Introduction
Cancer is a major public health problem worldwide.
Epidemiologic and animal studies have demonstrated an association
with the inclusion of vegetables and fruits containing
chemopreventive compounds in the diet and a reduced risk of cancer
development (1,2). Our laboratory has spent over 20 years
screening vegetables and fruits for natural phytochem-ical products
that inhibit DNA metabolic enzymes, primarily mammalian DNA
polymerases (Pols) and human DNA topoi-somerases (Topos).
Pols (DNA-dependent DNA polymerase, EC 2.7.7.7)
catalyze the addition of deoxyribonucleotides to the 3′-hydroxyl
terminus of primed double-stranded DNA (dsDNA) molecules (3). The human genome encodes at least 15
Pols that function in cellular DNA synthesis (4,5).
Eukaryotic cells contain three replicative Pols (α, δ and ɛ), a
single mitochon-drial Pol (γ), and at least 11 non-replicative Pols
[β, ζ, η, θ, ι, κ, λ, μ, ν, terminal deoxynucleotidyl transferase
(TdT), and REV1] (6,7). Pol structure is highly conserved, and
the catalytic subunit shows little variance among species. Sequence
homology classifies eukaryotic Pols into four main families: A, B,
X and Y (6). Family A includes
mitochondrial Pol γ as well as Pols θ and ν; family B includes the
three replicative Pols α, δ and ɛ and also Pol ζ; family X
comprises Pols β, λ and μ, as well as TdT; and family Y includes
Pols η, ι and κ, in addition to REV1 (7).
Topos are nuclear enzymes that alter DNA topology
and are required for the replication, transcription, recombination
and segregation of daughter chromosomes (8). Eukaryotic cells have two types of
Topos, I and II. Topo I catalyzes the passage of a DNA strand
through a transient single-strand break in the absence of any
high-energy cofactor. In contrast, Topo II catalyzes the passage of
dsDNA through a transient double-strand break in the presence of
ATP.
Selective inhibitors of Pols and Topos can suppress
the proliferation of normal and cancerous human cells or exhibit
cytotoxic effects (9–11). These inhibitors are potentially
useful as anticancer, antiviral, antiparasitic or antipregnancy
agents. In screening for these enzyme inhibitors in the ethanol
extracts of various food materials, we found that the extract of
mangosteen (Garcinia mangostana L.) from various parts of
the fruit, such as the fruit, pericarp, leaves and rind, strongly
inhibited the activities of mammalian Pols. We purified major
components and identified eight xanthones by NMR and mass spectra
analysis.
The mangosteen tree has been cultivated for
centuries in tropical areas of the world. The tree is presumed to
have originated in Southeast Asia or Indonesia and has largely
remained indigenous to Malay Peninsula, Myanmar, Thailand,
Cambodia, Vietnam and the Moluccas (12). The white, inner pulp of the
mangosteen fruit is highly praised as one of the best tasting of
all tropical fruits. This fruit has been dubbed the ‘queen of
fruit’ in its native Thailand. The mangosteen rind, leaves and bark
have been used as folk medicine for thousands of years (13). The thick mangosteen rind has been
and is used for treating catarrh, cystitis, diarrhea, dysentery,
eczema, fever, intestinal ailments, pruritis and other skin
ailments. The mangosteen leaves are also used by some natives in
teas and for diarrhea, dysentery, fever and thrush. Concentrates of
mangosteen bark are used for genito-urinary afflictions and
stomatosis.
Over 200 xanthones are currently known to exist in
nature and ~50 of them are found in the mangosteen. The xanthones
from mangosteen are gaining more interest because of their
remarkable pharmacological effects, including not only anticancer
activities (14,15), but also analgesic (16), antioxidant (17), anti-inflammatory (18), anti-allergy (19), antibacterial (20), anti-tuberculosis (21), antifungal (22), antiviral (23), cardioprotective (24), neuroprotective (25) and immunomodulation (26) effects. However, the underlying
molecular mechanisms of these compounds and their effects on the
activities of DNA metabolic enzymes have not been reported.
Therefore, the present study focused on the isolated
eight xanthones from mangosteen and investigated these compounds
for their inhibitory effects in vitro on mammalian Pol and
human Topo activities.
Materials and methods
Materials
A chemically synthesized DNA template, poly(dA), and
calf thymus DNA were purchased from Sigma-Aldrich Inc. and a
customized oligo(dT)18 DNA primer was produced by
Sigma-Aldrich Japan K.K. (Hokkaido, Japan). Radioactive nucleotide
[3H]-labeled 2′-deoxythymidine-5′-triphosphate (dTTP; 43
Ci/mmol) was obtained from Moravek Biochemicals Inc. (Brea, CA,
USA). Supercoiled pHOT-1 DNA was obtained from TopoGEN Inc. (Port
Orange, FL, USA). All other reagents were analytical grade from
Nacalai Tesque Inc. (Kyoto, Japan).
Isolation of xanthones from
mangosteen
Some xanthone compounds were isolated from the
leaves of mangosteen (Garcinia mangostana L.), which were
collected in Vietnam and provided by Dr Duy Hoang Le (Kobe
Pharmaceutical University). The chemical structures were determined
by spectroscopic analyses and comparison with reported data
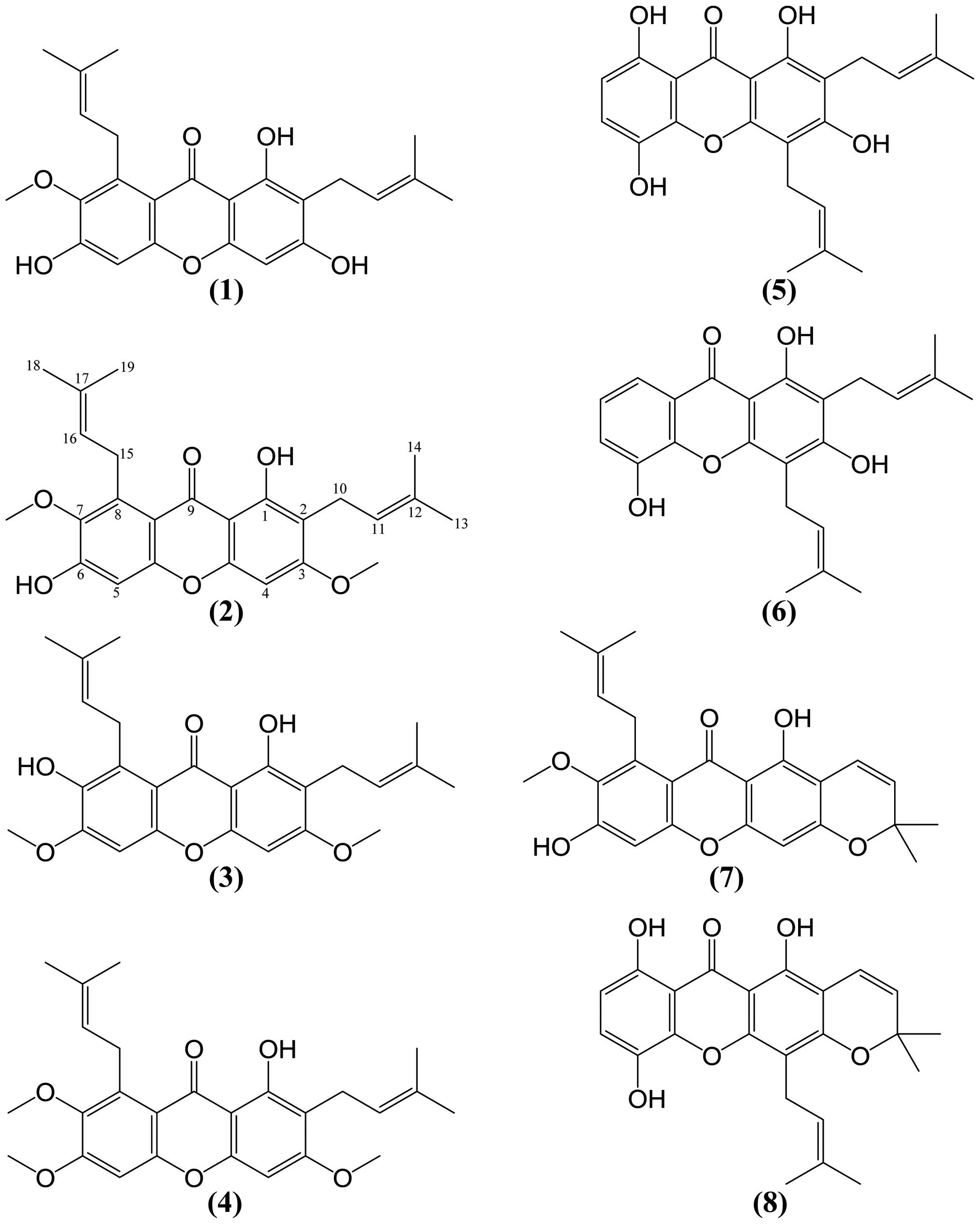
(27–30). The identified eight compounds are
α-mangostin (1), β-mangostin (2),
3,6-di-O-methyl-γ-mangostin (3), fuscax-anthone C
(4), gartanin (5), 8-deoxygartanin (6),
mangostanin (7) and morusignin I (8) (Fig. 1).
