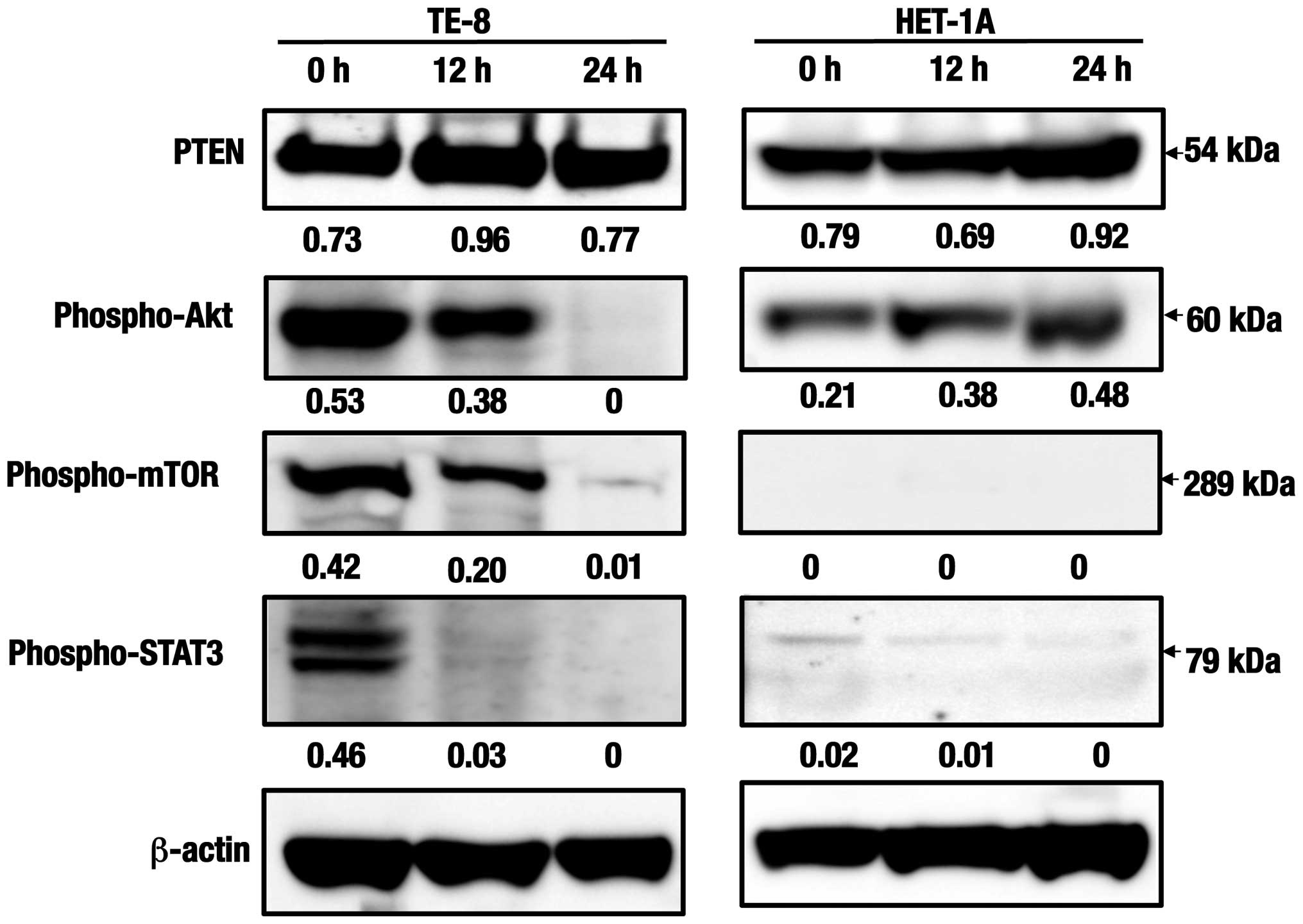
Western blot analyses of the cell
survival and proliferation-related proteins
Expression levels in the phosphatase and tensin
homolog deleted on chromosome ten (PTEN) were considerably detected
in TE-8 cells during 24 h and as maximum at 12 h after treatment
with the C. zedoaria extract (Fig. 4). While, expression levels of the
activated Akt, as measured by the serine 473 phosphorylation
(phospho-Akt), the activated mammalian target of rapamycin
(phospho-mTOR) were found to be suppressed as early as at 12 h, and
no clear expression was detected at 24 h. Further, expression of
the phosphorylated signal transducer and activator of transcription
3 (phospho-STAT3) seemed to be suppressed early. Even though
expression levels of PTEN and phospho-Akt seemed to increase in
HET-1A cells at 24 h with the same treatment, no significant
expression of phospho-mTOR or phospho-STAT3 was detected in the
same cells.
Effect of the C. zedoaria extract on
colony formation of TE-8 cells
Colony formation of TE-8 cells determined at 12 days
after incubation was shown clearly to be inhibited under the
presence of the C. zedoaria extract and survival rates of
colonies were found to decrease with the extract added in a
dose-dependent manner (Fig.
5).
