Introduction
Osteosarcoma is the most common form of primary bone
malignancy, which occurs predominantly in infants and adolescents
(1). It represents the prevalent
cause of cancer-related death for children with an incidence of 4–5
cases/108. Patients with osteosarcoma are routinely
treated by combining surgery and high-dose chemotherapy and this
has significantly improved the 5-year survival rate over time.
Nevertheless, osteosarcoma shows high propensity to metastasize and
invade surrounding tissue, the lung being the most common site of
initial metastatic disease or, less frequently, the other bones
(2). Therefore, it is important to
identify novel therapeutic strategies which can improve the general
conditions and the overall survival rate of patients with
osteosarcoma.
SPARC (secreted protein acidic and rich in cysteine)
also called Osteonectin or BM-40, is a non-structural matricellular
glycoprotein expressed in a variety of mammalian tissues (3,4). The
effects of SPARC on cell behaviour are highly tissue specific and
concern modification of cell shape, migration, proliferation,
differentiation and survival (5–9).
SPARC is also known to modulate cell-cell and cell-matrix
interactions, and to influence de-adhesive and cell growth
regulatory properties (10).
Moreover, its expression is related to the ability to regulate
processes such as bone formation, fibrosis and tissue repair
(11). SPARC is differentially
expressed in various tumors including breast and colorectal
cancers, melanoma or glioma (12–17).
Many studies show that in cancer cells SPARC modulates
proliferation, apoptosis, invasion and angiogenesis. Its
overexpression can promote tumor vascularization by interacting
with cytokines, such as VEGF and PDGF and stimulating the secretion
of metalloproteases (18). This
could lead to the conclusion that SPARC is a tumor promoter protein
with pro-invasive activity. However, the role of SPARC in
tumorigenesis is more complex and seems to be cell-type specific
owing to its diverse functions in a given micro-environment
(10). Indeed, in other tumors,
such as ovarian and gastric cancer cells, SPARC is significantly
downregulated and its restoring mediates the inhibition of cell
proliferation (19,20).
Recently, we have demonstrated that osteosarcoma
MG63 cells are sensitive to the synthetic cannabinoid WIN55,212-2,
an agonist of cannabinoid receptors (21). In these cells WIN is able to
increase the level of the main markers of endoplasmic reticulum
(ER) stress and mediate the overexpression of tumor suppressor
factor PAR-4. The consequence of these effects is the sensitization
to the action of the cytokine TRAIL and the induction of apoptotic
cell death. In the present study, we aimed to elucidate the role of
SPARC in the effects induced by WIN in osteosarcoma MG63 cells.
Materials and methods
Cell cultures
Human osteosarcoma MG63 cells were acquired from
Interlab Cell Line Collection (ICLC; Genova, Italy). Cells were
cultured at 37°C in Dulbecco's modified Eagle's medium (DMEM),
supplemented with 10% (v/v) heat-inactivated fetal bovine serum
(FBS), 2.0 mM L-glutamine, and antibiotic antimycotic solution (100
U/ml penicillin, 100 μg/ml streptomycin and 250 ng/ml amphotericin
B; Sigma) in a humidified atmosphere containing 5% CO2.
For the experiments, cells were seeded at 60–70% confluence. After
overnight incubation, culture medium was replaced with fresh DMEM
containing low percentage of FBS (2%) and cells were treated with
the cannabinoid WIN55,212-2 (WIN) (Sigma Aldrich S.R.L., Milan,
Italy). Control cells were cultured in the presence of vehicle
alone (DMSO).
Cell viability assay
Cell viability was determined by
3-[4,5-dimethylthiazolyl-2]2,5-diphenyl-tetrazolium bromide (MTT;
Sigma Aldrich S.R.L.) assay as previously reported (22).
Western blot analysis
Protein extracts were prepared by washing the cells
in phosphate-buffer saline (PBS) and incubating for 20 min in
ice-cold lysis buffer supplemented with protease inhibitor
cocktail, as previously reported (23). After sonication three times for 10
sec, proteins were quantified by Bradford method and equal amounts
(40 μg) were separated by SDS-PAGE and then electrotransferred to a
nitrocellulose membrane for the detection with specific antibodies.
The blots were developed using the alkaline phosphatase
colorimetric or enhanced chemiluminescence (ECL) labeling systems.
Optical densities of the bands were analyzed with Quantity One
Imaging software from Bio-Rad Laboratories. The correct protein
loading was verified by means of both red Ponceau staining and
immunoblotting for actin. The results shown in the figures are
representative of four independent experiments with similar
results.
Semi-quantitative RT-PCR analysis for
SPARC
RNA was isolated using RNeasy mini kit (Qiagen,
Milan, Italy). cDNA was amplified from 1 μg of RNA by using
QuantiTect reverse transcription kit (Qiagen) followed by
polymerase chain reaction (PCR). The reactions omitting reverse
transcriptase enzyme served as negative control. GAPDH was used as
a housekeeping gene to demonstrate equal loading of RNA.
