Esophageal cancer is the sixth leading cause of
cancer-related mortality and the eighth most common cancer with
more than 455,800 new esophageal cancer cases and 400,200 deaths
according to global cancer statistics (1). The incidence rates of EC have been
increasing (2). Tobacco use and
alcohol consumption are the main risks for EC (3). The two main types of EC are squamous
cell carcinoma (SCC) and adenocarcinoma. In the highest-risk area,
which stretches from Northern Iran through the Central Asian
republics to North-Central China, 90% of cases are squamous cell
carcinomas (4). Despite many
advances in EC treatment, over the last several decades, the
prognosis for patients with advanced EC remains poor. The overall
5-year survival ranges from 15% to 25% (5). Poor outcomes in patients with EC are
related to diagnosis at advanced (metastatic or disseminated)
stages. New treatment methods and strategies are necessary. Genome
instability with alterations in the expression of proteins is a
hallmark of cancer (6). Extensive
study in this field has led to a better understanding of the
molecular mechanism by which FBPs regulate cellular processes and
of how their deregulations contribute to carcinogenesis. In this
review, we summarize the related FBPs involved in EC, focusing on
the function and the substrates of each related F-box protein in
EC.
Intracellular protein degradation plays an important
role in the regulation of the cell cycle, signal transduction, and
disposal of improperly folded proteins. The ubiquitin-proteasome
pathway is the major system for protein degradation (7). Ubiquitin Proteasome System (UPS) is
an evolutionary conserved protein degradation mechanism that is
involved in various physiological responses such as cell cycle
control, DNA replication, transcription, and cell signaling
(8,9). Ubiquitin (Ub) is a 76-amino acid
protein that is covalently conjugated to a lysine residue in
proteins. Similarly to phosphorylation, ubiquitination is
reversible and linked to deubiquitination.
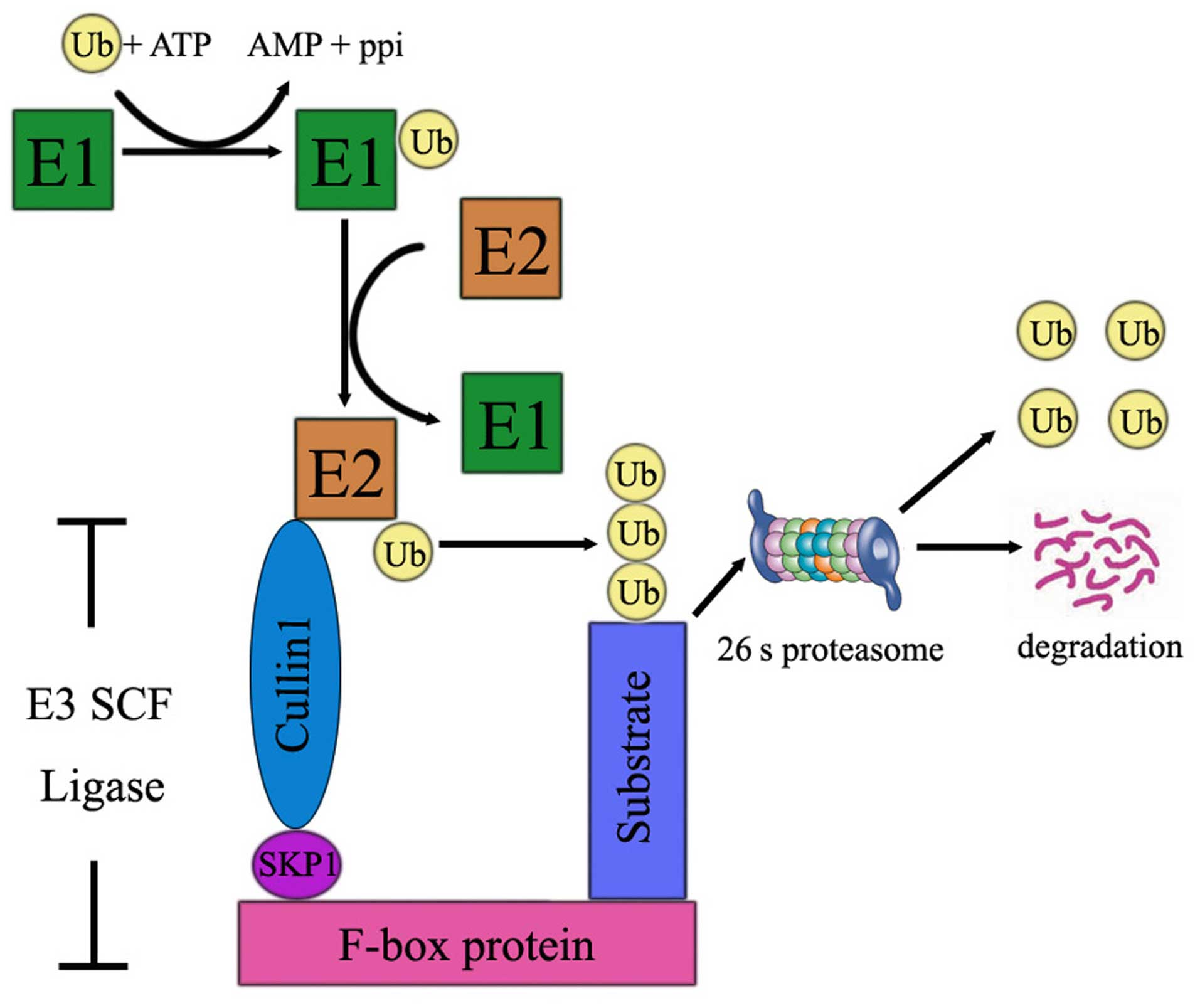
Ubiquitin attachment to substrates is accomplished
by the coordinated activity of three enzymes: ubiquitin-activating
enzyme (E1), ubiquitin conjugating enzyme (E2), and
ubiquitin-protein ligase (E3). The degradation of proteins by the
UPS is mainly comprised of three steps. The first step is that Ub
is activated by the E1 enzyme by creation of the thioester linkage
between Ub and the cysteine residues of E1 in an ATP and
Mg2+-dependent manner. Then, E2 accepts the activated
ubiquitin molecule from E1 and with a help from an E3 ubiquitin
ligase transfers it to the lysine residue of a target protein. In
the third step, the ubiquitin proteins are recognized and degraded
by 26 proteasome to several small peptides (Fig. 1). Ubiquitin-mediated proteolysis is
instigated by the attachment of K48 polyubiquitin chains to
substrates, which provides a signal for recognition and degradation
by the proteasome. E3 ubiquitin ligases are a large family of
proteins that are engaged in the regulation of the turnover and
activity of many target proteins. E3 determines the target protein
specificity, and it is the reason why the deregulation of E3 ligase
often leads to cancer development (10).