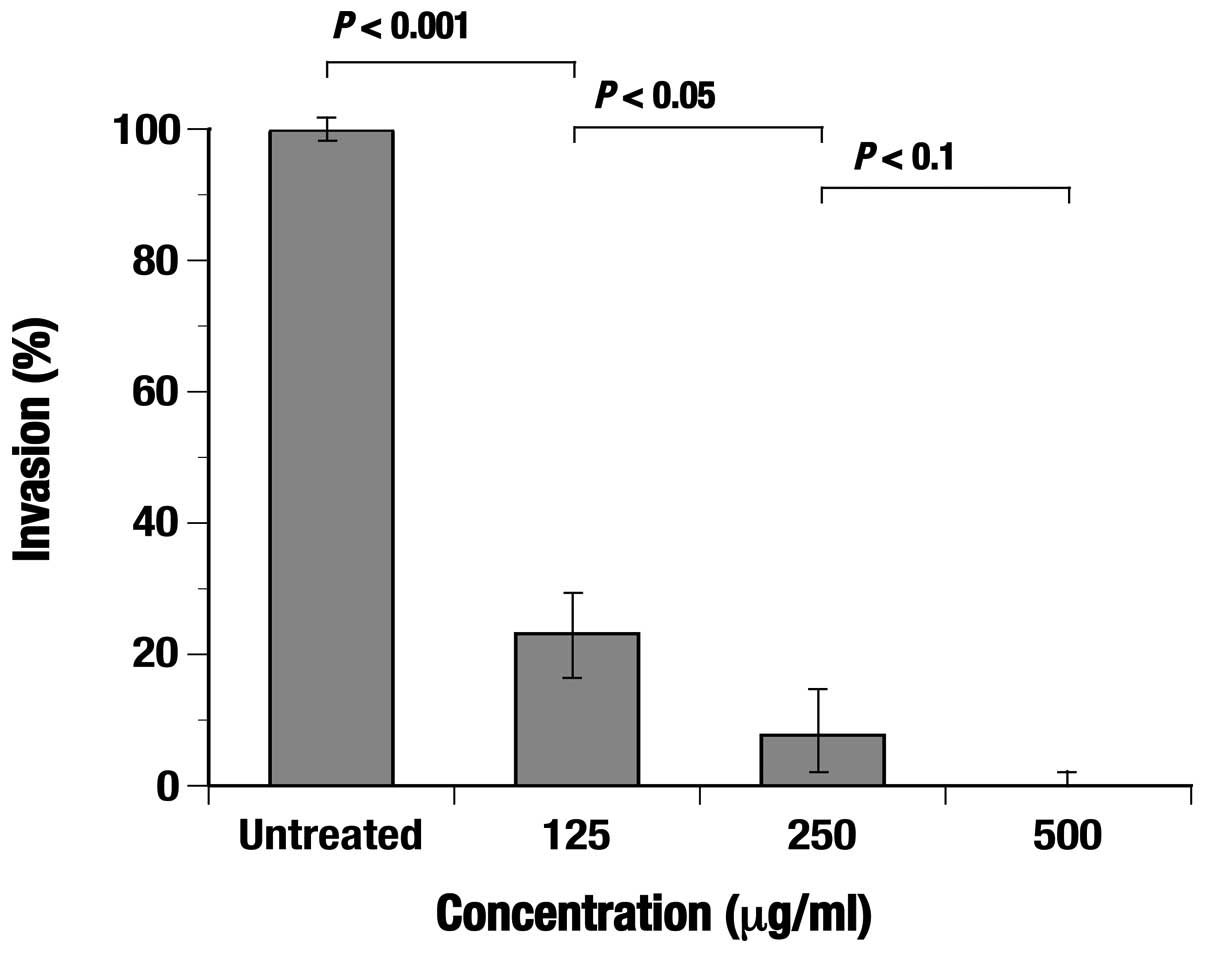
Effect of the C. zedoaria extract on
invasion capacity of TE-8 cells
The cell invasion was significantly (P<0.001)
inhibited under the presence of the extract and the invasion rate
was clearly shown to decrease depending on the concentration of the
extract added (Fig. 6).
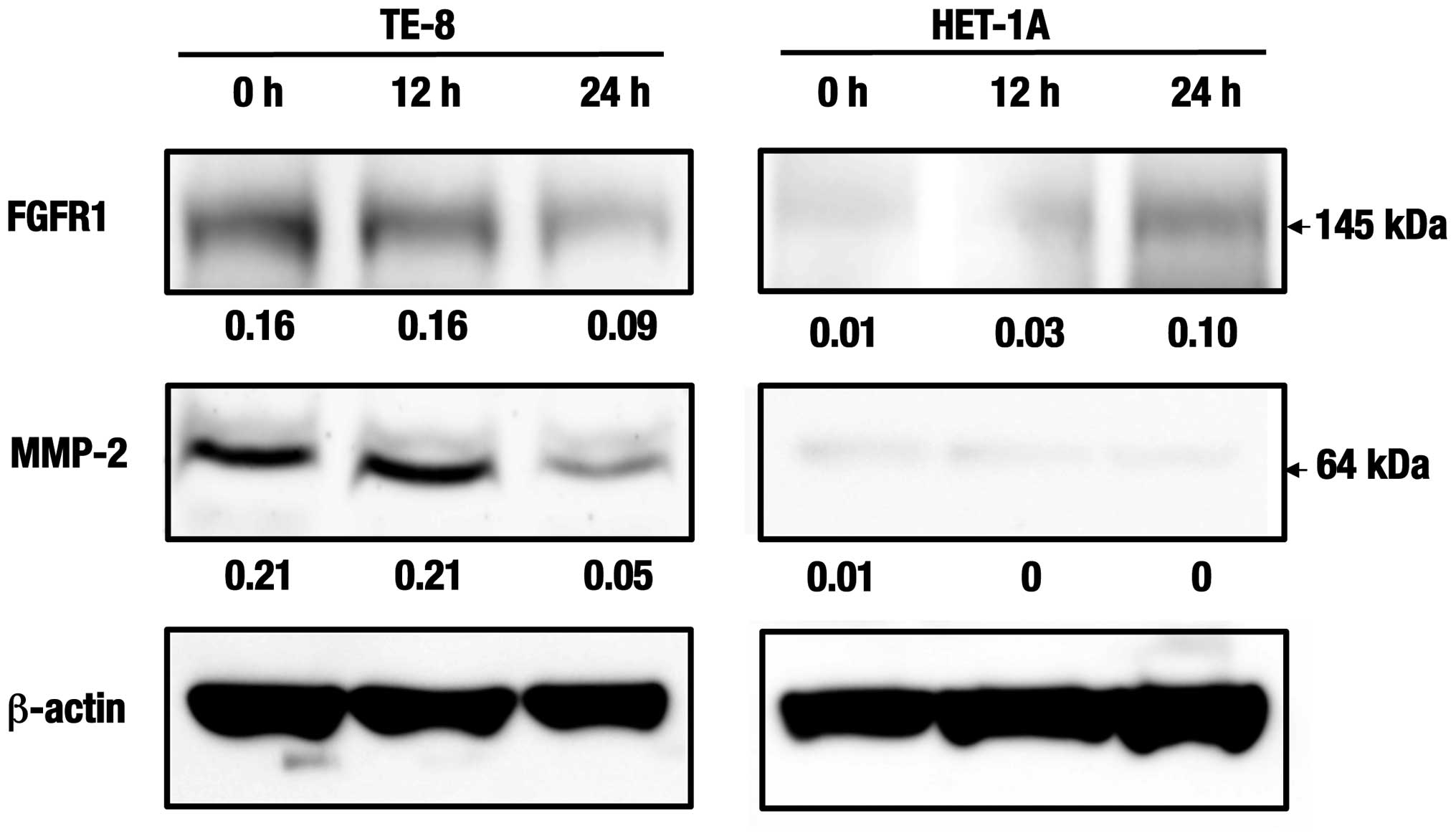
Western blot analyses of the cell
invasion and angiogenesis-related proteins
Expression levels of the fibroblast growth factor
receptor 1 (FGFR1) in TE-8 cells were suppressed at 24 h after
treatment with the C. zedoaria extract. Furthermore, those
