Introduction
The 5-year overall survival of breast cancer
patients with distant metastasis is 23.4% (http://seer.cancer.gov/ for details see ref. 1) and once detected, metastatic breast
cancer is incurable. Distant metastases arise from circulating
cancer cells that migrate from the blood stream to colonise a
distant organ (extravasation) (2).
Specific inhibition of cancer cell extravasation would therefore
potentially lead to a decrease in the incidence of metastasis.
Thus, delineating the molecular mechanisms involved in this process
is one of the crucial challenges for breast cancer treatment.
One such mechanism is the interaction between
sialyl-Lewis x (sLex) antigen expressed by cancer cells
and the selectins expressed by endothelial or circulating immune
cells (3). There is a compelling
body of evidence that sLex/selectin interaction is
involved in metastatic progression in several types of solid
tumours including gastric (4),
lung (5) and prostate (6) cancers. However, the relationship in
breast cancer metastasis remains controversial (7).
We have previously reported that sLex was
over-expressed in estrogen receptor (ER)-negative breast tumours
compared to ER-positive ones. Although ER-negative cancers are
known to typically be of higher grade and intrinsically more
aggressive and more often develop metastatic foci, we found no
evidence that sLex was correlated with metastatic
behaviour either in the ER-negative or the ER-positive group
(7).
This lack of association led us to question the
context of expression of sLex in breast cancer cells,
asking whether the scaffold carrying sLex (e.g.
glycosphingolipids or specific glycoproteins) influenced its role
in metastasis. Indeed, we reported that rolling on endothelial
cells was more efficient when sLex was carried by
glycoproteins rather than glycolipids (7). However, sLex was found to
be carried by several membrane bound glycoproteins in a breast
cancer cell line that displayed selectin-dependent rolling on
endothelial lining. We identified two of these glycoproteins as
tetherin, also known as bone marrow stromal antigen 2 (BST-2), and
the galectin-3-binding protein (also named MAC-2BP) encoded by the
LGALS3BP gene (7).
Subsequently, Shirure et al have shown that MAC-2BP carrying
sLex was functionally involved in ZR-75-1 rolling on
HUVECs cultured as monolayer (8).
In the present study, we assessed the expression of
sLex, BST-2 and LGALS3BP in a cohort of 249 invasive
breast cancer patients. We investigated whether the combination of
sLex with one or other of its known carriers has a
bearing on the prognostic value of sLex.
Materials and methods
Human samples
The samples used in the study were provided by the
King's Health Partners Cancer Biobank under their generic ethics
approval from National Research Ethics Service, Committee East of
England-Cambridge East, reference 12/EE/0493. Under this approval
the Biobank are also able to provide pseudo-anonymised samples for
research without informed consent if they were collected before
September 2006. All tissue samples used in the study were collected
prior to Human Tissue Act 2004.
Tissue microarray (TMA) construction and
analysis
Formalin-fixed paraffin wax embedded tissue from
consecutive cases of invasive breast carcinoma dating from 1990 to
1995 (n=400) were selected from the King's Health Partners Cancer
Biobank. Clinical data, including date and site of metastatic
recurrence, were collected and validated for each sample. From each
block 0.6-mm diameter cores were marked on haematoxylin and
eosin-stained slides and the invasive cancer subsequently sampled
using the Beecher TMArrayer (Wisconsin, MA, USA) and placed into
replicate, 100 core TMA blocks. Sections from two duplicate TMA
blocks were cut at 3 μm and dried overnight at 37°C. Sections were
baked for 2 h at 60°C, blocked using 10% bovine serum albumin in
