Introduction
2,3,5,6-tetramethylpyrazine (TMP) is an active
alkaloid extracted from the traditional Chinese medicinal herb
Ligusticum chuanxiong, which has been widely used in the
clinical treatment of neurovascular, cardiovascular and liver
diseases in China (1–6). In recent years, increasing number of
studies have suggested that TMP possesses anticancer properties
(7–11). However, the mechanisms have yet to
be elucidated. Epithelial-mesenchymal transition (EMT) is a
developmental process characterized by the acquisition of
invasiveness in epithelial tumors frequently accompanied by loss of
their polarity and acquiring migratory properties of mesenchymal
cells (12), which has been viewed
as a critical process of invasion and metastasis in various types
of cancer cells (13) associated
with drug resistance (14).
Natural killer (NK) cells are a type of cytotoxic
lymphocyte capable of mediating early innate immune responses to
viral infections and recognition of transformed malignant cells
(15,16). NK group 2, member D (NKG2D) is an
activating receptor expressed by NK and T cells that play a major
role in cancer immunosurveillance (17). MHC class I chain-related molecules
A and B (MICA/B) and UL16 binding protein (ULBP) 1–6 molecules are
the ligands of NKG2D (NKG2DLs), upregulation of MICA/B and ULBP in
tumor cells suggests that NKG2D may exerts a key role in response
to infection and transformation (18,19).
However, the regulation of the NKG2D signaling during the EMT of
carcinomas have yet to be elucidated.
Clear cell renal cell carcinoma (ccRCC) is one of
the most common kidney cancers, and there are several studies
showing that EMT is associated with carcinoma invasion and
metastasis, but the relationship between ccRCC and EMT is not yet
clarified (20). In our previous
study (21), we found that the
content of soluble MICA (sMICA) in serum of patients with renal
cell carcinoma was significantly higher than in healthy adults, the
percentage of membrane MICA (mMICA) were significantly increased in
human kidney cancer tissues and ccRCC cell lines. Furthermore, we
indicated that the relationship of expression of NKG2D and MICA/B
play an important role in mediation of the cytotoxic NK cells in
ccRCCs and sMICA-mediated tumor immune escape through
down-regulated NKG2D expression. In the present study, we tested
the hypothesis that TMP inhibits proliferation, migration and
invasion of ccRCC cells through regulation of NKG2D-MICA signaling
during the EMT by using the ccRCC cell lines 786-O and A498.
Materials and methods
Drugs and cell culture
Tetramethypyrazine (TMP Enterprises, St. Louis, MO,
USA) was dissolved in dimethyl sulfoxide (DMSO; Sinopharm Chemical
Reagent Co., Ltd., Shanghai, China) as a stock solution. The
dilutions of all of the reagents were freshly prepared before each
experiment. Hence, normal saline was used as a vehicle control in
all experiments. RCC cell lines (786-O, A498) were obtained from
the Chinese Academy of Medical Sciences and maintained in RPMI-1640
(HyClone Laboratories, Inc., Logan, UT, USA) supplemented with 10%
(v/v) heat-inactivated fetal bovine serum (FBS; Gibco, Carlsbad,
CA, USA), containing 100 U/ml penicillin and 100 μg/ml streptomycin
in a humidified atmosphere of 5% CO2 at 37°C. Cells were
harvested by brief incubation in 0.02% (w/v) EDTA in PBS. In
blocking experiments, anti-MICA/B mAbs were added to the RCC cell
suspension at 10 μg/ml and incubated at 37°C for 30 min before the
addition of target cells.
Growth and cell viability analysis
The viability of ccRCC cells was evaluated by
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphe-nyltetrazolium bromide
(MTT) assay. Briefly, cells (5×103/well) seeded in
96-well plates were incubated with increasing concentrations of TMP
for 24, 48 and 72 h, respectively. The controls were treated with
an equal volume of the drug vehicle DMSO, but the applied
concentration did not exhibit a modulating effect on cell growth.
Optical density (OD) of each culture was read by Universal
Microplate Reader at wavelength of 570 nm.
Reverse transcription polymerase chain
reaction (RT-PCR)
Total RNA was extracted from kidney tissue using a
Qiagen RNeasy kit (Qiagen, Basel, Switzerland). Complementary DNA
(cDNA) First Strand was produced using a SuperScript First-Strand
Synthesis system using oligo (dt) antisense primers (Invitrogen,
Lucerne, Switzerland). MICA and MICB transcripts were amplified
from cDNA by 30 cycles of polymerase chain reaction. The primer
sequences were as follows: MICA (517 bp) upstream
(5′-ACACCCAGCAGTGGGGGGAT-3′); downstream primer
(5′-GCAGGGAATTGAATCCCAGCT-3′); MICB (678 bp) upstream
′5-ACACCCAGCAGT GGGGGGAT-3′, downstream
5′-AGCAGTCGTGAGTTTGCCCAC-3′. The amplification conditions consisted
of denaturizing (95°C for 60 sec), followed by annealing (56°C for
60 sec), and extension (72°C for 60 sec). Amplified fragments were
analyzed in 1.5% agarose gel electrophoresis in the presence of
ethidium bromide (Sigma-Aldrich). β-actin (500 bp) was used as an
internal control for the amount of RNA input.
Flow cytometry
The Annexin V-FITC/PI apoptosis detection kit
according to the manufacturer’s instructions (Roche Diagnostics,
Indianapolis, IN, USA). Briefly, ccRCC cells were seeded in 6-well
plates and treated with 100 μM TMP for 48 h. Cells 1×106
were collected and suspended in 500 μl binding buffer, and 5 μl
Annexin V-FITC and 5 μl PI were added to each sample and incubated
in the dark for 15 min. The cell surface phosphatidylserine in
apoptotic cells was quantitatively estimated by using Annexin
V/FITC apoptosis detection kit according to manufacturer’s
instructions (Roche Diagnostics). Data were detected using FACScan
flow cytometry (FACS LSRFortessa; BD Biosciences, San Jose, CA,
USA). Triplicate experiments with triplicate samples were
performed.
Terminal deoxynucleotidyl
transferase-mediated deoxyuridine triphosphate nick-end
labelled
Apoptosis was detected using a One Step TUNEL
apoptosis assay kit (Beyotime Institute of Biotechnology, Shanghai,
China) according to the protocol. Briefly, 5×103 cells
were seeded on coverslips and cultured in RPMI-1640 with or without
100 μM TMP containing 10% FBS in 24-well plates for 24 h. After
fixing with 4% formaldehyde, the cells were washed with PBS and
permeabilized using 0.2% Triton X-100. Following equilibration,
cells were labeled using TdT reaction mix and incubated for 1 h at
37°C in a humidified chamber. Subsequently, 2X SSC was added for 15
min to stop the reaction. Apoptotic cells were detected using a
Nikon Eclipse 90i microscope (Nikon Corp., Tokyo, Japan) (22).
Wound healing assay
Cells (5×105) were plated onto 24-well
plates per well and were grown to >90% confluence for 48 h. The
medium was removed, and in the cell monolayers a wound line was
created by manually scraping the cells with a 10 μl plastic pipette
tip. Debris was removed from the culture by washing with PBS two
times, and then the cells cultured in RPMI-1640 containing 1% FBS
with TMP (at 100 μM). Images (magnification, ×40) were taken at 0
and 24 h using a Nikon Eclipse 90i microscope (Nikon Corp.). These
experiments were repeated three times.