Enzymes
Pol α was purified from calf thymus by
immunoaffinity column chromatography, as described by Tamai et
al (31). Recombinant rat Pol
β was purified from Escherichia coli JMpβ5, as described by
Date et al (32). The human
Pol γ catalytic gene was cloned into pFastBac, with the
histidine-tagged enzyme expressed using the BAC-TO-BAC HT
Baculovirus Expression System according to the supplier's
instructions (Life Technologies, Inc., Gaithersburg, MD, USA), and
purified using ProBound resin (Invitrogen Japan K.K, Tokyo, Japan)
(33). Human Pols δ and ɛ were
purified by nuclear fractionation of human peripheral blood cancer
cells (Molt-4) using the second subunit of Pols δ and ɛ-conjugated
affinity column chromatography, respectively (34). A truncated form of human Pol η
(residues 1–511) tagged with His6 at the C-terminus was
expressed in E. coli and purified as described by Kusumoto
et al (35). Recombinant
mouse Pol ι tagged with His6 at the C-terminus was
expressed and purified by Ni-NTA column chromatography (36). A truncated form of Pol κ (residues
1–560) with a His6-tag at the C-terminus was expressed
in E. coli and purified as described by Ohashi et al
(37). Recombinant human His-Pol λ
was expressed and purified according to a method described by
Shimazaki et al (38). Calf
TdT, T7 RNA polymerase and T4 polynucleotide kinase were purchased
from Takara Bio Inc. (Kyoto, Japan). Purified human placenta Topos
I and II were purchased from TopoGEN Inc. Bovine pancreas
deoxyribonuclease I was obtained from Stratagene Cloning Systems
(La Jolla, CA, USA).
Measurement of Pol activity
The reaction mixtures for calf Pol α and rat Pol β
have been described (39,40). Those for human Pol γ and for human
Pols δ and ɛ were as described by Umeda et al (33) and Ogawa et al (41), respectively. The reaction mixtures
for mammalian Pols η, ι and κ were the same as that for calf Pol α,
and the reaction mixture for human Pol λ was the same as for rat
Pol β. For Pols, poly(dA)/oligo(dT)18 (A/T=2/1) and
tritium-labeled 2′-deoxythymidine 5′-triphos-phate
([3H]-dTTP) were used as the DNA template-primer and
nucleotide [i.e., 2′-deoxynucleoside 5′-triphosphate (dNTP)]
substrates, respectively. For the TdT reactions,
oligo(dT)18 (3′-OH) and dTTP were used as the DNA primer
substrate and nucleotide substrate, respectively.
The eight isolated xanthone compounds were dissolved
in distilled dimethyl sulfoxide (DMSO) at various concentrations
and sonicated for 30 sec. Aliquots of 4 μl of sonicated samples
were mixed with 16 μl of each enzyme (final amount, 0.05 units) in
50 mM Tris-HCl (pH 7.5), containing 1 mM dithiothreitol, 50%
glycerol and 0.1 mM ethylenediaminetetraacetic acid (EDTA), and
kept at 0ºC for 10 min. These inhibitor-enzyme mixtures (8 μl) were
added to 16 μl of each of the enzyme standard reaction mixtures and
incubation carried out at 37ºC for 60 min. Activity without the
inhibitor was considered 100% and the remaining activity at each
inhibitor concentration was determined relative to this value. One
unit of Pol activity was defined as the amount of enzyme that
catalyzed incorporation of 1 nmol dNTP [measured using
[3H]-dTTP] into synthetic DNA template-primers in 60 min
at 37ºC under the normal reaction conditions for each enzyme
(39,40).
Measurement of Topo activity
Purified human placental Topos I and II were
purchased from TopoGen Inc. The catalytic activity of Topo I was
determined by detecting supercoiled plasmid DNA (Form I) in its
nicked form (Form II) (42). The
Topo I reaction was performed in a 20-μl reaction mixture that
contained 10 mM Tris-HCl (pH 7.9), pHOT-1 DNA (250 ng), 1 mM EDTA,
150 mM NaCl, 0.1% bovine serum albumin (BSA), 0.l mM spermidine, 5%
glycerol, 2 μl of one of the eight isolated xanthone compounds
dissolved in DMSO, and 2 units of Topo I. Topo II catalytic
activity was analyzed in the same manner, except the reaction
mixture contained 50 mM Tris-HCl (pH 8.0), 120 mM KCl, 10 mM
MgCl2, 0.5 mM ATP, 0.5 mM dithiothreitol, supercoiled
pHOT-1 DNA (250 ng), and 2 units of Topo II (42). Reaction mixtures were incubated at
37ºC for 30 min, followed by digestion with 1% sodium dodecyl
sulfate (SDS) and 1 mg/ml proteinase K. After digestion, 2 μl
loading buffer (5% sarkosyl, 0.0025% bromophenol blue and 25%
glycerol) was added. The same procedure was followed for mobility
shift assay assessment of enzyme DNA binding, except SDS
denaturation and proteinase K digestion were omitted. Reaction
mixtures were subjected to 1% agarose gel electrophoresis in
Tris/borate/EDTA buffer. Agarose gels were stained with ethidium
bromide (EtBr) and DNA band shifts from Form I to Form II were
detected using an enhanced chemiluminescence detection system
(Perkin-Elmer Life Sciences Inc., Waltham, MA, USA). Zero-D scan
(Version 1.0, M & S Instruments Trading Inc., Osaka, Japan) was
used for densitometric quantitation.
Other enzyme assays
The activities of T7 RNA polymerase, T4
polynucleotide kinase and bovine deoxyribonuclease I were measured
using standard assays according to the manufacturer's
specifications as described by Nakayama and Saneyoshi (43), Soltis and Uhlenbeck (44) and Lu and Sakaguchi (45), respectively.
Thermal transition of DNA
Thermal transition profiles of dsDNA to
single-stranded DNA (ssDNA) with or without α-mangostin were
obtained with a spectrophotometer (UV-2500; Shimadzu Corp., Kyoto,
Japan) equipped with a thermoelectric cell holder was used as
previously described (46). Calf
thymus DNA (6 μg/ml) was dissolved in 0.1 M sodium phosphate buffer
(pH 7.0) containing 1% DMSO. The solution temperature was
equilibrated at 75ºC for 10 min, and then increased by 1ºC at 2-min
intervals for each measurement point. Any change in the absorbance
(260 nm) of the test compound itself at each temperature point was
automatically subtracted from that of DNA plus the compound in the
spectrophotometer.
Cell culture and measurement of cancer
cell viability
The human cervical cancer cell line HeLa was
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). HeLa cells were cultured in Dulbecco's modified Eagle's
medium supplemented with 10% fetal bovine serum, 100 units/ml
penicillin and 100 μg/ml streptomycin. Cells were cultured at 37ºC
in a humidified incubator containing 5% CO2/95% air. For
assessment of cell growth, cells were plated at 2×103
cells/well in 96-well microplates, cultured for 12 h and various
concentrations of the eight isolated xanthone compounds added. The
compounds were dissolved in DMSO to produce 10-mM stock solutions,
which were further diluted to appropriate final concentrations with
growth medium containing 0.5% DMSO immediately prior to use. Cell
viability was determined by the WST-1 assay following 24-h
incubation (47).