of the matrix metalloproteinase-2 (MMP-2) were also suppressed at
24 h in the same cells (Fig. 7).
Whereas, no such expression or change was observed in HET-1A cells
at the same duration.
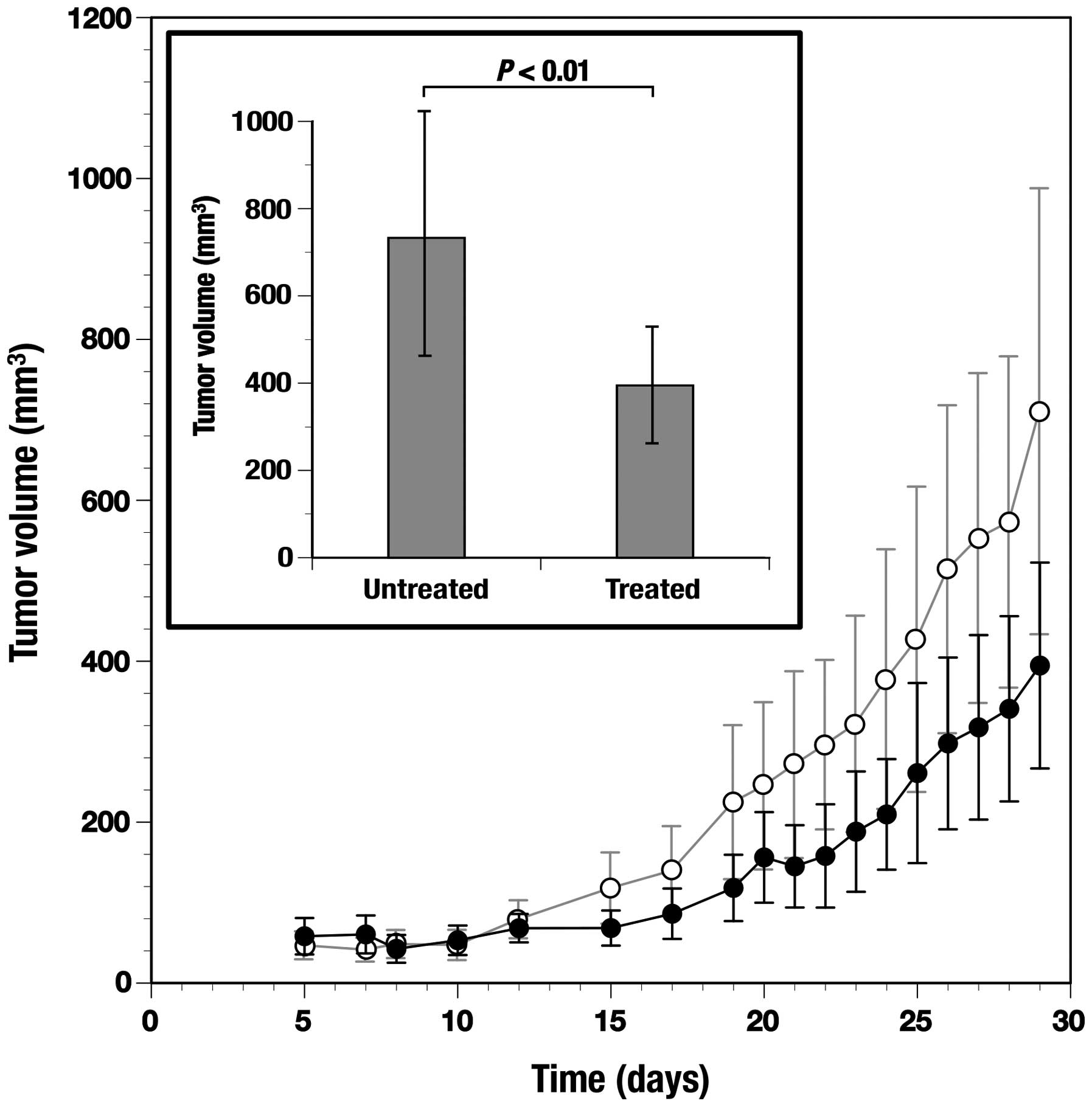
Antitumor effect of the C. zedoaria
extract in tumor bearing mice
After subcutaneous inoculation with TE-8 cells,
tumors were formed within 8 days in all the mice. Tumor formation
in treated and untreated mice was measured periodically up to 29
days (Fig. 8). Tumor formation
determined at day 29 was significantly (P<0.01) suppressed in
the mice treated through the oral administration of the C.