Transwell migration and invasion
assays
The invasion of RCC cells were determined using a
Transwell assay, briefly, experiments were performed in RPMI-1640
containing 10% FBS as a chemoattractant. Following 100 μM TMP
treatment for 48 h, cells were re-suspended in RPMI-1640 containing
1% FBS and added (1×106 cells/100 μl) to the upper
chamber consisting of a polycarbonate membrane (8-μm pore size;
Corning Life Science). After a 48-h incubation at 37°C under 5%
CO2, cells that had not migrated were removed, whereas
migrated cells were fixed in 4% paraformaldehyde for 10 min at room
temperature and stained with Crystal Violet Staining Solution kit
(Beijing Solarbio Science and Technology Co., Ltd., Beijing,
China). The number of migrated cells were counted using Nikon
Eclipse 90i microscope.
Western blotting
Western blotting was performed to analyze the
expression of apoptotic and EMT signal pathway-related proteins in
ccRCC cells. Briefly, 786-O and A498 cells seeded in 6-well plates
were exposed to 100 μM TMP for 48 h. The cells were harvested and
lysates were fractionated by 10% SDS-PAGE. The proteins were
electro-transferred onto PVDF membranes, and then the membranes
were blocked in 5% skimmed milk-Tris-buffered saline plus Tween-20
solution and incubated with primary antibodies E-cadherin,
vimentin, fibronectin, matrix metalloproteinase-9 (MMP-9) and
TGF-β1. Following incubation with peroxidase-conjugated AffiniPure
goat anti-rabbit IgG (1:5,000; ZSGB-Bio Co., Ltd., Beijing, China).
The bound antibodies were visualized by using an enhanced
chemiluminescence reagent (Millipore, Billerica, MA, USA) followed
by Bio-Rad Image Lab™. Data was expressed as the relative density
of the protein normalized to β-actin. The percentages of increase
or decrease of protein were estimated by comparison to vehicle
control (100%).
Immunofluorescence staining analysis
In order to investigate the inhibition mechanism of
TMP on ccRCC cell line, the epithelial-mesenchymal transition
signal pathway-related markers E-cadherin, vimentin and fibronectin
were detected by cell immunofluorescence techniques. Cells were
seeded on coverslips in 6-well plates (6×104/well) with
or without treated with 100 μM TMP for 24 h, and then fixed in 4%
paraformaldehyde for 30 min and washed three times with PBS. The
slips were permeabilized for 10 min with 0.1% Triton X-100 and
washed three times with PBS, and then cells were blocked with 10%
normal goat serum for 30 min. Cells were incubated overnight at 4°C
with primary antibodies against E-cadherin (1:100 dilution; Cell
Signaling Technology), vimentin (1:100, ab92547; Abcam). Later they
were stained in the dark with tetraethyl rhodamine isothiocyanate
(TRITC) (1:200; ZSGB-Bio Co.) as secondary antibodies. Images were
taken with a Nikon Eclipse 90i microscope. The staining was
analyzed with the image-analyzing system, Image-Pro Plus 6 (Media
Cybernetics, Inc., Rockville, MD, USA).
Isolation of natural killer cells
Primary human NK cells were were isolated with an NK
cell isolation kit (Miltenyi Biotec, Inc., Auburn, CA, USA) and
obtained as previously described (23). Briefly, peripheral blood
lymphocytes were isolated by density gradient centrifugation
(Biochrom GmbH, Berlin, Germany) from heparinized venous blood
obtained from normal healthy volunteer donors and depletion of
plastic-adherent monocytic cells. Isolation of highly pure NK cells
was achieved by depletion of magnetically labeled cells. The
percentage of NK cells after isolation was evaluated using
FITC-conjugated anti-CD3 mAbs and PE-conjugated anti-CD56 mAbs (BD
Biosciences) in flow cytometry, and routinely exceeded 95%, with
CD3 contamination in purified NK cells typically <1%.
Cytotoxicity assay
NK cell cytotoxicity against renal carcinoma cell
lines was assessed using the calcein-AM release assay, as
previously described (24). Renal
carcinoma target cells (T) were cultured in medium with or without
100 μM TMP for 48 h. Then, the NK cell cytotoxicity assay was
carried out by incubating these cells with NK effector cells (E) at
various T/E ratios (1/20–1/5) for 3 h at 37°C. In blocking
experiments, anti-MICA/B mAbs were added to the NK cell suspension
and incubated at 37°C for 30 min before the addition of target
cells. The number of dead cells was estimated by measuring the
fluorescence intensity in medium. The fluorescence intensity in
medium released spontaneously from target cells and the total
maximum fluorescence intensity released from all target cells by
treatment with 1% Triton X-100 were also determined.
Statistical analysis
Data of continuous variables are presented as means
± SD. Comparisons between the treatments were analyzed by one-way
ANOVA and performed with SPSS 10.0 software. P<0.05 was deemed
statistically significant.
Results
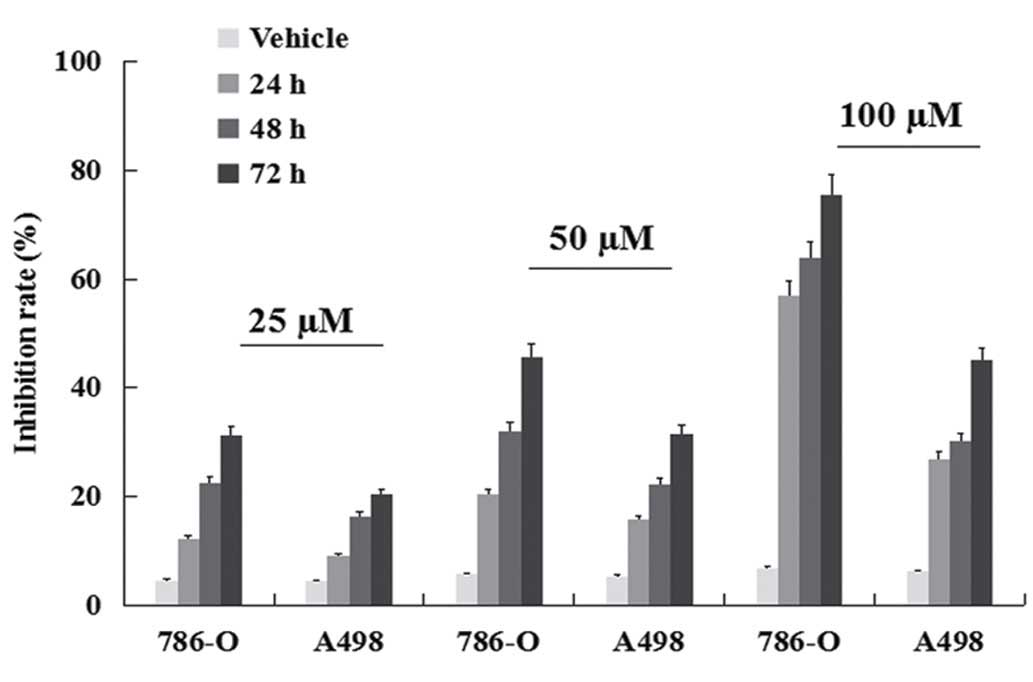
Inhibition of viability
ccRCC cell lines were treated with TMP for 24, 48
and 72 h, respectively and then subjected to the MTT assay. TMP
effectively inhibited the viability of human clear cell renal cell
carcinoma 786-O and A489 cells. As shown in Fig. 1, the inhibition rate increased from
24 to 72 h. The maximum inhibition rate of 75.63% was found with
use of 100 μM for 72-h treatment in 786-O. These results indicated
that TMP had a dose- and time-dependent antiproliferative effect on
ccRCC cells in the range of 25–100 μM for up to 24, 48 and 72 h of
exposure. Moreover, a better inhibition rate was shown in 786-O
than A498 cell line (P<0.05).
