Introduction
Hepatocellular carcinoma (HCC) is one of the most
common cancers worldwide and the third leading causes of
cancer-related death (1,2). Despite recent advances in clinical
and experimental treatment, the prognosis of HCC remains poor with
a dismal 5-year survival (3), and
the detailed mechanism underlying the development and progression
of HCC has not been fully elucidated (4). Therefore, it is of great importance
to clarify the molecular mechanisms of HCC and develop novel
strategies for the diagnosis and treatment of HCC.
MicroRNAs (miRNAs) are endogenous non-coding 20–22
nucleotide RNAs that regulate protein expression by interacting
with complementary sites within the 3′-untranslated region (UTR) of
target mRNAs (5–7). Increasing evidence suggests that
aberrant expression of miRNAs plays crucial roles in various
cellular processes, including cell proliferation, apoptosis,
differentiation, migration and invasion (8–13).
In addition, it has been widely recognized that deregulation of
miRNAs may lead to abnormal expression of oncogenes or tumor
suppressors and contribute to the development and progression of
human cancers.
miR-519a, which belongs to a large cluster of
miRNAs, C19MC, plays a critical role in the pathogenesis of human
cancers. It played an oncogenic role and could serve as a
diagnostic and prognostic biomarker in ovarian epithelial tumors
(14). Moreover, it was recently
found to confer tamoxifen resistance by targeting the phosphatase
and tensin homolog (PTEN), retinoblastoma protein (RB1), and
cyclin-dependent kinase inhibitor 1A (CDKN1A)/p21 in estrogen
receptor (ER)+ breast cancer (15). Studies have shown that miR-519a
functioned as a tumor suppressor through multiple p21-inducing
pathways in HeLa human cervical carcinoma (16). Moreover, miR-519a reduced cell
proliferation by lowering RNA-binding protein HuR levels in colon
carcinoma (17). Thus, the exact
roles of miR-519a in human cancers are cancer-type specific.
However, the functional role of miR-519a and the underlying
mechanisms in HCC are still unknown.
In this study, we demonstrated that upregulation of
miR-519a was associated with poor prognostic features and reduced
5-year survival of HCC patients. Gain- and loss-of-function studies
revealed that miR-519a promoted HCC cell proliferation and cell
cycle progression. Finally, we identified PTEN and PI3K/AKT pathway
as direct targets of miR-519a.
Materials and methods
Clinical tissues and data
Tumor samples and matched tumor-adjacent tissues
were obtained from 116 patients undergoing curative resection of
their primary HCC in the Department of Hepatobiliary Surgery at the
First Affiliated Hospital of Xi'an Jiaotong University during
January 2006 to December 2009. Clinical sample was used after
obtaining informed consent from each patient. None of the patients
had received any perioperative chemo- or radiotherapy. The
demographic and clinicopathological data of all enrolled patients
were obtained through review of hospital records, and are presented
in Table I. The protocols of this
study were approved by the Xi'an Jiaotong University Ethics
Committee according to the Declaration of Helsinki.
 | Table ICorrelation between the
clinicopathological characteristics and miR-519a expression in HCC
(n=116). |
Table I
Correlation between the
clinicopathological characteristics and miR-519a expression in HCC
(n=116).
| | Expression level | |
|---|
| |
| |
|---|
| Clinical
parameters | n |
miR-519ahigh (n=64) |
miR-519alow (n=52) | P-value |
|---|
| Age (years) |
| <65 years | 61 | 34 | 27 | 0.897 |
| ≥65 years | 55 | 30 | 25 | |
| Gender |
| Male | 89 | 49 | 40 | 0.964 |
| Female | 27 | 15 | 12 | |
| Tumor size
(cm) | | | | 0.005a |
| <5 | 78 | 36 | 42 | |
| ≥5 | 38 | 28 | 10 | |
| Tumor number | | | | 0.972 |
| Solitary | 98 | 54 | 44 | |
| Multiple | 18 | 10 | 8 | |
| Edmondson | | | | |
| I+II | 71 | 33 | 38 | 0.018a |
| III+IV | 45 | 31 | 14 | |
| TNM stage | | | | 0.013a |
| I+II | 93 | 46 | 47 | |
| III+IV | 23 | 18 | 5 | |
| Capsular
infiltration | | | | 0.599 |
| Present | 70 | 40 | 30 | |
| Absent | 46 | 24 | 22 | |
| Venous
infiltration | | | | 0.005a |
| Present | 19 | 16 | 3 | |
| Absent | 97 | 48 | 49 | |
| AFP (ng/ml) | | | | 0.947 |
| <400 | 42 | 23 | 19 | |
| ≥400 | 74 | 41 | 33 | |
| HBV | | | | 0.873 |
| Positive | 110 | 60 | 50 | |
| Negative | 6 | 4 | 2 | |
Cell culture and treatment
The human immortalized normal hepatic cell line LO2
and HCC cell lines (HepG2, Hep3B, Huh7, SMMC-7721 and Bel-7402)
were obtained from the Chinese Academy of Sciences (Shanghai,
China). The cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10%
fetal bovine serum (Gibco, Grand Island, NY, USA) at 37°C with 5%
CO2. Akt inhibitor MK-2206 (1 μM, Selleck Chemicals,
Houston, TX, USA) was used to treat HCC cells following the
manufacturer's instructions.
Quantitative real-time polymerase chain
reaction (qRT-PCR)
Total RNA was extracted from clinical specimens or
HCC cells using TRIzol reagent (Invitrogen) following
manufacturer's instruction. PCR amplification was performed using a
TaqMan human miRNA assay kit (Applied Biosystems, Foster City, CA,
USA) and a SYBR® Premix Ex Taq™ II (Perfect Real-Time)
kit (Takara Bio Inc., Shiga, Japan) with an ABI PRISM 7300 Sequence
Detection System (Applied Biosystems). qPCR primer against mature
miRNA hsa-miR-519a-3p (HmiRQP0588), Homo sapiens snRNA U6 qPCR
Primer (HmiRQP9001), PTEN (HQP015535) and GAPDH (HQP006940) were
purchased from Genecopoeia (Guangzhou, China).
Western blot analysis
Total protein was extracted from HCC cells and 40 μg
of isolated protein was separated by 10% SDS-PAGE and transferred
onto a PVDF membrane (Bio-Rad Laboratories, Hercules, CA, USA). The
membranes were probed with the following primary antibodies: Akt
(1:1000, Cell Signaling Technology, Inc., Danvers, MA, USA), p-Akt
(1:1000, Cell Signaling Technology, Inc.), PTEN (1:1000, Cell
Signaling Technology, Inc.), Cyclin D (1:1000, Cell Signaling
Technology, Inc.), and p27 (1:1000, Cell Signaling Technology,
Inc.) overnight. Then the membranes were incubated with the
HRP-conjugated goat anti-mouse or anti-rabbit IgG antibody
(ZSGB-BIO, China). Protein bands were visualized using an enhanced
chemiluminescence kit (Amersham, Little Chalfont, UK).
Immunohistochemical staining
Immunohistochemistry was performed on
paraformaldehyde-fixed paraffin sections. PTEN (1:100, Cell
Signaling Technology, Inc.) antibody was used in
immunohistochemistry by a streptavidin peroxidase-conjugated
(SP-IHC) method. Detailed procedure of immunohistochemistry was
performed as previously reported (18).
