Introduction
Although the 5-year survival rate of breast cancer
patients is increasing due to advanced therapeutic techniques,
lower survival rate and poor prognosis are still observed in
patients diagnosed with metastatic breast cancer (1). Actually, ~98% of breast cancer
patients with a localized tumor can survive 5 years, but just 27%
of patients diagnosed with metastatic breast cancer can live >5
years (2). This indicates the
importance of cancer metastasis prevention to improve therapeutic
efficacy in breast cancer patients.
To metastasize, cancer cells must be released from
the primary tumor and travel to a distant site by attachment and
invasion through degradation of biological barriers, such as
basement membrane (BM) and extracellular matrix (ECM). The
degradation is predominantly regulated by membrane-degrading
enzymes, such as matrix metalloproteinases (MMPs), urokinase
plasminogen activator (uPA), and uPA receptor (uPAR) (3). Among MMPs, the gelatinases MMP-2 and
-9 play important roles in breast cancer metastasis and
angiogenesis (4). In breast cancer
cell systems, several studies have revealed that metastatic
potential was suppressed by inhibition of MMP-2 and/or -9 (4–6).
Also, a clinical study showed that expression levels of MMP-2 and
-9 in metastatic breast cancer patients were higher than those in
non-metastatic breast cancer patients (7). uPA is also closely related to
metastatic potential. uPA accelerates ECM degradation and MMP-9
activation through the catalysis of plasmin production from
plasminogen and it is also a well-known prognostic factor in breast
cancer (8,9). In clinical evaluation, poor prognosis
and high rate of relapse were shown in node-negative breast cancer
patients with high levels of uPA in breast tumor tissue or high
concentrations of serum uPA, whereas patients with low levels of
tissue uPA had a low risk of recurrence (8,10,11).
Consequently, the inhibition of these proteases is a key strategy
to prevent breast cancer metastasis.
Proton beam therapy is a promising radiotherapeutic
tool due to the characteristic delivery of specifically designed
doses to tumor tissue with minimized damage to surrounding normal
tissues because the ionizing proton beam is deposited in an exact
region with a dramatic end point increase of the specific energy
per unit in a body (Bragg Peak) (12). Proton beam therapy is widely
applied to treat various types of cancer including breast cancer
(13). In general, the molecular
biological study of therapeutic effects in a proton beam has
focused on tumor cell apoptosis caused by direct or indirect DNA
damage (14,15). However, the effects on metastasis,
a major cause of recurrence and poor prognosis, have not been fully
investigated.
We previously showed that a proton beam diminished
the metastatic potential of MCF-7 and MDA-MB-231 human breast
cancer cells via the inhibition of MMP-9 activity and expression
(12,16). Furthermore, proton beam irradiation
suppressed angiogenic activity in MCF-7 human breast cancer cells
through the inhibition of vascular endothelial growth factor (VEGF)
expression, an important angiogenic factor (17). Here, we investigated the efficacy
of proton beam irradiation on breast cancer metastasis using
aggressive 4T1 murine breast cancer cells isolated from naturally
developed mammary tumor in BALB/c mice and its orthotopic breast
cancer model (18,19). We analyzed the effects of proton
beam irradiation on cell proliferation, viability and metastatic
potential in 4T1 murine breast cancer cells and also measured tumor
growth, lung metastasis and metastatic gene mRNA expression in 4T1
orthotopic animal models.
Materials and methods
Cell culture
The 4T1 murine breast cancer cell line was obtained
from the American Type Culture Collection (Rockville, MD, USA) and
routinely maintained in Dulbecco's modified Eagle's medium (DMEM,
Welgene, Daegu, Korea) supplemented with 10% fetal bovine serum
(FBS) (Invitrogen, Carlsbad, CA, USA) and 1% antibiotic-antimycotic
solution (Welgene) in 5% (v/v) CO2 atmosphere at
37ºC.
Cell proliferation assay
To evaluate the effect of proton beam irradiation on
cell proliferation, we conducted 3-(4,5-dimethyl-thiazol-2-yl)-2,5
diphenyltetrazolium bromide (MTT) assays. 4T1 murine breast cancer
cells were plated at 2,000 cells per well into 96-well plates and
incubated for 24 h. Cells were then irradiated with a dose of 2, 4,
8, or 16 Gy by spread-out Bragg peak (SOBP) with the 100 MeV proton
accelerator (Korea Multi-Purpose Accelerator Complex, Gyeongju,
Korea), grown for 24 or 48 h, and then incubated with MTT for 4 h
in the dark. Produced insoluble formazan was dissolved in dimethyl
sulfoxide (DMSO) and the absorbance of formazan was measured at 570
nm.
Clonogenic cell survival assay
Clonogenic cell viability was assessed by colony
forming assay. Three hundred cells were seeded in each well of a
6-well plate and attached for 24 h. Then, the cells were irradiated
with a proton beam in a dose-dependent manner and additionally
grown for 6 days. The colonies were fixed with 10% formalin for 20
min, stained with 1% crystal violet for 40 min and then
captured.
Wound healing assay
Cell migration was evaluated with a wound healing
assay. Cells were plated on 6-well plates coated with collagen IV
(Corning, Bedford, MA, USA) and cultured to confluence. Cell
monolayers were scratched with a blue tip and then detached cells
were removed by excluding media and washing with phosphate-buffered
saline. Media were replaced to DMEM supplemented with 2% FBS and
cells were irradiated with a proton beam in a dose of 2, 4, 8 or 16
Gy. The wound areas were observed and captured at 0 and 24 h after
proton beam irradiation, respectively.