The amplified products were resolved by agarose gel
electrophoresis (1% agarose, 0.5 μg/ml ethidium bromide;
Sigma-Aldrich), and the bands were visualized and photographed with
ChemiDoc XRS (Bio-Rad Laboratories Srl, Milan, Italy). The primer
sequences (Proligo USA, Milan, Italy) are as follows: SPARC,
forward 5′-TGATGATGGTGC AGAGGAAA-3′ and reverse
5′-GGGGGATGTATTTGCAA GG-3′; GAPDH, forward 5′-TGACATCAAGAAGGTGA-3′
and reverse 5′-TCCACCACCCTGTTGCTGTA-3′. For PCR analysis, the
following protocol was performed: initial denaturation at 94°C for
2 min; denaturation at 94°C for 15 sec, annealing at 60°C for 30
sec and extension at 72°C for 30 sec for 35 cycles and a final
extension at 72°C for 10 min.
Gene silencing using siRNAs
Small interfering RNAs (siRNAs) against SPARC
(5′-AACAAGACCUU CGACUCUU CC-3′) (siSPARC), CHOP (D-004819-01-0005
and D-004819-02-0005) (siCHOP), and scrambled siRNA (siScr), as a
negative non-silencing control, were purchased from Dharmacon RNA
Technologies (Chicago, IL, USA) and GRP78 (siGRP78) from Santa Cruz
Biotechnology (Santa Cruz, CA, USA).
Cells (105) were seeded in 6-well plates
and cultured in antibiotic-free DMEM supplemented with 2.0 mM
L-glutamine, until ~50% confluence. Then, cells were transfected
with 30 nM siSPARC in the presence of 5 μl Metafectene Pro (Biontex
Laboratories GmbH, Martinsried/Planegg, Germany), with 100 nM
siCHOP in the presence of 6.2 μl of Metafectene Pro or with 50 nM
siGRP78 in the presence of 4 μl Lipofectamine 2000 (Invitrogen Life
Technologies, Monza, Italy) in a final volume of 1 ml serum-free
medium. The reaction was stopped after 6 h replacing the culture
medium with fresh DMEM + 10% FBS. After 24 h from transfection,
silenced cells were treated with WIN for another 24 h. Only for
SPARC silencing, cells were treated after 48 h from
transfection.
Caspase-8/SPARC
coimmunoprecipitation
The caspase-8/SPARC complex was detected in MG63
