Introduction
The paired-box (PAX) family, initially described in
Drosophila but highly conserved among vertebrates,
encompasses nine DNA-binding proteins that function as
transcription factors. PAX proteins share a common structure: a
DNA-binding paired domain (PAired boX, hence the name of the
family), a PST homeodomain and an octapeptide; genes from this
family differ in the length of the first two and the presence or
absence of the last domain. During development, PAX genes are
expressed in different regions of the forming embryo, thus they
constitute important gene expression regulators (1,2).
PAX8 has a fairly well understood role in development: it is
associated to morphogenesis of the kidney, thyroid gland and
nervous system (3,4), and its expression is regulated by
alternative splicing (5).
The PAX8 gene, located at 2q13 and spanning 63 kb,
is transcribed, processed and translated to give rise to six
reported mRNAs: the originally described PAX8A, B, C, and D, which
are in turn translated to the corresponding protein isoforms
(6), and a more recently described
transcript, PAX8F, that shares the PAX8A ORF and bears an extended
5′UTR (7). The existence of PAX8B
has been questioned and its GenBank entry (NM_013951.3) is
currently suppressed. All PAX8 isoforms bind DNA with similar
affinity; however, PAX8A and PAX8B display higher transactivation
potential (6).
Recent studies suggest a role for PAX8 in
carcinogenesis that makes it a biomarker candidate; still, more
studies are necessary to completely understand this role. So far,
PAX8 has been associated to important cancer processes such as
Retinoblastoma/E2F1 transcription (8,9) and
telomerase activity (10). Another
study recently showed that by inhibiting PAX8 expression with
shRNAs in ovarian cancer cell lines, their viability diminished
(11); this suggests a direct
involvement of PAX8 in cell proliferation which, in turn, could
represent a potential therapeutic target.
An important fact that links PAX8 to carcinogenesis
is the PAX8/PPARγ genetic rearrangement found in 30–35% of
follicular thyroid carcinoma and up to 10% of follicular thyroid
adenoma (12–14). Hence, t(2;3)(q13;p25) fuses the
promoter and 5-coding portion of the transcription factor PAX8 that
involves DNA binding domains to the full-length coding sequence of
the nuclear receptor peroxisome proliferator-activated receptor-γ 1
gene (15).
As a biomarker, PAX8 is frequently detected in
epithelial tumors of the thyroid, parathyroid, kidney, thymus and
female genital tract (16,17). In thyroid tumors, PAX8
overexpression has been found in follicular and papillary adenomas
and carcinomas (16,18,19).
Wilms' tumors and nephrogenic adenomas, among other renal tumors,
show PAX8 overexpression owing to its role during renal
organogenesis (20–22). PAX8-expressing oviduct cells have
been pointed out as the probable origin of ovarian and endometrial
cancers, rendering PAX8 as an early gynecological cancer marker,
associated with poor disease outcome (23,24).
Only few groups have reported PAX8 overexpression in cervical
cancer employing distinct methodologies (25–27).
A genome-wide search for PAX8 binding sites using
ChIP-Seq technology was recently carried out on thyroid cells. The
authors identified binding sites that show that PAX8 regulates
genes involved in cell proliferation and differentiation, as well
as binding sites located in non-promoter regions, including
introns, that hint to a role as a distal transcriptional regulator
(28). However, none of the
aforementioned reports of PAX8 as a tumor marker or functional PAX8
studies specify whether its results concern any particular PAX8
isoform; therefore, we set out to investigate which isoform or
isoforms were expressed in a set of locally advanced cervical tumor
samples compared to normal cervical tissues. PAX8 isoforms could
possibly activate different genes contributing to carcinogenesis,
given their different in vitro transactivation properties
(6).
We had hypothesized that, since splicing is highly
altered in cancer (29), we would
find a particular PAX8 splicing pattern in cervical carcinoma tumor
samples and cervical carcinoma-derived cell lines, but RACE
experiments revealed the expression of previously unreported
aberrant transcripts instead. We also found evidence hinting of a
complex gene rearrangement in the genomic DNA from the cell lines,
providing a possible explanation for these transcripts. To our
knowledge, this is the first study showing that PAX8 gene undergoes
complex rearrangement processes that could contribute to establish
the CC tumor phenotype.
Materials and methods
Expression data Tissue samples
Patients were prospectively enrolled into the
National Cancer Institute of Mexico (INCAN) tumor-banking protocol
at the time of diagnosis. All patients included accepted and signed
informed consent; institutional ethics and scientific board
committees approved the protocol. Immediately after punch biopsy,
tumor samples were split into three pieces, one for the pathologic
confirmation of at least 80% of tumor cells, mandatory for this
type of molecular profiles, and the remaining two for RNA and DNA
isolation. RNA and DNA biopsies were frozen in liquid nitrogen
until nucleic acid extraction. Eligibility criteria were i)
patients with a confirmed pathologic diagnosis of CC staged IB2 up
to IIIB (LACC); ii) biopsies with pathology report with >80% of
tumors cells; hence, the genomic analysis is mainly addressed for
tumor cells; iii) age greater to 20 and less than 60 years; iv)
high-quality DNA and RNA; v) no presence of comor bidities; vi) and
without previous oncological treatment.
Healthy cervical tissues were obtained from patients
who had undergone hysterectomy by uterine myomatosis. Inclusion
criteria were: i) no previous cervical surgery (such as the loop
electrosurgical excision procedure or cone biopsy), ii) no HPV
infection, iii) no hormonal treatment, and iv) at last three
previous negative Pap smears.
PAX8 mRNA expression
We designed two sets of primers, one for detection
of PAX8 exons 3–4 and another for exons 10–11 in the mRNA
(NM_003466.3; see Fig. 1). RNA was
extracted from 18 tumoral and 20 healthy, HPV-free, cervical tissue
samples using the TRIzol reagent (Life Technologies cat. #
15596-026), following the manufacturer's recommendations. Its
integrity was verified through agarose gel electrophoresis and it
was quantitated by spectrophotometry (OD260/280>1.9). For first
strand synthesis, we mixed 500 ng RNA, 0.5 mM dNTPs, 10 mM DT, 1×
First-strand buffer and 180 ng random hexamers, heated them to 42°C
for 2 min; then we added 200 units of SuperScript II reverse
transcriptase (Invitrogen cat. #18064-022) and incubated the
reaction for 50 min at 42°C, followed by 15 min at 70°C. The
resulting cDNAs was probed for DNA contamination by performing
no-RT assays. Quantitative PCR (qPCR) reactions were performed in
triplicate using LightCycler® 480 SYBR Green I Master
Mix (Roche, cat. # 04 707 516 0081) in the LightCycler 480
instrument, following the manufacturer's recommendations (40
amplification cycles, Tm 58°C). End-point PCR was performed using
the PCR Master mix 2× (Thermo Scientific, cat. #K0171) 0.5 μM de
cada primer (35 cycles, Tm 56°C).
Western blotting
Total proteins were extracted from 80–85% confluent
cell cultures. Culture media was removed and cells were rinsed
twice with PBS, scraped off from culture dishes, and lysed using
the RIPA lysis reagent (Santa Cruz Biotechnology) following the
manufacturer's recommendations.
