Introduction
Human melanoma is a malignant tumor that has
increased in incidence over the past years worldwide. In the United
States, it is estimated that 144,860 new cases of melanoma will be
diagnosed in the year 2016 (1).
During the period between 1975 and 2012, the incidence of melanoma
has increased annually at a percentage of 3.2% in males and 2.4% in
females (1). During the early
stages of the disease, melanoma is curable through surgical
excision (2). However, the
majority of fatalities occur from secondary tumors originating from
the metastatic spread of the cancer, as a result of ineffective
chemotherapeutic treatment options, and eventually lead to a poor
prognosis and the reduced survival of patients (2,3). Of
note, the annual costs for the treatment of melanoma in the United
States have increased considerably from the year 2010 to 2015 (from
approximately 400 to 650 million USD) and are expected to increase
further in the years 2020 and 2030 (4). The therapeutic strategies that target
human melanoma cells focus on multiple signaling pathways that are
constitutively activated and play important roles in cell
proliferation, cell survival and the resistance of the cancer cells
to chemotherapeutic regimens. Phytochemical-related compounds have
attracted considerable attention, due to their low cost, low
toxicity and public acceptance as dietary chemopreventative agents
(5).
Resveratrol (chemical strucure shown in Fig. 1A, bottom panel) is a naturally
occurring phytoalexin found in grapes, cranberries and peanuts that
is generated in response to pathogenic attack. Resveratrol has been
shown to exert a wide diversity of biological effects, including
the inhibition of the initiation, promotion and progression of
cancer, the inhibition of cancer cell proliferation, the inhibition
of kinase enzyme activity and the induction of apoptosis (6–9).
Despite the multifaceted anticancer activity of resveratrol, the
potency of this compound is hampered by the poor pharmacokinetic
properties exhibited in vivo.
3,4,5,4′-trans-tetramethoxystilbene (3,4,5,4′-TMS) (Fig. 1A, top panel) is a resveratrol
analogue that contains methoxy group substitutions in place of the
hydroxyl groups of the stilbene moiety. 3,4,5,4′-TMS has shown
promising metabolic stability and bioavailability in a previous
study conducted on C57BL/6 mice (10). In addition, 3,4,5,4′-TMS has been
shown to exert more potent inhibitory effects than resveratrol on
the proliferation of cancer cells in vitro, whereas the
anti-proliferative action of the compound is attributed to the
induction of apoptosis and cell cycle arrest (10,11).
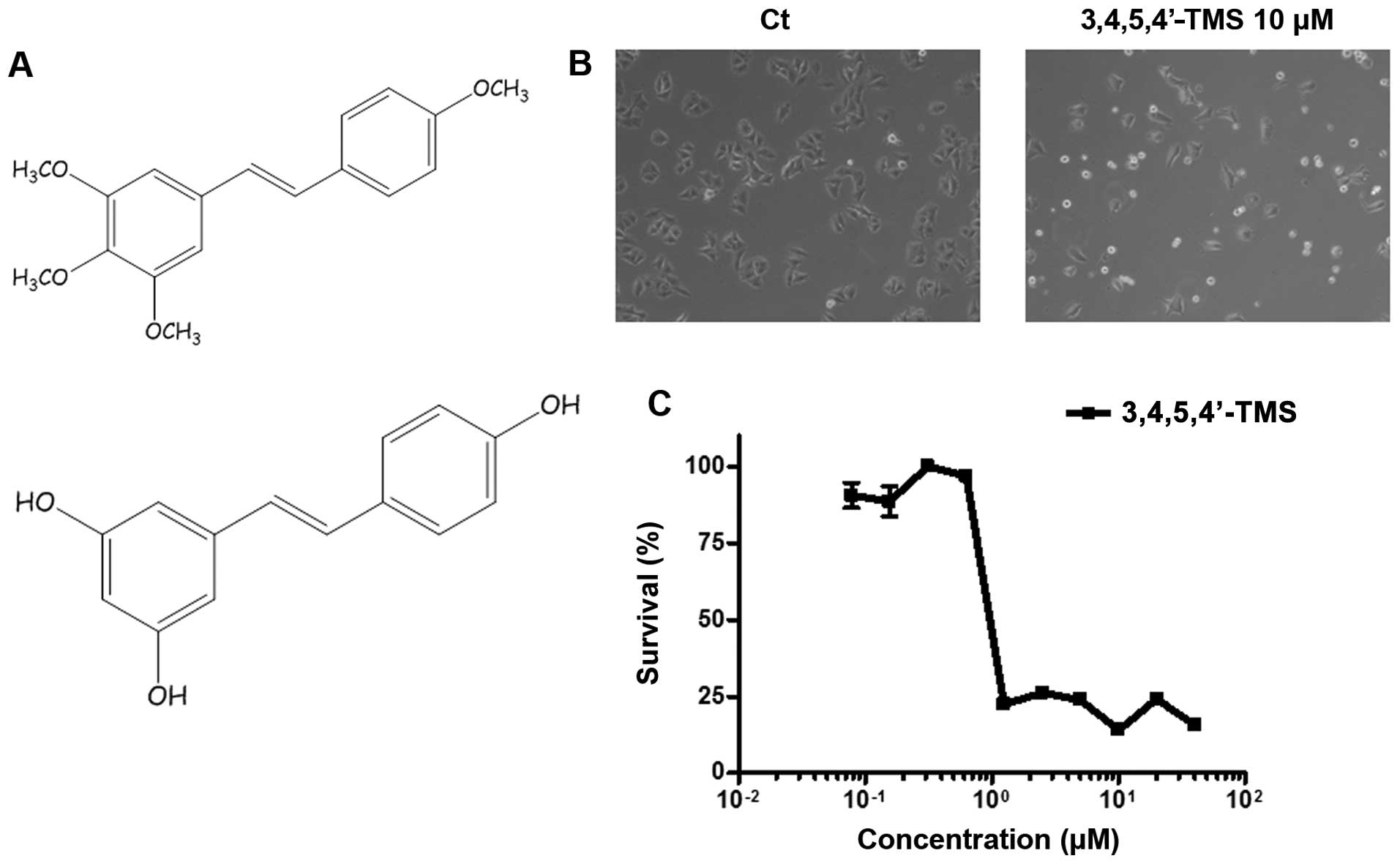 | Figure 1Anti-proliferative effect of the
resveratrol analogue, 3,4,5,4′-trans-tetramethoxystilbene
(3,4,5,4′-TMS), in A375 cells. (A) Chemical structures of
3,4,5,4′-TMS (upper panel) and resveratrol (bottom panel). (B)
Light microscopy of A375 cells treated for 24 h with 10 μM of
3,4,5,4′ TMS. (C) MTT cell viability assay of A375 cells incubated
with a concentration range of 3,4,5,4′-TMS (0.078, 0.156, 0.3125,
0,625, 1.25, 2.5, 5, 10, 20 and 40 μM) for 96 h. The experiments
were carried out at least 3 times and error bars indicate the means
± SD. |
A recent study conducted by our group demonstrated
that 3,4,5,4′-TMS may effectively inhibit the proliferation of
human melanoma cells by inducing apoptosis and cell cycle arrest,
through the activation of the mitogen-activated protein kinase
(MAPK), ERK1/2 (12). In the
present study, the activation of the MAPK protein, p38, by
3,4,5,4′-TMS was investigated using A375 human melanoma cells. The
induction of the expression of the mitosis-associated protein,
Aurora A, was also examined. The data demonstrate that 3,4,5,4′-TMS
possesses a pleiotropic spectrum of biological activities in human
melanoma cells that includes the modulation of the cell cycle and
cell signaling-associated proteins, the induction of apoptosis and
the inhibition of metastasis.
Materials and methods
Materials and chemicals
3,4,5,4′-TMS was purchased from Sigma-Aldrich (St.
Louis, MO, USA). DMSO, ethanol, formaldehyde, paraformaldehyde,
PBS, bovine serum albumin and propidium iodide were purchased from
Sigma-Aldrich. SB203580 was purchased from Calbiochem (San Diego,
CA, USA). Triton X-100 and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Reasearch Organics Inc. (Cleveland, OH, USA)
and RNAse was from Qiagen, Inc. (Valencia, CA, USA). Antibodies for
JNK (Cat no. 9252), p38 (Cat no. 9211), p-p38 (Cat no. 9212) and
Aurora A (Cat no. 3079) detection were from Cell Signaling
Technology, Inc. (Beverly, MA, USA). Antibodies for β-tubulin (Cat
no. sc-31782) and β-actin (Cat no. sc-47778) detection were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Peroxidase conjugated secondary antibodies [goat anti-rabbit IgG
(Cat no. 12-34) and rabbit anti-mouse IgG (Cat no. cat no. 06-371)]
were purchased from Millipore (Temecula, CA, USA).
Cell culture
A375 cells that were used for the experiments were
provided by the American Type Culture Collection (ATCC, Manassas,
VA, USA) and were maintained in RPMI-1640 containing 10% (v/v)
heat-inactivated (56°C for 45 min to inactivate complement) fetal
calf serum at 37°C, 5% CO2/95% air with 100% humidity
and were passaged using trypsin-EDTA. The cultured cells were
routinely passaged every 2–3 days. All cell culture reagents were
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA).