cells by immunoprecipitation either with the anti-caspase-8 or the
anti-SPARC antibodies. Cell lysates were subjected to
centrifugation at 14,000 rpm at 4°C for 15 min. Proteins
concentration was evaluated by using Bradford assay and then was
adjusted to 500 μg of protein in a final volume of 500 μl of RIPA
buffer. After pre-clearing phase by 1 h of incubation at room
temperature with 20 μl of protein A/G Plus-agarose beads (Santa
Cruz Biotechnology), lysates were spun at 14,000 rpm for 10
min.
Anti-SPARC or anti-caspase-8 antibodies or the
appropriate antibodies used as negative controls were added to the
supernatant and incubated overnight at 4°C, followed by
immunoprecipitation with protein A/G Plus-agarose beads for 2 h of
incubation at room temperature. The beads were washed three times
with PBS and the bound proteins were detached by boiling the beads
in the presence of sample buffer for 5 min. The supernatants were
subjected to electrophoresis on SDS-polyacrylamide gels and then
electroblotted on nitrocellulose filter for the detection of the
immunoprecipitate complex.
Statistical analysis
Cell viability data were expressed as the mean ± SE
and evaluated by the Student's t-test. Differences were considered
to indicate a statistically significant result when the P-value was
<0.01.
Results
WIN induces overexpression of SPARC
protein
In a previous study we demonstrated that WIN, a
synthetic ligand of cannabinoid receptors, is able to sensitize
osteosarcoma MG63 and Saos-2 cells to TRAIL action (21). In the present study we first
analysed the effect of WIN on modulating the level of SPARC, a
factor which plays different roles on cell proliferation and
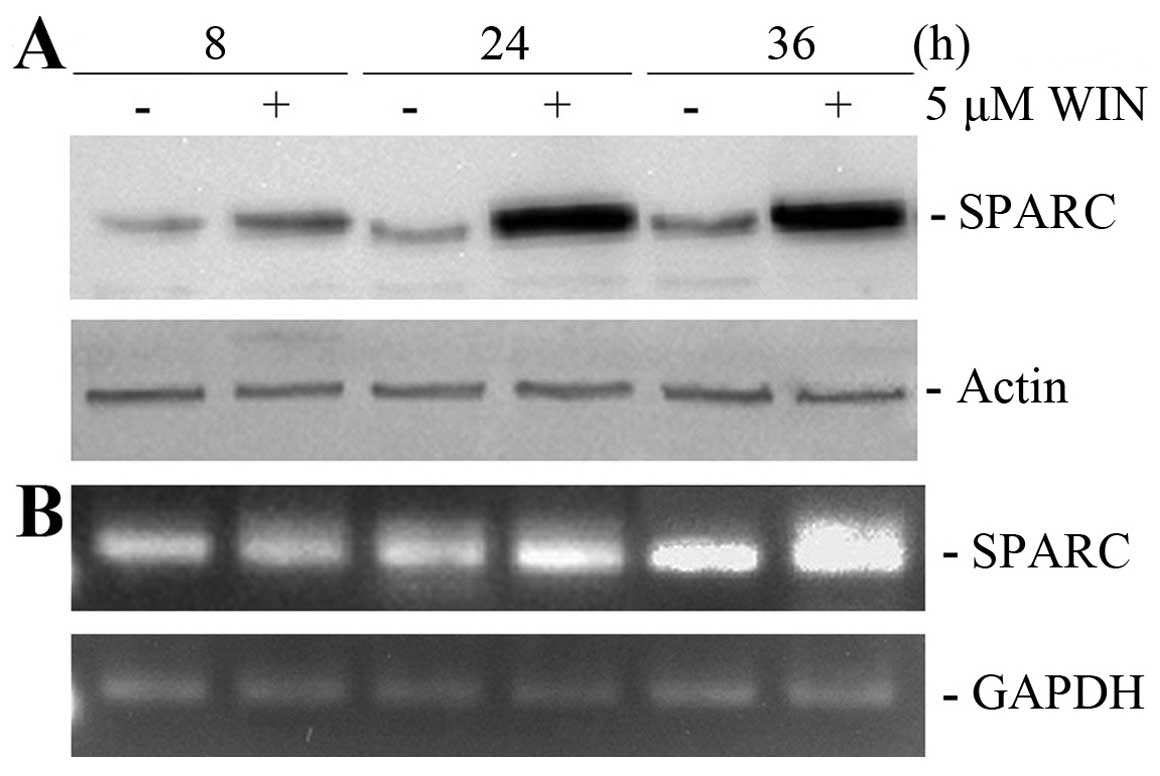
migration. As shown in Fig. 1A,
5 μM WIN, a concentration which is
responsible for the morphological and TRAIL-sensitizing effects,
induced a time-dependent increase in the level of SPARC in MG63