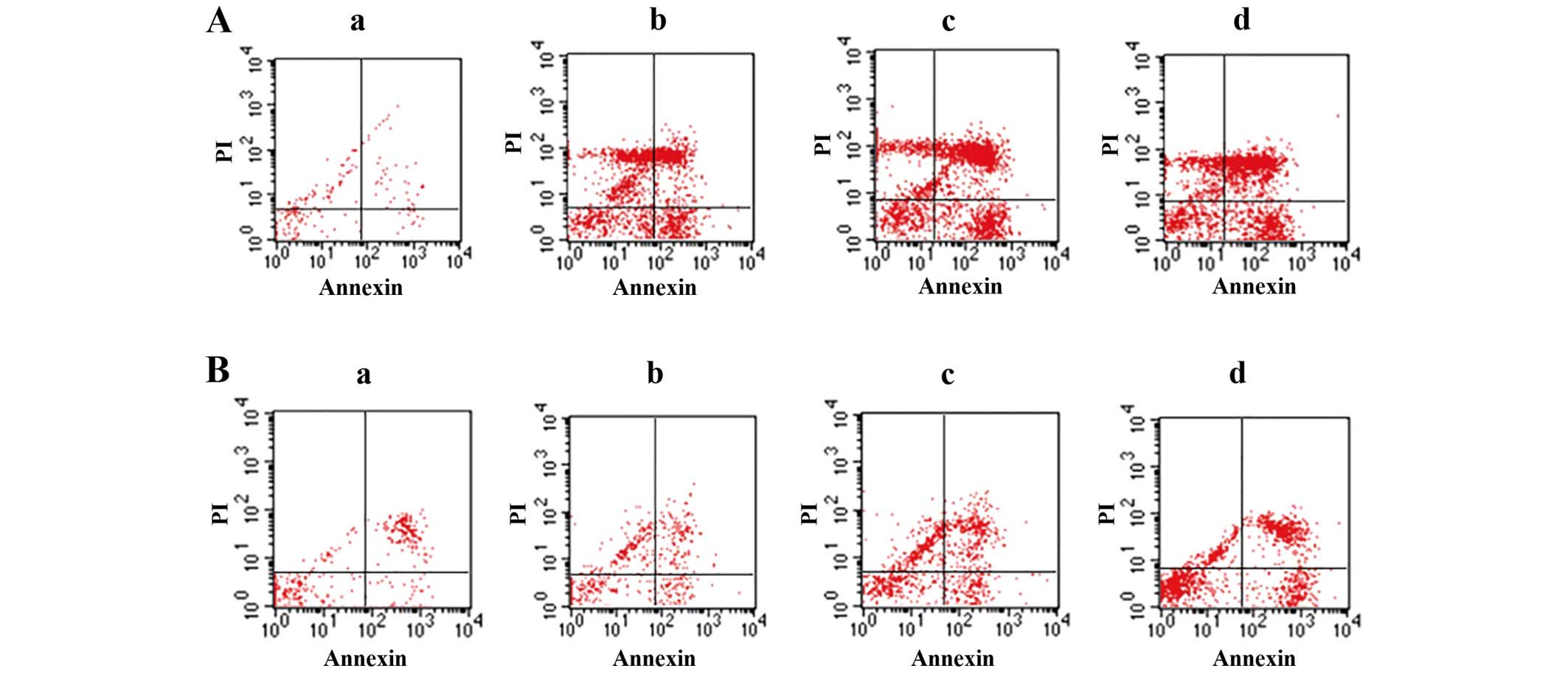
TMP induction of ccRCC cell
apoptosis
To evaluate the TMP-induced cell apoptosis, ccRCC
cells were then stained with Annexin V/FITC and were analyzed by
flow cytometry. As shown in Fig.
2, the percentage of apoptotic cells was gradually increased in
786-O and A498 after treatment 24 h with TMP at the concentrations
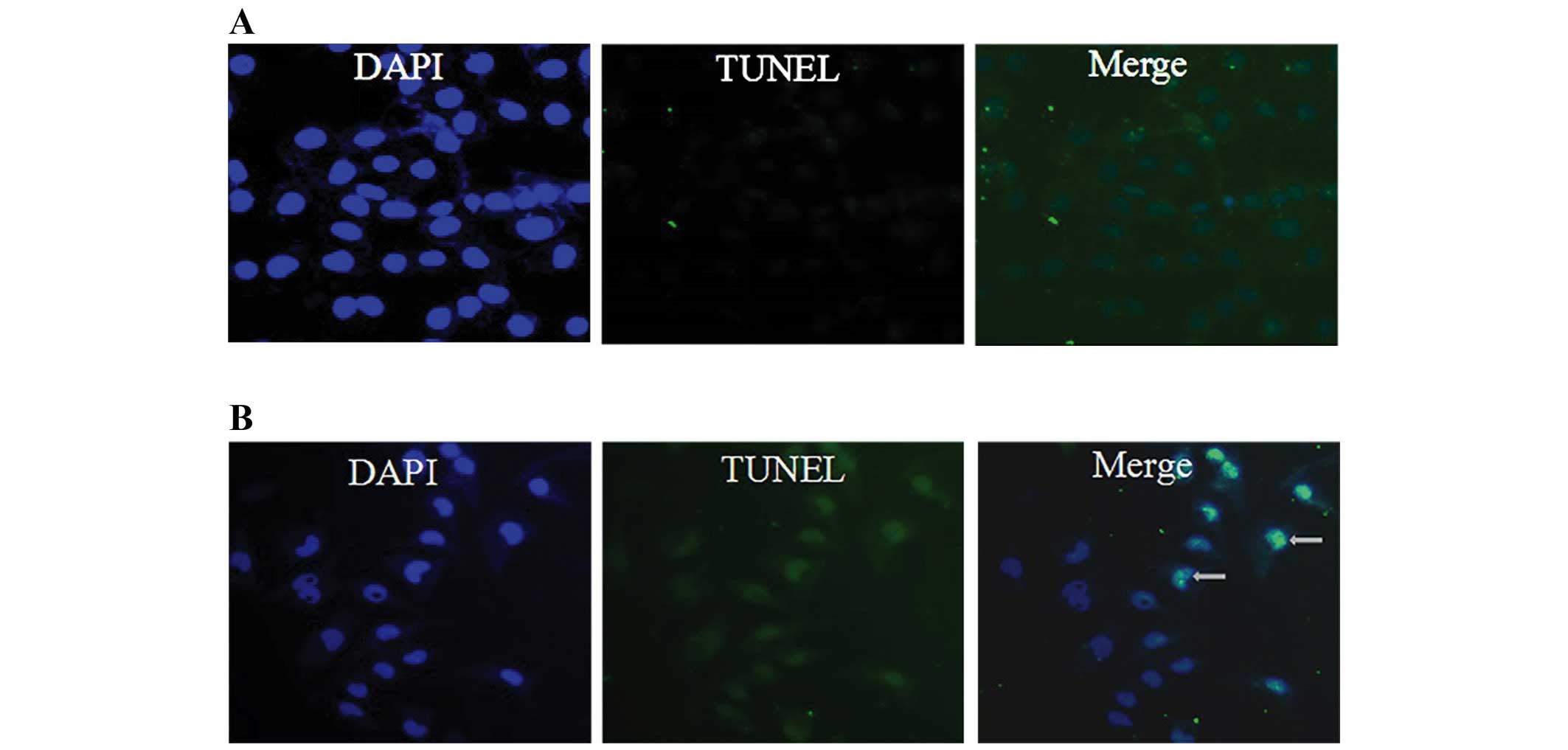
of 25, 50 and 100 μM. Furthermore, we examined the morphologic
changes in 786-O by TUNEL staining as shown in Fig. 3. When cells were cultured with 100
μM TMP for 24 h, the apoptotic morphologic changes were observed as
compared with the vehicle control.
