Introduction
Slits (Slit1-3) proteins and their cognate receptors
(Robo1-4) were originally identified as key regulators in neural
development, yet, their functions are not limited to neurons. The
Slit/Robo signaling pathway also functions in the development of
the heart (1), gonad (2), follicle (3) and kidney (4). Altered expression of Slits and Robo
is common in various pathological conditions, particularly in
cancer. Current research on the Slit/Robo signaling pathway in
tumors is focused on growth and metastasis, of which the
Slit2/Robo1 signaling axis is the most studied. Numerous studies
demonstrate that Slit2 exerts its functions through binding to the
Robo1 receptor. This induces controversial effects on two aspects
of tumor cells, by either promoting or suppressing tumor growth and
on metastasis, depending on cancer types. While the Slit2/Robo1
signaling axis suppressed growth or metastasis in breast cancer
(5,6), pancreatic ductal adenocarcinoma
(7), intrahepatic
cholangiocarcinoma (8),
medulloblastoma (9), and glioma
(10–12), it promoted growth and metastasis in
intestinal cancer (13),
colorectal carcinoma (14),
pancreatic islet tumors (15), and
chemically-induced squamous cell carcinoma (16). These results underline the multiple
effects of Slit2/Robo1 signal pathway on tumor progression.
For tumor metastasis to occur, tumor cells must
degrade the extracellular matrix and basement membrane to
facilitate invasion. MMPs, in particular, MMP2 and MMP9 are thought
to play a critical role in this process. To date, very few studies
have reported on the effect of the Slit2/Robo1 signaling pathway on
MMP2 and MMP9 expression. In Slit2-transgenic mice, MMP2,
but not MMP9 expression was upregulated in chemically-induced skin
tumors (17). Moreover, a Robo1
blocking monoclonal antibody inhibited the activity of MMP2 and
MMP9 in tongue cancer cells (18).
Conversely, Slit2 inhibited the CXCL12-induced activities of MMP2
and MMP9 in breast cancer cells (19). These results suggest that Slit2
could have both inhibitory and stimulatory effects on MMP2 and MMP9
expression and activity in different tumors. However, how the
Slit2/Robo1 signaling pathway modulates MMP2 and MMP9 expression
and activity currently remains unknown.
Although the effects of the Slit2/Robo1 signaling
axis on tumor growth and metastasis have been reported in various
cancers, very little is known of this pathway in hepatocellular
carcinoma (HCC). In the present study, we first examined the
expression of Slit2 and Robo1 in several HCC cell lines, and then,
explored the effects of Slit2/Robo1 on tumor growth and metastasis
of cells by lentivirus-mediated Slit2 RNA knockdown and
Robo1 overexpression. We found that, unlike the previously
reported either inhibitory or promoting effects of the Slit2/Robo1
signaling axis on tumor growth and metastasis, Slit2 and Robo1
induced opposing effects on these tumor characteristics.
Materials and methods
Cell lines, vector constructs and
reagents
All HCC cell lines, Sk-hep-1, SMMC-7721, HepG2 and
normal hepatic cell lines L-O2 were cultured in high-glucose
Dulbecco's modified Eagle's medium (DMEM; HyClone
Laboratories, Logan, UT, USA) supplemented with 10% fetal bovine
serum (FBS; Gibco-BRL, Carlsbad, CA, USA). The human full length
cDNA of Robo1 (4824 bp) was supplies by Professor Jiahuai Han
(State Key Laboratory of Cellular Stress Biology, School of Life
Sciences, Xiamen University, Xiamen, China) and subcloned into the
lentiviral expression vector pBobi (gift from Professor Jiahuai
Han) with a cMyc tag at the carboxyl terminus. Small haipin RNA
(shRNA) oligos directed against Slit2 which were designed and
screened with online service (http://katahdin.cshl.org/siRNA/RNAi.cgi?type=shRNA),
were annealed and cloned into the lentivirus vector pll3.7 (State
Key Laboratory of Cellular Stress Biology, School of Life
Sciences). MAPK inhibitors, SB203580, PD98059, SP600125 and PI3K
inhibitor, LY294002, were purchased from Calbiochem (San Diego, CA,
USA).
Establishment of stable cell lines
Virus stocks were prepared by co-transfecting pll3.7
or pBobi (cMyc tag) with two packaging plasmids (pHR and pVSVG)
into 293T cells. Viral supernatants were harvested after 48 h,
filtered and centrifuged (90 min at 75,000 × g). For Slit2
RNA knockdown, cell lines were infected with pll3.7-Slit2-shRNA in
the presence of polybrene (8 μg/ml; Sigma, St. Louis, MO, USA) for
2 days. Infected cells were screened by G418 (1,500 μg/ml for
Sk-hep-1; Sigma) treatment. The same pll3.7 vectors with scramble
sequence of Slit2 were used as a control
(pll3.7-Slit2-shctrl). For Robo1 overexpression, the
method is the same as mentioned above, except cell lines were
infected with pBobi-Robo1, and screened by puromycin (2.5 μg/ml;
Sigma), the same vector encoding GFP was used as a control
(pBobi-GFP). Stable transfectants were assayed for the expression
of Slit2 and Robo1 by real-time quantitative PCR (qPCR) and western
blot analysis.
PCR
Real-time qPCR and primers used were previously
described (20). For reverse
transcription PCR (RT-PCR), amplification conditions were initial
denaturation at 95ºC for 1 min, followed by 32 cycles of 95ºC for
30 sec, 58ºC for 30 sec and 72ºC for 1 min for MMPs and TIMPs, by
23 cycles and the same conditions for GAPDH. All primer sequences
are listed in Table I.
 | Table IPrimers for reverse transcriptional
PCR. |
Table I
Primers for reverse transcriptional
PCR.
| Primers |
|---|
| hMMP1-Forward |
5′-CATCCAAGCCATATATGGACGTTCC-3′ |
| hMMP1-Reverse |
5′-TCTGGAGAGTCAAAATTCTCTTCGT-3′ |
| hMMP2-Forward |
5′-TTTGACGGTAAGGACGGACTC-3′ |
| hMMP2-Reverse |
5′-TTGGTGTAGGTGTAAATGGGTG-3′ |
| hMMP3-Forward |
5′-TCAGTCCCTCTATGGACCTC-3′ |
| hMMP3-Reverse |
5′-GAGGGAAACCTAGGGTGTGG-3′ |
| hMMP8-Forward |
5′-AGTGCCTGACAGTGGTGGTTTT-3′ |
| hMMP8-Reverse | 5′-CCAGTAGGTTGG
ATAGGGTTGC-3′ |
| hMMP9-Forward |
5′-CGGAGCACGGAGACGGGTAT-3′ |
| hMMP9-Reverse |
5′-GCCGCCACGAGGAACAAACT-3′ |
| hMMP10-Forward |
5′-AGTTTGGCTCATGCCTACCC-3′ |
| hMMP10-Reverse |
5′-GGCCCAGAACTCATTTCCTTT-3′ |
| hMMP11-Forward |
5′-GGTGGCAGCCCATGAATTTG-3′ |
| hMMP11-Reverse |
5′-ACTGAGCACCTTGGAAGAACC-3′ |
| hMMP13-Forward |
5′-TGGAATTAAGGAGCATGGCG-3′ |
| hMMP13-Reverse |
5′-CCTCGGAGACTGGTAATGGC-3′ |
|
hMT1-MMP-Forward |
5′-AGGTGATCATCATTGAGGTGG-3′ |
|
hMT1-MMP-Reverse |
5′-ACAGAGAGAAGCAAGGAGGC-3′ |
| hTIMP1-Forward |
5′-TGCACCTGTGTCCCACCCCACCCACAGACG-3′ |
| hTIMP1-Reverse |
5′-GGCTATCTGGGACCGCAGGGACTGCCAGGT-3′ |
| hTIMP2-Forward |
5′-AGGGCCAAAGCGGTCAGT-3′ |
| hTIMP2-Reverse |
5′-CCTGCTTATGGGTCCTCGA-3′ |
| hGAPDH-Forward |
5′-ACCACAGTCCATGCCATCAC-3′ |
| hGAPDH-Reverse |
5′-TCCACCACCCTGTTGCTGTA-3′ |
Enzyme-linked immunosorbent assay
A total of 1×106 cells were seeded per
well in 6-well microplates (Shanghai Sunub Bio-Tech Development,
Inc., Shanghai, China). After cells became attached (8 h later),
the culture medium was replaced with 2 ml serum-free fresh
DMEM/well. Twenty-four hours later, culture supernatants were
collected and centrifuged to remove cell debris. Secreted Slit2
production in supernatants was measured by Enzyme-linked
immunosorbent assay (ELISA) kit (EIAab, #E06 72 h) in accordance
with the manufacturer's instructions. Optical densities of
supernatants were measured at 450 nm in a microplate reader
(MultiSkan MK3; Thermo Fifher Scientific, Waltham, MA, USA).
Cell proliferation, migration and
invasion assay
Cell proliferation, migration and invasion assays
were performed as previously described (20). Briefly, a total of 500 cells were
seeded per well in 96-well microplates (Shanghai Sunub Bio-Tech
Development) and cultured for 4 consecutive days. The medium was
changed every other day. On each day, one group of culture media
was replaced with 100 μl serum-free fresh medium containing 10% MTT
(Sigma) and maintained at 37ºC for 4 h. After discarding the
medium, 150 μl of DMSO was added to each well to dissolve MTT
formazans. Optical densities were measured at 560 nm with a
reference wavelength at 630 nm.