cells. The effect was already evident after 8 h of treatment and
reached the maximum at 24–36 h. Similar results were obtained using
semi-quantitative RT-PCR, thus, demonstrating that the increase in
SPARC protein was associated with transcriptional activation.
SPARC is responsible for
TRAIL-sensitization via the interaction with caspase-8
protease
We were interested to clarify whether SPARC can be a
critical mediator of the cytotoxic action induced by WIN/TRAIL
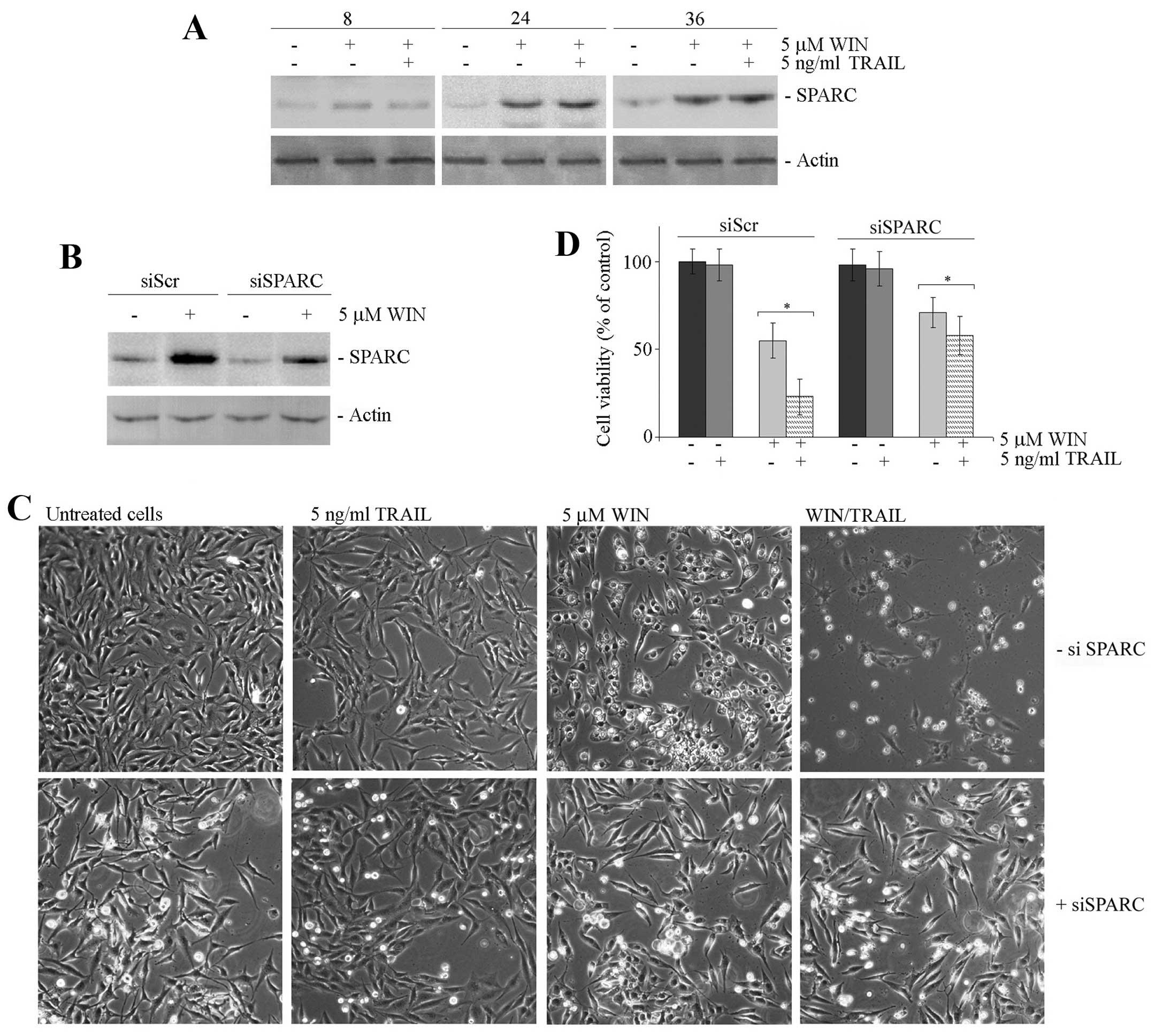
combined treatment. After confirming that the level of SPARC
remained high also in the presence of WIN/TRAIL combined treatment
(Fig. 2A), we analysed the effect
of the downregulation of SPARC expression by a specific siRNA which
knocked down the endogenous protein level by ~70% (Fig. 2B). In SPARC silenced cells,
WIN/TRAIL combined treatment exerted smaller effects than that
observed in non-silenced cells. After 24 h of treatment with WIN or
WIN/TRAIL combination, siSPARC-transfected cells showed a
morphology, which was very similar to that observed in control
cells (Fig. 2C). Moreover, MTT
assay evidenced that in siSPARC-transfected cells the reduction in
MG63 cell viability induced by WIN/TRAIL treatment was
approximately 40 vs. 80% of that observed in siSicr-transfected
cells exposed to the same treatment (Fig. 2D).
Caspase-8 is the main initiating protease activity
in extrinsic apoptotic pathway and we previously demonstrated that
its activation is a very probable event responsible for the
WIN/TRAIL-dependent cytotoxic effect (21). Moreover, as recently reported,
SPARC can potentiate the apoptotic pathway by enhancing the
signalling cascade in a caspase-8-dependent manner (24), therefore we investigated the
involvement of SPARC in caspase-8 activation in our experimental
model. First, we analysed the activation of this protease after
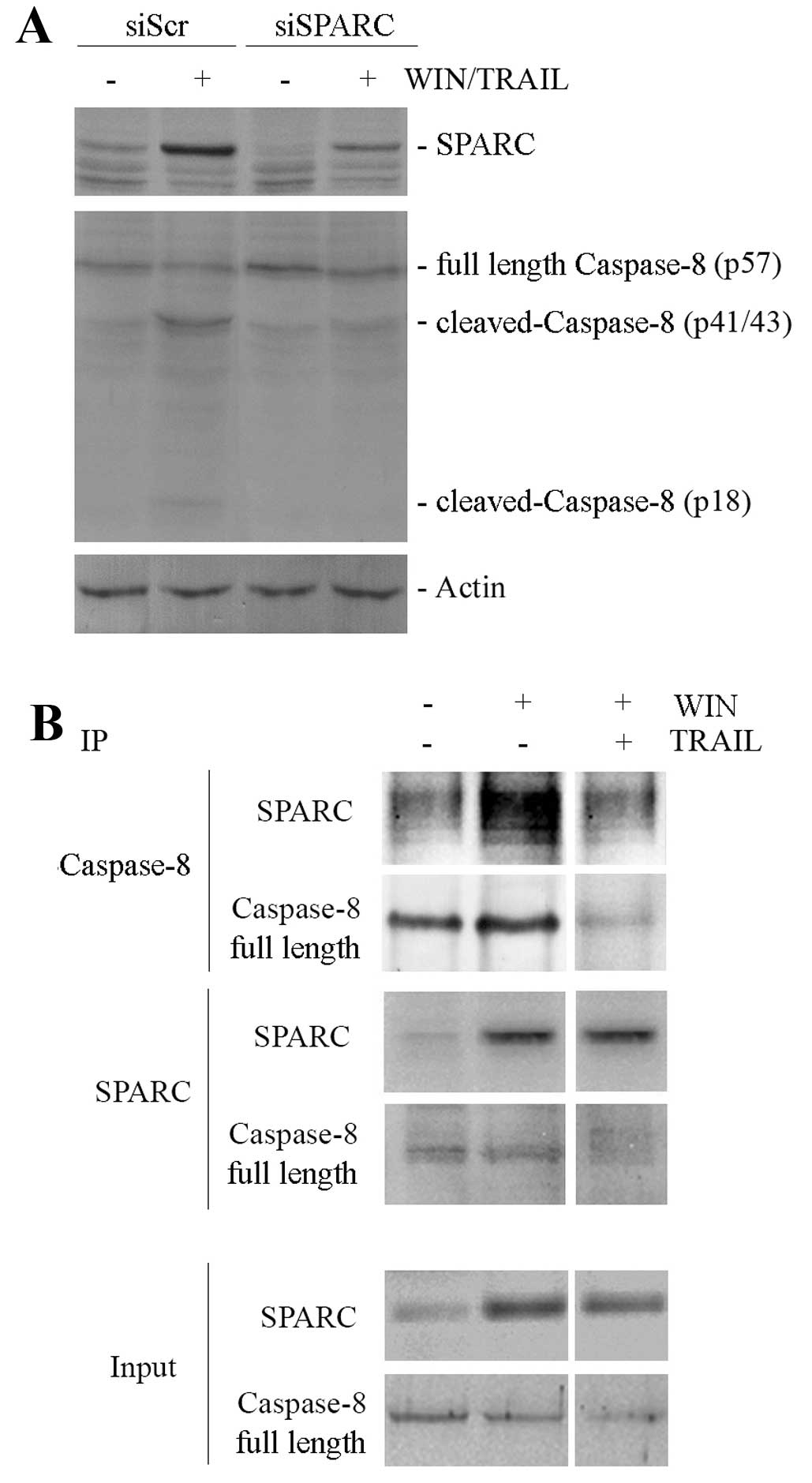
SPARC silencing. As shown in Fig.
