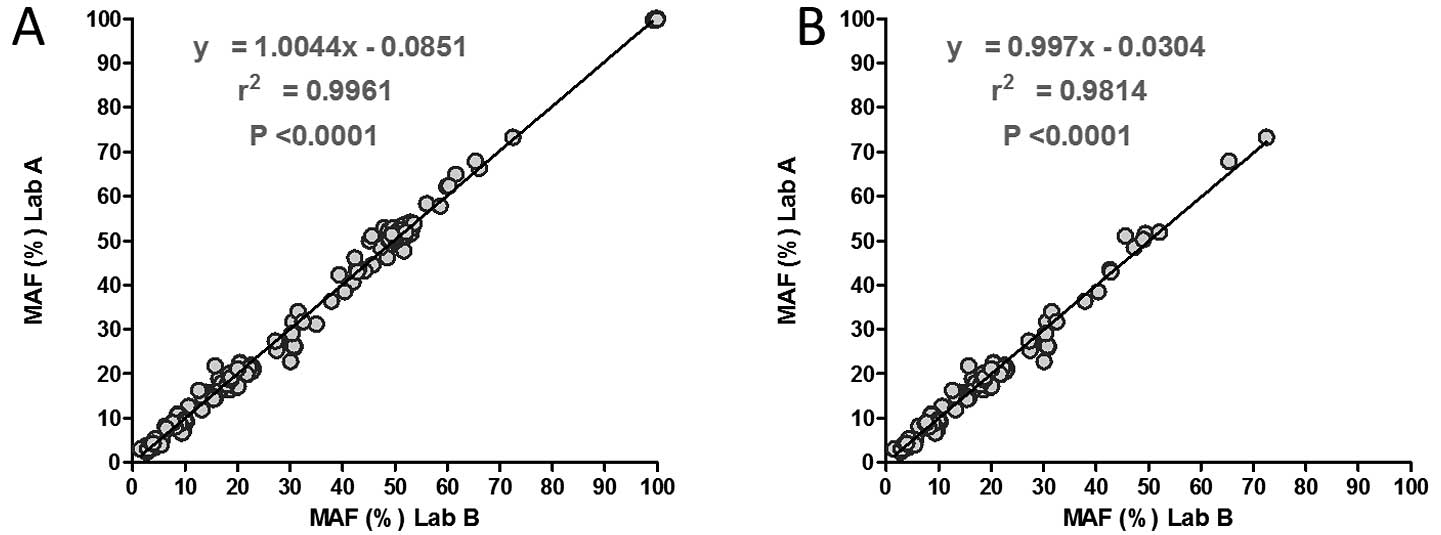
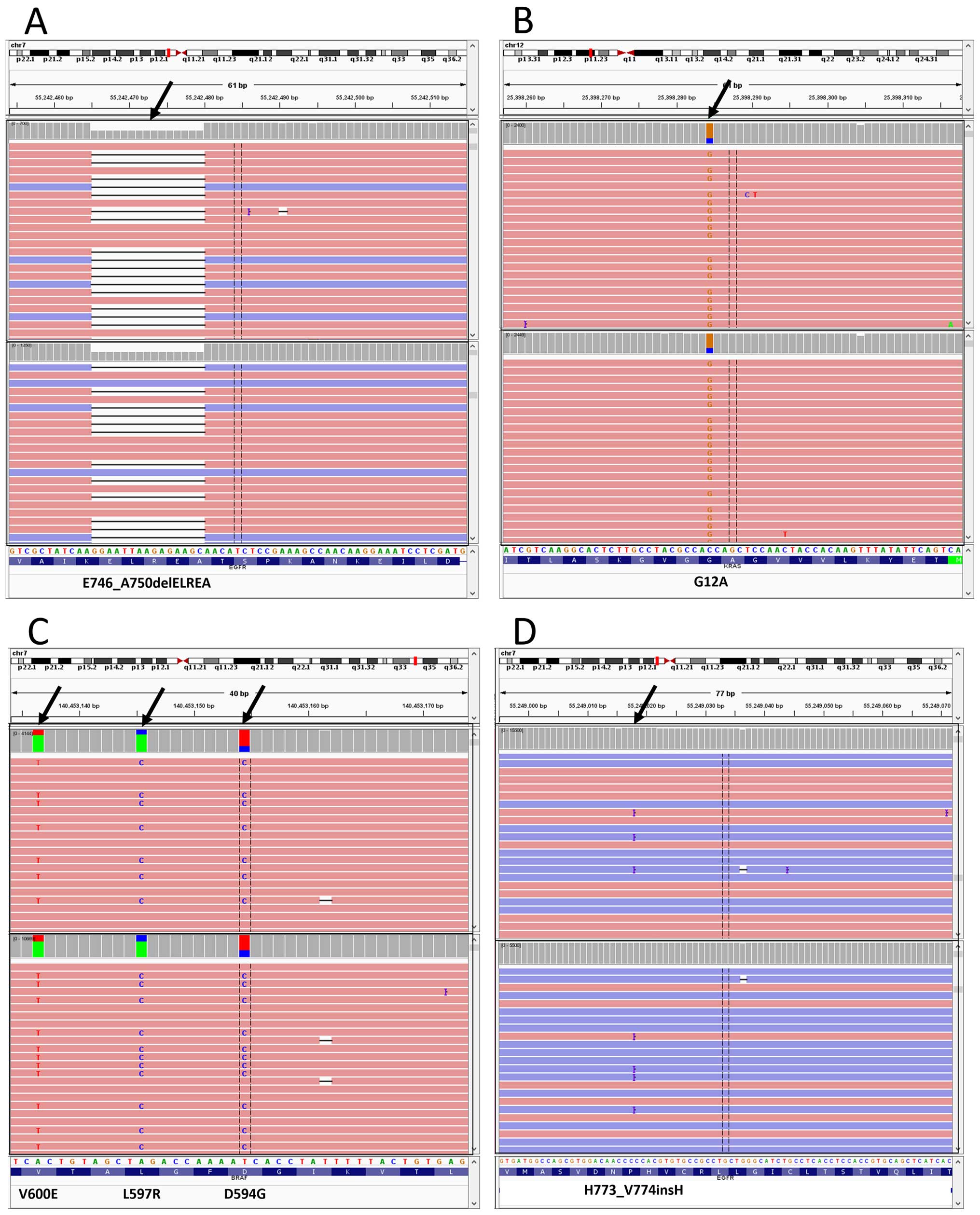
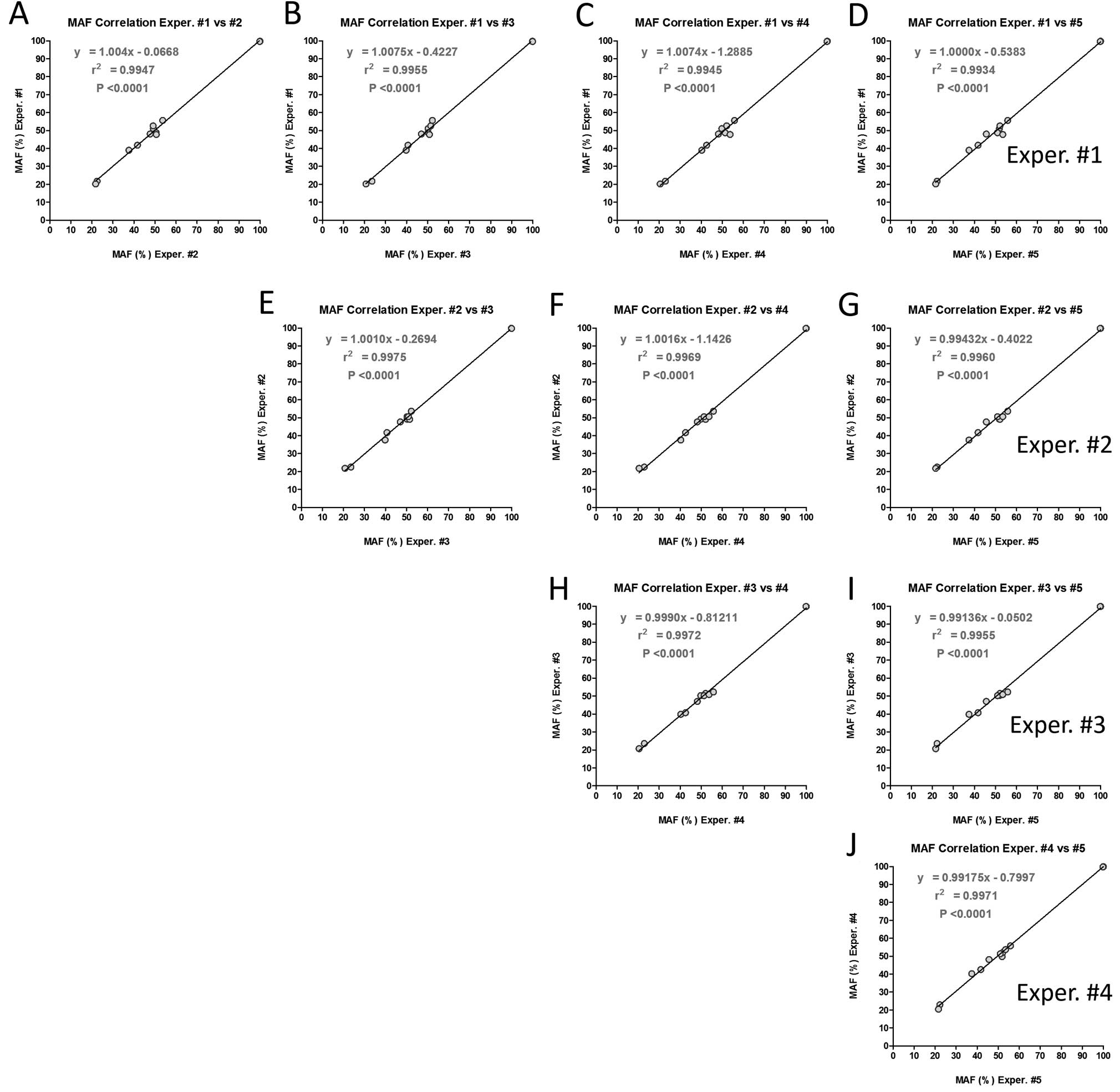
|
1
|
Cancer Genome Atlas Research Network.
Comprehensive molecular profiling of lung adenocarcinoma. Nature.
511:543–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
George J, Lim JS, Jang SJ, Cun Y, Ozretić
L, Kong G, Leenders F, Lu X, Fernández-Cuesta L, Bosco G, et al:
Comprehensive genomic profiles of small cell lung cancer. Nature.
524:47–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Govindan R, Ding L, Griffith M,
Subramanian J, Dees ND, Kanchi KL, Maher CA, Fulton R, Fulton L,
Wallis J, et al: Genomic landscape of non-small cell lung cancer in
smokers and never-smokers. Cell. 150:1121–1134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hammerman PS, Lawrence MS, Voet D, Jing R,
Cibulskis K, Sivachenko A, Stojanov P, McKenna A, Lander ES,
Gabriel S, et al; Cancer Genome Atlas Research Network.
Comprehensive genomic characterization of squamous cell lung
cancers. Nature. 489:519–525. 2012. View Article : Google Scholar
|
|
5
|
Network TCGA; Cancer Genome Atlas Network.