For cell migration, cells that were 90% confluent in
a 24-well plate (Shanghai Sunub Bio-Tech Development) were
scratched with a 200-μl pipette tip to form wounded gaps. After
washing out cell debris, the wounded gaps were photographed under a
microscope at ×40 magnification to acquire a baseline image. Cells
were then cultured in DMEM containing 10% FBS for 24 h and
photographed to obtain the second set of images. Gap width was
measured by Image-Pro Plus 6.0 software. Cell migration was
quantitatively analyzed by subtracting the gap width of the second
image from the baseline image.
For cell invasion, chambers (8 μm; Millipore,
Billerica, MA, USA) were coated with 20 μl diluted Matrigel (0.1 mg
protein/ml; BD Biosciences) for 30 min at 37ºC and inserted into a
24-well plate. A total of 1×104 cells/chamber in 100 μl
serum-free DMEM were plated on the upper side of Matrigel-coated
Transwell chambers. Medium in the lower chambers contained 10% FBS
as the source of chemo-attractants. The plates were incubated for
24 h at 37ºC. Cells that had invaded the lower surface at 37ºC were
fixed with methanol and stained with crystal violet. Three random
fields were counted under a light microscope.
Cell adhesion assay
Twenty-four-well plates were pre-coated with 0.2%
gelatin. Cells were then plated on coated culture plates and
incubated for 30 min. After medium and non-adherent cells were
removed, adherent cells were washed with PBS two times, and stained
with crystal violet. After being washed twice, the adherent cells
were counted by counting the cell numbers on eight random
fields.
Gelatin zymography
Gelatin zymography was performed as previously
described (20). A total of 2
million cells were cultured in a 6-cm dish (Shanghai Sunub Bio-Tech
Development) with 5 ml DMEM (10% FBS) for 8 h. Medium was replaced
with 2-ml serum-free DMEM and incubated for an additional 12 h. The
conditioned medium was collected and filtered through a 0.22-μm
filter (Millipore). A total of 10 μl of conditioned media was mixed
with 10 μl sample buffer (0.25 M Tris-HCl, pH 6.0, 8.5% glycerol,
4% sodium dodecyl sulfate and 0.01% bromophenol blue) and
electrophoresed in a 7.5% SDS-polyacrylamide gel containing 2 mg/ml
gelatin (Sigma). After electrophoresis, the gel was washed three
times for 10 min in 2.5% Triton X-100 and placed in incubation
buffer (50 mM Tris-HCl, pH 7.6, 10 mM CaCl2, 50 mM NaCl
and 0.05% Brij-35) overnight at 37ºC. After incubation, the gel was
stained with a solution of 0.25% Coomassie blue R250, 40% methanol
and 10% acetic acid for 1 h at room temperature and destained with
40% methanol and 10% acetic acid until protein bands were
apparent.
Western blot analysis
Western blot analysis was performed as previously
described (20). A total of 50 μg
of protein samples were examined for each protein. After
electrophoresis and membrane transfer, the membranes were incubated
with rabbit polyclonal primary antibody for Robo1 (#ab7279; Abcam)
at a 1:1,000 dilution, rabbit polyclonal primary antibody Akt
(#9272; Cell Signaling Technology), phospho-Akt (#9271; Cell
Signaling Technology) at a 1:1,000 dilution, mouse monoclonal
antibody for α-tubulin (#T6074; Sigma) at a 1:4,000 dilution and
appropriate horseradish peroxidase-linked secondary antibodies at a
1:2,000 dilution. After washing three times with TBST, bound
antibody was developed using the ECL plus Western Blotting
Detection system (Thermo Fisher Scientific).
In vivo tumor growth
Cells (2×107 cells/ml) were suspended in
PBS, and a 0.1 ml of suspension was inoculated subcutaneously
(s.c.) into the right flanks of 4-week-old Balb/c nude mice on day
0. All animals were sacrificed after 30 days, the resulting tumors
were completely dissected, and then the tumor volumes calculated
using the formula (length × width) 2/2.
Experimental metastasis
Cells (2×106/mouse) in 0.1 ml of PBS were
injected into 4-week-old Balb/c nude mice through the tail vein.
All animals were sacrificed after 30 days. The lungs were first
dissected and visible tumor nodules in all lung lobes counted using
a stereo fluorescence microscope. Nodules were then fixed in 4%
paraformaldehyde (PAF) solution, embedded in paraffin, sectioned
and stained with hematoxylin and eosin (H&E) for routine
histological examination by light microscopy.
Statistical analysis
Statistical analysis was carried out using GraphPad
Prism 5 software (GraphPad Software, Inc., Irvine, CA, USA) and
depicted as mean ± SD or SEM. Student's t-test or the two-way
analysis of variance (ANOVA) were performed and P<0.05 was
considered statistically significant.
Results
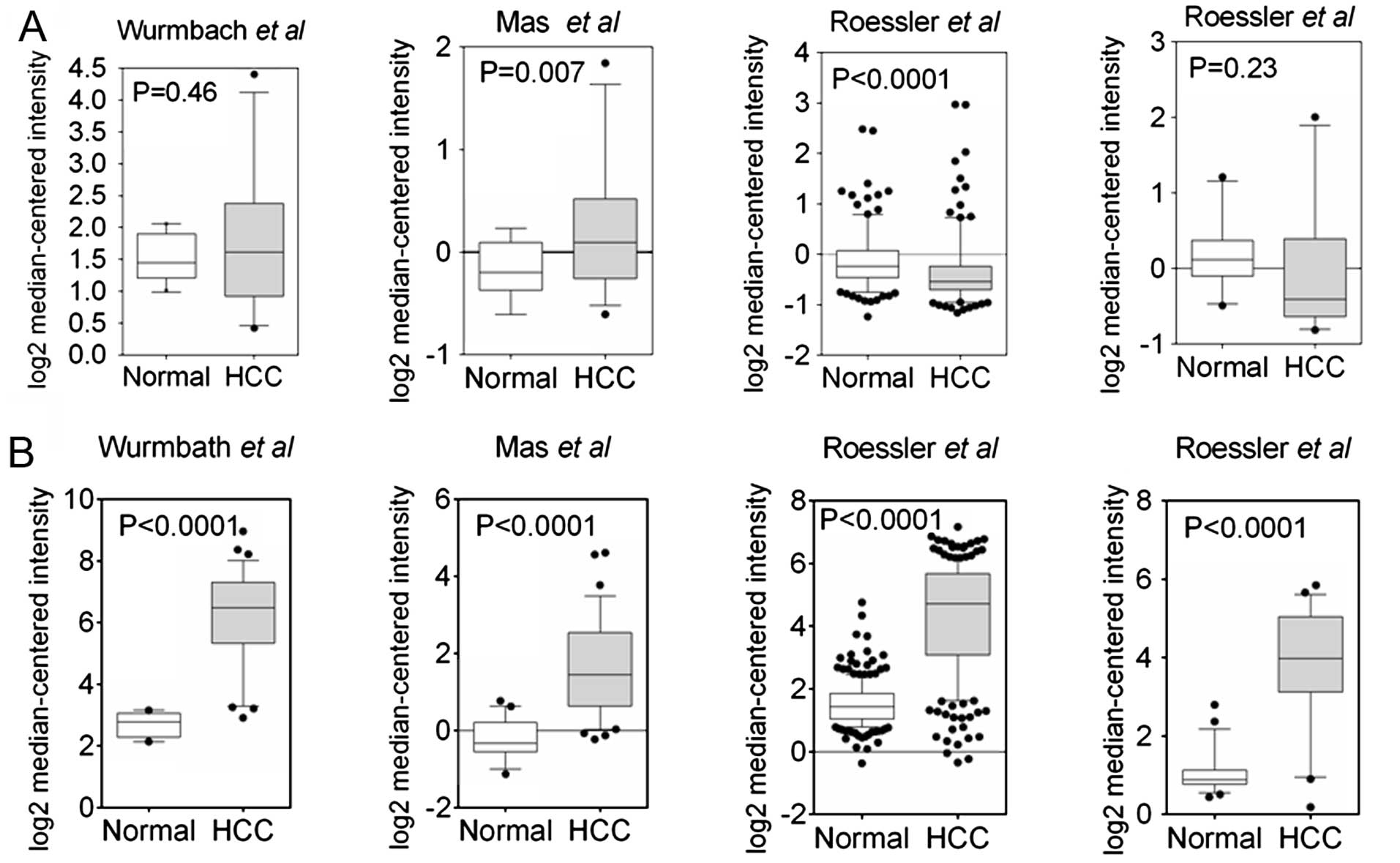
Slit2 is heterogeneously expressed in
HCC
Jin et al (21) reported the epigenetic inactivation
of Slit2 in 6 of 8 HCC cell lines and 45 of 54 tumor tissues. To
extend our understanding of Slit2 expression in HCC samples
on a large-scale, we explored the Slit2 expression using an
Oncomine database. Data mining revealed a heterogeneous expression
pattern of Slit2 in HCC samples (22–24).
