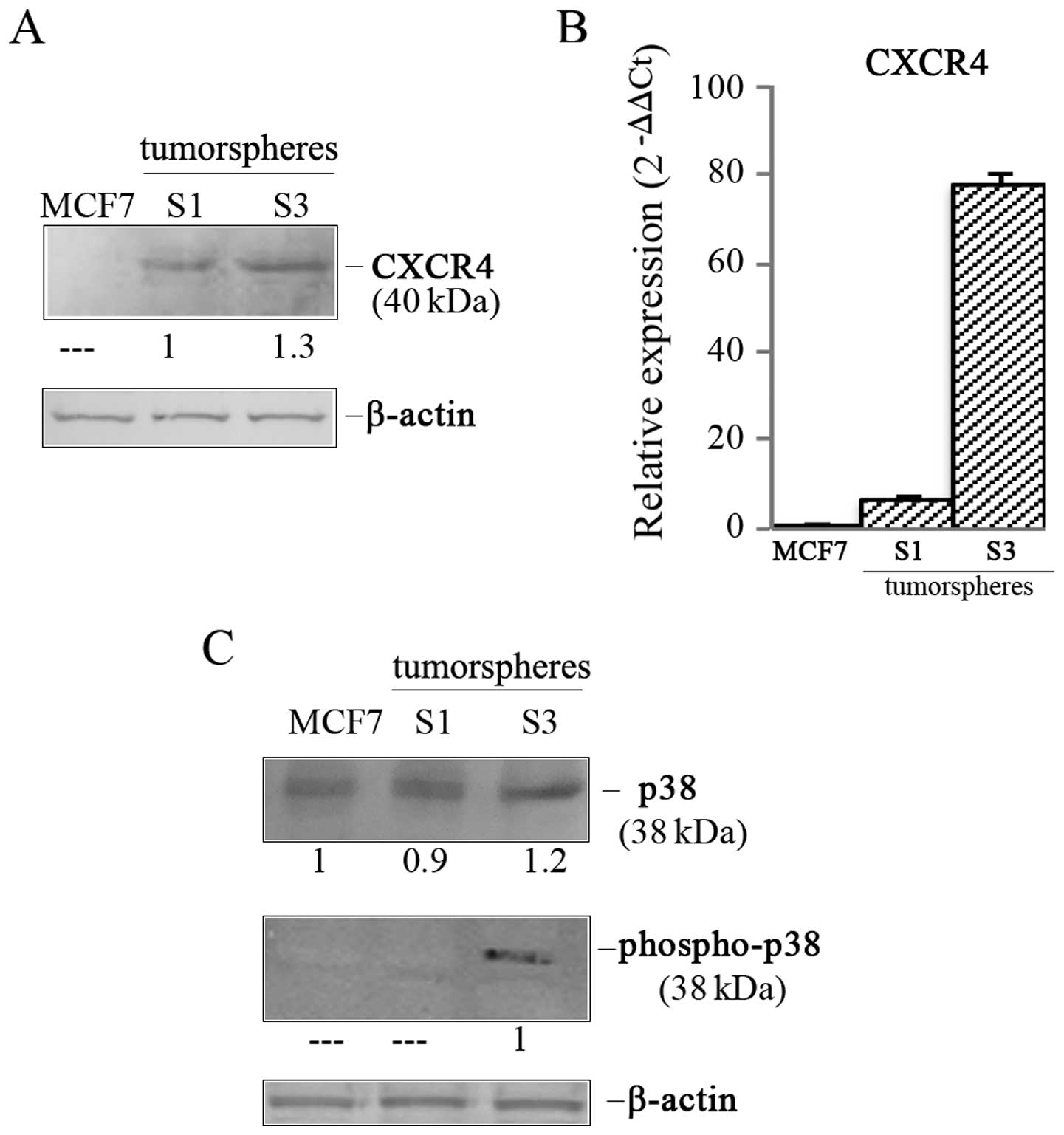
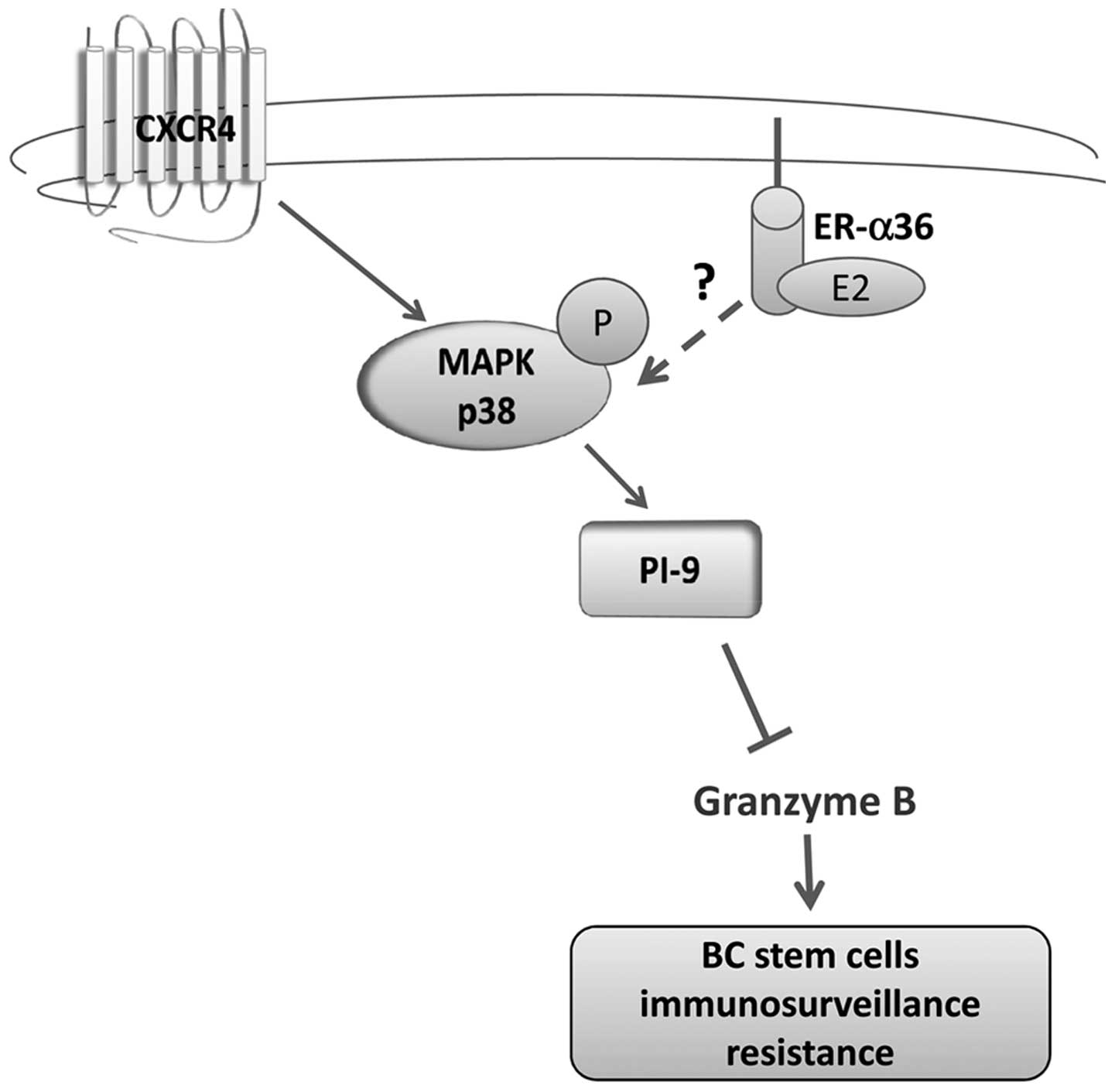
|
1
|
Forouzanfar MH, Foreman KJ, Delossantos
AM, Lozano R, Lopez AD, Murray CJ and Naghavi M: Breast and
cervical cancer in 187 countries between 1980 and 2010: A
systematic analysis. Lancet. 378:1461–1484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Key T, Appleby P, Barnes I and Reeves G;
Endogenous Hormones and Breast Cancer Collaborative Group.