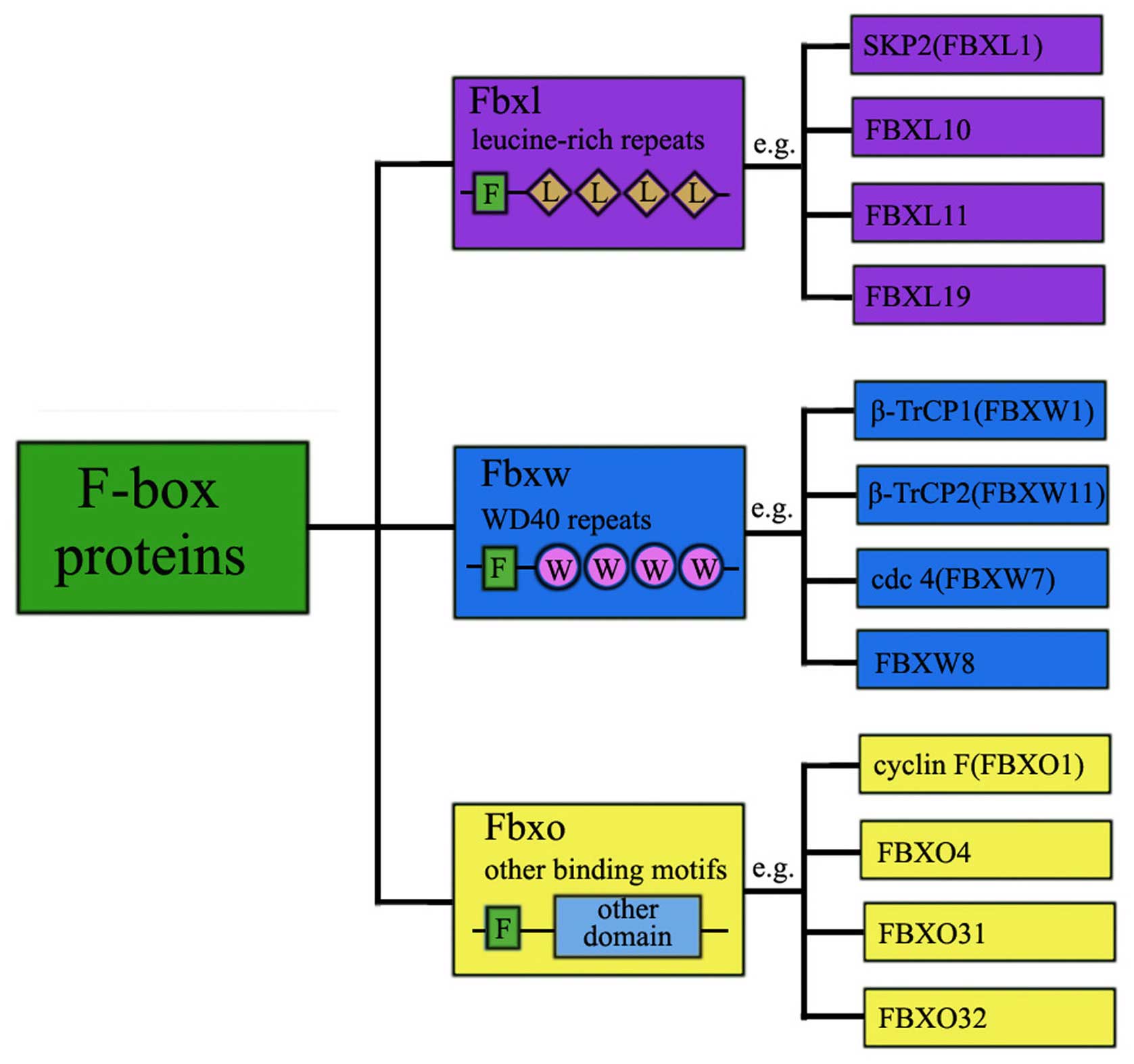
F-box is a widespread protein motif of ~40–50 amino
acids and it functions as a site of protein-protein interaction.
F-box proteins are categorized within three families based on the
recognizable domains beyond the F-box domain, which comprise the
Leu-rich repeat (L) family, WD40 domain (W) family and the F-box
only (O) family (Fig. 2). So far,
69 FBPs were identified in the human genome (14) and newer members of the F-box
protein family are being discovered. Extensive studies have been
heavily focused on only four FBPs SKP2, FBXW7, β-TrCP1, β-TrCP2,
and the other FBPs largely remain functionally mysterious.
The FBXW family is composed of 10 proteins, all the
members contain WD40 repeat domains. Proteins involved in
protein-protein interaction and containing WD40 repeats comprise
the first class of FBPs (FBXWs). There are 10 family members of
FBXWs. This class mainly targets proteins involved in cell cycle
regulation and tumorigenesis and thereby modulating their outcome.
The typical representatives of FBXW family are βTrCP1 (FBXW1),
βTrCP2 (FBXW11), hCdc4 (FBXW7). The roles of β-TrCP in cancer
differ according to its substrates. Loss of FBXW7 has been found in
many cancers and it is associated with poor prognosis. FBXW4 is
mutated, lost and underexpressed in a variety of human cancer cell
lines and clinical patient samples (15). FBXW8 is the only known F-box
protein to form an E3 complex with Skp1, Rbx1 and Cul7, which does
not bind SKP1 alone but selectively interacts with Skp1-FBXW8
heterodimer (16).
Some F-box proteins harbor an F-box and leucine-rich
repeats (LRRs). For this reason, the 22 FBPs with LRRs are
designated as FBXLs. These motifs are 20–29 residue sequences that
are frequent in many proteins providing a structural framework for
protein-protein recognition mechanism. SKP2 (also known as FBXL1)
can serve as an exemplified family member that has been
comprehensively studied and well characterized with respect to its
substrates. SKP2 is over-expressed in various types of human
cancers, which supports its role as a proto-oncoprotein. FBXL10
overexpression was observed in human pancreatic cancer (17). FBXL5 inhibited the metastasis of
gastric cancer (18).
FBXO proteins do not possess either WD40 or LRR
domains, but usually have other protein-protein interaction domains
such as zinc-finger, carbohydrate interacting (CASH), proline-rich
domains, Sec7, cyclinbox, Traf-domain-like and calponin homology
(CH) (19). FBXO family is the
most diverse subfamily of FBPs. Those proteins have other different
or unknown domain in the C-terminal region. There are 37 members in
the FBXO family. Currently, emerging experimental and clinical data
have begun to reveal some interesting biological functions on FBXO
proteins. FBXO5, an anaphase-promoting complex/cyclosome inhibitor,
can control tumor cell proliferation. FBXO5 overexpression causes
mitotic catastrophe and genomic instability and potentially
contributes to tumorigenesis (20). FBXO11 targets BCL6 for degradation
and functions as a tumor suppressor in diffuse large B-cell
lymphomas (21).
SKP2, located on 5p13 chromosome, was discovered in
1995 by Beach and his collaborators (22). The SCFskp2 E3 ligase
contains at the N-terminus an F-box domain which facilitates its
binding to SKP1 and CUL1 and at the C-terminus a leucine-rich
repeat domain for substrate recognition. SKP2, S-phase kinase
associated protein 2, plays a crucial role in cell cycle
progression by promoting the degradation of many key regulatory
proteins, including p27, p16, p21, p57, E2F-1, TOB1, RBL2, cyclin
D/E, BRCA2, FOXO1, RASSF1A. As the majority of the substrates are
tumor supressor proteins, it can be asserted that SKP2 functions as
an oncogene (23–26). P27 is a negative regulator of the
cell cycle that plays an important role in tumor suppression. Loss
of p27 results in uncontrolled proliferation and promotes tumor
progression (20,27). Taken together, these findings
indicate that the SKP2-mediated reduction, as a result of enhanced
degradation of tumor suppressor p27, contribute to the development
of cancers including esophageal cancer (28). Therefore, the p27 degradation
inhibitors present an attractive target for drugs. The involvement
of SKP2 overexpression in metastasis has been reported in many
tumors including melanoma (29),
nasopharyngeal carcinoma (30),
pancreatic cancer (31), breast
(32) and colorectal cancer
(33). Collectively, these data
suggest SKP2 to be a proto-oncoprotein.