As shown in Fig. 1A, only one
analysis showed significantly reduced Slit2 expression
compared to normal tissues (24),
suggesting that the observed reduction in Slit2 expression
does not apply to all HCC samples. In contrast, the expression of
Robo1 was markedly elevated in HCC samples (Fig. 1B). To verify the results in HCC
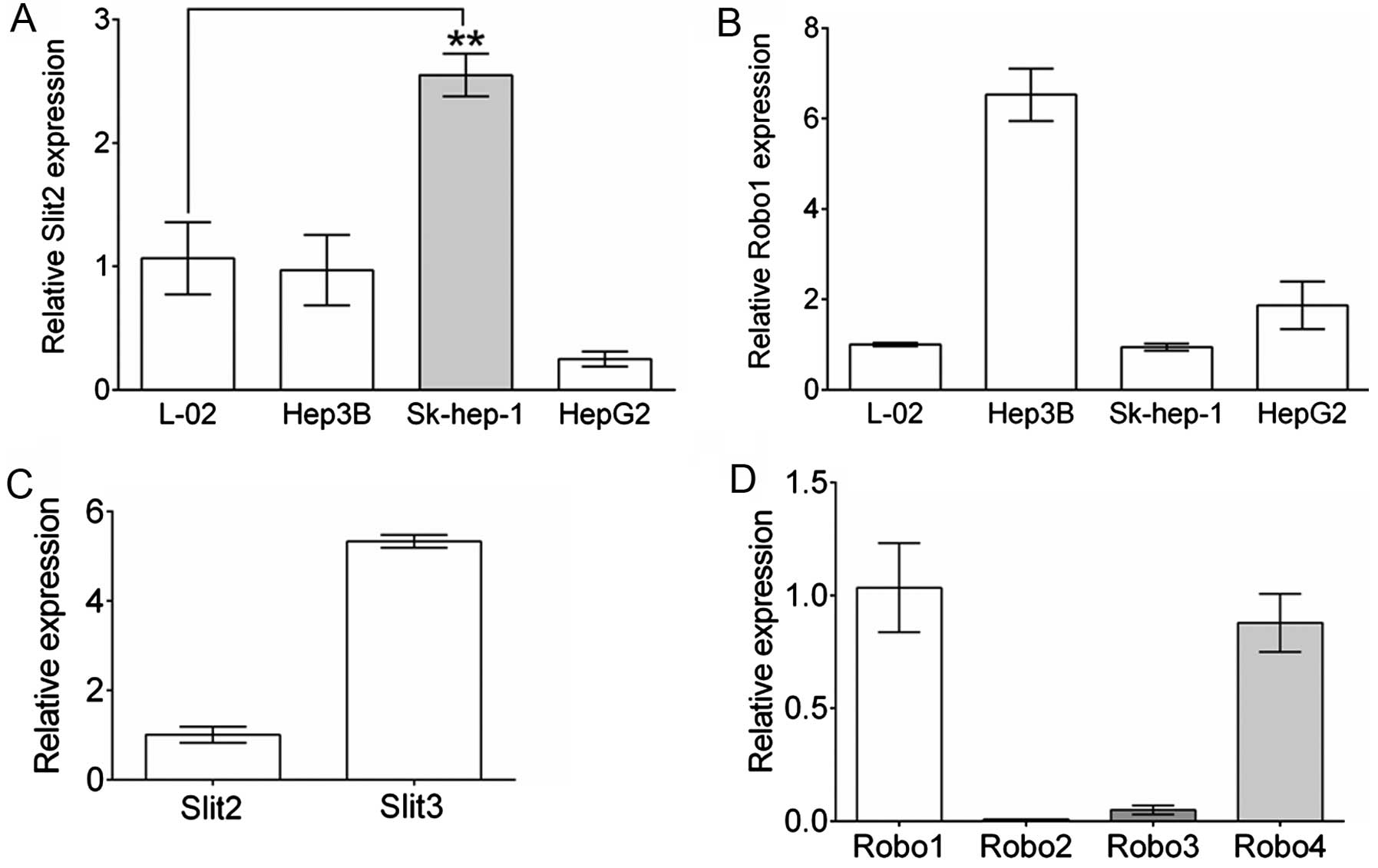
cell lines, we contrasted Slit2 and Robo1 gene expression in 3
commonly used HCC cell lines to those of a normal hepatic cell
line, L-O2 by real-time qPCR. As shown in Fig. 2A, compared to L-O2, Hep3B cells
showed a comparable level of Slit2 expression to L-O2, and
reduced Slit2 expression was observed in HepG2 cells.
However, Sk-hep-1 cells showed elevated Slit2 expression
relative to L-O2 (P=0.0016, n=3). Robo1 expression in Sk-hep-1 was
comparable to that of L-O2, while Hep3B and HepG2 cells maintained
a markedly elevated Robo1 expression level. Sk-hep-1 cells were
selected for further study by establishing the stable Slit2
knockdown or Robo1-overexpressing cell lines. Furthermore,
to understand the expression of other slit ligands and Robo
receptors in Sk-hep-1 cells, the expression of Slit3 and
Robo2, 3 and 4 were compared to that of
Slit2 or Robo1, respectively. As shown in Fig. 2C, the expression of Slit3
was nearly 5-fold greater than that of Slit2 in Sk-hep-1
cells, indicating a potentially important role for Slit3 in
Sk-hep-1 cells. Robo4 was slightly less expressed compared with
Robo1, while Robo2 and Robo3 expression levels were
extremely low (Fig. 2D).
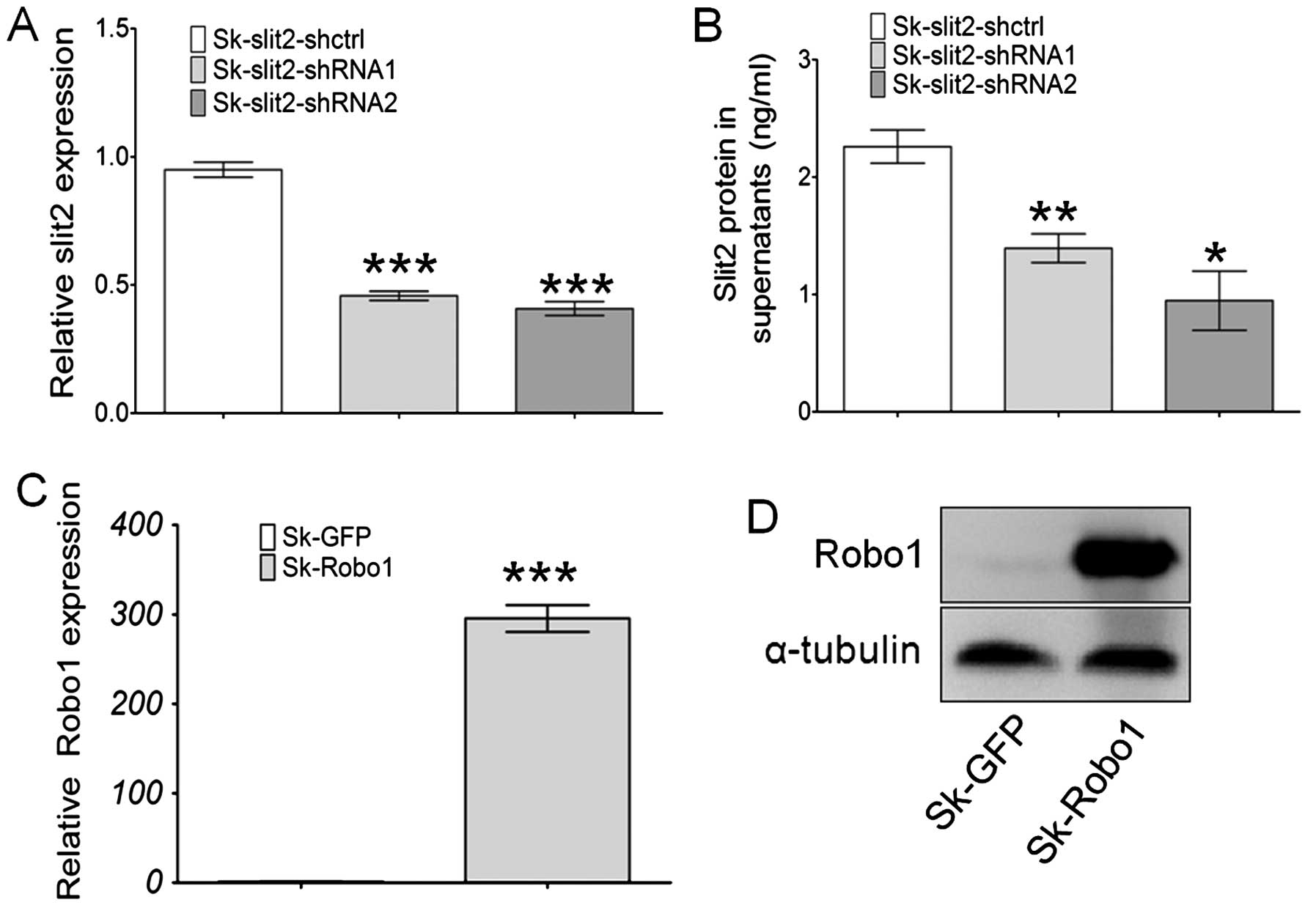
Generation of stable Slit2 knockdown and
Robo1 overexpressing cell lines
Sk-hep-1 cells were transduced with a VSV-G
pseudotyped lentivirus vector (pll3.7) for stable Slit2
knockdown (Sk-Slit2-shRNA), and with a pBobi vector for
stable Robo1 overexpression (Sk-Robo1). The
efficiency of gene knockdown and overexpression were assayed at
both mRNA and protein levels. Slit2 expression in Sk-hep-1
cells was significantly decreased when transduced with two pll3.7
vectors containing Slit2-shRNA, with knockdown efficiencies
of 55 and 60% for Slit2 in Sk-hep-1 cells (Fig. 3A), respectively. These mRNA results
were further confirmed at the protein level. As Slit2 is a secreted
soluble protein, an ELISA assay was performed to examine changes in
Slit2 proteins in the supernatant of Sk-hep-1 cells. The
concentration of Slit2 in the supernatant of Sk-Slit2-shctrl
cells was 2.26 ng/ml (n=3). After Slit2 mRNA knockdown, the
concentration of Slit2 reduced to 1.39 ng/ml (n=3, P=0.0098), 0.94
ng/ml (n=3, P=0.0105) in Sk-Slit2-shRNA1 and shRNA2 cells,
respectively (Fig. 3B).
