Introduction
Hepatocellular carcinoma (HCC) is the third leading
cause of cancer-related deaths worldwide (1). Many treatment options are available
for patients with HCC, including surgical resection, local ablation
therapy, chemoembolization, liver transplantation and molecular
target therapy. Nevertheless, the prognosis of HCC remains poor,
due to intrahepatic spread, postsurgical recurrence and
chemoresistance (2–4).
Cancer stem cells (CSCs) have emerged as a potential
cause of many malignant properties of tumors, including
tumorigenicity, chemoradiation resistance, metastasis and tumor
recurrence (5). CSCs are believed
to share unique characteristics with normal stem cells; for
example, they have the ability to self-renew and produce
differentiated cells. Subsequent to the identification of CSCs in
leukemia (6), CSCs have been
reported in various solid tumors, including breast cancer, melanoma
and colon cancer (7–9). In studies on HCC, the side population
(SP) fraction, CD133, CD90, CD44 and epithelial cell adhesion
molecules, were identified as CSC-specific markers (10–13).
Many CSC markers have been shown to be associated with disease
progression and outcome; however, no molecular target therapy for
these markers has been developed.
CD13/Aminopeptidase N (APN) is a zinc-binding, type
2 transmembrane ectopeptidase (150 kDa), which is expressed on
various cell types, such as kidney, intestinal epithelium, liver,
placenta and lung cells (14,15).
CD13 was first described as a marker for hematopoietic cells of
myeloid origin. Recent studies have indicated that CD13 has various
functions, including roles in inflammatory and immunological
responses, signal transduction, antigen processing, neuropeptide
and cytokine degradation, angiogenesis and extracellular matrix
degradation (16,17). However, high CD13 expression levels
have been detected in various solid tumors; additionally, CD13 was
reported to be correlated with malignant behavior in colon,
prostate and non-small cell lung cancers (18–20).
We previously reported that CD13 might be both a
marker of CSCs and a candidate therapeutic target in HCC (21). We showed that CD13-positive cells
exhibited strong chemoradiation resistance in vitro and
in vivo. Moreover, CD13 expression protected cells from DNA
damage by regulating the levels of reactive oxygen species (ROS).
Inhibition of CD13 induced tumor cell apoptosis and resulted in
tumor disruption, via blocking the ability of dormant CSCs to
self-renew and re-initiate a tumor.
Ubenimex is a CD13/APN inhibitor. Ubenimex has been
used as an adjuvant chemotherapy drug because of its function as an
immunoenhancer in oncotherapy and reported to prolong the survival
of patients with acute adult non-lymphocytic leukemia.
This drug was found to be cytotoxic to various tumor
cell lines (22–24). We previously reported that
combining ubenimex with 5-FU treatment, which is a ROS-inducing
chemotherapy, improved liver cancer treatment (21).
In the systemic treatment of HCC, only sorafenib has
been shown to provide overall survival benefit in a phase 3
randomized control trial (25).
However, as a locoregional chemotherapy, some conventional
cytotoxic agents were used in clinical practice. Transcatheter
arterial chemoembolization (TACE) is the golden standard for the
treatment of intermediate-stage HCC, and involves the
administration of chemotherapeutic drugs, such as cisplatin or
doxorubicin with or without lipiodol (26). In addition, we previously reported
the efficacy of 5-fluorouracil arterial infusion + interferon
therapy (FAIT) for advanced HCC (27). Thus, we thought that these
conventional cytotoxic agents were worth trying to evaluate the
effects of combination therapy with ubenimex.
In the present study, we explored the effects of
ubenimex in combination with various anticancer drugs, which were
used in the treatment of HCC, and we elucidated the mechanism
underlying the effects of these combinations.
Materials and methods
Cell culture
Two human liver cancer cell lines, HuH-7 and
PLC/PRF/5, were obtained from the Japan Cancer Research Resources
Bank (Tokyo, Japan). These cells were cultured and maintained in
Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum (FBS) and 500 μg/ml penicillin-streptomycin. Cells
were incubated at 37°C in a humidified atmosphere containing 5%
CO2 in air.
Cells were treated with 5-fluorouracil (5-FU),
cisplatin (CDDP), doxorubicin (DXR; Wako Pure Chemical Industries,
Osaka, Japan), sorafenib (SOR; BioVision, Tucson, AZ, USA), and
ubenimex (kindly supplied by Nihon Kayaku, Tokyo, Japan).
Flow cytometry for detecting expression
of CSC markers
To analyze CD13 expression, cells were incubated
with anticancer drugs with/without ubenimex (100 μg/ml) for 24, 48
and 72 h. At this concentration, ubenimex decreased the cell
viability to 78.4% in HuH7 and 81.2% in PLC/PRF/5 for 72 h. Next,
cells were resuspended at 106 cells/100 μl and incubated
for 60 min at room temperature with an anti-CD13 mouse monoclonal
antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After
incubation, the samples were washed twice with phosphate-buffered
saline (PBS) and resuspended in PBS containing 1% FBS. Labeled
samples were analyzed with flow cytometry on a FACSAria II (BD
Biosciences, San Jose, CA, USA). The cells were routinely sorted
twice and re-analyzed for purity.
To evaluate the change of other CSC marker after the
exposure to cytotoxic agents with/without ubenimex, an anti-CD44
mouse monoclonal antibody (Santa Cruz Biotechnology) was used as
described above.
Cell growth inhibition assay
Growth inhibition was tested with the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay (Sigma-Aldrich, St. Louis, MO, USA). Cells were cultured in
96-well culture plates with various concentrations of anticancer
drugs and ubenimex, alone or in combination. After 72-h
incubations, 10 μl (50 μg) of MTT was added to each well and
incubated for 4 h at 37°C. Next, the medium was removed, and 100 μl
of acid isopropanol was added to dissolve the resultant formazan
crystals. Plate absorbance was measured in a microplate reader at
570 nm, and absorbance at 650 nm was measured as reference. Results
were expressed as the percentage of absorbance relative to
untreated controls.
Assessment of combined drug effects with
isobologram analysis
Isobologram analyses were used to determine whether
the interactions between anticancer drugs and ubenimex were
additive, synergistic, or antagonistic (28,29).
Dose-dependent effects were determined for each compound; for
combinations, the dose of one compound was varied, and the dose of
the other compound was fixed. Data points on the isobologram were
evaluated according to their positions relative to the diagonal.
The lower left region indicated synergism, falling on the diagonal
line indicated additive effects, and the upper right region
indicated antagonism.
The combination index (CI) provided a means to
analyze the combined effects with a median-effect plot analysis.
The CI was calculated according to the following formula: CI =
(dA/D30A) + (dB/D30B), where D30A
is the concentration of drug A (ubenimex) required to produce 30%
of the effect, and dA is the concentration of drug A required to
produce 30% of the effect when combined with dB. Similarly,
D30B is the concentration of drug B (anticancer drug)
required to produce 30% of the effect, and dB is the concentration
of drug B required to produce 30% of the effect when combined with
dA. The CI values were defined as follows: <0.8 = synergism;
from 0.8 to 1.2 = additive effect; and >1.2 = antagonism.
Cell cycle analysis with propidium iodide
staining
For cell cycle analysis, cells were incubated with
each anticancer drug, alone or with ubenimex (100 μg/ml) for 48 h,
then fixed in 70% ethanol on ice. After centrifugation, cells were
stained with 50 mg/ml propidium iodide (PI) solution (Dojindo
Molecular Technologies, Kumamoto, Japan) and 0.1 mg/ml RNase A
(Invitrogen). Stained cells were analyzed with flow cytometry on a
FACSAria II. Each histogram was constructed with data from at least
20,000 events. Flow cytometric analyses were performed with the
FlowJo software (Digital Biology, Tokyo, Japan).
Apoptosis analysis with Annexin V
staining
The Annexin V-FITC apoptosis detection kit (BD
Biosciences) was used to detect cells undergoing apoptosis after
anticancer treatment, according to the manufacturer's protocol.
Firstly, cells were incubated for 48 h with each
anticancer drug or ubenimex (100 μg/ml) alone or in combination.
Cell suspension (100 μl) was mixed with 5 μl of Annexin V-FITC and
2.5 μl of PI and incubated for 30 min at room temperature in the
dark. The samples were analyzed with flow cytometry on a FACSAria
II (BD Biosciences). Cells stained with Annexin V were considered
apoptotic cells.