Plasmids and cell transfection
miRNA vectors, including miR-519a expression vector
(HmiR0342-MR04), the control vector for miR-519a (CmiR0001-MR04,
miR-control), miR-519a inhibitor (HmiR-AN0588-AM04, anti-miR-519a)
and the negative control for the miR-519a inhibitor
(CmiR-AN0001-AM04, anti-miR-NC), and PTEN expression plasmid
(EX-I0450-M02-5) were purchased from Genecopoeia (Guangzhou,
China). The PTEN siRNA duplex sequence, 5′-GUU AGC AGA AAC AAA AGG
AGA UAU CAA-3′ (sense)/5′-UUG AUA UCU CCU UUU GUU UCU GCU AAC-3′
(antisense) and a nonspecific duplex oligonucleotide as a negative
control were synthesized by Sangon Biotech Co., Ltd. (Shanghai,
China). Cells were transfected with oligonucleotides using
Lipofectamine 2000 Reagent (Invitrogen Life Technologies) following
the manufacturer's instructions.
Luciferase assay
Cells were seeded in triplicate in 24-well plate and
allowed to settle for ~12 h. pGL3-PTEN was co-transfected into HCC
cells with TK-Renilla plasmid as control signals using
Lipofectamine 2000. Luciferase and control signals were measured at
48 h after transfection using the Dual Luciferase Reporter Assay
kit (Promega, Madison, WI, USA), according to a protocol provided
by the manufacturer. Three independent experiments were performed
and the data are presented as the mean ± SD.
MTT and colony formation assays
At 48 h after transfection, 5×103 cells
were seeded into 96-well plates and stained with 0.5 mg/ml sterile
MTT (Sigma-Aldrich, St. Louis, MO, USA) for 4 h at 37°C. Then the
culture medium was discarded and an extra 150 μl DMSO
(Sigma-Aldrich) were added. The absorbance at 490 nm was measured
at 24, 48 and 72 h after transfection. The colony formation assay
was performed as previously described. Briefly, 500 cells per well
were seeded on six-well plates. After 2 weeks, the colonies were
stained with 1% crystal violet and the number of colonies was
counted.
Cell cycle and cell proliferation
assays
At 48 h after transfection, HCC cells were collected
for cell cycle analysis. After being washed with PBS three times,
the cells were fixed with 80% ethanol overnight at −20°C, and were
subsequently treated with RNaseA (Sigma) for 30 min at 37°C,
followed by incubation in 20 μg/ml of propidium iodide (Sigma) for
20 min at room temperature. After incubation, the cells were
subjected to flow cytometry analysis using a FACS Calibur (BD
Biosciences, Bedford, MA, USA). For proliferation,
5-bromo-deoxyuridine (BrdU) labeling and immunofluorescence was
used. Cells grown on cover slips (Fisher Scientific, Pittsburgh,
PA, USA) were incubated with BrdU for 1 h and stained with
anti-BrdU antibody (Sigma) according to the manufacturer's
instruction. Gray level images were acquired under a laser scanning
microscope (Axioskop 2 plus, Carl Zeiss Co. Ltd., Jena,
Germany).
Statistical analysis
Data are presented as the mean ± SD from at least
three independent replicates. SPSS software, 16.0 (SPSS, Inc,
Chicago, IL, USA) was used to conduct the analysis, and a
two-tailed Student t-test was employed to analyze the differences
between two groups. Pearson's correlation analysis was used to
analyze the correlation between two indices. Survival curves were
plotted by the Kaplan-Meier method and compared using the log-rank
test. Differences were considered statistically significant at
P<0.05.
Results
Expression of miR-519a is upregulated in
HCC
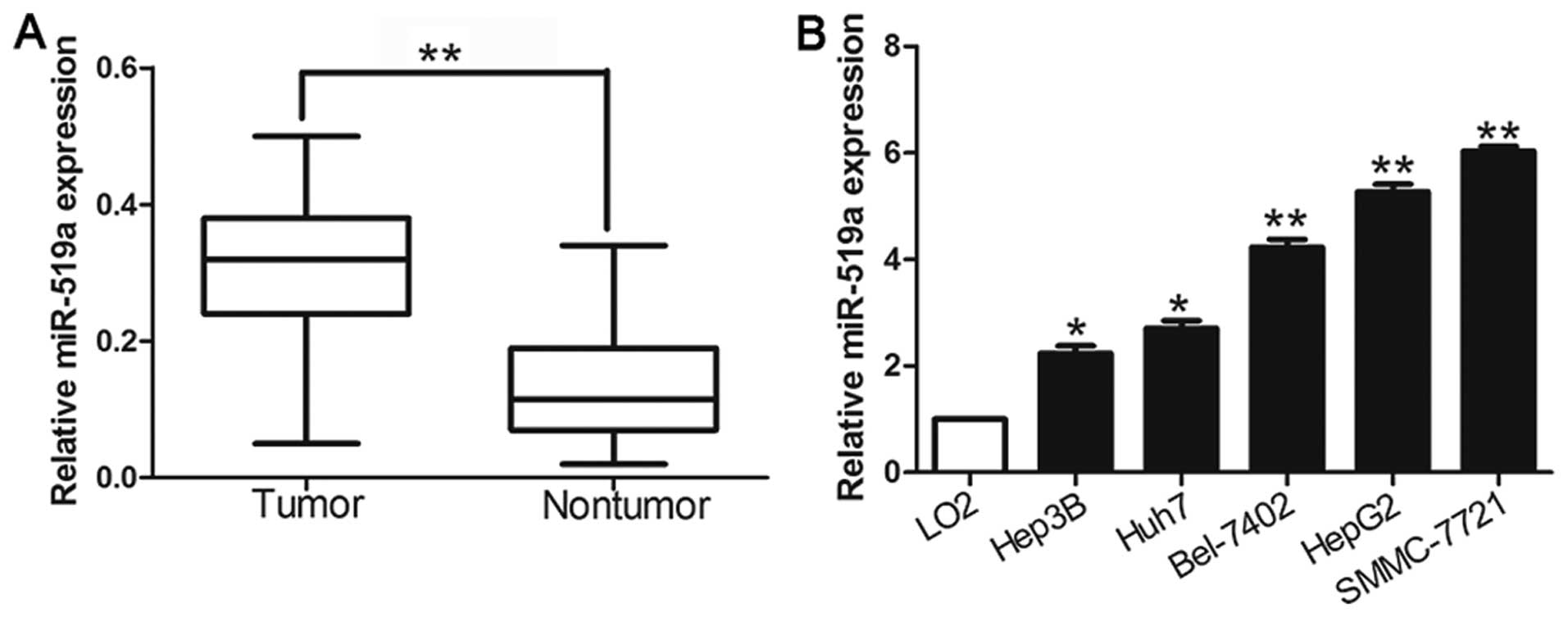
We investigated the expression level of miR-519a in
116 pairs of HCC tissues and matched tumor-adjacent tissues using
qRT-PCR. The results showed that the mean level of miR-519a
expression in HCC tissues was significantly higher than that in the
non-tumor tissues (P<0.05, Fig.