PBS and stained with anti-sLex monoclonal antibody (mAb)
HECA-452 (7) (BD Biosciences,
Oxford, UK) at a concentration of 1 μg.ml−1,
anti-LGALS3PB mAb 6B7 (Novus Biologicals, Cambridge, UK) at a
concentration of 0.2 μg.ml−1, or anti-BST-2 polyclonal
antibody (9) (Novus Biologicals)
at a concentration of 0.2 μg.ml−1. The staining was
performed using a Ventana Ultra automat (Roche, Rotkreuz,
Switzerland) running on Program U33. Primary antibodies were
incubated for 32 min on slides, without heating, followed with
appropriate secondary and tertiary reagents (UltraView universal
DAB Detection kit; Dako, Ely, UK). Staining conditions were
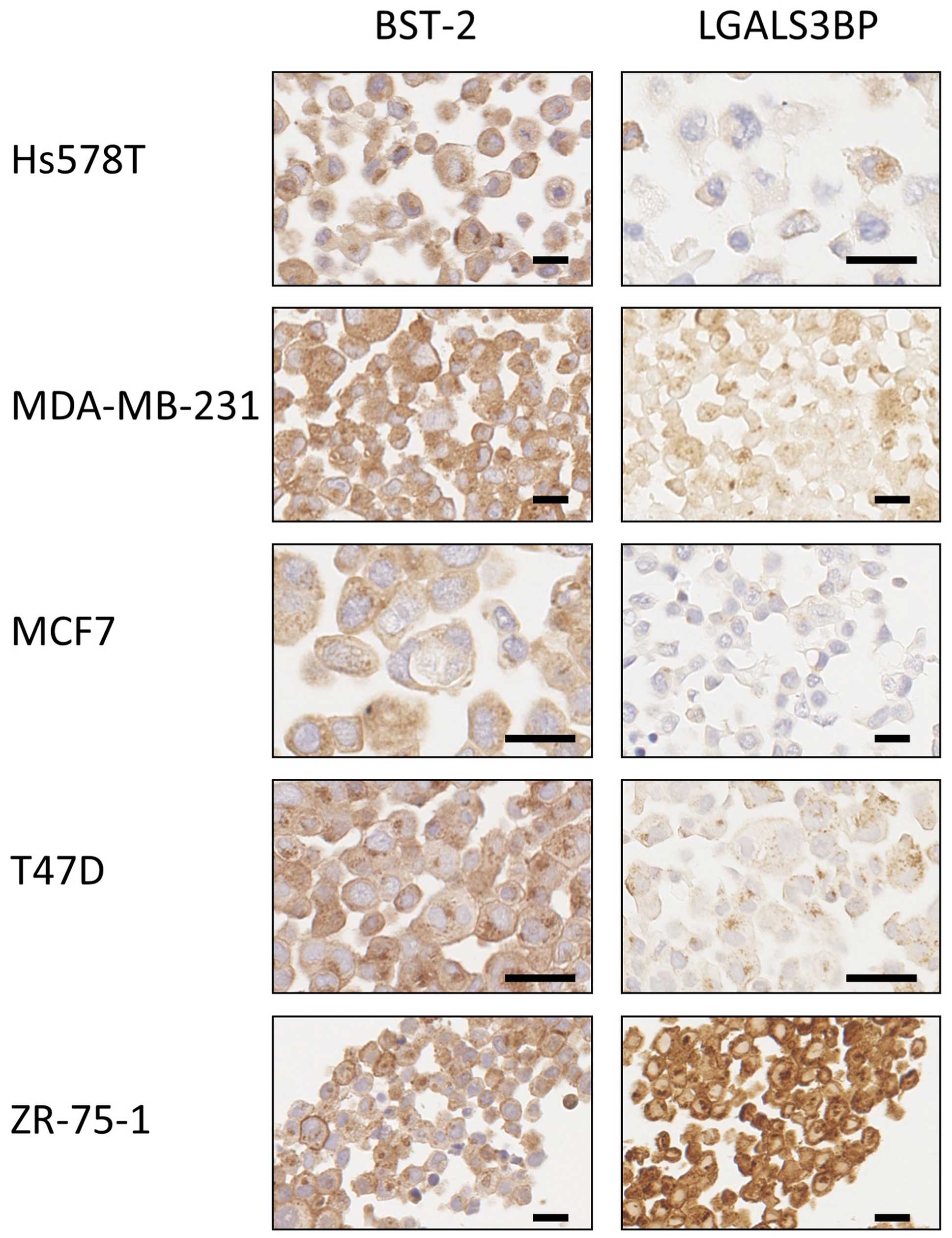
optimised for each antibody using a panel of pellets of breast
cancer cell lines that were formalin-fixed and paraffin-embedded
(Fig. 1). This panel included the
ZR-75-1 for which both BST-2 and LGALS3BP staining were positive,
according to the fact that these proteins were immuno-precipitated
from this particular cell line (7). The staining of biopsies was
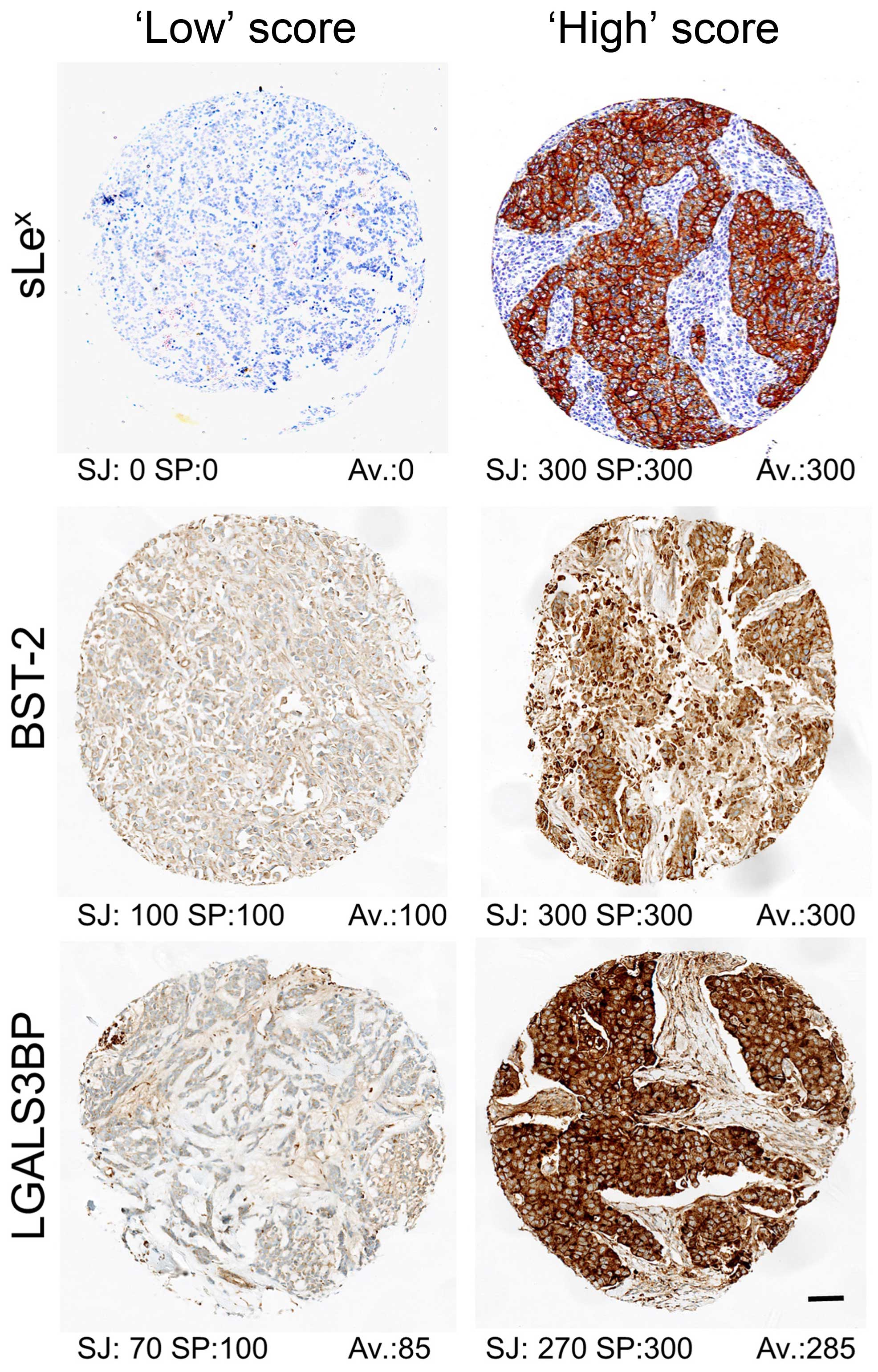
independently scored by two observers (S.J. and S.E.P., breast
pathologist). Score was calculated as the core average of the
product IxP, where I is the intensity of the staining (0, 1, 2 or
3) and P the percentage (0–100%) of stained tumour cells (see
Fig. 2 for illustration). Cases
where there was disagreement between the observers were reviewed
and consensus reached on a multiheaded microscope. Tumours were
classified as ‘low’ when ≤ to the median and ‘high’ otherwise.
Statistical analyses were carried on using Prism5 (Graph-Pad
software).
Depending on technical issues (e.g. cores damaged or
lost during process, quality of the tissue present in the cores)
unbiased observation could only be performed for about 350 samples
for each marker. To avoid any cohort bias, we chose to include in
the statistical analysis only the samples for which observation
could have been made for each of the three markers. When
cross-referenced, averaged scores for each of the targets were
validated for 249 tumours out of the 400 initially included. The
details of clinical and biological features of this cohort is given
in Table I. Overall, this cohort
fits the known statistics of the pathology regarding the rate of
expression biomarkers (10) or the
survival rates (1), thus excluding
bias from the cohort selection. Unfortunately, HER2 expression
status was only available for 134 (53%) of the samples rendering
difficult to specifically address the triple-negative group (17
samples, 12.7%) within this cohort. We thus decided to stratify our
cohort based on the sole ER expression for subsequent analysis.
 | Table IClinical and biological features of
the cohort of 249 patients. |
Table I
Clinical and biological features of
the cohort of 249 patients.
|
Characteristics | Data |
|---|
| Median age at
diagnosis: year | 57.0 (Min: 29.0,
Max 94.0) |
| Average tumour
size: cm | 2.64 |
| Histological grade:
n (%) |
| G1 | 41 (16.5) |
| G2 | 93 (37.3) |
| G3 | 97 (39.0) |
| ND | 18 (7.2) |
| Lymph node
Involvement: n (%) |
| Positive | 128 (51.4) |
| Negative | 108 (43.4) |
| ND | 13 (5.2) |
| Estradiol receptor
(ER): n (%) |
| Positive | 189 (75.9) |
| Negative | 57 (22.9) |
| ND | 3 (1.2) |
| Progesterone
receptor (PR): n (%) |
| Positive | 153 (61.4) |
| Negative | 93 (37.3) |
| ND | 3 (1.2) |
| HER2: n (%) |
| Positive | 110 (44.2) |
| Negative | 25 (10.0) |
| ND | 114 (45.8) |
| Median follow-up:
months | 148.0 |
| Local recurrences:
n (%) | 37 (14.9) |
| Distant
recurrences: n (%) | 73 (29.3) |
| Breast cancer
specific death: n (%) | 68 (27.3) |
Results
Staining and scoring of biopsies
For all three antibodies, the observed staining was
mainly cytoplasmic with membrane signal observable when the cells
were strongly stained (Fig. 2).