Total protein (50 μg) was mixed with Laemmli sample
buffer, boiled, separated in 12% or 15% SDS-PAGE and transferred
onto a Hybond-P PVDF membrane (Amersham-GE Healthcare). Membranes
were probed overnight using a 1:500 (v/v) dilution of the
appropriate antibody; for detection, 1:2500 (v/v) dilutions of HRP
anti-rabbit or anti-mouse conjugate antibodies (Santa Cruz
Biotechnology) were used. Finally, using the SuperSignal WestFemto
chemiluminescent substrate (Thermo Scientific), the membranes were
scanned in the C-Digit blot scanner (Li-Cor) and the images were
analyzed in the associated ImageStudio software (LiCor). Membranes
were stripped and re-probed for actin detection as a loading
control. The commercial antibodies used were anti-PAX8 (Cell
Signaling Technology #9857) and anti-β actin (SantaCruz
Biotechnology sc-1616). A representative image from three
independent experiments is shown.
RT-PCR, 5′ RACE and Exon PCR
DNA and RNA were isolated from tumor samples or from
the CaSki (ATCC, CRL1550) and SiHa (ATCC, HTB35) cells grown to
approximately 80–85% confluence, using the TRIzol reagent (Life
technologies) following the manufacturer's recommendations.
First strand cDNA synthesis was carried out for 90
min at 42°C. Total RNA (5 μg) was mixed with 1 mM oligo dT, 0.25 mM
of each dNTP, 1× First-strand buffer, 10 mM DTT and 200 units of
M-MLV reverse transcriptase (Promega). This reaction (1 μl) was
used as a template for 50 μl PCR reactions for amplification of
PAX8 transcripts using the PAX8Fw and PAX8Rv primers (Table I) 0.2 μM each, 25 μM of each dNTP,
1× Herculase II buffer and 1 unit of Herculase II fusion DNA
polymerase (Agilent).
 | Table IPrimers used for 5′ RACE and Exon PCR
experiments. |
Table I
Primers used for 5′ RACE and Exon PCR
experiments.
| Name | 5′-3′ | Sequence Nt
position |
|---|
| Primers for 5′
RACE |
| 1 | 5′ RACE adapter GCU
GAU GGC GAU GAA UGA ACA CUG CGU UUG CUG GCU UUG AUG AAA | NA |
| 2 | RACE Outer primer
GCT GAT GGC GAT GAA TGA ACA CTG | NA |
| 3 | RACE Inner primer
CGC GGA TCC GAA CAC TGC GTT TGC TGG CTT TGA TG | NA |
| 4 | PAX8UTR3′RV AGT CCT
CCT GTT GCT CAG TCG CT | 1561–1539a |
| 5 | PAX8 RV CTA CAG ATG
GTC AAA GGC CGT | 1519–1499a |
| Primers for
RT-PCR |
| 1 | PAX8 FW ATG CCT CAC
AAC TCC ATC AGA | 167–187a |
| 2 | PAX8 RV CTA CAG ATG
GTC AAA GGC CGT | 1519–1499a |
| 3 | GAPDH FW CCT CAA
GAT CAT CAG CAA TGC CT | 617–639b |
| 4 | GAPDH RV TCA CGC
CAC AGT TTC CCG GAG | 781–761b |
| Primers for Exon
PCR |
| 6 | PAX8EX1Fw GAT GCA
GGC ATC GAA TCT C | 399–417c |
| 7 | PAX8EX1Rv ACG CTC
TCG AGA TCC AAC C | 639–621c |
| 8 | PAX8EX2Fw ATC CCC
ACC CAA ACT CCT AC | 64185–64204c |
| 9 | PAX8EX2Rv TCA GCT
GGA GAA GTC AAG CC | 64468–64449c |
| 10 | PAX8EX3Fw TGT CTA
AAG ACC CCA CCT GC | 66487–66506c |
| 11 | PAX8EX3Rv AGC CAG
GCC TTT CTT GTC TC | 66824–66805c |
| 12 | PAX8EX4Fw GCC ATG
AGT TCT CTT TCC TCC | 68332–68352c |
| 13 | PAX8EX4Rv GGT ATG
CTG AAG GGG AGG TG | 68538–68519c |
| 14 | PAX8EX5Fw ACT ACC
CCA GAG TCA CCC AG | 68961–68980c |
| 15 | PAX8EX5Rv AAA GCC
TCA GCA AAC TGC TC | 69215–69196c |
| 15 | PAX8EX6Fw TTT GGC
CTA GAG CAT GAA TAG | 69376–69396c |
| 16 | PAX8EX6Rv GAG CAC
AGG CTC ATT TGG AG | 69692–69673c |
| 17 | PAX8EX7Fw CCT AAG
ACA CAG GCT CAG GG | 74363–74382c |
| 18 | PAX8EX7Rv AGC CAA
GCT CTT CAG TCC C | 74620–74602c |
| 19 | PAX8EX8Fw CTT GTG
CGT GTT CCC TCC | 75738–77555c |
| 20 | PAX8EX8Rv GTC TGC
CCT GAG GAC CC | 76060–76044c |
| 21 | PAX8EX9Fw AGC CTC
AGG AGA GTG AGA TG | 84030–84040c |
| 22 | PAX8EX9Rv GTC CCA
CCT TGC TCC AAT AC | 84269–84250c |
| 23 | PAX8EX10Fw CCT GCA
TTG ATG CCC TTC | 91115–91132c |
| 24 | PAX8EX10Rv AGG TAA
CCT TTG ACC CAC CC | 91335–91316c |
Amplification conditions were 5 min pre-incubation
at 95°C followed by 35 cycles of 20 sec at 95°C, 20 sec at 53°C and
70 sec at 68°C, followed by a final step of 4 min at 68°C. GAPDH
transcripts were detected as a loading control, using the
corresponding primers (Table I)
0.25 μM each, 0.2 mM of each dNTP, 2.5 mM MgCl2 and 0.75 units of
GoTaq DNA Polymerase (Promega) in a 25 μl volume. The amplification
conditions were: 5 min pre-incubation at 95°C, 35 cycles of 30 sec
at 95°C, 20 sec at 57°C and 20 sec at 72°C, followed by a final
elongation step of 7 min at 72°C.
Rapid amplification of cDNA ends (RACE)
was performed using the First Choice RLM-RACE kit (Life
Technologies-Ambion) according to the manufacturer's protocol
Briefly, 1 μg of RNA from HeLa cells, SiHa cells or
tumor samples was dephosphorylated with Calf intestine Acid
Phosphatase (CIP) to remove the available phosphates from rRNA,
tRNA, uncapped and partial transcripts; the cap structure was
subsequently removed from mature mRNAs with Tobacco Acid
Phosphatase (TAP) so that only these transcripts could acquire the
5′ RACE adapter (Table I) trough
ligation with T4 RNA ligase. Next, a random-primed RT reaction was
performed using M-MLV reverse transcriptase (Promega). The PAX8
transcripts were amplified from the cDNA pool through nested PCR,
the first round was performed using the 5′ RACE Outer Primer from
the kit in combination with the PAX8UTR3′Rv primer, while the 5′
RACE Inner Primer and the PAX8Rv primer were used for the second
round (Table I). The resulting
amplicons were resolved in ethidium bromide-stained 2% agarose
gels.
PAX8 exons were amplified from genomic DNA with
ad-hoc primers based on the PAX8 complete sequence (GenBank Gene
ID: 7849; locus NG_012384). These primers were designed using the
ExonPrimer server (ihg. helmholtz-muenchen.de/ihg/ExonPrimer.html)
and are shown in Table I. PCR was
performed using 25 ng gDNA template, 0.25 μM of each primer, 0.2 mM
of each dNTP, 2.5 mM MgCl2 and 0.75 units of GoTaq DNA
Polymerase (Promega) in a 25 μl volume. The amplification
conditions were as follows: 5 min pre-incubation at 92°C followed
by 35 cycles of 20 sec at 92°C, 20 sec at 67°C and 30 sec at 72°C,
followed by a final step of 7 min at 72°C. All the resulting
amplicons were resolved in ethidium bromide-stained 1.5% or 2%
agarose gels.