Overexpression of Robo1 in Sk-hep-1 cells induced a nearly
300-fold increase in mRNA levels (Fig.
3C), this was confirmed at the protein level by western
blotting (Fig. 3D). Stable
Slit2-knockdown and Robo1-overexpressing cell lines
were thus generated successfully.
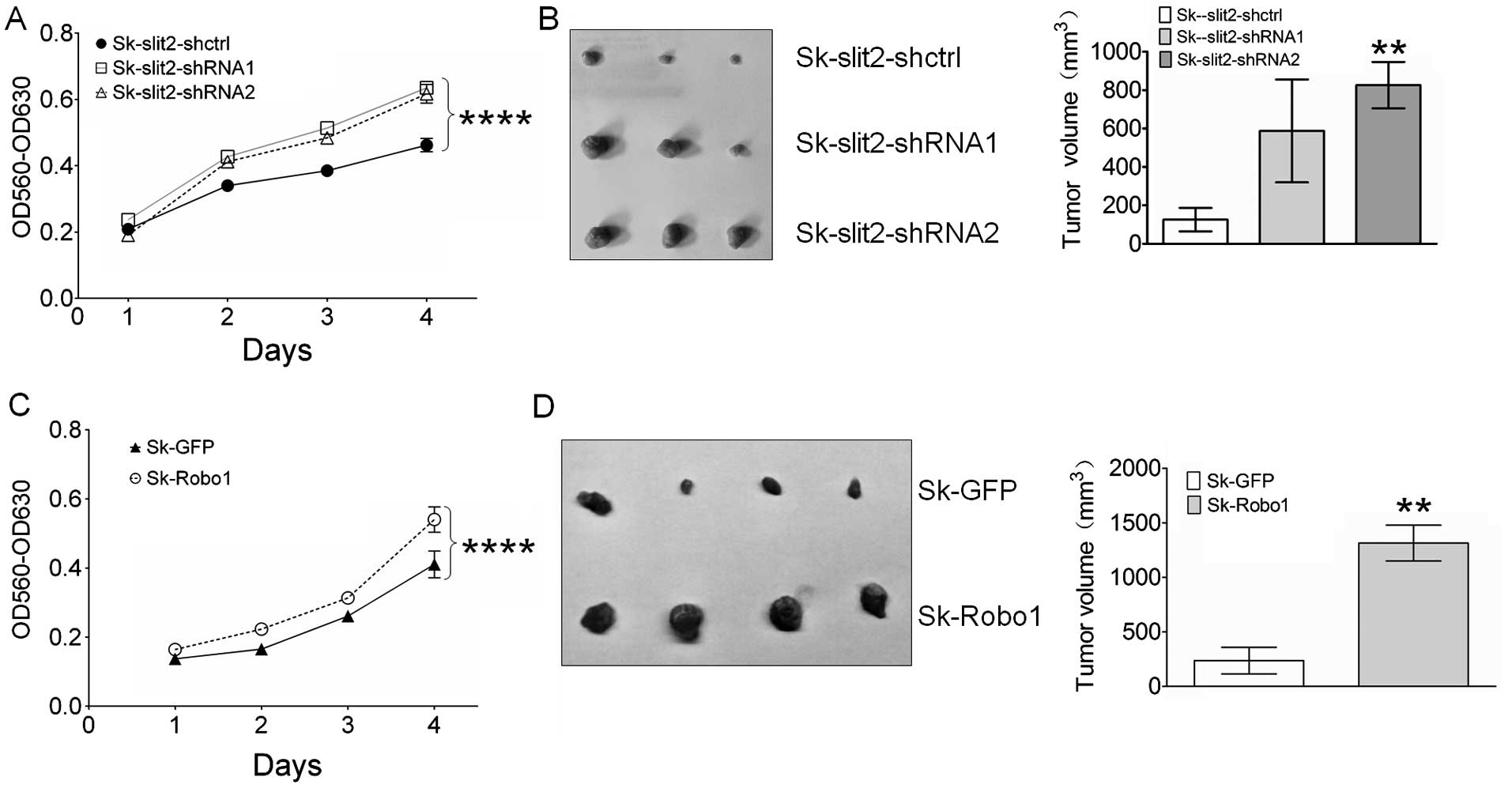
Slit2 knockdown and Robo1 overexpression
promote proliferation and tumor growth of Sk-hep-1 cells
To investigate the effects of Slit2 knockdown
and Robo1 overexpression on the proliferation of Sk-hep-1
cells, an MTT assay was performed. As shown in Fig. 4A, Slit2 knockdown in
Sk-hep-1 cells promoted cell proliferation, suggesting an innate
role for Slit2 in Sk-hep-1 cells as a tumor suppressor. Contrary to
Slit2, Robo1 appears to be a tumor promoter, since Robo1
overexpression in Sk-hep-1 cells resulted in enhanced cell
proliferation (Fig. 4C). To test
whether the effects of Slit2 and Robo1 on in vitro cell
proliferation can be reproduced in in vivo model, transduced
Sk-hep-1 cells were injected into nude mice, and 30 days later
tumor volumes measured. As expected, Slit2 knockdown
stimulated the growth of Sk-hep-1 tumors (Fig. 4B), although a significant
difference in tumor volume was not observed between Sk-Slit2-shctrl
(125.6±61 mm3) and Sk-Slit2-shRNA1 (587.3±267.3
mm3, P=0.168) xenografts due to individual differences
in mice, a significant increase in tumor volume was observed in
Sk-Slit2-shRNA2 tumors (825.7±120.9 mm3,
P=0.0067) relative to Sk-Slit2-shctrl tumors. Overexpression of
Robo1 in Sk-hep-1 cells also promoted tumor growth. As shown
in Fig. 4D, a significant increase
in tumor volume was observed in Sk-Robo1 xenografts
(1315±164.4 mm3, P=0.0015), when compared to Sk-GFP
control xenografts (237.5±121.7 mm3). These results
suggest that Slit2 and Robo1 exerted opposing effects on HCC
proliferation and tumor growth.
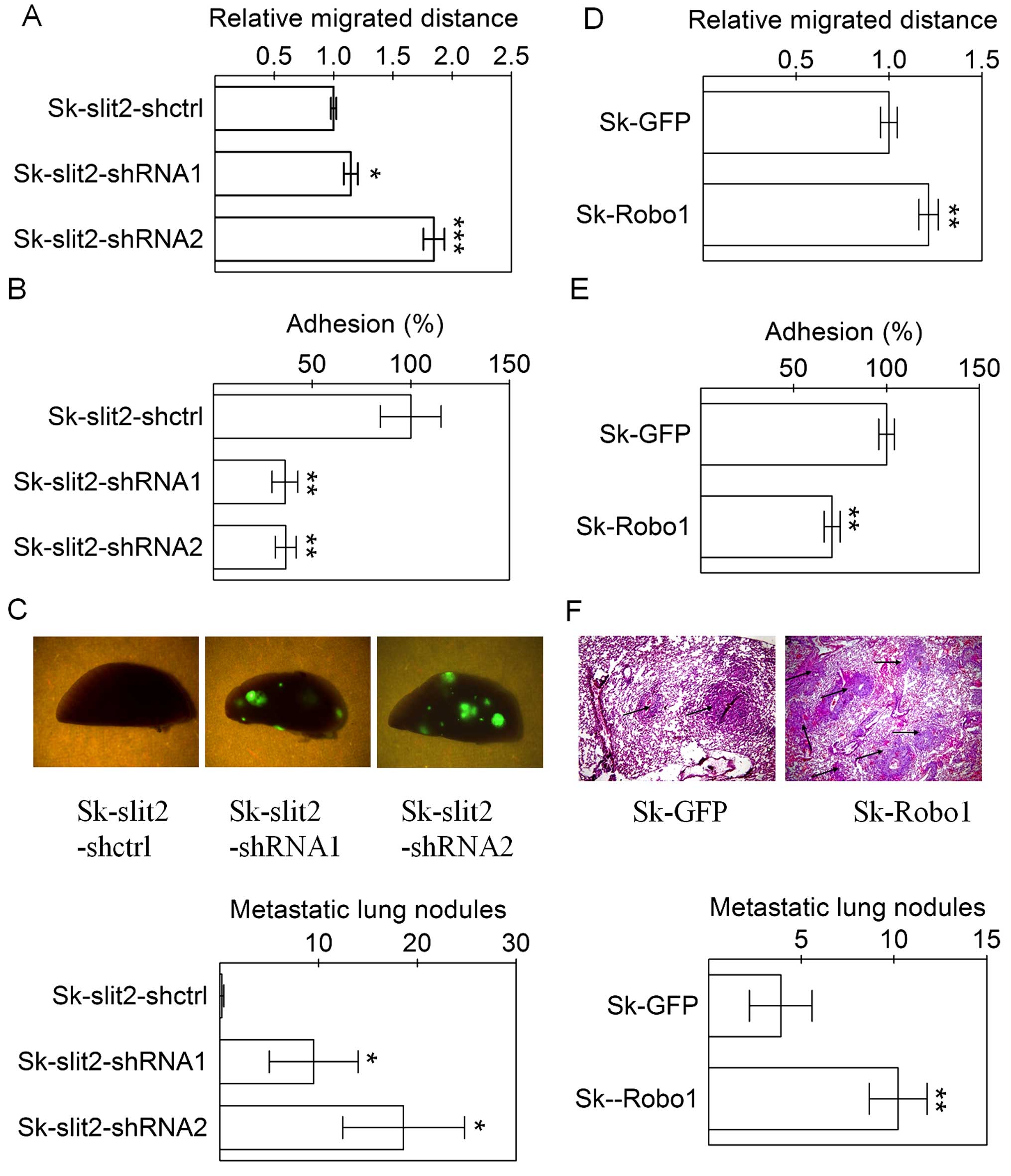
Slit2 knockdown and Robo1 overexpression
promote invasion and metastasis of Sk-hep-1 cells
Metastasis is the main cause of death for patients
with HCC, with cell migration, and adhesion affecting aspects of
tumor metastasis. We examine the effects of Slit2 knockdown
and Robo1 overexpression on tumor metastasis, using both
in vitro assays such as cell migration, adhesion and an
in vivo intravenous injection of Sk-hep-1 cells for an
experimental metastasis assay. As with cellular proliferation and
tumor growth, Slit2 and Robo1 also displayed contrasting functions
in aspects of metastasis. Both Slit2 knockdown and
Robo1 overexpression in Sk-hep-1 cells enhanced cell
migration (Fig. 5A and D) and
decreased cell adhesion (Fig. 5B and
E). The effects of Slit2 knockdown and Robo1
overexpression on metastasis of Sk-hep-1 cells were observed after
the intravenous injection of tumor cells, since all lentiviral
pll3.7 vectors for Slit2 knockdown carried a GFP marker, the
pulmonary metastasis of cells was conveniently analyzed by directly
counting fluorescent metastatic Sk-hep-1 tumor nodules on the
surface of lung tissues. Since the pBobi vectors for Robo1
overexpression did not have a GFP marker, the pulmonary metastasis
of cells was determined by counting tumor nodules on lung tissues
sections. Slit2 knockdown (Fig.