Cell cycle analysis
Cellular DNA content for cell cycle analysis was
determined as follows: aliquots of 3×105 HeLa cells were
added to a 35-mm dish and incubated with the medium that contained
the test compound for 0 to 24 h. Cells were then washed with
ice-cold PBS three times, fixed with 70% (v/v) ethanol, and stored
at −20ºC. DNA was stained with a PI
(3,8-diamino-5-[3-(diethylmethylammonio)propyl]-6-phenyl-phenanthridinium
diiodide) staining solution for at least 10 min at room temperature
in the dark. Fluorescence intensity was measured using a BD
FACSCalibur flow cytometer in combination with CellQuest software
(Becton-Dickinson Co., Tokyo, Japan).
Measurement of caspase-3 activity
The enzymatic activity of caspase-3 was measured
using EnzChek Caspase-3 Assay Kit #2 (Life Technologies Japan Co.,
Ltd., Tokyo, Japan), according to the instruction manual. HeLa
cells (1×106) after inducing apoptosis with test
compound were washed with PBS and lysed. Then, the caspase-3
activity in the extracts was determined with a fluorometric assay.
The fluorescent product of the substrate Z-DEVD-Rhodamine 110
generated by caspase-3 in the cell extract was detected in a
fluorescent microplate reader (SH-9000 Lab; Hitachi
High-Technologies Corp., Tokyo, Japan) using an excitation of 496
nm and emission of 520 nm. To determine the background fluorescence
of the substrate, test compound without the Z-DEVD-R110 substrate
for negative controls, and mixture of activity buffer and
Z-DEVD-R110 substrate for only substrate controls were also
executed.
4′,6-Diamidino-2-phenylindole (DAPI)
staining
Aliquots of 2.5×104 HeLa cells were
plated in each well of an 8-well chamber slide (Thermo Fisher
Scientific K.K., Tokyo, Japan). Then, the cells were incubated with
or without the test compound (27.2 and 54.4 μM) for 12 and 24 h at
37ºC with 5% CO2. After washed with PBS twice, the cells
were fixed with 4% paraformaldehyde (pH 7.4) for 15 min. Then, the
cells were stained with DAPI for 1 min, and the percentage of
apoptotic cells was counted under a fluorescence microscope
(Olympus IX70; Olympus, Tokyo, Japan).
Statistical analysis
The data are expressed as the mean value ± the
standard deviation (SD) of the mean of at least three independent
determinations for each experiment. Statistical significance
between each experimental group was analyzed using Student's
t-test, and a probability level of 0.01 and 0.05 was used as the
criterion of significance.
Results
Effect of the isolated xanthone compounds
1-8 on the activities of mammalian Pols
Selective inhibitors of mammalian Pols have been
investigated with the goal to develop chemothera-peutic drugs for
anticancer, antivirus and anti-inflammation treatments (48). In our ongoing screening for natural
inhibitors of mammalian Pols, we found that the extract of
mangosteen (Garcinia mangostana L.) had inhibitory activity
against mammalian Pols. The isolated xanthone compounds 1-8
(Fig. 1) were selected and
prepared for this study.
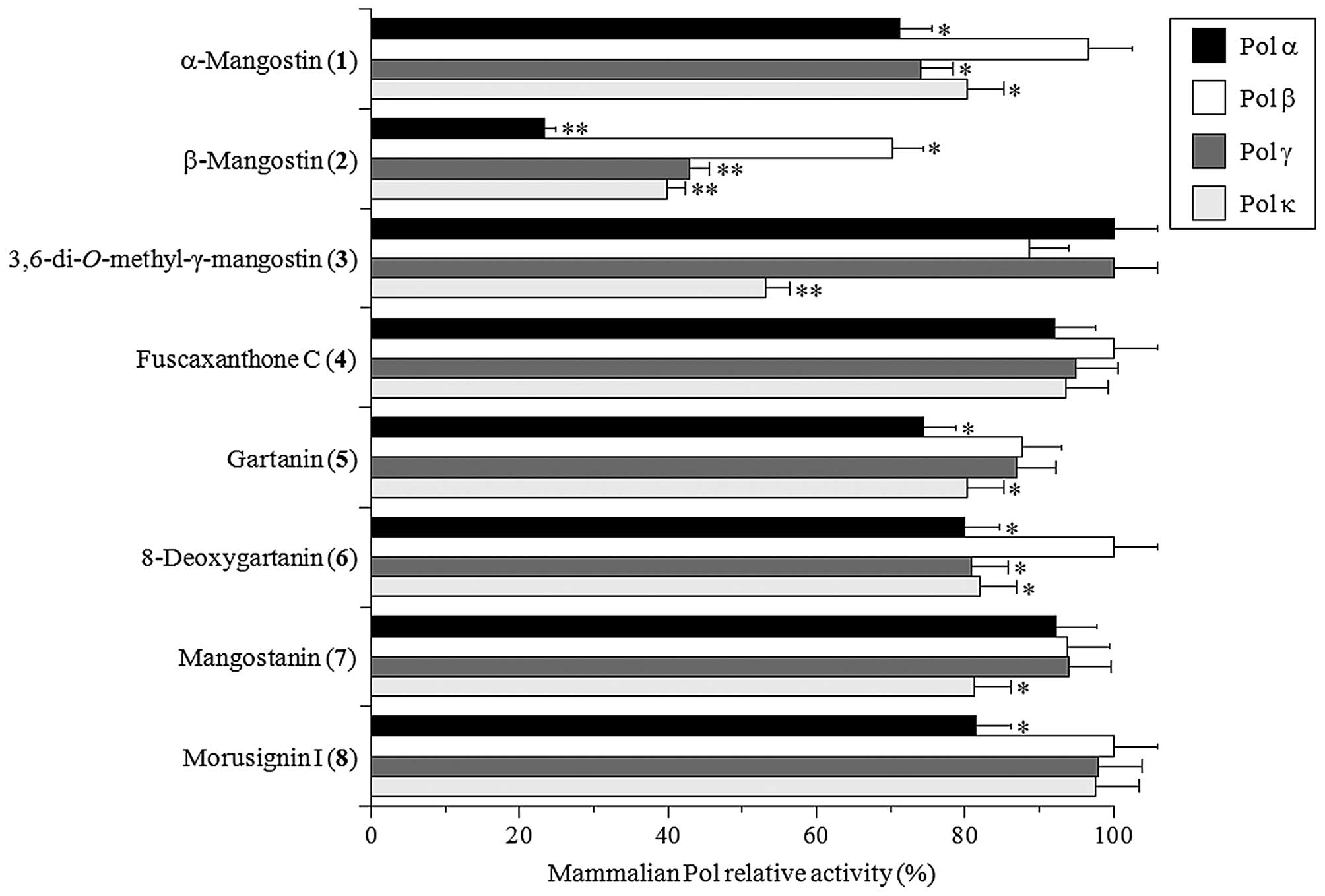
The inhibitory activity of each compound toward
mammalian Pols was investigated using calf Pol α, rat Pol β, and
human Pols γ and κ. For mammalian Pols, Pols α, β, γ and κ were
used as the representatives of Pol families B, X, A and Y,
respectively (6,7). Assessment of the relative activity of
each Pol at a set concentration (10 μM) of the eight test compounds
showed that β-mangostin (2) was the strongest inhibitor of
these Pol species among the compounds tested (Fig. 2). In particular, this compound
showed the strongest inhibitory activity against Pol α among the
four mammalian Pols. α-Mangostin (1) and
3,6-di-O-methyl-γ-mangostin (3) slightly inhibited
Pols α, γ, and κ activities and Pol κ activity, respectively. These
results suggested that the common backbone structure with prenyl
groups at C-2 and C-8 of compounds 1-3 might be important
for Pol inhibition, and both the hydroxyl group at C-6 and the
methoxy group at C-3, which are present in β-mangostin (2),
must be much more important for Pol inhibitory activity.