Cloning and sequence analysis
The RACE product bands were excised from the agarose
gels and purified using the QIAquick Spin kit (Qiagen) according to
the manufacturer's protocol. After purification, the RACE products
were cloned into the pGEM T-Easy plasmid (Promega). Plasmids from
positive colonies were analyzed by restriction; each clone was
sequenced in both chains using universal primers and the Big Dye
Terminator Ready Reaction kit (Perkin-Elmer) and analyzed in the
ABI PRISM 3130xl Genetic Analyzer System (Applied Biosystems).
Sequence information was analyzed using the CLC Bio Main Workbench
(CLC Bio; Qiagen) as well as the BLAT (genome.ucsc.edu) and BLAST
(blast.ncbi.nlm.nih.gov) algorithms.
In order to search for sequence similarity among
clones, we performed a CLUSTAL alignment and checked it manually.
Based on this alignment, we searched for the most adequate model of
evolution using the FindModel server (http://www.hiv.lanl.gov/content/sequence/findmodel/findmodel.html),
and the GTR model was selected to perform a maximum likelihood
phylogenetic analysis employing a 1000-replicate bootstrap
analysis. Finally, a tree was constructed using the neighborjoining
method.
Results
PAX8 mRNA expression
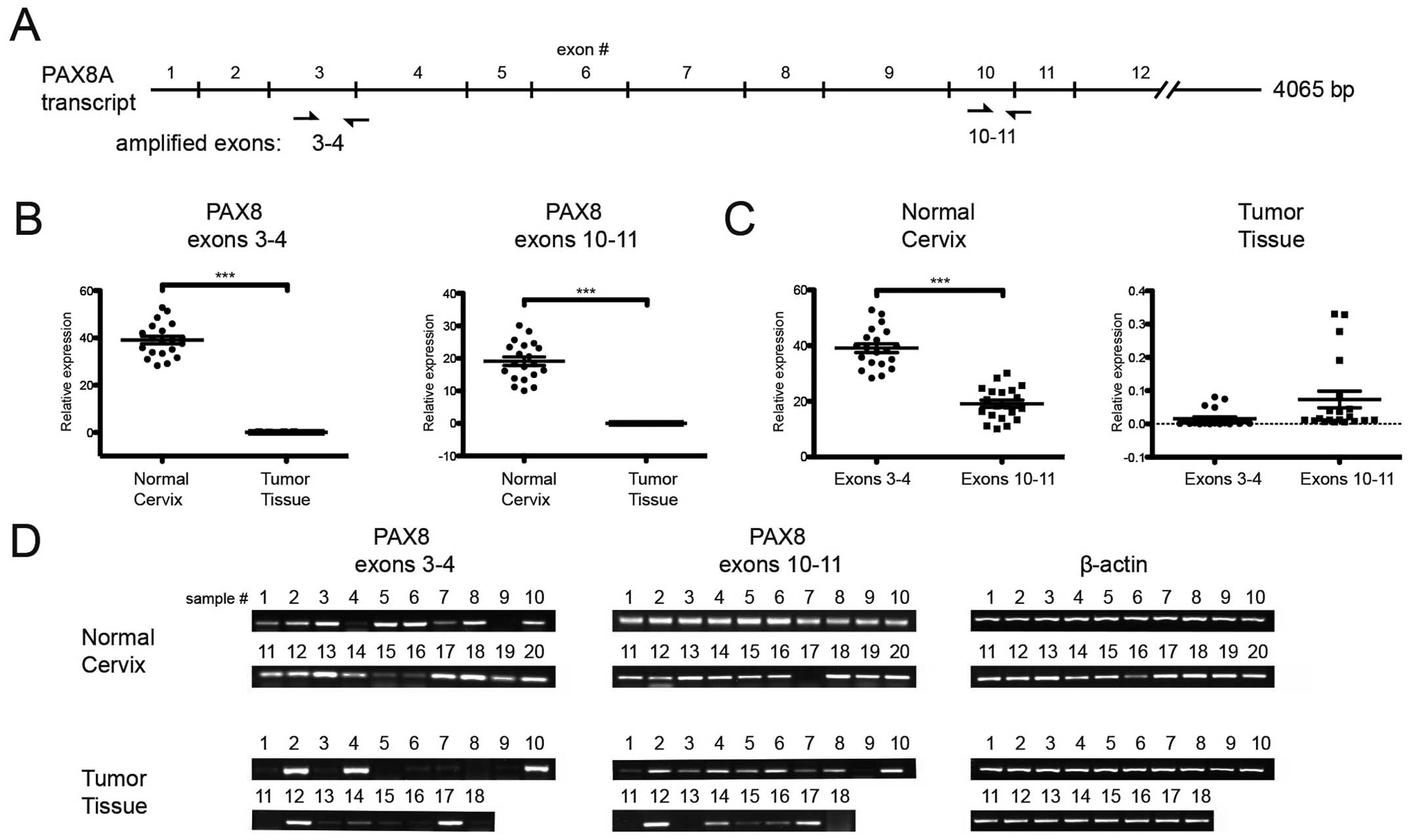
Since splicing events remove exons 8–10 (6), we designed the two sets of primers
depicted in Fig. 1A to assess the
PAX8 mRNA expression and integrity. We amplified independently
exons 3–4 and exons 10–11 from 20 normal cervix samples and 18
cervical tumor samples and found interesting results: Although
overall messenger expression was lower in tumors (Fig. 1B), the exon 3–4:10–11 ratio
(Fig. 1C) was different in tumors
when compared to normal cervical samples; which was more evident
upon agarose-gel separation of the amplicons (Fig. 1D). This observation suggested the
presence of transcripts containing only the 3′-most end of the PAX8
mRNA that may or may not correspond to reported splicing
products.
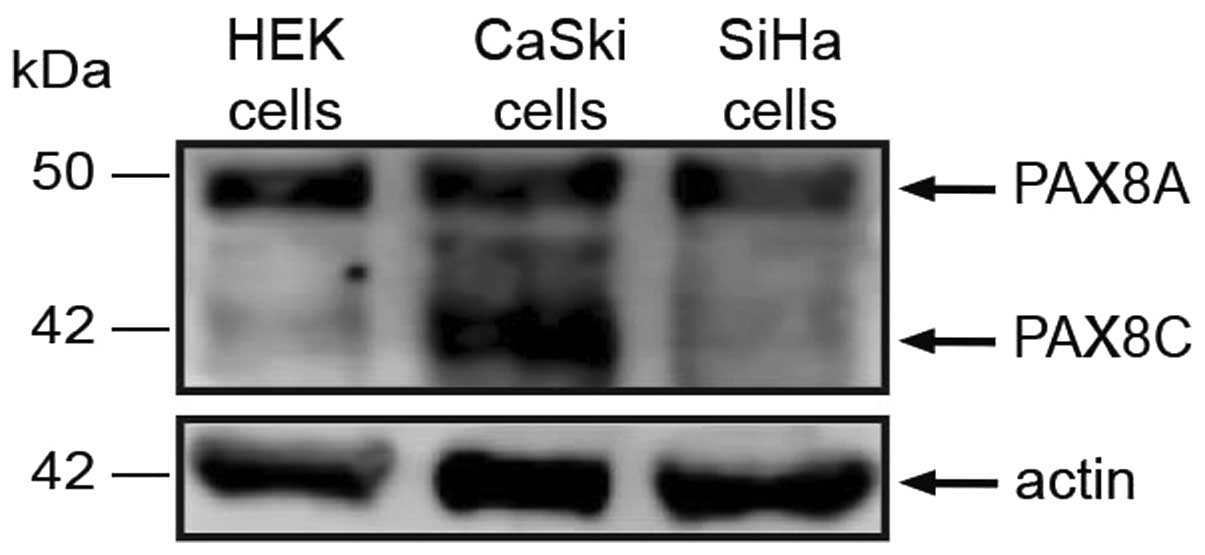
PAX8 protein expression
In order to detect the PAX8 isoforms possibly
expressed in cervical carcinoma-derived cell lines, we selected an
antibody raised against the carboxi-terminal region of PAX8, shared
by its isoforms. Previously, PAX8 has been detected through
immunocytochemistry but current cell imaging techniques are yet to
discriminate between isoforms sharing structural motifs.
