Introduction
Lung cancer is one of the most common malignancies
responsible for the high mortality of patients worldwide (1). Due to aggressive behavior of lung
cancer cells including high metastasis and chemotherapeutic
resistance, lung cancer patients experience tumor recurrence after
successful therapy (2,3). In terms of metastasis, anoikis, a
cellular process of detachment-induced apoptosis, has been accepted
as pivotal inhibitory mechanism (2). Metastatic cancer cells, therefore,
acquire molecular mechanisms to overcome anoikis by upregulating
their survival signals (4).
Likewise, resistance to anticancer drugs has been shown to strongly
associate with the augmentation of several survival pathways
(5–8), and among them phosphatidylinositol
3′-kinase/protein kinase B (PI3K/AKT) is a central signal in
mediating chemotherapeutic resistance in most cancers (9–12).
Not only the active AKT (p-AKT) predominantly stimulates the
survival of the cells, but it also acts through NF-κB to induce
proliferation, invasion, and metastasis (13). In terms of survival, p-AKT
stimulates the expression of anti-apoptotic B-cell lymphoma-2
(Bcl-2) family proteins, Bcl-2 and Mcl-1, while suppresses
proapoptotic proteins such as Bad, Bax, Bid and Bim (14). Bcl-2 regulates the intrinsic
apoptotic pathway by inhibiting with the release of cytochrome
c from the mitochondria or binding to apoptotic protease
activating factor 1 (Apaf-1) through the interaction with Bax
(15). Bcl-2 is overexpressed in
many cancers including small cell lung cancer (SCLC) and non-small
cell lung cancer (NSCLC) and its upregulation was reported to
associate with chemotherapeutic resistance (16,17).
Mounting evidence indicated that certain integrins
such as integrin β1 and β3 are important in triggering AKT
activation (18–21). Integrin β1 is a collagen receptor
that induces specific cell signal distinct from those activated by
other integrins. Adhesion of the cells by means of integrin β1 was
shown to induce the phosphorylation of AKT through PI3K-dependent
mechanism (18). Likewise,
integrin β3 was shown in highly metastatic prostate cancer cells to
induce migration through AKT (20). In addition to mentioned contexts,
the interaction of integrin to extracellular matrix (ECM) provides
cellular AKT survival signals through the activation of focal
adhesion kinase (FAK) (4).
Considerable attention has been paid in searching of
the safe natural compounds that can support chemotherapy as well as
prevent metastasis. Among them, α-lipoic acid (LA) has been
accepted as a promising natural compound to be used in cancer
patients due to its direct anticancer activity against various
cancers with sufficient safety information (22–26).
Previously, we have shown that LA induces lung cancer cell
apoptosis through ROS-dependent Bcl-2 downregulation (24). However, the effect of LA in
regulation of integrins as well as anoikis and cancer response to
chemotherapy are largely unknown. We hypothesize that LA may affect
the pattern of cellular integrins due to its ability to modify ROS
status of the cells. In detail, this compound may shift the redox
status toward increased specific ROS, and such specific ROS
regulate the integrin expression and signaling through AKT as well
as susceptibility to anoikis and drug-induced cell death. Such
knowledge might benefit further investigation and the development
of this natural compound for anticancer approach.
Materials and methods
Cell culture and reagents
Human lung cancer epithelial H460 cells were
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA). The cells were cultivated in Roswell Park Memorial
Institute (RPMI)-1640 medium supplemented with 2 mM L-glutamine,
10% fetal bovine serum (FBS) and 100 U/ml of
penicillin/streptomycin. Cell cultures were maintained in a 5%
CO2 environment at 37°C. Cells were routinely passaged
at preconfluent density using a 0.25% trypsin solution with 0.53 mM
EDTA. RPMI-1640 medium, FBS, L-glutamine, penicillin/streptomycin,
phosphate-buffered saline (PBS), trypsin, and EDTA were purchased
from Gibco (Grand Island, NY, USA). LA, cisplatin, catalase,
Hoechst33342, N-acetylcysteine (NAC), propidium iodide (PI),
dimethylsulfoxide (DMSO), absolute ethanol,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide
(MTT), 2,7-dichlorofluorescein diacetate (DCFH2-DA),
dihydroethidium (DHE) and hydroxyphenyl fluorescein (HPF) were
obtained from Sigma Chemical, Inc. (St. Louis, MO, USA). LA was
prepared by dissolving in absolute ethanol, and stock sample was
further diluted in culture medium. The final concentration of
absolute ethanol used in all of the experiments was 0.1%. The
results from the treated cells were compared with the non-treated
cell exposed to the 0.1% final concentration of absolute ethanol.
Paclitaxel, etoposide, Mn (III) tetrakis (4-benzoic acid) porphyrin
chloride (MnTBAP) were obtained from Calbiochem (San Diego, CA,
USA). Agarose was obtained from Bio-Rad (Hercules, CA, USA).
Antibodies for Bcl-2, Mcl-1, Bax, caspase-3, FAK, phosphorylated
FAK (Y397), AKT, phosphorylated AKT (S473), integrin α5, integrin
αv, integrin β1, integrin β3, β-actin and peroxidase-labeled
specific secondary antibodies were obtained from Cell Signaling
Technology, Inc. (Denver, MA, USA).
Assessment of cell viability
Cell viability was determined by MTT colorimetric
assay. Briefly, after specific treatments, cells in 96-well plates
were incubated with 0.4 mg/ml of MTT for 4 h at 37°C in the dark.
The supernatant was then removed and DMSO was added to dissolve the
formazan product. The intensity was measured at 570 nm using a
microplate reader (Anthros, Durham, NC, USA). The optical density
ratio of treated to non-treated control cells was calculated and
presented in terms of relative cell viability.
Nuclear staining assay
Apoptotic and necrotic cell death were detected by
Hoechst33342 and PI co-staining. After specific treatments, cells
were stained with 10 μM of the Hoechst33342 and 5 μM PI dyes for 30
min at 37°C. The apoptotic cells having condensed chromatin and/or
fragmented nuclei are presented as bright blue fluorescence of
Hoechst33342, while PI stained cells indicate necrosis. Mode of
cell death was visualized and scored under a fluorescent microscope
(Olympus IX51 with DP70).
Proliferation assay
Human lung cancer cells were seeded at a density of
2×103 cells/well in 96-well plates. Cell proliferation
was determined by MTT assay at 0, 24, 48, and 72 h after exposure
to LA at indicated concentrations (0–10 μM) for 48 h. The
absorbance of formazan product which was dissolved by DMSO was
measured by spectrophotometry at 570 nm using a microplate
reader.
Anchorage-independent growth assay
Anchorage-independent growth was analyzed by the
colony formation assay in soft agar. Briefly, 250 μl of the bottom
layer which was a mixture of 1% agarose and completed RPMI-1640
media at a ratio 1:1 was prepared in 24-well plates. A single cell
suspension of treated-H460 cells with non-toxic concentration (0–10
μM) of LA for 48 h was used to prepare the upper layer. The top
layer composed with 0.335% agarose containing the cells at
1×103 in 250 μl. Then completed RPMI medium (250
μl/well) was added and refilled every 3 days. The colony formation
was determined after 2 weeks using a phase-contrast microscope
(Olympus IX51 with DP70).
Western blot analysis
After specific treatments, the cells were incubated
in lysis buffer containing 20 mM Tris-HCl (pH 7.5), 1% Triton
X-100, 150 mM sodium chloride, 10% glycerol, 1 mM sodium
orthovanadate, 50 mM sodium fluoride, 100 mM phenylmethylsulfonyl
fluoride, and a protease inhibitor cocktail (Roche Molecular
Biochemicals, Indianapolis, IN, USA) for 1 h on ice. The cell
lysate was collected, and the protein content was determined using
the BCA protein assay kit (Thermo Scientific, Waltham, MA, USA).
Equal amount of protein from each sample were denatured by heating
at 95°C for 5 min and subsequently loaded onto 10% SDS-PAGE. After
separation, proteins were transferred onto 0.45-μm nitrocellulose
membranes (Bio-Rad). Transferred membranes were blocked for 1 h in
5% non-fat dry milk in TBST (25 mM Tris-HCl pH 7.5, 125 mM NaCl,
and 0.05% Tween-20) and incubated overnight with specific primary
antibodies at 4°C. Then, the membranes were washed three times with
TBST and incubated with appropriate horseradish peroxidase-labeled
secondary antibodies for 2 h at room temperature. The immune
complexes were detected by SuperSignal West Pico chemiluminescent
substrate (Pierce Biotechnology) and quantified using analyst/PC
densitometry software (Bio-Rad).