Endogenous sex hormones and breast cancer in postmenopausal women:
reanalysis of nine prospective studies. J Nat Cancer Inst.
94:606e6162002.
|
|
3
|
Welboren WJ, Sweep FC, Span PN and
Stunnenberg HG: Genomic actions of estrogen receptor alpha: What
are the targets and how are they regulated? Endocr Relat Cancer.
16:1073–1089. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Robertson JF: Oestrogen receptor: A stable
phenotype in breast cancer. Br J Cancer. 73:5–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nilsson S, Mäkelä S, Treuter E, Tujague M,
Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M and
Gustafsson JA: Mechanisms of estrogen action. Physiol Rev.
81:1535–1565. 2001.PubMed/NCBI
|
|
6
|
Katzenellenbogen BS and Katzenellenbogen
JA: Estrogen receptor transcription and transactivation: Estrogen
receptor alpha and estrogen receptor beta: regulation by selective
estrogen receptor modulators and importance in breast cancer.
Breast Cancer Res. 2:335–344. 2000. View
Article : Google Scholar
|
|
7
|
Björnström L and Sjöberg M: Mechanisms of
estrogen receptor signaling: Convergence of genomic and nongenomic
actions on target genes. Mol Endocrinol. 19:833–842. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deroo BJ and Buensuceso AV: Minireview:
Estrogen receptor-beta: mechanistic insights from recent studies.
Mol Endocrinol. 24:1703–1714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Z, Zhang X, Shen P, Loggie BW, Chang
Y and Deuel TF: Identification, cloning, and expression of human
estrogen receptor-alpha36, a novel variant of human estrogen
receptor-alpha66. Biochem Biophys Res Commun. 336:1023–1027. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chaudhri RA, Schwartz N, Elbaradie K,
Schwartz Z and Boyan BD: Role of ERα36 in membrane-associated
signaling by estrogen. Steroids. 81:74–80. 2014. View Article : Google Scholar
|
|
11
|
Zou Y, Ding L, Coleman M and Wang Z:
Estrogen receptor-alpha (ER-alpha) suppresses expression of its
variant ER-alpha 36. FEBS Lett. 583:1368–1374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Osborne CK: Tamoxifen in the treatment of
breast cancer. N Engl J Med. 339:1609–1618. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clarke R, Leonessa F, Welch JN and Skaar
TC: Cellular and molecular pharmacology of antiestrogen action and
resistance. Pharmacol Rev. 53:25–71. 2001.PubMed/NCBI
|
|
14
|
Clarke R, Liu MC, Bouker KB, Gu Z, Lee RY,
Zhu Y, Skaar TC, Gomez B, O'Brien K, Wang Y, et al: Antiestrogen
resistance in breast cancer and the role of estrogen receptor
signaling. Oncogene. 22:7316–7339. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Charafe-Jauffret E, Ginestier C, Iovino F,
Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F,
Dutcher J, et al: Breast cancer cell lines contain functional
cancer stem cells with metastatic capacity and a distinct molecular
signature. Cancer Res. 69:1302–1313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng H, Yin L, Zhang XT, Liu LJ, Wang ML
and Wang ZY: ER-α variant ER-α36 mediates antiestrogen resistance
in ER-positive breast cancer stem/progenitor cells. J Steroid
Biochem Mol Biol. 144B:417–426. 2014. View Article : Google Scholar
|
|
17
|
Massari F, Santoni M, Ciccarese C and
Santini D: The immunocheckpoints in modern oncology: The next 15
years. Expert Opin Biol Ther. 15:917–921. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mori S, Jewett A, Murakami-Mori K,
Cavalcanti M and Bonavida B: The participation of the Fas-mediated
cytotoxic pathway by natural killer cells is tumor-cell-dependent.