Consistently with reported detection in HEK cells, we observed a 50
kDa band, apparently corresponding to PAX8A in CaSki and SiHa
cells; a 43 kDa band was detected, corresponding to PAX8C (Fig. 2). The reported PAX8A and C
transcripts bear exons 3–4 and 10–11, therefore, their presence was
not likely to account for the imbalance between these mRNA regions
in cervical tumors.
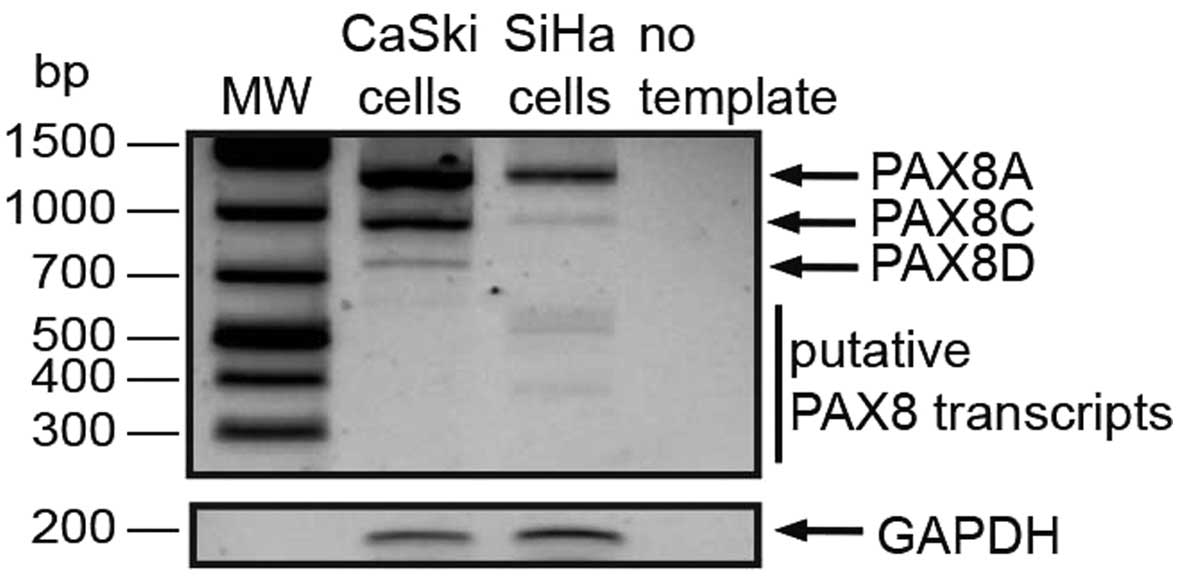
PAX8 aberrant transcripts
We next set out to identify the PAX8 transcripts
possibly expressed in cervical carcinoma-derived cell lines at the
mRNA level. The 1372-nt PAX8A/F ORF was present in both CaSki and
SiHa cells (Fig. 3), consistent
with our protein detection results. The PAX8C and PAX8D transcripts
were also detected in both cell lines. However, what really caught
our interest were the 400–500 nt products that we found in both
lines, more evident in the SiHa lane in Fig. 3.
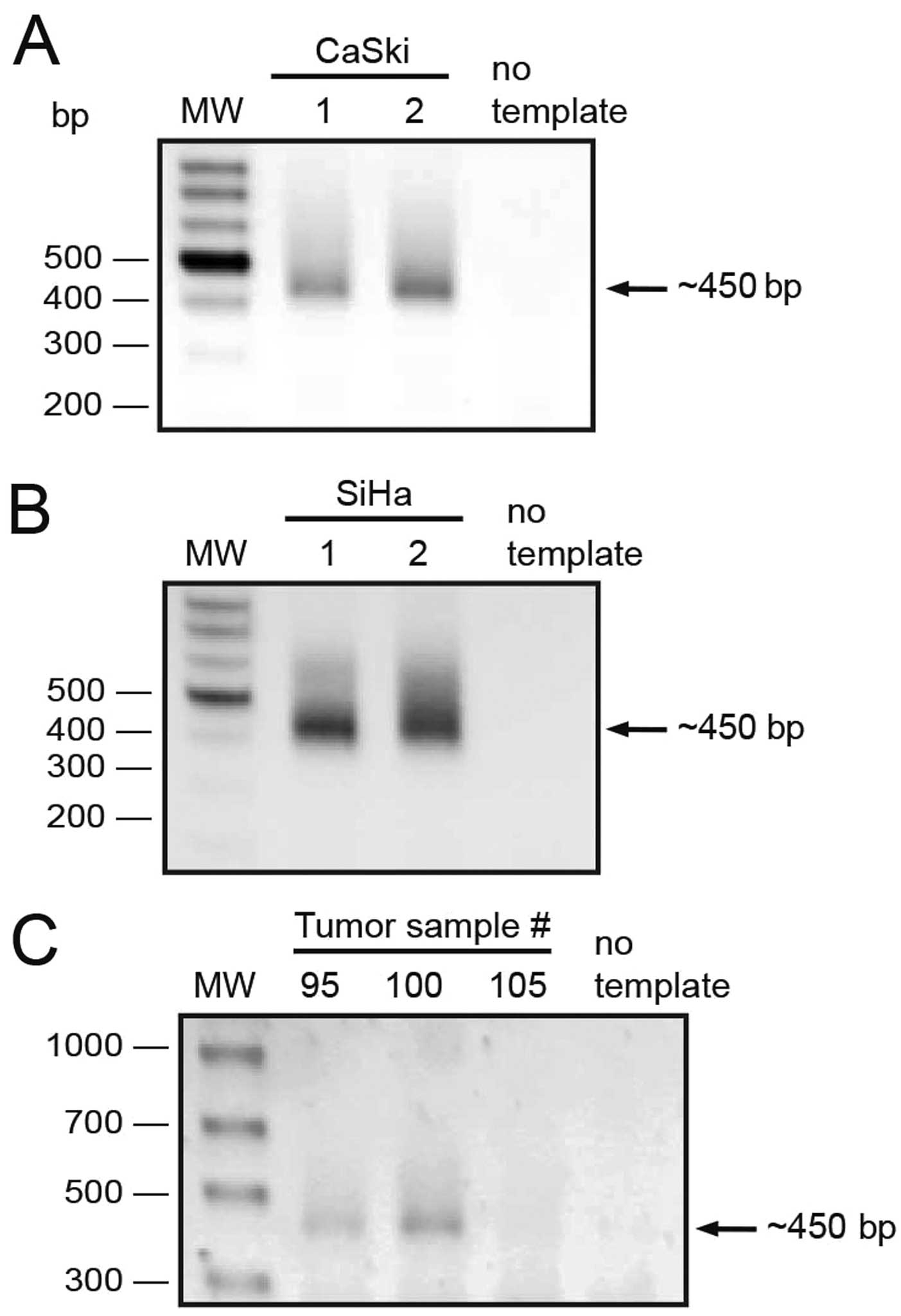
Due to the fact that exons 10–11 were detected at
higher levels in tumor samples, we reasoned there was a population
of transcripts sharing the 3′ end of the PAX8 ORF; so we set up a
5′ RACE strategy using a reverse primer located 20 nt downstream
from the end codon (PAX8UTR3′RV, Table
I). We performed two independent 5′ RACE experiments from CaSki
and SiHa cell lines (Fig. 4A and
B). A single band of about 450 nt was observed as product from
all these experiments. Furthermore, to rule out the possibility
that the detected transcripts were exclusive to cell lines, we
performed 5′ RACE experiments on three tumor samples and obtained
similar ~450 bp products from two of them (Fig. 4C). All these 5′ RACE products were
excised from the gel and cloned into a T-protruding vector.