5C) or Robo1 overexpression (Fig. 5F) in Sk-hep-1 cells significantly
increased the number of metastatic tumor nodules. Therefore, the
counter effect of Slit2 and the effect of Robo1, on tumor
metastasis were validated in an in vivo model.
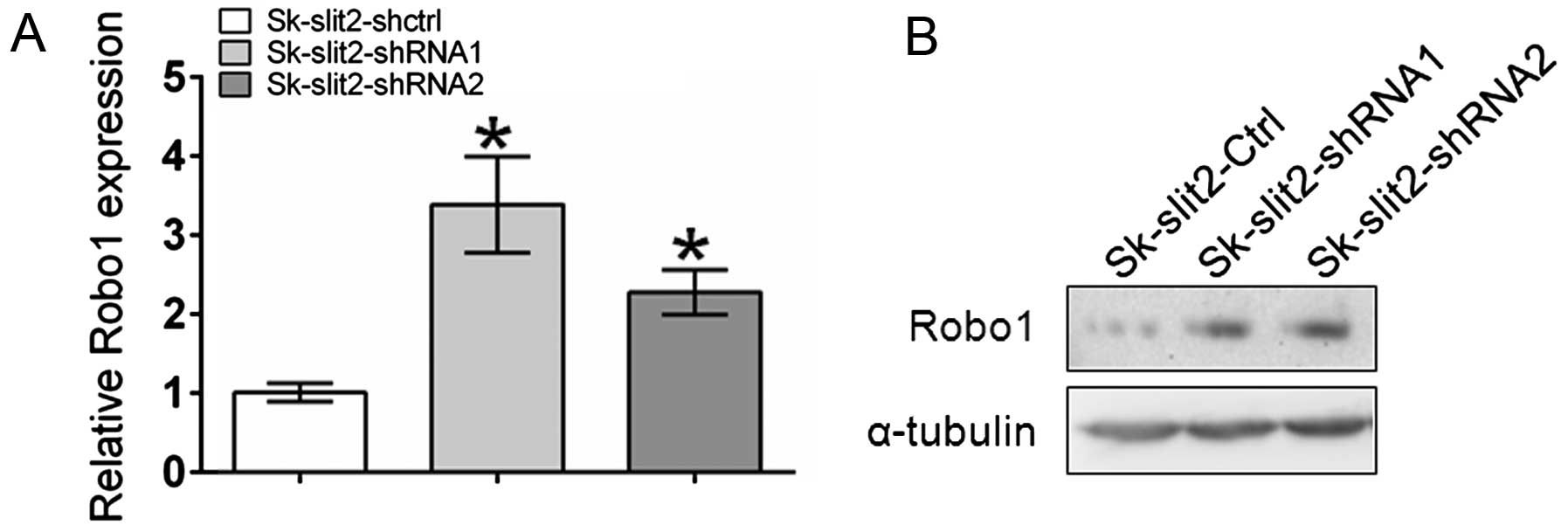
Slit2 knockdown induces upregulation of
Robo1 expression
An inverse correlation between Slit2 and
Robo1 expression has recently been reported (25–27).
To investigate the effects of Slit2 knockdown on
Robo1 expression, qPCR and western blot analysis were
performed. As shown in Fig. 6,
Slit2 knockdown induced the upregulation of Robo1 at both
the mRNA and protein level. Given the stimulatory effects of Robo1
overexpression on tumor growth and metastasis, the observation that
Slit2 knockdown promoted tumor growth and metastasis of
Sk-hep-1 cells could be partly ascribed to the upregulation of
Robo1.
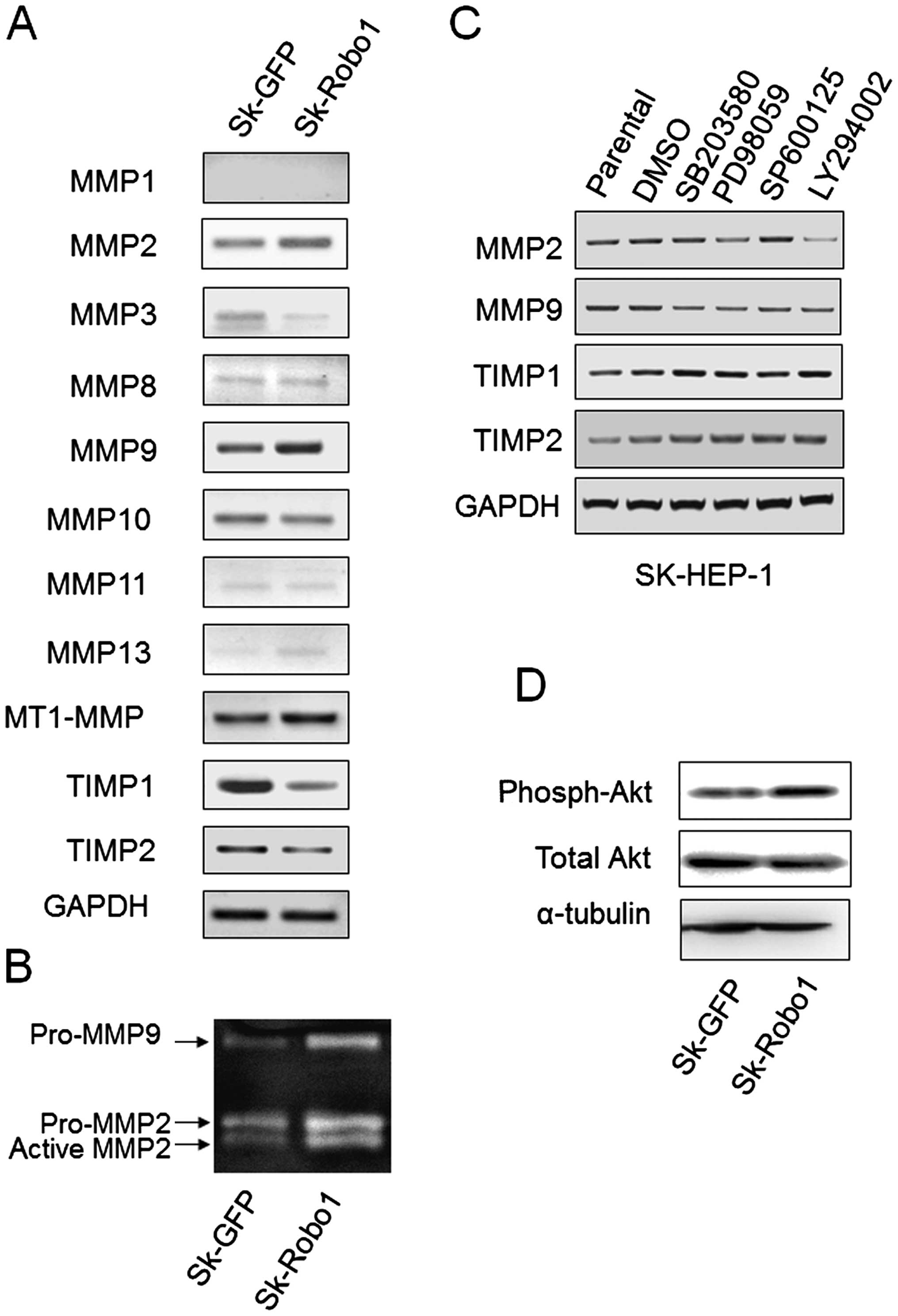
Robo1 overexpression increases MMP2, -9
and decreases TIMP1, -2 expression through different signaling
pathways
Most MMPs are secreted as inactive proenzymes whose
activities are tightly regulated by mRNA transcription and
stability control, and proenzyme activation via their activators
and inhibitors. MT1-MMP (MMP14, activator), and TIMP1 and -2
(inhibitors) are key molecules for pro-MMPs activation. To explore
the underlying correlations between Robo1 overexpression and
MMPs, the expression of several secreted MMPs and TIMP1, -2 was
examined by RT-PCR. As shown in Fig.