zedoaria extract when compared to control mice without such an
administration (Fig. 8,
inset).
Discussion
The active constituents isolated from the genus
Curcumin including C. longa and C. zedoaria have been
demonstrated to possess potent antitumor properties against various
human tumor cells. However, clinical applications have been limited
because of their poor aqueous solubility and bioavailability even
though a high dose administration and food intake has already been
accepted from a standpoint of safety (7,38).
Whereas, it has recently been developed for preparing new types of
formulations in order to improve their deliveries as anticancer
drugs (38,39). The C. zedoaria extract has
been prepared differently to date, and the methanol or ethanol
extract has been characterized to contain numbers of chemical
constituents including sesquiterpenes (26), curcuminoids (6) and ethyl p-methoxycinnamate
(40), which have also been
demonstrated to have strong cytotoxic activities against cancer
cells (13,14,41,42).
While, fractions prepared previously from C. zedoaria were
reported to possess anti-proliferation activity to varying degree
(16,43), which were likely to be caused by
the method used in their preparation. The cell proliferation
inhibition (CPI) assay against TE-8 and HET-1A cells using various
concentrations of the C. zedoaria extract indicated strong
cytotoxic activities to both esophageal cell types in a
dose-dependent manner (Fig. 1).
However, when minimum concentrations of the extract at
CPI50 were compared, the level in TE-8 cells were
1.8-fold lower than the HET-1A cells suggesting that cancer cells
seemed to be more susceptible to the extract resulting in induction
of apoptosis in TE-8 cells within a short period after treatment
with the C. zedoaria extract. In fact, morphological changes
of TE-8 cells (Fig. 2) were
observed within 5 h after treatment with the extract (data not
shown).
Western blot analyses in this study clearly
suggested that the induction of apoptosis in TE-8 cells treated
with the C. zedoaria extract occurred through the caspase
cascade-dependent pathways, which involved activation of caspase-9,
caspase-3 and PARP along with suppression of Bcl-2 during 24 h, and
resulted in the induction of cell death (Fig. 3). Involvement of caspase-3
activation in the induction of apoptotic cell death has also been
demonstrated recently in different cancer cells treated with C.
zedoaria (43). Interestingly,
such an induction of the caspase cascade causing apoptotic cell
death seemed to scarcely occur in non-cancerous HET-1A cells.
Delayed and poorly increased levels of caspase cascade pathways
causing apoptotic cell death were also found to occur in
non-cancerous esophageal (CHEK-1) cells when compared to esophageal
cancer (TE-2 and TE-13) cells treated with chemically synthesized
compounds in our previous study (29).
Mechanism of cell death has been accepted to be
governed not only by upregulation of the proapoptotic pathway or
downregulation of the anti-apoptotic pathway but also by modulation
of the survival signaling pathways (44). In the cell survival signaling
pathways, PTEN is one of the most common tumor suppressors and
negative regulators of downstream effectors (45,46).
Expression levels of PTEN were demonstrated to be elevated as early
as 12 h after treatment with C. zedoaria extract in TE-8
cells and suppression of Akt activation, which determined by
expression levels of phosphorylated Akt (phospho-Akt) on Ser473,
occurred at the same duration (Fig.
4). Accordingly, treatment of TE-8 cells with the C.
zedoaria extract induced PTEN activation accompanying by Akt
suppression within a short duration (<12 h).
As demonstrated previously, elevated levels of
phosphorylation occurred in Akt, mTOR, and their downstream
molecules to change p-mTOR and corresponding P-molecules were
detected in malignant tumor cells (47). The Akt/mTOR signaling pathway
comprising mTOR and the downstream Akt must play a crucial role in
the survival of their cells. It was clearly determined that
expression of p-mTOR was also suppressed within 12 h after
treatment with the C. zedoaria extract. Previously, it was
shown that the anti-proliferation effect of curcumin was mediated
by induction of suppressed levels of phosphorylation of mTOR but at
the same time suppression of expression levels of phosphorylation
of Akt on its Ser473 at a longer duration of the treatment (72 h)
(47). Further, overexpression of
PTEN has been demonstrated to induce apoptosis through
Akt-dependent and -independent pathways in breast cancer cells
(48). It still need confirmation,
but the presence of multiple feedback loops has been described
previously in the regulation of Akt/mTOR signaling pathway involved
in the phosphatase-dependent effects of curcumin against prostate
cancer cells (49). It must
therefore be plausible that the C. zedoaria extract mediated
upregulation of anti-proliferation signaling in TE-8 cells first
accompanied by apoptotic cell death via upregulation of caspase
cascade signaling. While in HET-1A cells, expressions of PTEN and
p-Akt were observed with a slight increase, but no significant
expression was found in p-mTOR or p-STAT3 during 24 h. It must
indicate that no clear change of the phosphatase- and caspase
cascade-dependent signaling pathways assumed in TE-8 cells occurred
in HET-1A cells after treatment with C. zedoaria
extract.