Measurement of intracellular ROS
levels
CellROX Deep Red reagent (Invitrogen, Gent, Belgium)
is a fluorogenic probe for measuring intracellular oxidative stress
in both live and fixed cells. The cell-permeant dye is
nonfluorescent while in a reduced state and exhibits fluorescence
upon oxidation by reactive oxygen species. After 48-h incubations
with each anticancer drug, with/without ubenimex (100 μg/ml), cells
were stained with 2 μM CellROX Deep Red reagent by adding the probe
to the complete medium and incubating the cells at 37°C for 30 min
in the dark. Samples were analyzed with flow cytometry on a
FACSAria II. The flow cytometric analysis was performed with FlowJo
software.
Statistical analysis
Data are expressed as means ± SD. The unpaired
Student's t-test was used to examine differences between groups in
cell proliferation, apoptosis, and cell cycle status. A P-value
<0.05 was taken as statistically significant. Statistical
analyses were performed with JMP Pro software, version 11.0 (SAS,
Institute, Inc., Cary, NC, USA).
Results
The expression of CD13 increases with
anticancer drugs and decreases with ubenimex
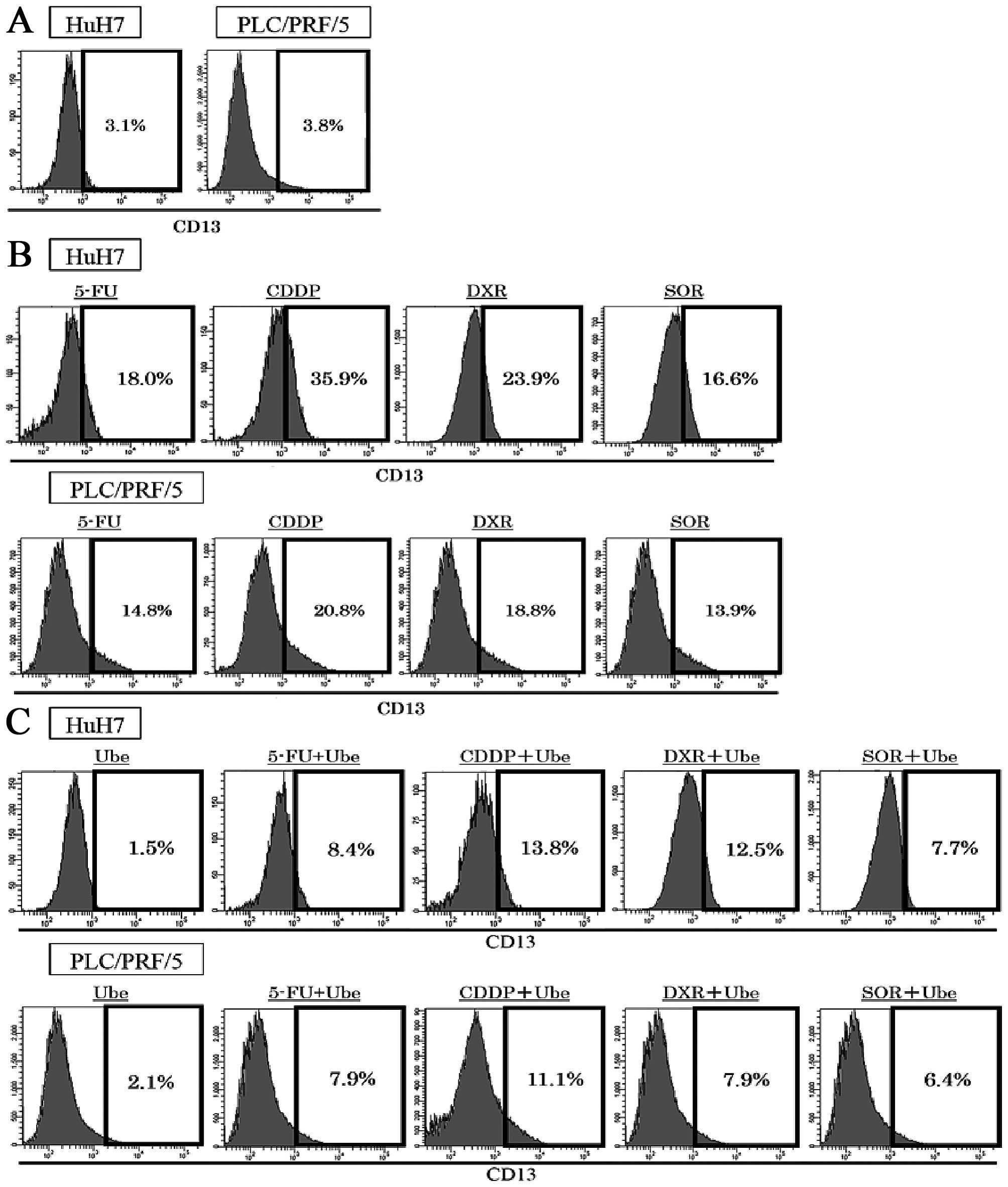
The expression of CD13 were assessed with FACS
analyses in two human HCC cell lines. The expression of CD13 was
3.5±0.5% in HuH7 and 3.9±0.4% in PLC/PRF/5 (Fig. 1A). Exposure to ubenimex decreased
the expression of CD13; 1.5±0.3% in HuH7, 2.1%±0.4% in PLC/PRF/5.
After 72-h exposure to each anticancer drug alone, the expression
of CD13 restrictively increased; in HuH7, expression of CD13 was
18.0±1.3% by 5-FU treatment, 35.9±3.2% by CDDP treatment, 23.9±2.6%
by DXR treatment, and 16.6±1.9% by SOR treatment; in PLC/PRF/5,
14.8±1.3% by 5-FU treatment, 20.8±1.9% by CDDP treatment, 18.8±1.6%
by DXR treatment, and 13.9±1.4% by SOR treatment (P<0.05;
Fig. 1B). When ubenimex was
combined with each anticancer drug, the expression of CD13
increased less than that observed with the anticancer drugs alone,
in both cell lines; in HuH7, the expression of CD13 decreased to
8.4±1.1% by 5-FU treatment, 13.8±1.9% by CDDP treatment, 12.5±1.8%
by DXR treatment, and 7.7±1.0% by SOR treatment; in PLC/PRF/5,
7.9±0.8% by 5-FU treatment, 11.1±0.9% by CDDP treatment, 7.9±0.8%
by DXR treatment, and 6.4±0.5% by SOR treatment (P<0.05;
Fig. 1C).