3A, the bands corresponding to the active cleaved forms of
caspase-8 observed in WIN/TRAIL-treated cells were almost invisible
in silenced cells.
Then, we assessed the possible interaction between
SPARC and caspase-8 in MG63 cells by co-immunoprecipitation studies
using a caspase-8 antibody (against an epitope to C-terminal
region) that recognizes both the full length and the cleaved forms
of the protein. As shown in Fig.
3B, caspase-8 and SPARC co-immunoprecipitated in a reciprocal
fashion after WIN treatment. Instead, this interaction disappeared
when the cells were treated with WIN/TRAIL combination,
demonstrating that the cleavage of caspase-8 induced by the
combined treatment prevents the SPARC immunoprecipitation.
The SPARC increase is related to
WIN-induced ER stress
Based on the previously demonstrated role of
WIN-induced ER stress and autophagic process on MG63 cell viability
(21), we hypothesized a possible
relationship between ER stress induction and the SPARC level.
Recently, it has been reported that the overexpression of SPARC
induces apoptosis in neuroblastoma cells mediated by the induction
of ER stress (25). To establish
whether a similar relationship occurs in our experimental system,
we analysed the level of CHOP, a marker of ER stress, in
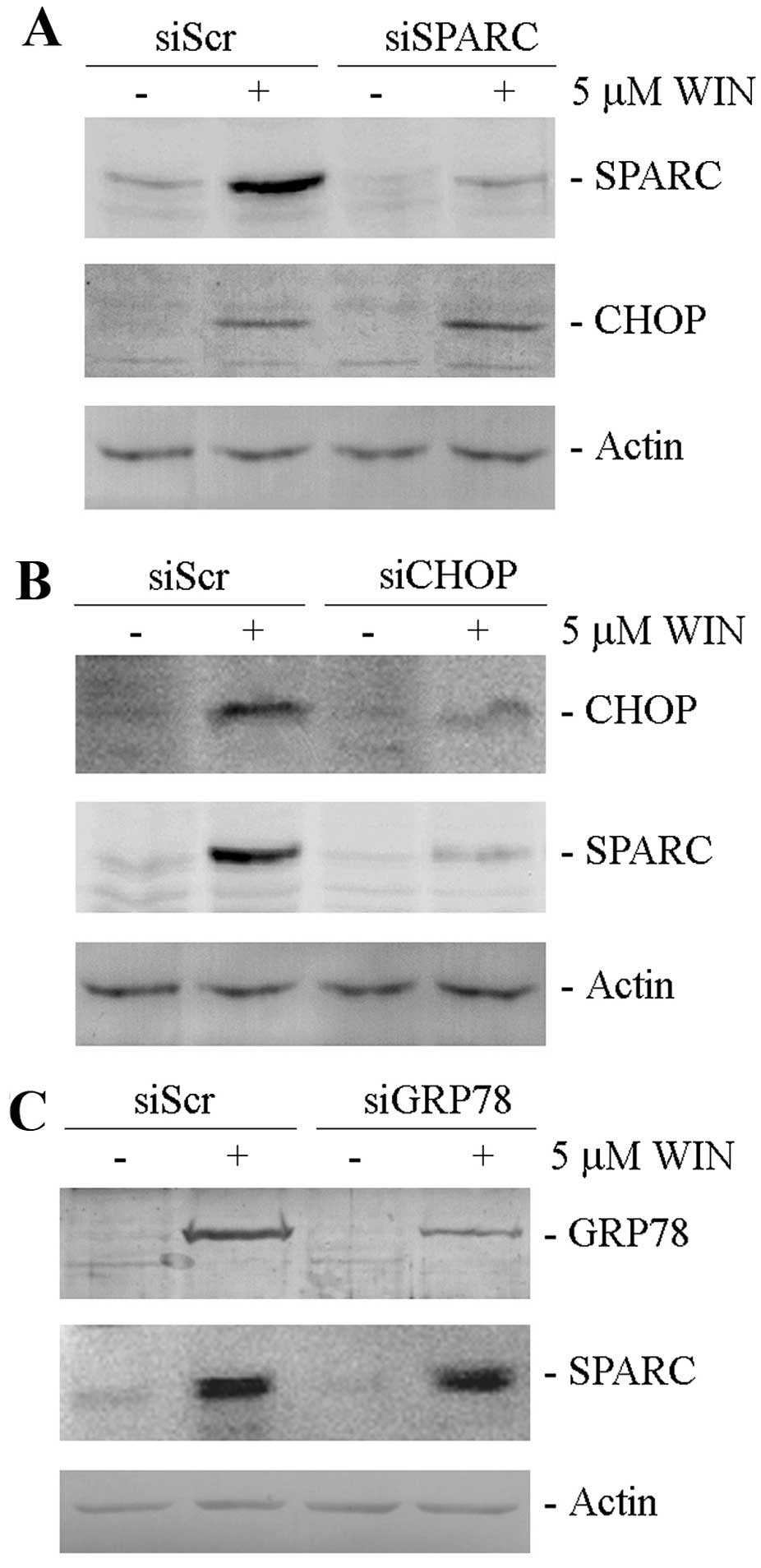
WIN-treated SPARC silenced cells. As shown in Fig. 4A, the increase in the level of
CHOP, observed after WIN-treatment, was not modified by the reduced
SPARC expression in silenced cells. Notably, we demonstrated that
SPARC increase was a consequence of the activation of the
transcriptional factor CHOP induced by WIN. In fact, CHOP silencing
which almost completely suppressed the basal levels of CHOP,
markedly reduced WIN-induced SPARC increase (Fig. 4B) while the downregulation of
GRP78/Bip, another ER stress marker, whose levels are positively
regulated by the cannabinoid, did not modify SPARC upregulation
induced by WIN treatment (Fig.
4C).
Discussion
The importance to know the expression protein
profile of tumor cells has been widely demonstrated as the response
of cells to antineoplastic drugs strongly depends on its specific