Tissue culture flasks, multi-well plates and cell culture dishes
were purchased from Corning Life Sciences (Tewksbury, MA, USA), and
glass coverslips from Knittel-Gläser (Braunschweig, Germany).
MTT assay
A375 cells were plated in 96-well flat-bottomed
plates, treated with 3,4,5,4′-TMS at a concentration range of
0.078–40 μM and incubated for 96 h at 37°C. The control cells were
treated with DMSO (0.1% v/v). Cell viability was determined
spectrophotometrically using MTT as a substrate, as previously
described (13).
Cell cycle analysis
A375 cells were incubated with 3,4,5,4′-TMS (10 μM),
SB203580 (4 μM) or 0.1% DMSO (control cells) for 24, 36 or 48 h.
The cells were washed twice with PBS, detached with trypsin-EDTA
and centrifuged at 2,000 rpm for 5 min. The cells were then washed
twice with PBS and resuspended in 70% ethanol at −20°C for 24 h.
The cells were then incubated with PI (50 μg/ml) containing RNase
(40 μg/ml) for 30 min at 37°C. The fluorescence intensity was
determined using a Beckman Coulter flow cytometer (Beckman Coulter
International SA, Nyon, Switzerland), using FL3 as the channel for
fluorescence emission. At least 10,000 events were acquired and
analysis was carried out using CXP multicomp cytometer analysis
software (Beckman Coulter International SA).
Determination of mitotic index
A375 cells were seeded in 18×18 mm coverslips at a
density of 4×104 cells/ml, and incubated at 37°C for 24
h in the presence of 3,4,5,4′-TMS (10–30 μM). The cells were washed
with PBS and fixed with 3.7% formaldehyde in PBS for 10 min.
Nuclear staining of the cells was carried out by incubation with 1
μM TO-PRO-3 iodide (Molecular Probes; Invitrogen Life Technologies)
for 10 min. The cells were mounted using UltraCruz Mounting Medium
with DAPI (Santa Cruz Biotechnology, Inc.) and analyzed using a
confocal microscope (Leica Microsystems GmbH, Heidelberg, Germany).
The mitotic index was determined according to the following
formula: number of cells in mitosis/total number of cells ×100. The
experiment was carried out at least 3 times, and the results are
expressed as the mean values ± standard deviation (SD).
Confocal immunofluorescence
A375 cells were seeded in 18×18 mm coverslips at a
density of 5×104 cells/ml, and incubated at 37°C for 24
h in the presence of 3,4,5,4′-TMS (10–30 μM). The cells were washed
3 times with PBS, fixed with 4% paraformaldehyde/PBS and
permeabilized in 0.1% Triton X-100/PBS for 10 min. Following 3
washes with PBS, the cells were blocked with 1% BSA/PBS for 30 min
and incubated with a primary antibody against β-tubulin and/or
Aurora A (diluted 1:200 in 0.1% BSA/PBS) overnight at 4°C. The
cells were then incubated with Alexa Fluor 555 goat anti-mouse IgG
(Cat no. A-21422; Thermo Fisher Scientific, Waltham, MA, USA)
secondary antibody in the case of β-tubulin, or with CF 488A goat
anti-rabbit IgG secondary antibody (Cat no. 20012; Biotium, Inc.,
Hayward, CA, USA), in the case of p-p38 and for Aurora A (diluted
1:200 in 1% BSA/PBS) for 1 h. The cells were finally washed 3 times
with PBS, stained with 1 μM TO-PRO-3 iodide for 10 min, and mounted
with UltraCruz Mounting Medium with DAPI. Where appropriate, the
cytoplasm was stained with rhodamine phalloidin (Invitrogen Life
Technologies) for 40 min at room temperature (diluted 1:150 in in
0.2% BSA/PBS), prior to incubation with the primary antibody.
Wound healing assay
A375 cells were seeded in 24-well plates at a
density of 1×105 cells/ml and incubated at 37°C for 24 h
to reach 80–90% confluency. Wound healing was conducted by
scratching the surface of each well with a sterile 10 μl pipette
tip. The detached cells were removed by washing the cell layer
twice with PBS. The wound closure was monitored in the presence of
5 μM 3,4,5,4′-TMS at 12 or 24 h, at different positions of the
wound. The cellular motility was quantified using image analysis
(ImageJ 1.4.3.67 Launcher Symmetry Software). Wound healing assays
were carried out in triplicate.
Attachment assay
The 24-well plates were coated overnight at 4°C with
collagen type IV (50 μg/ml; Sigma-Aldrich) and were washed 3 times
with PBS the following morning. The wells were blocked with 1% BSA
for 2 h prior to the experiment. The cells were seeded in each well
at a density of 4×105 cells/ml in the presence or
absence of 5 μM of 3,4,5,4′-TMS and incubated at 37°C for the time
periods of 45 min, and 2 and 3 h. The cells were washed 3 times
with PBS and the cellular density was quantified by staining with
trypan blue and counting using a haemocytometer. The attachment
assays were conducted in triplicate.
Western blot analysis
A375 cells were plated out in T25 flasks at a
density of 1.5×105 cells/ml and incubated at 37°C for 24
h in the presence of 3,4,5,4′-TMS (20–50 μM). The medium was
removed and the cells were lysed with 100 μl of RIPA buffer
(Sigma-Aldrich) containing protease inhibitor cocktail (Roche
Diagnostics, Basel, Switzerland). The extraction of the protein was
carried out by centrifugation at 13,000 rpm for 10 min, at 4°C. A
total of 30 μg of protein was loaded in 10% SDS-polyacrylamide mini
gels and transferred by electroblotting onto PVDF membranes
(Bio-Rad Laboratories, Inc., Hercules, CA). The membranes were
blocked with 5% milk/0.1% Tris-buffered saline with Tween-20
(TBS-T) at room temperature for 1 h by continuous shaking. The
incubation with the primary antibodies (JNK, p38, p-p38, β-actin,
Aurora A, diluted 1:500 or 1:200 in 1% milk/0.1% TBS-T) was carried
out overnight at 4°C. The membranes were washed 3 times with 0.1%
TBS-T and incubated with secondary antibody (HRP; diluted 1:2,000
in 5% milk/0.1% TBS-T) at room temperature for 1 h. The
membrane-bound antibodies were visualized by the use of ECL western
blotting detection reagent (Amersham Corp., Arlington Heights, IL,
USA) on X-ray films (FujiFilm, Valhalla, NY, USA).
Statistical analysis
The data are presented as the average of at least 3
independent measurements and analyzed by a paired t-test and
one-way ANOVA, using Microsoft Excel version 2007. P-values
<0.05 were considered to indicate statistically significant
differences.
Results
3,4,5,4′-TMS inhibits the growth of A375
melanoma cells at micromolar concentrations through a mechanism
involving mitotic arrest at the prometaphase stage
3,4,5,4′-TMS has been shown to possess promising
anti-proliferative activity in vitro in breast, ovarian and
colon cancer cells (10,11,14,15).
Recently, we published a study that demonstrates the efficacy of
3,4,5,4′-TMS in human melanoma in vitro (12). In the present study, the results of
the latter study were verified. The A375 cell line was selected in
order to further examine the anti-proliferative and anticancer
activity of 3,4,5,4′-TMS in human melanoma. An initial
investigation of the A375 cells by phase contrast light microscopy
revealed doublets of cells floating on the surface of the
Petri-dish following 24 h of treatment with 10 μM of 3,4,5,4′-TMS,
compared with the control cells treated with the solvent alone
(DMSO) (Fig. 1B). The incubation
of the cells with 3,4,5,4′-TMS for longer periods of time (96 h),
indicated that cellular growth was effectively inhibited at
concentrations <1 μM (IC50=0.7 μM), as demonstrated
by MTT cell viability assay (Fig.