Cleavage of PTEN through phosphorylation was found
to occur by caspase-3 indicating that PTEN was also targeted by
caspase-3 due to a potential regulatory mechanism under some
physiological conditions (50).
Since caspase-9 is a one of downstream substrates of Akt and
phosphorylated Akt targets a number of downstream substrates
including caspases, suppressed expressions of p-Akt may induce the
inhibition of phosphorylation of caspase(s) resulting in apoptotic
cell death.
Effects of curcumin were investigated previously on
inhibition of STAT3 phosphorylation in human multiple myeloma cells
and it was demonstrated that inhibition of STAT3 phosphorylation
induced by curcumin was reversible and occurred rapidly (51). In this study, downregulation of
STAT3 occurred as early as 12 h after treatment with the extract in
TE-8 cells (Fig. 4). Further
investigation to determine the precise mechanisms and other
signaling pathways involved in the cell death is required to be
conducted; this should comprise a series of complex signaling
pathways, some of which should cross-talk each other in cancer
cells possessing large numbers of dysregulated proteins.
In receptor tyrosine kinases, the fibroblast growth
factor receptor (FGFR) consists of four highly conserved receptor
tyrosine kinases (52). It was
suggested previously that FGFR could also be a target for
anticancer agent development, and that FGFR inhibitors have
potential for therapeutic application as anticancer agents
(53,54). In TE-8 cells, it was found that
expression of FGFR1 reduced at 24 h after treatment with the C.
zedoaria extract (Fig. 7), but
the predicted effects of inhibition effects on the downstream
signaling pathways such as the Akt signaling pathway occurred as
early as 12 h.
Angiogenesis has been demonstrated to be essential
for solid tumor growth because tumor progression and metastasis
depend on the existence of a functional blood supply system as
reported previously (55). The
MMP-2, one of the matrix metalloproteinases, involved in an
extracellular matrix degrader has an important role in the
endothelial cell migration, organization and angiogenesis (56). The active form of MMP-2 (62 kDa)
might facilitate penetration of cancer cells through the blood
vessel walls allowing them to metastasize to other tissues or
organs (57). Since extremely
reduced expression of MMP-2 preferentially occurred in TE-8 cells
treated with the C. zedoaria extract at 24 h, it was
suggested that the extract has potentially diverse effects on
anti-proliferation, survival, angiogenesis, migration and
organization. In fact, some of these activities in the extract were
also shown clearly through the colony assay (Fig. 5) and the cell invasion assay
(Fig. 6) indicating that the
extract possessed significant inhibiting activity against colony
formation and cell migration of TE-8 cells. Finally, tumor
formation with esophageal TE-8 cancer cells was significantly
suppressed in the mice orally administered with the C.
zedoaria extract (Fig. 8).
Taken together, therefore, the C. zedoaria extract could be
promising as an antitumor agent against esophageal cancer.
Presence of constituents involving antitumor
activities has been reported in the same ethanol extract from C.
zedoaria as described above. Identification of the active
constituents in the C. zedoaria extract is now in progress
along with determination of the best route for administration of
the extract with appropriate dose and duration in order to apply
this natural product into a novel therapy against esophageal
cancer.
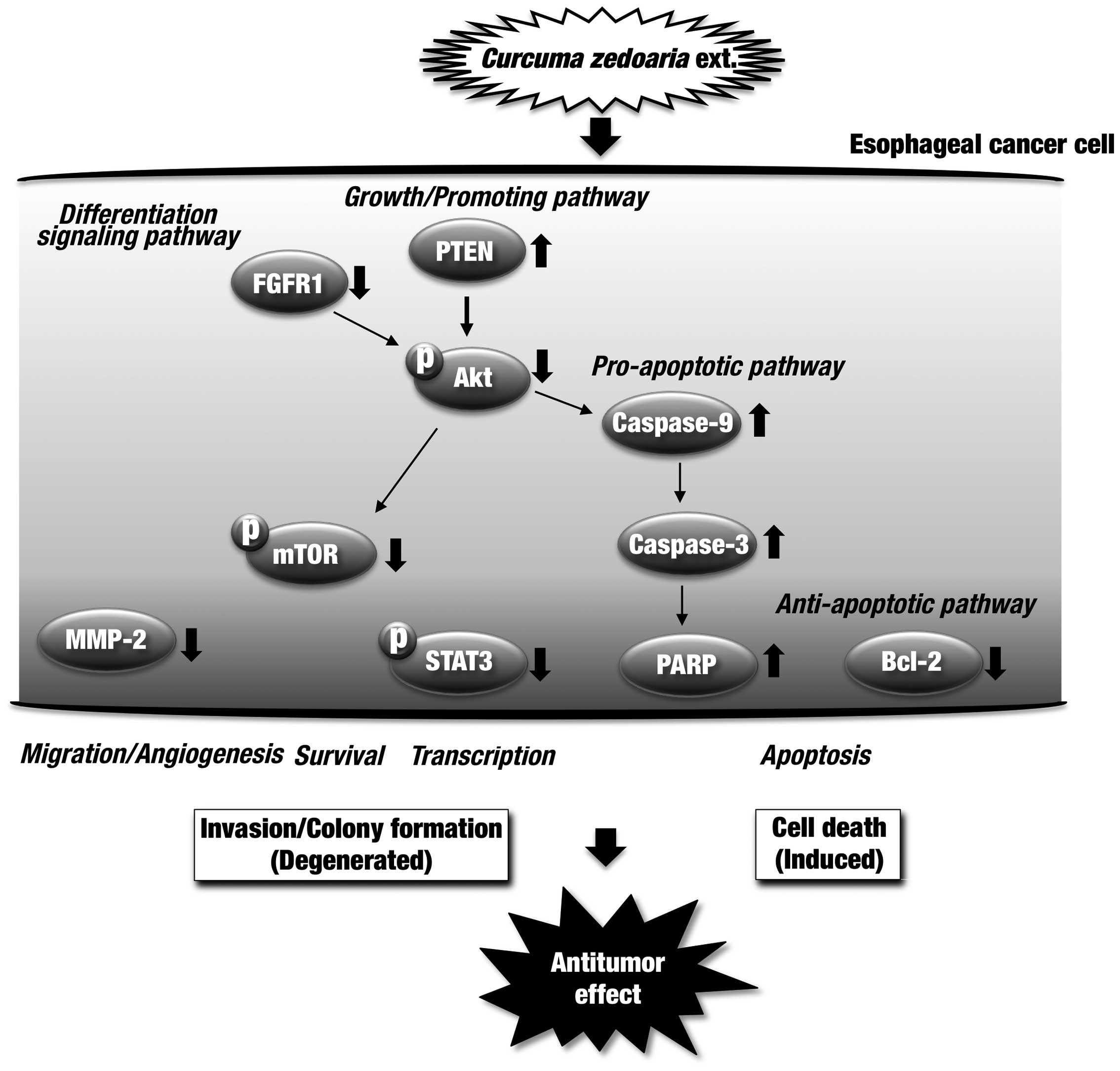
In conclusion, treatment of esophageal cancer cells
with the C. zedoaria extract was shown to have antitumor
action in in vitro and in vivo experiments. In