The involvement of SKP2 as a common driving factor
in carcinogenesis has been proven. Fukuchi et al (24) first elucidated the role of SKP2 in
tumor progression, in which they suggested that SKP2 might be a
prognostic factor in early stage ESCC. They analyzed SKP2 and p27
expression in surgical specimens obtained from 32 patients, and
they found that SKP2 expression showed an inverse location and
correlation to p27 expression in early compared to advanced ESCC.
There was an inverse relationship between SKP2 and p27 in 6 ESCC
cell lines, but not cyclin E, cyclin D1 and E2F-1. DNA
amplification is one of the mechanisms activating SKP2 gene in
ESCC. Amplification and elevated expression of SKP2 was correlated
significantly with tumor stage and positive lymph node metastasis
in ESCC (34). Downregulation of
SKP2 by antisense treatment induced apoptosis and inhibited
invasion and migration in lung cancer cells (35). Wang et al (36) found that SKP2 expression levels was
increased in ESCC tissues. Elevated expression of SKP2 correlated
significantly with tumor stage and positive lymph node metastasis,
and high expression of SKP2 promoted the radioresistance of ESCC
cancer cells in part through Rad51 pathway. These alterations in
the various ways of the carcinogenesis appeared in different stages
of EC. The expression of SKP2 protein increased during esophageal
squamous cell cancer progression from normal esophageal tissues to
esophageal intraepithelial dysplasia (EID) and ESCC, which
indicated SKP2 as a potential diagnostic mark in clinical settings
(37). Liang et al
(38) showed that SKP2 expression
was not correlated with lymph node metastasis, but correlated with
local tumor invasion, which was not consistent with the observation
of Wang et al (36). SKP2
expression might be a new prognostic biomarker for tumor recurrence
in ESCC patients.
Molecular mechanisms by which SKP2 induces tumor
growth have not been fully elucidated. Some studies on the
relationship between PI3K/Akt pathway and SKP2 have been reported.
Akt is a downstream molecule of PI3K in response to growth factor
stimulation, and activated Akt promotes cell survival by
suppressing apoptosis (39).
PI3-K/Akt pathway leads to elevated SKP2 expression and subsequent
enhanced p27 destruction in human cancers. Inhibition of Akt1
activity in breast cancer cells could downregulate SKP2 expression
(40). SKP2 is the main
determinant in the PI3K/Akt-dependent regulation of p27(kip1) in
the prostate cancer cell lines PC3 and DU145 (41). Reichert et al (42) showed that in pancreatic ductal
adenocarcinoma cells, PI3K/Akt signaling was linked to SKP2 gene
transcription via control of a cis-acting element, E2F1, binding to
the proximal human SKP2 gene promoter. Whereas, the degradation of
E2F1 in S-G2 phase was mediated by SCFSKP2 complex
(43). Others have also reported
that PI3K/Akt signaling controls SKP2 transcription in different
cellular systems (44). However,
whether SKP2 also can affect the PI3K-Akt pathway still remains
unclear. Wang et al (34)
showed that decreased SKP2 reduced pAkt expression, and that the
PI3K/AKT pathway is the downstream target of SKP2. These results
suggest a possible negative feedback loop between PI3K/Akt and SKP2
that may help to maintain the balance between cell survival and
apoptosis. Further research exploring the molecular mechanisms by
which SKP2 affects the PI3K/Akt pathway awaits further
investigation.
FBXW7, F-box and WD40 repeat domain-containing 7, is
an F-box protein that is responsible for substrate recognition by
an SCF (Skp1-Cul1-F-box protein)-type ubiquitin ligase complex.
FBXW7 was first identified in budding yeast in 1973 (45). FBXW7 is localized to chromosome
region 4q32 and has three isoforms (FBXW7α, FBXW7β, FBXW7γ)
(46). All three isoforms contain
conserved interaction domains in the C-terminus and various
isoform-specific domains in the N-terminal region. FBXW7 has
pivotal roles in cell division, growth, and differentiation.
Burgeoning amounts of literature strongly supported FBXW7 was a
tumor suppressor in human cancers based on the following evidence:
i) FBXW7 low expression was frequently found in various human
cancers (47). ii) FBXW7 mediates
the degradation of several major oncoproteins such as cyclin E
(48), c-Myc (49), Notch (50) and c-Jun (50), which function in proliferation,
differentiation, apoptosis, and metabolism. iii) Decreased FBXW7
protein is associated with poor prognosis as well as tumor
metastasis (51–55). iv) FBXW7β is a p53 target gene and
p53 mutations may reduce FBXW7 expression (56). Furthermore, loss of FBXW7 in cancer
cells might promote resistance to taxol and ABT-737 (57,58).
These observations suggest that by upregulating FBXW7, drug
resistance could be reversed. It is worth mentioning that most
studies focus on discovering the ubiquitin targets of FBXW7
ubiquitin ligase pathway.
Increased number of substrates have deepened our
understanding of the diverse oncogenic pathways that FBXW7
regulates. It is widely accepted that the FBXW7 gene mutations and
allelic loss are the main mechanisms which downregulate FBXW7
protein expression and its tumor suppressor functions in cancer.
Proteasome-mediated FBXW7 protein degradation is another mechanism
(59,60). Over the past 5 years, our
understanding of the FBXW7 has increased enormously. The first to
explore FBXW7 in EC was Sterian et al (61), they found that 54% of the
esophageal adenocarcinomas showed allelic deletion in the
chromosome 4q region. This was confirmed by exome sequencing on 113
ESCC tumor-normal pairs. The link between the expression of FBXW7
protein and prognosis has been verified in many cancers. In ESCC,
decreased FBXW7 protein level may contribute to tumor progression
and local invasiveness (62). In
support of this notion, FBXW7 mRNA was significantly lower in ESCC
cancer tissues than in non-cancer tissues, which is correlated with
poor prognosis in ESCC (63).
RNAi-mediated knockdown of FBXW7 in ESCC cells promotes
proliferation in ESCC cell line KYSE70 (63). FBXW7 expression is under the
control of several oncogenic micro-RNAs such as miR-27, miR-92, and
miR-223 in numerous cancers. Overexpression of miR-223 increases
genomic instability by modulating expression of FBXW7 (64). There was a significant inverse
relationship between the expression levels of miR-223 and FBXW7
protein in ESCC, which indicated FBXW7 as a functional downstream
target of miR-223 (54).
Human β-Transducin repeat-containing protein
(β-TrCP), first identified in 1998 as a binding partner of HIV-1
Vpu protein in a yeast two-hybrid screening. The role of β-TrCP in
cancers is tissue-dependent. Overexpressed β-TrCP1 or β-TrCP2 has
been detected in multiple cancers, including gastrointestinal
cancers, hepato-blastoma, colorectal cancer, pancreatic cancer,
breast cancer and melanoma, suggesting a carcinogenic function for
these two proteins. β-TrCP also participates in cell adhesion and
migration (70). One typical
substrate of β-TrCP is the IκB protein, the inhibitor of the NFκB
pathway (71). Another substrate
is β-catenin in Wnt pathway (72).