1A). Further results confirmed that miR-519a was upregulated in
a panel of HCC cell lines (HepG2, Huh7, Hep3B, SMMC-7721 and
Bel-7402) compared with that in non-transformed LO2 hepatic cell
line (P<0.05, Fig. 1B). The
results suggest that elevated expression of miR-519a may contribute
to the development of HCC.
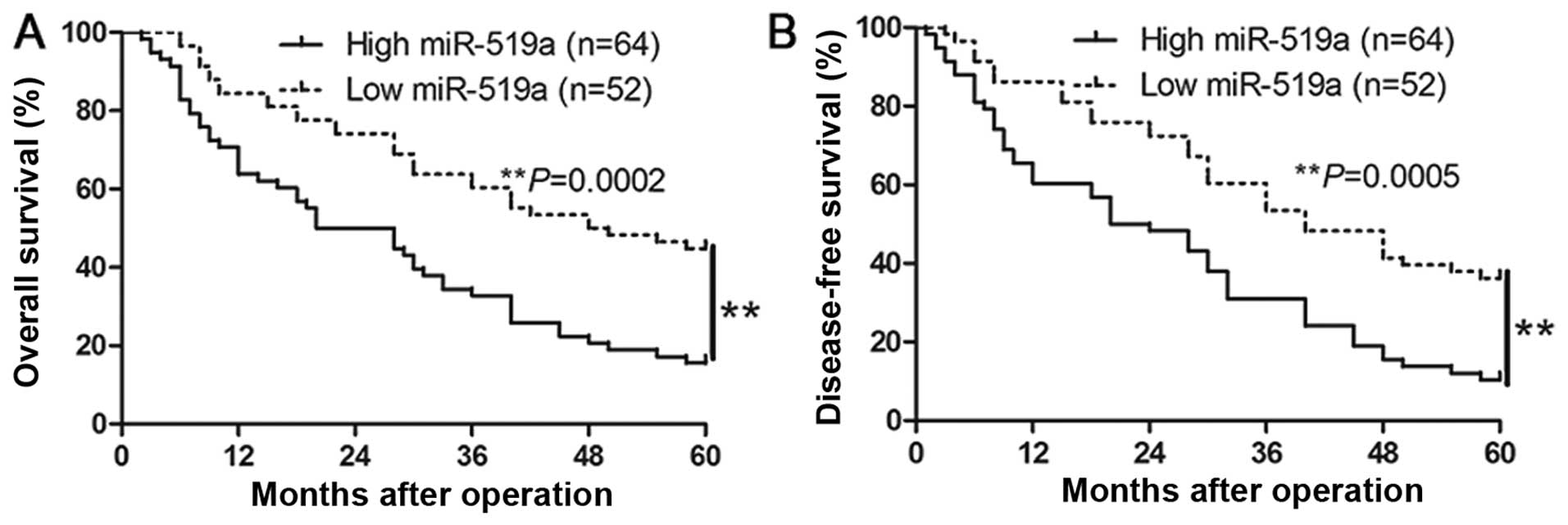
Clinical significance of miR-519a
expression in HCC specimens
We determined the mean level of miR-519a as a
cut-off value to investigate the correlation between miR-519a level
and the clinical features and prognosis of HCC patients. As shown
in Table I, the high expression of
miR-519a was prominently associated with large tumor size
(P=0.005), high Edmondson-Steiner grading (P=0.018), advanced
tumor-node-metastasis (TNM) tumor stage (P=0.013) and venous
infiltration (P=0.005). Furthermore, Kaplan-Meier analysis showed
that high miR-519a expression was closely associated with shorter
overall survival (P=0.0002, Fig.
2A) and disease-free survival (P=0.0005, Fig. 2B), which highlights the potential
value of miR-519a as a predictive biomarker for the outcome of
HCC.
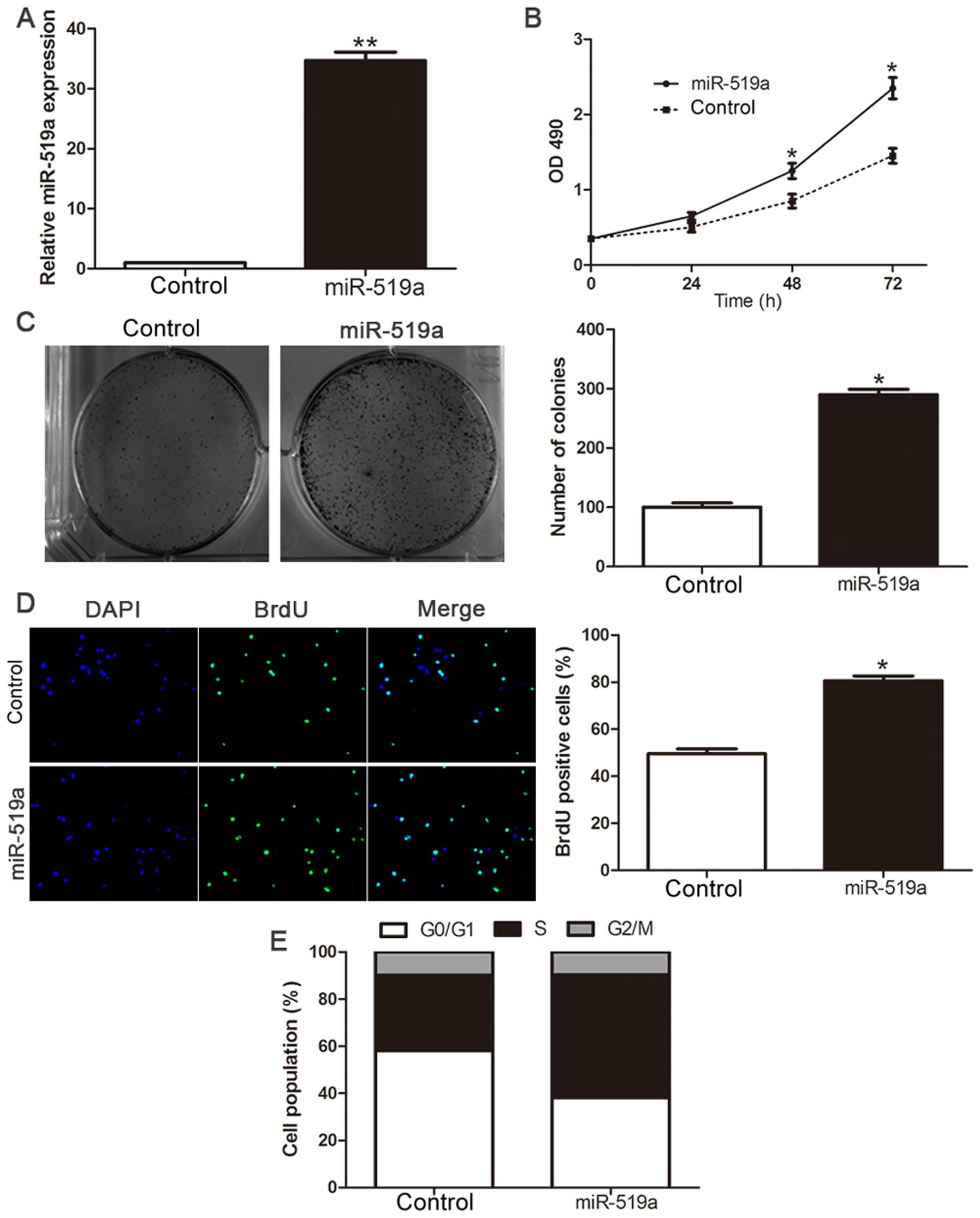
Overexpression of miR-519a promotes
proliferation and cell cycle progression of HCC cells
To investigate the biological function of miR-519a
in the development and progression of HCC, we transfected HCC cell
line Hep3B with miR-519a mimic (P<0.01, Fig. 3A). Cell viability was measured
using MTT assays and we observed that ectopic expression of
miR-519a had more viability over time compared with NC group
(P<0.05, Fig. 3B).