Animal model and tumorigenesis
Animal experiments were performed with the approval
of the Institutional Animal Care and Use Committee at Dongguk
University (IACUC-2014-008). Female BALB/c mice were purchased from
Orient Bio Inc. (Gapyung, Korea) and housed under a 12 h light/dark
cycle at 25±2ºC with a relative humidity of 50±5%. After adaptation
of one week, 1×105 4T1 murine breast cancer cells were
implanted into the mammary fat pad of 5-week-old female BALB/c mice
to establish the orthotopic animal breast cancer model. When the
tumor volumes reached ~200 mm3, mice were randomly
divided into 4 groups: 0 (control), 10, 20 and 30 Gy. Mice were
protected by an acryl plate (50 mm thickness) and mammary tumors
were selectively irradiated with a proton beam at a dose of 10, 20
or 30 Gy using the 100 MeV proton accelerator. Tumor sizes and body
weights were measured twice a week. Tumor volume (mm3)
was calculated with the following formula: tumor volume =
width2 × length × 0.5. Mice were sacrificed after 14
days and breast tumors and lungs were dissected.
Quantitative RT-PCR
The proton beam irradiation-mediated changes in
metastasis-regulating genes, such as MMP-9, MMP-2, uPA, uPAR,
cyclooxygenase (COX)-2, and VEGF, were analyzed. Total RNA was
extracted from breast tumor tissues using the easy-BLUE™ Total RNA
Extraction kit (iNtRON Biotechnology Inc., Sungnam, Korea)
according to the manufacturer's protocol. cDNA was synthesized with
Goscript™ Reverse Transcriptase (Promega, Madison, WI, USA).
Real-time PCR reactions were performed with QGreen 2X SybrGreen
Master Mix (Cellsafe, Suwon, Korea) using Eco™ Real-Time PCR
(Illumina, San Diego, CA, USA). The reaction was preheated at 95ºC
for 10 min, followed by 40 cycles of 90ºC for 10 sec, 60ºC for 15
sec, and 72ºC for 20 sec; products were verified by melting curve
analysis. Relative expression levels compared with control were
automatically evaluated by Eco Software v3.1.7. Glyceraldehyde
3-phosphate dehydrogenase (GAPDH) was used as an internal control.
Sequences of the primers used for the reaction were as follows:
MMP-9: forward,
5′-GCGAGTGTCTCCCTCAAACG-3′;reverse,5′-TCCTCACGGTGATGCTGTTC-3′.
MMP-2: forward, 5′-GCTGATACTGACACTGGTACTG-3′; reverse,
5′-CAATCTTTTCTGGGAGCTC-3′. uPA: forward,
5′-ATGCATGGTGCATGACTGCTCT-3′; reverse,
5′-ATTCGTGCAAGATGAGCTGCTC-3′. uPAR: forward,
5′-CAGAGCACAGAAAGGAGCTTGA-3′; reverse,
5′-CTGAAAGGTCTGGTTGCTATGGA-3′. COX-2: forward,
5′-CCTGCTGCCCGACACCTTCA-3′; reverse, 5′-AGCAACCCGGCCAGCAATCT-3′.
VEGF: forward, 5′-GTACCTCCACCATGCCAAGT-3′; reverse,
5′-GCATTCACATCTGCTGTGCT-3′. GAPDH: forward,
5′-GTATGACTCCACTCACGGCAAA-3′; reverse,
5′-GGTCTCGCTCCTGGAAGATG-3′.
Pathological analysis
To observe metastasized tumor cells in the lung,
lung tissues were collected from mice, fixed with 10% formalin, and
embedded in paraffin. Then the paraffin-embedded lung tissues were
cut into 4-mm-thick slices and stained with haematoxylin and eosin
(H&E). Tissue sections were randomly selected and tumor nodules
were counted using optical microscopes; the sizes of the tumor
nodules were also measured. The sizes of the tumor nodules were
calculated with the following formula: size of tumor nodule = (the
longest diameter + the shortest diameter)/2.
Statistical analysis
Statistical significance was determined using the
Student's t-test or one-way ANOVA. All cell experiments were
conducted in triplicate, and results are presented as mean ± SD.
P-values of <0.05 were considered significant.
Results
Effects on proliferation and survival of
4T1 murine breast cancer cells
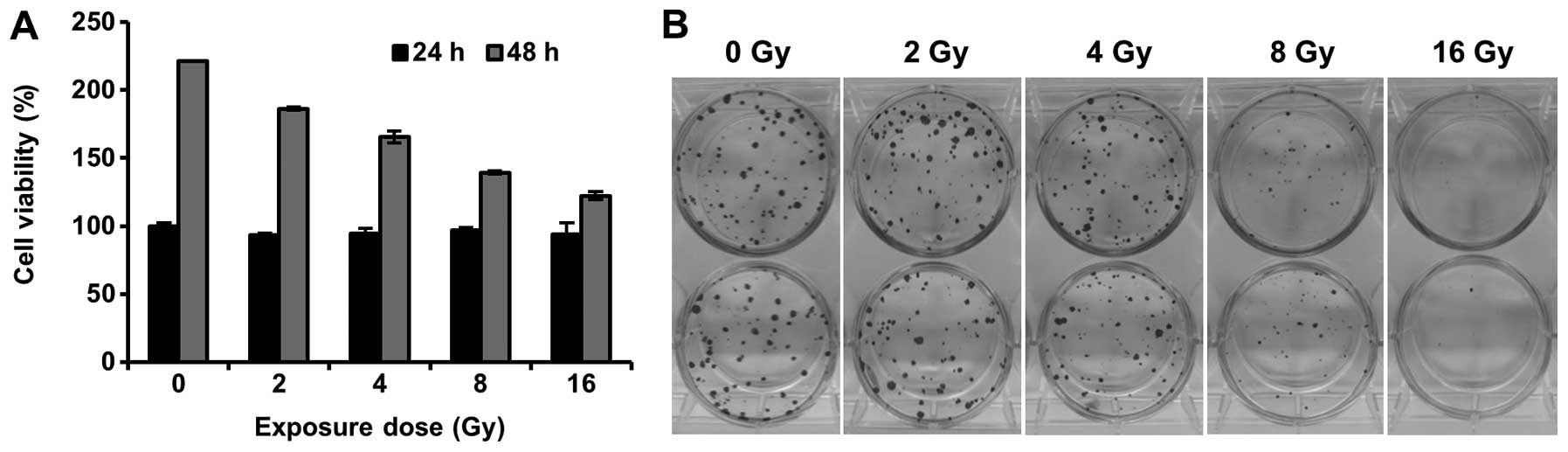
We assessed the effect of a proton beam on cell
proliferation and clonogenic survival in 4T1 murine breast cancer
cells. Although cell proliferation was not affected by proton beam
irradiation within 24 h, the proton beam inhibited the
proliferation at 48 h in MTT assay (Fig. 1A). In addition, the colony forming
assay showed that clonogenic viability was significantly decreased
by proton beam irradiation (Fig.