IκB functions as a tumor suppressor. β-catenin has been identified
to be upregulated in various types of human cancer, and is always
correlated with poor prognosis and short survival (73,74).
IκB and β-catenin exerting antagonistic functions make it hard to
be an ideal drug target. β-TrCP is a member of the SCF family with
both oncogenic and tumor suppressor properties. In most cases,
β-TrCPs function as oncoproteins, whereas in a few others, they
have displayed tumor suppressive functions. Studies investigating
the role of β-TrCP in esophageal tumorigenesis are limited. Li
et al (75) first evaluated
the significance of β-TrCP in ESCC. Reduced expression of β-TrCP
was observed in 24.4% (29/119) ESCC specimens. There was no
correlation among the expressions of β-catenin and β-TrCP. No
correlation between immunoexpression of β-TrCP and
clinicopathological parameters was found in ESCC patients (75).
Cyclin-dependent kinases (CDK) drive cell-cycle
progression, control transcriptional processes, and thus, regulate
cell proliferation. Cyclin D1, the allosteric regulator of CDK4/6,
is an integral mediator of growth factor-dependent G1 phase
progression. Cyclin D1 overexpression occurs frequently in human
cancer including esophageal carcinomas. Both CDK4 and CDK6, when
complexed with cyclin D1, promote cell cycle progression (89). FBXO4 is an F-box constituent of SCF
ubiquitin ligases that directs ubiquitylation of cyclin D1
(90), therefore, FBXO4 was
considered as a tumor suppressor. FBXO4 promotes the ubiquitination
and degradation of TRF1, which is important for maintaining
telomere length. This role might make FBXO4 a factor in extending
the lifespan of nascent transforming cells (91). A study from Barbash et al
detected 14% hemizygous, missense mutations in the primary human
esophageal carcinoma (92). FBXO4
loss predisposes mice to NMBA (an esophageal carcinogen) induced
papilloma formation, which could be reversed by CDK4/6 inhibitors.
This results comfirmed the suppressor role of FBXO4 and
FBXO4/cyclin D1 pathway in esophageal tumorigenesis (91).
FBXO31. FBXO31 is a candidate breast tumor
suppressor encoded in 16q24.3 and plays a crucial role in DNA
damage response (93,94). The expression and function of
FBXO31 is controversial in different type of cancers. Studies in
breast cancer (95),
hepatocellular carcinoma (96) and
gastric cancer (97) indicated
that FBXO31 functioned as a tumor suppressor. FBXO31
mediated-degradation of MDM2 increased the levels of p53 and led to
growth arrest, suggesting FBXO31 as a tumor suppressor (98). Recently, a study showed a
conflicting result in lung cancer (99). Higher expression of FBXO31
significantly correlated to tumor size and infiltration, clinical
stages and metastasis. In addition, exogenous expression of FBXO31
promoted cell growth, metastasis and invasion in lung cancer cell
line A549. Moreover, tumorigenicity assays in nude mice showed
FBXO31 promoted tumor growth in vivo. This result was
consistent with the report by Kogo et al (100). They demonstrated the expression
of FBXO31 in 68 ESCC cases. High expression of FBXO31 was related
to depth of tumor invasion and clinical stage, but showed no
significant differences in lymph node metastasis, lymphatic and
venous invasion. Furthermore, FBXO31 mRNA expression in ESCC cancer
tissue may be promising novel prognostic marker. The previously
identified substrate for SCFFbxo31 in melanoma cells is
Cyclin D1 (93). However, in ESCC
cancer cells, FBXO31 mediates MKK6 but not cyclin D1 for
degradation, and FBXO31 exerts anti-apoptotic effects on cancer
cells in response to stress stimuli (94).
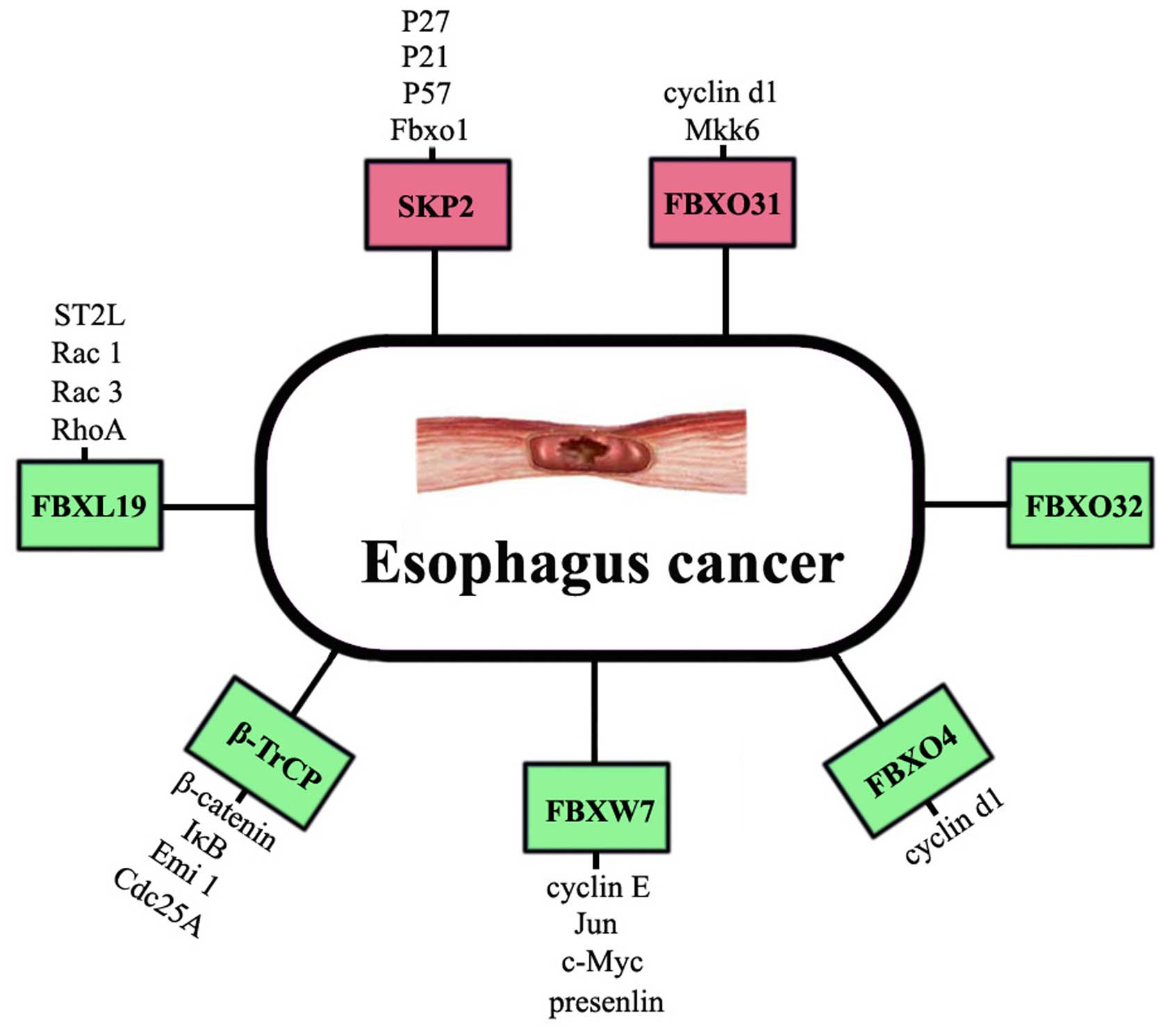
Extensive study of UPS has led to a better
understanding of the molecular mechanism by which E3 ligases
contribute to EC. FBPs are the core components of PBPs. Two F-box
proteins SKP2 and FBXW7 have showed strong clinical relevance in
the initiation and development of EC. Other FBPs such as β-TrCP,
FBXL19, FBXO4, FBXO31 and FBXO32 have been proved to be involved in
EC (Fig. 3) (101). Considering that F-box proteins
can bind with a diversity of substrates and that each substrate may
be regulated by many different E3 ligases depending on cell type.