Consistently, the upregulated expression of miR-519a markedly
enhanced the colony formation as suggested by the increase in
colony number (P<0.05, Fig.
3C). Furthermore, the level of DNA synthesis, examined with
BrdU incorporation assay, was significantly elevated in miR-519a
transduced Hep3B cells, whereas the vector control cells displayed
relatively lower BrdU incorporation rates (P<0.05, Fig. 3D). Cell cycle analysis revealed
that overexpression of miR-519a decreased the number of Hep3B cells
in G0/G1 phase while increased the proportion of Hep3B cells in
S-phase (P<0.05, Fig. 3E).
Collectively, these results demonstrate that miR-519a functions to
enhance proliferation, tumorigenicity and cell cycle progression of
HCC cells.
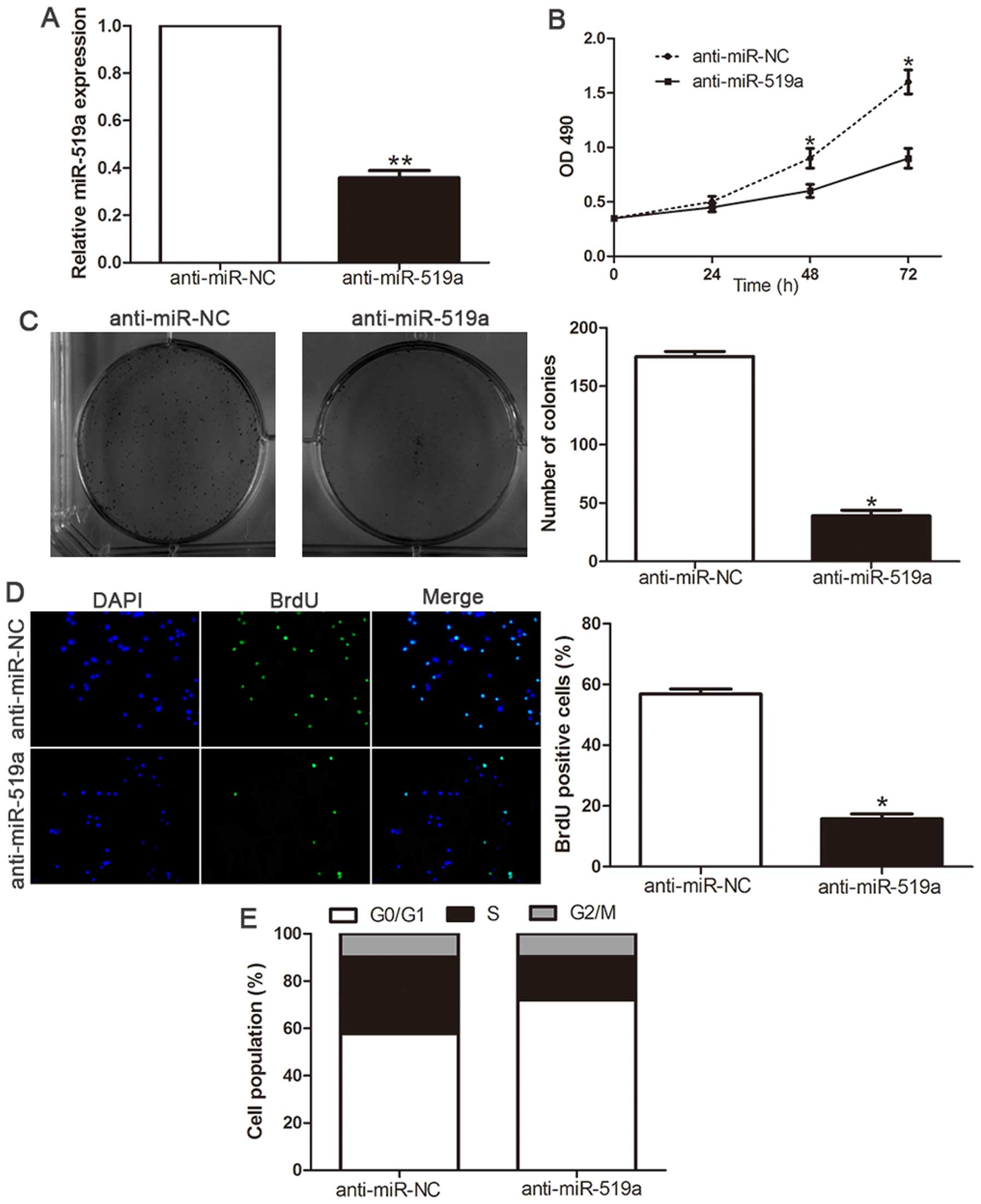
Inhibition of miR-519a attenuates
proliferation and cell cycle progression of HCC cells
Loss-of-function studies were further performed to
confirm the biological function by anti-miR-519a vector. The
expression of miR-519a was significantly decreased in SMMC-7721
cells (P<0.01, Fig. 4A)
following transfection of miR-519a inhibitors. As shown in Fig. 4B, downregulation of miR-519a led to
a significant reduction of cell viability (P<0.05). Furthermore,
suppression of miR-519a significantly inhibited the colony
formation ability (P<0.05, Fig.
4C). Moreover, BrdU incorporation assay confirmed that
downregulation of miR-519a significantly inhibited the
proliferation in SMMC-7721 cells (P<0.05, Fig. 4D). In addition, flow cytometry
showed inhibition of miR-519a in SMMC-7721 cells significantly
increased the percentage of cells in G1/G0 phase and decreased the
percentage of cells in S phase (P<0.05, Fig. 4E). These results demonstrated that
miR-519a regulates the proliferation, tumorigenicity and cell cycle
of HCC cells.