1B). These results are consistent with several studies that
revealed inhibition of cell growth by proton beam irradiation in
human solid tumors, such as pancreas, prostate, and breast cancer
(14,15,20).
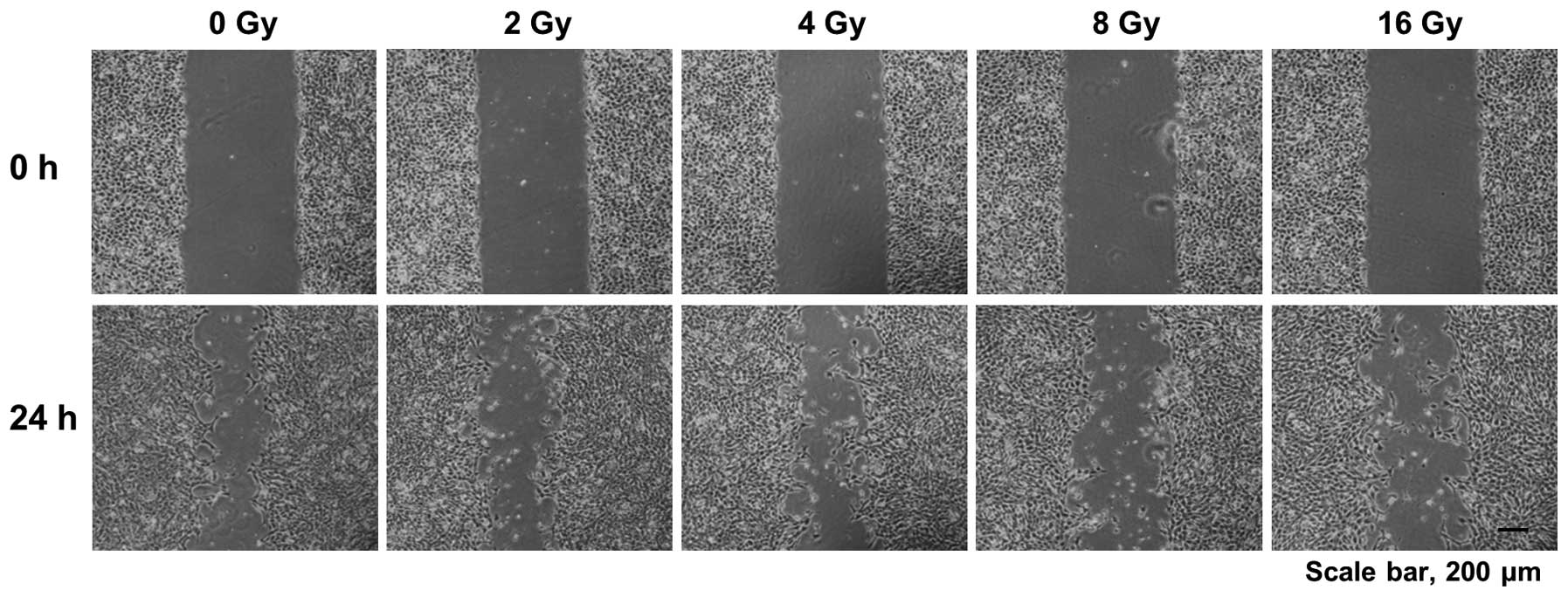
Effects on cell migration in 4T1 murine
breast cancer cells
Cell migration is crucial for cancer cells to
metastasize from a primary tumor tissue to distant sites in the
body. Therefore, we also evaluated the effects of proton beam
irradiation on cell migration of 4T1 murine breast cancer cells in
the wound healing assay. We found that cell migration of 4T1 murine
breast cancer cells was effectively prevented by proton beam
irradiation ranging from 2 to 16 Gy (Fig. 2). This result demonstrates that a
proton beam may suppress the metastatic potential of 4T1 murine
breast cancer cells.