ROS detection
Intracellular hydrogen peroxide
(H2O2), superoxide anion
(O2·−) and hydroxyl radical (OH·)
were determined by flow cytometry using DCFH2-DA, DHE
and HPF as fluorescent probes, respectively. Cells at
1.5×105 cells/well were seeded overnight into 6-well
plates. Before LA treatment, the cells were incubated either with
10 μM DCFH2-DA, 10 μM DHE or 10 μM HPF for 30 min at
4°C, after which they were washed with PBS and treated with 10 μM
of LA for 1–3 h. After the indicated time, cells were washed,
resuspended in PBS, and immediately analyzed for fluorescence
intensity using FACSCalibur (Beckton-Dickinson, Rutheford, NJ, USA)
at the excitation and emission wavelengths of 488 and 538 nm,
respectively, for detecting DCF fluorescence, at 488 and 610 nm for
DHE and 490 and 515 nm for HPF. Mean fluorescence intensity was
quantified by CellQuest software (Becton-Dickinson, Franklin Lakes,
NJ, USA) analysis of the recorded histograms. Relative fluorescence
was calculated as a ratio of the treated to the non-treated control
fluorescence intensity.
Statistical analysis
All data were expressed as the means ± SD from three
or more independent experiments. Multiple comparisons were examined
for significant differences of multiple groups, using analysis of
variance (ANOVA), followed by individual comparisons with the
Scheffe’s post hoc test. Statistical significance was set at
p<0.05.
Results
Effects of LA on viability of human lung
cancer H460 cells
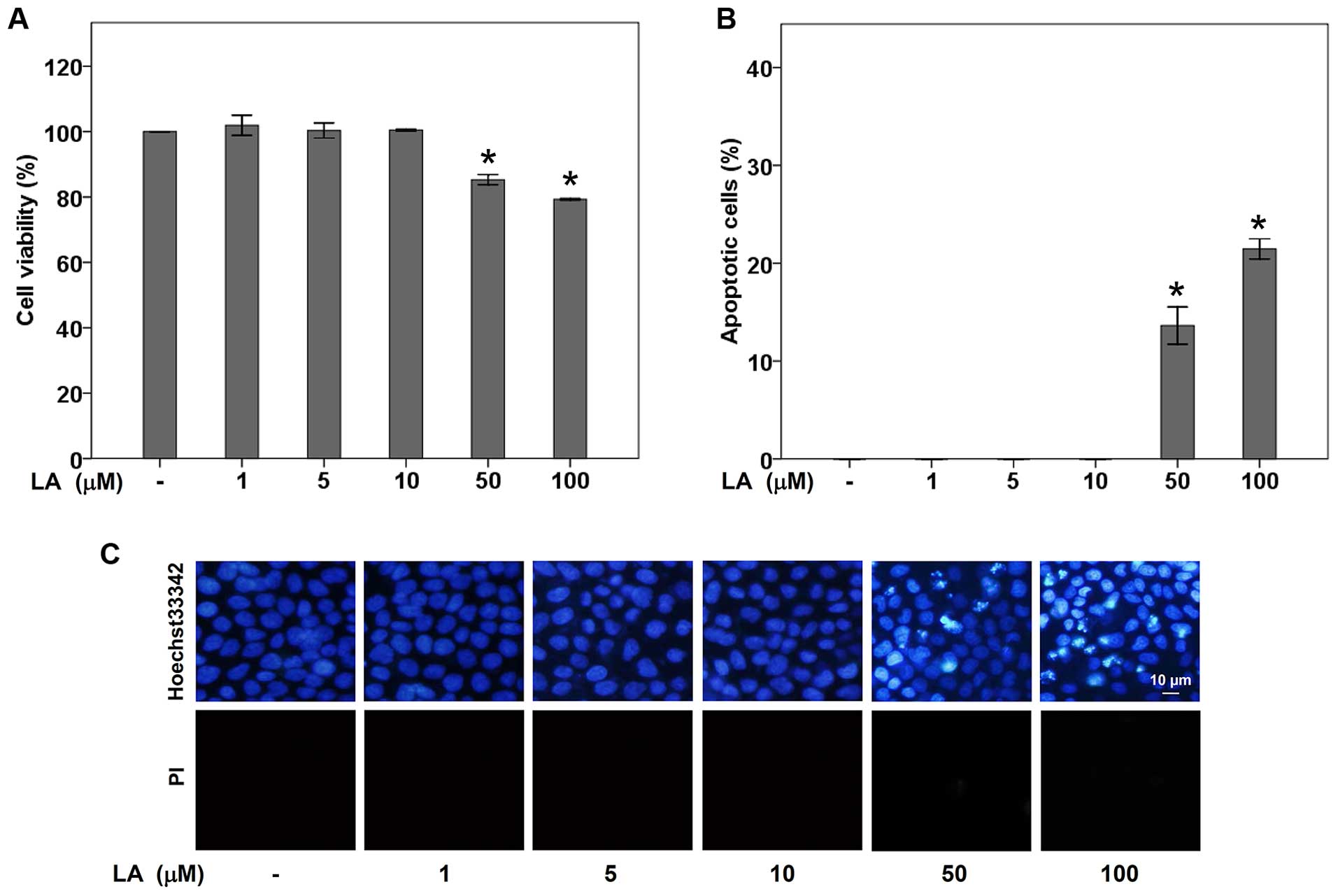
The non-toxic concentrations of LA were first
determined. Human lung cancer H460 cells were cultured in RPMI
medium in the absence or presence of LA (0–100 μM) for 48 h, and
cell viability was determined by MTT viability assay. The results
indicated that treatment with 0–10 μM of LA caused no significant
difference of % cell viability in H460 lung cancer cells compared
with non-treated control cells (Fig.
1A). The cytotoxicity effect of LA was early observed in the
presence of 50 μM LA with ~85% viable cells. To confirm the effect
of LA on cell toxicity, mode of cell death was evaluated by
Hoechst33342 and PI co-staining assay. Fig. 1B and C demonstrate that apoptotic
cells containing condensed and/or fragmented nuclei were not
detectable in response to LA treatment at the concentrations of
0–10 μM. Treatment doses of 50 and 100 μM caused a significant
increase in cell apoptosis over the control, while necrosis was
barely detected in any of the concentrations.
LA sensitizes anoikis and inhibits
anchorage-independent growth
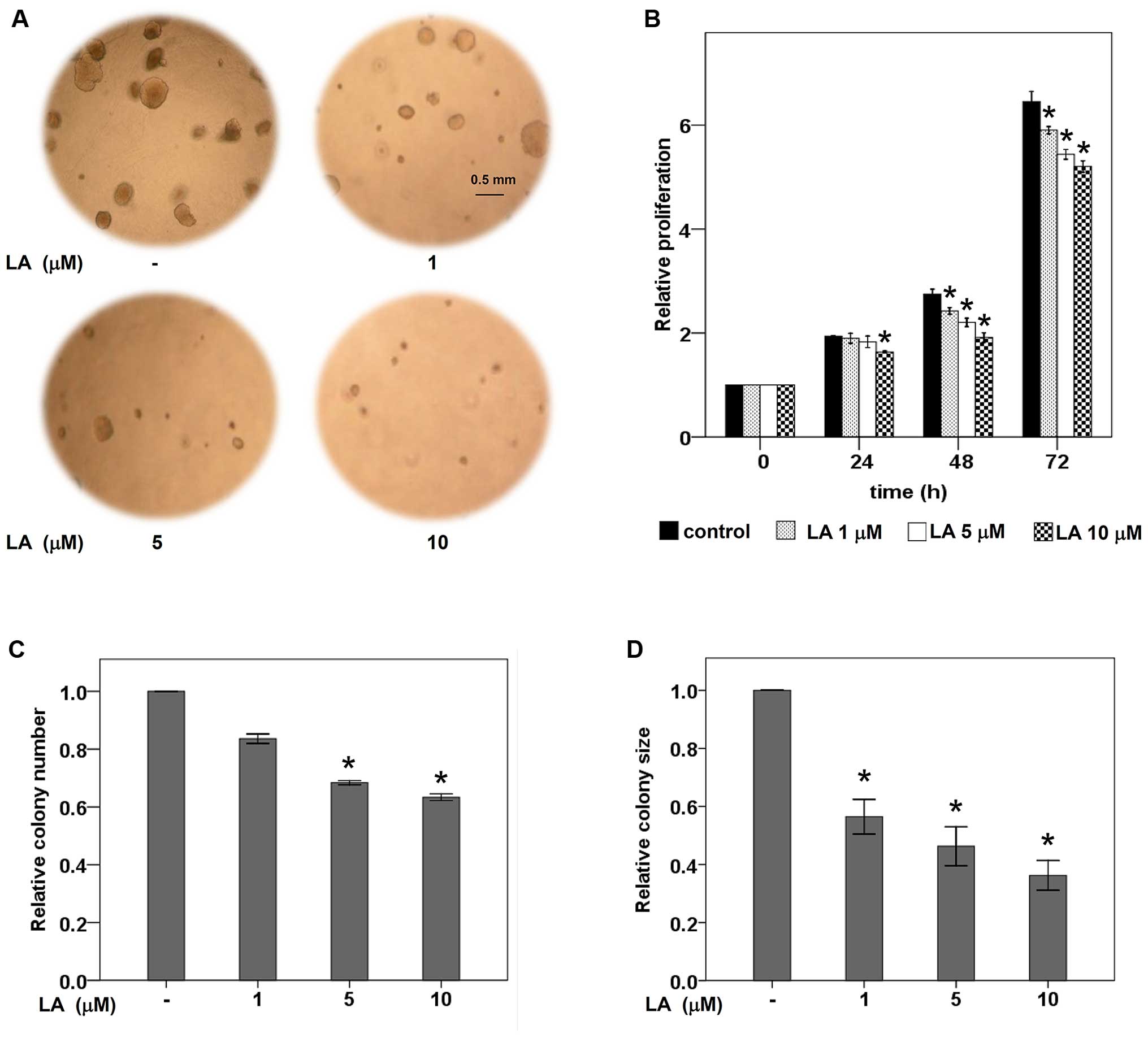
The ability to grow independently of cell adhesion
of tumor cells has been shown to be an important hallmark of
aggressive metastatic cells (4,14).