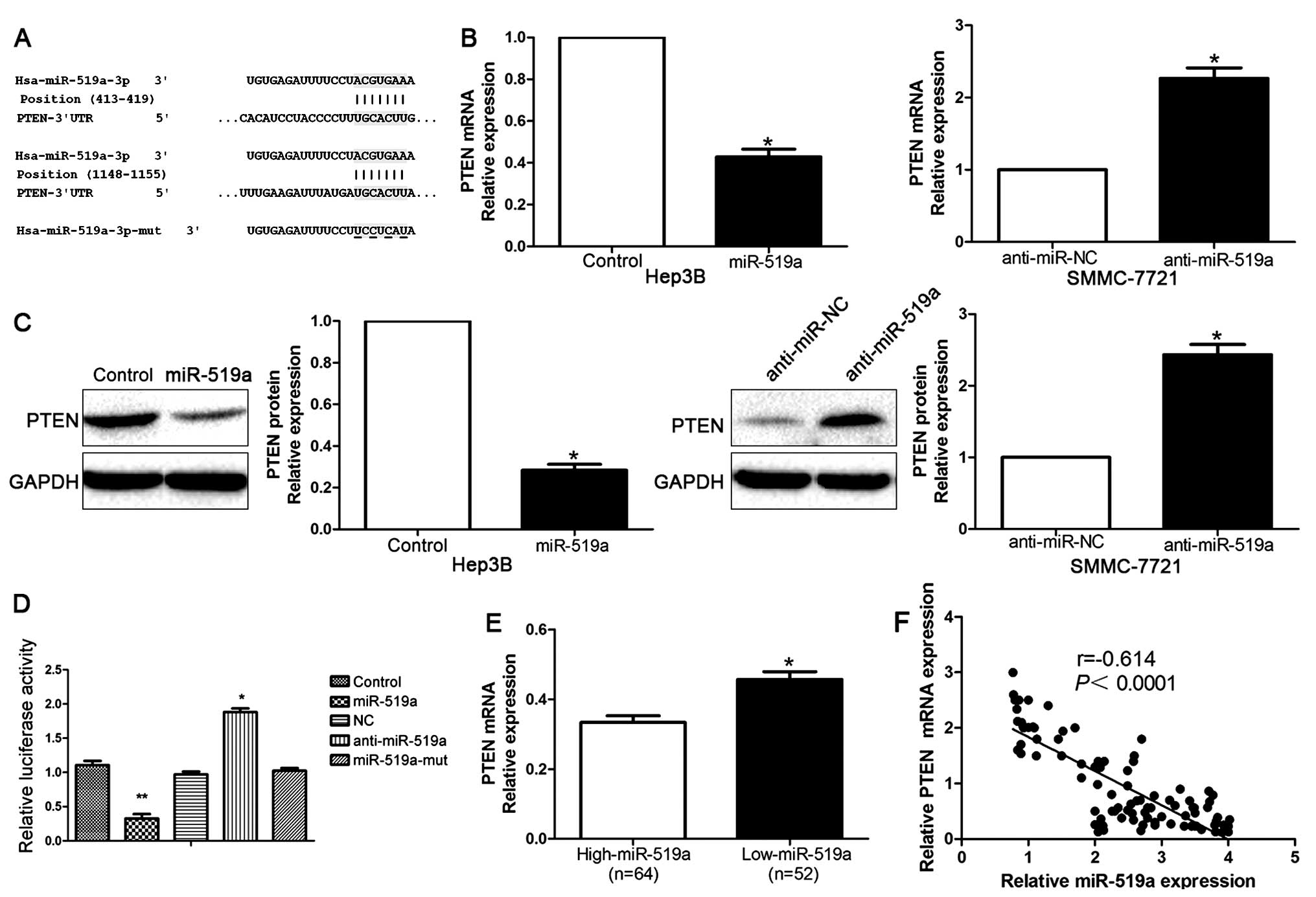
PTEN was a direct target of miR-519a in
HCC
To further explore the underlying mechanisms by
which miR-519a exerted its functional effects on HCC, we used
TargetScan to search for potential downstream targets of miR-519a.
As shown in Fig. 5A, we found that
3′-UTR of PTEN contained the highly conserved putative miR-519a
binding sites. qRT-PCR and western blot analysis showed that
ectopic expression of miR-519a markedly decreased, whereas
inhibition of miR-519a increased the mRNA (P<0.05, Fig. 5B) and protein (P<0.05, Fig. 5C) expression of PTEN. Moreover,
when co-transfected with PTEN-3′ UTR luciferase reporter plasmid,
as shown in Fig. 5D, miR-519a
mimics led to significant reduction of luciferase activity of PTEN
(P<0.01), while transfection of miR-519a inhibitors resulted in
significant increase of luciferase activity of PTEN (P<0.01,
Fig. 5D). In addition, the
luciferase activity was unaffected by the miR-519a-mut (Fig. 5D). To further validate that PTEN
was a direct downstream target of miR-519a, we examined the
correlation between miR-519a level and PTEN expression in HCC
tissues. We found PTEN mRNA level in the miR-519a high-expressing
tumors was significantly lower than those in the miR-519a
low-expressing tumors (P<0.05, Fig.
5E). Moreover, miR-519a level was inversely correlated with the
level of PTEN mRNA in HCC tissues (r=−0.614, P<0.0001, Fig. 5F). Consistently, as indicated by
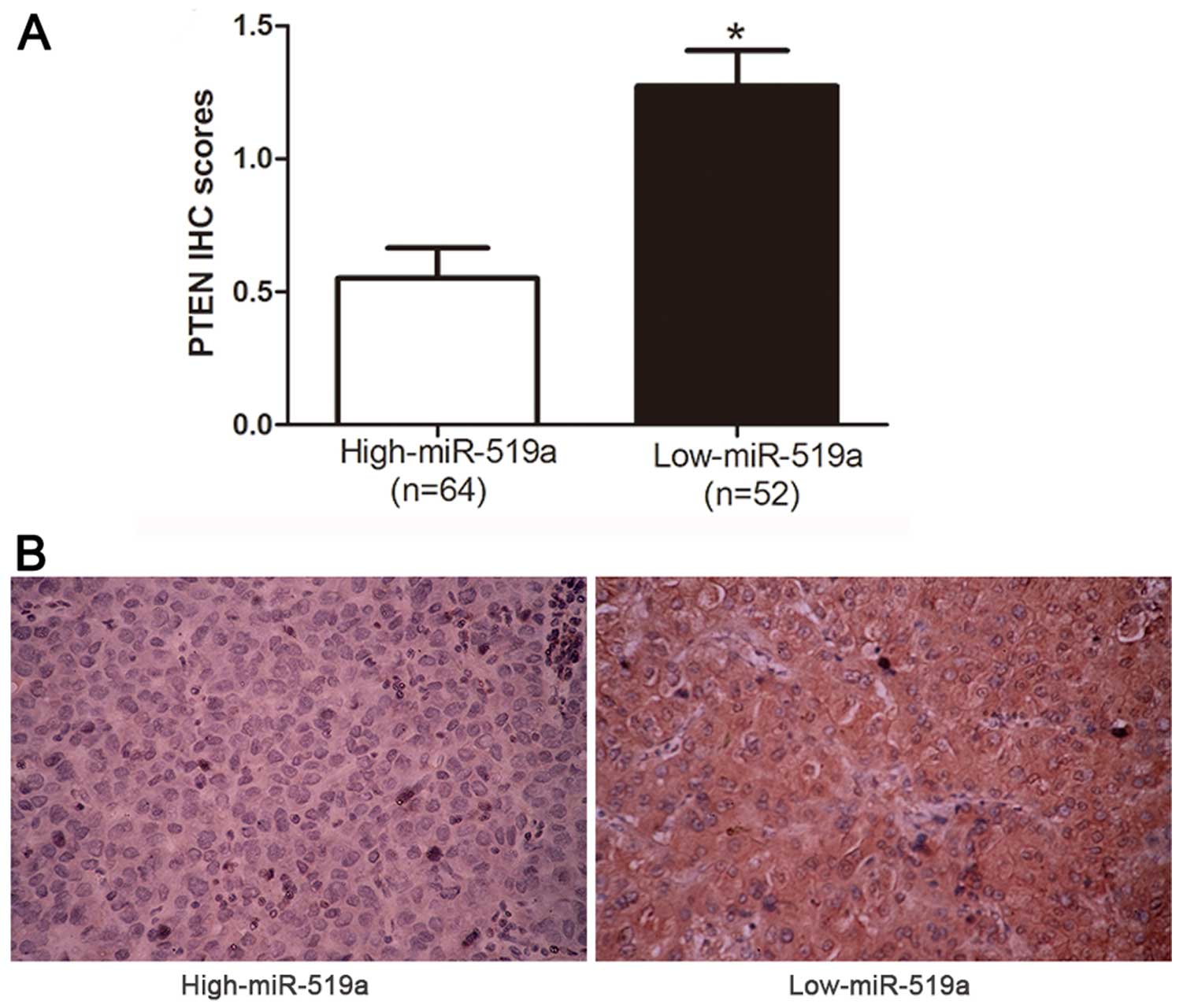
the IHC results, the protein expression of PTEN was obviously
decreased in patients with high level of miR-519a (P<0.05,
Fig. 6). Collectively, these
results strongly suggest that PTEN is a downstream target of
miR-519a in HCC.