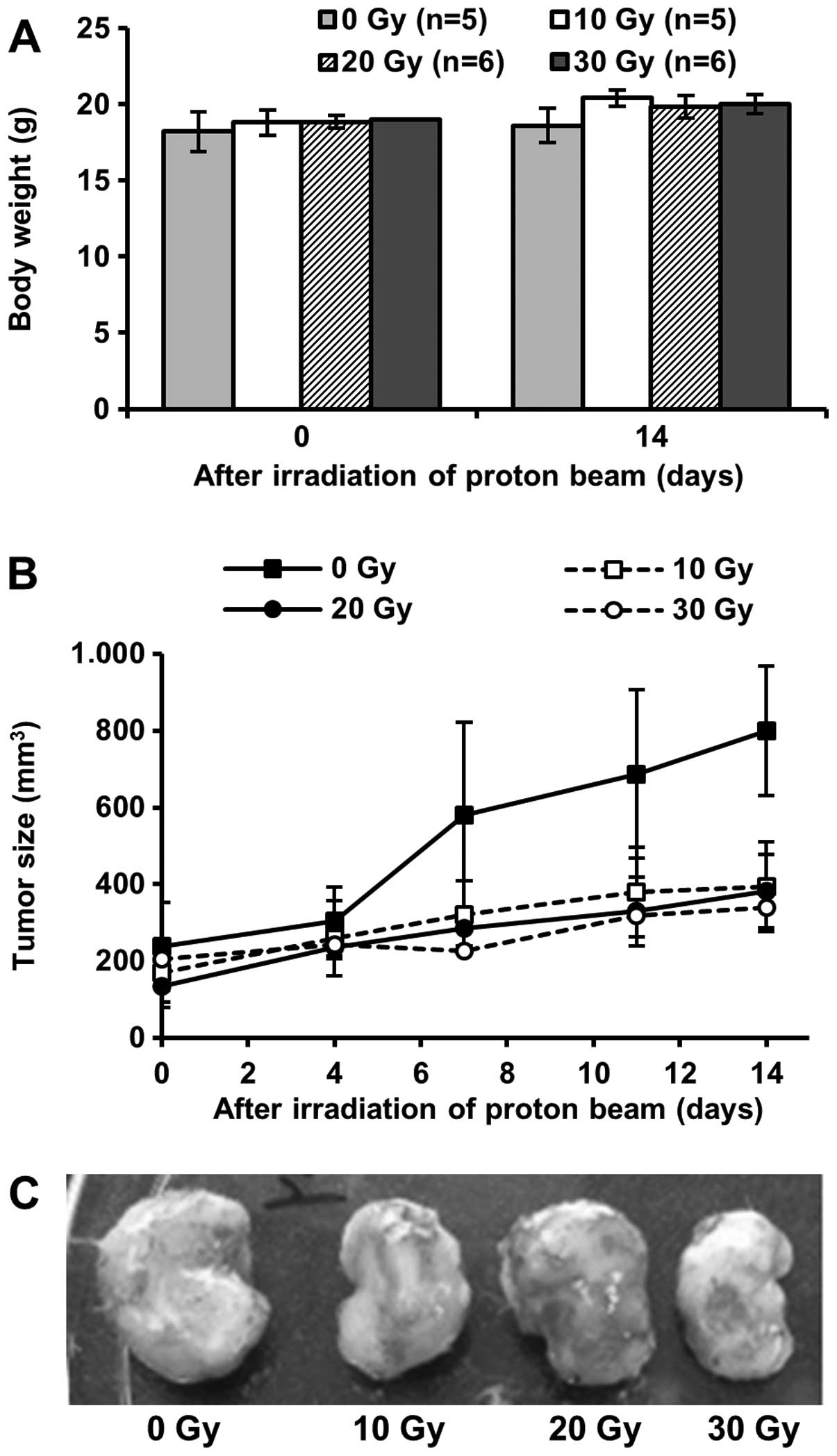
Effects on breast tumor growth and lung
metastasis in 4T1 orthotopic animal model
In vitro experiments showed the suppressive
effects of proton beam irradiation against cell proliferation,
clonogenic survival and cell migration in 4T1 murine breast cancer
cells (Figs. 1 and 2). Based on the results, we assessed the
effects of proton beam irradiation on breast tumor growth and lung
metastasis in BALB/c mice implanted with 4T1 murine breast cancer
cells. The growth of breast tumors irradiated by a proton beam was
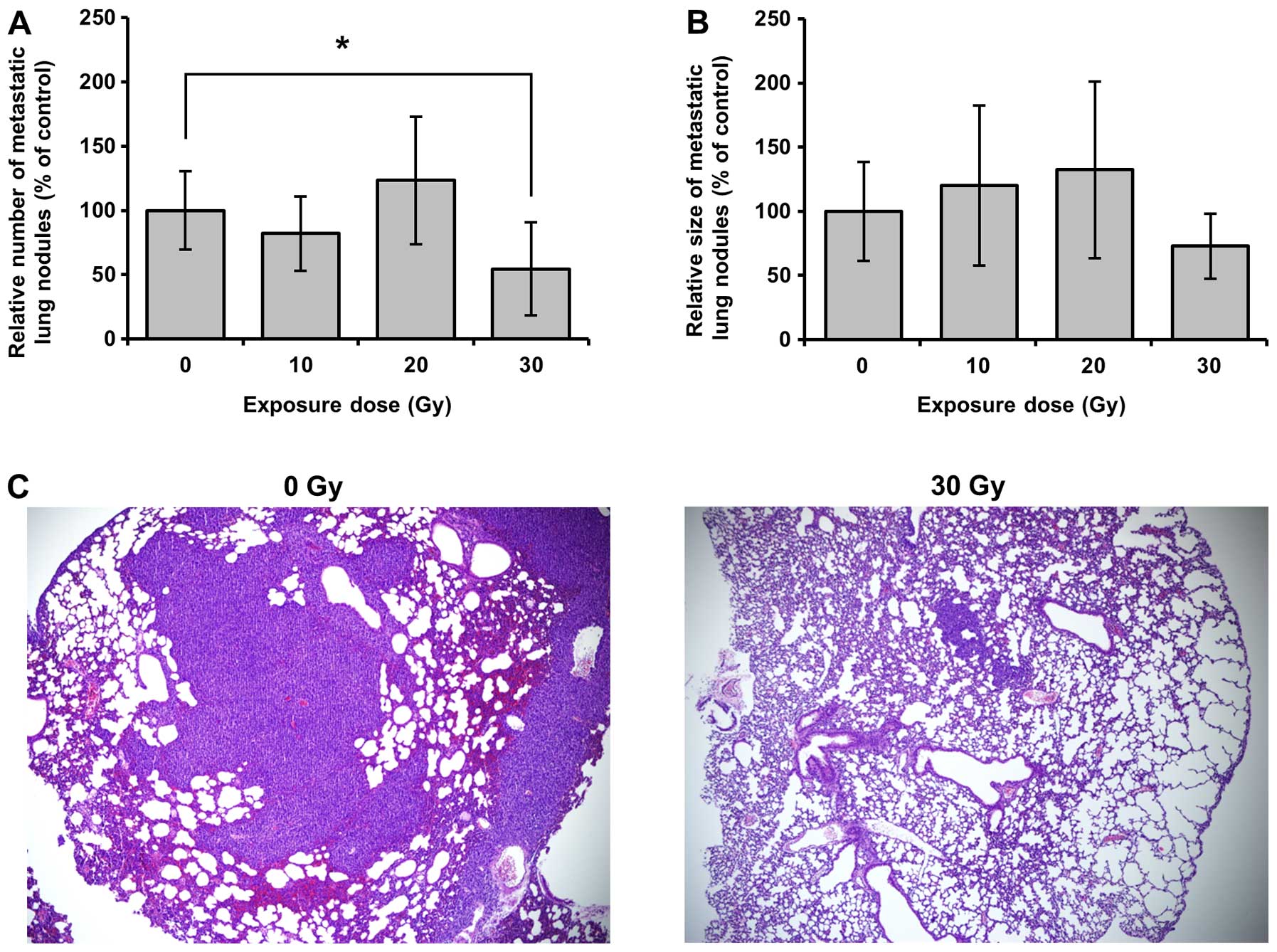
significantly repressed without body weight changes (Fig. 3A and B). Furthermore, fewer tumor
nodules were found in lungs of mice irradiated with 30 Gy proton