Next, the effect of LA on anchorage-independent survival and growth
was investigated by soft agar colony formation assay. The cells
were pretreated with LA at non-toxic concentrations for 48 h prior
to subject in the layer of agarose containing RPMI medium as
described in Materials and methods. Fig. 2A, C and D show that LA treatment
significantly inhibited survival and growth of the lung cancer
cells in a dose-dependent manner. Both the number and size of
colonies were significantly suppressed in LA-treated cells in
comparison to those of non-treated control. Our results revealed
that LA treatment sensitizes anoikis and inhibits growth of these
cells in detached condition as indicated by the significant
decrease in the number and size of colonies, respectively.
Additionally, anti-proliferative effect of LA at
non-toxic concentrations (0–10 μM) was further evaluated in normal
cell adherent condition. Fig. 2B
indicates that 10 μM of LA obviously suppressed proliferation of
human lung cancer cells. Taken together, our results have
demonstrated the effects of LA in sensitization of anoikis,
inhibition of tumor growth in both anchorage-dependent and
-independence conditions.
LA depletes integrin β1, and β3
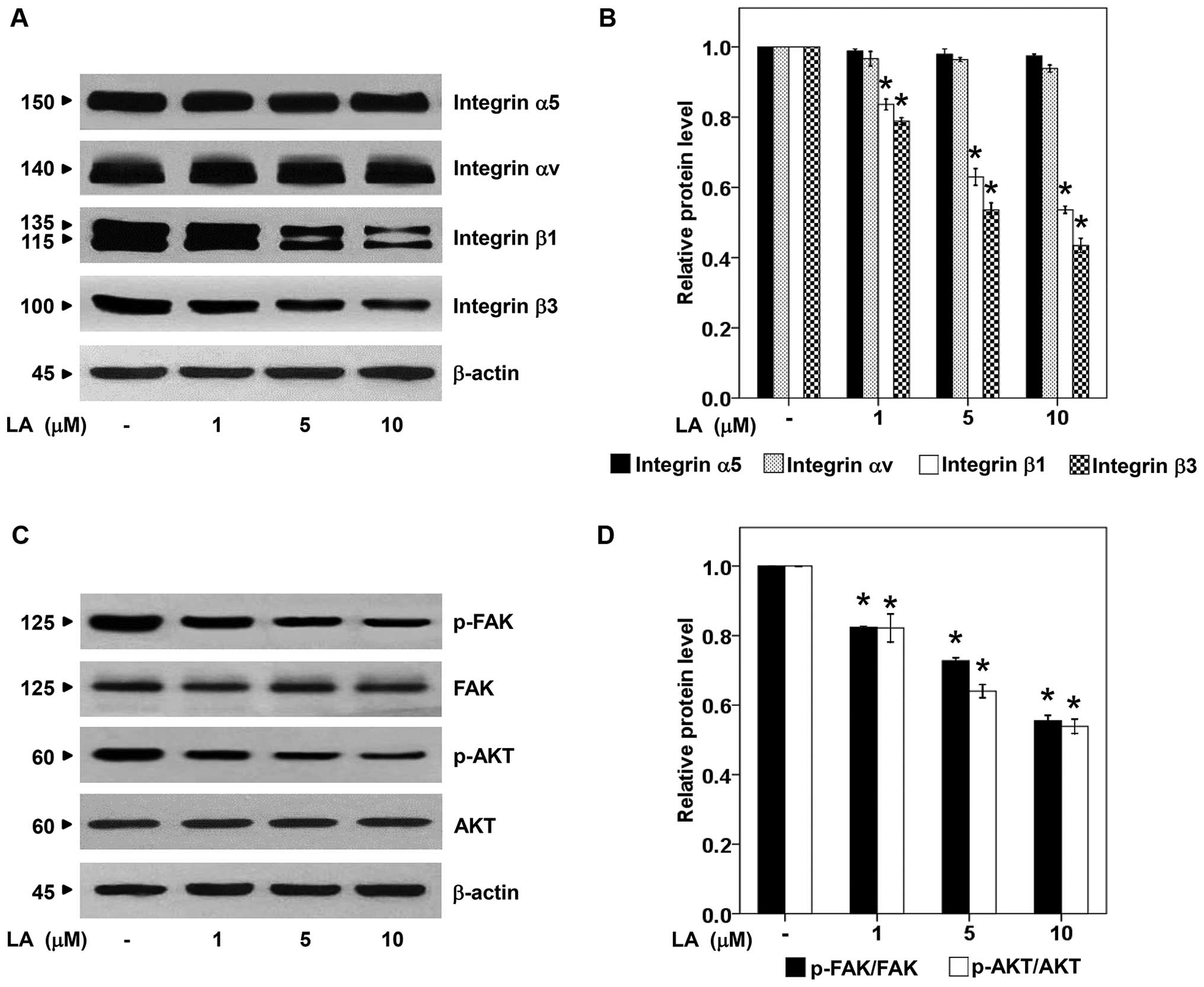
As recent evidence has reported the role of certain
integrins such as integrin α5, αv, β1 and β3 in potentiating lung
cancer anoikis resistance, migration, and metastasis (4,27,28),
the expression of integrins in response to LA treatment was
analyzed. The lung cancer cells were exposure to non-toxic
concentrations of LA as previously described and the levels of
integrin α5, αv, β1 and β3 were examined by western blotting.
Fig. 3A and B illustrate the
dramatic reduction of integrin β1 and β3 in response to LA
treatment. In terms of integrin α5 and αv, we found no
alteration.
Integrins were shown to mediate anoikis resistance
in various cancer cells by increasing cellular survival signals
such as FAK and PI3K/AKT pathways (4,29).
We further tested such downstream molecular targets of integrins
and found that in correlation to integrin β1 and β3 depletion,
p-FAK and p-AKT were strongly downregulated in the presence of LA
at the concentrations of 0–10 μM, whereas, there was no difference
in the level of total FAK and AKT (Fig. 3C and D). These data suggested the
possible mechanism of LA in attenuation of anoikis resistance and
growth via the suppression of integrins and their related
downstream pro-survival pathway.
LA sensitizes chemotherapy-induced
apoptosis in human lung cancer cells
Substantial evidence has indicated the role of
integrins in regulation of chemotherapy resistance (27,30–32).