Comprehensive molecular characterization of human colon and rectal
cancer. Nature. 487:330–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cancer Genome Atlas Network. Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abstract G and Brief I; Cancer Genome
Atlas Research Network. The Molecular Taxonomy of Primary Prostate
Cancer. Cell. 163:1011–1025. 2015. View Article : Google Scholar
|
|
8
|
Cancer Genome Atlas Research Network.
Comprehensive molecular characterization of urothelial bladder
carcinoma. Nature. 507:315–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hodis E, Watson IR, Kryukov GV, Arold ST,
Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C,
et al: A landscape of driver mutations in melanoma. Cell.
150:251–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bass AJ, Thorsson V, Shmulevich I,
Reynolds SM, Miller M, Bernard B, Hinoue T, Laird PW, Curtis C,
Shen H, Weisenberger DJ, et al; Cancer Genome Atlas Research
Network. Comprehensive molecular characterization of gastric
adenocarcinoma. Nature. 513:202–209. 2014. View Article : Google Scholar :
|
|
11
|
Andersson AK, Ma J, Wang J, Chen X, Gedman
AL, Dang J, Nakitandwe J, Holmfeldt L, Parker M, Easton J, et al;
St. Jude Children's Research Hospital-Washington University
Pediatric Cancer Genome Project. The landscape of somatic mutations
in infant MLL-rearranged acute lymphoblastic leukemias. Nat Genet.
47:330–337. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brennan CWW, Verhaak RGWGW, McKenna A,
Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn
JZ, Berman SH, et al: The somatic genomic landscape of
glioblastoma. Cell. 155:462–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cancer Genome Atlas Research Network.
Genomic and epigenomic landscapes of adult de novo acute myeloid
leukemia. N Engl J Med. 368:2059–2074. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cancer T and Atlas G; Cancer Genome Atlas
Research Network. Integrated genomic analyses of ovarian carcinoma.
Nature. 474:609–615. 2011. View Article : Google Scholar
|
|
15
|
Chan-On W, Nairismägi M-L, Ong CK, Lim WK,
Dima S, Pairojkul C, Lim KH, McPherson JR, Cutcutache I, Heng HL,
et al: Exome sequencing identifies distinct mutational patterns in
liver fluke-related and non-infection-related bile duct cancers.