beam (Fig. 4A). Also, although not
significant, the size of tumor nodules in mice irradiated with 30
Gy proton beam was smaller than others (Fig. 4B and C). However, 10 and 20 Gy
proton beam could not prevent lung metastasis of breast cancer. The
results correspond with those of in vitro cell proliferation
and cell migration assays (Figs. 1
and 2).
Effects on metastatic gene expression in
4T1-implanted breast tumors
We determined the proton beam irradiation-mediated
change in transcription of metastatic genes, such as COX-2, uPA,
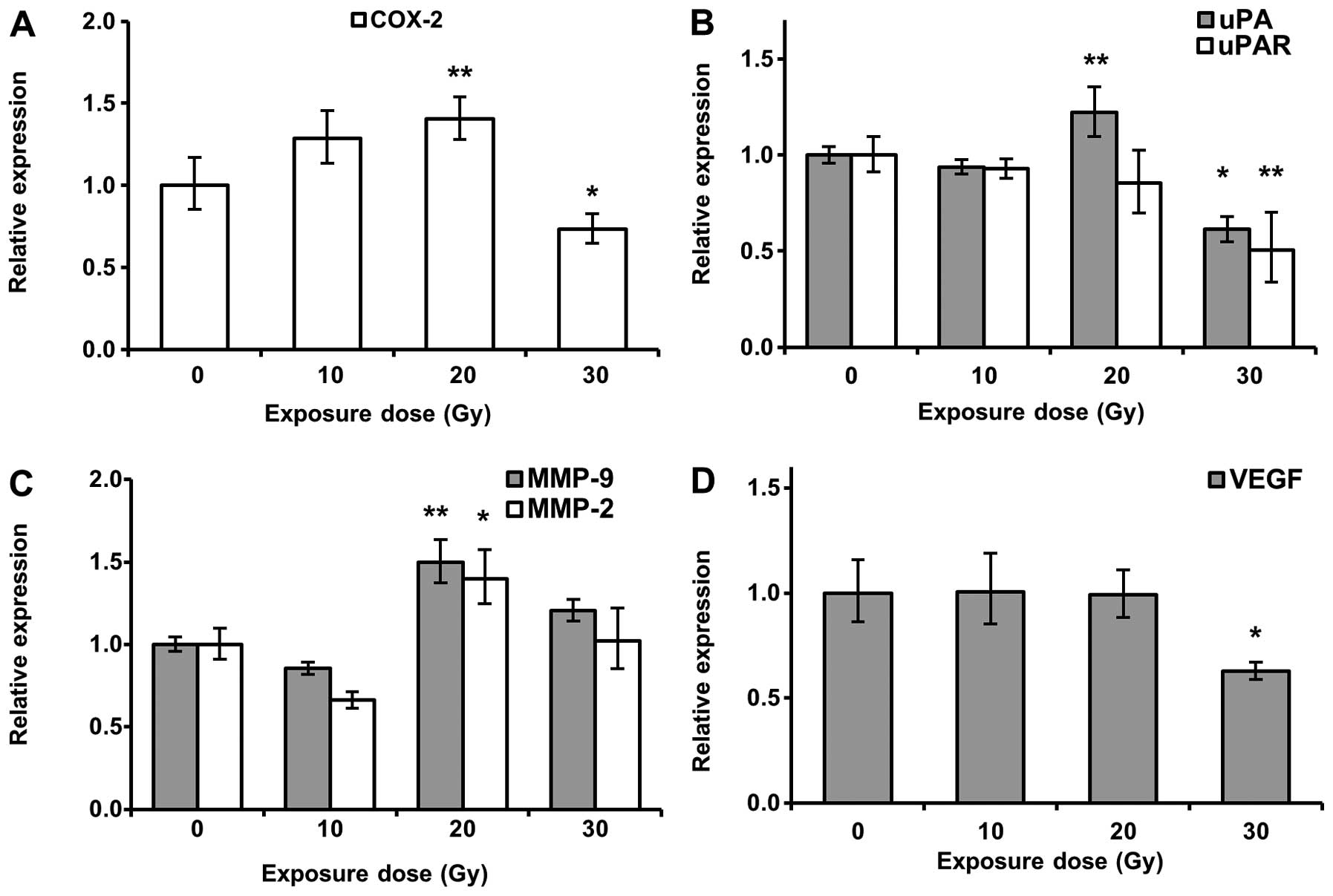
uPAR, MMP-9 and MMP-2, in breast tumors. As shown in Fig. 5A and B, COX-2, uPA and uPAR
expressions were inhibited by 30 Gy proton beam irradiation.