Effect of the isolated xanthone compounds
1-8 on the activities of human Topos I and II
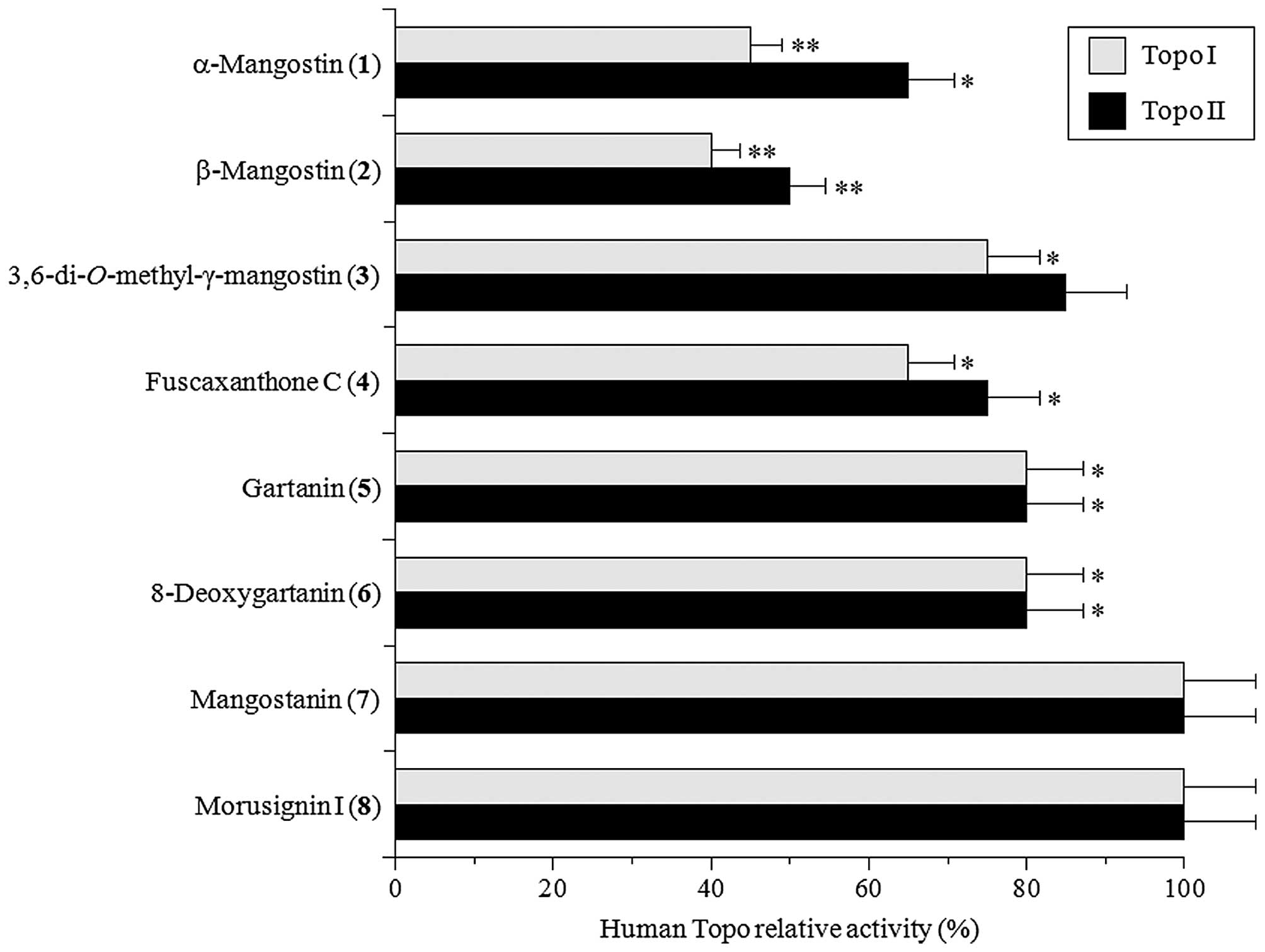
Next, the inhibitory effects of the eight isolated
xanthones (10 μM each) were examined against human Topos I and II,
which have ssDNA and dsDNA nicking activity, respectively (8). β-Mangostin (2) was the
strongest inhibitor of both Topos I and II, and the inhibitory
effect of this compound on Topo II was more potent than that on
Topo I (Fig. 3). In contrast,
mangostanin (7) and morusignin I (8) did not
influence the activities of these Topos. Topo inhibition could be
ranked as β-mangostin (2) >α-mangostin (1)
>3,6-di-O-methyl-γ-mangostin (3) = fuscaxanthone C
(4) = gartanin (5) = 8-deoxygartanin (6)
>mangostanin (7) = morusignin I (8). Since
β-mangostin (2) showed the strongest inhibitory activities
on both mammalian Pols and human Topos, we focused on this compound
in the subsequent study.
Effects of β-mangostin on the activities
of various mammalian Pols and other DNA metabolic enzymes
Evaluation of the inhibition of DNA metabolic enzyme
activities in vitro by β-mangostin revealed that this
compound inhibited the activities of all eleven mammalian Pols
tested from all four families. Calf Pol α and calf TdT were the
strongest and weakest inhibited, respectively, with 50% inhibitory
concentration (IC50) values of 6.4 and 39.6 μM,
respectively (Table I). The
inhibition by β-mangostin against mammalian Pol families could be
ranked as B-family Pols >A-family Pol >Y-family Pols >
X-family Pols. By comparison, the IC50 values of
aphidicolin, a known eukaryotic DNA replicative Pols α, δ and ɛ
inhibitor, were 20, 13 and 16 μM, respectively (49), showing that the Pol inhibitory
activity of β-mangostin was >1.5-fold higher than that of
aphidicolin. β-Mangostin inhibited the activity of human Topos I
and II with IC50 values of 10.0 and 8.5 μM,
respectively. Topotecan and doxorubicin, which are Topo I and Topo
II inhibitors, respectively, also inhibited the nicking activities
of Topos I and II with IC50 values of 45 and 60 μM,
respectively (data not shown). The data show that the inhibitory
effect on Topos I and II of β-mangostin was more potent than that
of topotecan and doxorubicin, respectively. The inhibition of human
Topos activities by β-mangostin was approximately the same order of
potency compared with that of the activities of mammalian Pols
except for X-family of Pols (Table
I).
 | Table IIC50 values of β-mangostin
on the activities of mammalian Pols, human Topos and various DNA
metabolic enzymes. |
Table I
IC50 values of β-mangostin
on the activities of mammalian Pols, human Topos and various DNA
metabolic enzymes.
| Enzyme | IC50
values (μM) |
|---|
| Mammalian Pols |
| A-Family of
Pol |
| Human Pol γ | 8.6±0.51 |
| B-Family of
Pols |
| Calf Pol α | 6.4±0.38 |
| Human Pol δ | 7.4±0.44 |
| Human Pol ɛ | 8.5±0.50 |
| X-Family of
Pols |
| Rat Pol β | 34.8±2.1 |
| Human Pol λ | 28.5±1.7 |
| Human Pol μ | 38.2±2.2 |
| Calf TdT | 39.6±2.3 |
| Y-Family of
Pols |
| Human Pol η | 9.3±0.55 |
| Mouse Pol ι | 8.7±0.52 |
| Human Pol κ | 9.2±0.54 |
| Human Topos |
| Human Topo I | 10±1.0 |
| Human Topo II | 8.5±0.9 |
| Other DNA metabolic
enzymes |
| T7 RNA
polymerase | >200 |
| T4 polynucleotide
kinase | >200 |
| Bovine
deoxyribonuclease I | >200 |
In contrast, β-mangostin did not influence the
activities of other DNA metabolic enzymes, such as T7 RNA
polymerase, T4 polynucleotide kinase, and bovine deoxyribonuclease
I (Table I). These results
indicate that β-mangostin can be specifically classified as an
inhibitor of mammalian Pols and Topos.