1C). Furthermore, FACS analysis was carried out following 24 h
of treatment of the cells with 10 μM of the drug, in order to
provide additional insight into the mechanisms of action of
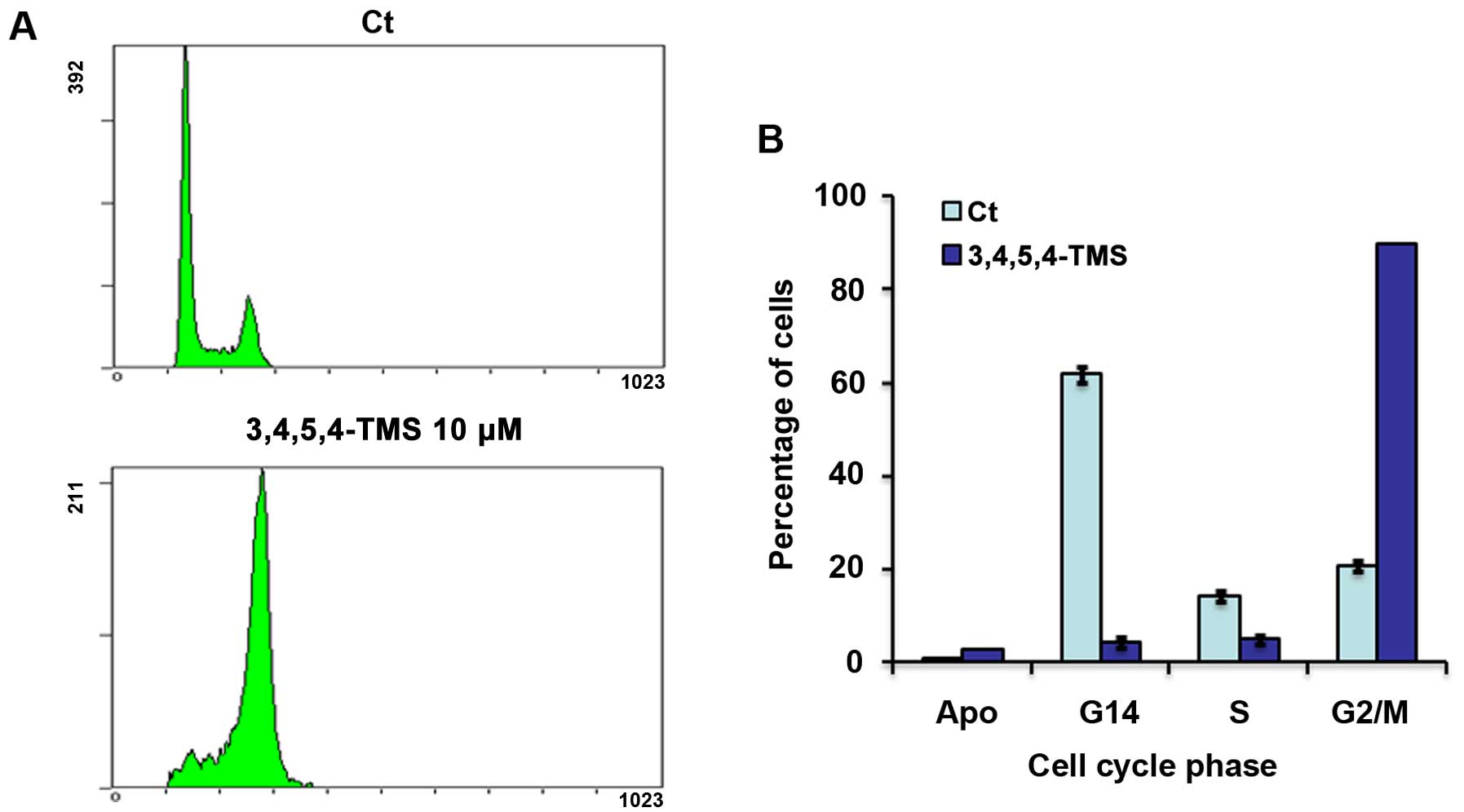
3,4,5,4′-TMS in human melanoma. Treatment with 3,4,5,4′-TMS
arrested A375 cells at the G2/M phase of the cell cycle (90±0.2%),
with a simultaneous decrease in the number of cells in the G1 phase
(4.25±1%) and S phase (4.75±1%), compared with the control cells
(Fig. 2). In addition to G2/M
arrest, 3,4,5,4′-TMS induced the apoptotic cascade of a small
fraction of the A375 cell population (2.6±0.1 vs. 0.7±0% for the
control cells) (Fig. 2).
Furthermore, experiments were carried out in order to determine
whether the effects caused by 3,4,5,4′-TMS were attributed to G2 or
M phase arrest. The cells were visualized by confocal microscopy
following nuclear staining with TO-PRO-3. A large increase in the
mitotic index (22–23±4.8–5.3 vs. 4.25±0.6% for control) was
observed following incubation of A375 cells with 10 and/or 20 μM of
3,4,5,4′-TMS for 24 h that was similar in ratio with the G2/M
blockage noted in the flow cytometry experiments (Fig. 3B). In parallel, visualization of
the A375 nuclei indicated the blockage of cell mitotic division at
the prometaphase stage (Fig.
3A).
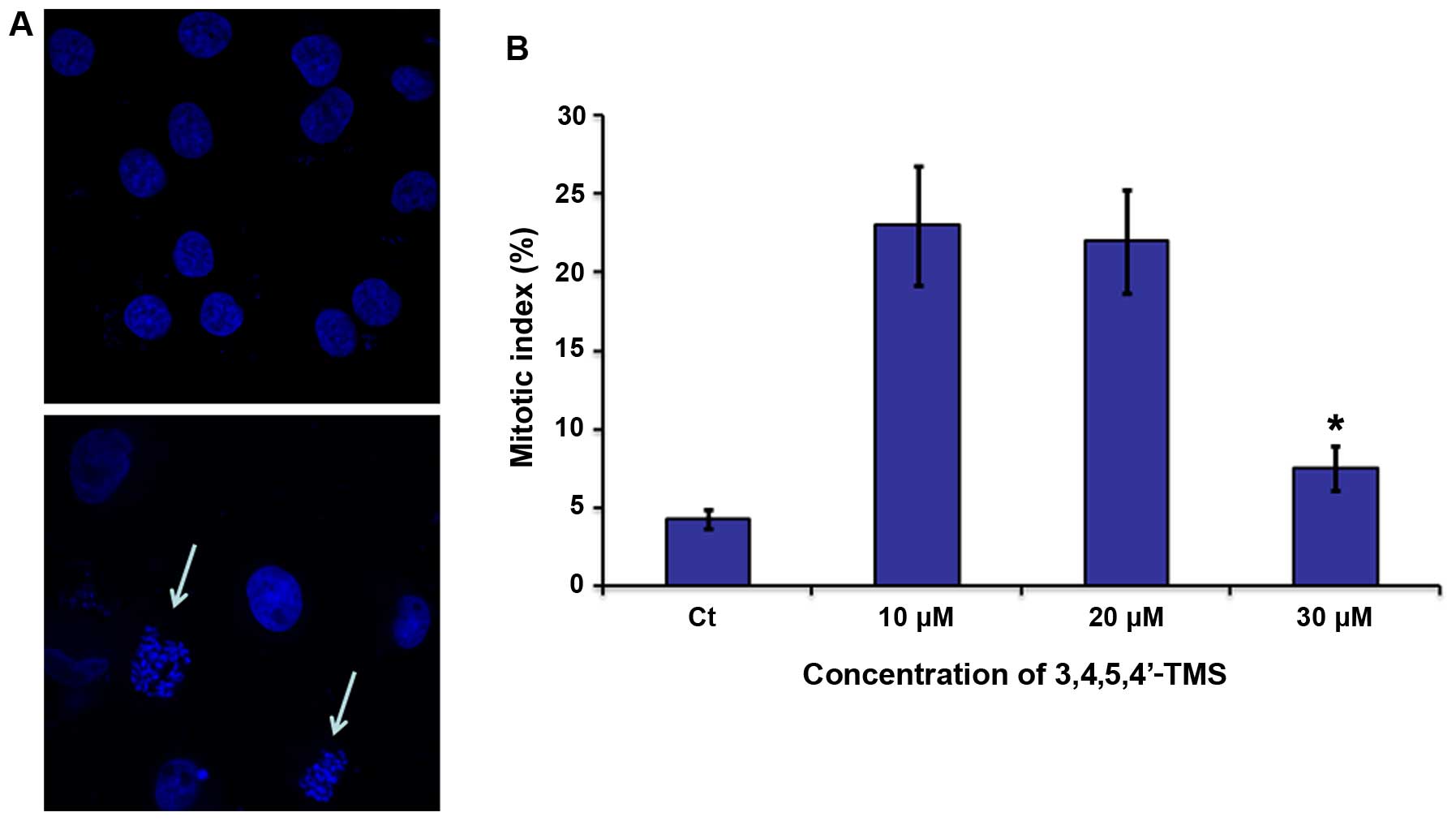 | Figure 33,4,5,4′-trans-tetramethoxystilbene
(3,4,5,4′-TMS) induces mitotic arrest at the prometaphase in A375
cells. (A) Nuclear staining of A375 cells using TO-PRO-3 iodide in
the presence/absence of 3,4,5,4′-TMS (10 μM) for 24 h. White arrows
indicate cells arrested at the prometaphase stage. (B) Percentage
of cells undergoing mitosis in the presence of 3,4,5,4′-TMS (10–30
μM). Control cells were treated with 0.1% DMSO for 24 h. Error bars
indicate means ± SD from at least 3 independent determinations.
*P<0.05, significant difference compared to the
control. |
3,4,5,4′-TMS induces the activation of
the cell signaling protein, p38, and requires active p38 for
maximum potency
Based on the initial observation regarding the
anti-mitotic activity of 3,4,5,4′-TMS, western blot analysis was
carried out in order to examine the expression levels of key target
proteins involved in cell signaling following incubation of A375
cells with the drug. The primary objective of the study was to
examine whether MAPK signaling is important for the potency of
3,4,5,4′-TMS in A375 cells. Initially, an increase in the total
levels of JNK was observed that was concentration-dependent.
Furthermore, the levels of the phosphorylated form of p38 were
significantly increased following treatment of the cells with 30
and 50 μM of 3,4,5,4′-TMS (Fig. 4A and
B). The protein expression levels of p38 exhibited a similar
increasing pattern (Fig. 4A and
B). Additional experiments using confocal microscopy were
conducted, in order to confirm that p38 is activated based on the
localization of the phosphorylated form of the protein in the cell.
p-p38 was translocated to the nucleus of the A375 cells following
treatment with 3,4,5,4′-TMS, as demonstrated by intense staining of
the protein in the nuclear regions of the cells. In contrast to
these findings, the cells that were treated with DMSO alone
exhibited weaker staining of p-p38 that was localized across the
nuclear and cytoplasmic regions (Fig.