We expected to obtain a number of positive colonies
carrying a 450 bp insert but instead, we surprisingly obtained
colonies carrying differently sized inserts. Plasmids from these
colonies were purified and sequenced. In total, we obtained 33
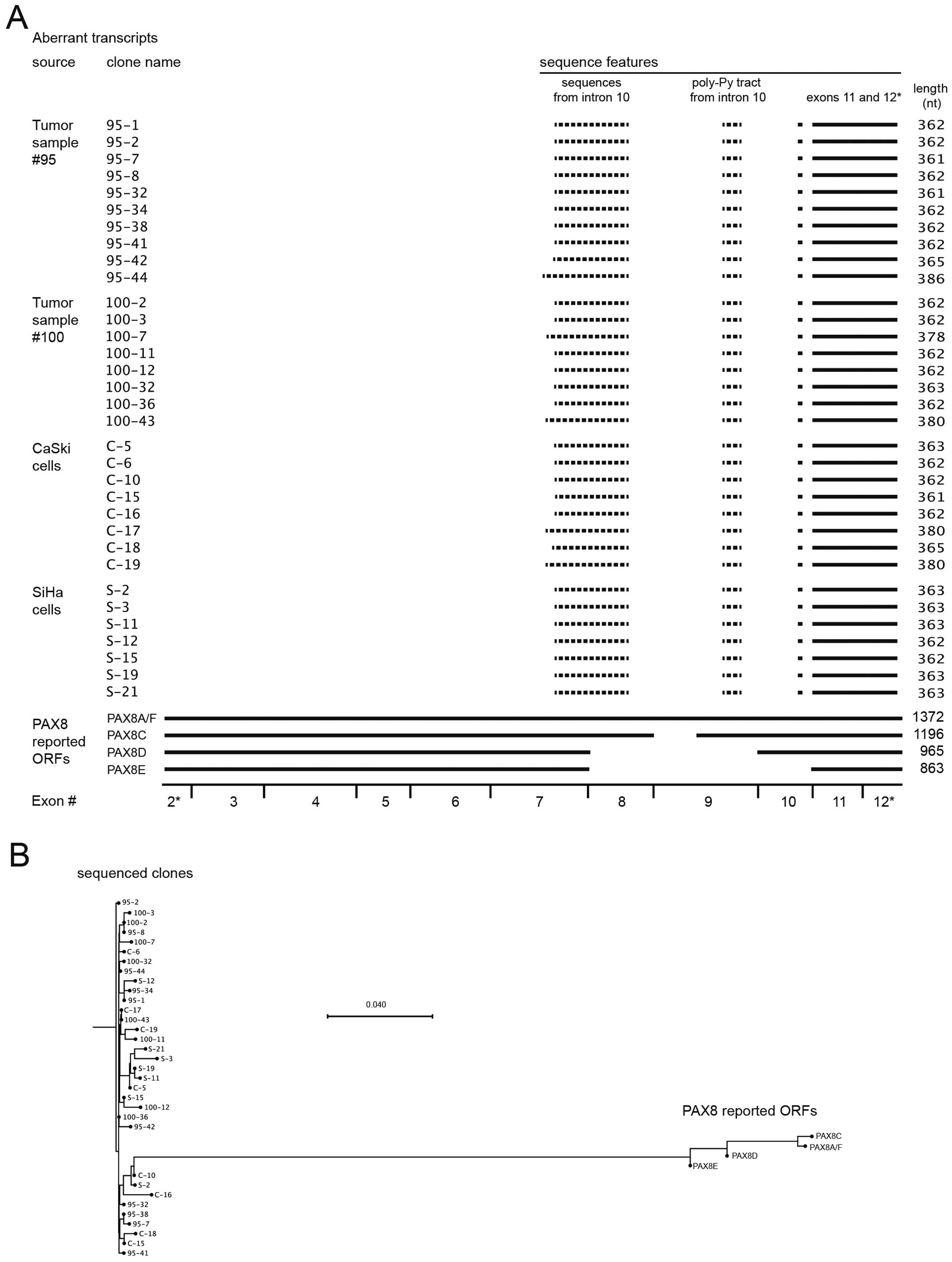
sequences that are shown in Fig.
5A, aligned with the four reported PAX8 transcripts. Solid
lines represent identical sequences, whereas sequences represented
by dotted lines did not align to the PAX8 transcripts. The
sequences of all transcripts were submitted to GenBank under
accessions KJ545852 to KJ545885. The clones obtained form tumor
samples and cervical carcinoma-derived cell lines form a single
pool of PAX8 aberrant transcripts. The reported transcripts and our
sequenced clones clustered separately from each other, and no
evident clustering was found within our clone group (Fig. 5B; Table II).
 | Table IIExpected amplicon sizes from Exon PCR
experiments. |
Table II
Expected amplicon sizes from Exon PCR
experiments.
| Exon no. | Exon PCR amplicon
size (bp) |
|---|
| 2 | 241 |
| 3 | 284 |
| 4 | 338 |
| 5 | 207 |
| 6 | 255 |
| 7 | 317 |
| 8 | 258 |
| 9 | 323 |
| 10 | 240 |
| 11 | 221 |
Upon analysis of the sequences from our collection
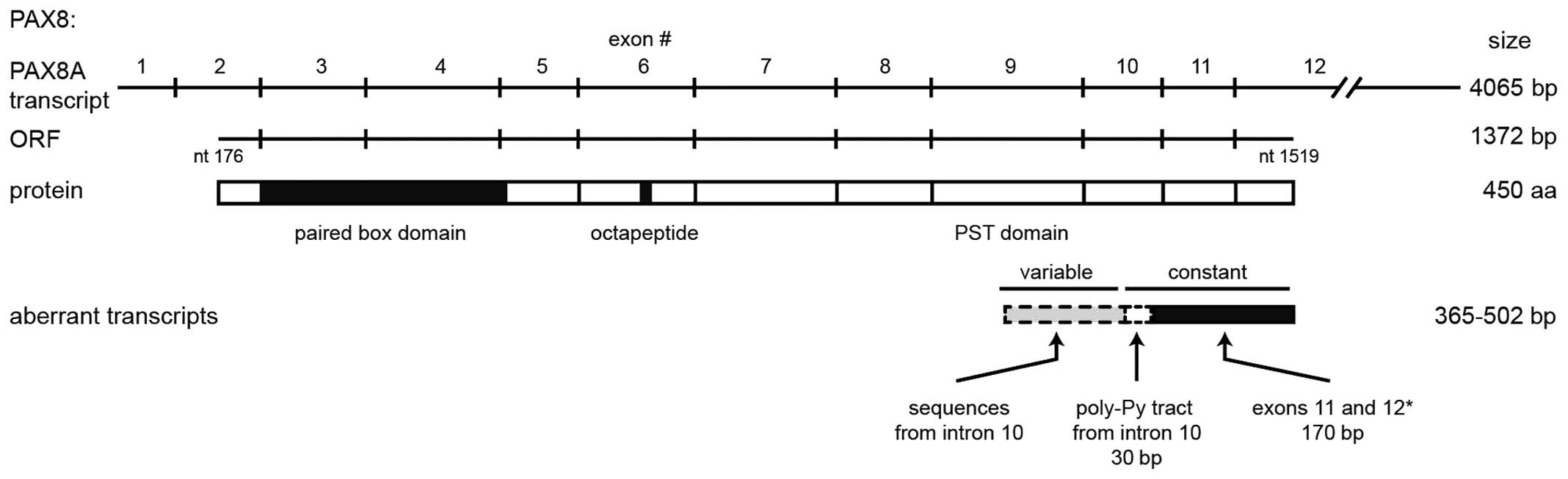
of cloned transcripts we found that they only shared the 3′-most
170 nt with the PAX8 coding sequence. This region only represents
two (11 and 12) of the ten exons that constitute the PAX8 ORF,
encoded in exons 2–12 (see the representation in Fig. 6). Upstream from that region, the 33
clones share a 30-nt sequence from intron 10, which contains the
poly pyrimidine tract and is located 5 nt upstream of the py-AG
splicing acceptor site, adjacent to exon 11. The remaining 5′
region of the cloned transcripts was variable among them,
comprising sequences that align to other regions of exon 10 with
multiple single nucleotide mismatches. Performing a BLAST search
with our clones produced variable results pointing at contigs or
chromosome assemblies (data not shown). Taken together, the results
from both analyses suggested genomic instability in the PAX8 gene,
because the aberrant transcripts that we were able to isolate
contain sequences from different non-contiguous genomic locations.