However, proton beams did not suppress MMP-2 and -9 transcription
(Fig. 5C). Surprisingly, mRNA
expression of COX-2, uPA, MMP-2 and MMP-9 genes were enhanced in
breast tumor irradiated with 20 Gy proton beam (Fig. 5A–C), suggesting that optimized dose
irradiation of a proton beam is important for improving cancer
treatment and preventing metastasis. We also clarified the effect
of proton beam irradiation on VEGF gene expression. Lower levels of
VEGF mRNA were also observed in breast tumors irradiated with 30 Gy
proton beam (Fig. 5D). The
suppression of VEGF mRNA expression corresponded with the change of
metastatic gene expression by proton beam irradiation.
Discussion
Radiation therapy is a treatment technique to kill
cancer cells and prevent tumor growth using high energy beams, such
as X-ray, gamma ray or proton beam. X-ray is the most common energy
source for radiation therapy. However, this technique can induce
various side effects, such as radionecrosis, blood dyscrasia,
inappropriate hormone secretion and so on, as a result of normal
tissues damage caused by the physical characteristics of X-ray.
Proton beam therapy is a new high technology radiation therapy. A
proton beam enters the body, releasing the highest energy when it
reaches the target and then the energy quickly fades away. Because
of these physical properties, proton beam irradiation is used to
treat various cancers, including breast, bladder, head and neck, to
minimize normal tissue damage. Global proton beam facilities to
treat cancer are increasing (21).
We found in previous studies that metastatic
potential in MCF-7 and MDA-MB-231 human breast cancer cells was
diminished by proton beam irradiation and cell viabilities in both
were also decreased (12,16,17,22).
The results demonstrated that a proton beam should increase the
efficacy of breast cancer treatment while inhibiting metastasis.
However, previous studies did not elucidate the in vivo
anti-metastatic potential of a proton beam. Here, we assessed the
effects of proton beam irradiation on in vivo breast cancer
metastasis by using the 4T1 orthotopic breast cancer model. 4T1
cells are known as an adequate cancer cell model to research human
breast cancer because the cells can easily develop into a primary
tumor in the mammary gland and spontaneously metastasize with a
similar pattern as human breast cancer metastasis (23). Therefore, we used 4T1 cells to
clarify the anti-metastatic effects of a proton beam. Prior to
in vivo analysis, we found that proton beam irradiation
prevented cell proliferation and clonogenic survival in an in
vitro system with 4T1 murine breast cancer cells (Fig. 1). Also, cell migration of 4T1
murine breast cancer cells was suppressed by proton beam
irradiation in the wound healing assay (Fig. 2). The results show that metastatic
activity in 4T1 murine breast cancer cells should be prevented by
proton beam irradiation. Therefore, the data support that 4T1
murine breast cancer cells are suitable to investigate the in
vivo anti-metastatic effects of a proton beam. Based on these
results, we established the BLAB/c 4T1 orthotopic breast cancer
model and then irradiated the breast cancers with 10, 20 and 30 Gy
proton beams.
The 5-year survival rate in breast cancer patients
is predominantly determined by whether or not the cancer
metastasizes (24). Therefore,
inhibition of metastatic potential in breast cancer is important to
improve prognosis of patients. Wang et al in 2012 showed
that endothelial-mesenchymal transition (EMT) in human epithelial
cells was enhanced by proton beam irradiation of <2 Gy (25). Although not all cancer cells
undergo EMT prior to metastasis, many cancers go through EMT for
successful metastasis (26–28).
Therefore, the data demonstrate that <2 Gy proton beam should
enhance metastatic potential in cancer. Here, we found that breast
tumor growth was significantly suppressed by proton beam
irradiation without changes of body weight (Fig. 3), and 30 Gy proton beam
significantly inhibited lung metastasis in the animal model
(Fig. 4A and C). However, although
10 and 20 Gy proton beams critically delayed breast tumor growth,
neither dose of proton beams prevented lung metastasis of breast
cancer in the animal model (Figs.
3 and 4A and C). Consequently,
both studies implicate the importance of appropriate exposure dose
to improve therapeutic efficacy in proton beam therapy.