Therefore, identifying critical substrates of each F-box protein is
paramount for understanding tumorigenesis and discovering
therapeutic targets. However, it should be stressed that FBPs
deregulate an entire network of proteins. Two main substrates of
β-TrCP are IκB in the NF-κB pathway and β-catenin in Wnt pathway
(71,72). IκB, inhibitor of NF-κB, functions
as a tumor suppressor. β-catenin has been identified to be an
oncogene in cancers. Intriguingly, some FBPs can be controlled by
their substrates. More complicated substrates feed back to control
FBPs. FBXW7α interacts with C/EBPδ and targets it for degradation
(62), but C/EBPδ inhibits FBXW7
expression and promotes mammary tumour metastasis (102). Consistent with these findings is
a recent study by Sancho and colleagues which show that, FBXW7
repression by hes5 (a Notch target gene) creates a feedback loop
that modulates Notch-mediated intestinal and neural stem cell fate
decisions (103).
In conclusion, this review provides only a glimpse
into the mechanisms through which FBPs are involved in EC. However,
all the current understanding is just the tip of the iceberg. The
growing details in understanding of SCF-based protein targeting of
a diverse array of fundamental substrates provide a great
opportunity for cancer therapeutic development targeting FBPs. It
has become clear that some FBPs, such as SKP2 and FBXW7, are
promising targets for EC therapy. The proteasome inhibitor
bortezomib, which blocks the entire protein degradation,
highlighted the therapeutic potential of targeting this protein
degradation system (104).
However, bortezomib has been used in clinical trial for cancer
treatment but with limited success. Selective inhibitors targeting
a particular E3 ligase or a certain F-box protein may be more
effective, but extensive research is to be continued (105). To this end, there are still many
important remaining questions to be resolved. We need to identify
the physiological substrates for many orphan F-box proteins. To
assess whether there is crosstalk between individual F-box proteins
calls for research in this emerging area involving functional
delineation of FBPs and their cellular context-specific substrates
in human EC. A better mechanistic understanding of FBP-regulated
network of proteins in initiation and progression of cancer will be
a research hotpot in the future.
J.G. and J.-R.H. are grateful for the Fundamental
Research Funds from the Central Universities of Central South
University (no. 2015zzts119).
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gammon MD, Schoenberg JB, Ahsan H, Risch
HA, Vaughan TL, Chow WH, Rotterdam H, West AB, Dubrow R, Stanford
JL, et al: Tobacco, alcohol, and socioeconomic status and
adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer
Inst. 89:1277–1284. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang WR, Fang JY, Wu KS, Shi XJ, Luo JY
and Lin K: Epidemiological characteristics and prediction of
esophageal cancer mortality in China from 1991 to 2012. Asian Pac J
Cancer Prev. 15:6929–6934. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hershko DD: Oncogenic properties and
prognostic implications of the ubiquitin ligase Skp2 in cancer.
Cancer. 112:1415–1424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao M and Karin M: Regulating the
regulators: Control of protein ubiquitination and ubiquitin-like
modifications by extra-cellular stimuli. Mol Cell. 19:581–593.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thrower JS, Hoffman L, Rechsteiner M and
Pickart CM: Recognition of the polyubiquitin proteolytic signal.
EMBO J. 19:94–102. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kitagawa K, Kotake Y and Kitagawa M:
Ubiquitin-mediated control of oncogene and tumor suppressor gene
products. Cancer Sci. 100:1374–1381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Y and Sun Y: Cullin-RING Ligases as
attractive anti-cancer targets. Curr Pharm Des. 19:3215–3225. 2013.
View Article : Google Scholar
|
|
12
|
Reed SI: Ratchets and clocks: The cell
cycle, ubiquitylation and protein turnover. Nat Rev Mol Cell Biol.
4:855–864. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peters J-M: The anaphase promoting
complex/cyclosome: A machine designed to destroy. Nat Rev Mol Cell
Biol. 7:644–656. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Z, Liu P, Inuzuka H and Wei W: Roles
of F-box proteins in cancer. Nat Rev Cancer. 14:233–247. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lockwood WW, Chandel SK, Stewart GL,
Erdjument-Bromage H and Beverly LJ: The novel ubiquitin ligase
complex, SCF(Fbxw4), interacts with the COP9 signalosome in an
F-box dependent manner, is mutated, lost and under-expressed in
human cancers. PLoS One. 8:e636102013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huber C, Dias-Santagata D, Glaser A,
O'Sullivan J, Brauner R, Wu K, Xu X, Pearce K, Wang R, Uzielli ML,
et al: Identification of mutations in CUL7 in 3-M syndrome. Nat
Genet. 37:1119–1124. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tzatsos A, Paskaleva P, Ferrari F,
Deshpande V, Stoykova S, Contino G, Wong KK, Lan F, Trojer P, Park
PJ, et al: KDM2B promotes pancreatic cancer via Polycomb-dependent
and -independent transcriptional programs. J Clin Invest.
123:727–739. 2013.PubMed/NCBI
|
|
18
|
Wu W, Ding H, Cao J and Zhang W: FBXL5
inhibits metastasis of gastric cancer through suppressing Snail1.
Cell Physiol Biochem. 35:1764–1772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cardozo T and Pagano M: The SCF ubiquitin
ligase: Insights into a molecular machine. Nat Rev Mol Cell Biol.
5:739–751. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao Y, Tang Q, Ni R, Huang X, Wang Y, Lu
C, Shen A, Wang Y, Li C, Yuan Q, et al: Early mitotic inhibitor-1,
an anaphase-promoting complex/cyclosome inhibitor, can control
tumor cell proliferation in hepatocellular carcinoma: Correlation
with Skp2 stability and degradation of p27(Kip1). Hum Pathol.
44:365–373. 2013. View Article : Google Scholar
|
|
21
|
Duan S, Cermak L, Pagan JK, Rossi M,
Martinengo C, di Celle PF, Chapuy B, Shipp M, Chiarle R and Pagano
M: FBXO11 targets BCL6 for degradation and is inactivated in
diffuse large B-cell lymphomas. Nature. 481:90–93. 2012. View Article : Google Scholar :
|
|
22
|
Demetrick DJ, Zhang H and Beach DH:
Chromosomal mapping of the genes for the human CDK2/cyclin
A-associated proteins p19 (SKP1A and SKP1B) and p45 (SKP2).