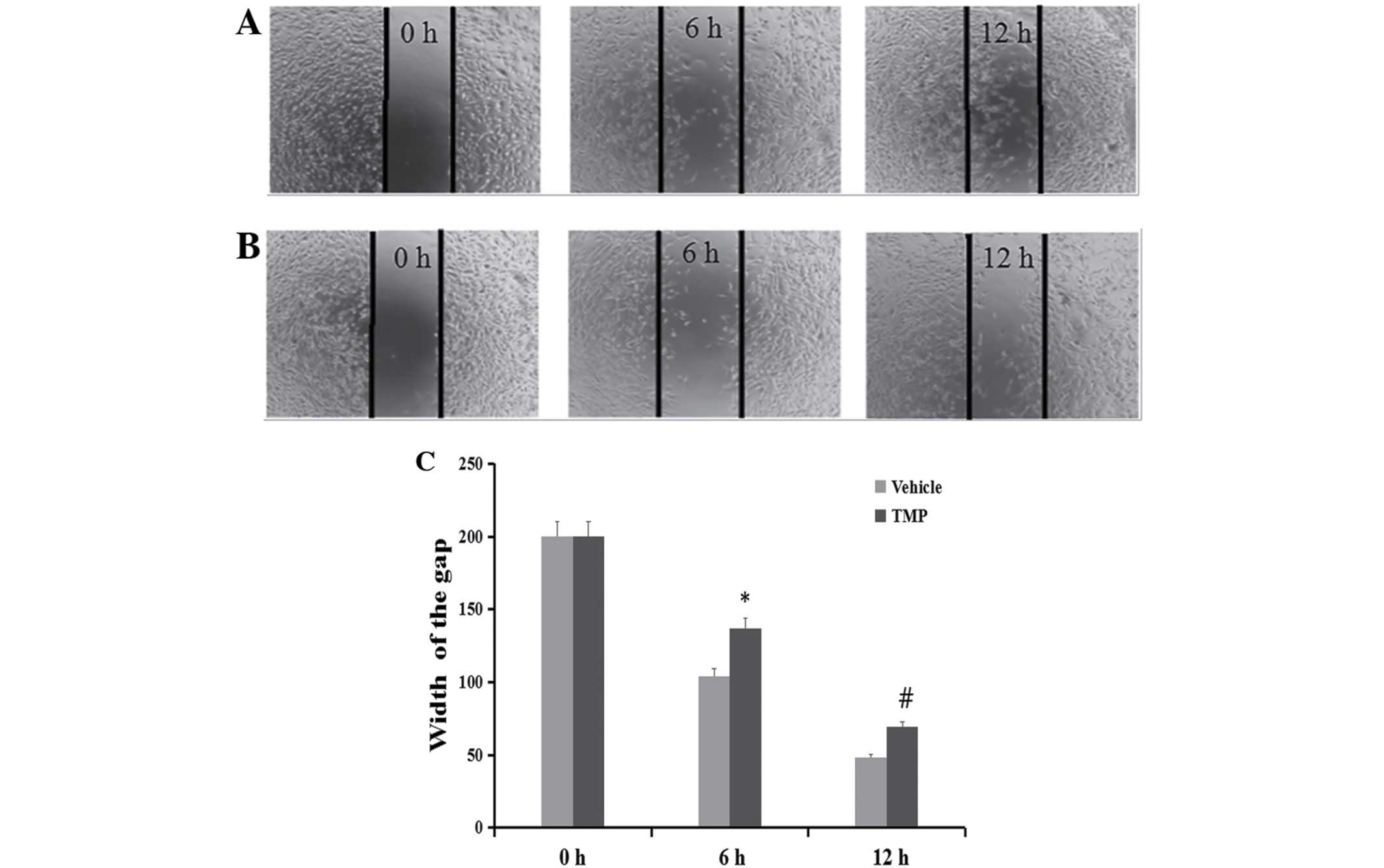
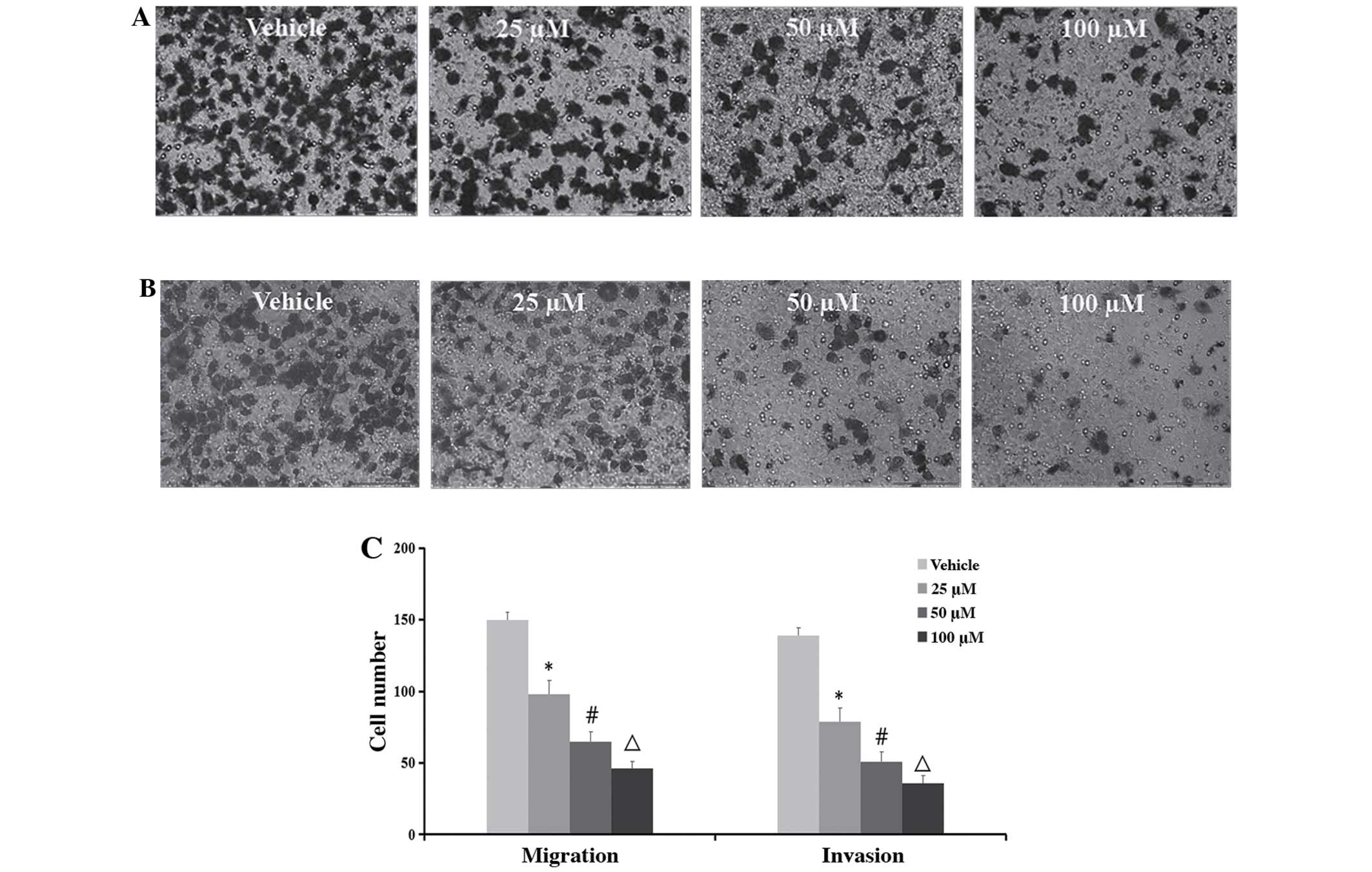
Inhibition of cell migration and
invasion
To determine whether TMP has effects on the
migration and invasion of ccRCC cells, the wound healing and
Transwell assays were performed. As demonstrated in Figs. 4 and 5, the migratory ability of the 786-O was
significantly inhibited after treatment of the cells with 100 μM of
TMP at 6 and 12 h through wound healing assay. On the other hand,
in the concentrations of 25, 50 and 100 μM TMP clearly inhibited
migration and invasion ability of cells through a Transwell insert
filter at 24 h. Together, these results demonstrated that TMP
inhibition of migration and invasion of ccRCC cells was dose- and
time-dependent.
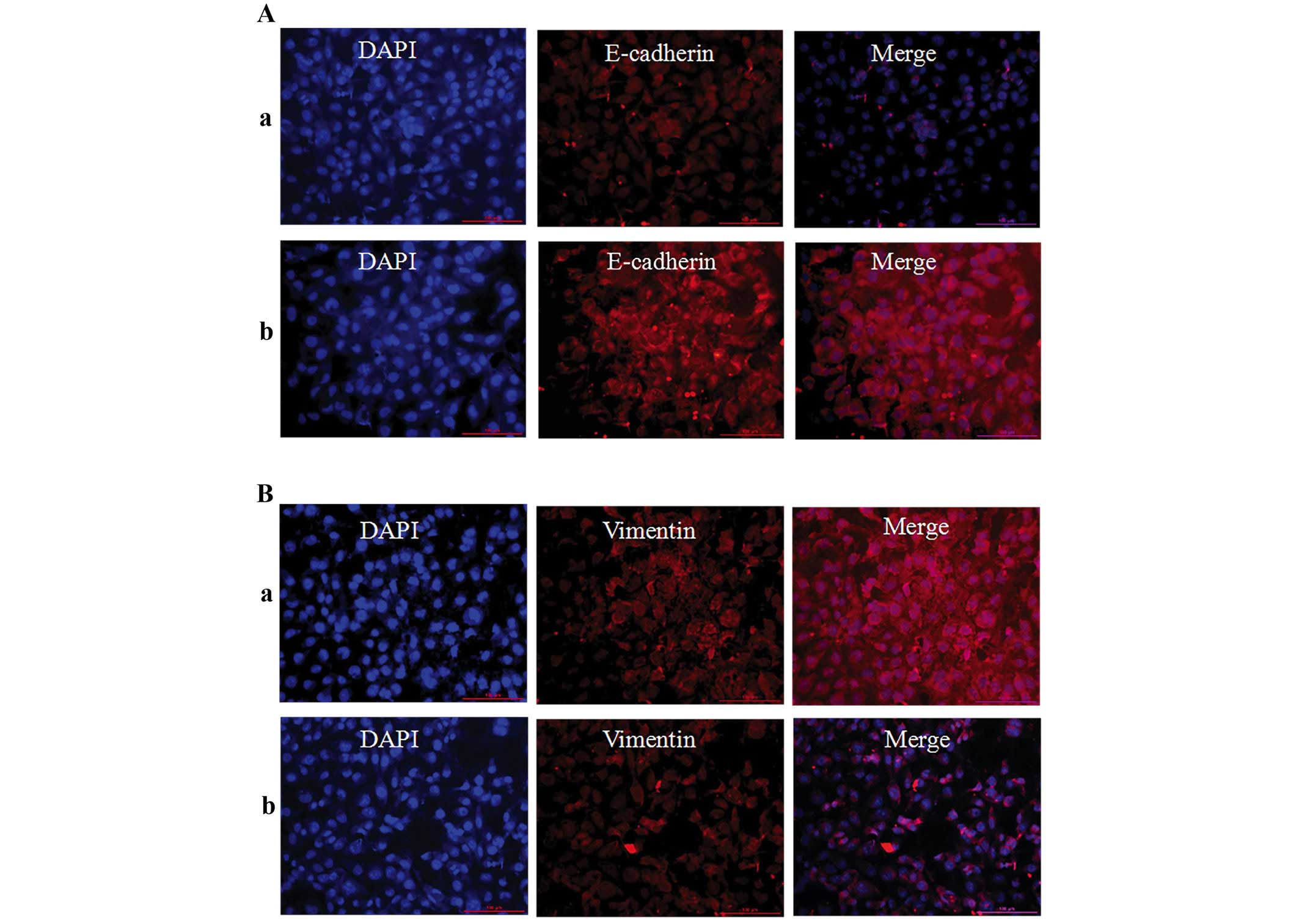