7A, MMP2, -9 and MT1-MMP expression were upregulated, and TIMP1
and 2 expression were downregulated in Robo1 overexpressing
Sk-hep-1 cells. Other MMPs, except MMP10, were either not detected
or detected in minute quantities, suggesting MMP2 and 9 to be the
key secreted MMPs involved in the metastasis of these cells. As a
pro-MMP activity assay, gelatin zymography was performed.
Robo1 overexpression increased the production of pro-MMP2
and 9, and induced pro-MMP2, but not pro-MMP9, activation (Fig. 7B).
Robo1 overexpression stimulated the
phosphorylation of Akt
The MAPK (28–30)
and PI3k/Akt (31–33) signaling pathways have been widely
reported to be involved in regulating the expression of MMPs in
HCC. To determine whether the alteration in MMP and TIMP expression
induced by Robo1 overexpression in Sk-hep-1 cells was
mediated by these two signaling pathways, three MAPK inhibitors
(SB203580 for p38 blockage, PD98059 for ERK blockage, and SP600125
for JNK blockage) and a PI3k inhibitor (LY294002) were used to
block the corresponding signal transduction pathways. The
expression of MMPs and TIMPs was assayed by RT-PCR. As shown in
Fig. 7C, LY294002 significantly
inhibited MMP2 expression. SB203580 inhibited MMP9 and PD98059
inhibited MMP2 and 9 expression slightly. Significant changes were
not observed in TIMPs expression upon inhibitor treatment (Fig. 7C). This suggested that varied
signaling pathways for the modulation of MMP2 and 9 expression
exist in cells. It was observed that p38 MAPK only slightly
affected MMP2 expression, and that ERK MAPK slightly affected MMP9.
The PI3k/Akt signaling pathway, however, significantly affected
MMP2 expression. Combined with the observation that Robo1
overexpression stimulated the phosphorylation of Akt (Fig. 7D), we postulated that the PI3K/Akt
signaling pathway mediates Robo1-induced metastasis of Sk-hep-1
cells, at least partly, by upregulating MMP2.
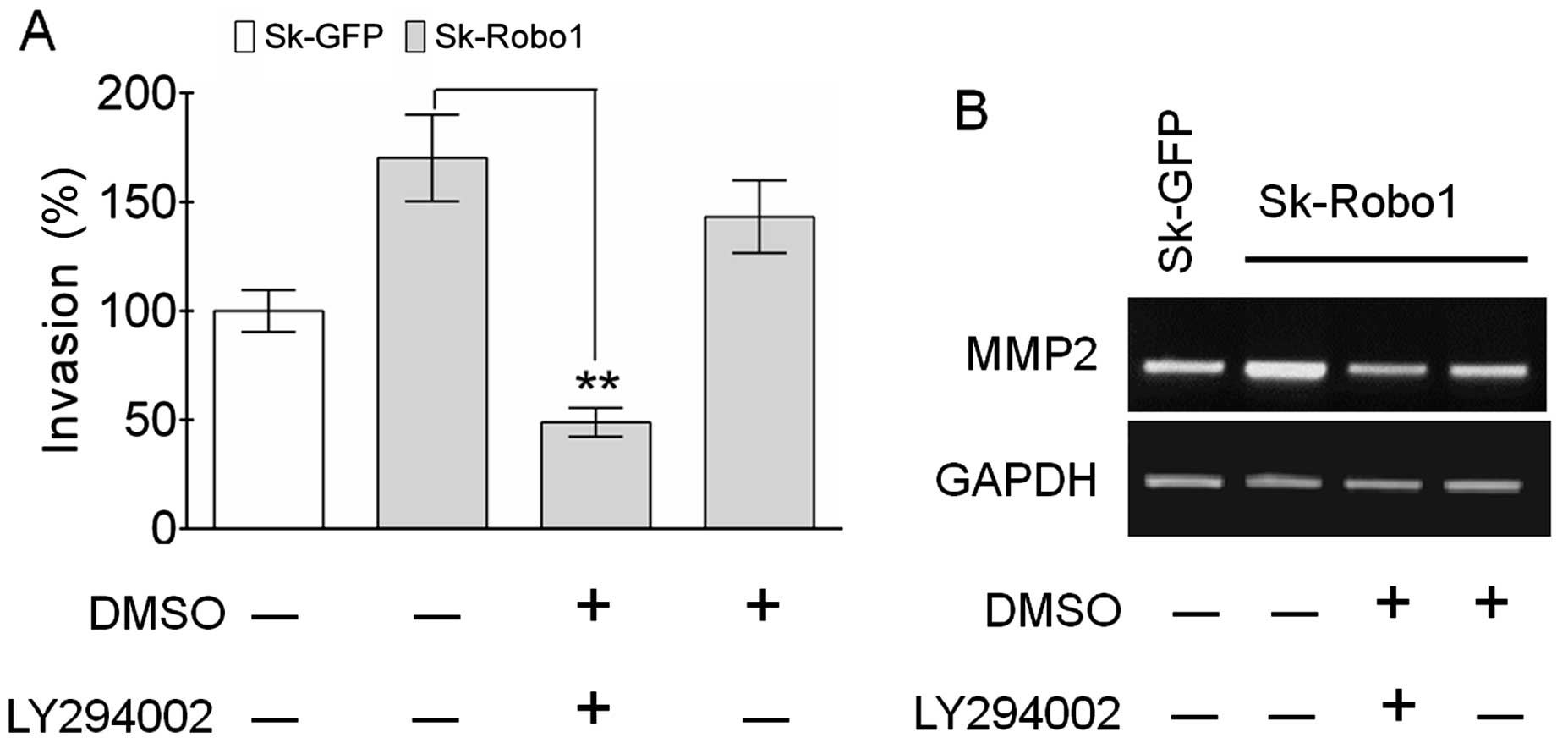
LY294002 antagonizes the enhancement of
cell invasion and MMP2 expression induced by Robo1
overexpression
To test the theory that the PI3k/Akt signaling
pathway is involved in metastasis of Sk-hep-1 cells, the effects of
LY294002 on in vitro tumor invasion were examined. As shown
in Fig. 8A, LY294002 neutralized
the enhanced invasive ability of Sk-hep-1 cells induced by
Robo1 overexpression, concomitant with blocking the
upregulation of MMP2 (Fig. 8B).
These results confirmed the crucial role of the PI3k/Akt signaling
pathway in Robo1-mediated tumor metastasis.
Discussion
Reduced Slit2 expression has been reported in
a great variety of cancers, and is predominantly due to the
hypermethylation of the Slit2 promoter (34–39).
Although Slit2 downregulation has also been reported in HCC
tissues (21), large-scale mining
of microarray data from the Oncomine database revealed a
heterogeneous expression pattern for Slit2 in HCC clinical
samples (Fig. 1A). Conversely,
expression of the Slit2 cognate receptor, Robo1, is aberrantly
upregulated in HCC samples (Fig.
1B). Interestingly, a negative regulatory loop between Slit2
and Robo1 mediated by miR-218, which targets the 3′
untranslated region (3′UTR) of Robo1 and resides in the intron of
the Slit2 genome, has recently been confirmed (25–27).
The significance of the negative regulatory loop is still elusive.
In the present study, a similar inverse correlation between Slit2
and Robo1 was found in Sk-hep-1, which maintain a high level of
Slit2 and a low level of Robo1, and in Hep3B and HepG2
cells, which maintain a high level of Robo1 but a low level of
Slit2 (Fig. 2A and B). Notably, we
found, Slit2 knockdown led to the upregulation of
Robo1 (Fig. 6). As the
target of RNA knockdown is a final sliced Slit2 mRNA,
not a genomic Slit2 sequence, effects are not exerted on the
miR-218 localized within an intron. Thus, the upregulation
of Robo1 caused by Slit2 knockdown is presumed to be via an
as yet unknown mechanism distinct from one that is miR-218
mediated. We examined the functions of Slit2 and Robo1 by knockdown
of Slit2 or by overexpression of Robo1, respectively,
in Sk-hep-1 cells. Unexpectedly, these alteration in Slit2
and Robo1 expression induced opposing effects on tumor
growth and metastasis.
Though still controversial, reports on the
inhibitory effects of Slit2 on the progression of various tumors
are more numerous than those describing its stimulatory effects.