Influence of β-mangostin on the
hyperchromicity of dsDNA
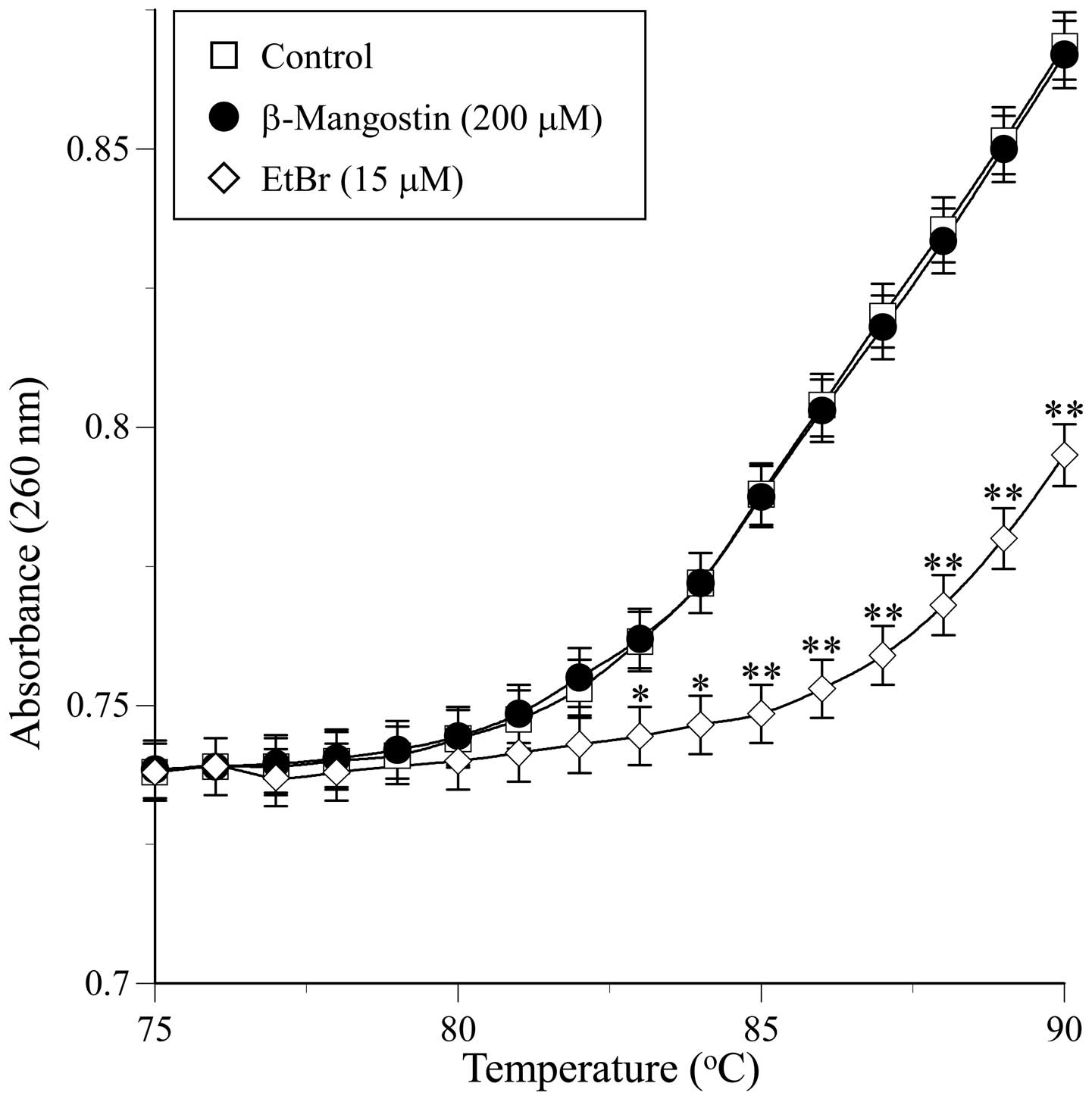
Specific assays were performed to determine whether
β-mangostin-induced inhibition resulted from the ability of the
compound to bind to DNA or the enzyme. The interaction of
β-mangostin with dsDNA was investigated by studying its thermal
transition. The melting temperature (Tm) of dsDNA in the presence
of an excess of β-mangostin (200 μM) was observed using a
spectrophotometer equipped with a thermoelectric cell holder. As
shown in Fig. 4, a thermal
transition (i.e., Tm) from 75 to 90ºC was not observed within the
concentration range used in the assay, whereas when a typical
intercalating compound, such as EtBr (15 μM), was used as a
positive control, an obvious thermal transition was observed.
We then assessed whether the inhibitory effect of
β-mangostin on mammalian Pols and Topos resulted from non-specific
adhesion to the enzyme or from selective binding to specific sites.
Neither excessive nucleic acid [in the form of Poly(rC)] nor
protein (BSA) significantly influenced β-mangostin-induced both Pol
and Topo inhibition (data not shown). This suggests that
β-mangostin selectively bound to the Pol and Topo molecules. These
observations reveal that β-mangostin does not act as a DNA
intercalating agent, and rather it directly binds the enzyme to
inhibit its activity.
Collectively, these results suggested that
β-mangostin might be a potent and selective inhibitor of mammalian
Pols and Topos. We next investigated whether Pol and/or Topo
inhibition by β-mangostin resulted in decreases in human cancer
cell proliferation.
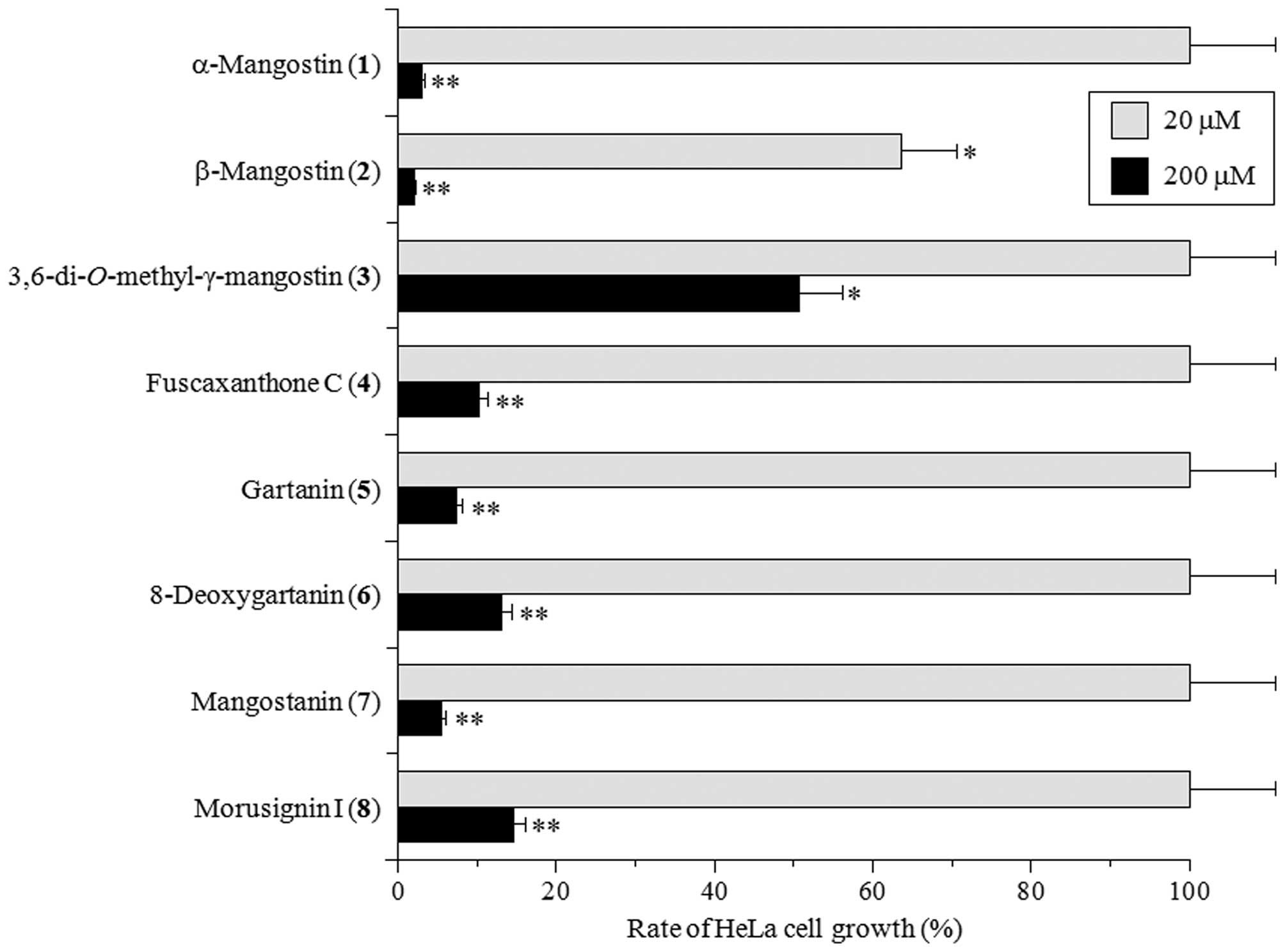
Effect of the isolated xanthone compounds
1-8 on cultured human cancer cells
Pols and Topos have recently emerged as important
cellular targets for the development of anticancer agents.