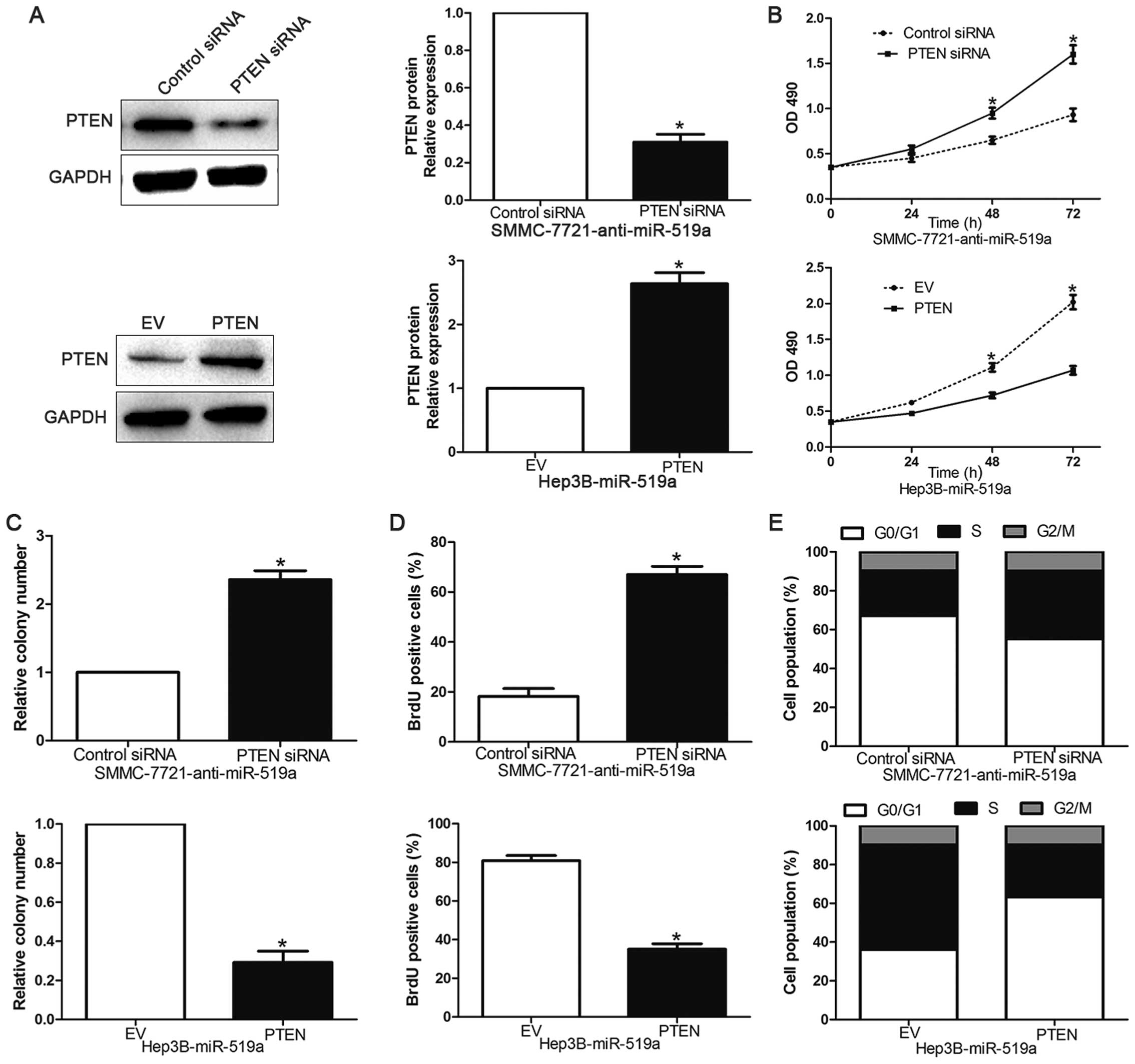
PTEN is a downstream mediator of the
biological function of miR-519a in HCC
To evaluate whether PTEN could mediate the
biological function of miR-519a in HCC, we inhibited PTEN
expression with siRNAs in miR-519a-suppressive SMMC-7721 cells
(P<0.05, Fig. 7A). We found
that cell viability (P<0.05, Fig.
7B), colony formation (P<0.05, Fig. 7C) and proliferation (P<0.05,
Fig. 7D) was significantly
increased after PTEN downregulation. Furthermore, PTEN knockdown
markedly reversed the prevention of cell cycle progression induced
by miR-519a suppression (P<0.05, Fig. 7E). Accordingly, PTEN overexpression
inhibited cell viability, colony formation, proliferation and
promoted G0/G1 phase in miR-519a-overexpressing Hep3B cells
(P<0.05, Fig. 7A–E,
respectively). These data showed that PTEN is a functional
downstream mediator of miR-519a in HCC.