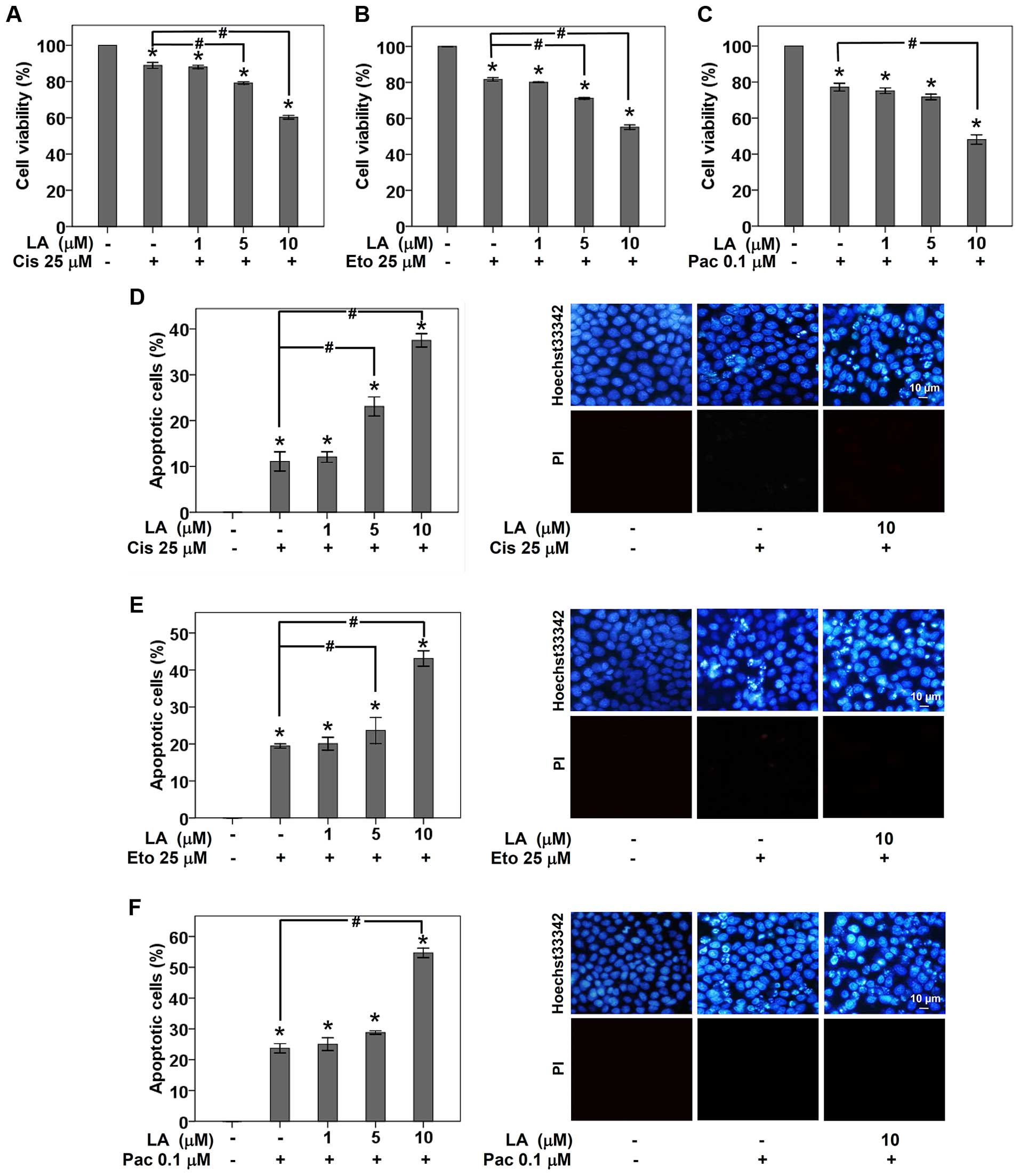
Therefore, we evaluated the effect of LA treatment on the
susceptibility to current chemotherapeutic drugs used for lung
cancer treatment. H460 lung cancer cells were cultured in completed
RPMI medium with or without non-toxic concentrations (0–10 μM) of
LA for 48 h. Cisplatin, etoposide or paclitaxel was then added into
pretreated cells for 24 h, before viable cells were detected by MTT
assay. As presented in Fig. 4A–C,
the incubation of H460 cells with either 25 μM cisplatin, 25 μM
etoposide, or 0.1 μM paclitaxel for 24 h significantly reduced cell
viability to 88.89, 81.62 and 77.17%, respectively. Importantly,
pretreatment of the cells with non-toxic concentration of LA (10
μM) for 48 h prior to drug treatment remarkably sensitized the lung
cancer cells to drug-induced apoptosis (Fig. 4D–F). It is worth noting herein that
the sensitization to cisplatin- and etoposide-induced apoptosis was
also found in lung cancer cell pretreated with of LA at low dose (5
μM) (Fig. 4D and E). These
findings add the information that the observed depletion of
integrin β1 and β3 and their survival counterparts mediated by LA
influence drug susceptibility in these lung cancer cells.
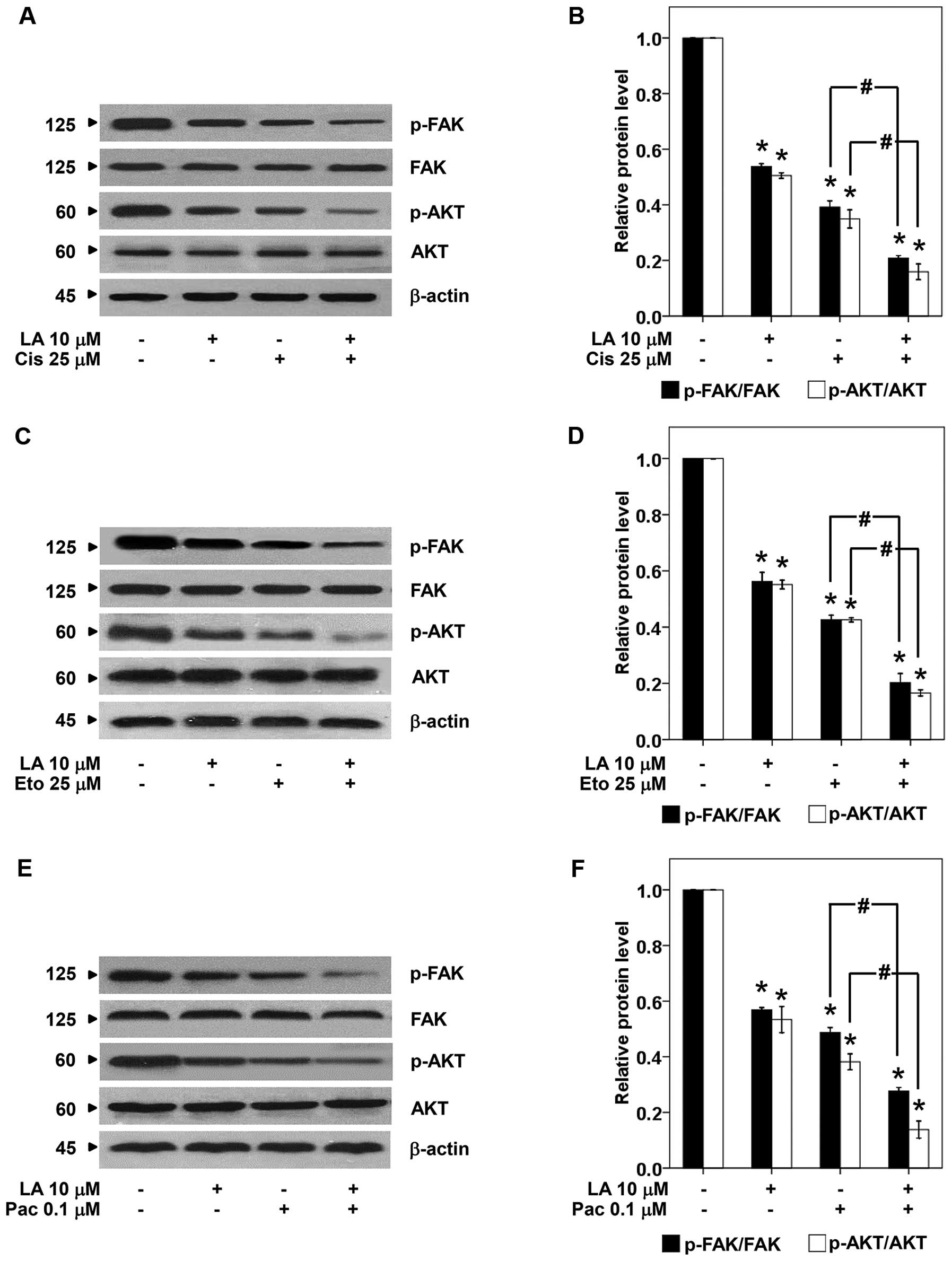
Furthermore, we evaluated the effect of LA on
survival and apoptosis regulatory proteins namely p-FAK, FAK,
p-AKT, AKT, Mcl-1, Bcl-2, Bax, and caspase-3. The cells were
pretreated with 10 μM of LA for 48 h, treated with cisplatin,