addition, tumor formation was significantly suppressed in the
xenograft mouse model of human esophageal cancer through the oral
administration of the extract. Overall results from the effects of
the C. zedoaria extract are summarized in Fig. 9. The C. zedoaria extract
possesses useful constituents to modulate multi-targets against
dysregulated proteins in esophageal tumor cells, and shows promise
as an agent against esophageal cancer.
Acknowledgements
This study was supported in part by the following
grants: Grants-in-Aid for Scientific Research from the Japan
Society for the Promotion of Science (JSPS nos. 22591450 and
23591857).
Abbreviations:
|
CPI
|
cell proliferation inhibition
|
|
PARP
|
poly(ADP-ribose) polymerase
|
|
PTEN
|
phosphatase and tensin homolog deleted
on chromosome ten
|
|
mTOR
|
mammalian target of rapamycin
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
FGFR1
|
fibroblast growth factor receptor
1
|
|
MMP-2
|
matrix metalloproteinase-2
|
References
|
1
|
Mishra BB and Tiwari VK: Natural products:
An evolving role in future drug discovery. Eur J Med Chem.
46:4769–4807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuttan R, Bhanumathy P, Nirmala K and
George MC: Potential anticancer activity of turmeric (Curcuma
longa). Cancer Lett. 29:197–202. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Negi PS, Jayaprakasha GK, Jagan Mohan Rao
L and Sakariah KK: Antibacterial activity of turmeric oil: A
byproduct from curcumin manufacture. J Agric Food Chem.
47:4297–4300. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kunnumakkara AB, Anand P and Aggarwal BB:
Curcumin inhibits proliferation, invasion, angiogenesis and
metastasis of different cancers through interaction with multiple
cell signaling proteins. Cancer Lett. 269:199–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hasima N and Aggarwal BB: Cancer-linked
targets modulated by curcumin. Int J Biochem Mol Biol. 3:328–351.
2012.
|
|
6
|
Kuroyanagi M and Natori S: Some
observation on curcuminoids from Zingiberaceae plants. Yakugaku
Zasshi. 90:1467–1470. 1970.(In Japanese). PubMed/NCBI
|
|
7
|
Sharma RA, Gescher AJ and Steward WP:
Curcumin: The story so far. Eur J Cancer. 41:1955–1968. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuo ML, Huang TS and Lin JK: Curcumin, an
antioxidant and anti-tumor promoter, induces apoptosis in human
leukemia cells. Biochim Biophys Acta. 1317:95–100. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mehta K, Pantazis P, McQueen T and
Aggarwal BB: Anti-proliferative effect of curcumin
(diferuloylmethane) against human breast tumor cell lines.
Anticancer Drugs. 8:470–481. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen H, Zhang ZS, Zhang YL and Zhou DY:
Curcumin inhibits cell proliferation by interfering with the cell
cycle and inducing apoptosis in colon carcinoma cells. Anticancer
Res. 19:3675–3680. 1999.
|
|
11
|
Motterlini R, Foresti R, Bassi R and Green
CJ: Curcumin, an anti-oxidant and anti-inflammatory agent, induces
heme oxygenase-1 and protects endothelial cells against oxidative
stress. Free Radic Biol Med. 28:1303–1312. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kunnumakkara AB, Guha S, Krishnan S,
Diagaradjane P, Gelovani J and Aggarwal BB: Curcumin potentiates
antitumor activity of gemcitabine in an orthotopic model of
pancreatic cancer through suppression of proliferation,
angiogenesis, and inhibition of nuclear factor-kappaB-regulated
gene products. Cancer Res. 67:3853–3861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen CC, Chen Y, Hsi YT, Chang CS, Huang
LF, Ho CT, Way TD and Kao JY: Chemical constituents and anticancer
activity of Curcuma zedoaria roscoe essential oil against non-small
cell lung carcinoma cells in vitro and in vivo. J Agric Food Chem.