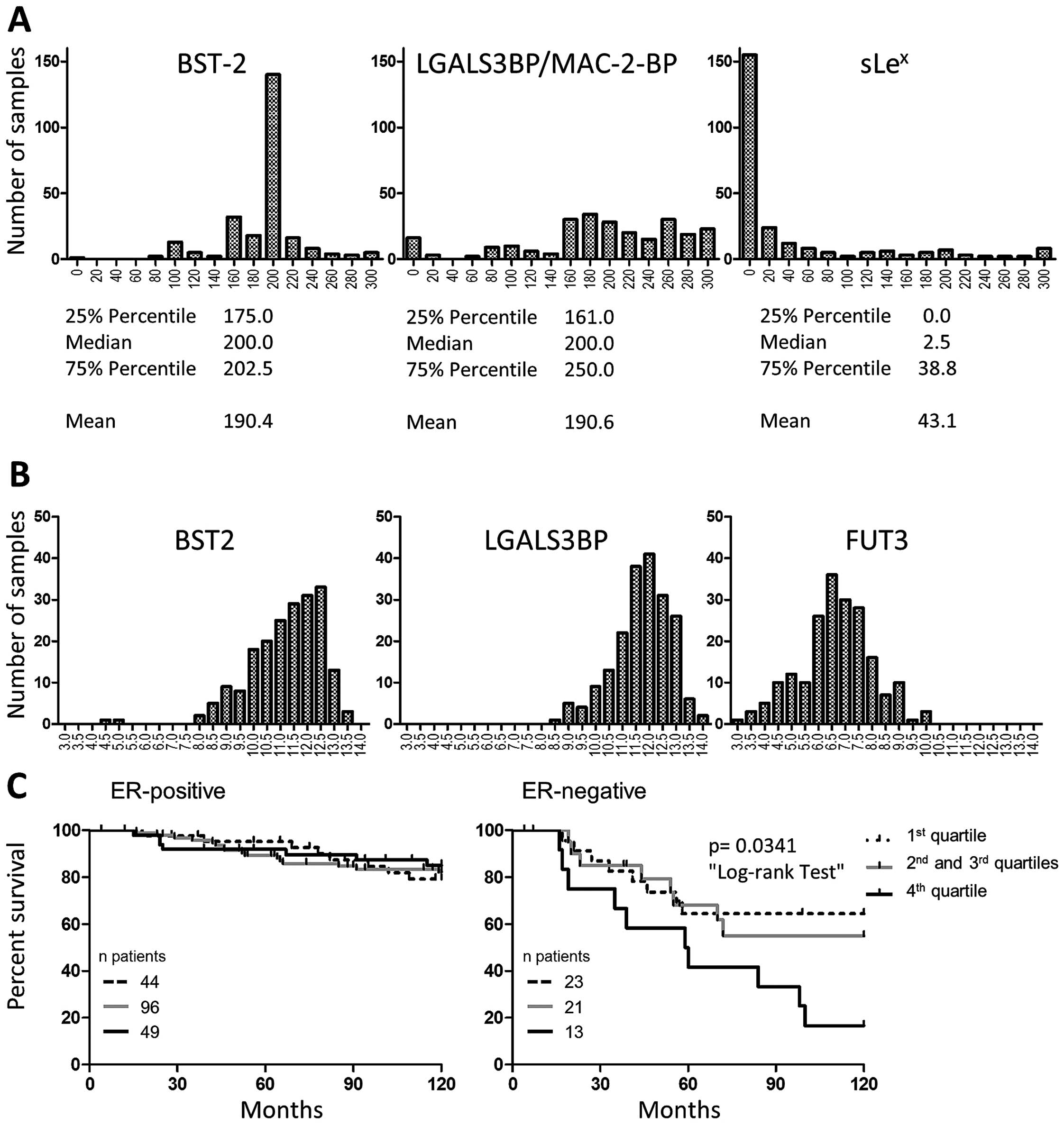
Variation of staining intensities allowed to classify each sample
according to the level of expression of each marker (11). The frequency distributions of the
scores were distinct when comparing the three markers (Fig. 3A) and quite different from the
Gaussian-type frequency distribution commonly observed when
measuring gene expression (Fig.
3B). Due to the asymmetrical score distributions, we chose to
use median to discriminate between ‘Low’ and ‘High’ scores and
compare group of tumours of the same size if possible. Median
scores were 2.5 for sLex and 200 for LGALS3BP. Median
was also 200 for the BST-2 group, but the very homogeneous staining
in this group resulted in 91 tumours being scored 200, making the
use of the median inappropriate to split the series in two equal
groups. After comparing the survival of the patients according to
the 25 and 75% percentile scores (Fig.
3C) we chose to use the highest quartile (score >202.5) to
define our ‘High’ BST-2 group. Although we identified both proteins
as carriers of sLex in a breast cancer cell line
(ZR-75-1), scores of sLex did not significantly
correlate with either BST-2 (Pearson r2=0.017, p=0.042)
or LGALS3BP (Pearson r2=0.002, p=0.445). This suggests
that in breast tumours, the occurrence of the high expression of
either carrier is independent of the occurrence of high
sLex expression and vice versa.
Correlation with clinical features
We first analysed the association of each of the
three markers with several histological and clinical features of
the tumours (Table II). As
previously reported (12),
sLex high expression correlated with ER-negativity,
lymph node (LN) involvement, and high grade. We also observed a
correlation with progesterone receptor (PR) negativity. When
considering the entire cohort, BST-2 and LGALS3BP did not correlate
with any of the tested features.
 | Table IICorrelation of antigen expression
with biological features of the tumours. |
Table II
Correlation of antigen expression
with biological features of the tumours.
|
sLex | BST-2 | LGALS3BP |
|---|
|
|
|
|
|---|
| Lowa | High | | Low | High | | Low | High | |
|---|
|
|
| |
|
| |
|
| |
|---|
| Features | nb | %c | n | % | p-value | n | % | n | % | p-value | n | % | n | % | p-value |
|---|
| ERd |