etoposide, or paclitaxel for 24 h, and the proteins were determined
by western blotting. As expected treatment of the cells with either
LA or drug alone significantly reduced p-FAK and p-AKT (Fig. 5). The combination of LA and drug
caused further reduction of such survival proteins. Also, the
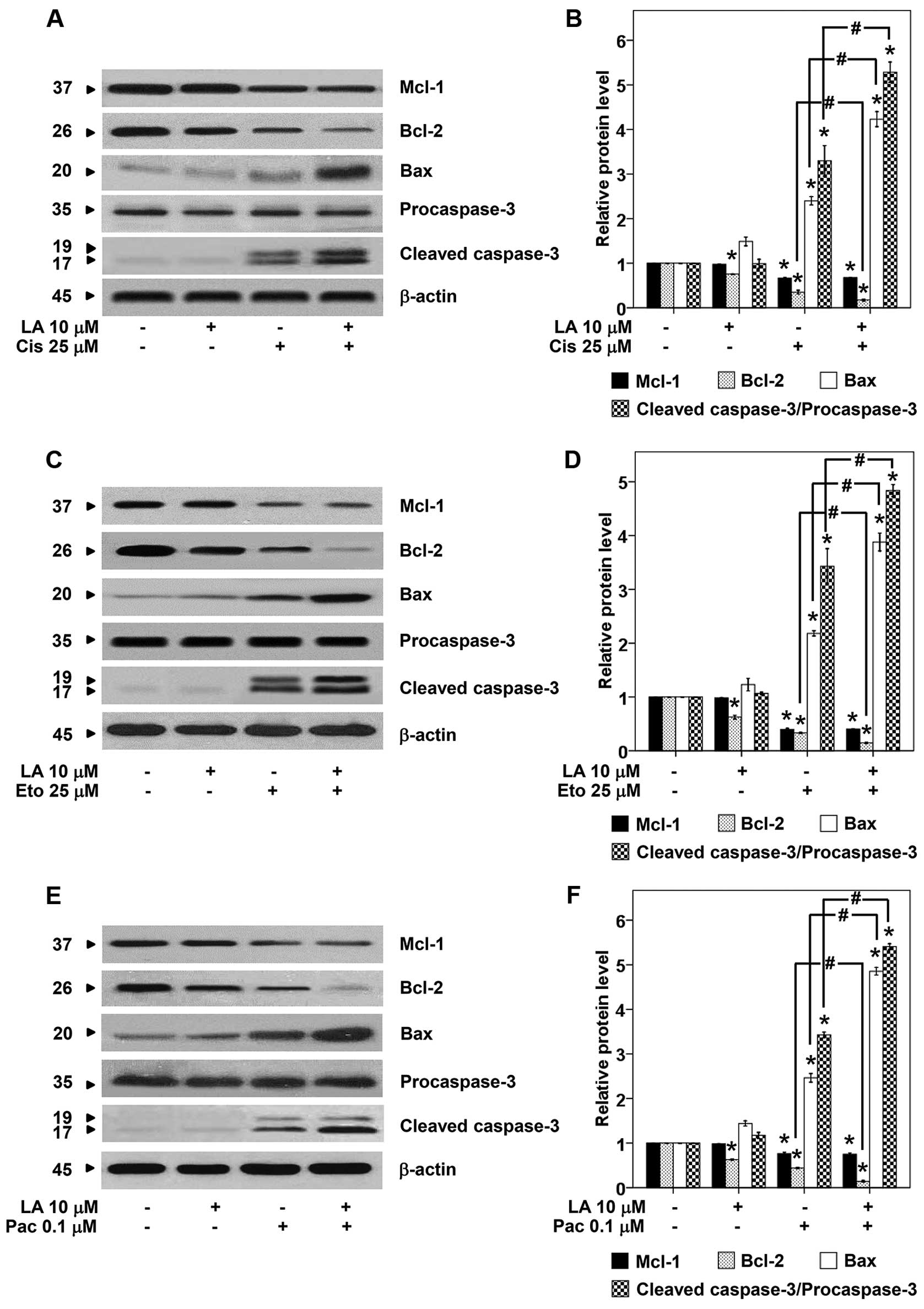
downstream anti- and proapoptotic members of Bcl-2 family protein
were evaluated. We found the key anti-apoptotic proteins Bcl-2 and
Mcl-1 significantly depleted in response to LA and drug treatment,
while the proapoptotic Bax significantly increased (Fig. 6). Also, the cleavage form of
caspase-3 significantly increased in response to the combination
treatment. Taken together, we provide supportive information that
LA sensitizes the cell response to chemotherapeutic drugs via FAK
and AKT-dependent mechanism.
O2·− and
H2O2 regulate integrin expression in LA
treated-lung cancer cells
Reactive oxygen species (ROS) are crucial signaling
molecules in cell biology. They have been shown to regulate protein
expression in many steps of protein processing including
transcription, translation, and degradation (24,33–37).
In order to clarify the underlying mechanism of LA in
downregulation of integrins, we determined the change in cellular
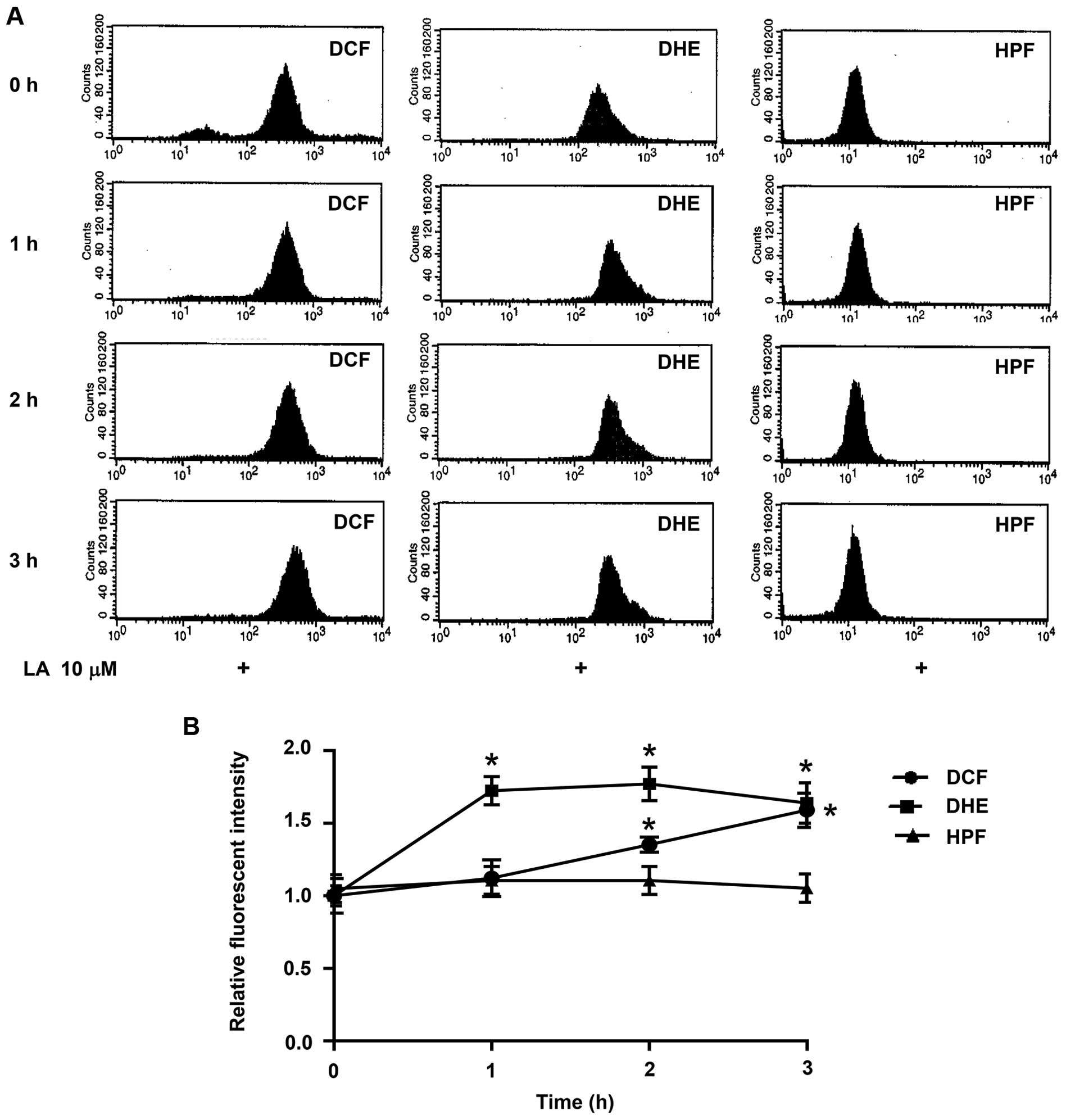
ROS in response to LA treatment. Flow cytometry was used to
evaluate cellular ROS level with the specific ROS fluorescence
dyes. Fluorescent intensity of DCFH2-DA, DHE and HPF
reflects the amounts of H2O2,
O2·− and OH·, respectively.