Cancer Immunol Immunother. 44:282–290. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goping IS, Barry M, Liston P, Sawchuk T,
Constantinescu G, Michalak KM, Shostak I, Roberts DL, Hunter AM,
Korneluk R, et al: Granzyme B-induced apoptosis requires both
direct caspase activation and relief of caspase inhibition.
Immunity. 18:355–365. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fritsch K, Finke J and Grüllich C:
Suppression of granzyme B activity and caspase-3 activation in
leukaemia cells constitutively expressing the protease inhibitor 9.
Ann Hematol. 92:1603–1609. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dontu G, Abdallah WM, Foley JM, Jackson
KW, Clarke MF, Kawamura MJ and Wicha MS: In vitro propagation and
transcriptional profiling of human mammary stem/progenitor cells.
Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
D'Anneo A, Carlisi D, Lauricella M, Puleio
R, Martinez R, Di Bella S, Di Marco P, Emanuele S, Di Fiore R,
Guercio A, et al: Parthenolide generates reactive oxygen species
and autophagy in MDA-MB231 cells. A soluble parthenolide analogue
inhibits tumour growth and metastasis in a xenograft model of
breast cancer. Cell Death Dis. 4:e8912013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Di Fiore R, Drago-Ferrante R, Pentimalli
F, Di Marzo D, Forte IM, D'Anneo A, Carlisi D, De Blasio A,
Giuliano M, Tesoriere G, et al: MicroRNA-29b-1 impairs in vitro
cell proliferation, self-renewal and chemoresistance of human
osteosarcoma 3AB-OS cancer stem cells. Int J Oncol. 45:2013–2023.
2014.PubMed/NCBI
|
|
24
|
Carlisi D, D'Anneo A, Martinez R, Emanuele
S, Buttitta G, Di Fiore R, Vento R, Tesoriere G and Lauricella M:
The oxygen radicals involved in the toxicity induced by
parthenolide in MDA-MB-231 cells. Oncol Rep. 32:167–172.
2014.PubMed/NCBI
|
|
25
|
Arif K, Hussain I, Rea C and El-Sheemy M:
The role of Nanog expression in tamoxifen-resistant breast cancer
cells. Onco Targets Ther. 8:1327–1334. 2015.PubMed/NCBI
|
|
26
|
Bilal I, Chowdhury A, Davidson J and
Whitehead S: Phytoestrogens and prevention of breast cancer: The
contentious debate. World J Clin Oncol. 5:705–712. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krieg AJ, Krieg SA, Ahn BS and Shapiro DJ:
Interplay between estrogen response element sequence and ligands
controls in vivo binding of estrogen receptor to regulated genes. J
Biol Chem. 279:5025–5034. 2004. View Article : Google Scholar
|
|
28
|
Jiang X, Ellison SJ, Alarid ET and Shapiro
DJ: Interplay between the levels of estrogen and estrogen receptor
controls the level of the granzyme inhibitor, proteinase inhibitor
9 and susceptibility to immune surveillance by natural killer
cells. Oncogene. 26:4106–4114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Furusato B, Mohamed A, Uhlén M and Rhim
JS: CXCR4 and cancer. Pathol Int. 60:497–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Trautmann F, Cojoc M, Kurth I, Melin N,
Bouchez LC, Dubrovska A and Peitzsch C: CXCR4 as biomarker for
radioresistant cancer stem cells. Int J Radiat Biol. 90:687–699.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lourenco S, Teixeira VH, Kalber T, Jose
RJ, Floto RA and Janes SM: Macrophage migration inhibitory
factor-CXCR4 is the dominant chemotactic axis in human mesenchymal
stem cell recruitment to tumors. J Immunol. 194:3463–3474. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rhodes LV, Short SP, Neel NF, Salvo VA,
Zhu Y, Elliott S, Wei Y, Yu D, Sun M, Muir SE, et al: Cytokine
receptor CXCR4 mediates estrogen-independent tumorigenesis,
metastasis, and resistance to endocrine therapy in human breast
cancer. Cancer Res. 71:603–613. 2011. View Article : Google Scholar :
|
|
33
|
Bots M, de Bruin E, Rademaker-Koot MT and
Medema JP: Proteinase inhibitor-9 expression is induced by
maturation in dendritic cells via p38 MAP kinase. Hum Immunol.