61:11418–11427. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Syu WJ, Shen CC, Don MJ, Ou JC, Lee GH and
Sun CM: Cytotoxicity of curcuminoids and some novel compounds from
Curcuma zedoaria. J Nat Prod. 61:1531–1534. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen X, Pei L, Zhong Z, Guo J, Zhang Q and
Wang Y: Anti-tumor potential of ethanol extract of Curcuma
phaeocaulis Valeton against breast cancer cells. Phytomedicine.
18:1238–1243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Roy SS, Nebie RHC, Zhang Y and Nair
MG: Functional food quality of Curcuma caesia, Curcuma zedoaria and
Curcuma aeruginosa endemic to Northeastern India. Plant Foods Hum
Nutr. 68:72–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanai M, Otsuka Y, Otsuka K, Sato M,
Nishimura T, Mori Y, Kawaguchi M, Hatano E, Kodama Y, Matsumoto S,
et al: A phase I study investigating the safety and
pharmacokinetics of ®) in cancer patients. Cancer
Chemother Pharmacol. 71:1521–1530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Belcaro G, Hosoi M, Pellegrini L,
Appendino G, Ippolito E, Ricci A, Ledda A, Dugall M, Cesarone MR,
Maione C, et al: A controlled study of a lecithinized delivery
system of curcumin (Meriva®) to alleviate the adverse
effects of cancer treatment. Phytother Res. 28:444–450. 2014.
View Article : Google Scholar
|
|
19
|
Lee H and Lin JY: Antimutagenic activity
of extracts from anti-cancer drugs in Chinese medicine. Mutat Res.
204:229–234. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng CH, Chiu WT, Juan CW, Mau JL, Chen
CC, Peng CC, Lai EY and Chyau CC: Pivotal role of curcuminoids on
the anti-mutagenic activity of Curcuma zedoaria extracts. Drug Chem
Toxicol. 33:64–76. 2010. View Article : Google Scholar
|
|
21
|
Kim KI, Kim JW, Hong BS, Shin DH, Cho HY,
Kim HK and Yang HC: Antitumor, genotoxicity and anticlastogenic
activities of polysaccharide from Curcuma zedoaria. Mol Cells.
10:392–398. 2000.PubMed/NCBI
|
|
22
|
Seo WG, Hwang JC, Kang SK, Jin UH, Suh SJ,
Moon SK and Kim CH: Suppressive effect of Zedoariae rhizoma on
pulmonary metastasis of B16 melanoma cells. J Ethnopharmacol.
101:249–257. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mau J, Lai EYC, Wang N, Chen C, Chang C
and Chyau C: Composition and antioxidant activity of the essential
oil from Curcuma zedoaria. Food Chem. 82:583–591. 2003. View Article : Google Scholar
|
|
24
|
Lai EY, Chyau CC, Mau JL, Chen CC, Lai YJ,
Shih CF and Lin LL: Antimicrobial activity and cytotoxicity of the
essential oil of Curcuma zedoaria. Am J Chin Med. 32:281–290. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wilson B, Abraham G, Manju VS, Mathew M,
Vimala B, Sundaresan S and Nambisan B: Antimicrobial activity of
Curcuma zedoaria and Curcuma malabarica tubers. J Ethnopharmacol.
99:147–151. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Makabe H, Maru N, Kuwabara A, Kamo T and
Hirota M: Anti-inflammatory sesquiterpenes from Curcuma zedoaria.
Nat Prod Res. 20:680–685. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen W, Lu Y, Gao M, Wu J, Wang A and Shi
R: Anti-angiogenesis effect of essential oil from Curcuma zedoaria
in vitro and in vivo. J Ethnopharmacol. 133:220–226. 2011.
View Article : Google Scholar
|
|
28
|
Zhou L, Zhang K, Li J, Cui X, Wang A,
Huang S, Zheng S, Lu Y and Chen W: Inhibition of vascular
endothelial growth factor-mediated angiogenesis involved in
reproductive toxicity induced by sesquiterpenoids of Curcuma
zedoaria in rats. Reprod Toxicol. 37:62–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Faried A, Faried LS, Nakagawa T, Yamauchi
T, Kitani M, Sasabe H, Nishimura T, Usman N, Kato H, Asao T, et al:
Chemically synthesized sugar-cholestanols possess a preferential
anticancer activity involving promising therapeutic potential
against human esophageal cancer. Cancer Sci. 98:1358–1367. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hashimoto S, Yazawa S, Asao T, Faried A,
Nishimura T, Tsuboi K, Nakagawa T, Yamauchi T, Koyama N, Umehara K,
et al: Novel sugar-cholestanols as anticancer agents against
peritoneal dissemination of tumor cells. Glycoconj J. 25:531–544.
2008. View Article : Google Scholar
|
|
31
|
Faried A, Arifin MZ, Ishiuchi S, Kuwano H
and Yazawa S: Enhanced expression of proapoptotic and autophagic
proteins involved in the cell death of glioblastoma induced by
synthetic glycans. J Neurosurg. 120:1298–1308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Napier KJ, Scheerer M and Misra S:
Esophageal cancer: A Review of epidemiology, pathogenesis, staging
workup and treatment modalities. World J Gastrointest Oncol.
6:112–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nishihira T, Hashimoto Y, Katayama M, Mori
S and Kuroki T: Molecular and cellular features of esophageal
cancer cells. J Cancer Res Clin Oncol. 119:441–449. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stoner GD, Kaighn ME, Reddel RR, Resau JH,
Bowman D, Naito Z, Matsukura N, You M, Galati AJ and Harris CC:
Establishment and characterization of SV40 T-antigen immortalized
human esophageal epithelial cells. Cancer Res. 51:365–371.