Noteworthy, we did not find a definite sequence or pattern
corresponding to either tumor samples or cell lines, reaffirming
the idea that PAX8 produces similar aberrant transcripts in both
cervical tumors and derived cell lines.
Some of the aberrant transcripts contain open
reading frames, but none of them comprises its full length. If
translated, these ORFs should lead to short (12–22 kDa) proteins
sharing the carboxi-terminus and thus the PST transactivation
domain from PAX8 (Fig. 6).
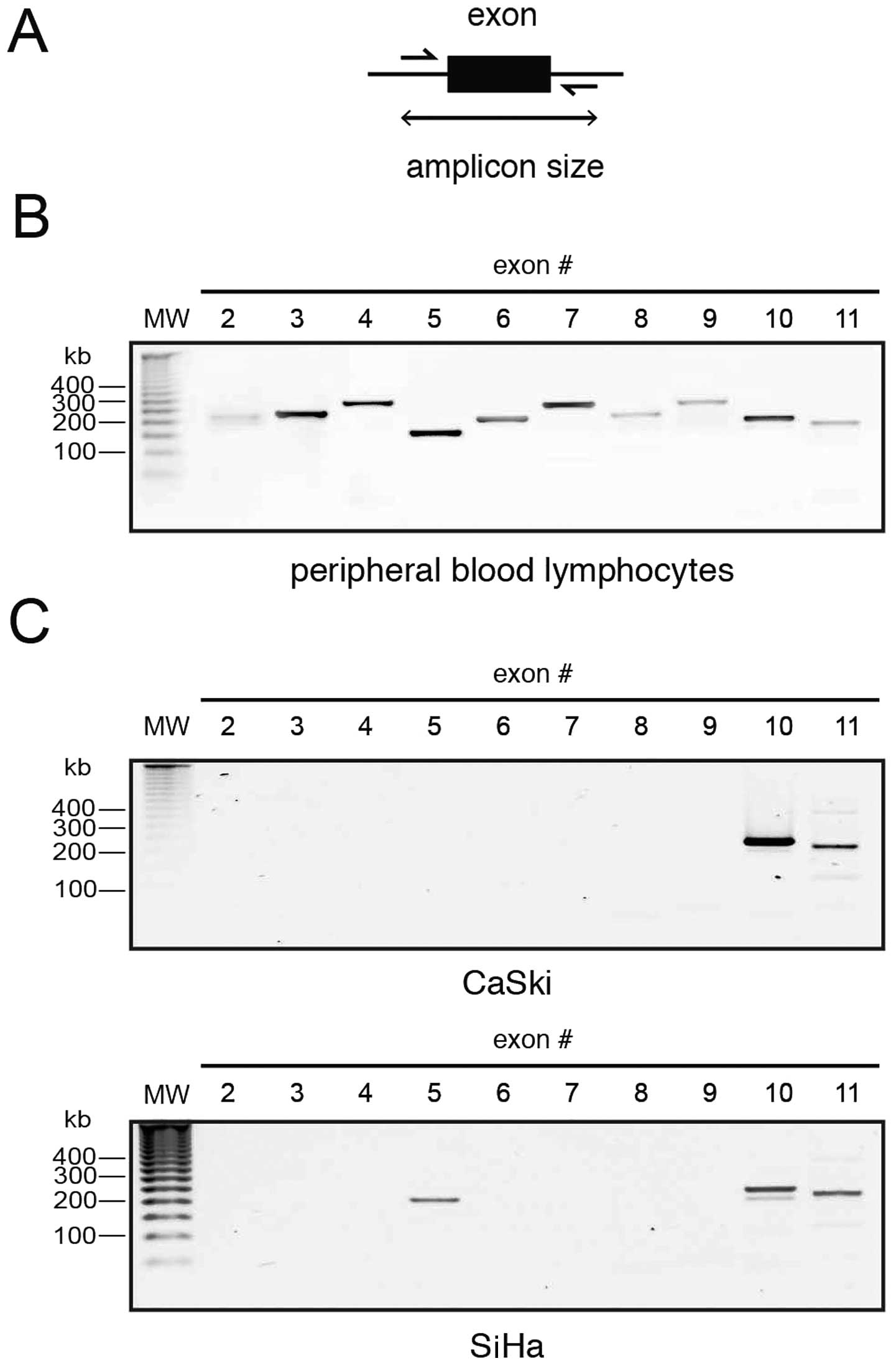
PAX8 exon amplification
Since all the detected transcripts conserved only
the 3′-most introns, we wanted to know whether the missing introns
were actually present in the genomic DNA of the cells. As an
initial approach to assess the integrity of the PAX8 gene, we
designed primers along the intronic regions flanking each exon
(Fig. 7A) with the aid of the
ExonPrimer software tool. We targeted only exons 2–11, since exon 1
is not translated in any reported PAX8 mRNA and exon 12, while
protein coding, is more than 2.5 kb long. A validation experiment
was performed using DNA isolated from peripheral blood lymphocytes
from a healthy patient, it yielded the expected amplicon sizes, as
seen in Fig. 7B.
The results using CaSki and SiHa cell DNA were very
interesting: amplicons corresponding to exons 2–9 failed to amplify
in both lines, save for exon 5 that was detected only in SiHa DNA
(Fig. 7C). Notably, only exons 10
and 11, those identified in the aberrant transcripts, were detected
through exon PCR. This suggests, together with the sequences
present in the cloned transcripts, that the PAX8 gene may be
altered in cervical carcinoma.
Discussion
In this study, we analyzed the expression levels of
two regions of the PAX8 mRNA in a set of tumoral and healthy
cervical tissue samples, and found an imbalance between their
expression levels. This imbalance can be attributed to the presence
of aberrant transcripts containing the 3′-most region of the mRNA.