4C).
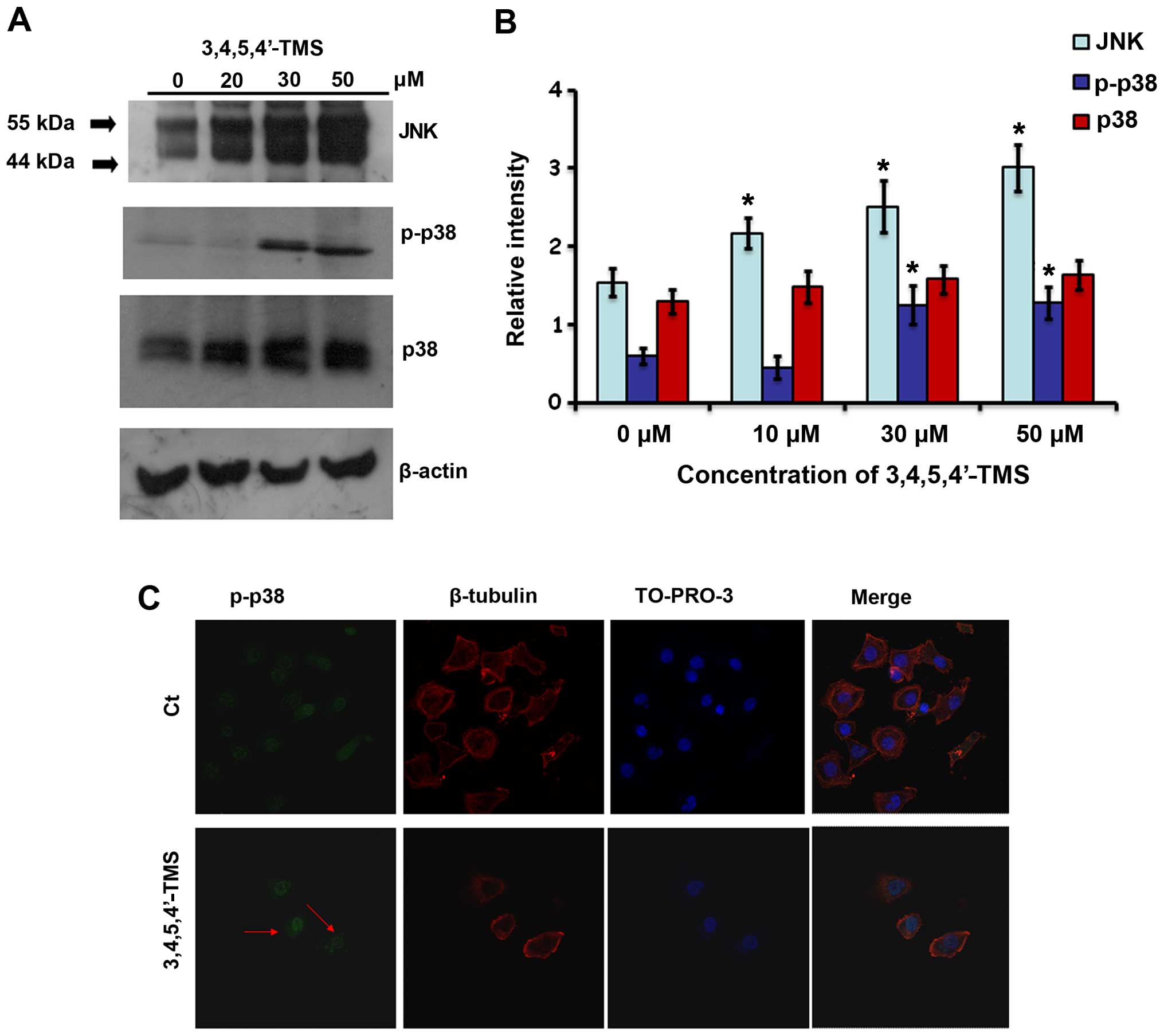 | Figure 43,4,5,4′-trans-tetramethoxystilbene
(3,4,5,4′-TMS) induces the upregulation of the MAPK proteins, JNK
and p38, in A375 cells. (A) Western blot analysis of cell cycle
protein markers in cells pre-treated with 20, 30 and 50 μM of
3,4,5,4′-TMS for 24 h. (B) Computer densitometry indicating
relative expression of the proteins in A375 cells following
treatment with 3,4,5,4′-TMS. (C) Confocal microscopy indicating
nuclear translocation of p-p38 in A375 cells pre-treated with 30 μM
of 3,4,5,4′-TMS. Red arrows indicate more intense green staining in
the nuclear region. Blue, TO-PRO-3 iodide staining; red, CF 488A
staining; green, Alexa Fluor 555 staining. *P<0.05,
significant difference compared to the control. |
Given that p38 is activated in response to treatment
with 3,4,5,4′-TMS, the present investigation was expanded, in order
to determine whether the inhibition of p38 activity is essential
for the potency of the drug. A375 cells were incubated with the p38
inhibitor (p38i), SB203580, at 4 μM in the presence or absence of
3,4,5,4′-TMS. The pharmacological inhibition of p38 caused the
arrest of A375 cells at the G1 phase along with G2/M phase arrest
caused by 3,4,5,4′-TMS (Fig. 5A).
The p38i, SB203580, attenuated the potent effects of 3,4,5,4′-TMS
and concomitant treatment with SB203580 and 3,4,5,4′-TMS decreased
the percentage of cells arrested in the G2/M phase to 51.5±4.7% as
opposed to 82±5.2% in the case of treatment with 3,4,5,4′-TMS alone
(Fig. 5B). The incubation of A375
cells with SB203580 (4 μM) and 20 μM of 3,4,5,4′-TMS for longer
periods of time (36 h), resulted in a lower number of cells
undergoing G1 phase arrest, compared with the treatment of the
cells with 10 μM of the drug for 24 h (Fig. 5A). In contrast to these
observations, the inhibition of p38 did not reduce the percentage
of A375 cells undergoing late apoptosis in the presence of
3,4,5,4′-TMS for 24 h (3.2±0.2 vs. 3.5±0.3%), as demonstrated by
FACS analysis (Fig. 5A and B).
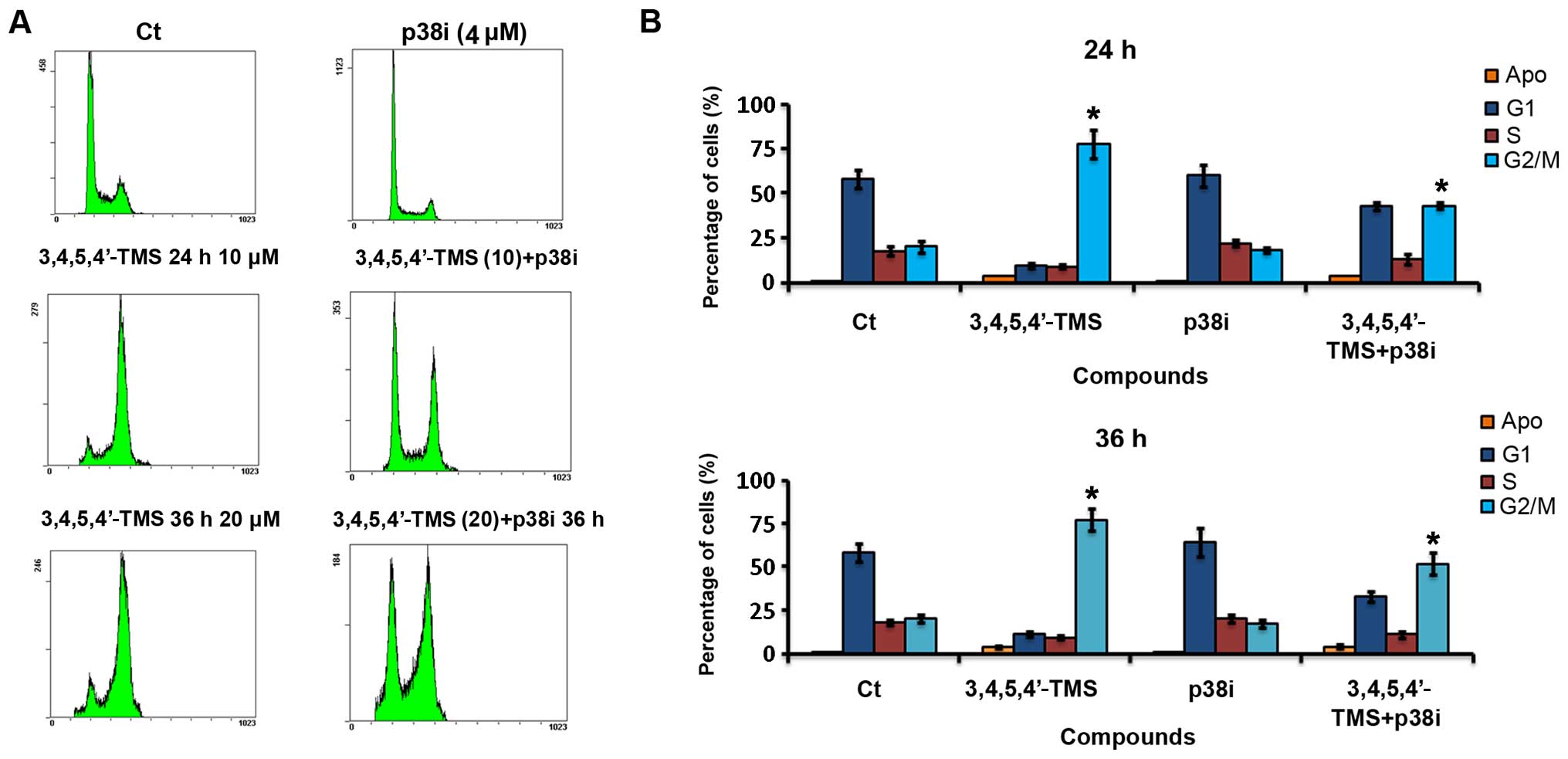 | Figure 5Pharmacological inhibition of p38
attenuates 3,4,5,4′-trans-tetramethoxystilbene
(3,4,5,4′-TMS)-mediated cell cycle arrest of A375 cells. (A) Cell
cycle histograms of A375 cells treated with: i) 0.1% DMSO for 24 h;
ii) 4 μM of the p38i SB203580 for 24 h; iii) 10 μM of 3,4,5,4′ TMS
alone for 24 h; iv) SB203580 (4 μM) and 3,4,5,4′ TMS (10 μM) for 24
h; v) 20 μM of 3,4,5,4′ TMS alone for 36 h; and vi) SB203580 (4 μM)
and 3,4,5,4′ TMS (20 μM) for 36 h. (B) Percentages of cells
pre-treated with DMSO (0.1%), 3,4,5,4′ TMS (10 or 20 μM), p38i (4
μM) or p38i (4 μM) + 3,4,5,4′-TMS (10 or 20 μM) for 24 or 36 h in
each phase of the cell cycle. Apoptosis was quantified by the
percentage of cells at the subG1 peak of the histogram. Experiments
were carried out in triplicate. *P<0.05, significant difference
compared to the 3,4,5,4′ TMS-treated group. |
3,4,5,4′-TMS induces the upregulation of
Aurora A and causes a localization shift from the spindle
poles
The expression of the mitosis-associated protein,
Aurora A, was examined in order to provide insight into the
mechanisms responsible for the anti-mitotic action of 3,4,5,4′-TMS.