molecular pattern. It is now evident that a protein can act as an
oncogene in a specific tumor model, but as a tumor suppressor in
another one and, consequently, its induced downregulation or
upregulation can lead to different cell responses. An example of
this assumption is SPARC with its different tissue-specific
behaviour, which has been examined in a variety of cancer
cells.
In this study we analysed the role of SPARC in
osteosarcoma cells and, in particular, its involvement in
WIN/TRAIL-induced cell death. In MG63 cells, although WIN is not
able to induce cell death, it sensitizes them to apoptosis induced
by WIN/TRAIL combined treatment (21). Our present data strongly indicate
for the first time that the synthetic cannabinoid WIN induced a
clear increase in the level of SPARC which remained high also in
cells treated with WIN/TRAIL combination. Silencing of SPARC
counteracted WIN/TRAIL-induced cell death as verified through both
morphological and cytotoxicity assays. Thus, we conclude that in
all likelihood in this tumor model SPARC behaves as a tumor
suppressor factor.
In several cell death mechanisms, the role exerted
by the activation of caspase-8, a canonical membrane upstream
protease, in sensitizing cancer cells to apoptosis induced by TRAIL
has been demonstrated. In a previous study we demonstrated the
activation of caspase-8 in osteosarcoma cells as a consequence of a
marked increase in PAR-4 level after WIN or WIN/TRAIL combined
treatment and a concomitant translocation of GRP78, a marker of ER
stress, to cell surface, thus correlating ER-stress induction and
the activation of extrinsic apoptotic pathway (21). In this study we identified further
components of membrane microdomain responsible for the cytotoxic
effect of WIN/TRAIL treatment. In fact, in our experimental model,
similarly to that observed by Tang and Tai (24) in colorectal cancer, the activation
of caspase-8 seemed to be related with the interaction with SPARC
as demonstrated by us through co-immunoprecipitation analysis. The
interaction was abrogated after WIN/TRAIL treatment owing to the
cleavage and activation of caspase-8.