Cytogenet Cell Genet. 73:104–107. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hershko D, Bornstein G, Ben-Izhak O,
Carrano A, Pagano M, Krausz MM and Hershko A: Inverse relation
between levels of p27(Kip1) and of its ubiquitin ligase subunit
Skp2 in colorectal carcinomas. Cancer. 91:1745–1751. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fukuchi M, Masuda N, Nakajima M, Fukai Y,
Miyazaki T, Kato H and Kuwano H: Inverse correlation between
expression levels of p27 and the ubiquitin ligase subunit Skp2 in
early esophageal squamous cell carcinoma. Anticancer Res. 24(2B):
777–783. 2004.PubMed/NCBI
|
|
25
|
Yang G, Ayala G, De Marzo A, Tian W,
Frolov A, Wheeler TM, Thompson TC and Harper JW: Elevated Skp2
protein expression in human prostate cancer: Association with loss
of the cyclin-dependent kinase inhibitor p27 and PTEN and with
reduced recurrence-free survival. Clin Cancer Res. 8:3419–3426.
2002.PubMed/NCBI
|
|
26
|
Traub F, Mengel M, Lück HJ, Kreipe HH and
von Wasielewski R: Prognostic impact of Skp2 and p27 in human
breast cancer. Breast Cancer Res Treat. 99:185–191. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Masuda TA, Inoue H, Sonoda H, Mine S,
Yoshikawa Y, Nakayama K, Nakayama K and Mori M: Clinical and
biological significance of S-phase kinase-associated protein 2
(Skp2) gene expression in gastric carcinoma: Modulation of
malignant phenotype by Skp2 overexpression, possibly via p27
proteolysis. Cancer Res. 62:3819–3825. 2002.PubMed/NCBI
|
|
28
|
Frescas D and Pagano M: Deregulated
proteolysis by the F-box proteins SKP2 and beta-TrCP: Tipping the
scales of cancer. Nat Rev Cancer. 8:438–449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rose AE, Wang G, Hanniford D, Monni S, Tu
T, Shapiro RL, Berman RS, Pavlick AC, Pagano M, Darvishian F, et
al: Clinical relevance of SKP2 alterations in metastatic melanoma.
Pigment Cell Melanoma Res. 24:197–206. 2011. View Article : Google Scholar
|
|
30
|
Xu HM, Liang Y, Chen Q, Wu QN, Guo YM,
Shen GP, Zhang RH, He ZW, Zeng YX, Xie FY, et al: Correlation of
Skp2 overexpression to prognosis of patients with nasopharyngeal
carcinoma from South China. Chin J Cancer. 30:204–212. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schüler S, Diersch S, Hamacher R, Schmid
RM, Saur D and Schneider G: SKP2 confers resistance of pancreatic
cancer cells towards TRAIL-induced apoptosis. Int J Oncol.
38:219–225. 2011.
|
|
32
|
Wang Z, Fukushima H, Inuzuka H, Wan L, Liu
P, Gao D, Sarkar FH and Wei W: Skp2 is a promising therapeutic
target in breast cancer. Front Oncol. 1:187022012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shapira M, Ben-Izhak O, Linn S, Futerman
B, Minkov I and Hershko DD: The prognostic impact of the ubiquitin
ligase subunits Skp2 and Cks1 in colorectal carcinoma. Cancer.
103:1336–1346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang XC, Wu YP, Ye B, Lin DC, Feng YB,
Zhang ZQ, Xu X, Han YL, Cai Y, Dong JT, et al: Suppression of
anoikis by SKP2 amplification and overexpression promotes
metastasis of esophageal squamous cell carcinoma. Mol Cancer Res.
7:12–22. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yokoi S, Yasui K, Saito-Ohara F, Koshikawa
K, Iizasa T, Fujisawa T, Terasaki T, Horii A, Takahashi T,
Hirohashi S, et al: A novel target gene, SKP2, within the 5p13
amplicon that is frequently detected in small cell lung cancers. Am
J Pathol. 161:207–216. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang XC, Tian LL, Tian J and Jiang XY:
Overexpression of SKP2 promotes the radiation resistance of
esophageal squamous cell carcinoma. Radiat Res. 177:52–58. 2012.
View Article : Google Scholar
|
|
37
|
Bai P, Xiao X, Zou J, Cui L, Bui Nguyen
TM, Liu J, Xiao J, Chang B, Wu J and Wang H: Expression of
p14(ARF), p15(INK4b), p16(INK4a) and skp2 increases during
esophageal squamous cell cancer progression. Exp Ther Med.
3:1026–1032. 2012.PubMed/NCBI
|
|
38
|
Liang Y, Hou X, Cui Q, Kang T-B, Fu J-H,
Zhang L-J, Luo R-Z, He J-H, Zeng Y-X and Yang H-X: Skp2 expression
unfavorably impacts survival in resectable esophageal squamous cell
carcinoma. J Transl Med. 10:732012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hennessy BT, Smith DL, Ram PT, Lu Y and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Discov. 4:988–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gao D, Inuzuka H, Tseng A, Chin RY, Toker
A and Wei W: Phosphorylation by Akt1 promotes cytoplasmic
localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction.
Nat Cell Biol. 11:397–408. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
van Duijn PW and Trapman J: PI3K/Akt
signaling regulates p27(kip1) expression via Skp2 in PC3 and DU145
prostate cancer cells, but is not a major factor in p27(kip1)
regulation in LNCaP and PC346 cells. Prostate. 66:749–760. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Reichert M, Saur D, Hamacher R, Schmid RM
and Schneider G: Phosphoinositide-3-kinase signaling controls
S-phase kinase-associated protein 2 transcription via E2F1 in
pancreatic ductal adenocarcinoma cells. Cancer Res. 67:4149–4156.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nakayama KI and Nakayama K: Ubiquitin
ligases: Cell-cycle control and cancer. Nat Rev Cancer. 6:369–381.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Andreu EJ, Lledó E, Poch E, Ivorra C,
Albero MP, Martínez-Climent JA, Montiel-Duarte C, Rifón J,
Pérez-Calvo J, Arbona C, et al: BCR-ABL induces the expression of
Skp2 through the PI3K pathway to promote p27Kip1 degradation and
proliferation of chronic myelogenous leukemia cells. Cancer Res.
65:3264–3272. 2005.PubMed/NCBI
|
|
45
|
Hartwell LH, Mortimer RK, Culotti J and
Culotti M: Genetic Control of the Cell Division Cycle in Yeast: V.
Genetic Analysis of cdc Mutants. Genetics. 74:267–286.
1973.PubMed/NCBI
|
|
46
|
Sionov RV, Netzer E and Shaulian E:
Differential regulation of FBXW7 isoforms by various stress
stimuli. Cell Cycle. 12:3547–3554. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Davis RJ, Welcker M and Clurman BE: Tumor
suppression by the Fbw7 ubiquitin ligase: Mechanisms and
opportunities. Cancer Cell. 26:455–464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Minella AC, Welcker M and Clurman BE: Ras
activity regulates cyclin E degradation by the Fbw7 pathway. Proc
Natl Acad Sci USA. 102:9649–9654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yada M: Hat ediated by the F-box protein
Fbw7. EMBO J. 23:2116–2125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hoeck JD, Jandke A, Blake SM, Nye E,
Spencer-Dene B, Brandner S and Behrens A: Fbw7 controls neural stem
cell differentiation and progenitor apoptosis via Notch and c-Jun.
Nat Neurosci. 13:1365–1372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Enkhbold C, Utsunomiya T, Morine Y, Imura
S, Ikemoto T, Arakawa Y, Kanamoto M, Iwahashi S, Saito Y, Ishikawa
D, et al: Loss of FBXW7 expression is associated with poor
prognosis in intrahepatic cholangiocarcinoma. Hepatol Res.