Therefore, we also investigated whether the eight isolated
xanthones had any cytotoxic effect on HeLa cells. At 100 μM, all
the compounds except for 3,6-di-O-methyl-γ-mangostin
(3) potently suppressed the rate of HeLa cell growth to
<80% that of control (Fig. 5).
At 10 μM, β-mangostin (2) could moderately suppress HeLa
cell growth, but other compounds did not influence cell
proliferation. The 50% lethal dose (LD50) of β-mangostin
(2) was 27.2 μM, which is ~2-fold higher than the
IC50 for DNA replicative Pols (X-family Pols α, δ and ɛ)
and Topos. This suggests that β-mangostin may be able to penetrate
the cell membrane and reach the nucleus, where it may then inhibit
Pols and/or Topos activities to suppress cell growth. Similar
findings for all eight xanthones were observed in the HCT116 human
colon carcinoma cell line (data not shown). These results reveal
that Pol and Topo inhibition (Figs.
2 and 3) and human cancer cell
cytotoxicity (Fig. 5) can be
induced by xanthones from mangosteen. Therefore, Pol/Topo
inhibition by β-mangostin represents a potentially useful
anticancer effect.
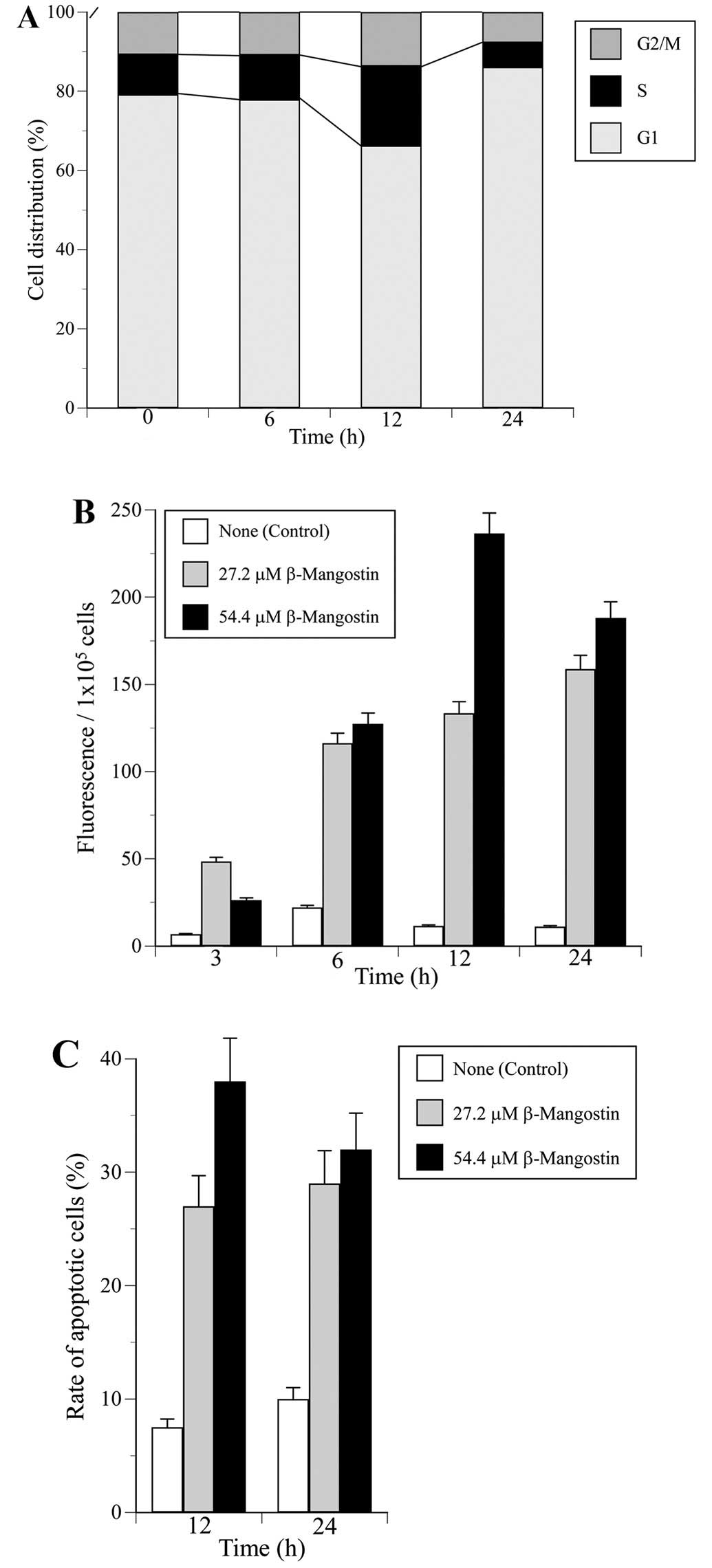
Next we analyzed whether β-mangostin could affect
the cell cycle distribution of HeLa cells. The cell cycle fraction
was recorded after 0, 6, 12 and 24 h of treatment with a
concentration of β-mangostin at 54.4 μM (the double density of
LD50). The ratio of cells in each of the three cell
cycle phases (G1, S and G2/M) is shown in Fig. 6A. Treatment with β-mangostin
significantly increased the population of cells in S and G1 phase
at 12 and 24 h, respectively, but did not influence the rate of
G2/M phase. Dehydroaltenusin, which is a mammalian Pol α specific
inhibitor, arrested the cell cycle in the G1 and S phases (50), and etoposide, which is a classical
Topo II inhibitor, arrested the cell cycle in the G2/M phase (data
not shown). These results suggest that β-mangostin may be an
effective inhibitor of DNA replicative Pols, such as Pol α, that
arrests the cell cycle at the G1 and S phases.
To examine whether the cell cycle arrest of HeLa
cells treated with β-mangostin was due to apoptosis, caspase-3
activity was analyzed using fluorescent substrate (Fig. 6B). When HeLa cells were treated
with the compound at 27.2 (=LD50 value) and 54.4 μM, the
cells maximally increased caspase-3 activity for 24 and 12 h,
respectively (Fig. 6B). In
addition, preincubation with a caspase-3 inhibitor, DEVD-CHO,
completely blocked this activity by β-mangostin (data not shown),
suggesting that the apoptosis by this compound was mediated through
caspase-3 activation.
Fig. 6C and D shows
the extent of apoptotic cells by DAPI staining. The percentage of
HeLa apoptotic cells after treatment with 27.2 and 54.4 μM of
β-mangostin was markedly high for 24 and 12 h, respectively
(Fig. 6C). These findings suggest
that the human cancer cytotoxic effect of β-mangostin must involve
a combination of cell proliferation arrest and apoptotic cell
death.
Discussion
This is the first study to examine the inhibitory
effects of xanthones isolated from mangosteen (G. mangostana
L.) (i.e., compounds 1-8 in Fig. 1) on the activities of mammalian Pol
species. β-Mangostin was the strongest inhibitor of mammalian Pols
α, β, γ and κ as the representatives of Pol families B, X, A and Y,
respectively (Fig. 2), and human
Topos I and II (Fig. 3) among the
compounds investigated. Its human cancer cytotoxicity (Fig. 5) was realized through the
inhibition of the DNA metabolic enzymes Pols and Topos, which are
essential for DNA replication, repair and recombination as well as
cell division.