Previous work on female genital tract tumors has shown a consistent
PAX8 expression in ovary tumors, such as non-mucinous carcinomas,
including serous, endometroid, clear cell and transitional cell
carcinomas (30,31). Only one investigation has reported
PAX8 expression in carcinomas of uterine cervix, but the results
are in the context of epithelial tumors (20).
We aimed to assess the possible differential
splicing of the PAX8 transcripts in cervical carcinoma-derived cell
lines and tumors. Upon performing these experiments, we found a
completely different scenario, rather than the expected protein
isoforms, we detected a single protein and several shorter putative
isoforms. Consequently, we aimed to detect the corresponding
shorter transcripts.
Notably, while we did detect a band of the expected
molecular weight by western blotting, the full-length ORF was only
amplified with a high sensitivity polymerase and was not detected
through 5′ RACE experiments (compare Figs. 3 and 4). This suggests a very low abundance of
this transcripts and, thus, high translation efficiency of this
mRNA. A high translation efficiency has been already suggested for
this transcript by Szczepanek-Parulska and co-workers (32), who amplified a 587/684 bp fragment
to detect PAX8A/F and hypothesized that the extra 97 bases at the
5′UTR participate in translational regulation.
The 5′ RACE experiments yielded a collection of
transcripts ranging from 378 to 542 (Fig. 4), out of the roughly 1300 bases of
the full-length PAX8 ORF. Shorter-than-expected transcripts from a
10-intron gene suggest alternative splicing, so it was puzzling
when the sequencing results only revealed the two 3′-most coding
exons from the ORF preceded by unknown and intronic sequences. The
fact that similar transcripts were obtained from different
independent sources strongly suggests that the aberrant transcripts
that we found are product of a recurring process, present in both
cervical carcinoma-derived cell lines and tumor samples.
The production of novel transcripts through
alterations of alternative splicing is a known trait of cancer
cells (29, reviewed in ref.
33) but only a few genes have
been reported to produce aberrant transcripts containing sequences
other than exons, such as FHIT and WWOX; this phenomenon has been
attributed to their proximity to the chromosome fragile sites FRA3B
and FRA16D, respectively (34,35).
PAX8, in turn, is located at the 2q13 locus (36,37),
close to the FRA2B fragile site (38,39),
which prompted us to try and assess the integrity of the PAX8
gene.
Rather than trying to establish the genomic status,
our exon amplification experiments show that it is a worthwhile
venture. These results suggest that the genomic sequence of the
PAX8 gene is at least partially disrupted, or that it harbors a
considerable amount of mutations that impede amplification of exons
2 to 10. Noteworthy, these results seem to correspond to the
sequences found in the aberrant transcripts, since only exons 10
and 11 were present in them, only these same exons were detectable
through Exon PCR. The PAX8 gene has previously proven to be
unstable in tumor cells, the (2;3)(q13;p25) translocation, detected
in both follicular thyroid carcinomas and adenomas (40), leads to the formation of a chimeric
PAX8-peroxisome proliferator-activated receptor (PPAR-γ) oncogene
(15). Moreover, cervical
carcinoma is prone to chromosome abnormalities as human
papillomavirus has been demonstrated to produce specific
chromosomal imbalances in transfected keratinocytes (41). In any case, we are conducting
further research to establish the nature of the suggested genomic
alterations in the PAX8 gene.
Taking the data together, we find it reasonable to
speculate that the cDNA aberrations that we found are the result of
genomic modifications characteristic of cervical carcinoma. Even
so, it is notable that these probable genomic modifications are
likely not present in every cell from our samples; otherwise we
would not have been able to detect the full-length PAX8 in total
protein extracts.
Here we report the presence of a number of aberrant
transcripts produced by the PAX8 gene cervical cancer tissues.
These transcripts are present in cervical carcinoma-derived cell
lines and tumors and likely encode shorter isoforms. Further
studies would shed light on the implications of these transcripts
in carcinogenesis and their role as cause and/or consequence of
other molecular phenomena.
Acknowledgements
E.L.U. is indebted to CONACyT México for a Retention
grant (RETENCION-191616). This work was partially supported by
CONACyT (SALUD-2009-01-113948 and SALUD-2014-1-233733). We thank
Jorge E. Campos for assistance in phylogenetic analysis.
References
|
1
|
Gruss P and Walther C: Pax in development.
Cell. 69:719–722. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goulding MD, Lumsden A and Gruss P:
Signals from the notochord and floor plate regulate the
region-specific expression of two Pax genes in the developing
spinal cord. Development. 117:1001–1016. 1993.PubMed/NCBI
|
|
3
|
Mansouri A, Hallonet M and Gruss P: Pax
genes and their roles in cell differentiation and development. Curr
Opin Cell Biol. 8:851–857. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mansouri A, Goudreau G and Gruss P: Pax
genes and their role in organogenesis. Cancer Res. 59(Suppl):
S1707–S1710. 1999.
|
|
5
|
Poleev A, Fickenscher H, Mundlos S,
Winterpacht A, Zabel B, Fidler A, Gruss P and Plachov D: PAX8, a
human paired box gene: Isolation and expression in developing
thyroid, kidney and Wilms' tumors. Development. 116:611–623.
1992.PubMed/NCBI
|
|
6
|
Kozmik Z, Kurzbauer R, Dörfler P and
Busslinger M: Alternative splicing of Pax-8 gene transcripts is
developmentally regulated and generates isoforms with different
transactivation properties. Mol Cell Biol. 13:6024–6035. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Szczepanek-Parulska E, Szaflarski W,
Piątek K, Budny B, Jaszczyńska-Nowinka K, Biczysko M, Wierzbicki T,
Skrobisz J, Zabel M and Ruchała M: Alternative 3′acceptor site in
the exon 2 of human PAX8 gene resulting in the expression of
unknown mRNA variant found in thyroid hemiagenesis and some types
of cancers. Acta Biochim Pol. 60:573–578. 2013.
|
|
8
|
Miccadei S, Provenzano C, Mojzisek M,
Natali PG and Civitareale D: Retinoblastoma protein acts as Pax 8
transcriptional coactivator. Oncogene. 24:6993–7001. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li CG, Nyman JE, Braithwaite AW and Eccles
MR: PAX8 promotes tumor cell growth by transcriptionally regulating
E2F1 and stabilizing RB protein. Oncogene. 30:4824–4834. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y-J, Campbell HG, Wiles AK, Eccles
MR, Reddel RR, Braithwaite AW and Royds JA: PAX8 regulates
telomerase reverse transcriptase and telomerase RNA component in
glioma. Cancer Res. 68:5724–5732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheung HW, Cowley GS, Weir BA, Boehm JS,
Rusin S, Scott JA, East A, Ali LD, Lizotte PH, Wong TC, et al:
Systematic investigation of genetic vulnerabilities across cancer
cell lines reveals lineage-specific dependencies in ovarian cancer.
Proc Natl Acad Sci USA. 108:12372–12377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marques AR, Espadinha C, Catarino AL,
Moniz S, Pereira T, Sobrinho LG and Leite V: Expression of
PAX8-PPAR gamma 1 rearrangements in both follicular thyroid
carcinomas and adenomas. J Clin Endocrinol Metab. 87:3947–3952.