44:E346–E352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ibusuki M, Yamamoto Y, Shinriki S, Ando Y
and Iwase H: Reduced expression of ubiquitin ligase FBXW7 mRNA is
associated with poor prognosis in breast cancer patients. Cancer
Sci. 102:439–445. 2011. View Article : Google Scholar
|
|
53
|
Iwatsuki M, Mimori K, Ishii H, Yokobori T,
Takatsuno Y, Sato T, Toh H, Onoyama I, Nakayama KI, Baba H, et al:
Loss of FBXW7, a cell cycle regulating gene, in colorectal cancer:
Clinical significance. Int J Cancer. 126:1828–1837. 2010.
|
|
54
|
Kurashige J, Watanabe M, Iwatsuki M,
Kinoshita K, Saito S, Hiyoshi Y, Kamohara H, Baba Y, Mimori K and
Baba H: Overexpression of microRNA-223 regulates the ubiquitin
ligase FBXW7 in oesophageal squamous cell carcinoma. Br J Cancer.
106:182–188. 2012. View Article : Google Scholar :
|
|
55
|
Yokobori T, Mimori K, Iwatsuki M, Ishii H,
Onoyama I, Fukagawa T, Kuwano H, Nakayama KI and Mori M:
p53-altered FBXW7 expression determines poor prognosis in gastric
cancer cases. Cancer Res. 69:3788–3794. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kimura T, Gotoh M, Nakamura Y and Arakawa
H: hCDC4b, a regulator of cyclin E, as a direct transcriptional
target of p53. Cancer Sci. 94:431–436. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Inuzuka H, Shaik S, Onoyama I, Gao D,
Tseng A, Maser RS, Zhai B, Wan L, Gutierrez A, Lau AW, et al:
SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for
ubiquitylation and destruction. Nature. 471:104–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wertz IE, Kusam S, Lam C, Okamoto T,
Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, et al:
Sensitivity to antitubulin chemotherapeutics is regulated by MCL1
and FBW7. Nature. 471:110–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen J, Shin JH, Zhao R, Phan L, Wang H,
Xue Y, Post SM, Ho Choi H, Chen JS, Wang E, et al: CSN6 drives
carcinogenesis by positively regulating Myc stability. Nat Commun.
5:53842014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ji S, Qin Y, Shi S, Liu X, Hu H, Zhou H,
Gao J, Zhang B, Xu W, Liu J, et al: ERK kinase phosphorylates and
destabilizes the tumor suppressor FBW7 in pancreatic cancer. Cell
Res. 25:561–573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sterian A, Kan T, Berki AT, Mori Y, Olaru
A, Schulmann K, Sato F, Wang S, Paun B, Cai K, et al: Mutational
and LOH analyses of the chromosome 4q region in esophageal
adenocarcinoma. Oncology. 70:168–172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Balamurugan K, Sharan S, Klarmann KD,
Zhang Y, Coppola V, Summers GH, Roger T, Morrison DK, Keller JR and
Sterneck E: FBXW7α attenuates inflammatory signalling by
downregulating C/EBPδ and its target gene Tlr4. Nat Commun.
4:16622013. View Article : Google Scholar
|
|
63
|
Yokobori T, Mimori K, Iwatsuki M, Ishii H,
Tanaka F, Sato T, Toh H, Sudo T, Iwaya T, Tanaka Y, et al: Copy
number loss of FBXW7 is related to gene expression and poor
prognosis in esophageal squamous cell carcinoma. Int J Oncol.
41:253–259. 2012.PubMed/NCBI
|
|
64
|
Xu Y, Sengupta T, Kukreja L and Minella
AC: MicroRNA-223 regulates cyclin E activity by modulating
expression of F-box and WD-40 domain protein 7. J Biol Chem.
285:34439–34446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gomes MD, Lecker SH, Jagoe RT, Navon A and
Goldberg AL: Atrogin-1, a muscle-specific F-box protein highly
expressed during muscle atrophy. Proc Natl Acad Sci USA.
98:14440–14445. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hanai J, Cao P, Tanksale P, Imamura S,
Koshimizu E, Zhao J, Kishi S, Yamashita M, Phillips PS, Sukhatme
VP, et al: The muscle-specific ubiquitin ligase atrogin-1/MAFbx
mediates statin-induced muscle toxicity. J Clin Invest.
117:3940–3951. 2007.PubMed/NCBI
|
|
67
|
Guo W, Zhang M, Shen S, Guo Y, Kuang G,
Yang Z and Dong Z: Aberrant methylation and decreased expression of
the TGF-β/Smad target gene FBXO32 in esophageal squamous cell
carcinoma. Cancer. 120:2412–2423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Guo W, Zhang M, Guo Y, Shen S, Guo X and
Dong Z: FBXO32, a new TGF-β/Smad signaling pathway target gene, is
epigenetically inactivated in gastric cardia adenocarcinoma.
Neoplasma. 62:646–657. 2015. View Article : Google Scholar
|
|
69
|
Chou JL, Su HY, Chen LY, Liao YP,
Hartman-Frey C, Lai YH, Yang HW, Deatherage DE, Kuo CT, Huang YW,
et al: Promoter hypermethylation of FBXO32, a novel TGF-beta/SMAD4
target gene and tumor suppressor, is associated with poor prognosis
in human ovarian cancer. Lab Invest. 90:414–425. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM
and Zhou BP: Stabilization of snail by NF-kappaB is required for
inflammation-induced cell migration and invasion. Cancer Cell.
15:416–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shirane M, Hatakeyama S, Hattori K and
Nakayama K and Nakayama K: Common pathway for the ubiquitination of
IkappaBalpha, IkappaBbeta, and IkappaBepsilon mediated by the F-box
protein FWD1. J Biol Chem. 274:28169–28174. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Spiegelman VS, Slaga TJ, Pagano M,
Minamoto T, Ronai Z and Fuchs SY: Wnt/beta-catenin signaling
induces the expression and activity of betaTrCP ubiquitin ligase
receptor. Mol Cell. 5:877–882. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang N, Wei P, Gong A, Chiu WT, Lee HT,
Colman H, Huang H, Xue J, Liu M, Wang Y, et al: FoxM1 promotes
β-catenin nuclear localization and controls Wnt target-gene
expression and glioma tumorigenesis. Cancer Cell. 20:427–442. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mokkapati S, Niopek K, Huang L, Cunniff
KJ, Ruteshouser EC, deCaestecker M, Finegold MJ and Huff V:
β-catenin activation in a novel liver progenitor cell type is
sufficient to cause hepatocellular carcinoma and hepatoblastoma.
Cancer Res. 74:4515–4525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Li AF, Hsu PK, Tzao C, Wang YC, Hung IC,
Huang MH and Hsu HS: Reduced axin protein expression is associated
with a poor prognosis in patients with squamous cell carcinoma of
esophagus. Ann Surg Oncol. 16:2486–2493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Katoh M and Katoh M: Identification and
characterization of FBXL19 gene in silico. Int J Mol Med.
14:1109–1114. 2004.PubMed/NCBI
|
|
77
|
O'Rielly DD and Rahman P: Genetics of
psoriatic arthritis. Best Pract Res Clin Rheumatol. 28:673–685.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chandran V: The genetics of psoriasis and
psoriatic arthritis. Clin Rev Allergy Immunol. 44:149–156. 2013.