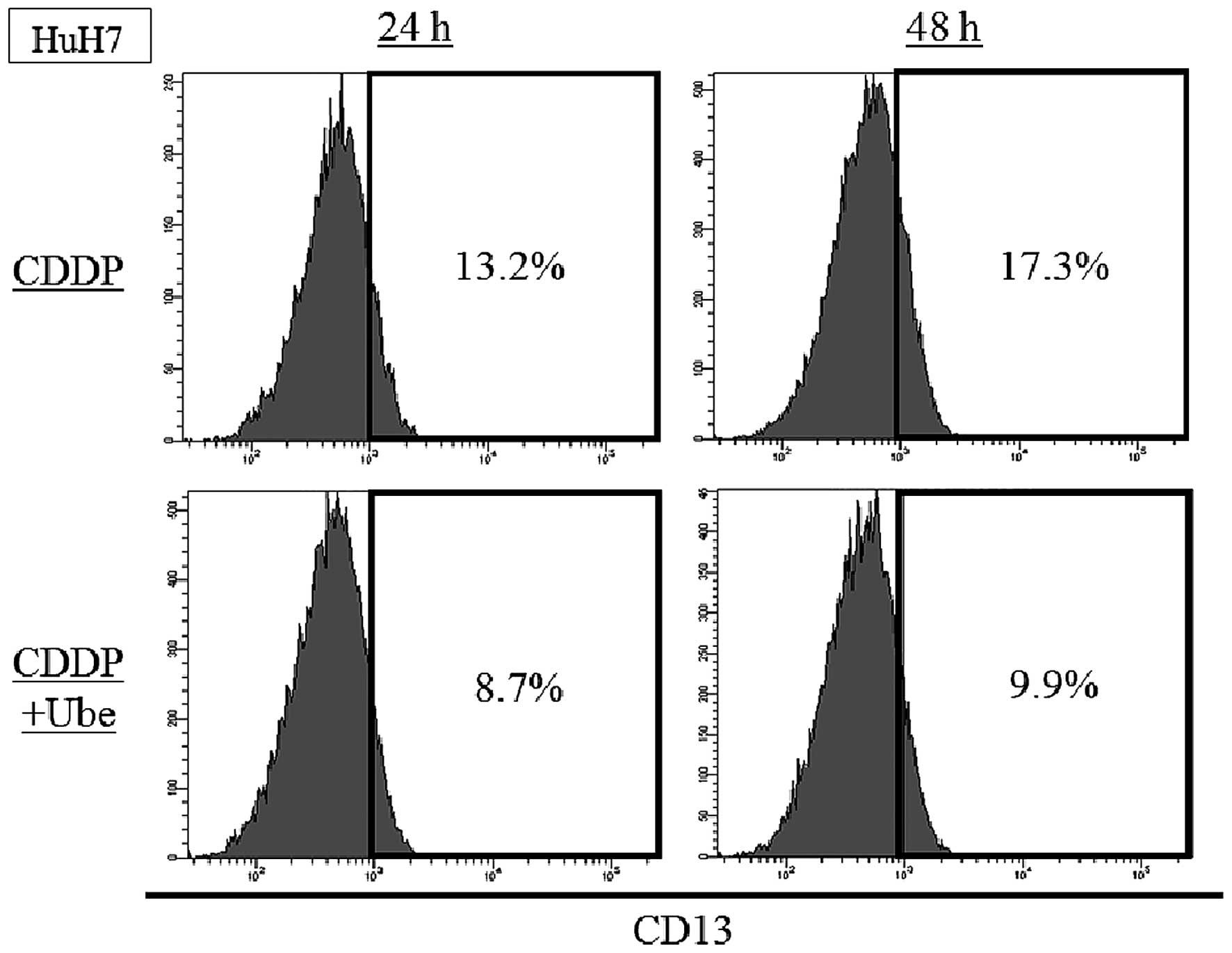
We evaluated the expression of CD13 at earlier
time-point. In HuH7, the expression of CD13 was 13.2±0.4% by 24-h
CDDP treatment and 17.3±1.1% by 48-h CDDP treatment. When ubenimex
was combined with CDDP, the expression of CD13 was decreased to
8.6±0.5% by 24-h treatment and 9.9±0.8% by 48-h treatment (Fig. 2).
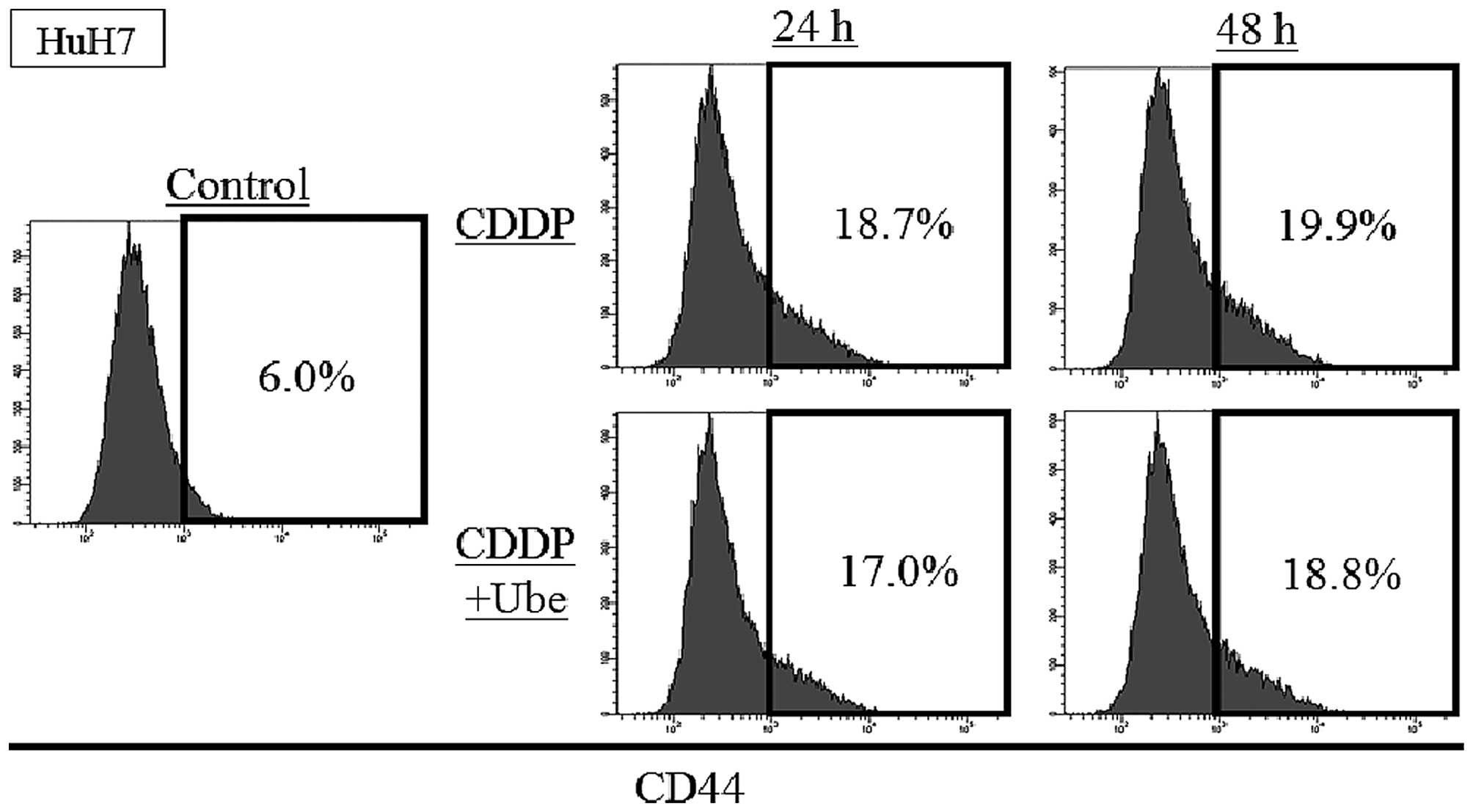
The expression of CD44 increases by
anticancer drugs and slightly decreases by ubenimex
To evaluate the change of other CSC markers after
the exposure to cytotoxic agents with/without ubenimex, the
expression of CD44 was assessed with FACS analysis in HuH7. The
expression of CD44 was 6.0±0.1%, and the increase after exposure to
CDDP; 18.7±0.5% at 24-h and 19.9±0.3% at 48-h. When ubenimex was
combined with CDDP, the expression of CD44 slightly decreased
relative to that observed with CDDP alone; 17.0±0.8% at 24-h and
18.8±0.3% at 48-h (P<0.05) (Fig.
3).