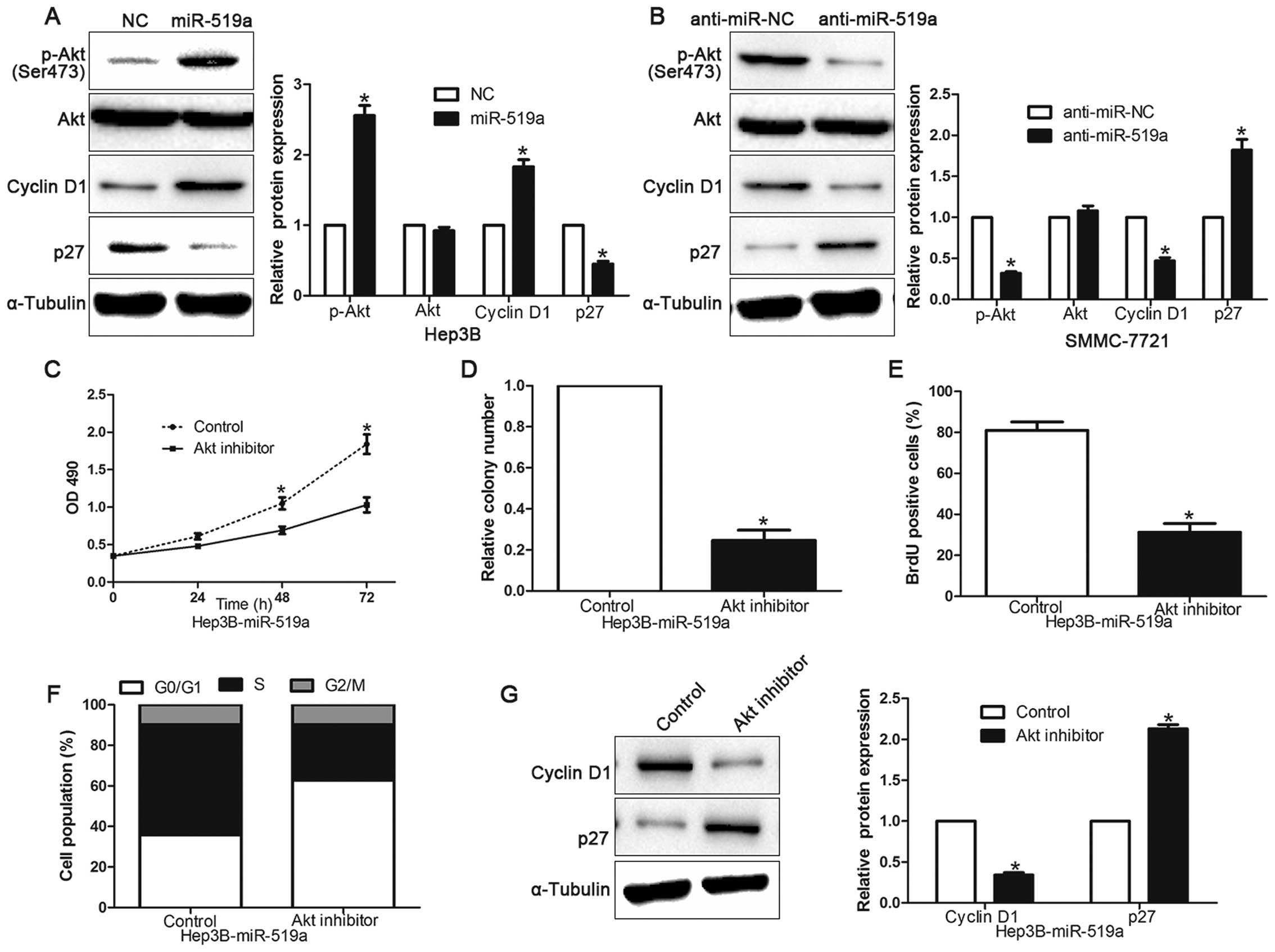
PI3K/Akt signaling is essential for the
biological function of miR-519a in HCC
Previous studies have demonstrated that PTEN was a
negative regulator of PI3K/Akt signaling (19), so we further investigated whether
dysregulation miR-519a altered the activity of PI3K/Akt signaling
in HCC cells. As shown in Fig. 8A and
B, overexpressing miR-519a significantly increased, but
silencing miR-519a decreased, the Akt activity and the expression
of phosphorylated Akt (Ser473) in HCC cells (P<0.05).
Consistently, the levels of Cyclin D1 and p27, two downstream
effectors of PI3K/Akt signaling, were also significantly altered in
the miR-519a-deregulated HCC cells (P<0.05). These results
indicate that miR-519a activates PI3K/Akt signaling. Furthermore,
we examined whether activation of PI3K/Akt signaling contributed to
miR-519a-mediated HCC cell proliferation and cell cycle
progression. In Hep3B cells overexpressing miR-519a, inactivation
of PI3K/Akt signaling by Akt inhibitor significantly decreased the
cell viability (P<0.05, Fig.
8C), colony formation (P<0.05, Fig. 8D), proliferation (P<0.05,
Fig. 8E) and promoted the
percentage of G0/G1 phase (P<0.05, Fig. 8F). Moreover, the Cyclin D1
expression was significantly decreased, but p27 was increased after
treating Hep3B cells overexpressing miR-519a with Akt inhibitor
(P<0.05, Fig. 8G). Taken
together, our results demonstrate that PI3K/Akt signaling plays an
essential function during miR-519a-induced HCC cell proliferation
and cell cycle progression.
Discussion
Increasing evidence demonstrates that aberrant
expression or function of miRNAs plays a crucial role in
carcinogenesis (20,21). Therefore, identification of novel
cancer-related miRNAs and their functional targets may provide
promising therapeutic targets for HCC (22). In previous studies, miR-519a has
been found to promote DNA damage, alter Ca2+ homeostasis
and enhance energy production via p21-induced pathways, resulting
in growth inhibition of HeLa cells (16,23).
Abdelmohsen et al (24)
indicated that miR-519 reduced cell proliferation by lowering
RNA-binding protein HuR levels in colon cancer. However, Kim et
al found that miR-519a was upregulated and correlated
significantly with pathological grade in ovarian epithelial tumors
(14). Moreover, miR-519a promoted
cell viability, cell cycle progression and resistance to apoptosis
in ER+ breast cancer (15).
In the present study, we identified that miR-519a
was upregulated in HCC tissues compared to matched tumor-adjacent
tissues, and this was also confirmed in HCC cells. Elevated
expression of miR-519a was significantly correlated with
clinicopathological features of HCC, including large tumor size,
high histological grade and TNM stage and venous infiltration. In
addition, patients with higher miR-519a had a poorer prognosis.
Functionally, ectopic expression of miR-519a promoted cell
viability, colony formation, proliferation and cell cycle
progression in HCC cells. Moreover, we identified PTEN as a novel
direct target of miR-519a. The functional effects of miR-519a
alteration on HCC cells could be reversed by PTEN modulation.
Moreover, the expression of PTEN was downregulated in HCC cases and
its expression level was inversely correlated with miR-519a.
Additionally, we found miR-519a led to upregulation of cell cycle
regulator Cyclin D1 and downregulation of p27 through activation of
PI3K/Akt pathway. Collectively, our results demonstrate that
miR-519a promotes HCC progression through activating Akt signaling
pathway by inhibiting PTEN.
Numerous studies showed that activation of the
PI3K/Akt signaling pathway was essential to the development and
progression of HCC and could modulate the malignant behavior of HCC
(25,26), such as cell proliferation,
invasiveness, angiogenesis and metastasis (27,28).
Of note, PTEN, which acts as an inhibitor of PI3K/Akt pathway, has
been found to be inhibited in HCC due to gene deletion or mutation
during oncogenesis (29,30). PTEN was an essential regulator of
cell proliferation, differentiation, growth and apoptosis, and its
deficiency was closely associated with HCC development and
progression (31). In addition,
PTEN could inhibited tumor cell growth and invasion by suppressing
the PI3k/Akt pathway (32).
Herein, we discovered that miR-519a activated PI3K/Akt signaling
through targeting PTEN, suggesting miR-519a may represent as a
potential therapeutic target for HCC treatment.