The effects of the Slit2/Robo1 signaling axis in HCC, however, have
rarely been studied. Jin et al (21) reported that adenovirus mediated
Slit2 overexpression in an SMMC-7721 cell line suppressed
cell growth, migration and invasion. However, the author did not
state whether the suppressive effects of Slit2 were mediated by the
Robo1 receptor. Our results strengthened the above (21) findings by further providing
evidence that lentivirus-mediated Slit2 knockdown in
Sk-hep-1 cells, promoted cell proliferation, highlighting the
potential tumor suppressor role of Slit2 in cells. Moreover,
Robo1 overexpression in Sk-hep-1 cells also promoted cell
proliferation. These results were also confirmed in our tumor
xenograft model, suggesting Slit2 and Robo1 induce opposing effects
on tumor growth (Fig. 4).
Cell migration, adhesion and invasion are closely
associated with tumor metastasis. Weakened cell adhesion, and
enhanced cell migration and invasion, are characteristics of
metastatic tumor cells. We found, both Slit2 knockdown and
Robo1 overexpression in Sk-hep-1 cells promoted tumor
metastasis, concomitant with decreased cell adhesion and enhanced
migration, indicating Slit2 and Robo1 induce opposing effects on
tumor metastasis (Fig. 5).
MMPs are critical participants in degrading the
extracellular matrix to pave the way for tumor metastasis, of which
MMP2 and 9 are key molecules due to their unique ability to degrade
type IV collagens, major components of the basement membrane.
Notably, MMP2 and 9 are secreted as latent proenzymes whose
activities rely on proteolytic activation. TIMP1 and 2 are
inhibitors of MMP activation, with MT1-MMP a specific activator of
MMP2 activation. The balance between MT1-MMP and TIMP2 determines
MMP2 activation. The activation of MMP9 is more complex, as many
molecules, including MMP2, are involved in this process (40). We found that only MMP2, -9 and
regulators of their activity such as MT1-MMP and TIMP1 and 2 were
dominantly expressed in Sk-hep-1 cells, suggesting the alterations
of MMP2 and -9 expression and their activities are important for
cellular metastasis. Robo1 overexpression stimulated
MMP2, -9 and MT1-MMP, but suppressed expression of
TIMP1 and 2, thereby promoting the activation of
MMP2, but not MMP9, and causally resulted in the promotion of tumor
metastasis (Fig. 7A and B).
The expression of MMP2 and 9 is regulated
heterogeneously by different signaling pathways in HCC tissues
(41–43). We found that MMP2, but not MMP9,
was regulated by the PI3K/Akt signaling pathway (Fig. 7C). Robo1 overexpression
enhanced the phosphorylation of Akt and SK-hep-1 cell invasion
which was antagonized by PI3K-specific inhibitor, LY294002
(Figs. 7D and 8). This led us to postulate that Robo1
promoted tumor invasion partly by the upregulation of MMP2 after
activating the PI3K/Akt signaling pathway. These results
strengthened the importance place on the PI3K/Akt signaling pathway
in the metastasis of HCC as frequently reported by others (31–33).
From results reported to date, the confirmed
involvement of the Slit2/Robo1 signaling pathway on tumor
progression either induces inhibitory or stimulatory effects. In
our experiments, the effects of Slit2 knockdown in Sk-hep-1
cells suggested a negative role for Slit2 in tumor metastasis,
whereas Robo1 overexpression in Sk-hep-1 cells pointed to a
positive role for Robo1 in tumor metastasis. As a result, a
question arises: how does the Robo1 receptor, which plays a
positive role on tumor metastasis, mediate the negative effects of
Slit2 in tumor metastasis if the Slit2/Robo1 signaling axis
mediates the effects observed in Sk-hep-1 cells? Although we did
not explore the exact mechanisms for the contrasting effects of
Slit2 and Robo1 on HCC growth and metastasis, the observation that
Slit2 knockdown caused the upregulation of Robo1
expression provides an indication to the possible mechanism.
Basically, the Slit2/Robo1 signaling axis induces suppressive
effects on HCC metastasis, while the knockdown of Slit2
removes the suppressive effects, resulting in the enhancement of
the metastasis. Furthermore, the upregulation of Robo1 induced by
Slit2 knockdown might induce stimulatory effects upon the
binding of other Slit ligands such as Slit3. We found that, while
Sk-hep-1 cells maintained a relatively high expression of
Slit2 and low expression of Robo1 compared levels in
with normal cells, an even higher expression of Slit3 was
detected (Fig. 1C). Our previous
study demonstrated that Slit3 knockdown promoted
proliferation, migration and invasion of A549 cells, indicating a
tumor suppressor role for Slit3 in lung adenocarcinoma (20). However, the effects of Slit3 might
depend on either the Robo receptors it interacts with, or the cell
context. Among the four members of Robo receptors, Robo4 is
structurally distinct from the others, indicating it may be
involved in signaling differently to that of Robo1. The Slit3/Robo4
signaling pathway has been shown to play a pro-angiogenic role in
embryonic angiogenesis (44) and
human engineered tissues (45). In
Sk-hep-1 cells, Robo4 was second in expression to Robo1, although
at a low level (Fig. 1D). Whether
Slit3 can induce the stimulatory effects on tumor metastasis by
binding to Robo1 that has been upregulated by Slit2
knockdown or to Robo4, deserves further study.
In summary, our results provide evidence that Slit2
and Robo1 induce opposing effects on tumor growth and metastasis.
Slit2 knockdown and Robo1 overexpression in Sk-hep-1
cells promotes tumor growth and metastasis, suggesting a negative
and positive role for Slit2 and Robo1, respectively, in tumor
progression. These results contradict published reports to date
that indicate the Slit2/Robo1 signaling axis induces either a
suppressive or stimulatory effect on tumor progression. Our finding
that Slit2 knockdown leads to the upregulation of Robo1,
presumably not mediated by miR-218, provides a potential new
mechanism for these contradictory results.
Acknowledgements
The present study was supported by Grants by the
National Natural Science Foundation of China (grant nos. 30971210
and 30671021) and the Key Project of Chinese Ministry of Education
(grant no. 109093).
References
|
1
|
Mommersteeg MT, Yeh ML, Parnavelas JG and
Andrews WD: Disrupted Slit-Robo signalling results in membranous
ventricular septum defects and bicuspid aortic valves. Cardiovasc
Res. 106:55–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weyers JJ, Milutinovich AB, Takeda Y, Jemc
JC and Van Doren M: A genetic screen for mutations affecting gonad
formation in Drosophila reveals a role for the slit/robo pathway.
Dev Biol. 353:217–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dickinson RE, Hryhorskyj L, Tremewan H,
Hogg K, Thomson AA, McNeilly AS and Duncan WC: Involvement of the
SLIT/ROBO pathway in follicle development in the fetal ovary.
Reproduction. 139:395–407. 2010. View Article : Google Scholar :
|
|
4
|
Piper M, Georgas K, Yamada T and Little M:
Expression of the vertebrate Slit gene family and their putative
receptors, the Robo genes, in the developing murine kidney. Mech
Dev. 94:213–217. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang PH, Hwang-Verslues WW, Chang YC,
Chen CC, Hsiao M, Jeng YM, Chang KJ, Lee EY, Shew JY and Lee WH:
Activation of Robo1 signaling of breast cancer cells by Slit2 from
stromal fibroblast restrains tumorigenesis via blocking
PI3K/Akt/β-catenin pathway. Cancer Res. 72:4652–4661. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marlow R, Strickland P, Lee JS, Wu X,
Pebenito M, Binnewies M, Le EK, Moran A, Macias H, Cardiff RD, et
al: SLITs suppress tumor growth in vivo by silencing Sdf1/Cxcr4
within breast epithelium. Cancer Res. 68:7819–7827. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Göhrig A, Detjen KM, Hilfenhaus G, Körner
JL, Welzel M, Arsenic R, Schmuck R, Bahra M, Wu JY, Wiedenmann B,
et al: Axon guidance factor SLIT2 inhibits neural invasion and
metastasis in pancreatic cancer. Cancer Res. 74:1529–1540. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mano Y, Aishima S, Fukuhara T, Tanaka Y,
Kubo Y, Motomura T, Toshima T, Iguchi T, Shirabe K, Maehara Y, et
al: Decreased roundabout 1 expression promotes development of
intrahepatic cholangiocarcinoma. Hum Pathol. 44:2419–2426. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Werbowetski-Ogilvie TE, Seyed Sadr M,
Jabado N, Angers-Loustau A, Agar NY, Wu J, Bjerkvig R, Antel JP,
Faury D, Rao Y, et al: Inhibition of medulloblastoma cell invasion
by Slit. Oncogene. 25:5103–5112. 2006.PubMed/NCBI
|
|
10
|
Mertsch S, Schmitz N, Jeibmann A, Geng JG,
Paulus W and Senner V: Slit2 involvement in glioma cell migration
is mediated by Robo1 receptor. J Neurooncol. 87:1–7. 2008.
View Article : Google Scholar
|
|
11
|
Xu Y, Li WL, Fu L, Gu F and Ma YJ:
Slit2/Robo1 signaling in glioma migration and invasion. Neurosci
Bull. 26:474–478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yiin JJ, Hu B, Jarzynka MJ, Feng H, Liu
KW, Wu JY, Ma HI and Cheng SY: Slit2 inhibits glioma cell invasion
in the brain by suppression of Cdc42 activity. Neuro Oncol.