2002.PubMed/NCBI
|
|
13
|
French CA, Alexander EK, Cibas ES, Nose V,
Laguette J, Faquin W, Garber J, Moore F Jr, Fletcher JA, Larsen PR,
et al: Genetic and biological subgroups of low-stage follicular
thyroid cancer. Am J Pathol. 162:1053–1060. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dwight T, Thoppe SR, Foukakis T, Lui WO,
Wallin G, Höög A, Frisk T, Larsson C and Zedenius J: Involvement of
the PAX8/peroxisome proliferator-activated receptor gamma
rearrangement in follicular thyroid tumors. J Clin Endocrinol
Metab. 88:4440–4445. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kroll TG, Sarraf P, Pecciarini L, Chen CJ,
Mueller E, Spiegelman BM and Fletcher JA: PAX8-PPARgamma1 fusion
oncogene in human thyroid carcinoma [corrected]. Science.
289:1357–1360. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Laury AR, Perets R, Piao H, Krane JF,
Barletta JA, French C, Chirieac LR, Lis R, Loda M, Hornick JL, et
al: A comprehensive analysis of PAX8 expression in human epithelial
tumors. Am J Surg Pathol. 35:816–826. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ordóñez NG: Value of PAX 8 immunostaining
in tumor diagnosis: A review and update. Adv Anat Pathol.
19:140–151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nonaka D, Tang Y, Chiriboga L, Rivera M
and Ghossein R: Diagnostic utility of thyroid transcription factors
Pax8 and TTF-2 (FoxE1) in thyroid epithelial neoplasms. Mod Pathol.
21:192–200. 2008.
|
|
19
|
Scouten WT, Patel A, Terrell R, Burch HB,
Bernet VJ, Tuttle RM and Francis GL: Cytoplasmic localization of
the paired box gene, Pax-8, is found in pediatric thyroid cancer
and may be associated with a greater risk of recurrence. Thyroid.
14:1037–1046. 2004. View Article : Google Scholar
|
|
20
|
Ozcan A, Shen SS, Hamilton C, Anjana K,
Coffey D, Krishnan B and Truong LD: PAX 8 expression in
non-neoplastic tissues, primary tumors, and metastatic tumors: A
comprehensive immuno histochemical study. Mod Pathol. 24:751–764.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tong G-X, Yu WM, Beaubier NT, Weeden EM,
Hamele-Bena D, Mansukhani MM and O'Toole KM: Expression of PAX8 in
normal and neoplastic renal tissues: An immunohistochemical study.
Mod Pathol. 22:1218–1227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Albadine R, Schultz L, Illei P, Ertoy D,
Hicks J, Sharma R, Epstein JI and Netto GJ: PAX8 (+)/p63 (−)
immunostaining pattern in renal collecting duct carcinoma (CDC): A
useful immunoprofile in the differential diagnosis of CDC versus
urothelial carcinoma of upper urinary tract. Am J Surg Pathol.
34:965–969. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mhawech-Fauceglia P, Wang D, Samrao D,
Godoy H, Pejovic T, Liu S and Lele S: Pair-Box (PAX8)
protein-positive expression is associated with poor disease outcome
in women with endometrial cancer. Br J Cancer. 107:370–374. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Wang Y, Li J, Yuan Z, Yuan B,
Zhang T, Cragun JM, Kong B and Zheng W: PAX8: A sensitive and
specific marker to identify cancer cells of ovarian origin for
patients prior to neoadjuvant chemotherapy. J Hematol Oncol.
6:602013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tacha D, Zhou D and Cheng L: Expression of
PAX8 in normal and neoplastic tissues: A comprehensive
immunohistochemical study. Appl Immunohistochem Mol Morphol.
19:293–299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kenny SL, McBride HA, Jamison J and
McCluggage WG: Mesonephric adenocarcinomas of the uterine cervix
and corpus: HPV-negative neoplasms that are commonly PAX8, CA125,
and HMGA2 positive and that may be immunoreactive with TTF1 and
hepatocyte nuclear factor 1-β. Am J Surg Pathol. 36:799–807. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shukla A, Thomas D and Roh MH: PAX8 and
PAX2 expression in endocervical adenocarcinoma in situ and
high-grade squamous dysplasia. Int J Gynecol Pathol. 32:116–121.
2013. View Article : Google Scholar
|
|
28
|
Ruiz-Llorente S, Carrillo Santa de Pau E,
Sastre-Perona A, Montero-Conde C, Gómez-López G, Fagin JA, Valencia
A, Pisano DG and Santisteban P: Genome-wide analysis of Pax8
binding provides new insights into thyroid functions. BMC Genomics.
13:1472012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
David CJ and Manley JL: Alternative
pre-mRNA splicing regulation in cancer: Pathways and programs
unhinged. Genes Dev. 24:2343–2364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nonaka D, Chiriboga L and Soslow RA:
Expression of pax8 as a useful marker in distinguishing ovarian
carcinomas from mammary carcinomas. Am J Surg Pathol. 32:1566–1571.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao L, Guo M, Sneige N and Gong Y: Value
of PAX8 and WT1 immunostaining in confirming the ovarian origin of
metastatic carcinoma in serous effusion specimens. Am J Clin
Pathol. 137:304–309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Szczepanek-Parulska E, Szaflarski W,
Piątek K, Budny B, Jaszczyńska-Nowinka K, Biczysko M, Wierzbicki T,
Skrobisz J, Zabel M and Ruchała M: Alternative 3′ acceptor site in
the exon 2 of human PAX8 gene resulting in the expression of
unknown mRNA variant found in thyroid hemiagenesis and some types
of cancers. Acta Biochim Pol. 60:573–578. 2013.
|
|
33
|
Kalnina Z, Zayakin P, Silina K and Linē A:
Alterations of pre-mRNA splicing in cancer. Genes Chromosomes
Cancer. 42:342–357. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ohta M, Inoue H, Cotticelli MG, Kastury K,
Baffa R, Palazzo J, Siprashvili Z, Mori M, McCue P, Druck T, et al:
The FHIT gene, spanning the chromosome 3p14.2 fragile site and
renal carcinoma-associated t(3;8) breakpoint, is abnormal in
digestive tract cancers. Cell. 84:587–597. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bednarek AK, Keck-Waggoner CL, Daniel RL,
Laflin KJ, Bergsagel PL, Kiguchi K, Brenner AJ and Aldaz CM: WWOX,
the FRA16D gene, behaves as a suppressor of tumor growth. Cancer
Res. 61:8068–8073. 2001.PubMed/NCBI
|
|
36
|
Stapleton P, Weith A, Urbánek P, Kozmik Z
and Busslinger M: Chromosomal localization of seven PAX genes and
cloning of a novel family member, PAX-9. Nat Genet. 3:292–298.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Karolchik D, Barber GP, Casper J, Clawson
H, Cline MS, Diekhans M, Dreszer TR, Fujita PA, Guruvadoo L,
Haeussler M, et al: The UCSC Genome Browser database: 2014 update.
Nucleic Acids Res. 42(D1): D764–D770. 2014. View Article : Google Scholar :
|
|
38
|
Hecht F, Ramesh KH and Lockwood DH: A
guide to fragile sites on human chromosomes. Cancer Genet
Cytogenet. 44:37–45. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
IJdo JW, Baldini A, Wells RA, Ward DC and
Reeders ST: FRA2B is distinct from inverted telomere repeat arrays
at 2q13. Genomics. 12:833–835. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cheung L, Messina M, Gill A, Clarkson A,
Learoyd D, Delbridge L, Wentworth J, Philips J, Clifton-Bligh R and
Robinson BG: Detection of the PAX8-PPARγ fusion oncogene in both
follicular thyroid carcinomas and adenomas. J Clin Endocrinol
Metab. 88:354–357. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Solinas-Toldo S, Dürst M and Lichter P:
Specific chromosomal imbalances in human papillomavirus-transfected
cells during progression toward immortality. Proc Natl Acad Sci
USA. 94:3854–3859. 1997. View Article : Google Scholar : PubMed/NCBI
|