In conclusion, we demonstrated that miR-519a was
frequently overexpressed in HCC tissues and cell lines. miR-519a
played a crucial role in the malignant progression of HCC cells
through activation of PI3K/Akt by inhibiting PTEN. Therefore,
miR-519a has the potential to be a valuable diagnostic and
prognostic biomarker and a novel therapeutic target for HCC.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81402039) and the Natural
Science Basic Research Plan in Shaanxi Province of China (Program
no. 2015JM8409).
References
|
1
|
Bosch FX, Ribes J, Díaz M and Cléries R:
Primary liver cancer: Worldwide incidence and trends.
Gastroenterology. 127(Suppl 1): S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Di Bisceglie AM, Bruix J,
Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M,
Talwalkar J, et al: Panel of Experts in HCC-Design Clinical Trials:
Design and endpoints of clinical trials in hepatocellular
carcinoma. J Natl Cancer Inst. 100:698–711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Talwalkar JA and Gores GJ: Diagnosis and
staging of hepatocellular carcinoma. Gastroenterology. 127(Suppl
1): S126–S132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosa A and Brivanlou AH: MicroRNAs in
early vertebrate development. Cell Cycle. 8:3513–3520. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vasudevan S, Tong Y and Steitz JA:
Switching from repression to activation: microRNAs can up-regulate
translation. Science. 318:1931–1934. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tu K, Zheng X, Dou C, Li C, Yang W, Yao Y
and Liu Q: MicroRNA-130b promotes cell aggressiveness by inhibiting
peroxisome proliferator-activated receptor gamma in human
hepatocellular carcinoma. Int J Mol Sci. 15:20486–20499. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dou C, Wang Y, Li C, Liu Z, Jia Y, Li Q,
Yang W, Yao Y, Liu Q and Tu K: MicroRNA-212 suppresses tumor growth
of human hepatocellular carcinoma by targeting FOXA1. Oncotarget.
6:13216–13228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang RM, Yang H, Fang F, Xu JF and Yang
LY: MicroRNA-331-3p promotes proliferation and metastasis of
hepatocellular carcinoma by targeting PH domain and leucine-rich
repeat protein phosphatase. Hepatology. 60:1251–1263. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su H, Yang JR, Xu T, Huang J, Xu L, Yuan Y
and Zhuang SM: MicroRNA-101, down-regulated in hepatocellular
carcinoma, promotes apoptosis and suppresses tumorigenicity. Cancer
Res. 69:1135–1142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang X, Zhang XF, Lu X, Jia HL, Liang L,
Dong QZ, Ye QH and Qin LX: MicroRNA-26a suppresses angiogenesis in
human hepatocellular carcinoma by targeting hepatocyte growth
factor-cMet pathway. Hepatology. 59:1874–1885. 2014. View Article : Google Scholar
|
|
13
|
Santhekadur PK, Das SK, Gredler R, Chen D,
Srivastava J, Robertson C, Baldwin AS Jr, Fisher PB and Sarkar D:
Multi-function protein staphylococcal nuclease domain containing 1
(SND1) promotes tumor angiogenesis in human hepatocellular
carcinoma through novel pathway that involves nuclear factor κB and
miR-221. J Biol Chem. 287:13952–13958. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim TH, Kim YK, Kwon Y, Heo JH, Kang H,
Kim G and An HJ: Deregulation of miR-519a, 153, and 485-5p and its
clinicopathological relevance in ovarian epithelial tumours.
Histopathology. 57:734–743. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ward A, Shukla K, Balwierz A, Soons Z,
König R, Sahin O and Wiemann S: MicroRNA-519a is a novel oncomir
conferring tamoxifen resistance by targeting a network of
tumour-suppressor genes in ER+ breast cancer. J Pathol.
233:368–379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abdelmohsen K, Srikantan S, Tominaga K,
Kang MJ, Yaniv Y, Martindale JL, Yang X, Park SS, Becker KG,
Subramanian M, et al: Growth inhibition by miR-519 via multiple
p21-inducing pathways. Mol Cell Biol. 32:2530–2548. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abdelmohsen K, Srikantan S, Kuwano Y and
Gorospe M: miR-519 reduces cell proliferation by lowering
RNA-binding protein HuR levels. Proc Natl Acad Sci USA.
105:20297–20302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang W, Dou C, Wang Y, Jia Y, Li C, Zheng
X and Tu K: MicroRNA-92a contributes to tumor growth of human
hepatocellular carcinoma by targeting FBXW7. Oncol Rep.
34:2576–2584. 2015.PubMed/NCBI
|
|
19
|
Jiang L, Wang C, Lei F, Zhang L, Zhang X,
Liu A, Wu G, Zhu J and Song L: miR-93 promotes cell proliferation
in gliomas through activation of PI3K/Akt signaling pathway.
Oncotarget. 6:8286–8299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in Cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Croce CM and Calin GA: miRNAs, cancer, and
stem cell division. Cell. 122:6–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marasa BS, Srikantan S, Martindale JL, Kim
MM, Lee EK, Gorospe M and Abdelmohsen K: MicroRNA profiling in
human diploid fibroblasts uncovers miR-519 role in replicative
senescence. Aging (Albany NY). 2:333–343. 2010.
|
|
24
|
Abdelmohsen K, Kim MM, Srikantan S,
Mercken EM, Brennan SE, Wilson GM, Cabo R and Gorospe M: miR-519
suppresses tumor growth by reducing HuR levels. Cell Cycle.
9:1354–1359. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Courtney KD, Corcoran RB and Engelman JA:
The PI3K pathway as drug target in human cancer. J Clin Oncol.
28:1075–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng GZ, Park S, Shu S, He L, Kong W,
Zhang W, Yuan Z, Wang LH and Cheng JQ: Advances of AKT pathway in
human oncogenesis and as a target for anti-cancer drug discovery.
Curr Cancer Drug Targets. 8:2–6. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cappellini A, Tabellini G, Zweyer M,
Bortul R, Tazzari PL, Billi AM, Falà F, Cocco L and Martelli AM:
The phosphoinositide 3-kinase/Akt pathway regulates cell cycle
progression of HL60 human leukemia cells through cytoplasmic
relocalization of the cyclin-dependent kinase inhibitor p27(Kip1)
and control of cyclin D1 expression. Leukemia. 17:2157–2167. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song MS, Salmena L and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor. Nat Rev Mol
Cell Biol. 13:283–296. 2012.PubMed/NCBI
|
|
30
|
Cantley LC and Neel BG: New insights into
tumor suppression: PTEN suppresses tumor formation by restraining
the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA.
96:4240–4245. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rahman MA, Kyriazanos ID, Ono T, Yamanoi
A, Kohno H, Tsuchiya M and Nagasue N: Impact of PTEN expression on
the outcome of hepatitis C virus-positive cirrhotic hepatocellular
carcinoma patients: Possible relationship with COX II and inducible
nitric oxide synthase. Int J Cancer. 100:152–157. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tamura M, Gu J, Tran H and Yamada KM: PTEN
gene and integrin signaling in cancer. J Natl Cancer Inst.
91:1820–1828. 1999. View Article : Google Scholar : PubMed/NCBI
|