Aurora A was detected at the prometaphase stage of A375 mitotic
cell division and the protein was localized to both spindle poles
(Fig. 6A). In the presence of
3,4,5,4′-TMS, Aurora A was localized to the middle of the cell,
while the mitotic spindle poles were absent. The staining of
β-tubulin in the cytoplasm was very weak, indicating mitotic
catastrophe caused by the drug (Fig.
6A). When the expression was monitored by western blot
analysis, a clear upregulation of the protein was noted following
treatment with 3,4,5,4′-TMS when compared with the control cells
treated with DMSO (Fig. 6B and
C).
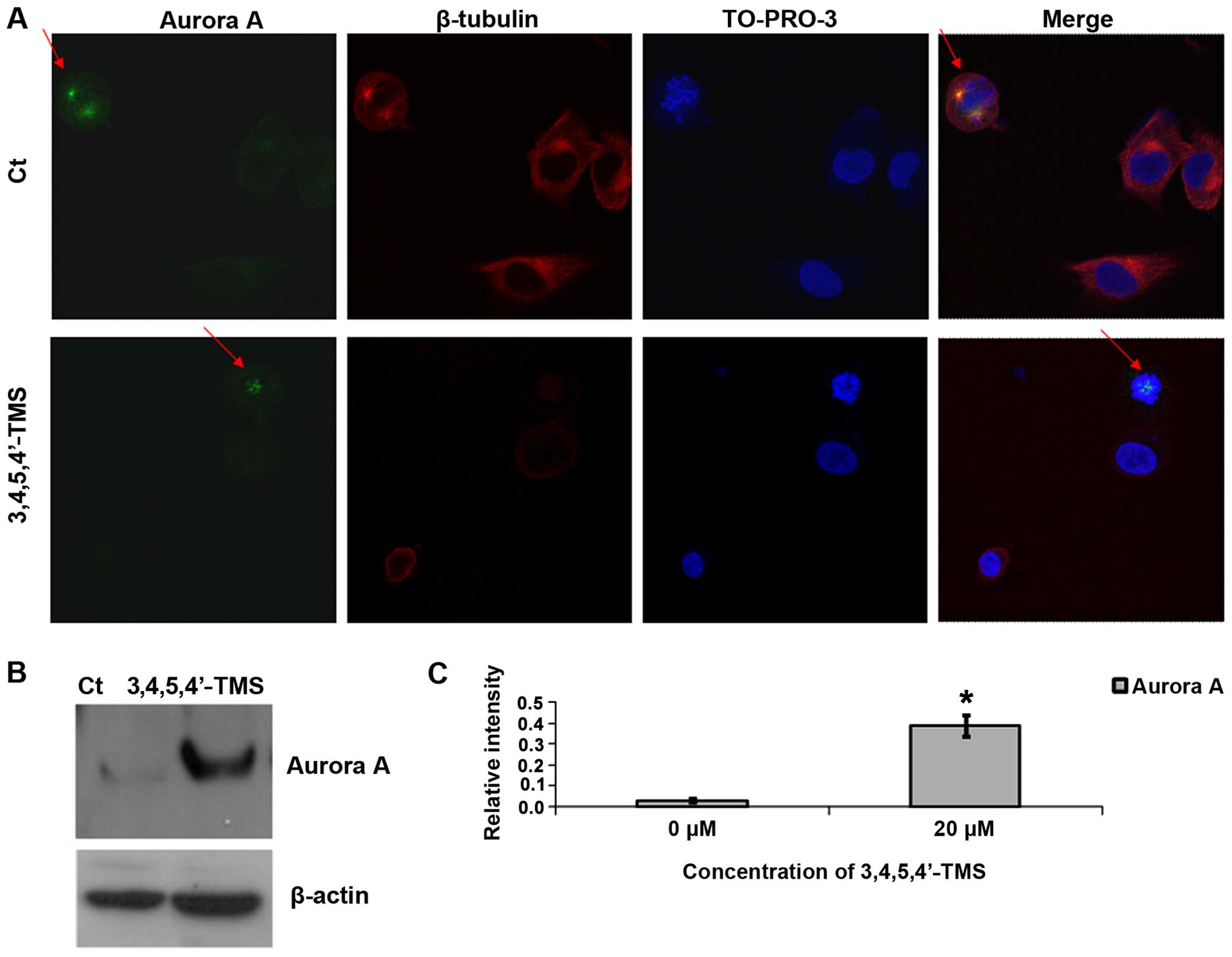 | Figure 63,4,5,4′-trans-tetramethoxystilbene
(3,4,5,4′-TMS) induces the upregulation of Aurora A and the
dissociation of the protein from the spindle poles. (A) Confocal
immunofluorescence of A375 cells pre-treated with 10 μM of
3,4,5,4′-TMS for 12 h. Blue, TO-PRO-3 iodide staining; red, CF 488A
staining; green, Alexa Fluor 555 staining. Red arrows indicate the
localization of Aurora A in the pressure and/or absence of
3,4,5,4′-TMS. (B) Representative blots indicating Aurora A protein
expression levels following treatment with 3,4,5,4′ TMS (10 μM) for
24 h. (C) Relative intensity of Aurora A expression. Experiments
were carried out in triplicate. *P<0.05, significant
difference compared to the control. |
3,4,5,4′-TMS exhibits anti-metastatic
activity in A375 cells
Having established that 3,4,5,4′-TMS effectively
inhibits the growth and proliferation of A375 cells, the effects of
the drug on melanoma cell migration and attachment were further
examined. For this purpose, wound healing and cell attachment
assays were employed. The compound 3,4,5,4′-TMS (5 μM) reduced the
wound-healing ability of A375 cells following 12 h of treatment
compared to the control samples (Fig.
7A). Similar results were obtained for 24 h of treatment (data
not shown). Specifically, 3,4,5,4′-TMS reduced the migration of the
cells at 12 h to 12±2%, compared with 36±4% corresponding to the
control samples (Fig. 7B). In
addition 3,4,5,4′-TMS significantly inhibited the attachment of the
cells on collagen type IV-coated 6-well plates at 45 min, and at
the 2- and 3-h time points (Fig.
7C). The effect was more profound at earlier time points when
compared with the control cells treated with DMSO alone (Fig. 7C).