We further investigated the effects of proton beam
irradiation on the expression of metastasis-regulating genes, such
as COX-2, uPA and uPAR, in 4T1 orthotopic breast cancer model. The
results showed lower levels of COX-2, uPA and uPAR transcription in
breast cancer in mice irradiated with 30 Gy proton beam (Fig. 5A and B). Furthermore, 30 Gy proton
beam downregulated VEGF mRNA expression (Fig. 5D). However, the expression of MMP-2
and -9 genes was not changed (Fig.
5C). The importance of these factors in metastasis has been
well explained in previous studies. Enhanced COX-2 expression
triggers metastasis with the induction of VEGF expression, and
anti-metastatic agents inhibit COX-2 and MMPs expression (29–31).
Other reports demonstrated that the uPA system facilitates cell
invasion, adhesion and metastasis in breast cancer (32,33).
Furthermore, Andres et al suggested that the uPA system
should be a useful biomarker for predicting the prognosis of breast
cancer patients (34). Based on
these reports, metastatic potential is closely correlated with the
expression level of these factors and should be suppressed by their
downregulation. Therefore, the present results demonstrate that 30
Gy proton beam prevents lung metastasis of breast cancer through
the suppression of COX-2, uPA, uPAR and VEGF in the 4T1 orthotopic
breast cancer animal model.
In conclusion, we found that cell proliferation,
viability and migration of 4T1 breast cancer cells were
significantly decreased by proton beam irradiation. Also, in
vivo research showed that tumor growths in 4T1 orthotopic
breast cancer model were drastically delayed by proton beam
irradiation. Furthermore, 30 Gy proton beam inhibited lung
metastasis via suppression of COX-2, uPA, uPAR and VEGF gene
expression. However, prevention of lung metastasis was not observed
in mice irradiated with 10 and 20 Gy proton beams. Taken together,
our data suggest that a proton beam is a useful tool in metastatic
breast cancer treatment and demonstrate that selection of a
suitable exposure dose is important to improve therapeutic efficacy
through the inhibition of metastasis to distant organs in breast
cancer.
Acknowledgements
This study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Ministry of Science,
ICT and future Planning (2014M2B2A4030083).
References
|
1
|
Weigelt B, Peterse JL and van't Veer LJ:
Breast cancer metastasis: Markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Howlader NNA, Krapcho M, Garshell J,
Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,
Mariotto A, et al: SEER Cancer Statistics Review, 1975–2013.
http://seer.cancer.gov/csr/1975_2013.
Accessed April 15, 2016
|
|
3
|
Westermarck J and Kahari VM: Regulation of
matrix metalloproteinase expression in tumor invasion. FASEB J.
13:781–792. 1999.PubMed/NCBI
|
|
4
|
Lee KS, Shin JS and Nam KS: Starfish
polysaccharides downregulate metastatic activity through the MAPK
signaling pathway in MCF-7 human breast cancer cells. Mol Biol Rep.
40:5959–5966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang F, Hu M, Lei Q, Xia Y, Zhu Y, Song X,
Li Y, Jie H, Liu C, Xiong Y, et al: Nifuroxazide induces apoptosis
and impairs pulmonary metastasis in breast cancer model. Cell Death
Dis. 6:e17012015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Kong X, Wang Y and Yang Q: BRCC2
inhibits breast cancer cell growth and metastasis in vitro and in
vivo via down-regulating AKT pathway. Cell Death Dis. 4:e7572013.
View Article : Google Scholar
|
|
7
|
Daniele A, Zito AF, Giannelli G, Divella
R, Asselti M, Mazzocca A, Paradiso A and Quaranta M: Expression of
metal-loproteinases MMP-2 and MMP-9 in sentinel lymph node and
serum of patients with metastatic and non-metastatic breast cancer.
Anticancer Res. 30:3521–3527. 2010.PubMed/NCBI
|
|
8
|
Harbeck N, Schmitt M, Kates RE, Kiechle M,
Zemzoum I, Jänicke F and Thomssen C: Clinical utility of
urokinase-type plasminogen activator and plasminogen activator
inhibitor-1 determination in primary breast cancer tissue for
individualized therapy concepts. Clin Breast Cancer. 3:196–200.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang S, New L, Pan Z, Han J and Nemerow
GR: Urokinase plasminogen activator/urokinase- specific surface
receptor expression and matrix invasion by breast cancer cells
requires constitutive p38alpha mitogen-activated protein kinase
activity. J Biol Chem. 275:12266–12272. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Janicke F, Prechtl A, Thomssen C, Harbeck
N, Meisner C, Untch M, Sweep CGJF, Selbmann H-K, Graeff H and
Schmitt M: Randomized adjuvant chemotherapy trial in high-risk,
lymph node-negative breast cancer patients identified by
urokinase-type plasminogen activator and plasminogen activator
inhibitor type 1. J Natl Cancer Inst. 93:913–920. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Look MP: Pooled analysis of uPA and PAI-1
for prognosis in primary breast cancer patients. EORTC Receptor and
Biomarker Study Group. Int J Biol Markers. 15:70–72.
2000.PubMed/NCBI
|
|
12
|
Lee KS, Lee DH, Chun SY and Nam KS:
Metastatic potential in MDA-MB-231 human breast cancer cells is
inhibited by proton beam irradiation via the Akt/nuclear
factor-kappaB signaling pathway. Mol Med Rep. 10:1007–1012.
2014.PubMed/NCBI
|
|
13
|
Foote RL, Stafford SL, Petersen IA, Pulido
JS, Clarke MJ, Schild SE, Garces YI, Olivier KR, Miller RC, Haddock
MG, et al: The clinical case for proton beam therapy. Radiat Oncol.