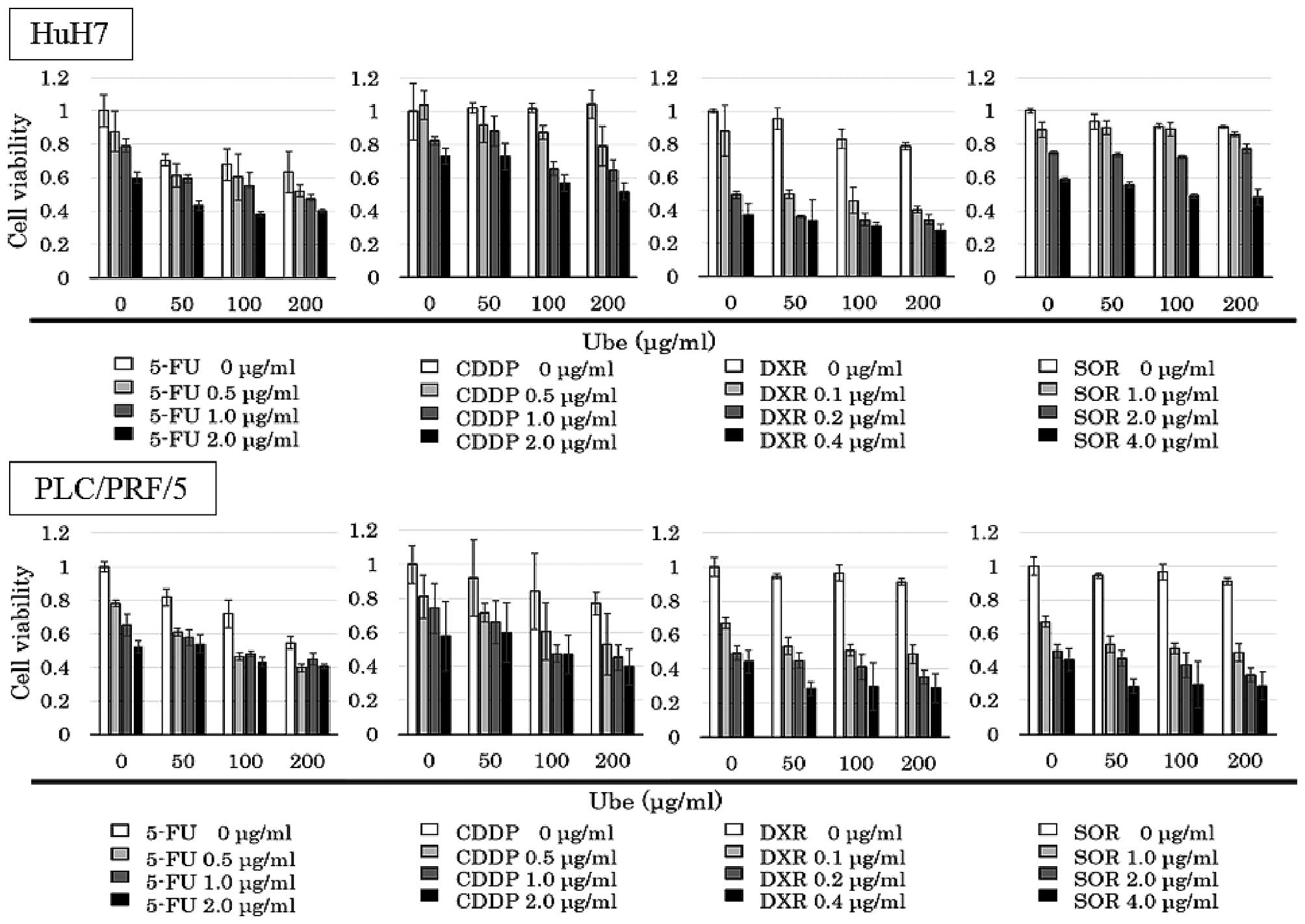
Interactions between ubenimex and
anticancer drugs
The IC30 values of ubenimex alone was
394.8 μg/ml for HuH7, and 498.8 μg/ml for PLC/PRF/5. The MTT assay
was performed with various concentrations of anticancer drugs
combined with different fixed concentrations of ubenimex (0, 50,
100 and 200 μg/ml; Fig. 4).
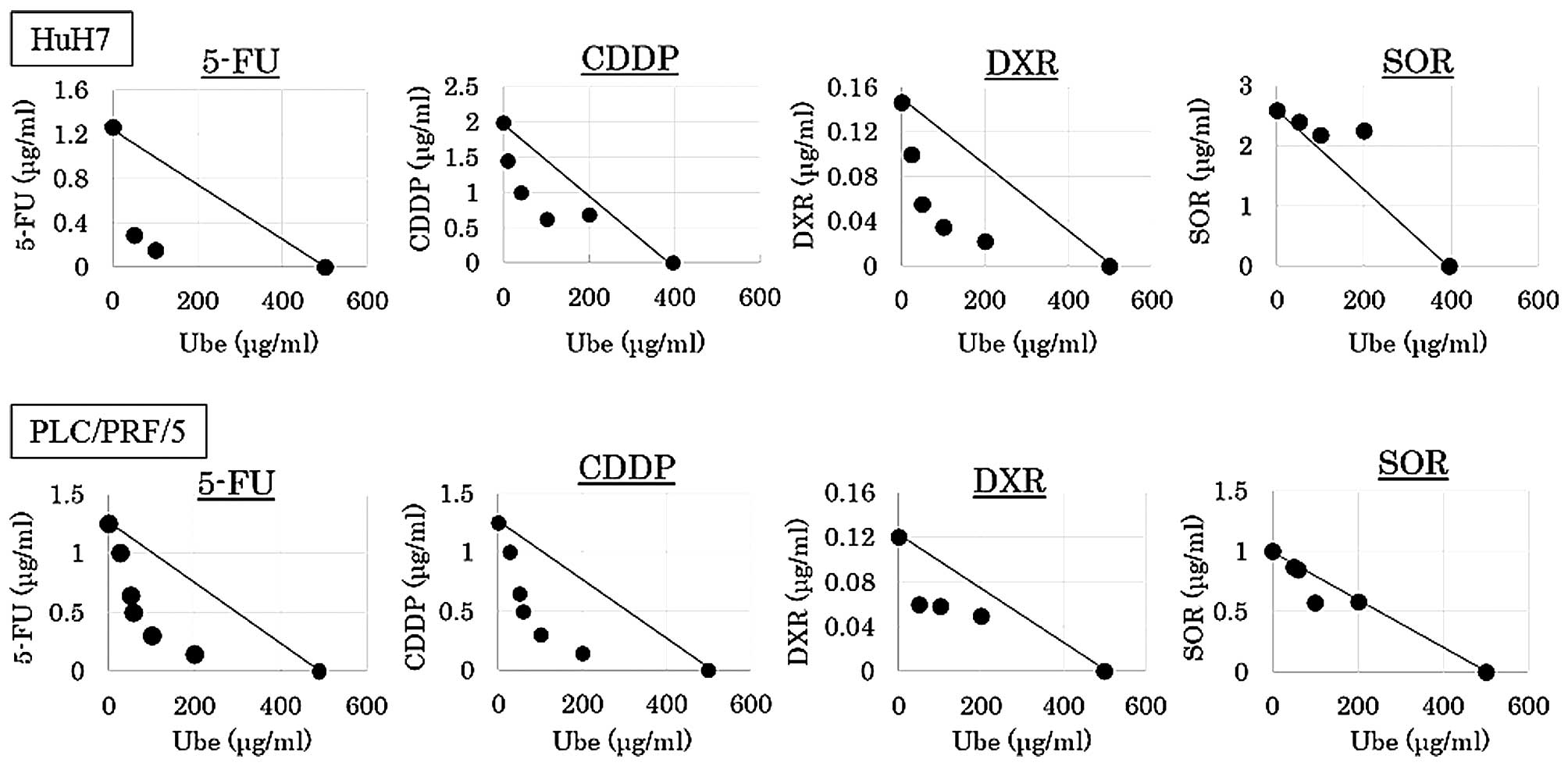
Isobologram analyses were performed, and CI values
were calculated. The data points fell in the lower left region of
the isobologram for combinations of ubenimex with 5-FU, CDDP and
DXR (Fig. 3); all these CI values
were <0.8 (Table I). The data
points fell approximately on the diagonal for combinations of
ubenimex with SOR, in both cell lines (Fig. 5); these CI values were between 0.8
and 1.2 in both cell lines (Table
I).
 | Table IThe combination index for ubenimex
combined with anticancer drugs. |
Table I
The combination index for ubenimex
combined with anticancer drugs.
| Cell line | 5-FU | CDDP | DXR | SOR |
|---|
| HuH7 | 0.49±0.36 | 0.59±0.13 | 0.68±0.13 | 1.17±0.18 |
| PLC/PRF/5 | 0.64±0.28 | 0.59±0.16 | 0.70±0.10 | 0.92±0.10 |
The results indicated that, when ubenimex was
combined with 5-FU, CDDP and DXR, the effect was synergistic,
respectively, and combined with SOR, the effect was additive.