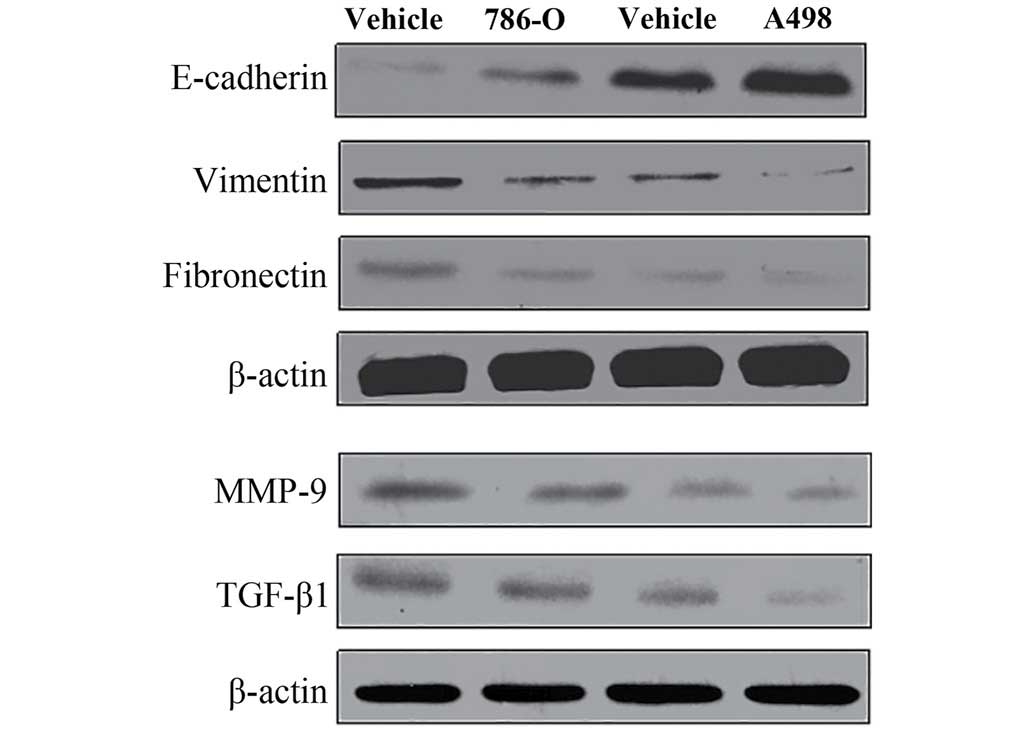
Inhibition of epithelial-to-mesenchymal
transition markers, MMP-9 and TGF-β1
Immunofluorescence techniques and western blotting
were used to assess EMT-related proteins (E-cadherin, vimentin and
fibronectin) in 786-O cells. As shown in Figs. 6 and 7, the protein expression level of
E-cadherin was significantly upregulated, but vimentin and
fibronectin were significantly downregulation when the cells were
treatment with 100 μM of TMP for 24 h. These results revealed that
TMP was effective in inhibiting EMT process. Furthermore, western
blotting results showed that the protein expression of MMP-9 and
TGF-β1 were significantly decreased after treatment with 100 μM TMP
for 24 h. These results indicated the collapse of MMP and the