The relationship between SPARC increase and
WIN-dependent ER stress induction was also particularly
interesting. To our knowledge only one study reports that SPARC
overexpression triggered ER-stress and thereby unfolded protein
response (UPR) in neuroblastoma cells (25). In our experimental conditions the
reduction in SPARC level did not modify the WIN-dependent increase
in the level of CHOP, a main transcription factor mediator of the
cell response to ER-stress. Instead, CHOP downregulation was
accompanied by the reduction in SPARC level, thus, indicating an
inverse relationship between ER-stress induction and SPARC
increase. Overall, data reported in the present study, and
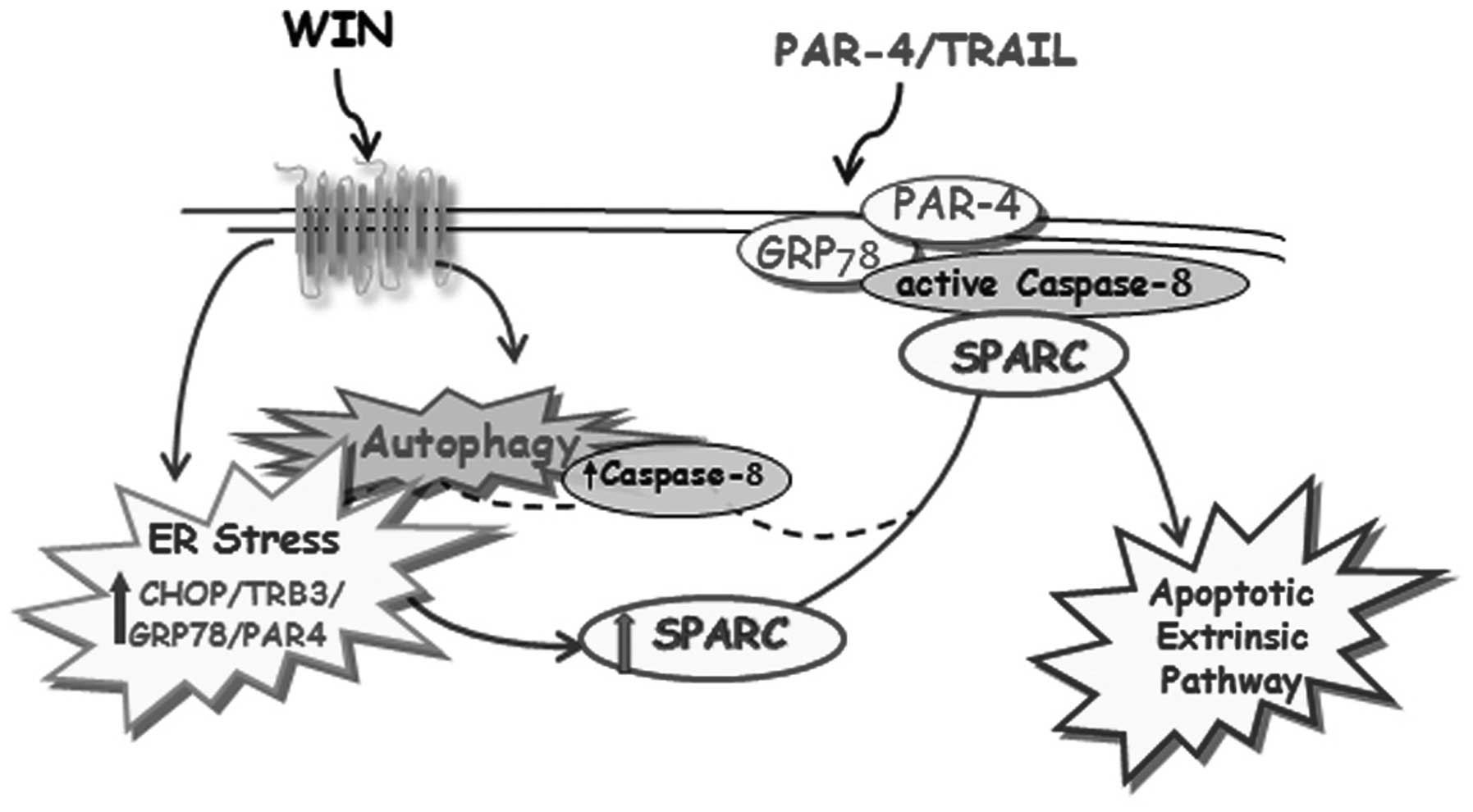
summarized in Fig. 5, demonstrate
for the first time that in osteosarcoma cells WIN induces a marked
increase in the level of SPARC and that in these cells SPARC
cooperates for the sensitization to TRAIL action by modulating the
translocation of caspase-8 in the plasma membrane. Caspase-8 forms
a microdomain together with PAR-4 and GRP-78 which, acting as TRAIL
receptor, induce caspase-8 cleavage after the addition of the
cytokine with the consequent triggering of the extrinsic apoptotic
pathway.
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
2
|
Letourneau PA, Xiao L, Harting MT, Lally
KP, Cox CS Jr, Andrassy RJ and Hayes-Jordan AA: Location of
pulmonary metastasis in pediatric osteosarcoma is predictive of
outcome. J Pediatr Surg. 46:1333–1337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brekken RA and Sage EH: Sparc a
matricellular protein: A crossroad of cell-matrix communication.
Med Sci Monit. 13:25–30. 2001.
|
|
4
|
Lane TF and Sage EH: The biology of SPARC,
a protein that modulates cell-matrix interactions. FASEB J.
8:163–173. 1994.PubMed/NCBI
|
|
5
|
Motamed K, Funk SE, Koyama H, Ross R,
Raines EW and Sage EH: Inhibition of PDGF-stimulated and
matrix-mediated proliferation of human vascular smooth muscle cells
by SPARC is independent of changes in cell shape or
cyclin-dependent kinase inhibitors. J Cell Biochem. 84:759–771.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Delany AM, Kalajzic I, Bradshaw AD, Sage
EH and Canalis E: Osteonectin-null mutation compromises osteoblast
formation, maturation, and survival. Endocrinology. 144:2588–2596.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alford AI and Hankenson KD: Matricellular
proteins: Extracellular modulators of bone development, remodeling,
and regeneration. Bone. 38:749–757. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kunigal S, Gondi CS, Gujrati M, Lakka SS,
Dinh DH, Olivero WC and Rao JS: SPARC-induced migration of
glioblastoma cell lines via uPA-uPAR signaling and activation of
small GTPase RhoA. Int J Oncol. 29:1349–1357. 2006.PubMed/NCBI
|
|
9
|
Rentz TJ, Poobalarahi F, Bornstein P, Sage
EH and Bradshaw AD: SPARC regulates processing of procollagen I and
collagen fibrillogenesis in dermal fibroblasts. J Biol Chem.