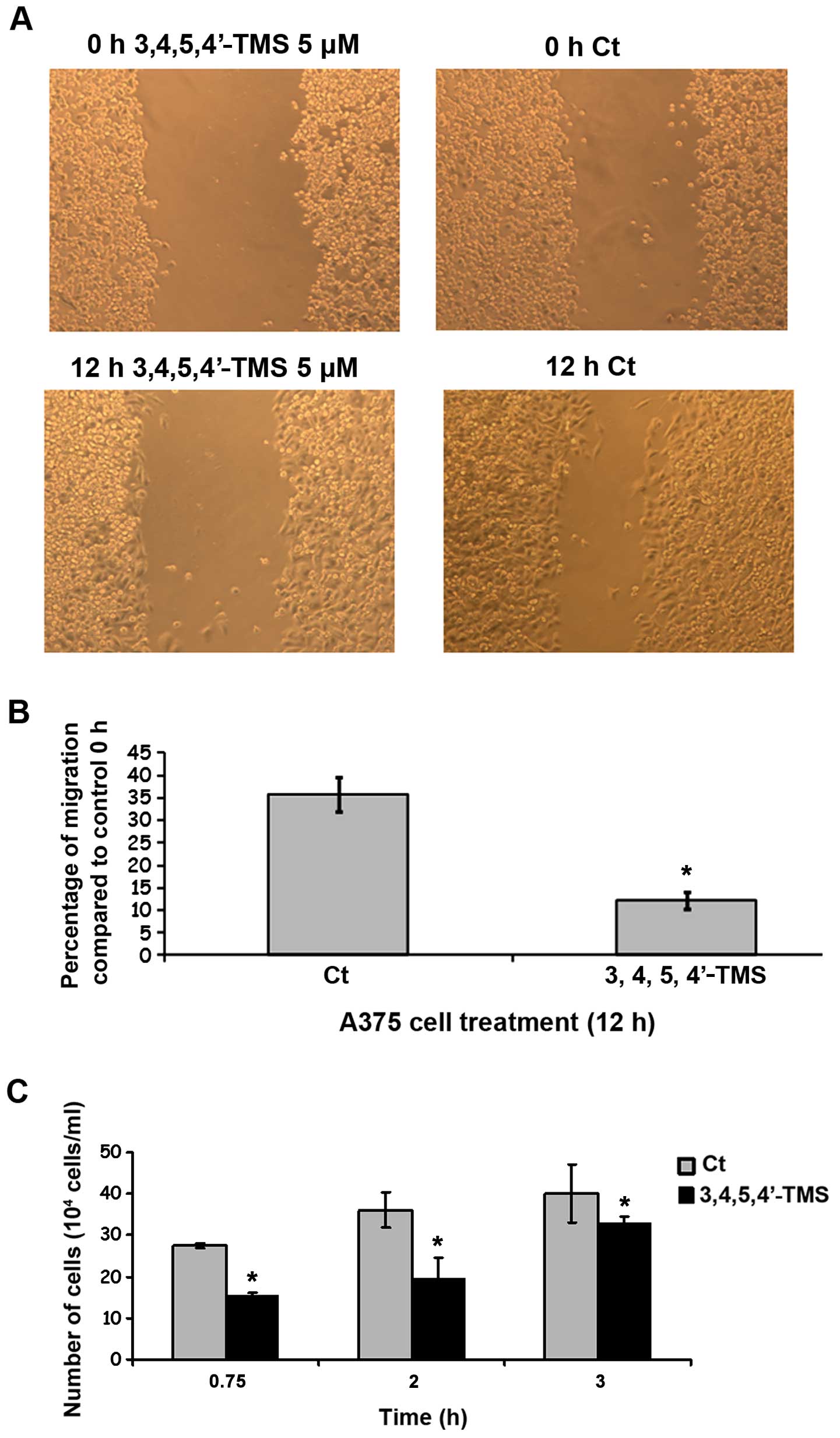 | Figure 73,4,5,4′-trans-tetramethoxystilbene
(3,4,5,4′-TMS) inhibits the migration and attachment of A375 cells.
(A) Wound healing assay of A375 cells that were treated with
3,4,5,4′-TMS (5 μM) for 12 h. (B) Percentage of inhibition of cell
migration mediated by 3,4,5,4′-TMS, compared to control cells
containing DMSO. (C) Inhibition of A375 cellular attachment to
collagen type IV-coated surface by 5 μM of 3,4,5,4′-TMS at 45 min,
2 and 3 h. *P<0.05, significantly different than
control. Images are traces of 1 out of 3 independent
determinations. |
Discussion
Natural products have played a pivotal role in the
identification of new treatment strategies for cancer. The
enhancement of the potency and efficacy of natural products by
utilizing synthetic strategies is an attractive avenue for the
design of effective anticancer drugs. 3,4,5,4′-TMS is a small
molecular weight anticancer drug that possesses enhanced potency
and bioavailability compared with the parent molecule, resveratrol.
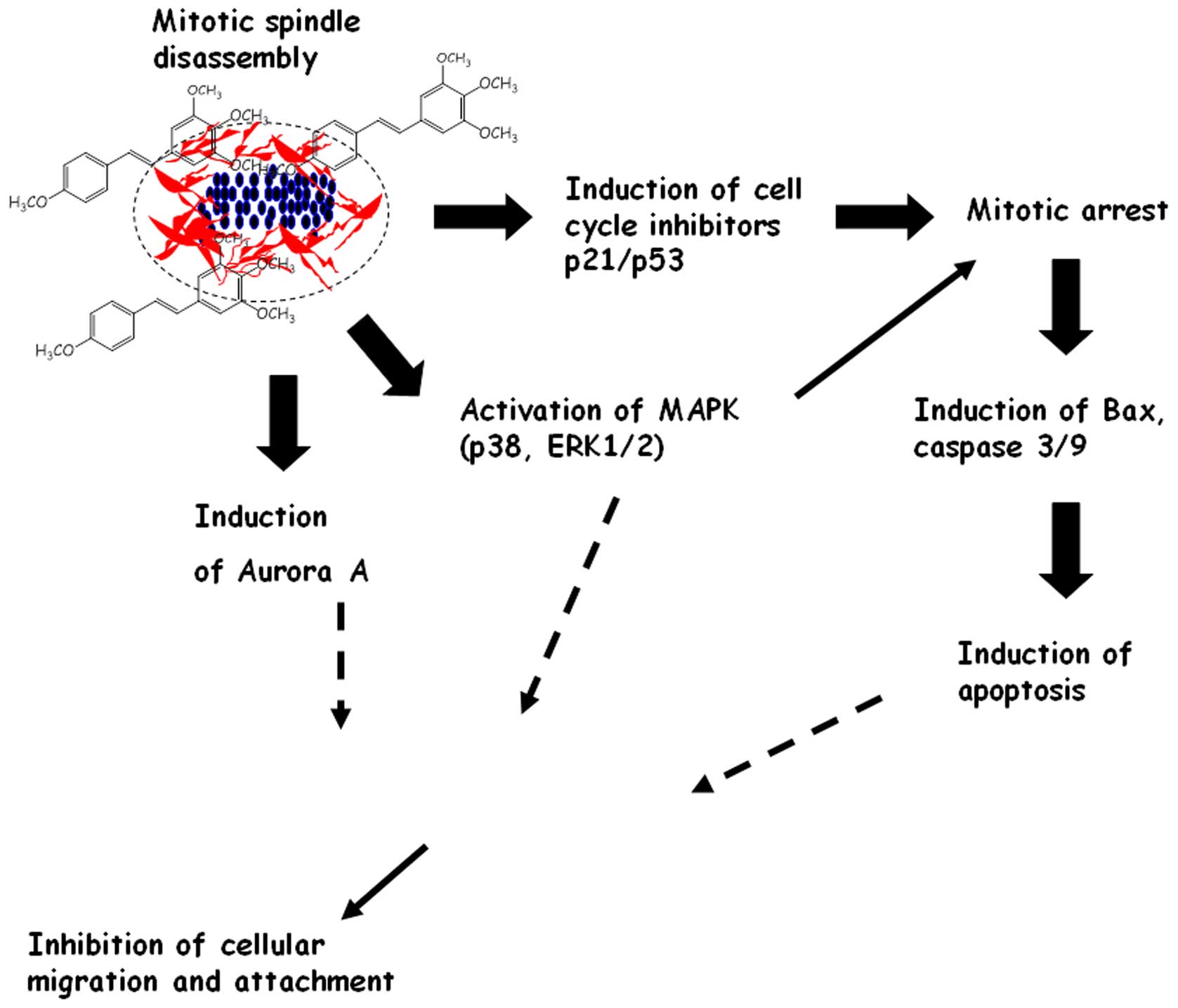
The current study presents a comprehensive report of 3,4,5,4′-TMS
activity in A375 human melanoma cells. In addition to the
inhibition of cell proliferation, 3,4,5,4′-TMS impeded the
migration of A375 cells, thus presenting an effective therapeutic
strategy for the treatment of human metastatic melanoma. The
mechanism of action of 3,4,5,4′-TMS involves mitotic arrest, the
induction of apoptosis and the activation of the p38 and Aurora A
proteins. The molecular events associated with the anticancer
effects of 3,4,5,4′-TMS in human melanoma cells are summarized in
Fig. 8.
3,4,5,4′-TMS has been examined in vitro in
terms of cytotoxicity in breast, liver, ovarian, colon cancer and
melanoma cell lines. The cytotoxicity of 3,4,5,4′-TMS is estimated
at submicromolar concentrations, in terms of IC50
values, for breast liver and ovarian cancer cells (0.3–0.7 μM for
MCF7 breast, HepG2 liver and A2780 ovarian cancer cells), whereas
in colon cancer cells, the compound appears less potent (11.5 μM
for HT-29 colon cancer cells) (10,11,15).
In human melanoma cells, 3,4,5,4′-TMS exhibited an IC50
value in the range of 0.5–1.25 μM (Bro and A375 cells, 0.5 μM; MeWo
and M5 melanoma cells, 1.25 μM) (12). It is evident from these studies
that some cell lines appear to be more sensitive to 3,4,5,4′-TMS
anti-proliferative activity compared to others. In the present
study 3,4,5,4′-TMS exhibited submicromolar toxicity in A375 cells,
which indicates that the latter cell line shows selectivity towards
the drug, as in the case of Bro, HepG2, A2780 and MCF7 cells.