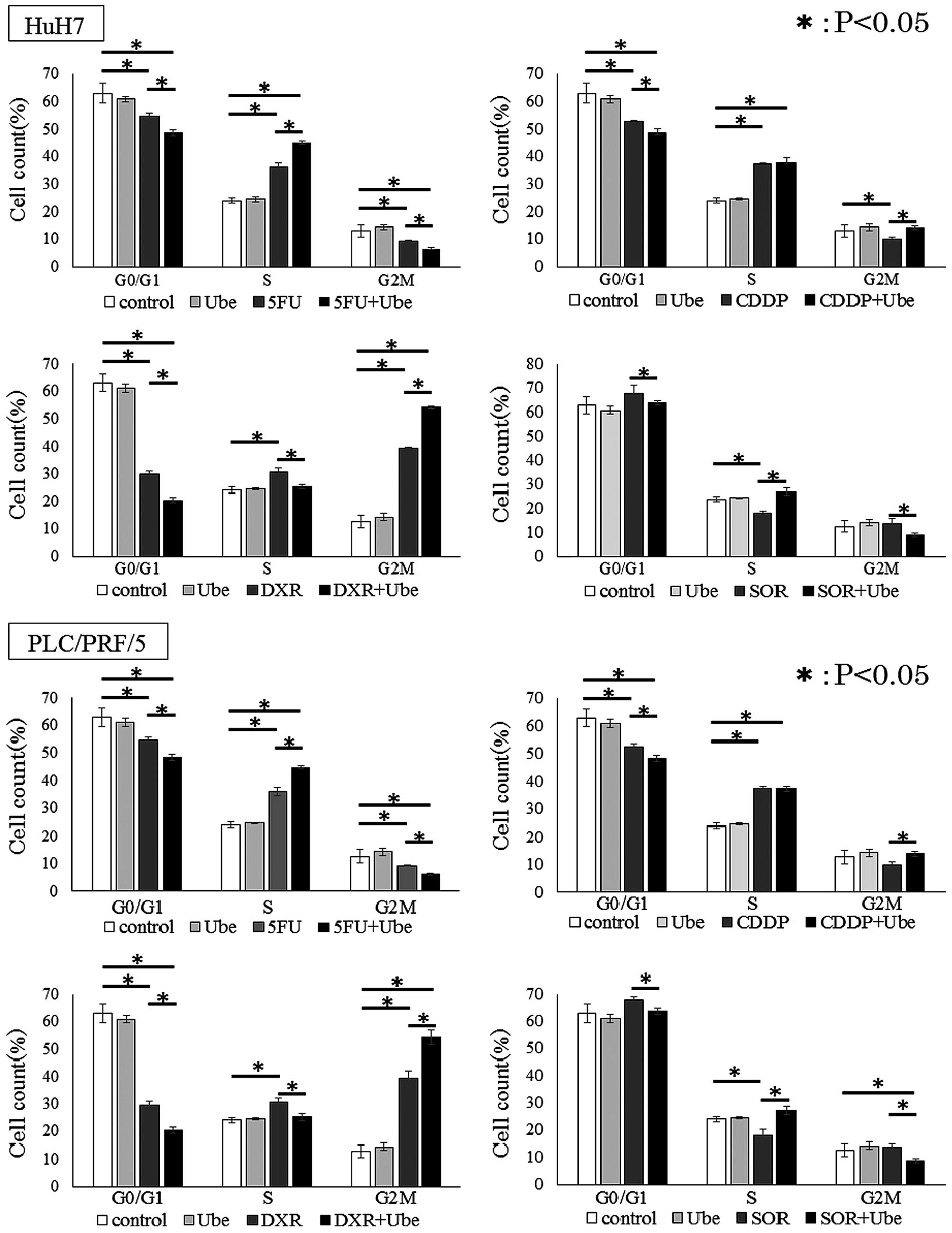
Ubenimex reduces the proportion of cells
in G0/G1 phase
We further explored the mechanism of synergistic
effects in combination with ubenimex and anticancer drugs by
examining the distribution of cells in different stages of the cell
cycle. In both cell lines, ubenimex alone did not affect the cell
cycle. Cell lines treated with 5-FU, CDDP and DXR showed
significantly smaller percentages of cells in the G0/G1 phase, and
a larger percentage of cells in the S/G2/M phase, compared to
control untreated conditions. In contrast, cells treated with SOR
showed a larger percentage of cells in the G0/G1 phase and a
smaller percentage of cells in the S/G2/M phases, compared to
control conditions (P<0.05 for each; Fig. 6). Moreover, in each cell line, when
5-FU, CDDP, DXR or SOR was combined with ubenimex, a significantly
smaller percentage of cells were in the G0/G1 phase and a larger
percentage of cells were in the S/G2/M phase compared to when cells
were exposed to the anticancer drug alone.
These results indicated that ubenimex reduced the
proportion of cells in G0/G1 phase.
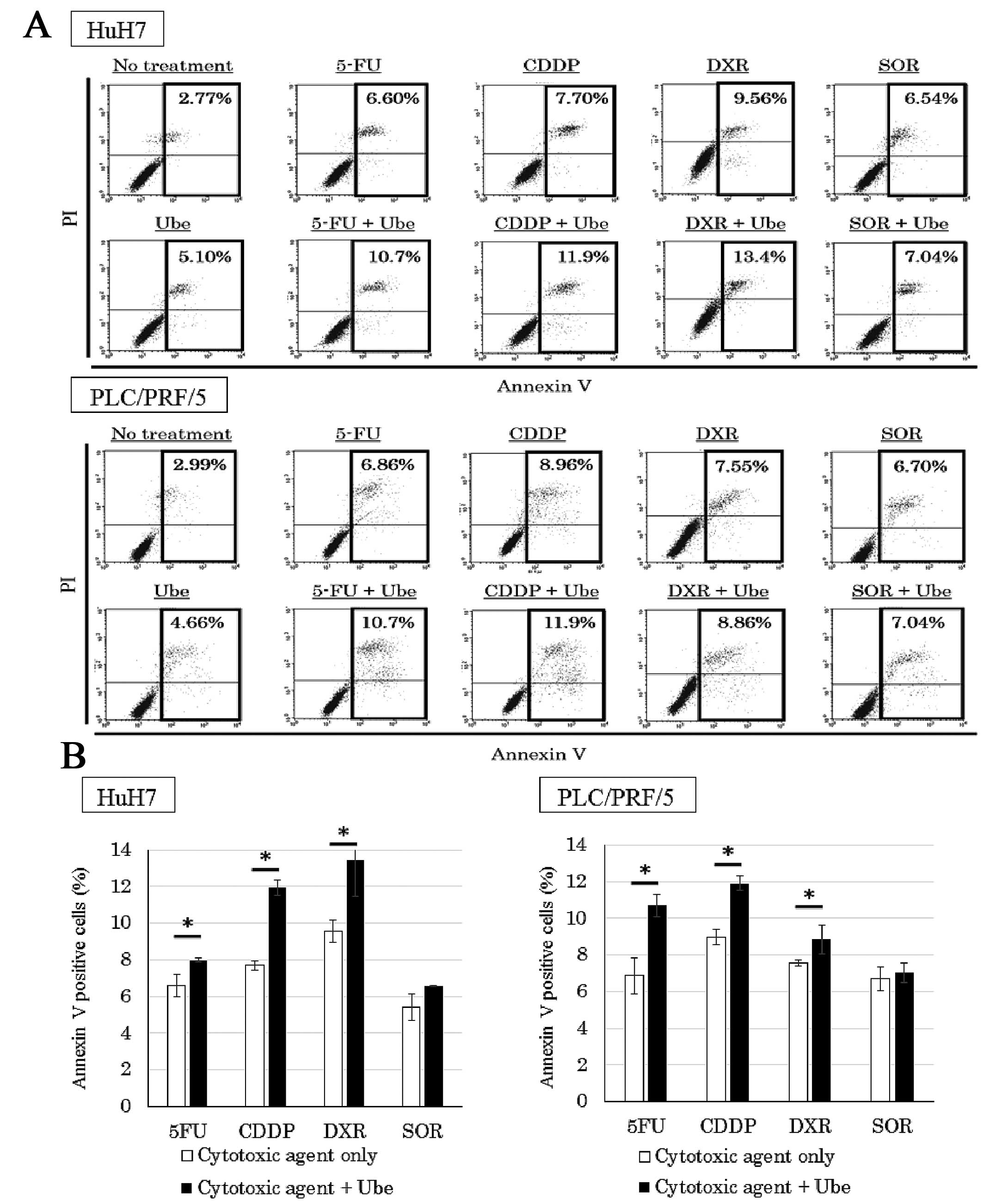
Ubenimex enhances apoptosis when combined
with 5-FU, CDDP and DXR
To quantify the percentage of cells undergoing
apoptosis, we performed the Annexin V assay (Fig. 7A). Cell lines treated with each
anticancer drug alone showed a larger percentage of Annexin
V-positive cells than that observed in control conditions.