282:22062–22071. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tai IT and Tang MJ: SPARC in cancer
biology: Its role in cancer progression and potential for therapy.
Drug Resist Updat. 11:231–246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bradshaw AD and Sage EH: SPARC, a
matricellular protein that functions in cellular differentiation
and tissue response to injury. J Clin Invest. 107:1049–1054. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bellahcène A and Castronovo V: Increased
expression of osteonectin and osteopontin, two bone matrix
proteins, in human breast cancer. Am J Pathol. 146:95–100.
1995.PubMed/NCBI
|
|
13
|
Horie K, Tsuchihara M and Nakatsura T:
Silencing of secreted protein acidic and rich in cysteine inhibits
the growth of human melanoma cells with G arrest induction. Cancer
Sci. 101:913–919. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yunker CK, Golembieski W, Lemke N, Schultz
CR, Cazacu S, Brodie C and Rempel SA: SPARC-induced increase in
glioma matrix and decrease in vascularity are associated with
reduced VEGF expression and secretion. Int J Cancer. 122:2735–2743.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi Q, Bao S, Maxwell JA, Reese ED,
Friedman HS, Bigner DD, Wang XF and Rich JN: Secreted protein
acidic, rich in cysteine (SPARC), mediates cellular survival of
gliomas through AKT activation. J Biol Chem. 279:52200–52209. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mateo F, Meca-Cortés O, Celià-Terrassa T,
Fernández Y, Abasolo I, Sánchez-Cid L, Bermudo R, Sagasta A,
Rodríguez-Carunchio L, Pons M, et al: SPARC mediates metastatic
cooperation between CSC and non-CSC prostate cancer cell
subpopulations. Mol Cancer. 13:2372014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheetham S, Tang MJ, Mesak F, Kennecke H,
Owen D and Tai IT: SPARC promoter hypermethylation in colorectal
cancers can be reversed by 5-Aza-2'deoxycytidine to increase SPARC
expression and improve therapy response. Br J Cancer. 98:1810–1819.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen ZY, Zhang JL, Yao HX, Wang PY, Zhu J,
Wang W, Wang X, Wan YL, Chen SW, Chen GW, et al: Aberrant
methylation of the SPARC gene promoter and its clinical implication
in gastric cancer. Sci Rep. 4:70352014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mok SC, Chan WY, Wong KK, Muto MG and
Berkowitz RS: SPARC, an extracellular matrix protein with
tumor-suppressing activity in human ovarian epithelial cells.
Oncogene. 12:1895–1901. 1996.PubMed/NCBI
|
|
20
|
Zhang JL, Chen GW, Liu YC, Wang PY, Wang
X, Wan YL, Zhu J, Gao HQ, Yin J, Wang W, et al: Secreted protein
acidic and rich in cysteine (SPARC) suppresses angiogenesis by
down-regulating the expression of VEGF and MMP-7 in gastric cancer.
PLoS One. 7:e446182012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Notaro A, Sabella S, Pellerito O, Di Fiore
R, De Blasio A, Vento R, Calvaruso G and Giuliano M: Involvement of
PAR-4 in cannabinoid-dependent sensitization of osteosarcoma cells
to TRAIL-induced apoptosis. Int J Biol Sci. 10:466–478. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pellerito O, Notaro A, Sabella S, De
Blasio A, Vento R, Calvaruso G and Giuliano M: WIN induces
apoptotic cell death in human colon cancer cells through a block of
autophagic flux dependent on PPARγ down-regulation. Apoptosis.
19:1029–1042. 2014.PubMed/NCBI
|
|
23
|
Portanova P, Notaro A, Pellerito O,
Sabella S, Giuliano M and Calvaruso G: Notch inhibition restores
TRAIL-mediated apoptosis via AP1-dependent upregulation of DR4 and
DR5 TRAIL receptors in MDA-MB-231 breast cancer cells. Int J Oncol.
43:121–130. 2013.PubMed/NCBI
|
|
24
|
Tang MJ and Tai IT: A novel interaction
between procaspase 8 and SPARC enhances apoptosis and potentiates
chemotherapy sensitivity in colorectal cancers. J Biol Chem.
282:34457–34467. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sailaja GS, Bhoopathi P, Gorantla B,
Chetty C, Gogineni VR, Velpula KK, Gondi CS and Rao JS: The
secreted protein acidic and rich in cysteine (SPARC) induces
endoplasmic reticulum stress leading to autophagy-mediated
apoptosis in neuroblastoma. Int J Oncol. 42:188–196. 2013.
|