68:959–964. 2007. View Article : Google Scholar
|
|
34
|
Lakshmi Narendra B, Eshvendar Reddy K,
Shantikumar S and Ramakrishna S: Immune system: A double-edged
sword in cancer. Inflamm Res. 62:823–834. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Krasnova Y, Putz EM, Smyth MJ and
Souza-Fonseca-Guimaraes F: Bench to bedside: NK cells and control
of metastasis. Clin Immunol. 15:30050–30054. 2015.
|
|
36
|
Medema JP, de Jong J, Peltenburg LT,
Verdegaal EM, Gorter A, Bres SA, Franken KL, Hahne M, Albar JP,
Melief CJ, et al: Blockade of the granzyme B/perforin pathway
through overexpression of the serine protease inhibitor PI-9/SPI-6
constitutes a mechanism for immune escape by tumors. Proc Natl Acad
Sci USA. 98:11515–11520. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Igney FH and Krammer PH: Immune escape of
tumors: Apoptosis resistance and tumor counterattack. J Leukoc
Biol. 71:907–920. 2002.PubMed/NCBI
|
|
38
|
Martínez-Lostao L, Anel A and Pardo J: How
do cytotoxic lymphocytes kill cancer cells? Clin Cancer Res.
21:5047–5056. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cunningham TD, Jiang X and Shapiro DJ:
Expression of high levels of human proteinase inhibitor 9 blocks
both perforin/granzyme and Fas/Fas ligand-mediated cytotoxicity.
Cell Immunol. 245:32–41. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ray M, Hostetter DR, Loeb CR, Simko J and
Craik CS: Inhibition of Granzyme B by PI-9 protects prostate cancer
cells from apoptosis. Prostate. 72:846–855. 2012. View Article : Google Scholar :
|
|
41
|
Soriano C, Mukaro V, Hodge G, Ahern J,
Holmes M, Jersmann H, Moffat D, Meredith D, Jurisevic C, Reynolds
PN, et al: Increased proteinase inhibitor-9 (PI-9) and reduced
granzyme B in lung cancer: Mechanism for immune evasion? Lung
Cancer. 77:38–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Levin ER: Cellular functions of plasma
membrane estrogen receptors. Steroids. 67:471–475. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee LM-J, Cao J, Deng H, Chen P, Gatalica
Z and Wang ZY: ER-alpha36, a novel variant of ER-alpha, is
expressed in ER-positive and -negative human breast carcinomas.
Anticancer Res. 28B:479–483. 2008.
|
|
44
|
Deng H, Zhang XT, Wang ML, Zheng HY, Liu
LJ and Wang ZY: ER-α36-mediated rapid estrogen signaling positively
regulates ER-positive breast cancer stem/progenitor cells. PLoS
One. 9:e880342014. View Article : Google Scholar
|
|
45
|
Zhang X, Deng H and Wang ZY: Estrogen
activation of the mitogen-activated protein kinase is mediated by
ER-α36 in ER-positive breast cancer cells. J Steroid Biochem Mol
Biol. 143:434–443. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mao L, Yuan L, Slakey LM, Jones FE, Burow
ME and Hill SM: Inhibition of breast cancer cell invasion by
melatonin is mediated through regulation of the p38
mitogen-activated protein kinase signaling pathway. Breast Cancer
Res. 12:R1072010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang M, Liu HX, Teng XD, Wang HB, Cui J,
Jia SS, Gu XY and Li ZG: The differences in CXCR4 protein
expression are significant for the five molecular subtypes of
breast cancer. Ultrastruct Pathol. 36:381–386. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dubrovska A, Hartung A, Bouchez LC, Walker
JR, Reddy VA, Cho CY and Schultz PG: CXCR4 activation maintains a
stem cell population in tamoxifen-resistant breast cancer cells
through AhR signalling. Br J Cancer. 107:43–52. 2012. View Article : Google Scholar : PubMed/NCBI
|