Moreover, when 5-FU, CDDP and DXR were combined with ubenimex, a
higher percentage of Annexin V-positive cells was observed compared
to that observed with the anticancer drugs alone (P<0.05).
However, the percentage of Annexin V-positive cells in cell lines
treated with SOR alone was not significantly different from the
percentage observed when SOR was combined with ubenimex (Fig. 7B).
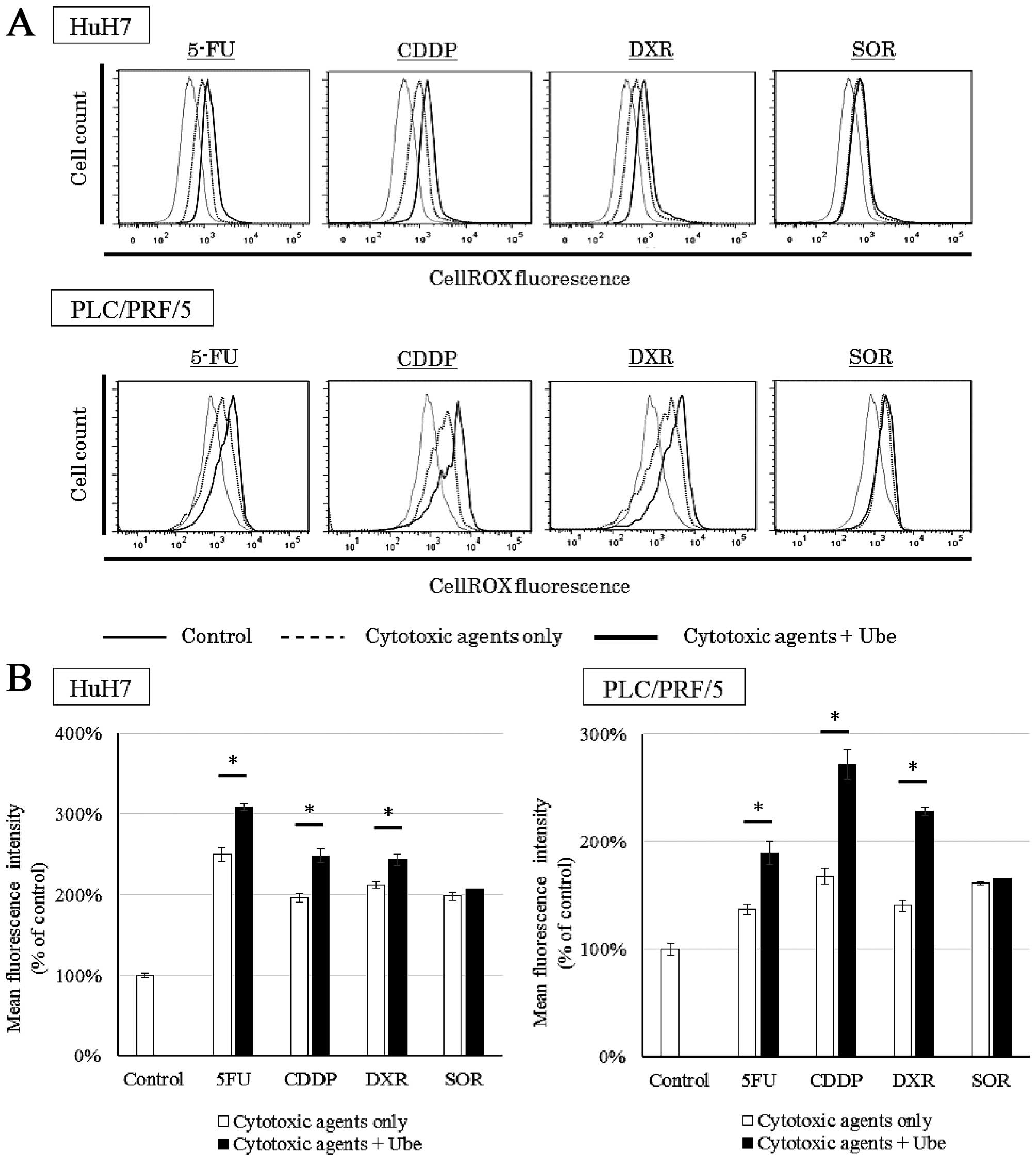
Ubenimex increases the intracellular ROS
when combined with 5-FU, CDDP and DXR
We evaluated the intracellular ROS levels when cells
were treated with each anticancer drug, with or without ubenimex
(Fig. 8A). Cell lines treated with
each anticancer drug alone demonstrated an increase in ROS compared
to control conditions. Additionally, in both cell lines, when 5-FU,
CDDP or DXR was combined with ubenimex, the intracellular ROS
increased above that observed with each drug alone (P<0.05).
However, when SOR was combined with ubenimex, the intracellular ROS
was not significantly different from that observed with SOR alone
(Fig. 8B).
Discussion
CD13 is a transmembrane ectopeptidase to degrade
peptides expressing on various organs and cell types. Previous
studies also indicated that CD13 played an important role in
controlling cancer cell growth and differentiation. Inhibition of
CD13 expression reduced proliferation in various types of cancer
cells (30,31). In addition, we previously
demonstrated that CD13 was a CSC marker in HCC and a therapeutic
target (21). In the present
investigation, we examined the effect of combining ubenimex with
conventional anticancer treatments, in vitro and in
vivo.
The expression of CD13 increased by treatment with
anticancer drug compared to untreated condition. From the fact that
the expression of CD13 increased in time-dependent manner, the
results suggested the increase of CD13 expression was the results
of selection by chemotherapy. However, the expression of CD13
increased by 24-h exposure of the anti-cancer drug, so these
results also indicate that anticancer drugs themselves upregulated
CD13 expression. Ubenimex, which inhibited CD13 activity, reduced
the number of CD13-positive cells by sensitizing cells to the
anticancer drugs.
To evaluate the influence of ubenimex to other CSC
markers, we examined the expression of CD44 after chemotherapy with
ubenimex. The expression of CD44 increased by anticancer treatment.
The combined therapy with ubenimex slightly decreased the
expression of CD44, and the result of reduction ratio was
statistically significant. The CSC marker positive cells included
cancer stem cells and CD13-positive cells and CD44-positive cells
could overlap each other partially. Ubenimex mainly reduces the
expression of CD13 and might reduce the population of the
overlapped CSC marker positive cells.
Isobologram analyses revealed that, when ubenimex
was combined with 5-FU, CDDP and DXR, the effects were synergistic,
and when combined with SOR, the effect was additive. To explore the
mechanism underlying the synergistic effects, we focused on the
cell cycle, apoptosis and intracellular ROS levels, because
previous studies suggested that CSCs were resistant to various
anticancer drugs, due to cell cycle dormancy, activated DNA-repair
mechanisms, and low intracellular ROS levels (32).
The cell cycle analyses revealed that both 5-FU and
CDDP alone could cause S-phase arrest or delay, and DXR alone
caused G2/M-phase arrest. These results were consistent with
previous reports (33–35). Ubenimex alone did not affect the
cell cycle. However, when combined with anticancer drugs, the
presence of ubenimex enhanced the cell cycle changes induced by the
anticancer drugs. In the presence of ubenimex, 5-FU caused a
greater increase in the proportion of cells in S phase, and DXR
caused more cells to accumulate in G2M phase. These results
suggested that ubenimex enhanced the effect of 5-FU on S-phase
arrest and the effect of DXR on G2/M arrest. However, ubenimex did
not enhance the effect of CDDP on S-phase accumulation. This result
might be explained by the fact that platinum compounds mainly act
on cancer cells by randomly damaging DNA, regardless of the cell
cycle phase. The cell cycle analyses also revealed that ubenimex
reduced the proportion of cells in G0/G1 phase after exposure to
anticancer drugs. Our previous report demonstrated that the largest
CD13-positive fraction was in the G0/G1 phase (21). These results indicated that
inhibition of CD13 with ubenimex abrogated CD13-positive cell
dormancy by inducing these cells to leave G0 and enter the cell
cycle; this induction may sensitize CSCs to the effects of
anticancer drugs.