Mechanistically, in this study, 3,4,5,4′-TMS was
shown to inhibit the cell cycle of A375 cells by inhibiting mitotic
cell division at the prometaphase stage, as a result of the
disassembly of the mitotic spindle. In contrast to the studies of
Ma et al (11) and
Piotrowska et al (15), in
this study, the A375 human melanoma cell line was shown to be
somewhat resistant to the apoptosis induced by 3,4,5,4′-TMS
(approximately 5% of the cells underwent apoptosis), compared with
the MCF7, HepG2 and A2780 cells in previous studies, where a
greater percentage of cells was shown to undergo apoptosis (10–20%)
(11,14,15).
Methoxylated stilbenes containing ≥3 methoxy substitutions induce
the apoptotic cascade and the mitotic catastrophe of cancer cells
(16,17). The potency of each drug is
dependent on the cis or trans configuration. The
stilbenes bearing a cis configuration possess greater
potency than the corresponding stilbenes bearing a trans
configuration (16,18). The mitotic catastrophe that is
induced by methoxylated stilbenes precedes the induction of
apoptosis, which is a secondary phenomenon that occurs due to the
perturbed cellular division. With regard to the activity of
3,4,5,4′-TMS in A375 cells, there are two possible explanations for
the diminished capacity of the drug to induce apoptosis: i)
3,4,5,4′-TMS is a trans analogue, thus it is not as potent
as cis methoxy stilbenes, such as combretastatin A4 (CA4) in
inducing mitotic arrest and consequently apoptosis; and ii) A375
and melanoma cells in general, are resistant to the induction of
apoptosis when exposed to chemotherapeutic agents via the
activation of different protective mechanisms (19). Furthermore, human melanoma cells
show decreased apoptosis at the sites of tumor lesions that may
explain their high metastatic potential (20). In concordance with the results
presented in the present study, a recent study demonstrated that a
structurally similar stilbene to 3,4,5,4′-TMS, bearing the
cis orientation
(cis-3,4′,5-trimethoxy-3′-aminostilbene), was capable of
inducing apoptosis in HCT116 colon cancer cells, but not in B16/F10
melanoma cells (21).
In an effort to identify plausible targets of
3,4,5,4′-TMS in A375 cells, the study focused on the MAPKs, p38 and
JNK. Initial experiments revealed that 3,4,5,4′-TMS induced the
phosphorylation of p38 and the upregulation of JNK proteins.
Furthermore, the p38 pathway was shown to be essential for the
potency of 3,4,5,4′-TMS, as the pharmacological inhibition of p38
led to a decrease in the percentage of human melanoma cells
arrested in the G2/M phase. The p38 MAPKs are a class of protein
kinases that have been characterized to respond to stress stimuli,
such as UV radiation, cytokines and osmotic shock. Previous studies
have highlighted the interplay of p38 with the intracellular events
triggered by microtubule-interfering agents (22,23).
It has been shown that CA4, a highly potent methoxylated stilbene
bearing the cis orientation, is capable of stimulating the
activation of both p38 and ERK kinases in the BEL-7402
hepatocellular carcinoma cell line (24). The p38 kinase is involved in the
reassembly of microtubules following exposure to CA4. However, in
contrast to the present study where the inhibition of p38
attenuated the cell cycle arrest of human melanoma cells caused by
3,4,5,4′-TMS, the inhibition of p38 in hepatocellular carcinoma
cells was reported to enhance CA4 cytotoxicity (24). The reason for this apparent
contradiction possibly lies on the structural dissimilarity of the
compounds 3,4,5,4′-TMS and CA4, that determines the precise
mechanism of the antimitotic action. 3,4,5,4′-TMS has been shown to
enhance tubulin polymerization and is predicted to bind to tubulin
at the paclitaxel binding site, whereas CA4 inhibits tubulin
polymerization and binds to tubulin at the colchicine binding site
(11,24). Despite this discrepancy, the data
presented in the current study are in alignment with previous
studies that have identified the p38 pathway as a crucial signaling
network that determines the activity of antimitotic drugs.
In this study, in addition to p38 activation,
3,4,5,4′-TMS was shown to induce the upregulation of Aurora A
expression. The finding that the total levels of Aurora A protein
are increased following treatment with certain antimitotic drugs
has been demonstrated in a recent study in the cases of the
microtubule stabilizers, taccalonolide and laulimalide (25). Aurora A and related
microtubule-associated proteins have been reported to be activated
in response to treatment with antimitotic drugs (25). 3,4,5,4′-TMS exhibited a similar
pattern of Aurora A upregulation. However, although the protein
levels were increased following treatment with 3,4,5,4′-TMS, Aurora
A did not follow the same localization pattern as in the control
cells, while the intensity of the protein expression was weak, as
demonstrated by immunofluoresence experiments. This occurred due to
the extensive mitotic spindle damage caused by 3,4,5,4′-TMS. The
protein Aurora A associates with the mitotic poles and adjacent
spindle microtubules during mitosis and is critical for the proper
formation of the mitotic spindle via the recruitment of several
microtubule-associated proteins. As 3,4,5,4′-TMS causes the
enhancement of tubulin polymerization and as a result, unstable
microtubules and spindle formation, Aurora A was not detected at
the spindle poles during the pro-metaphase and/or metaphase stages.
The increase in the total levels of the protein is consistent with
the incidence of the delayed progression of the cells through
mitosis. The expression of Aurora A was increased in the
3,4,5,4′-TMS-treated A375 cells, possibly as a mechanism to
overcome the mitotic arrest caused by the compound at the
prometaphase stage of cell division.
In addition to the aforementioned findings, the
present study demonstrated the ability of 3,4,5,4′-TMS to inhibit
migration and attachment of A375 human melanoma cells on collagen
type IV-coated plates. This, to the best of our knowledge, provides
the first preliminary evidence of the anti-metastatic activity of
3,4,5,4′-TMS. Human melanoma is a highly metastatic malignancy and
a high percentage of melanoma-associated deaths occur as a result
of secondary metastases of the tumors (26). Molecules resembling 3,4,5,4′ TMS
structure have been characterized as antimetastatic, although the
mechanisms underlying their action remain somewhat enigmatic. One
example includes the compound CA4 that has entered clinical phase
II/III trials for the treatment of several malignancies (27,28).
A second molecule with anti-angiogenic and vascular-disrupting
properties is stilbene 5c, a cis tri-methoxylated
aminostilbene with similar potency to that of CA4 (21). In addition, two recent studies have
documented that the compounds, 4-4′-dihydroxy-trans stilbene and
3,5,4′-trimethoxy trans stilbene (the methoxylated analogue of
resveratrol), exert anti-invasive effects via the downregulation of
MMP-9 and MMP-2 activities, as well as the modulation of the
adhesion molecule, E-cadherin (29,30).
It is possible that 3,4,5,4′-TMS modulates the activities of the
aforementioned proteins, although further research is required to
establish such a hypothesis. To our knowledge, only one study has
addressed the anti-invasive effects of 3,4,5,4′-TMS or DMU-212 on
VEGF-stimulated HUVEC migration (30). 3,4,5,4′-TMS has been reported to be
effective at concentrations between 5 and 80 μM at the 24- and 48-h
time points (31), which is in
agreement with the data presented in this study.
Recent studies conducted in the field of cancer
research and experimental therapeutics have highlighted the
importance of the MAPK signaling pathways in the development of
novel chemotherapeutic drugs for the treatment of cutaneous and/or
metastatic melanoma (2,32). An example includes the compound,
vemurafenib, that is currently undergoing clinical phase II/III
evaluation for the treatment of advanced and/or metastatic melanoma
(32). Vemurafenib inhibits the
kinase domain of the B-RAFV600E mutant protein and results in the
inhibition of MEK and ERK phosphorylation (32). In addition to these promising
findings, MAPK inhibitors are tested in combination therapy in
patients that have developed resistance to standard melanoma
therapy (32). In the present
study, 3,4,5,4′-TMS demonstrated an antagonistic action with the
p38i, SB203580, in A375 cells and additional research is required
to fully elucidate the exact mechanism of action of this compound.
Nevertheless, the results of our current and previous studies
(12), conducted on 3,4,5,4′-TMS
and human melanoma, have indicated that the compound targets the
p38 and the ERK1/2 pathways, and thus a potential interaction
between mitosis inhibition and MAPK signaling is expected. Future
studies are required to focus on the interaction of 3,4,5,4′-TMS
with B-RAF inhibitors in human melanoma.
In conclusion, the current study examined the
anticancer activity of 3,4,5,4′-TMS in A375 human melanoma cells.
The data indicate that 3,4,5,4′-TMS effectively inhibits the
proliferation of melanoma cells through a mechanism involving
mitotic arrest at the prometaphase stage of cell division, via the
disassembly of the mitotic spindle and the induction of apoptosis.
Importantly, the activation of the p38 and Aurora A proteins is
required for the growth-inhibiting action of 3,4,5,4′-TMS, while
the latter compound exhibits anti-invasive and anti-metastatic
properties. Taken together, the results suggest that 3,4,5,4′-TMS
is an effective therapeutic drug for use in the treatment of human
melanoma that warrants further investigation in vivo.