Fig. 7B indicates that treatment
of the cells with 10 μM LA significantly increased intracellular
O2·− and H2O2. However,
there was no alteration of OH· level in the cell treated
with LA (Fig. 7).
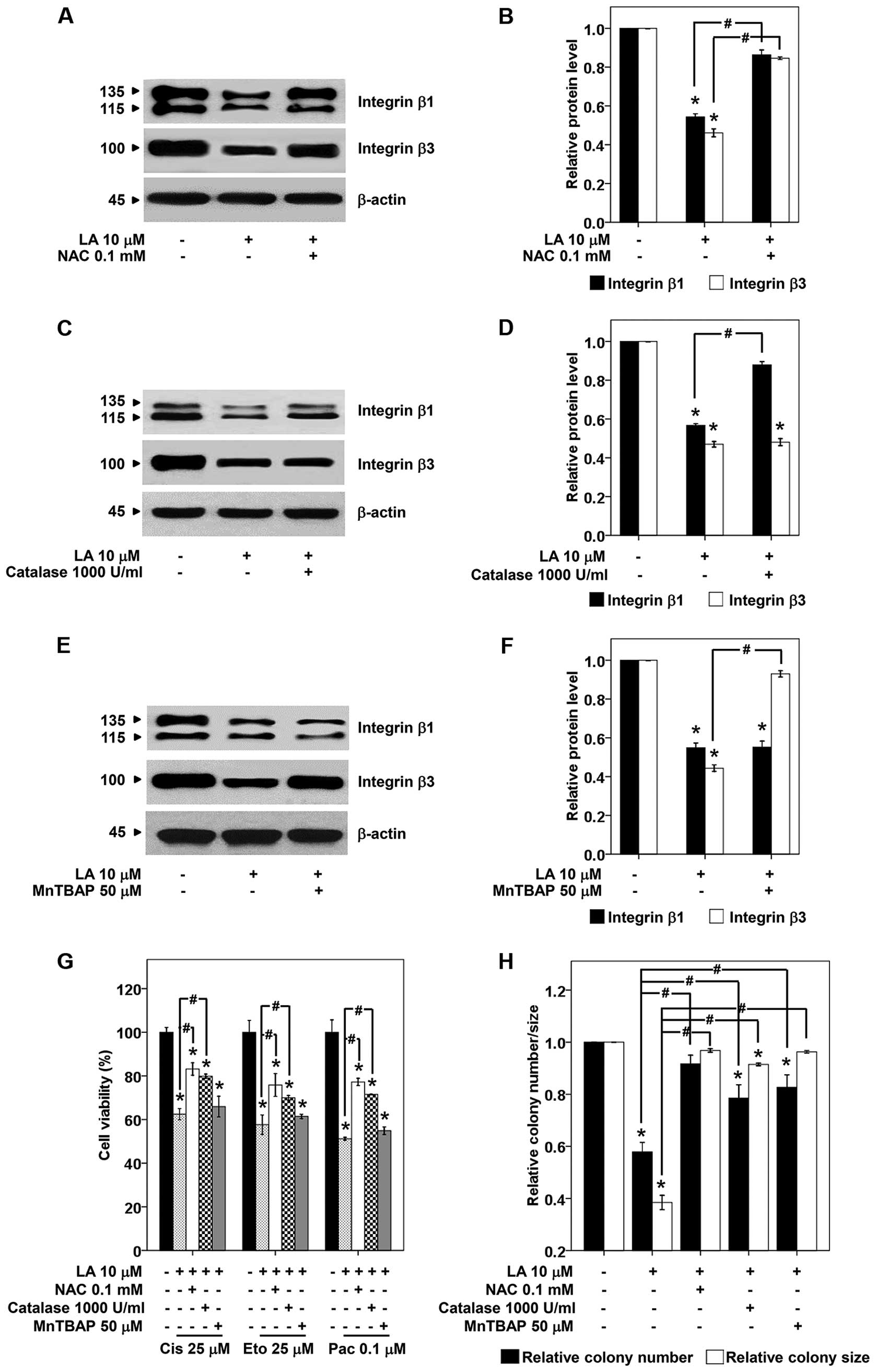
In order to link that such ROS-induced by LA
regulate integrin alteration, broad and specific ROS scavengers
were added prior to LA treatment, and the level of integrin β1 and
β3 was determined by western blot analysis. Fig. 8A and B show that treatment of the
cells with NAC, a broad ROS scavenger successfully restored the
expression of integrin β1 and β3. Attractively,
H2O2 scavenger, catalase specifically
prevented the reduction of integrin β1 but not β3 in LA-treated
H460 cells (Fig. 8C and D).
Integrin β3 was preserved by the pretreatment with
O2·− scavenger (MnTBAP), but such scavenger
has no effect on integrin β1 (Fig. 8E
and F). Thus, our results suggested that LA decreases integrin
β1 via H2O2 induction, while reduces integrin
β3 via O2·−.
To provide supportive information regarding the role
of specific ROS-mediating effect of LA on integrin alteration,
anoikis, and drug responses, the cells were pretreated with
specific ROS scavengers, treated with LA, and subjected to
anchorage-independent growth or drug sensitization assays as
previously described. Results indicated that the pan ROS scavenger
NAC and H2O2 scavenger, catalase abolished
effects of LA on anoikis as well as drug-mediated apoptosis
(Fig. 8G and H). Moreover, MnTBAP,
O2·− scavenger, inhibited anoikis induction
effect but had only slightly effect on chemosensitization (Fig. 8G and H). These results confirmed
the mechanistic roles of specific ROS on LA sensitization of lung
cancer cells to anoikis and chemotherapeutic agents.
Discussion
Due to its safety profile and anticancer activity,
LA has garnered interests as a potential compound for cancer
therapy. LA was shown to sensitize cancer cells to apoptosis by
modulating cellular redox status resulting in the downregulation of
anti-apoptotic and pro-survival proteins (24,26,38).
Herein we provide novel information regarding regulatory effect of
LA on intergrin pattern through specific ROS induction. The
H2O2 and O2·− induced
by LA treatment caused the decrease of integrin β1 and β3,
respectively. As integrins are the key initiators for AKT through
the interaction with ECM proteins (39), and AKT provides major cell survival
signals (40), the decline of such
integrins are likely to weakening the cancer cells.
Substantial evidence indicates that the abilities of
cells to resist anoikis as well as chemotherapeutic drug-induced
apoptosis are major obstacles for the positive clinical outcome in
lung cancer patients (2,3). In general, resistance to
chemotherapeutic agents in cancers is caused by many possible ways
including active drug efflux, high level of survival proteins,
modification of drug targets, and mutation in cellular checkpoint
signals (41). In lung cancer, the
role of AKT on drug resistance has been highlighted as it was shown
to be constitutively active in NSCLC cells and such an activation
status of the protein strongly promotes cellular survival and
resistance to chemotherapy and radiation (9). As generators of cellular AKT signal,
integrins, transmembrane receptors, have garnered increasing
attention in the cancer research field. In particular, the increase
of certain integrins in the cancer cells was shown to be tightly
associated with the augmented metastasis and drug resistance.
Integrin β1 causes chemotherapy resistance through the activation
of PI3K/AKT pathway, and the depletion of such an integrin regained
apoptosis response to cisplatin and gefitinib in lung cancer cells
(27,31,42,43).
Besides, overexpression of integrin β1 is required for anoikis
resistance and anchorage-independent growth (44), suggesting a positive role of this
integrin on cancer metastasis. Likewise, previous study
demonstrated that integrin β3 positively affects
anchorage-independent growth of lung cancer cells (45). Consistent with these findings, we
found that downregulation of integrins β1 and β3 mediated by LA
reversed drug and anoikis resistance (Figs. 2Figure 3–4).
Roles of ROS in cancer pathology are well
established especially in terms of survival and death (46–48),
and aggressive behavior such as chemotherapeutic resistance,
survival in detachment condition, migration and invasion (33,35,49).
LA has been shown to act as a prooxidant (50,51)
as well as an anti-oxidant (52,53)
depending on cell type and cellular redox status. Various studies
have reported on ROS induction activity of LA in cancer cells
(24,25). Our results showed that treatment of
the lung cancer cells with LA resulted in the significant increase
of intracellular O2·− and
H2O2 (Fig.