We also showed that ubenimex alone induced
apoptosis; moreover, combinations with 5-FU, CDDP, or DXR enhanced
apoptosis compared to the effects of the anticancer drug alone.
Several previous studies demonstrated that ubenimex induced cancer
cell apoptosis by activating the pathway involving caspase-3,
mitogen-activated protein kinase (MAPK), phos-phatidylinositol
3-kinase (PI3K), and glycogen synthase kinase-3β (GSK-3β) (22,36,37).
However, the detailed mechanism of the induction has not been
elucidated. We reasoned that ubenimex may induce apoptosis through
its inhibition of CD13 activity; previously, CD13 was found to be
involved in ROS excretion, and elevated ROS levels induced DNA
oxidative damage and triggered apoptosis (38,39).
ROS are oxygen-derived free radicals, such as
hydroxyl radicals, peroxides and superoxides and are known to lead
to DNA lesions, protein oxidation and lipid peroxidation (40). Currently, chemotherapeutic agents
are used to cause DNA damage via ROS accumulation which leads to
apoptosis induction in different tumor cells (41). The detailed mechanism of apoptosis
via ROS formation remains to be clarified. For instance, some drugs
have induced apoptosis through oxidative stress along with
activation of the MAPK signaling pathways (42–44),
that play an important role in the regulation of many cellular
processes including cell growth and proliferation, differentiation,
and apoptosis and downregulation of PI3K/AKT signaling pathways,
that play a pivotal role in cell survival and the enhanced
protection of cancer cells from apoptosis (45–47).
In our evaluations of intracellular ROS, ubenimex
elevated intracellular ROS in combination with 5-FU, CDDP and DXR,
but not with SOR. These results suggested that ROS played a key
role in the synergistic effects of ubenimex on the mechanism of
apoptosis induction. Also, they supported our hypothesis that 5-FU,
CDDP and DXR induced DNA injury and increased ROS levels in
CD13-positive cells, and that some of the increased ROS was
excreted via CD13 (48–50). Therefore, inhibition of CD13 by
ubenimex increased intracellular ROS levels in CD13-positive cells
and induced apoptosis. Our findings suggested that only anticancer
drugs that directly caused DNA damage and cell cycle entry would
show synergistic effects when combined with ubenimex. Further
studies are needed to determine the molecular mechanism of
ubenimex.
Three of the tested cytotoxic agents showed
synergism with ubenimex. However, SOR showed only an additive
effect with ubenimex. SOR merely inhibits several tyrosine protein
kinases (VEGFR and PDGFR) and Raf kinase (51); therefore, this drug did not
directly induce DNA damage or cell cycle changes. Moreover, SOR was
reported to be useful, both for treating HCC and for killing CSCs
derived from HCC tumors (52).
Although we demonstrated that exposure to SOR increased cellular
CD13 expression, and that exposure to SOR combined with ubenimex
decreased CD13 expression, SOR might affect HCC-derived CSCs
through a different pathway. We previously demonstrated the effects
of the combination therapy of 5-FU with ubenimex in vivo
(21). Our results suggested that
ubenimex may enhance the effect of transarterial chemoembolization
using cisplatin or doxorubicin.
In conclusion, the present study demonstrated that
combinations of 5-FU, CDDP and DXR with ubenimex synergistically
enhanced their antitumor effects on cell cycle regulation and
apoptosis induction, by increasing intracellular ROS levels in HCC
cell lines. In clinical studies, ubenimex was shown to have
beneficial effects in treatments for several types of malignancy,
including leukemia, non-small cell lung and gastric cancer
(53–55). Furthermore, our results provided
novel insight into a chemotherapeutic strategy for HCC by adding
ubenimex to chemotherapies currently in use.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tomimaru Y, Wada H, Eguchi H, Tomokuni A,
Hama N, Kawamoto K, Marubashi S, Umeshita K, Doki Y, Mori M, et al:
Clinical significance of surgical resection of metastatic lymph
nodes from hepatocellular carcinoma. Surg Today. 45:1112–1120.
2015. View Article : Google Scholar
|
|
4
|
Kobayashi T, Ishiyama K and Ohdan H:
Prevention of recurrence after curative treatment for
hepatocellular carcinoma. Surg Today. 43:1347–1354. 2013.
View Article : Google Scholar
|
|
5
|
Valent P, Bonnet D, De Maria R, Lapidot T,
Copland M, Melo JV, Chomienne C, Ishikawa F, Schuringa JJ, Stassi
G, et al: Cancer stem cell definitions and terminology: The devil
is in the details. Nat Rev Cancer. 12:767–775. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang D, Nguyen TK, Leishear K, Finko R,
Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE and Herlyn M: A
tumorigenic subpopulation with stem cell properties in melanomas.
Cancer Res. 65:9328–9337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar
|
|
10
|
Ma S, Lee TK, Zheng BJ, Chan KW and Guan
XY: CD133+ HCC cancer stem cells confer chemoresistance
by preferential expression of the Akt/PKB survival pathway.
Oncogene. 27:1749–1758. 2008. View Article : Google Scholar
|
|
11
|
Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai
P, Chu PW, Lam CT, Poon RT and Fan ST: Significance of
CD90+ cancer stem cells in human liver cancer. Cancer
Cell. 13:153–166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamashita T, Ji J, Budhu A, Forgues M,
Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, et al:
EpCAM-positive hepatocellular carcinoma cells are tumor-initiating
cells with stem/progenitor cell features. Gastroenterology.
136:1012–1024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J and
Li J: Cancer stem/progenitor cells are highly enriched in
CD133+CD44+ population in hepatocellular
carcinoma. Int J Cancer. 126:2067–2078. 2010. View Article : Google Scholar
|
|
14
|
Stange T, Kettmann U and Holzhausen HJ:
Immunoelectron microscopic single and double labelling of
aminopeptidase N (CD 13) and dipeptidyl peptidase IV (CD 26). Acta
Histochem. 98:323–331. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dixon J, Kaklamanis L, Turley H, Hickson
ID, Leek RD, Harris AL and Gatter KC: Expression of
aminopeptidase-n (CD 13) in normal tissues and malignant neoplasms
of epithelial and lymphoid origin. J Clin Pathol. 47:43–47. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bhagwat SV, Lahdenranta J, Giordano R,
Arap W, Pasqualini R and Shapiro LH: CD13/APN is activated by
angiogenic signals and is essential for capillary tube formation.
Blood. 97:652–659. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kehlen A, Egbert I, Thiele K, Fischer K,
Riemann D and Langner J: Increased expression of interleukin-8 and
amino-peptidase N by cell-cell contact: Interleukin-8 is resistant
to degradation by aminopeptidase N/CD13. Eur Cytokine Netw.
12:316–324. 2001.PubMed/NCBI
|
|
18
|
Ishii K, Usui S, Sugimura Y, Yoshida S,
Hioki T, Tatematsu M, Yamamoto H and Hirano K: Aminopeptidase N
regulated by zinc in human prostate participates in tumor cell
invasion. Int J Cancer. 92:49–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hashida H, Takabayashi A, Kanai M, Adachi
M, Kondo K, Kohno N, Yamaoka Y and Miyake M: Aminopeptidase N is
involved in cell motility and angiogenesis: Its clinical
significance in human colon cancer. Gastroenterology. 122:376–386.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tokuhara T, Hattori N, Ishida H, Hirai T,
Higashiyama M, Kodama K and Miyake M: Clinical significance of
aminopeptidase N in non-small cell lung cancer. Clin Cancer Res.
12:3971–3978. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haraguchi N, Ishii H, Mimori K, Tanaka F,
Ohkuma M, Kim HM, Akita H, Takiuchi D, Hatano H, Nagano H, et al:
CD13 is a therapeutic target in human liver cancer stem cells. J
Clin Invest. 120:3326–3339. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sekine K, Fujii H and Abe F: Induction of
apoptosis by bestatin (ubenimex) in human leukemic cell lines.