| Pos | 110 | 58.2 | 79 | 41.8 | 0.010 | 140 | 74.1 | 49 | 25.9 | NS | 102 | 54.0 | 87 | 46.0 | NS |
| Neg | 22 | 38.6 | 35 | 61.4 | | 45 | 78.9 | 12 | 21.1 | | 33 | 57.9 | 24 | 42.1 | |
| PRd |
| Pos | 92 | 60.1 | 61 | 39.9 | 0.012 | 111 | 72.5 | 42 | 27.5 | NS | 85 | 55.6 | 68 | 44.4 | NS |
| Neg | 40 | 43.0 | 53 | 57.0 | | 74 | 79.6 | 19 | 20.4 | | 50 | 53.8 | 43 | 46.2 | |
| HER2d |
| Pos | 13 | 52.0 | 12 | 48.0 | NS | 17 | 68.0 | 8 | 32.0 | NS | 12 | 48.0 | 13 | 52.0 | NS |
| Neg | 55 | 50.0 | 55 | 50.0 | | 81 | 73.6 | 29 | 26.4 | | 61 | 55.5 | 49 | 44.5 | |
| LNd |
| Pos | 60 | 47.6 | 66 | 52.4 | 0.039 | 95 | 75.4 | 31 | 24.6 | NS | 71 | 56.3 | 55 | 43.7 | NS |
| Neg | 71 | 61.2 | 45 | 38.8 | | 89 | 76.7 | 27 | 23.3 | | 59 | 50.9 | 57 | 49.1 | |
| TSd |
| <2 cm | 54 | 54.5 | 45 | 45.5 | NS | 69 | 69.7 | 30 | 30.3 | NS | 58 | 58.6 | 41 | 41.4 | NS |
| >2 cm | 78 | 53.1 | 69 | 46.9 | | 117 | 79.6 | 30 | 20.4 | | 77 | 52.4 | 70 | 47.6 | |
| DMd |
| Pos | 34 | 46.6 | 39 | 53.4 | NS | 56 | 76.7 | 17 | 23.3 | NS | 41 | 56.2 | 32 | 43.8 | NS |
| Neg | 100 | 56.8 | 76 | 43.2 | | 132 | 75.0 | 44 | 25.0 | | 96 | 54.5 | 80 | 45.5 | |
| Gradee |
| 1 | 23 | 56.1 | 18 | 43.9 | <0.001 | 33 | 80.5 | 8 | 19.5 | NS | 25 | 61 | 16 | 39.0 | NS |
| 2 | 61 | 65.6 | 32 | 34.4 | | 68 | 73.1 | 25 | 26.9 | | 50 | 53.8 | 43 | 46.2 | |
| 3 | 36 | 37.1 | 61 | 62.9 | | 76 | 78.4 | 21 | 21.6 | | 51 | 52.6 | 46 | 47.4 | |
| Agee |
| <50 yrs. | 34 | 47.9 | 37 | 52.1 | NS | 49 | 69.0 | 22 | 31.0 | NS | 38 | 53.5 | 33 | 46.5 | NS |
| 50–70 yrs. | 72 | 57.6 | 53 | 42.4 | | 97 | 77.6 | 28 | 22.4 | | 69 | 55.2 | 56 | 44.8 | |
| >70 yrs. | 28 | 52.8 | 25 | 47.2 | | 42 | 79.2 | 11 | 20.8 | | 30 | 56.6 | 23 | 43.4 | |
Both BST-2 and LGALS3BP high expression
correlated with earlier time of metastasis and poor prognosis
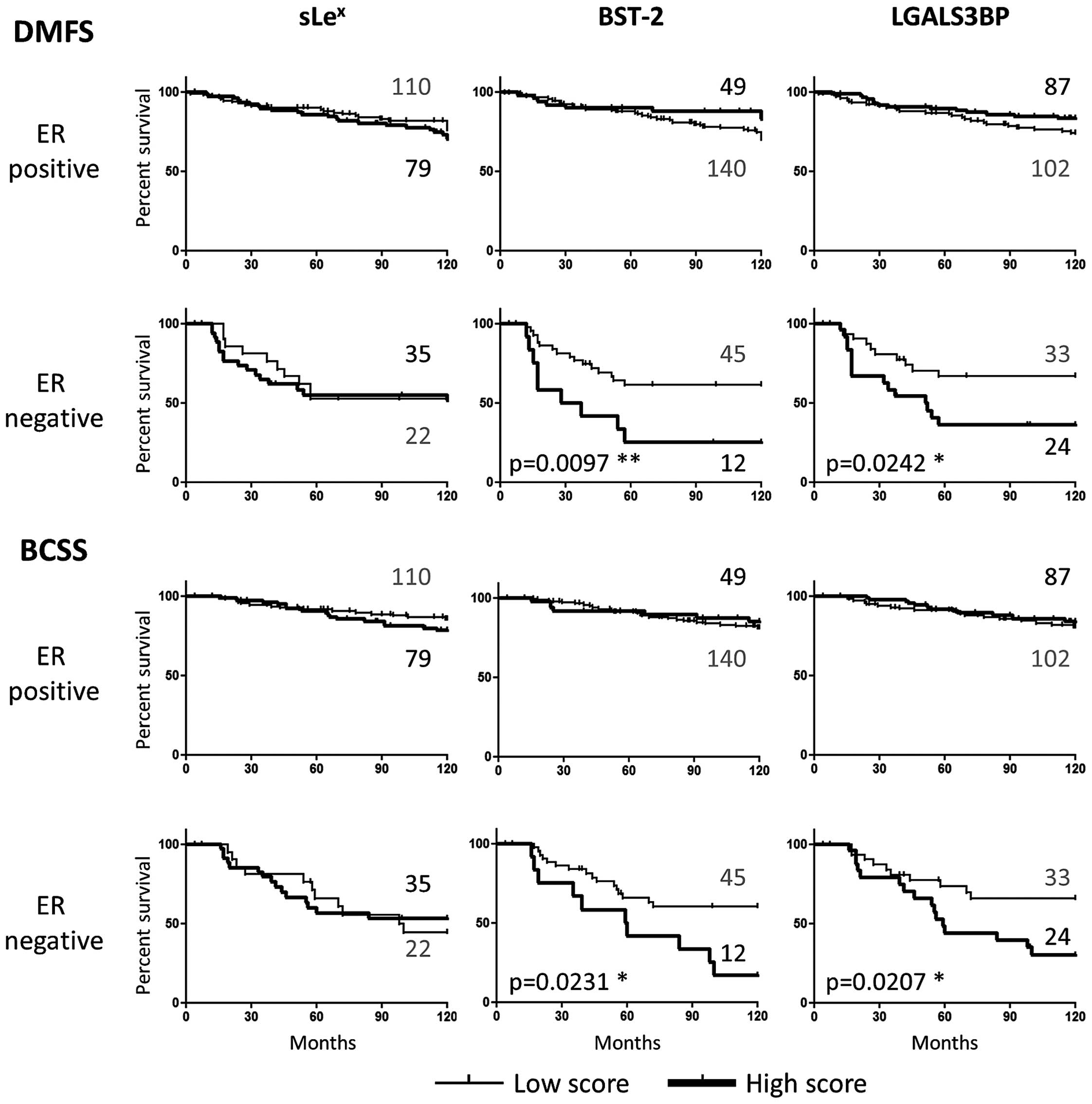
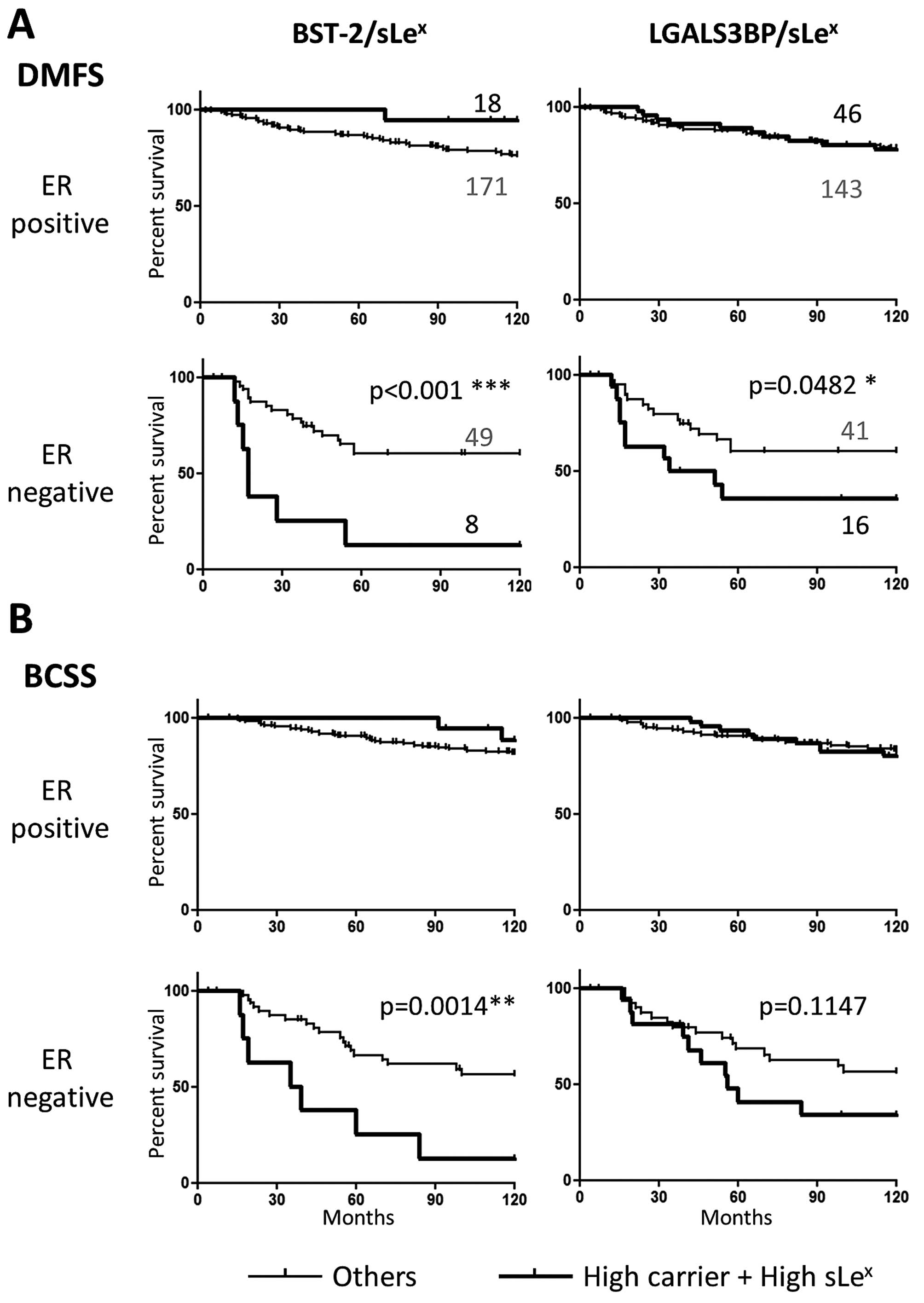
Kaplan-Meier analyses were performed for each marker
individually after ER stratification (Fig. 4). sLex on its own did
not associate with either distant metastasis-free survival (DMFS)
or breast cancer specific survival (BCSS), as we have previously
reported. However, strikingly, both BST-2 and LGALS3BP high
expression predicted earlier development of distant metastasis and
shorter patient survival in the ER-negative group, but not in the
ER-positive subset of patients (Fig.