Acknowledgements
This study was funded by the Hellenic non-profitable
company of Dermatological Research.
References
|
1
|
Tripp MK, Watson M, Balk SJ, Swetter SM
and Gershenwald JE: State of the science on prevention and
screening to reduce melanoma incidence and mortality: The time is
now. CA Cancer J Clin. May 27–2016.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Russo AE, Torrisi E, Bevelacqua Y,
Perrotta R, Libra M, McCubrey JA, Spandidos DA, Stivala F and
Malaponte G: Melanoma: Molecular pathogenesis and emerging target
therapies (Review). Int J Oncol. 34:1481–1489. 2009.PubMed/NCBI
|
|
3
|
Wong CY, Helm MA, Kalb RE, Helm TN and
Zeitouni NC: The presentation, pathology, and current management
strategies of cutaneous metastasis. N Am J Med Sci. 5:499–504.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guy GP Jr, Thomas CC, Thompson T, Watson
M, Massetti GM and Richardson LC: Centers for Disease Control and
Prevention (CDC): Vital signs: Melanoma incidence and mortality
trends and projections - United States, 1982–2030. MMWR Morb Mortal
Wkly Rep. 64:591–596. 2015.PubMed/NCBI
|
|
5
|
Pal HC, Hunt KM, Diamond A, Elmets CA and
Afaq F: Phytochemicals for the management of melanoma. Mini Rev Med
Chem. Feb 11–2016.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sale S, Tunstall RG, Ruparelia KC, Potter
GA, Steward WP and Gescher AJ: Comparison of the effects of the
chemopreventive agent resveratrol and its synthetic analog trans
3,4,5,4′-tetra-methoxystilbene (DMU-212) on adenoma development in
the Apc(Min+) mouse and cyclooxygenase-2 in human-derived colon
cancer cells. Int J Cancer. 115:194–201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Atten MJ, Attar BM, Milson T and Holian O:
Resveratrol-induced inactivation of human gastric adenocarcinoma
cells through a protein kinase C-mediated mechanism. Biochem
Pharmacol. 62:1423–1432. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG, et al: Cancer chemopreventive activity of resveratrol, a
natural product derived from grapes. Science. 275:218–220. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mahyar-Roemer M, Köhler H and Roemer K:
Role of Bax in resveratrol-induced apoptosis of colorectal
carcinoma cells. BMC Cancer. 2:272002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sale S, Verschoyle RD, Boocock D, Jones
DJ, Wilsher N, Ruparelia KC, Potter GA, Farmer PB, Steward WP and
Gescher AJ: Pharmacokinetics in mice and growth-inhibitory
properties of the putative cancer chemopreventive agent resveratrol
and the synthetic analogue trans 3,4,5,4′-tetramethoxystilbene. Br
J Cancer. 90:736–744. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma Z, Molavi O, Haddadi A, Lai R, Gossage
RA and Lavasanifar A: Resveratrol analog trans
3,4,5,4′-tetrame-thoxystilbene (DMU-212) mediates anti-tumor
effects via mechanism different from that of resveratrol. Cancer
Chemother Pharmacol. 63:27–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Androutsopoulos VP, Fragiadaki I and Tosca
A: Activation of ERK1/2 is required for the antimitotic activity of
the resveratrol analogue 3,4,5,4′-tetramethoxystilbene (DMU-212) in
human melanoma cells. Exp Dermatol. 24:632–634. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Androutsopoulos V, Arroo RR, Hall JF,
Surichan S and Potter GA: Antiproliferative and cytostatic effects
of the natural product eupatorin on MDA-MB-468 human breast cancer
cells due to CYP1-mediated metabolism. Breast Cancer Res.
10:R392008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Androutsopoulos VP, Ruparelia KC,
Papakyriakou A, Filippakis H, Tsatsakis AM and Spandidos DA:
Anticancer effects of the metabolic products of the resveratrol
analogue, DMU-212: Structural requirements for potency. Eur J Med
Chem. 46:2586–2595. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Piotrowska H, Myszkowski K, Ziółkowska A,
Kulcenty K, Wierzchowski M, Kaczmarek M, Murias M,
Kwiatkowska-Borowczyk E and Jodynis-Liebert J: Resveratrol analogue
3,4,4′,5-tetramethoxystilbene inhibits growth, arrests cell cycle
and induces apoptosis in ovarian SKOV-3 and A-2780 cancer cells.
Toxicol Appl Pharmacol. 263:53–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mazué F, Colin D, Gobbo J, Wegner M,
Rescifina A, Spatafora C, Fasseur D, Delmas D, Meunier P, Tringali
C, et al: Structural determinants of resveratrol for cell
proliferation inhibition potency: Experimental and docking studies
of new analogs. Eur J Med Chem. 45:2972–2980. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schneider Y, Chabert P, Stutzmann J,
Coelho D, Fougerousse A, Gossé F, Launay JF, Brouillard R and Raul
F: Resveratrol analog (Z)-3,5,4′-trimethoxystilbene is a potent
anti-mitotic drug inhibiting tubulin polymerization. Int J Cancer.
107:189–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen CH, Shee JJ, Wu JY, Lin YW, Wu JD and
Liu YW: Combretastatin A-4 inhibits cell growth and metastasis in
bladder cancer cells and retards tumour growth in a murine
orthotopic bladder tumour model. Br J Pharmacol. 160:2008–2027.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grossman D and Altieri DC: Drug resistance
in melanoma: Mechanisms, apoptosis, and new potential therapeutic
targets. Cancer Metastasis Rev. 20:3–11. 2001. View Article : Google Scholar
|
|
20
|
Mooney EE, Ruis Peris JM, O’Neill A and
Sweeney EC: Apoptotic and mitotic indices in malignant melanoma and
basal cell carcinoma. J Clin Pathol. 48:242–244. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alotaibi MR, Asnake B, Di X, Beckman MJ,
Durrant D, Simoni D, Baruchello R, Lee RM, Schwartz EL and Gewirtz
DA: Stilbene 5c, a microtubule poison with vascular disrupting
properties that induces multiple modes of growth arrest and cell
death. Biochem Pharmacol. 86:1688–1698. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Teng M, Jiang XP, Zhang Q, Zhang JP, Zhang
DX, Liang GP and Huang YS: Microtubular stability affects
pVHL-mediated regulation of HIF-1alpha via the p38/MAPK pathway in
hypoxic cardiomyocytes. PLoS One. 7:e350172012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Y, Zhu X, Chen Y, Wang X and Chen R:
p38 and JNK MAPK, but not ERK1/2 MAPK, play important role in
colchicine-induced cortical neurons apoptosis. Eur J Pharmacol.
576:26–33. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Quan H, Xu Y and Lou L: p38 MAPK, but not
ERK1/2, is critically involved in the cytotoxicity of the novel
vascular disrupting agent combretastatin A4. Int J Cancer.
122:1730–1737. 2008. View Article : Google Scholar
|
|
25
|
Rohena CC, Peng J, Johnson TA, Crews P and
Mooberry SL: Chemically diverse microtubule stabilizing agents
initiate distinct mitotic defects and dysregulated expression of
key mitotic kinases. Biochem Pharmacol. 85:1104–1114. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aires DJ, Wick J, Shaath TS, Rajpara AN,
Patel V, Badawi AH, Li C, Fraga GR, Doolittle G and Liu DY:
Economic costs avoided by diagnosing melanoma six months earlier
justify >100 benign biopsies. J Drugs Dermatol. 15:527–532.
2016.PubMed/NCBI
|
|
27
|
Roman BI, De Coen LM, Thérèse FC, Mortier
S, De Ryck T, Vanhoecke BW, Katritzky AR, Bracke ME and Stevens CV:
Design, synthesis and structure-activity relationships of some
novel, highly potent anti-invasive (E)- and (Z)-stilbenes. Bioorg
Med Chem. 21:5054–5063. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zweifel M, Jayson GC, Reed NS, Osborne R,
Hassan B, Ledermann J, Shreeves G, Poupard L, Lu SP, Balkissoon J,
et al: Phase II trial of combretastatin A4 phosphate, carboplatin
and paclitaxel in patients with platinum-resistant ovarian cancer.
Ann Oncol. 22:2036–2041. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maccario C, Savio M, Ferraro D, Bianchi L,
Pizzala R, Pretali L, Forti L and Stivala LA: The resveratrol
analog 4,4′-dihydroxy-trans-stilbene suppresses transformation in
normal mouse fibroblasts and inhibits proliferation and invasion of
human breast cancer cells. Carcinogenesis. 33:2172–2180. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weng CJ, Wu CF, Huang HW, Wu CH, Ho CT and
Yen GC: Evaluation of anti-invasion effect of resveratrol and
related methoxy analogues on human hepatocarcinoma cells. J Agric
Food Chem. 58:2886–2894. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen LK, Qiang PF, Xu QP, Zhao YH, Dai F
and Zhang L: Trans-3,4,5,4′-tetramethoxystilbene, a resveratrol
analog, potently inhibits angiogenesis in vitro and in vivo. Acta
Pharmacol Sin. 34:1174–1182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Russo A, Ficili B, Candido S, Pezzino FM,
Guarneri C, Biondi A, Travali S, McCubrey JA, Spandidos DA and
Libra M: Emerging targeted therapies for melanoma treatment
(Review). Int J Oncol. 45:516–524. 2014.PubMed/NCBI
|