Leukemia. 13:729–734. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ezawa K, Minato K and Dobashi K: Induction
of apoptosis by ubenimex (Bestatin) in human non-small-cell lung
cancer cell lines. Biomed Pharmacother. 50:283–289. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Terauchi M, Kajiyama H, Shibata K, Ino K,
Nawa A, Mizutani S and Kikkawa F: Inhibition of APN/CD13 leads to
suppressed progressive potential in ovarian carcinoma cells. BMC
Cancer. 7:1402007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al; SHARP Investigators Study Group. Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lencioni R, Petruzzi P and Crocetti L:
Chemoembolization of hepatocellular carcinoma. Semin Intervent
Radiol. 30:3–11. 2013. View Article : Google Scholar :
|
|
27
|
Nagano H, Wada H, Kobayashi S, Marubashi
S, Eguchi H, Tanemura M, Tomimaru Y, Osuga K, Umeshita K, Doki Y,
et al: Long-term outcome of combined interferon-α and
5-fluorouracil treatment for advanced hepatocellular carcinoma with
major portal vein thrombosis. Oncology. 80:63–69. 2011. View Article : Google Scholar
|
|
28
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bijnsdorp IV, Giovannetti E and Peters GJ:
Analysis of drug interactions. Methods Mol Biol. 731:421–434. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wickström M, Larsson R, Nygren P, Gullbo J
and Aminopeptidase N: Aminopeptidase N (CD13) as a target for
cancer chemotherapy. Cancer Sci. 102:501–508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hitzerd SM, Verbrugge SE, Ossenkoppele G,
Jansen G and Peters GJ: Positioning of aminopeptidase inhibitors in
next generation cancer therapy. Amino Acids. 46:793–808. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Naka K, Muraguchi T, Hoshii T and Hirao A:
Regulation of reactive oxygen species and genomic stability in
hematopoietic stem cells. Antioxid Redox Signal. 10:1883–1894.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maeda S, Wada H, Naito Y, Nagano H,
Simmons S, Kagawa Y, Naito A, Kikuta J, Ishii T, Tomimaru Y, et al:
Interferon-α acts on the S/G2/M phases to induce apoptosis in the
G1 phase of an IFNAR2-expressing hepatocellular carcinoma cell
line. J Biol Chem. 289:23786–23795. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qin LF and Ng IO: Induction of apoptosis
by cisplatin and its effect on cell cycle-related proteins and cell
cycle changes in hepatoma cells. Cancer Lett. 175:27–38. 2002.
View Article : Google Scholar
|
|
35
|
Fan C, Zheng W, Fu X, Li X, Wong YS and
Chen T: Strategy to enhance the therapeutic effect of doxorubicin
in human hepatocellular carcinoma by selenocystine, a synergistic
agent that regulates the ROS-mediated signaling. Oncotarget.
5:2853–2863. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sawafuji K, Miyakawa Y, Weisberg E,
Griffin JD, Ikeda Y and Kizaki M: Aminopeptidase inhibitors inhibit
proliferation and induce apoptosis of K562 and STI571-resistant
K562 cell lines through the MAPK and GSK-3beta pathways. Leuk
Lymphoma. 44:1987–1996. 2003. View Article : Google Scholar
|
|
37
|
Liang W, Gao B, Xu G, Weng D, Xie M and
Qian Y: Possible contribution of aminopeptidase N (APN/CD13) to
migration and invasion of human osteosarcoma cell lines. Int J
Oncol. 45:2475–2485. 2014.PubMed/NCBI
|
|
38
|
Prasad V, Chandele A, Jagtap JC, Sudheer
Kumar P and Shastry P: ROS-triggered caspase 2 activation and
feedback amplification loop in beta-carotene-induced apoptosis.
Free Radic Biol Med. 41:431–442. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Madesh M, Zong WX, Hawkins BJ, Ramasamy S,
Venkatachalam T, Mukhopadhyay P, Doonan PJ, Irrinki KM, Rajesh M,
Pacher P, et al: Execution of superoxide-induced cell death by the
proapoptotic Bcl-2-related proteins Bid and Bak. Mol Cell Biol.
29:3099–3112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Balaban RS, Nemoto S and Finkel T:
Mitochondria, oxidants, and aging. Cell. 120:483–495. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chetram MA, Bethea DA, Odero-Marah VA,
Don-Salu-Hewage AS, Jones KJ and Hinton CV: ROS-mediated activation
of AKT induces apoptosis via pVHL in prostate cancer cells. Mol
Cell Biochem. 376:63–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
McCubrey JA, Lahair MM and Franklin RA:
Reactive oxygen species-induced activation of the MAP kinase
signaling pathways. Antioxid Redox Signal. 8:1775–1789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
El-Najjar N, Chatila M, Moukadem H,
Vuorela H, Ocker M, Gandesiri M, Schneider-Stock R and
Gali-Muhtasib H: Reactive oxygen species mediate
thymoquinone-induced apoptosis and activate ERK and JNK signaling.
Apoptosis. 15:183–195. 2010. View Article : Google Scholar
|
|
44
|
Navarro R, Busnadiego I, Ruiz-Larrea MB
and Ruiz-Sanz JI: Superoxide anions are involved in
doxorubicin-induced ERK activation in hepatocyte cultures. Ann NY
Acad Sci. 1090:419–428. 2006. View Article : Google Scholar
|
|
45
|
Massaoka MH, Matsuo AL, Figueiredo CR,
Farias CF, Girola N, Arruda DC, Scutti JA, Romoff P, Favero OA,
Ferreira MJ, et al: Jacaranone induces apoptosis in melanoma cells
via ROS-mediated downregulation of Akt and p38 MAPK activation and
displays antitumor activity in vivo. PLoS One. 7:e386982012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yu CC, Wu PJ, Hsu JL, Ho YF, Hsu LC, Chang
YJ, Chang HS, Chen IS and Guh JH: Ardisianone, a natural
benzoquinone, efficiently induces apoptosis in human
hormone-refractory prostate cancers through mitochondrial damage
stress and survivin downregulation. Prostate. 73:133–145. 2013.
View Article : Google Scholar
|
|
47
|
Hao W, Yuan X, Yu L, Gao C, Sun X, Wang D
and Zheng Q: Licochalcone A-induced human gastric cancer BGC-823
cells apoptosis by regulating ROSmediated MAPKs and PI3K/AKT
signaling pathways. Sci Rep. 18:52015.
|
|
48
|
Hartmann KU and Heidelberger C: Studies on
fluorinated pyrimidines. XIII. Inhibition of thymidylate
synthetase. J Biol Chem. 236:3006–3013. 1961.PubMed/NCBI
|
|
49
|
Wang D and Lippard SJ: Cellular processing
of platinum anti-cancer drugs. Nat Rev Drug Discov. 4:307–320.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lenaz L, Necco A, Dasdia T and Di Marco A:
Biologic activity of some adriamycin (NSC-123127) derivatives.
Cancer Chemother Rep. 58:769–776. 1974.PubMed/NCBI
|
|
51
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43-9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hashimoto N, Tsunedomi R, Yoshimura K,
Watanabe Y, Hazama S and Oka M: Cancer stem-like sphere cells
induced from de-differentiated hepatocellular carcinoma-derived
cell lines possess the resistance to anti-cancer drugs. BMC Cancer.
14:7222014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wakita A, Ohtake S, Takada S, Yagasaki F,
Komatsu H, Miyazaki Y, Kubo K, Kimura Y, Takeshita A, Adachi Y, et
al: Randomized comparison of fixed-schedule versus
response-oriented individualized induction therapy and use of
ubenimex during and after consolidation therapy for elderly
patients with acute myeloid leukemia: The JALSG GML200 Study. Int J
Hematol. 96:84–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ichinose Y, Genka K, Koike T, Kato H,
Watanabe Y, Mori T, Iioka S, Sakuma A and Ohta M; NK421 Lung Cancer
Surgery Group. Randomized double-blind placebo-controlled trial of
bestatin in patients with resected stage I squamous-cell lung
carcinoma. J Natl Cancer Inst. 95:605–610. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xu JW, Li CG, Huang XE, Li Y and Huo JG:
Ubenimex capsule improves general performance and chemotherapy
related toxicity in advanced gastric cancer cases. Asian Pac J
Cancer Prev. 12:985–987. 2011.PubMed/NCBI
|