4). While none of the markers were associated with DM when
analysing the cohort as a whole, both BST-2 and LGALS3BP correlated
with DM within the ER-negative group (p=0.0270 and p=0.0353
Fisher's exact test, respectively). This suggests that both
glycoproteins are involved in ER-negative tumour progression
independently of sLex expression.
BST-2 significantly alters the
organotropism of ER-negative metastasis
We previously reported that ‘very-high’ (i.e. score
of >60) expression of sLex in ER-positive tumours
drove metastasis to the bone (12). In the present study metastatic
ER-positive tumours with very-high sLex similarly
colonised bone more frequently than those with low sLex
expression (87.5 and 52.6%, respectively).
ER-negative tumours colonised distant organs at
various frequencies: lung (8.9%), liver (12.5%), bone (14.3%),
brain (17.9%) and other distant organs (23.2%) such as distant
lymph nodes (contra-lateral or mediastinal), pleura or skin. These
frequencies were compared between groups displaying high or low
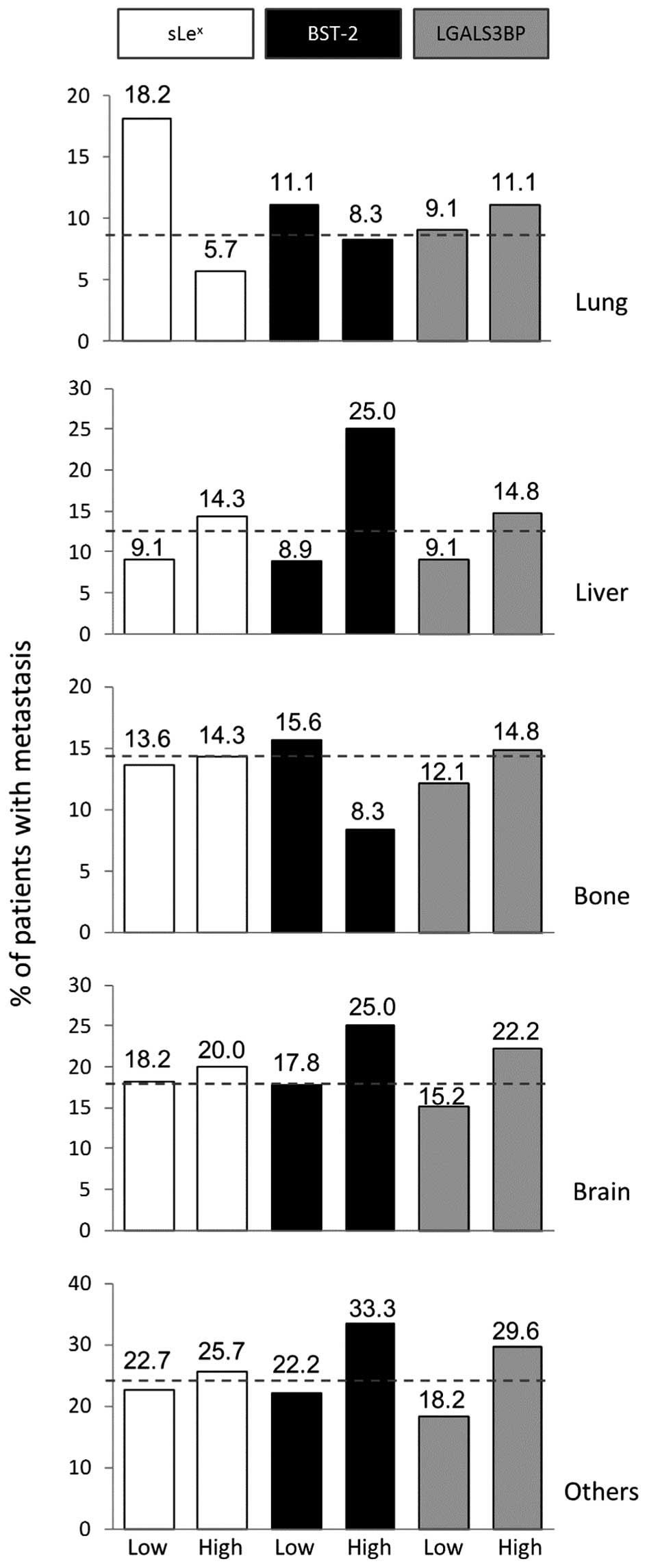
expression of sLex, BST-2 and LGALS3BP (Fig. 5). Taken independently, variation in
occurrence of metastasis for each site was not found to be
significantly associated to the expression of any of the tested
markers. However, high expression of BST-2 seemed to generally
affect the distribution of metastasis across the board. High
expression of BST-2 was associated with increased metastasis in
liver (2.8-fold) and brain (1.4-fold) while metastasis was
decreased in lung (1.3-fold) and bone (1.9-fold). By comparison,
the two other markers had limited effects. High sLex
expression seemed to follow the same trend as high BST-2,
especially for liver and lung metastasis. High expression of
LGALS3BP tended to be associated with an overall higher frequency
of metastasis without affecting the tropism. As a result, we found
that expression of BST-2 significantly altered the pattern of
metastasis when compared to the two other markers (Chi-square test,
p=0.0246).
Combined expression of BST-2 and
sLex further improves BST-2 prognostic value
Although we did not find sLex correlation
with its carriers in tumours, there is, nonetheless, a discrete
fraction of patients whose tumours displayed high sLex
expression together with high expression of BST-2 (10.6% of
patients) and/or LGALS3BP (24.4%). Due to the documented function
of sLex carried by these proteins as E-selectin ligand
(7,8), we investigated the prognostic value
of these combinations of markers in our series.
We analysed metastasis-free and breast cancer
specific survival of patients within groups displaying high
sLex expression together with high expression of one or
the other of its carriers (BST-2 or LGALS3BP) compared to the rest
of the patients (Fig. 6).
This stratification did not bear any significance
for the patients with ER-positive tumours. In the ER-negative
group, the combination of sLex with LGALS3BP did not
improve, and actually reduced, the prognostic value of LGALS3BP.
This suggests that the LGALS3BP function in ER-negative breast
cancer metastasis is independent of sLex expression.
This was surprising since sLex bearing LGALS3BP has been
shown to be involved in the rolling of the ZR-75-1 breast cancer
cell line on endothelial cells (8). However, LGALS3BP, as a secreted
protein, has been linked to cancer metastasis through interactions
with other proteins such as Tem1 (13) or galectins 1 and 3 (14–16).
These multiple mechanisms may explain why LGALS3BP on its own bears
some prognostic value, as previously reported (17), while the sLex/LGALS3BP
does not.
In contrast, the sLex/BST-2 combination
retained (and even increased) statistical significance in analysis
for both metastasis and patient survival, despite the fact the
sLex/BST-2 group was reduced to 8 patients. Computation
of Hazard Ratio showed an increase of risk when sLex was
combined to BST-2, for both BCSS (from 3.654 to 4.566) and DMFS
(from 3.961 to 7.070), indicating that the patients displaying both
markers concomitantly were more rapidly at risk than the others
(Table III). By contrast, the
same comparison performed using LGALS3BP with sLex did
not show any increase. The combination of BST-2 with
sLex identified a sub-group (14%) of ER-negative
patients with an 80% risk of metastasis at 5 years, twice the level
in the patients with tumours not over-expressing both markers
simultaneously. The patients with ER-negative carcinomas expressing
both sLex and BST-2 had only a 20% survival at 10 years,
about three times less than other patients with ER-negative
cancers. This may be due to the metastatic tropism associated with
BST-2, possibly enforced by sLex, since brain and liver
metastasis are more rapidly lethal than bone and lung metastasis
(18).
 | Table IIIHazard ratios of survival according
to single or combined variables. |
Table III
Hazard ratios of survival according
to single or combined variables.