11:779–789. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang QQ, Zhou DL, Lei Y, Zheng L, Chen
SX, Gou HJ, Gu QL, He XD, Lan T, Qi CL, et al: Slit2/Robo1
signaling promotes intestinal tumorigenesis through Src-mediated
activation of the Wnt/β-catenin pathway. Oncotarget. 6:3123–3135.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou WJ, Geng ZH, Chi S, Zhang W, Niu XF,
Lan SJ, Ma L, Yang X, Wang LJ, Ding YQ, et al: Slit-Robo signaling
induces malignant transformation through Hakai-mediated E-cadherin
degradation during colorectal epithelial cell carcinogenesis. Cell
Res. 21:609–626. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang XM, Han HX, Sui F, Dai YM, Chen M and
Geng JG: Slit-Robo signaling mediates lymphangiogenesis and
promotes tumor lymphatic metastasis. Biochem Biophys Res Commun.
396:571–577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang LJ, Zhao Y, Han B, Ma YG, Zhang J,
Yang DM, Mao JW, Tang FT, Li WD, Yang Y, et al: Targeting
Slit-Roundabout signaling inhibits tumor angiogenesis in
chemical-induced squamous cell carcinogenesis. Cancer Sci.
99:510–517. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qi C, Lan H, Ye J, Li W, Wei P, Yang Y,
Guo S, Lan T, Li J, Zhang Q, et al: Slit2 promotes tumor growth and
invasion in chemically induced skin carcinogenesis. Lab Invest.
94:766–776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao R, Zhao Y, Wang LJ and Li WP: Effects
of Roundabout 5 on adhesion, invasion and potential motility of
human tongue carcinoma Tb cells. Chin Med J (Engl). 124:2367–2371.
2011.
|
|
19
|
Prasad A, Fernandis AZ, Rao Y and Ganju
RK: Slit protein-mediated inhibition of CXCR4-induced chemotactic
and chemoinvasive signaling pathways in breast cancer cells. J Biol
Chem. 279:9115–9124. 2004. View Article : Google Scholar
|
|
20
|
Zhang C, Guo H, Li B, Sui C, Zhang Y, Xia
X, Qin Y, Ye L, Xie F, Wang H, et al: Effects of Slit3 silencing on
the invasive ability of lung carcinoma A549 cells. Oncol Rep.
34:952–960. 2015.PubMed/NCBI
|
|
21
|
Jin J, You H, Yu B, Deng Y, Tang N, Yao G,
Shu H, Yang S and Qin W: Epigenetic inactivation of SLIT2 in human
hepatocellular carcinomas. Biochem Biophys Res Commun. 379:86–91.
2009. View Article : Google Scholar
|
|
22
|
Wurmbach E, Chen YB, Khitrov G, Zhang W,
Roayaie S, Schwartz M, Fiel I, Thung S, Mazzaferro V, Bruix J, et
al: Genome-wide molecular profiles of HCV-induced dysplasia and
hepatocellular carcinoma. Hepatology. 45:938–947. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mas VR, Maluf DG, Archer KJ, Yanek K, Kong
X, Kulik L, Freise CE, Olthoff KM, Ghobrial RM, McIver P, et al:
Genes involved in viral carcinogenesis and tumor initiation in
hepatitis C virus-induced hepatocellular carcinoma. Mol Med.
15:85–94. 2009. View Article : Google Scholar
|
|
24
|
Roessler S, Jia HL, Budhu A, Forgues M, Ye
QH, Lee JS, Thorgeirsson SS, Sun Z, Tang ZY, Qin LX, et al: A
unique metastasis gene signature enables prediction of tumor
relapse in early-stage hepatocellular carcinoma patients. Cancer
Res. 70:10202–10212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S,
Guo X, Wang B, Gang Y, Zhang Y, et al: MiR-218 inhibits invasion
and metastasis of gastric cancer by targeting the Robo1 receptor.
PLoS Genet. 6:e10008792010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alajez NM, Lenarduzzi M, Ito E, Hui AB,
Shi W, Bruce J, Yue S, Huang SH, Xu W, Waldron J, et al: MiR-218
suppresses nasopharyngeal cancer progression through downregulation
of survivin and the SLIT2-ROBO1 pathway. Cancer Res. 71:2381–2391.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gu JJ, Gao GZ and Zhang SM: miR-218
inhibits the migration and invasion of glioma U87 cells through the
Slit2-Robo1 pathway. Oncol Lett. 9:1561–1566. 2015.PubMed/NCBI
|
|
28
|
Cho SB, Park YL, Park SJ, Park SY, Lee WS,
Park CH, Choi SK, Heo YH, Koh YS, Cho CK, et al: KITENIN is
associated with activation of AP-1 target genes via MAPK cascades
signaling in human hepatocellular carcinoma progression. Oncol Res.
19:115–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang X, Huang S, Zhang F, Han X, Miao L,
Liu Z, Fan Z and Ji G: Lentiviral-mediated Smad4 RNAi promotes
SMMC-7721 cell migration by regulation of MMP-2, VEGF and MAPK
signaling. Mol Med Rep. 3:295–299. 2010.
|
|
30
|
Zhu B, Shi S, Ma YG, Fan F and Yao ZZ:
Lysophosphatidic acid enhances human hepatocellular carcinoma cell
migration, invasion and adhesion through P38 MAPK pathway.
Hepatogastroenterology. 59:785–789. 2012.
|
|
31
|
Chen JS, Wang Q, Fu XH, Huang XH, Chen XL,
Cao LQ, Chen LZ, Tan HX, Li W, Bi J, et al: Involvement of
PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in
hepatocellular carcinoma: Association with MMP-9. Hepatol Res.
39:177–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li X, Yang Z, Song W, Zhou L, Li Q, Tao K,
Zhou J, Wang X, Zheng Z, You N, et al: Overexpression of Bmi-1
contributes to the invasion and metastasis of hepatocellular
carcinoma by increasing the expression of matrix metalloproteinase
(MMP)-2, MMP-9 and vascular endothelial growth factor via the
PTEN/PI3K/Akt pathway. Int J Oncol. 43:793–802. 2013.PubMed/NCBI
|
|
33
|
Xu J, Jia L, Ma H, Li Y, Ma Z and Zhao Y:
Axl gene knockdown inhibits the metastasis properties of
hepatocellular carcinoma via PI3K/Akt-PAK1 signal pathway. Tumour
Biol. 35:3809–3817. 2014. View Article : Google Scholar
|
|
34
|
Maiti GP, Ghosh A, Mondal P, Ghosh S,
Chakraborty J, Roy A, Roychowdhury S and Panda CK: Frequent
inactivation of SLIT2 and ROBO1 signaling in head and neck lesions:
Clinical and prognostic implications. Oral Surg Oral Med Oral
Pathol Oral Radiol. 119:202–212. 2015. View Article : Google Scholar
|
|
35
|
Nones K1, Waddell N, Song S, Patch AM,
Miller D, Johns A, Wu J, Kassahn KS, Wood D, Bailey P, et al:
Genome-wide DNA methylation patterns in pancreatic ductal
adenocarcinoma reveal epigenetic deregulation of SLIT-ROBO, ITGA2
and MET signaling. Int J Cancer. 135:1110–1118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma WJ, Zhou Y, Lu D, Dong D, Tian XJ, Wen
JX and Zhang J: Reduced expression of Slit2 in renal cell
carcinoma. Med Oncol. 31:7682014. View Article : Google Scholar
|
|
37
|
Kim GE1, Lee KH, Choi YD, Lee JS, Lee JH,
Nam JH, Choi C, Park MH and Yoon JH: Detection of Slit2 promoter
hypermethylation in tissue and serum samples from breast cancer
patients. Virchows Archiv. 459:383–390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dunwell TL, Dickinson RE, Stankovic T,
Dallol A, Weston V, Austen B, Catchpoole D, Maher ER and Latif F:
Frequent epigenetic inactivation of the SLIT2 gene in chronic and
acute lymphocytic leukemia. Epigenetics. 4:265–269. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dallol A, Krex D, Hesson L, Eng C, Maher
ER and Latif F: Frequent epigenetic inactivation of the SLIT2 gene
in gliomas. Oncogene. 22:4611–4616. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Toth M, Chvyrkova I, Bernardo MM,
Hernandez-Barrantes S and Fridman R: Pro-MMP-9 activation by the
MT1-MMP/MMP-2 axis and MMP-3: Role of TIMP-2 and plasma membranes.
Biochem Biophys Res Commun. 308:386–395. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chung TW, Lee YC and Kim CH: Hepatitis B
viral HBx induces matrix metalloproteinase-9 gene expression
through activation of ERK and PI-3K/AKT pathways: Involvement of
invasive potential. FASEB J. 18:1123–1125. 2004.PubMed/NCBI
|
|
42
|
Chen F, Deng J, Liu X, Li W and Zheng J:
HCRP-1 regulates cell migration and invasion via EGFR-ERK mediated
up-regulation of MMP-2 with prognostic significance in human renal
cell carcinoma. Sci Rep. 5:134702015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheng JC, Chou CH, Kuo ML and Hsieh CY:
Radiation-enhanced hepatocellular carcinoma cell invasion with
MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction
pathway. Oncogene. 25:7009–7018. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang B, Dietrich UM, Geng JG, Bicknell R,
Esko JD and Wang L: Repulsive axon guidance molecule Slit3 is a
novel angiogenic factor. Blood. 114:4300–4309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Paul JD, Coulombe KL, Toth PT, Zhang Y,
Marsboom G, Bindokas VP, Smith DW, Murry CE and Rehman J:
SLIT3-ROBO4 activation promotes vascular network formation in human
engineered tissue and angiogenesis in vivo. J Mol Cell Cardiol.
64:124–131. 2013. View Article : Google Scholar : PubMed/NCBI
|