inhibition of TGF-β1 signaling in ccRCC cells when treated with
TMP.
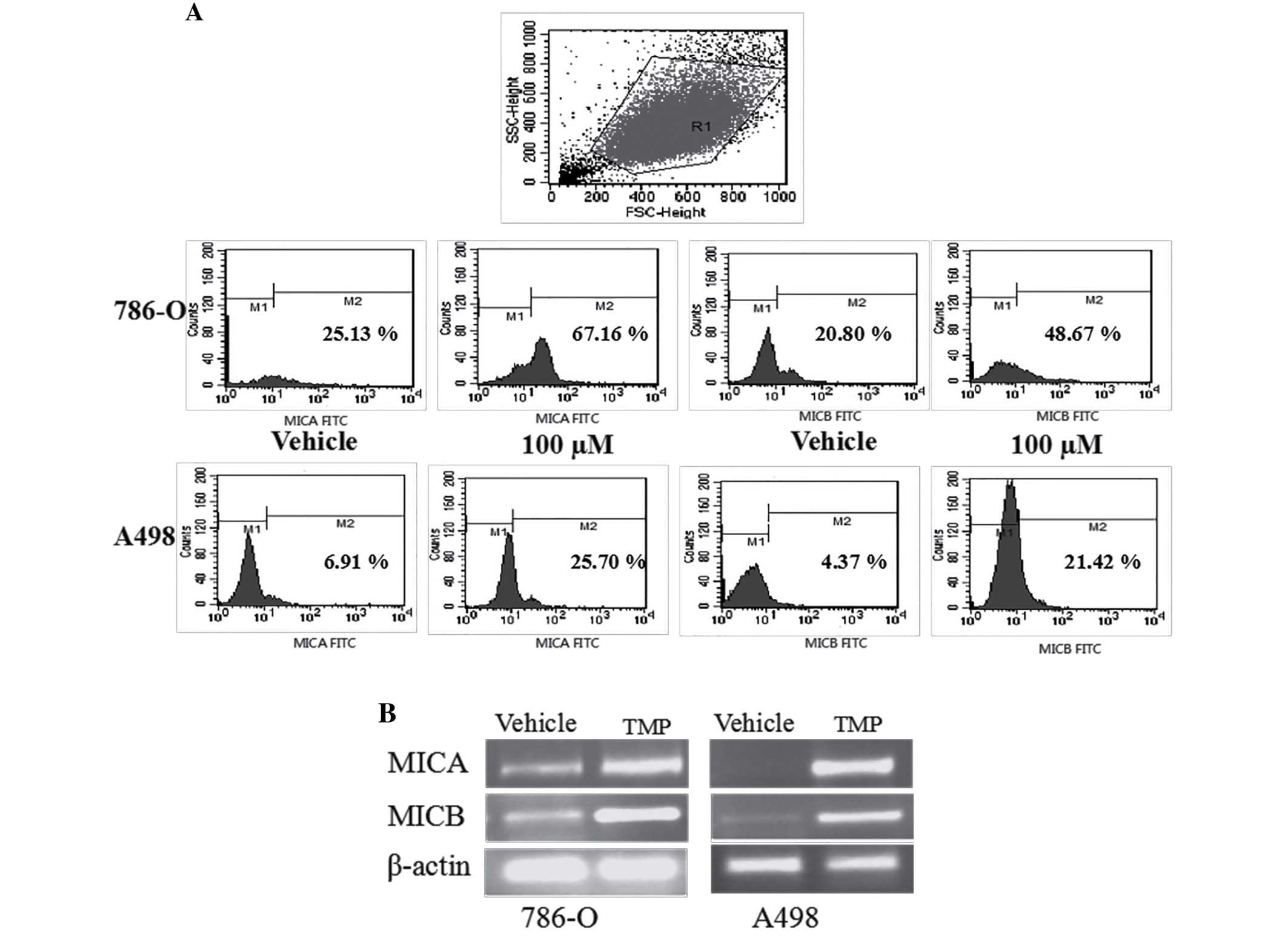
TMP upregulates the expression of
MICA/MICB on the surface of ccRCC cell lines
In our previously report, the expression of MICA/B
was significantly higher in 786-O than normal renal cells, but
there was no different in A498 cells. To investigate the effect of
TMP on the surface expression of NKG2D ligands in renal carcinoma
cells, we initially performed a flow cytometric analysis and
reverse transcription polymerase chain reaction (RT-PCR) analysis
on 786-O and A498 cells. As shown in Fig. 8, 786-O and A498 cells showed
apparent upregulation of MICA and MICB after treated with TMP,
indicating that the inhibition of TMP on renal cancer cells may be
associated with NKG2D-MICA signaling.