| BCSSa | DMFSb |
|---|
|
|
|
|---|
| Variables | Hazard ratio | 95% Confidence
interval | Hazard ratio | 95% Confidence
interval |
|---|
| BST-2 | 3.654 | 1.362–9.801 | 3.961 | 1.379–11.380 |
|
BST-2/sLex | 4.566 | 1.468–14.200 | 7.070 | 2.077–24.070 |
| LGALS3BP | 2.541 | 1.153–5.597 | 2.554 | 1.130–5.772 |
|
LGALS3BP/sLex | 2.048 | 0.840–4.990 | 2.569 | 1.008–6.549 |
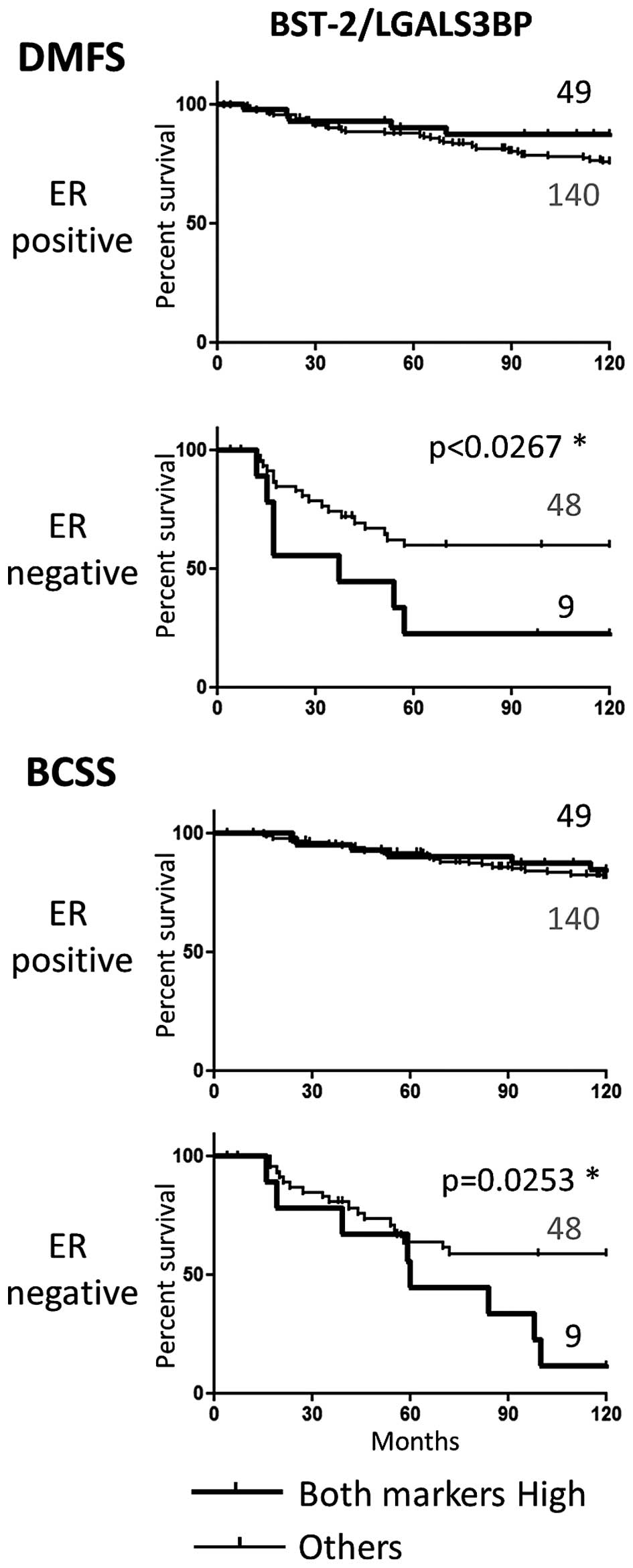
By comparison, stratification based on the high
expression of both BST-2 and LGALS3BP did not result in a better
prediction of metastasis or survival than each of the markers taken
individually (Fig. 7).
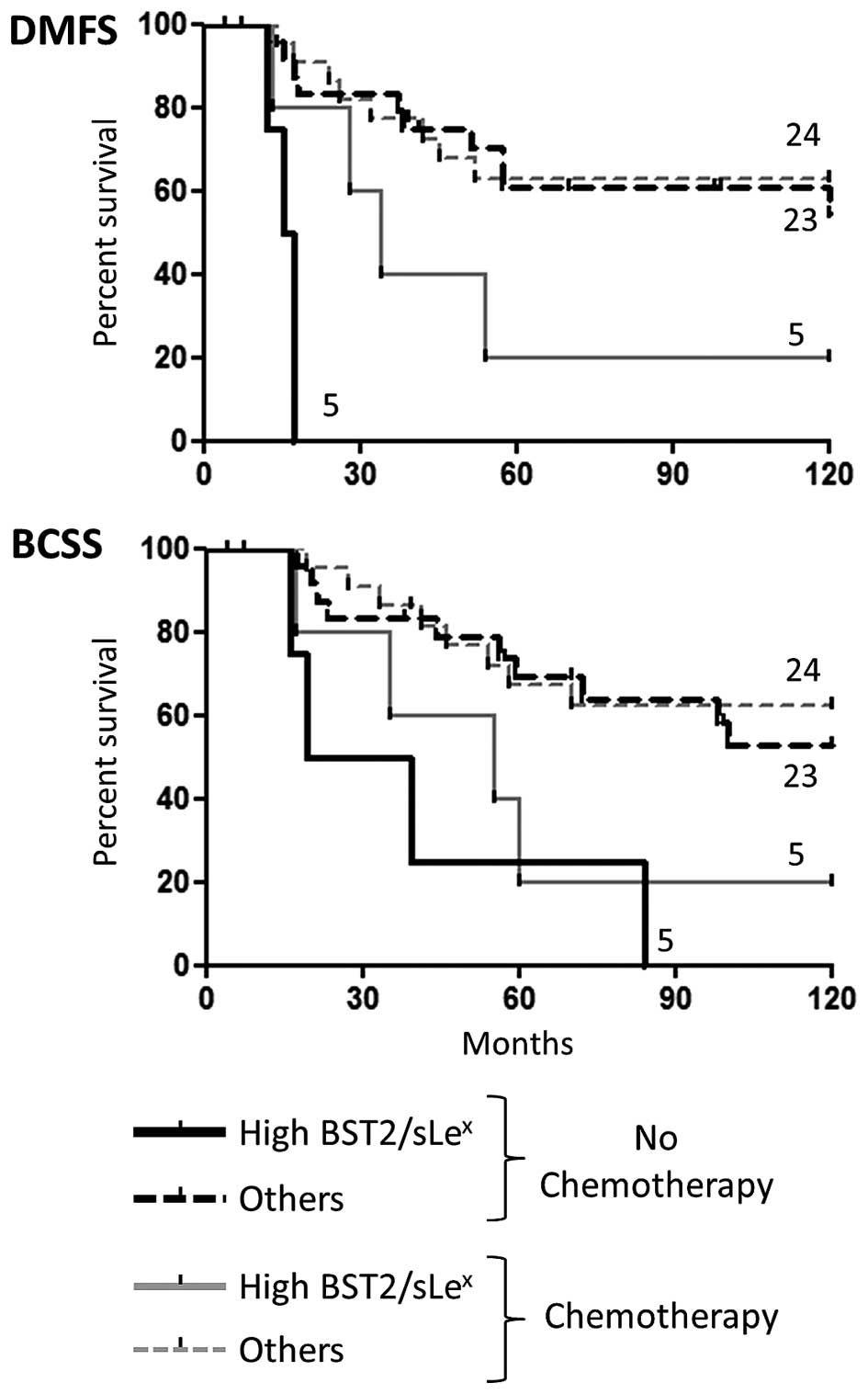
Influence of treatment of ER-negative
patients on survival analyses
Detailed review of the adjuvant therapy given to the
ER-negative groups revealed that half of the patients (28) received chemotherapy drugs, while
the other half (29) was only
treated with Tamoxifen as it was routinely performed during the
nineties (see Table IV for
details). We found no differences in DMFS or BCSS when comparing
these two arms, thus excluding any treatment bias in the results
reported above. We further explored the effect of each protocol (no
chemotherapy vs. chemotherapy) on the aggressive High
BST-2/sLex sub-population. The results seemed to
indicate that the patients belonging to the poor prognosis group
fared better when treated with chemotherapy compared to those
treated only with Tamoxifen (Fig.