7:1742012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alan Mitteer R, Wang Y, Shah J, Gordon S,
Fager M, Butter P-P, Jun Kim H, Guardiola-Salmeron C,
Carabe-Fernandez A and Fan Y: Proton beam radiation induces DNA
damage and cell apoptosis in glioma stem cells through reactive
oxygen species. Sci Rep. 5:139612015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Narang H, Kumar A, Bhat N, Pandey BN and
Ghosh A: Effect of proton and gamma irradiation on human lung
carcinoma cells: Gene expression, cell cycle, cell death,
epithelial-mesenchymal transition and cancer-stem cell trait as
biological end points. Mutat Res. 780:35–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee KS, Shin JS and Nam KS: Effect of
proton beam irradiation on the regulation of metastasis-enhancing
factors in MCF-7 human breast cancer cells. J Korean Phys Soc.
63:1373–1378. 2013. View Article : Google Scholar
|
|
17
|
Lee KS, Shin JS, Shon YH and Nam KS:
Anti-angiogenic activity in metastasis of human breast cancer cell
irradaited by a proton beam. J Korean Phys Soc. 61:268–272. 2012.
View Article : Google Scholar
|
|
18
|
Miller FR: Tumor subpopulation
interactions in metastasis. Invasion Metastasis. 3:234–242.
1983.PubMed/NCBI
|
|
19
|
Miller FR, Miller BE and Heppner GH:
Characterization of metastatic heterogeneity among subpopulations
of a single mouse mammary tumor: Heterogeneity in phenotypic
stability. Invasion Metastasis. 3:22–31. 1983.PubMed/NCBI
|
|
20
|
Lee KB, Lee JS, Park JW, Huh TL and Lee
YM: Low energy proton beam induces tumor cell apoptosis through
reactive oxygen species and activation of caspases. Exp Mol Med.
40:118–129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jermann M: Particle therapy statistics in
2014. Int J Particle Ther. 2:50–54. 2015. View Article : Google Scholar
|
|
22
|
Lee KS, Mo JY, Shon YH and Nam KS:
Inhibition of metastatic activities in human breast cancer cells
irradiated by a proton beam. J Korean Phys Soc. 59:653–656. 2011.
View Article : Google Scholar
|
|
23
|
Pulaski BA and Ostrand-Rosenberg S: Mouse
4T1 breast tumor model. Curr Protoc Immunol. Chapter 20(Unit 20):
222001. View Article : Google Scholar
|
|
24
|
Nieves-Alicea R, Colburn NH, Simeone AM
and Tari AM: Programmed cell death 4 inhibits breast cancer cell
invasion by increasing tissue inhibitor of metalloproteinases-2
expression. Breast Cancer Res Treat. 114:203–209. 2009. View Article : Google Scholar :
|
|
25
|
Wang M, Hada M, Saha J, Sridharan DM,
Pluth JM and Cucinotta FA: Protons sensitize epithelial cells to
mesenchymal transition. PLoS One. 7:e412492012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heerboth S, Housman G, Leary M, Longacre
M, Byler S, Lapinska K, Willbanks A and Sarkar S: EMT and tumor
metastasis. Clin Transl Med. 4:62015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wallerand H, Cai Y, Wainberg ZA, Garraway
I, Lascombe I, Nicolle G, Thiery J-P, Bittard H, Radvanyi F and
Reiter RR: Phospho-Akt pathway activation and inhibition depends on
N-cadherin or phospho-EGFR expression in invasive human bladder
cancer cell lines. Urol Oncol. 28:180–188. 2010. View Article : Google Scholar
|
|
28
|
Bailey JM, Singh PK and Hollingsworth MA:
Cancer metastasis facilitated by developmental pathways: Sonic
hedgehog, Notch, and bone morphogenic proteins. J Cell Biochem.
102:829–839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kundu N and Fulton AM: Selective
cyclooxygenase (COX)-1 or COX-2 inhibitors control metastatic
disease in a murine model of breast cancer. Cancer Res.
62:2343–2346. 2002.PubMed/NCBI
|
|
30
|
Morita Y, Hata K, Nakanishi M, Nishisho T,
Yura Y and Yoneda T: Cyclooxygenase-2 promotes tumor
lymphangiogenesis and lymph node metastasis in oral squamous cell
carcinoma. Int J Oncol. 41:885–892. 2012.PubMed/NCBI
|
|
31
|
Kang JH, Song KH, Jeong KC, Kim S, Choi C,
Lee C and Oh S: Involvement of Cox-2 in the metastatic potential of
chemotherapy-resistant breast cancer cells. BMC Cancer. 11:3342011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stillfried GE, Saunders DN and Ranson M:
Plasminogen binding and activation at the breast cancer cell
surface: The integral role of urokinase activity. Breast Cancer
Res. 9:R142007. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Cremoux P, Grandin L, Dieras V,
Savignoni A, Degeorges A, Salmon R, Bollet MA, Reyal F,
Sigal-Zafrani B, Vincent-Salomon A, et al: Urokinase-type
plasminogen activator and plasminogen-activator-inhibitor type 1
predict metastases in good prognosis breast cancer patients.
Anticancer Res. 29:1475–1482. 2009.PubMed/NCBI
|
|
34
|
Andres SA, Edwards AB and Wittliff JL:
Expression of urokinase-type plasminogen activator (uPA), its
receptor (uPAR), and inhibitor (PAI-1) in human breast carcinomas
and their clinical relevance. J Clin Lab Anal. 26:93–103. 2012.
View Article : Google Scholar : PubMed/NCBI
|