Topo inhibitors, such as adriamycin, amsacrine,
ellipticine, saintopin, streptonigrin and terpentecin, are
intercalating agents that are thought to bind directly to the DNA
molecule and subsequently indirectly inhibit both types of Topo
activity (51). These chemicals
inhibit the DNA chain rejoining reactions catalyzed by Topos by
stabilizing a tight Topo protein-DNA complex termed the ‘cleavable
complex’ (52,53). The possible binding of β-mangostin
to DNA was examined by measuring the Tm of dsDNA, and no
β-mangostin was found to bind to dsDNA (Fig. 4). Topo inhibitors are categorized
into two classes, ‘suppressors’, which are believed to interact
directly with the enzyme, and ‘poisons’, which stimulate DNA
cleavage and intercalation (54,55).
β-Mangostin may be considered a suppressor of Topo functions rather
than a conventional poison as this compound does not appear to
stabilize Topo protein-DNA covalent complexes, as do the
above-mentioned agents. Therefore, β-mangostin could be a new type
of Topo inhibitor.
The fifteen Pols encoded by mammalian genomes are
specialized for different functions, including DNA replication, DNA
repair, recombination and translesion synthesis (6). Some Pol inhibitors, such as cytosine
arabinoside (AraC), are anti-cancer drugs, because these compounds
inhibit the activities of DNA replicative Pols α, δ and ɛ, suppress
DNA synthesis, arrest cells at S phase, and induce apoptosis in
cancer cells that have higher cellular Pol activity than normal
cells (56). β-Mangostin
suppressed proliferation of HeLa cell growth (Fig. 5) and arrested the cell cycle at S
phase (Fig. 6A). These effects
occur via the inhibition of Pols, especially DNA replicative
B-family Pol species (Fig. 2 and
Table I), suggesting that this
compound affects the cellular DNA synthesis of cancer cells.
Together this suggests that foods containing β-mangostin have
potential positive effects in the prevention of cancer and
promotion of health.
Acknowledgements
The present study was supported in part by the
Ministry of Education, Culture, Sports, Science and Technology
(MEXT), Japan, and the Japan-Supported Program for the Strategic
Research Foundation at Private Universities, 2012–2016. T.T.
acknowledges Kobe Pharmaceutical University Collaboration Fund.
Abbreviations:
|
Pol
|
DNA polymerase (E.C.2.7.7.7)
|
|
Topo
|
DNA topoisomerase
|
|
dsDNA
|
double-stranded DNA
|
|
TdT
|
terminal deoxynucleotidyl
transferase
|
|
dTTP
|
2′-deoxythymidine-5′-tripho-sphate
|
|
dNTP
|
2′-deoxynucleoside 5′-triphosphate
|
|
DMSO
|
dimethyl sulfoxide
|
|
EDTA
|
ethylenediaminetetraacetic acid
|
|
BSA
|
bovine serum albumin
|
|
SDS
|
sodium dodecyl sulfate
|
|
EtBr
|
ethidium bromide
|
|
ssDNA
|
single-stranded DNA
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
SD
|
standard deviation
|
|
IC50
|
50% inhibitory concentration
|
|
Tm
|
melting temperature
|
|
LD50
|
50% lethal dose
|
References
|
1
|
Liu RH: Potential synergy of
phytochemicals in cancer prevention: Mechanism of action. J Nutr.
134(Suppl): 3479S–3485S. 2004.PubMed/NCBI
|
|
2
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kornberg A and Baker TA: DNA replication.
2nd edition. W.D. Freeman and Co; New York: pp. 197–225. 1992
|
|
4
|
Hubscher U, Maga G and Spadari S:
Eukaryotic DNA polymerases. Annu Rev Biochem. 71:133–163. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bebenek K and Kunkel TA: DNA repair and
replication. Advances in Protein Chemistry. Yang W: Elsevier; San
Diego: pp. 137–165. 2004, View Article : Google Scholar
|
|
6
|
Lange SS, Takata K and Wood RD: DNA
polymerases and cancer. Nat Rev Cancer. 11:96–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Loeb LA and Monnat RJ Jr: DNA polymerases
and human disease. Nat Rev Genet. 9:594–604. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang JC: DNA topoisomerases. Annu Rev
Biochem. 65:635–692. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu LF: DNA topoisomerase poisons as
antitumor drugs. Annu Rev Biochem. 58:351–375. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakaguchi K, Sugawara F and Mizushina Y:
Inhibitors of eukaryotic DNA polymerases. Seikagaku. 74:244–251.
2002.(In Japanese). PubMed/NCBI
|
|
11
|
Berdis AJ: DNA polymerases as therapeutic
targets. Biochemistry. 47:8253–8260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ji X, Avula B and Khan IA: Quantitative
and qualitative determination of six xanthones in Garcinia
mangostana L. by LC-PDA and LC-ESI-MS. J Pharm Biomed Anal.
43:1270–1276. 2007. View Article : Google Scholar
|
|
13
|
Wexler B: Mangosteen. Woodland Publishing;
Utah: 2007
|
|
14
|
Akao Y, Nakagawa Y, Iinuma M and Nozawa Y:
Anti-cancer effects of xanthones from pericarps of mangosteen. Int
J Mol Sci. 9:355–370. 2008. View Article : Google Scholar
|
|
15
|
Yoo JH, Kang K, Jho EH, Chin YW, Kim J and
Nho CW: α- and γ-Mangostin inhibit the proliferation of colon
cancer cells via β-catenin gene regulation in Wnt/cGMP signalling.
Food Chem. 129:1559–1566. 2011. View Article : Google Scholar
|
|
16
|
Cui J, Hu W, Cai Z, Liu Y, Li S, Tao W and
Xiang H: New medicinal properties of mangostins: Analgesic activity
and pharmacological characterization of active ingredients from the
fruit hull of Garcinia mangostana L. Pharmacol Biochem Behav.
95:166–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung HA, Su BN, Keller WJ, Mehta RG and
Kinghorn AD: Antioxidant xanthones from the pericarp of Garcinia
mangostana (Mangosteen). J Agric Food Chem. 54:2077–2082. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen LG, Yang LL and Wang CC:
Anti-inflammatory activity of mangostins from Garcinia mangostana.
Food Chem Toxicol. 46:688–693. 2008. View Article : Google Scholar
|
|
19
|
Nakatani K, Atsumi M, Arakawa T, Oosawa K,
Shimura S, Nakahata N and Ohizumi Y: Inhibitions of histamine
release and prostaglandin E2 synthesis by mangosteen, a Thai
medicinal plant. Biol Pharm Bull. 25:1137–1141. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakagami Y, Iinuma M, Piyasena KG and
Dharmaratne HR: Antibacterial activity of α-mangostin against
vancomycin resistant Enterococci (VRE) and synergism with
antibiotics. Phytomedicine. 12:203–208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suksamrarn S, Suwannapoch N, Phakhodee W,
Thanuhiranlert J, Ratananukul P, Chimnoi N and Suksamrarn A:
Antimycobacterial activity of prenylated xanthones from the fruits
of Garcinia mangostana. Chem Pharm Bull (Tokyo). 51:857–859. 2003.
View Article : Google Scholar
|
|
22
|
Kaomongkolgit R, Jamdee K and Chaisomboon
N: Antifungal activity of alpha-mangostin against Candida albicans.
J Oral Sci. 51:401–406. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen SX, Wan M and Loh BN: Active
constituents against HIV-1 protease from Garcinia mangostana.
Planta Med. 62:381–382. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Devi Sampath P and Vijayaraghavan K:
Cardioprotective effect of alpha-mangostin, a xanthone derivative
from mangosteen on tissue defense system against
isoproterenol-induced myocardial infarction in rats. J Biochem Mol
Toxicol. 21:336–339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weecharangsan W, Opanasopit P, Sukma M,
Ngawhirunpat T, Sotanaphun U and Siripong P: Antioxidative and
neuroprotective activities of extracts from the fruit hull of
mangosteen (Garcinia mangostana Linn.). Med Princ Pract.