8). Albeit statistically significant, this analysis was
performed with a very limited number of samples and should be
considered with appropriate reserve.
 | Table IVAdjuvant treatments of ER-negative
patients. |
Table IV
Adjuvant treatments of ER-negative
patients.
| Treatment | No. of
patients |
|---|
No
chemotherapy
n=29 | None | 1 |
| Tamoxifen | 28 |
Chemotherapy
n=28 | CMFa + Tamoxifen | 6 |
| CMF | 13 |
| EFCb | 5 |
| Other
chemotoxicsc | 4 |
Discussion
In this study, we retrospectively assessed the
prognostic significance of two membrane bound glycoproteins, BST-2
and LGALS3BP, in breast cancer metastasis and survival. Both
proteins were chosen based on the fact that they were found to be
E-selectin ligands, when carrying the carbohydrate determinant
known as sialyl-Lewis x (sLex) antigen, and potentially
involved in blood borne metastasis. We previously investigated the
expression of sLex in breast cancer and found this
glycan overexpressed in ER-negative breast cancers (7). To detect sLex expression
we chose the widely used HECA-452 monoclonal antibody that, in our
hands, was able to recognize sLex carried by multiple
glycoproteins and/or glycolipids (7). HECA-452 binding is therefore
non-discriminative of the carrier of the glycan. This is why we
wanted to examine the relevance of sLex together with
two of its described protein carriers in our statistical analyses.
Since carbohydrate structures, or glycans, result from the combined
expression of several synthesizing enzymes (glycosyltransferases),
one of the most efficient way to assess their expression in tumour
sample is to use specific probes (monoclonal antibodies or lectins)
to detect them on tissue sections. Thus, to analyse both proteins
and glycans from the same material, we chose to assess the
expression of sLex and its putative carriers by
immunohistochemistry. Another aim of our study was to investigate
the possible correlation of our markers or combinations of markers
with the organotropism of the metastases. To achieve this, we
exploited a series of formalin-fixed paraffin-embedded samples
archived by the King's Health Partners Cancer Biobank, which
consist of 400 patient biopsies all associated with the extensive
clinical history (e.g. date and site of metastasis and treatment
protocols) of the corresponding patients. From this series, we
managed to validate scoring for all three markers for 249 samples.
Although this cohort was of modest size, the quality of the
associated clinical data made it suitable for the purpose of our
study.
Of note, we found that both BST-2 and LGALS3BP
glycoproteins were indeed associated with the development of
subsequent distant metastasis and also patient survival, albeit
only in the patients with ER-negative tumours. Conversely, the
expression of these proteins was not dependent on the ER-status of
the tumours. This suggests that both proteins act on the pathway of
metastasis in partnership with other factors that are potentially
specific to ER-negative cancer cells.
One such factor could be the sLex
antigen, which correlates with ER negativity (7). We found that combining
sLex and LGALS3BP high expression to stratify patients
did not improve the prognostic significance of LGALS3BP. This
suggests that the pro-metastatic function of LGALS3BP is not
primarily due the ability of this protein to be recognised by
E-selectin. Indeed, LGALS3BP (MAC-2BP) was experimentally shown to
be involved in pro-metastatic interactions (15,16)
independently of sLex expression or selectin
involvement. One previous study also reported LGALS3BP to be
associated with poor survival in node-negative breast cancer
patients (17). On the contrary,
another large study showed no correlation of the cytosolic
expression of LGALS3BP with prognosis in breast cancer (19). However, the authors of this study
argued that the cytosolic, immature, form of LGALS3BP may not be
appropriate to predict prognosis due to the possible lack of
biological activity. While implication of LGALS3BP in tumour
progression and metastasis is now well documented in a number of
cancers, it is also clear that the galectins/galectin binding
protein/ligand networks involved are intricate and plastic
(20). The present study confirms
the clinical relevance of LGALS3BP by showing its association with
occurrence of metastasis in ER-negative breast cancer, but
dismisses sLex antigen as a mediator of this effect.
On the contrary, high-sLex/high-BST-2
combined expression was a better predictor of distant metastasis
and survival than BST-2 alone. High expression of BST-2 mRNA was
previously associated with tumour aggressiveness and decreased
survival in breast cancer by others (21). Cai et al also reported BST-2
protein expression to be associated with breast cancer bone
metastasis in a smaller cohort (n=50) of breast samples (22). Experimental data, based on cell
lines and in vivo models, have hinted that BST-2 was
involved in increased proliferation and reduced apoptosis (23), as well as increased migration,
invasion and metastasis (21,22,24).
The data we are presenting imply that the role of BST-2 in
metastasis is specific to patients with ER-negative tumours, and
may be further enhanced by sLex expression. The reason
why BST-2 appears to specifically influence the tropism of
ER-negative breast cancers is unclear. It could be related to the
described function of BST-2 as an organiser of membrane
microdomains (i.e. lipid rafts) (25). Indeed, by acting on the
organization of the cell membrane, oligomers of BST-2 may promote
the function of other molecules, specifically expressed in
ER-negative cancer cells compared to ER-positive ones, which are
involved in brain or liver metastasis. Identifying such partners of
BST-2 would thus warrant further investigations. Clusterisation of
BST-2 around the lipid rafts would also create patches of BST-2
associated glycans, including sLex (25). Such organization would indeed
enhance the ability of BST-2 borne sLex to be recognised
by selectin by improving the avidity of the interaction. Whether
these putative mechanisms actually participate in the metastatic
process in breast cancer would require further experimental
demonstration.
The prognostic value of combined expression of BST-2
and LGALS3BP was not improved compared to each marker individually.
Thus, the synergistic effect of sLex and BST-2
expression on prognosis is specific of these particular two
markers, and not random. Importantly, due to the complex
biosynthesis of glycans, such a combination of markers could not
have been easily detected using gene profiling strategies on its
own. Supporting this, one very recent study has demonstrated that
integrated analysis of glycosylation genes and their glycan
products indeed resulted in significant prognostic data (26). In the same line, our present study
demonstrates the potential of the widely available technique of
immunohistochemistry to investigate combinations of glycans
together with relevant protein carriers as prognosis markers.
The combination of BST-2 expression and
sLex positivity identified a small subset of patients
that fared significantly worse than the other ER-negative breast
cancer patients. Although sub-stratification ended up producing
small groups of patients, the statistical analysis retained a high
degree of significance. Whilst patients with ER-negative tumours
may receive adjuvant chemotherapy, depending on other biological
and patient variables, it is clear that not all of these women do
equally badly (27). Based on new
biomarkers, such as our BST-2/sLex combination, one may
therefore consider improving ER-negative patients follow-up and
treatment protocols with possibly more aggressive therapy. On the
other hand, such biomarkers, presumably involved in
selectin-mediated metastasis (28), could also serve as molecular target
for tailored therapy (29). In
that regard, we believe both BST-2 and LGALS3BP could be considered
as putative targets to treat ER-negative metastatic breast
cancer.
Acknowledgements
This work was supported by the Conseil Regional du
Nord - Pas de Calais (SJ), La Ligue contre le Cancer (SJ and PD),
Centre National de Recherche Scientifique (CNRS) through the Projet
International de Coopération Scientifique (PICS) grant ‘GLYCOMET’
(PICS06165) (S.J., P.D., J.M.B.) and the Medical Research Council
(V.T.).