1991.PubMed/NCBI
|
|
35
|
Franken NAP, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar
|
|
36
|
Pretlow TG, Delmoro CM, Dilley GG,
Spadafora CG and Pretlow TP: Transplantation of human prostatic
carcinoma into nude mice in Matrigel. Cancer Res. 51:3814–3817.
1991.PubMed/NCBI
|
|
37
|
Gorelik B, Ziv I, Shohat R, Wick M,
Hankins WD, Sidransky D and Agur Z: Efficacy of weekly docetaxel
and bevacizumab in mesenchymal chondrosarcoma: A new theranostic
method combining xenografted biopsies with a mathematical model.
Cancer Res. 68:9033–9040. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mach CM, Mathew L, Mosley SA, Kurzrock R
and Smith JA: Determination of minimum effective dose and optimal
dosing schedule for liposomal curcumin in a xenograft human
pancreatic cancer model. Anticancer Res. 29:1895–1899.
2009.PubMed/NCBI
|
|
39
|
Kim TH, Jiang HH, Youn YS, Park CW, Tak
KK, Lee S, Kim H, Jon S, Chen X and Lee KC: Preparation and
characterization of water-soluble albumin-bound curcumin
nanoparticles with improved antitumor activity. Int J Pharm.
403:285–291. 2011. View Article : Google Scholar
|
|
40
|
Gupta SK, Banerjee AB and Achari B:
Isolation of Ethyl p-methoxycinnamate, the major antifungal
principle of Curcuma zedoaria. Lloydia. 39:218–222. 1976.PubMed/NCBI
|
|
41
|
Carvalho FR, Vassao RC, Nicoletti MA and
Maria DA: Effect of Curcuma zedoaria crude extract against tumor
progression and immunomodulation. J Venom Anim Toxins Incl Trop
Dis. 16:324–341. 2010. View Article : Google Scholar
|
|
42
|
Shin Y and Lee Y: Cytotoxic activity from
Curcuma zedoaria through mitochondrial activation on ovarian cancer
cells. Toxicol Res. 29:257–261. 2013. View Article : Google Scholar
|
|
43
|
Syed Abdul Rahman SN, Abdul Wahab N and
Abd Malek SN: In vitro morphological assessment of apoptosis
induced by anti-proliferative constituents from the rhizomes of
Curcuma zedoaria. Evid Based Complement Alternat Med.
2013:2571082013. View Article : Google Scholar
|
|
44
|
Fraser M, Leung B, Jahani-Asl A, Yan X,
Thompson WE and Tsang BK: Chemoresistance in human ovarian cancer:
The role of apoptotic regulators. Reprod Biol Endocrinol. 1:662003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Blanco-Aparicio C, Renner O, Leal JF and
Carnero A: PTEN, more than the AKT pathway. Carcinogenesis.
28:1379–1386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Georgescu MM: PTEN tumor suppressor
network in PI3K-Akt pathway control. Genes Cancer. 1:1170–1177.
2010. View Article : Google Scholar
|
|
47
|
Johnson SM, Gulhati P, Arrieta I, Wang X,
Uchida T, Gao T and Evers BM: Curcumin inhibits proliferation of
colorectal carcinoma by modulating Akt/mTOR signaling. Anticancer
Res. 29:3185–3190. 2009.PubMed/NCBI
|
|
48
|
Weng L, Brown J and Eng C: PTEN induces
apoptosis and cell cycle arrest through
phosphoinositol-3-kinase/Akt-dependent and -independent pathways.
Hum Mol Genet. 10:237–242. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yu S, Shen G, Khor TO, Kim JH and Kong AN:
Curcumin inhibits Akt/mammalian target of rapamycin signaling
through protein phosphatase-dependent mechanism. Mol Cancer Ther.
7:2609–2620. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Torres J, Rodriguez J, Myers MP, Valiente
M, Graves JD, Tonks NK and Pulido R: Phosphorylation-regulated
cleavage of the tumor suppressor PTEN by caspase-3: Implications
for the control of protein stability and PTEN-protein interactions.
J Biol Chem. 278:30652–30660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bharti AC, Donato N and Aggarwal BB:
Curcumin (diferuloylmethane) inhibits constitutive and
IL-6-inducible STAT3 phosphorylation in human multiple myeloma
cells. J Immunol. 171:3863–3871. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liang G, Liu Z, Wu J, Cai Y and Li X:
Anticancer molecules targeting fibroblast growth factor receptors.
Trends Pharmacol Sci. 33:531–541. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Katoh M and Nakagama H: FGF receptors:
Cancer biology and therapeutics. Med Res Rev. 34:280–300. 2014.
View Article : Google Scholar
|
|
54
|
Hu Y, Lu H and Zhang J, Chen J, Chai Z and
Zhang J: Essential role of AKT in tumor cells addicted to FGFR.
Anticancer Drugs. 25:183–188. 2014. View Article : Google Scholar
|
|
55
|
Jubb AM, Oates AJ, Holden S and Koeppen H:
Predicting benefit from anti-angiogenic agents in malignancy. Nat
Rev Cancer. 6:626–635. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li A, Varney ML, Valasek J, Godfrey M,
Dave BJ and Singh RK: Autocrine role of interleukin-8 in induction
of endothelial cell proliferation, survival, migration and MMP-2
production and angiogenesis. Angiogenesis. 8:63–71. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Peng JM, Chen YH, Hung SW, Chiu CF, Ho MY,
Lee YJ, Lai TC, Hsiao M, Liang CM and Liang SM: Recombinant viral
protein promotes apoptosis and suppresses invasion of ovarian
adeno-carcinoma cells by targeting α5β1 integrin to down-regulate
Akt and MMP-2. Br J Pharmacol. 165:479–493. 2012. View Article : Google Scholar :
|