7). Using specific anti-oxidants, broad ROS scavenger NAC,
O2·− scavenger MnTBAP, and
H2O2 scavenger catalase, successfully
reversed the effects of LA on integrin β1 and β3 expression
(Fig. 8A–F), drug sensitization
(Fig. 8G), anchorage-independent
colony formation (Fig. 8H).
Nevertheless, O2·− and
H2O2 are reactive molecules produced in the
stressed cells and/or in crosstalk between cells and inflammation
within the tumor microenvironment (54). The knowledge gained from this study
may improve the understanding of the molecular basis of ROS on
integrins in lung cancer.
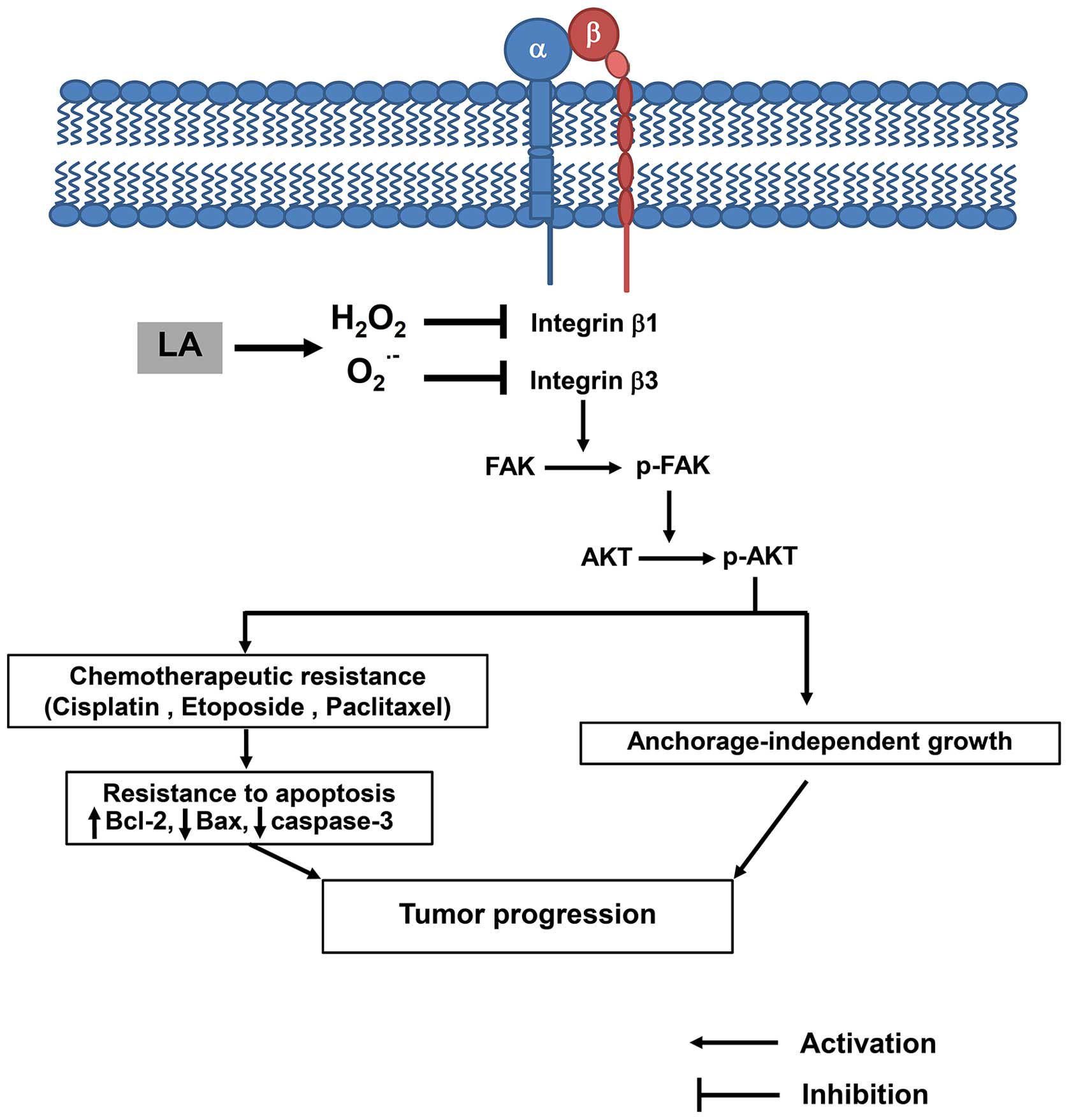
In conclusion, our data provide evidence that LA
regulates integrin expression pattern and sensitizes human lung
cancer cells to anoikis and chemotherapy. LA decreases integrin β1
and β3 via specific ROS induction (Fig. 9). These data provide novel
anticancer approaches that may support the development of LA to
benefit anticancer therapy.
Acknowledgements
This study was supported by Grant for International
Research Integration: Chula Research Scholar, Ratchadaphiseksomphot
Endowment Fund.
References
|
1
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hunter KW, Crawford NPS and Alsarraj J:
Mechanisms of metastasis. Breast Cancer Res. 10(Suppl 1): S2. 2008.
View Article : Google Scholar :
|
|
3
|
Merk J, Rolff J, Dorn C, Leschber G and
Fichtner I: Chemoresistance in non-small-cell lung cancer: Can
multidrug resistance markers predict the response of xenograft lung
cancer models to chemotherapy? Eur J Cardiothorac Surg. 40:e29–e33.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paoli P, Giannoni E and Chiarugi P:
Anoikis molecular pathways and its role in cancer progression.
Biochim Biophys Acta. 1833:3481–3498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Igney FH and Krammer PH: Death and
anti-death: Tumour resistance to apoptosis. Nat Rev Cancer.
2:277–288. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Q-H, Shi M-L, Sun C, Bai J and Zheng
J-N: Role of the ERK1/2 pathway in tumor chemoresistance and tumor
therapy. Bioorg Med Chem Lett. 25:192–197. 2015. View Article : Google Scholar
|
|
7
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar
|
|
8
|
Wilson TR, Longley DB and Johnston PG:
Chemoresistance in solid tumours. Ann Oncol. 17(Suppl 10):
x315–x324. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brognard J, Clark AS, Ni Y and Dennis PA:
Akt/protein kinase B is constitutively active in non-small cell
lung cancer cells and promotes cellular survival and resistance to
chemotherapy and radiation. Cancer Res. 61:3986–3997.
2001.PubMed/NCBI
|
|
10
|
Pommier Y, Sordet O, Antony S, Hayward RL
and Kohn KW: Apoptosis defects and chemotherapy resistance:
Molecular interaction maps and networks. Oncogene. 23:2934–2949.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
West KA, Castillo SS and Dennis PA:
Activation of the PI3K/Akt pathway and chemotherapeutic resistance.
Drug Resist Updat. 5:234–248. 2002. View Article : Google Scholar
|
|
12
|
Wongvaranon P, Pongrakhananon V, Chunhacha
P and Chanvorachote P: Acquired resistance to chemotherapy in lung
cancer cells mediated by prolonged nitric oxide exposure.
Anticancer Res. 33:5433–5444. 2013.PubMed/NCBI
|
|
13
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-κB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chiarugi P and Giannoni E: Anoikis: A
necessary death program for anchorage-dependent cells. Biochem
Pharmacol. 76:1352–1364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adams JM and Cory S: Bcl-2-regulated
apoptosis: Mechanism and therapeutic potential. Curr Opin Immunol.