Abbreviations:
|
BCSS
|
breast cancer specific survival
|
|
BST-2
|
bone marrow stromal antigen 2
|
|
DM
|
distant metastasis
|
|
ER
|
estrogen receptor
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
LGALS3BP
|
galectin-3-binding protein
|
|
LN
|
lymph node
|
|
mAb
|
monoclonal antibody
|
|
NHS
|
national health service
|
|
PR
|
progesterone receptor
|
|
sLex
|
sialyl-Lewis x antigen
|
|
TMA
|
tissue microarray
|
References
|
1
|
Howlader N, Noone A, Krapcho M, et al:
SEER Cancer Statistics Review. National Cancer Institute; 2011
|
|
2
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
St Hill CA: Interactions between
endothelial selectins and cancer cells regulate metastasis. Front
Biosci (Landmark Ed). 16:3233–3251. 2011. View Article : Google Scholar
|
|
4
|
Kawamura YI, Adachi Y, Curiel DT,
Kawashima R, Kannagi R, Nishimoto N and Dohi T: Therapeutic
adenoviral gene transfer of a glycosyltransferase for prevention of
peritoneal dissemination and metastasis of gastric cancer. Cancer
Gene Ther. 21:427–433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Komatsu H, Mizuguchi S, Izumi N, Chung K,
Hanada S, Inoue H, Suehiro S and Nishiyama N: Sialyl Lewis X as a
predictor of skip N2 metastasis in clinical stage IA non-small cell
lung cancer. World J Surg Oncol. 11:3092013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gakhar G, Navarro VN, Jurish M, Lee GY,
Tagawa ST, Akhtar NH, Seandel M, Geng Y, Liu H, Bander NH, et al:
Circulating tumor cells from prostate cancer patients interact with
E-selectin under physiologic blood flow. PLoS One. 8:e851432013.
View Article : Google Scholar
|
|
7
|
Julien S, Ivetic A, Grigoriadis A, QiZe D,
Burford B, Sproviero D, Picco G, Gillett C, Papp SL, Schaffer L, et
al: Selectin ligand sialyl-Lewis x antigen drives metastasis of
hormone-dependent breast cancers. Cancer Res. 71:7683–7693. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shirure VS, Reynolds NM and Burdick MM:
Mac-2 binding protein is a novel E-selectin ligand expressed by
breast cancer cells. PLoS One. 7:e445292012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Crother TR, Schröder NWJ, Karlin J, Chen
S, Shimada K, Slepenkin A, Alsabeh R, Peterson E and Arditi M:
Chlamydia pneumoniae infection induced allergic airway
sensitization is controlled by regulatory T-cells and plasmacytoid
dendritic cells. PLoS One. 6:e207842011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patani N, Martin L-A and Dowsett M:
Biomarkers for the clinical management of breast cancer:
International perspective. Int J Cancer. 133:1–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pinder SE, Brown JP, Gillett C, Purdie CA,
Speirs V, Thompson AM and Shaaban AM: The manufacture and
assessment of tissue microarrays: Suggestions and criteria for
analysis, with breast cancer as an example. J Clin Pathol.
66:169–177. 2013. View Article : Google Scholar
|
|
12
|
Julien S, Ivetic A, Grigoriadis A, QiZe D,
Burford B, Sproviero D, Picco G, Gillett C, Papp SL, Schaffer L, et
al: Selectin ligand sialyl-Lewis x antigen drives metastasis of
hormone-dependent breast cancers. Cancer Res. 71:7683–7693. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Becker R, Lenter MC, Vollkommer T, Boos
AM, Pfaff D, Augustin HG and Christian S: Tumor stroma marker
endosialin (Tem1) is a binding partner of metastasis-related
protein Mac-2 BP/90K. FASEB J. 22:3059–3067. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tinari N, Kuwabara I, Huflejt ME, Shen PF,
Iacobelli S and Liu FT: Glycoprotein 90K/MAC-2BP interacts with
galectin-1 and mediates galectin-1-induced cell aggregation. Int J
Cancer. 91:167–172. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Piccolo E, Tinari N, Semeraro D, Traini S,
Fichera I, Cumashi A, La Sorda R, Spinella F, Bagnato A, Lattanzio
R, et al: LGALS3BP, lectin galactoside-binding soluble 3 binding
protein, induces vascular endothelial growth factor in human breast
cancer cells and promotes angiogenesis. J Mol Med Berl. 91:83–94.
2013. View Article : Google Scholar
|
|
16
|
Inohara H, Akahani S, Koths K and Raz A:
Interactions between galectin-3 and Mac-2-binding protein mediate
cell-cell adhesion. Cancer Res. 56:4530–4534. 1996.PubMed/NCBI
|
|
17
|
Tinari N, Lattanzio R, Querzoli P, Natoli
C, Grassadonia A, Alberti S, Hubalek M, Reimer D, Nenci I, Bruzzi
P, et al; Consorzio Interuniversitario Nazionale per la
Bio-Oncologia (CINBO). High expression of 90K (Mac-2 BP) is
associated with poor survival in node-negative breast cancer
patients not receiving adjuvant systemic therapies. Int J Cancer.
124:333–338. 2009. View Article : Google Scholar
|
|
18
|
Largillier R, Ferrero J-M, Doyen J,
Barriere J, Namer M, Mari V, Courdi A, Hannoun-Levi JM, Ettore F,
Birtwisle-Peyrottes I, et al: Prognostic factors in 1,038 women
with metastatic breast cancer. Ann Oncol. 19:2012–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Foekens JA, Klijn JG, Natoli C, van Putten
WL, Di Stefano P, Look MP, Portengen H and Iacobelli S: Expression
of tumor-associated 90K-antigen in human breast cancer: No
correlation with prognosis and response to first-line therapy with
tamoxifen. Int J Cancer. 64:130–134. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grassadonia A, Tinari N, Iurisci I,
Piccolo E, Cumashi A, Innominato P, D'Egidio M, Natoli C, Piantelli
M and Iacobelli S: 90K (Mac-2 BP) and galectins in tumor
progression and metastasis. Glycoconj J. 19:551–556. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mahauad-Fernandez WD, DeMali KA, Olivier
AK and Okeoma CM: Bone marrow stromal antigen 2 expressed in cancer
cells promotes mammary tumor growth and metastasis. Breast Cancer
Res. 16:4932014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai D, Cao J, Li Z, Zheng X, Yao Y, Li W
and Yuan Z: Up-regulation of bone marrow stromal protein 2 (BST2)
in breast cancer with bone metastasis. BMC Cancer. 9:1022009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sayeed A, Luciani-Torres G, Meng Z,
Bennington JL, Moore DH and Dairkee SH: Aberrant regulation of the
BST2 (Tetherin) promoter enhances cell proliferation and apoptosis
evasion in high grade breast cancer cells. PLoS One. 8:e671912013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yi EH, Yoo H, Noh KH, Han S, Lee H, Lee
JK, Won C, Kim BH, Kim MH, Cho CH, et al: BST-2 is a potential
activator of invasion and migration in tamoxifen-resistant breast
cancer cells. Biochem Biophys Res Commun. 435:685–690. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Billcliff PG, Rollason R, Prior I, Owen
DM, Gaus K and Banting G: CD317/tetherin is an organiser of
membrane micro-domains. J Cell Sci. 126:1553–1564. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Milde-Langosch K, Schütze D,
Oliveira-Ferrer L, Wikman H, Müller V, Lebok P, Pantel K, Schröder
C, Witzel I and Schumacher U: Relevance of βGal-βGalNAc-containing
glycans and the enzymes involved in their synthesis for invasion
and survival in breast cancer patients. Breast Cancer Res Treat.
151:515–528. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Teschendorff AE and Caldas C: A robust
classifier of high predictive value to identify good prognosis
patients in ER-negative breast cancer. Breast Cancer Res.
10:R732008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stübke K, Wicklein D, Herich L, Schumacher
U and Nehman N: Selectin-deficiency reduces the number of
spontaneous metastases in a xenograft model of human breast cancer.
Cancer Lett. 321:89–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eccles SA, Aboagye EO, Ali S, Anderson AS,
Armes J, Berditchevski F, Blaydes JP, Brennan K, Brown NJ, Bryant
HE, et al: Critical research gaps and translational priorities for
the successful prevention and treatment of breast cancer. Breast
Cancer Res. 15:R922013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Desmedt C, Piette F, Loi S, Wang Y,
Lallemand F, Haibe-Kains B, Viale G, Delorenzi M, Zhang Y,
D'Assignies MS, et al: Strong time dependence of the 76-gene
prognostic signature for node-negative breast cancer patients in
the TRANSBIG multicenter independent validation series. Clin Cancer
Res. 13:3207–3214. 2007. View Article : Google Scholar : PubMed/NCBI
|