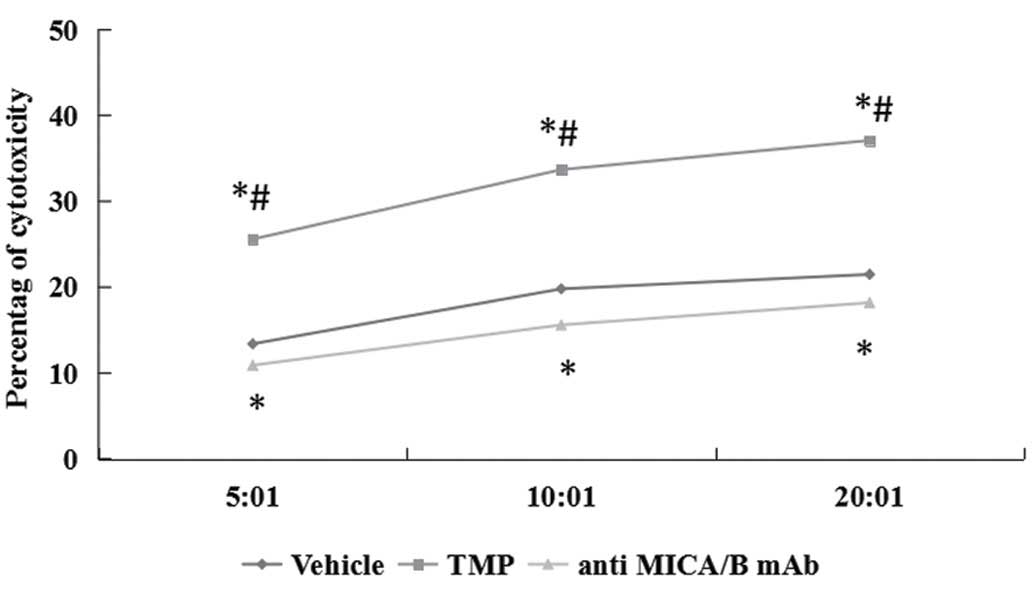
TMP enhances susceptibility of ccRCC
cells to NK cell-mediated cytotoxicity
In order to detect the correlation of NKG2D
signaling pathway, the interactions were blocked with saturating
amounts of anti-MICA/B mAb. The effect of TMP on ccRCC cells to NK
cell-mediated cytotoxicity was detected. Two renal carcinoma cell
lines were cultured for 24 h in the presence of 100 μM TMP and the
susceptibility to cytotoxicity of NK cells was examined. As shown
in Fig. 9, TMP significantly
increased the susceptibility of ccRCC cells to NK cells.
Furthermore, NK cells pretreatment with MICA/B blocking antibody
could partially prevent the cytotoxic activity of NK cells against
all target cells treated with 100 μM TMP.
Discussion
The present study demonstrated that TMP inhibited
growth and induction of apoptosis in clear cell renal cell
carcinoma (ccRCC) cell lines in a concentration- and time-dependent
manner. Based on the results, the invasion and migration were
significantly suppressed when treated with 100 μM TMP through wound
healing and Transwell assays in 786-O cells and A498 cells.
Moreover, TMP suppressed the EMT pathway through downregulation the
protein expression levels of epithelial marker E-cadherin and
upregulation of mesenchymal markers vimentin and fibronectin in
ccRCC cell lines 786-O and A498. Clear cell renal cell carcinoma is
one of the most common kidney cancers, and increasing number of
studies have demonstrated that epithelial-mesenchymal transition is
associated with renal cell carcinoma invasion and metastasis
(25–27). Therefore, the anticancer effect of
TMP on ccRCC cells might be mediated through suppressing the EMT
pathway, thus supporting the potential of TMP to be developed as a
promising agent for treatment of cancer. In agreement with previous
studies, our findings contribute to the studies on biomarkers of
kidney cancer and the mechanism of renal cancer progression driven
by EMT pathway. Although there have been several studies on the
molecular regulation of EMT, the mechanism is still not clear.
Major histocompatibility complex class I-related
chains A and B (MICA/B), a ligand of Natural killer group 2, member
D (NKG2D) receptor, is broadly upregulated in epithelial originated
tumor cells, which play a critical role in the immune surveillance
against tumor cells and are associated with the prognosis of
several malignancies (28). MICA/B
is a ligand for the activating receptor NKG2D, the binding of
NKG2DLs to NKG2D supports the cytotoxic effect of NK cells and T
cells against tumor cells. Mounting evidence indicates that
malignant epithelial cell polarization loss during the EMT allows
the interaction of NKG2DL with NKG2D, leading to the activation of
antitumor immune responses, which suggest that the correlation of
NKG2D and NKG2DLs expression with the presence of EMT
characteristics (20). However,
the regulation of the NKG2D signaling during the EMT of carcinomas
have yet to be elucidated. EMT is a morphogenetic process
characterized by the acquisition of mesenchymal properties linked
with an invasive phenotype and metastasis of tumor cells. Induction
of EMT by TGF-β stimulation strongly upregulated the expression of
NKG2D ligands (NKG2DLs), MICA/B and ULBP1–3 in several epithelial
tumor cell lines and by Snail1 overexpression in colorectal cancer
cells (29). Loss of epithelial
integrity observed during the EMT process may be an important point
of control of cancer progression by NKG2D-mediated immune
mechanisms. Accordingly, with the increased expression of NKG2DLs,
triggering of EMT rendered cancer cells more susceptible to
NKG2D-mediated killing by NK cells (23,30).
Decrease of MICA/B expression was associated with a dramatic
increase of NKG2D(+)-tumor infiltrating lymphocytes in vivo.
In our previous study (21), we
found that the content of soluble MICA (sMICA) in serum of patients
with renal cell carcinoma was significantly higher than in healthy
adults, the percentage of membrane MICA (mMICA) were significantly
increased in human kidney cancer tissues and ccRCC cell line 786-O
and Ketr-3, but no higher in A498 cells. Furthermore, we indicated
that the relationship of expression of NKG2D and MICA/B play an
important role in mediation of the cytotoxic NK cells in ccRCCs and
sMICA mediated tumor immune escape through downregulated NKG2D
expression. However, the underlying molecular mechanisms in the
tumor microenvironment in ccRCC lesions is not yet clarified. In
the present study, based on the results of PCR and FACS, the
expression of MICA and MICB were higher in 786-O, but not A498,
which is in agreement with our previous results. Interestingly, the
antitumor effects of TMP were more significant in MICA high
expression RCC cell lines 786-O than low expression RCC cell lines
A498. Thus, we hypothesize that TMP inhibit proliferation,
migration and invasion of ccRCC cells through regulation of
NKG2D-NKGDLs signaling during the EMT. In order to further revealed
the mechanism, the interactions were blocked with saturating
amounts of anti-MICA/B mAb, the effect of TMP on ccRCC cells to NK
cell-mediated cytotoxicity were detected and the results showed
that TMP significantly increased the susceptibility of ccRCC cells
to NK cells. EMT is primarily mediated by local production and
activation of TGF-β1, EMT is also a relevant checkpoint in the
control of tumor progression through NKG2D-mediated immune
responses. Many studies have been conducted on the relationship
between matrix metalloproteinases (MMPs) expression in cancer
patients. MMP-9 due to their proteolytic nature degrade proteins
that regulate various cellular behaviors related to cancer cell
differentiation, migration, invasion, and surveillance of the
immune system (31). In our
results, treatment with TMP, the protein levels of TGF-β1 and MMP-9
were lower in ccRCC cells indicating that the mechanism of
suppression EMT may be related through TGF-β1 and MMP signal
pathway. Previous studies reported that TGF-β downregulates the
expression of NKG2D and its ligands in different tumor types. Taken
together, the antitumor effect of TMP is a complicated process, in
which the NKG2D-NKG2DL signaling plays a key role.