15:281–287. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang YP, Li PG, Kondo M, Ji HP, Kou Y and
Ou B: Effect of a mangosteen dietary supplement on human immune
function: A randomized, double-blind, placebo-controlled trial. J
Med Food. 12:755–763. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ryu HW, Curtis-Long MJ, Jung S, Jin YM,
Cho JK, Ryu YB, Lee WS and Park KH: Xanthones with neuraminidase
inhibitory activity from the seedcases of Garcinia mangostana.
Bioorg Med Chem. 18:6258–6264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ito C, Itoigawa M, Takakura T, Ruangrungsi
N, Enjo F, Tokuda H, Nishino H and Furukawa H: Chemical
constituents of Garcinia fusca: Structure elucidation of eight new
xanthones and their cancer chemopreventive activity. J Nat Prod.
66:200–205. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hano Y, Okamoto T, Suzuki K, Negishi M and
Nomura T: Constituents of the Moraceae plants. 17 Components of the
root bark of Morus insignis Bur 3 Structures of three new
isopre-nylated xanthones morusignins I, J, and K and an
isoprenylated flavone morusignin L. Heterocycles. 36:1359–1366.
1993. View Article : Google Scholar
|
|
30
|
Bennett GJ, Lee HH and Das NP:
Biosynthesis of mangostin. Part 1 The origin of the xanthone
skeleton. J Chem Soc, Perkin Trans. 1(10): 2671–2676. 1990.
View Article : Google Scholar
|
|
31
|
Tamai K, Kojima K, Hanaichi T, Masaki S,
Suzuki M, Umekawa H and Yoshida S: Structural study of
immunoaffinity-purified DNA polymerase α-DNA primase complex from
calf thymus. Biochim Biophys Acta. 950:263–273. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Date T, Yamaguchi M, Hirose F, Nishimoto
Y, Tanihara K and Matsukage A: Expression of active rat DNA
polymerase β in Escherichia coli. Biochemistry. 27:2983–2990. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Umeda S, Muta T, Ohsato T, Takamatsu C,
Hamasaki N and Kang D: The D-loop structure of human mtDNA is
destabilized directly by 1-methyl-4-phenylpyridinium ion
(MPP+), a parkinsonism-causing toxin. Eur J Biochem.
267:200–206. 2000. View Article : Google Scholar
|
|
34
|
Oshige M, Takeuchi R, Ruike T, Kuroda K
and Sakaguchi K: Subunit protein-affinity isolation of Drosophila
DNA polymerase catalytic subunit. Protein Expr Purif. 35:248–256.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kusumoto R, Masutani C, Shimmyo S, Iwai S
and Hanaoka F: DNA binding properties of human DNA polymerase et
al: Implications for fidelity and polymerase switching of
translesion synthesis. Genes Cells. 9:1139–1150. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Biertümpfel C, Zhao Y, Kondo Y,
Ramón-Maiques S, Gregory M, Lee JY, Masutani C, Lehmann AR, Hanaoka
F and Yang W: Structure and mechanism of human DNA polymerase eta.
Nature. 465:1044–1048. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ohashi E, Murakumo Y, Kanjo N, Akagi J,
Masutani C, Hanaoka F and Ohmori H: Interaction of hREV1 with three
human Y-family DNA polymerases. Genes Cells. 9:523–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shimazaki N, Yoshida K, Kobayashi T, Toji
S, Tamai K and Koiwai O: Over-expression of human DNA polymerase
lambda in E. coli and characterization of the recombinant enzyme.
Genes Cells. 7:639–651. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mizushina Y, Tanaka N, Yagi H, Kurosawa T,
Onoue M, Seto H, Horie T, Aoyagi N, Yamaoka M, Matsukage A, et al:
Fatty acids selectively inhibit eukaryotic DNA polymerase
activities in vitro. Biochim Biophys Acta. 1308:256–262. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mizushina Y, Yoshida S, Matsukage A and
Sakaguchi K: The inhibitory action of fatty acids on DNA polymerase
β. Biochim Biophys Acta. 1336:509–521. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ogawa A, Murate T, Suzuki M, Nimura Y and
Yoshida S: Lithocholic acid, a putative tumor promoter, inhibits
mammalian DNA polymerase β. Jpn J Cancer Res. 89:1154–1159. 1998.
View Article : Google Scholar
|
|
42
|
Yonezawa Y, Tsuzuki T, Eitsuka T, Miyazawa
T, Hada T, Uryu K, Murakami-Nakai C, Ikawa H, Kuriyama I, Takemura
M, et al: Inhibitory effect of conjugated eicosapentaenoic acid on
human DNA topoisomerases I and II. Arch Biochem Biophys.
435:197–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nakayama C and Saneyoshi M: Inhibitory
effects of 9-β-D-xylofuranosyladenine 5′-triphosphate on
DNA-dependent RNA polymerase I and II from cherry salmon
(Oncorhynchus masou). J Biochem. 97:1385–1389. 1985.PubMed/NCBI
|
|
44
|
Soltis DA and Uhlenbeck OC: Isolation and
characterization of two mutant forms of T4 polynucleotide kinase. J
Biol Chem. 257:11332–11339. 1982.PubMed/NCBI
|
|
45
|
Lu BC and Sakaguchi K: An endo-exonuclease
from meiotic tissues of the basidiomycete Coprinus cinereus. Its
purification and characterization. J Biol Chem. 266:21060–21066.
1991.PubMed/NCBI
|
|
46
|
Mizushina Y, Murakami C, Ohta K, Takikawa
H, Mori K, Yoshida H, Sugawara F and Sakaguchi K: Selective
inhibition of the activities of both eukaryotic DNA polymerases and
DNA topoisomerases by elenic acid. Biochem Pharmacol. 63:399–407.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ishiyama M, Tominaga H, Shiga M, Sasamoto
K, Ohkura Y and Ueno K: A combined assay of cell viability and in
vitro cytotoxicity with a highly water-soluble tetrazolium salt,
neutral red and crystal violet. Biol Pharm Bull. 19:1518–1520.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mizushina Y: Specific inhibitors of
mammalian DNA polymerase species. Biosci Biotechnol Biochem.
73:1239–1251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mizushina Y, Kamisuki S, Mizuno T,
Takemura M, Asahara H, Linn S, Yamaguchi T, Matsukage A, Hanaoka F,
Yoshida S, et al: Dehydroaltenusin, a mammalian DNA polymerase α
inhibitor. J Biol Chem. 275:33957–33961. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Murakami-Nakai C, Maeda N, Yonezawa Y,
Kuriyama I, Kamisuki S, Takahashi S, Sugawara F, Yoshida H,
Sakaguchi K and Mizushina Y: The effects of dehydroaltenusin, a
novel mammalian DNA polymerase α inhibitor, on cell proliferation
and cell cycle progression. Biochim Biophys Acta. 1674:193–199.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gatto B, Capranico G and Palumbo M: Drugs
acting on DNA topoisomerases: Recent advances and future
perspectives. Curr Pharm Des. 5:195–215. 1999.PubMed/NCBI
|
|
52
|
Larsen AK, Escargueil AE and Skladanowski
A: Catalytic topoisomerase II inhibitors in cancer therapy.
Pharmacol Ther. 99:167–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Teicher BA: Next generation topoisomerase
I inhibitors: Rationale and biomarker strategies. Biochem
Pharmacol. 75:1262–1271. 2008. View Article : Google Scholar
|
|
54
|
Bailly C: Topoisomerase I poisons and
suppressors as anticancer drugs. Curr Med Chem. 7:39–58. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pommier Y, Leo E, Zhang H and Marchand C:
DNA topoisomerases and their poisoning by anticancer and
antibacterial drugs. Chem Biol. 17:421–433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Park JK, Lee JS, Lee HH, Choi IS and Park
SD: Accumulation of polycyclic aromatic hydrocarbon-induced single
strand breaks is attributed to slower rejoining processes by DNA
polymerase inhibitor, cytosine arabinoside in CHO-K1 cells. Life
Sci. 48:1255–1261. 1991. View Article : Google Scholar : PubMed/NCBI
|