19:488–496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sartorius UA and Krammer PH: Upregulation
of Bcl-2 is involved in the mediation of chemotherapy resistance in
human small cell lung cancer cell lines. Int J Cancer. 97:584–592.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harada T, Ogura S, Yamazaki K, Kinoshita
I, Itoh T, Isobe H, Yamashiro K, Dosaka-Akita H and Nishimura M:
Predictive value of expression of P53, Bcl-2 and lung
resistance-related protein for response to chemotherapy in
non-small cell lung cancers. Cancer Sci. 94:394–399. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Velling T, Nilsson S, Stefansson A and
Johansson S: beta1-Integ-rins induce phosphorylation of Akt on
serine 473 independently of focal adhesion kinase and Src family
kinases. EMBO Rep. 5:901–905. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhattacharya S, Ray RM and Johnson LR:
Integrin beta3-mediated Src activation regulates apoptosis in IEC-6
cells via Akt and STAT3. Biochem J. 397:437–447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng DQ, Woodard AS, Tallini G and
Languino LR: Substrate specificity of alpha(v)beta(3)
integrin-mediated cell migration and phosphatidylinositol
3-kinase/AKT pathway activation. J Biol Chem. 275:24565–24574.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ivaska J, Nissinen L, Immonen N, Eriksson
JE, Kähäri VM and Heino J: Integrin alpha 2 beta 1 promotes
activation of protein phosphatase 2A and dephosphorylation of Akt
and glycogen synthase kinase 3 beta. Mol Cell Biol. 22:1352–1359.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dozio E, Ruscica M, Passafaro L, Dogliotti
G, Steffani L, Marthyn P, Pagani A, Demartini G, Esposti D,
Fraschini F, et al: The natural antioxidant alpha-lipoic acid
induces p27(Kip1)-dependent cell cycle arrest and apoptosis in
MCF-7 human breast cancer cells. Eur J Pharmacol. 641:29–34. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feuerecker B, Pirsig S, Seidl C, Aichler
M, Feuchtinger A, Bruchelt G and Senekowitsch-Schmidtke R: Lipoic
acid inhibits cell proliferation of tumor cells in vitro and in
vivo. Cancer Biol Ther. 13:1425–1435. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moungjaroen J, Nimmannit U, Callery PS,
Wang L, Azad N, Lipipun V, Chanvorachote P and Rojanasakul Y:
Reactive oxygen species mediate caspase activation and apoptosis
induced by lipoic acid in human lung epithelial cancer cells
through Bcl-2 down-regulation. J Pharmacol Exp Ther. 319:1062–1069.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Simbula G, Columbano A, Ledda-Columbano
GM, Sanna L, Deidda M, Diana A and Pibiri M: Increased ROS
generation and p53 activation in α-lipoic acid-induced apoptosis of
hepatoma cells. Apoptosis. 12:113–123. 2007. View Article : Google Scholar
|
|
26
|
Wenzel U, Nickel A and Daniel H: α-Lipoic
acid induces apoptosis in human colon cancer cells by increasing
mitochondrial respiration with a concomitant
O2−·-generation. Apoptosis. 10:359–368. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maiuthed A and Chanvorachote P: Cisplatin
at sub-toxic levels mediates integrin switch in lung cancer cells.
Anticancer Res. 34:7111–7117. 2014.PubMed/NCBI
|
|
28
|
Caccavari F, Valdembri D, Sandri C,
Bussolino F and Serini G: Integrin signaling and lung cancer. Cell
Adhes Migr. 4:124–129. 2010. View Article : Google Scholar
|
|
29
|
Guadamillas MC, Cerezo A and Del Pozo MA:
Overcoming anoikis - pathways to anchorage-independent growth in
cancer. J Cell Sci. 124:3189–3197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aoudjit F and Vuori K: Integrin signaling
inhibits paclitaxel-induced apoptosis in breast cancer cells.
Oncogene. 20:4995–5004. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hodkinson PS, Mackinnon AC and Sethi T:
Extracellular matrix regulation of drug resistance in small-cell
lung cancer. Int J Radiat Biol. 83:733–741. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meadows GG: Integration/Interaction of
Oncologic Growth. Springer Science & Business Media; 2005,
View Article : Google Scholar
|
|
33
|
Luanpitpong S, Talbott SJ, Rojanasakul Y,
Nimmannit U, Pongrakhananon V, Wang L and Chanvorachote P:
Regulation of lung cancer cell migration and invasion by reactive
oxygen species and caveolin-1. J Biol Chem. 285:38832–38840. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pongrakhananon V, Nimmannit U, Luanpitpong
S, Rojanasakul Y and Chanvorachote P: Curcumin sensitizes non-small
cell lung cancer cell anoikis through reactive oxygen
species-mediated Bcl-2 downregulation. Apoptosis. 15:574–585. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang L, Chanvorachote P, Toledo D, Stehlik
C, Mercer RR, Castranova V and Rojanasakul Y: Peroxide is a key
mediator of Bcl-2 down-regulation and apoptosis induction by
cisplatin in human lung cancer cells. Mol Pharmacol. 73:119–127.
2008. View Article : Google Scholar
|
|
36
|
Rungtabnapa P, Nimmannit U, Halim H,
Rojanasakul Y and Chanvorachote P: Hydrogen peroxide inhibits
non-small cell lung cancer cell anoikis through the inhibition of
caveolin-1 degradation. Am J Physiol Cell Physiol. 300:C235–C245.
2011. View Article : Google Scholar :
|
|
37
|
Chanvorachote P, Pongrakhananon V,
Wannachaiyasit S, Luanpitpong S, Rojanasakul Y and Nimmannit U:
Curcumin sensitizes lung cancer cells to cisplatin-induced
apoptosis through superoxide anion-mediated Bcl-2 degradation.
Cancer Invest. 27:624–635. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi DY, Liu HL, Stern JS, Yu PZ and Liu
SL: Alpha-lipoic acid induces apoptosis in hepatoma cells via the
PTEN/Akt pathway. FEBS Lett. 582:1667–1671. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Giancotti FG and Ruoslahti E: Integrin
signaling. Science. 285:1028–1032. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zahreddine H and Borden KL: Mechanisms and
insights into drug resistance in cancer. Front Pharmacol. 4:282013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sethi T, Rintoul RC, Moore SM, MacKinnon
AC, Salter D, Choo C, Chilvers ER, Dransfield I, Donnelly SC,
Strieter R, et al: Extracellular matrix proteins protect small cell
lung cancer cells against apoptosis: A mechanism for small cell
lung cancer growth and drug resistance in vivo. Nat Med. 5:662–668.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Morello V, Cabodi S, Sigismund S,
Camacho-Leal MP, Repetto D, Volante M, Papotti M, Turco E and
Defilippi P: β1 integrin controls EGFR signaling and tumorigenic
properties of lung cancer cells. Oncogene. 30:4087–4096. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schooley AM, Andrews NM, Zhao H and
Addison CL: β1 integrin is required for anchorage-independent
growth and invasion of tumor cells in a context dependent manner.
Cancer Lett. 316:157–167. 2012. View Article : Google Scholar
|
|
45
|
Ninsontia C and Chanvorachote P: Ouabain
mediates integrin switch in human lung cancer cells. Anticancer
Res. 34:5495–5502. 2014.PubMed/NCBI
|
|
46
|
Clerkin JS, Naughton R, Quiney C and
Cotter TG: Mechanisms of ROS modulated cell survival during
carcinogenesis. Cancer Lett. 266:30–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Luanpitpong S, Chanvorachote P, Nimmannit
U, Leonard SS, Stehlik C, Wang L and Rojanasakul Y: Mitochondrial
super-oxide mediates doxorubicin-induced keratinocyte apoptosis
through oxidative modification of ERK and Bcl-2 ubiquitination.
Biochem Pharmacol. 83:1643–1654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Luanpitpong S, Nimmannit U, Chanvorachote
P, Leonard SS, Pongrakhananon V, Wang L and Rojanasakul Y: Hydroxyl
radical mediates cisplatin-induced apoptosis in human hair follicle
dermal papilla cells and keratinocytes through Bcl-2-dependent
mechanism. Apoptosis. 16:769–782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Songserm T, Pongrakhananon V and
Chanvorachote P: Sub-toxic cisplatin mediates anoikis resistance
through hydrogen peroxide-induced caveolin-1 up-regulation in
non-small cell lung cancer cells. Anticancer Res. 32:1659–1669.
2012.PubMed/NCBI
|
|
50
|
Dicter N, Madar Z and Tirosh O: α-lipoic
acid inhibits glycogen synthesis in rat soleus muscle via its
oxidative activity and the uncoupling of mitochondria. J Nutr.
132:3001–3006. 2002.PubMed/NCBI
|
|
51
|
Çakatay U, Kayali R, Sivas A and Tekeli F:
Prooxidant activities of alpha-lipoic acid on oxidative protein
damage in the aging rat heart muscle. Arch Gerontol Geriatr.
40:231–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Biewenga GP, Haenen GR and Bast A: The
pharmacology of the antioxidant lipoic acid. Gen Pharmacol.
29:315–331. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Packer L, Witt EH and Tritschler HJ:
alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med.
19:227–250. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ziech D, Franco R, Georgakilas AG,
Georgakila S, Malamou-Mitsi V, Schoneveld O, Pappa A and
Panayiotidis MI: The role of reactive oxygen species and oxidative
stress in environmental carcinogenesis and biomarker development.
Chem Biol Interact. 188:334–339. 2010. View Article : Google Scholar : PubMed/NCBI
|