View Article : Google Scholar
|
|
79
|
Cabaleiro T, Prieto-Pérez R, Navarro R,
Solano G, Román M, Ochoa D, Abad-Santos F and Daudén E: Paradoxical
psoria-siform reactions to anti-TNFα drugs are associated with
genetic polymorphisms in patients with psoriasis. Pharmacogenomics
J. Jul 21–2015, (Epub ahead of print) http://dx.doi.org/10.1038/tpj.2015.53.
View Article : Google Scholar
|
|
80
|
Kurowska-Stolarska M, Hueber A, Stolarski
B and McInnes IB: Interleukin-33: A novel mediator with a role in
distinct disease pathologies. J Intern Med. 269:29–35. 2011.
View Article : Google Scholar
|
|
81
|
Zhao J, Wei J, Mialki RK, Mallampalli DF,
Chen BB, Coon T, Zou C, Mallampalli RK and Zhao Y: F-box protein
FBXL19-mediated ubiquitination and degradation of the receptor for
IL-33 limits pulmonary inflammation. Nat Immunol. 13:651–658. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhao J, Mialki RK, Wei J, Coon TA, Zou C,
Chen BB, Mallampalli RK and Zhao Y: SCF E3 ligase F-box protein
complex SCF (FBXL19) regulates cell migration by mediating Rac1
ubiquitination and degradation. FASEB J. 27:2611–2619. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
ten Klooster JP, Leeuwen I, Scheres N,
Anthony EC and Hordijk PL: Rac1-induced cell migration requires
membrane recruitment of the nuclear oncogene SET. EMBO J.
26:336–345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Su J and Li H: RAC1 overexpression
promotes the proliferation, migration and epithelial-mesenchymal
transition of lens epithelial cells. Int J Clin Exp Pathol.
8:10760–11767. 2015.PubMed/NCBI
|
|
85
|
Filippi MD, Szczur K, Harris CE and
Berclaz PY: Rho GTPase Rac1 is critical for neutrophil migration
into the lung. Blood. 109:1257–1264. 2007. View Article : Google Scholar
|
|
86
|
Lao-Sirieix P and Fitzgerald RC: Role of
the micro-environment in Barrett's carcinogenesis. Biochem Soc
Trans. 38:327–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Dong S, Zhao J, Wei J, Bowser RK, Khoo A,
Liu Z, Luketich JD, Pennathur A, Ma H and Zhao Y: F-box protein
complex FBXL19 regulates TGFβ1-induced E-cadherin down-regulation
by mediating Rac3 ubiquitination and degradation. Mol Cancer.
13:762014. View Article : Google Scholar
|
|
88
|
Wei J, Mialki RK, Dong S, Khoo A,
Mallampalli RK, Zhao Y and Zhao J: A new mechanism of RhoA
ubiquitination and degradation: roles of SCF(FBXL19) E3 ligase and
Erk2. Biochim Biophys Acta. 1833:2757–2764. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sheppard KE and McArthur GA: The
cell-cycle regulator CDK4: An emerging therapeutic target in
melanoma. Clin Cancer Res. 19:5320–5328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lian Z, Lee EK, Bass AJ, Wong KK,
Klein-Szanto AJ, Rustgi AK and Diehl JA: FBXO4 loss facilitates
carcinogen induced papilloma development in mice. Cancer Biol Ther.
16:750–755. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lee TH, Perrem K, Harper JW, Lu KP and
Zhou XZ: The F-box protein FBX4 targets PIN2/TRF1 for
ubiquitin-mediated degradation and regulates telomere maintenance.
J Biol Chem. 281:759–768. 2006. View Article : Google Scholar
|
|
92
|
Barbash O, Zamfirova P, Lin DI, Chen X,
Yang K, Nakagawa H, Lu F, Rustgi AK and Diehl JA: Mutations in Fbx4
inhibit dimerization of the SCF(Fbx4) ligase and contribute to
cyclin D1 overexpression in human cancer. Cancer Cell. 14:68–78.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Santra MK, Wajapeyee N and Green MR: F-box
protein FBXO31 mediates cyclin D1 degradation to induce G1 arrest
after DNA damage. Nature. 459:722–725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Jia L and Sun Y: F-box proteins FBXO31 and
FBX4 in regulation of cyclin D1 degradation upon DNA damage.
Pigment Cell Melanoma Res. 22:518–519. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kumar R, Neilsen PM, Crawford J, McKirdy
R, Lee J, Powell JA, Saif Z, Martin JM, Lombaerts M, Cornelisse CJ,
et al: FBXO31 is the chromosome 16q24.3 senescence gene, a
candidate breast tumor suppressor, and a component of an SCF
complex. Cancer Res. 65:11304–11313. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Huang HL, Zheng WL, Zhao R, Zhang B and Ma
WL: FBXO31 is down-regulated and may function as a tumor suppressor
in hepatocellular carcinoma. Oncol Rep. 24:715–720. 2010.PubMed/NCBI
|
|
97
|
Zhang X, Kong Y, Xu X, Xing H, Zhang Y,
Han F, Li W, Yang Q, Zeng J, Jia J, et al: F-box protein FBXO31 is
down-regulated in gastric cancer and negatively regulated by miR-17
and miR-20a. Oncotarget. 5:6178–6190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Malonia SK, Dutta P, Santra MK and Green
MR: F-box protein FBXO31 directs degradation of MDM2 to facilitate
p53-mediated growth arrest following genotoxic stress. Proc Natl
Acad Sci USA. 112:8632–8637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Huang HL, Jiang Y, Wang YH, Chen T, He HJ,
Liu T, Yang T, Yang LW, Chen J, Song ZQ, et al: FBXO31 promotes
cell proliferation, metastasis and invasion in lung cancer. Am J
Cancer Res. 5:1814–1822. 2015.PubMed/NCBI
|
|
100
|
Kogo R, Mimori K, Tanaka F, Komune S and
Mori M: FBXO31 determines poor prognosis in esophageal squamous
cell carcinoma. Int J Oncol. 39:155–159. 2011.PubMed/NCBI
|
|
101
|
Liu J, Han L, Li B, Yang J, Huen MS, Pan
X, Tsao SW and Cheung AL: F-box only protein 31 (FBXO31) negatively
regulates p38 mitogen-activated protein kinase (MAPK) signaling by
mediating lysine 48-linked ubiquitination and degradation of
mitogen-activated protein kinase kinase 6 (MKK6). J Biol Chem.
289:21508–21518. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Balamurugan K, Wang JM, Tsai HH, Sharan S,
Anver M, Leighty R and Sterneck E: The tumour suppressor C/EBPδ
inhibits FBXW7 expression and promotes mammary tumour metastasis.
EMBO J. 29:4106–4117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Sancho R, Blake SM, Tendeng C, Clurman BE,
Lewis J and Behrens A: Fbw7 repression by hes5 creates a feedback
loop that modulates Notch-mediated intestinal and neural stem cell
fate decisions. PLoS Biol. 11:e10015862013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Kane RC, Bross PF, Farrell AT and Pazdur
R: Velcade: U.S. FDA approval for the treatment of multiple myeloma
progressing on prior therapy. Oncologist. 8:508–513. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Skaar JR, Pagan JK and Pagano M: SCF
ubiquitin ligase-targeted therapies. Nat Rev Drug Discov.
13:889–903. 2014. View Article : Google Scholar : PubMed/NCBI
|