Nat Genet. 45:1474–1478. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen K, Yang D, Li X, Sun B, Song F, Cao
W, Brat DJ, Gao Z, Li H, Liang H, et al: Mutational landscape of
gastric adenocarcinoma in Chinese: Implications for prognosis and
therapy. Proc Natl Acad Sci USA. 112:1107–1112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johnson BE, Mazor T, Hong C, Barnes M,
Aihara K, McLean CY, Fouse SD, Yamamoto S, Ueda H, Tatsuno K, et
al: Mutational analysis reveals the origin and therapy-driven
evolution of recurrent glioma. Science. 343:189–193. 2014.
View Article : Google Scholar :
|
|
18
|
Jones DTW, Jäger N, Kool M, Zichner T,
Hutter B, Sultan M, Cho YJ, Pugh TJ, Hovestadt V, Stütz AM, et al:
Dissecting the genomic complexity underlying medulloblastoma.
Nature. 488:100–105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lawrence MS, Sougnez C, Lichtenstein L,
Cibulskis K, Lander E, Gabriel SB, Getz G, Ally A, Balasundaram M,
Birol I, et al; Cancer Genome Atlas Network. Comprehensive genomic
characterization of head and neck squamous cell carcinomas. Nature.
517:576–582. 2015. View Article : Google Scholar
|
|
20
|
Li M, Zhang Z, Li X, Ye J, Wu X, Tan Z,
Liu C, Shen B, Wang XA, Wu W, et al: Whole-exome and targeted gene
sequencing of gallbladder carcinoma identifies recurrent mutations
in the ErbB pathway. Nat Genet. 46:872–876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin DC, Hao JJ, Nagata Y, Xu L, Shang L,
Meng X, Sato Y, Okuno Y, Varela AM, Ding LW, et al: Genomic and
molecular characterization of esophageal squamous cell carcinoma.
Nat Genet. 46:467–473. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lohr JG, Stojanov P, Carter SL,
Cruz-Gordillo P, Lawrence MS, Auclair D, Sougnez C, Knoechel B,
Gould J, Saksena G, et al; Multiple Myeloma Research Consortium.
Widespread genetic heterogeneity in multiple myeloma: Implications
for targeted therapy. Cancer Cell. 25:91–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Creighton CJ, Morgan M, Gunaratne PH,
Wheeler DA, Gibbs RA, Gordon Robertson A, Chu A, Beroukhim R,
Cibulskis K, Signoretti S, et al; Cancer Genome Atlas Research
Network. Comprehensive molecular characterization of clear cell
renal cell carcinoma. Nature. 499:43–49. 2013. View Article : Google Scholar
|
|
24
|
Tirode F, Surdez D, Ma X, Parker M, Le
Deley MC, Bahrami A, Zhang Z, Lapouble E, Grossetête-Lalami S,
Rusch M, et al; St. Jude Children's Research Hospital-Washington
University Pediatric Cancer Genome Project and the International
Cancer Genome Consortium. Genomic landscape of Ewing sarcoma
defines an aggressive subtype with co-association of STAG2 and TP53
mutations. Cancer Discov. 4:1342–1353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carrick DM, Mehaffey MG, Sachs MC,
Altekruse S, Camalier C, Chuaqui R, Cozen W, Das B, Hernandez BY,
Lih CJ, et al: Robustness of next generation sequencing on older
formalin-fixed paraffin-embedded tissue. PLoS One. 10:e01273532015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fujita S, Masago K, Takeshita J, Okuda C,
Otsuka K, Hata A, Kaji R, Katakami N and Hirata Y: Validation of an
ion torrent sequencing platform for the detection of gene mutations
in biopsy specimens from patients with non-small-cell lung cancer.
PLoS One. 10:e01302192015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Newman AM, Bratman SV, To J, Wynne JF,
Eclov NC, Modlin LA, Liu CL, Neal JW, Wakelee HA, Merritt RE, et
al: An ultrasensitive method for quantitating circulating tumor DNA
with broad patient coverage. Nat Med. 20:548–554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dawson SJ, Tsui DWY, Murtaza M, Biggs H,
Rueda OM, Chin SF, Dunning MJ, Gale D, Forshew T, Mahler-Araujo B,
et al: Analysis of circulating tumor DNA to monitor metastatic
breast cancer. N Engl J Med. 368:1199–1209. 2013. View Article : Google Scholar : PubMed/NCBI
|