In summary, our results suggested that TMP possess
the activity of anti-proliferation and apoptosis induction in human
clear cell renal cell carcinoma (ccRCC) cell lines, 786-O and A498
cells. Interestingly, the inhibition was more significantly
increased in 786-O than in A498 cells. Our following studies
indicated that the molecular mechanisms may be mediated through
inhibition of NKG2D related signaling pathway to further suppress
EMT progression. The binding of NKG2D to its ligands activates NK
cells, which gives the rationale for studies on the utilization of
TMP as a potential cancer therapeutic compound to increase NK
cell-mediated cytotoxicity against high NKG2DL expressing cancer
cells.
Acknowledgements
We are grateful to Central Research Laboratory, the
Second Hospital of Shandong University for technical assistance and
the generous support. The present study was supported by a grant
from the National Natural Science Foundation of China (grant no.
81500042), the Natural Science Foundation of Shandong Province
(grant nos. ZR2014HQ045 and ZR2013HM103) and the Medical and Health
Science Technology Development Plan Project of Shandong Province
(grant no. 2015WS0307).
References
|
1
|
Yan Y, Zhao J, Cao C, Jia Z, Zhou N, Han
S, Wang Y, Xu Y, Zhao J, Yan Y, et al: Tetramethylpyrazine promotes
SH-SY5Y cell differentiation into neurons through epigenetic
regulation of Topoisomerase IIβ. Neuroscience. 278:179–193. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang L, Zhang X, Cui G, Chan JY, Wang L,
Li C, Shan L, Xu C, Zhang Q, Wang Y, et al: A novel agent exerts
antitumor activity in breast cancer cells by targeting
mitochondrial complex II. Oncotarget. Mar 27–2016.(Epub ahead of
print).
|
|
3
|
Kao TK, Chang CY, Ou YC, Chen WY, Kuan YH,
Pan HC, Liao SL, Li GZ and Chen CJ: Tetramethylpyrazine reduces
cellular inflammatory response following permanent focal cerebral
ischemia in rats. Exp Neurol. 247:188–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu X, Liu H, Zeng Z, Zhou W, Liu J and He
Z: Pharmacokinetics of ligustrazine ethosome patch in rats and
anti-myocardial ischemia and anti-ischemic reperfusion injury
effect. Int J Nanomed. 6:1391–1398. 2011. View Article : Google Scholar
|
|
5
|
Jia Y, Wang Z, Zang A, Jiao S, Chen S and
Fu Y: Tetramethylpyrazine inhibits tumor growth of lung cancer
through disrupting angiogenesis via BMP/Smad/Id-1 signaling. Int J
Oncol. 48:2079–2086. 2016.PubMed/NCBI
|
|
6
|
Kim M, Kim SO, Lee M, Lee JH, Jung WS,
Moon SK, Kim YS, Cho KH, Ko CN and Lee EH: Tetramethylpyrazine, a
natural alkaloid, attenuates pro-inflammatory mediators induced by
amyloid β and interferon-γ in rat brain microglia. Eur J Pharmacol.
740:504–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang XJ, Xu YH, Yang GC, Chen HX and Zhang
P: Tetramethylpyrazine inhibits the proliferation of acute
lymphocytic leukemia cell lines via decrease in GSK-3β. Oncol Rep.
33:2368–2374. 2015.PubMed/NCBI
|
|
8
|
Zhang P, Zheng BB, Wang HY, Chen JH, Liu
XY and Guo XL: DLJ14, a novel chemo-sensitization agent, enhances
therapeutic effects of adriamycin against MCF-7/A cells both in
vitro and in vivo. J Pharm Pharmacol. 66:398–407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yi B, Liu D, He M, Li Q, Liu T and Shao J:
Role of the ROS/AMPK signaling pathway in
tetramethylpyrazine-induced apoptosis in gastric cancer cells.
Oncol Lett. 6:583–589. 2013.PubMed/NCBI
|
|
10
|
Chen Z, Pan X, Georgakilas AG, Chen P, Hu
H, Yang Y, Tian S, Xia L, Zhang J, Cai X, et al:
Tetramethylpyrazine (TMP) protects cerebral neurocytes and inhibits
glioma by down regulating chemokine receptor CXCR4 expression.
Cancer Lett. 336:281–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng CY, Xiao W, Zhu MX, Pan XJ, Yang ZH
and Zhou SY: Inhibition of cyclooxygenase-2 by tetramethylpyrazine
and its effects on A549 cell invasion and metastasis. Int J Oncol.
40:2029–2037. 2012.PubMed/NCBI
|
|
12
|
Pan H, Jiang T, Cheng N, Wang Q, Ren S, Li
X, Zhao C, Zhang L, Cai W and Zhou C: Long non-coding RNA BC087858
induces non-T790M mutation acquired resistance to EGFR-TKIs by
activating PI3K/AKT and MEK/ERK pathways and EMT in non-small-cell
lung cancer. Oncotarget. Jul 9–2016.(Epub ahead of print).
|
|
13
|
Bonyadi Rad E, Hammerlindl H, Wels C,
Popper U, Ravindran Menon D, Breiteneder H, Kitzwoegerer M, Hafner
C, Herlyn M, Bergler H, et al: Notch4 signaling induces a
mesenchymal-epithelial-like transition in melanoma cells to
suppress malignant behaviors. Cancer Res. 76:1690–1697. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raza U, Saatci Ö, Uhlmann S, Ansari SA,
Eyüpoğlu E, Yurdusev E, Mutlu M, Ersan PG, Altundağ MK and Zhang J:
The miR-644a/CTBP1/p53 axis suppresses drug resistance by
simultaneous inhibition of cell survival and epithelial-mesenchymal
transition in breast cancer. Oncotarget. Jul 8–2016.(Epub ahead of
print). PubMed/NCBI
|
|
15
|
Matusali G, Tchidjou HK, Pontrelli G,
Bernardi S, D’ Ettorre G, Vullo V, Buonomini AR, Andreoni M,
Santoni A, Cerboni C and Doria M: Soluble ligands for the NKG2D
receptor are released during HIV-1 infection and impair NKG2D
expression and cytotoxicity of NK cells. FASEB J. 27:2440–2450.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guerra N, Tan YX, Joncker NT, Choy A,
Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM and Raulet
DH: NKG2D-deficient mice are defective in tumor surveillance in
models of spontaneous malignancy. Immunity. 28:571–580. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
López-Larrea C, Suárez-Alvarez B,
López-Soto A, López-Vázquez A and Gonzalez S: The NKG2D receptor:
Sensing stressed cells. Trends Mol Med. 14:179–189. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cosman D, Müllberg J, Sutherland CL, Chin
W, Armitage R, Fanslow W, Kubin M and Chalupny NJ: ULBPs, novel MHC
class I-related molecules, bind to CMV glycoprotein UL16 and
stimulate NK cytotoxicity through the NKG2D receptor. Immunity.
14:123–133. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
López-Soto A, Zapico LH, Acebes-Huerta A,
Rodrigo L and Gonzalez S: Regulation of NKG2D signaling during the
epithelial-to-mesenchymal transition. Oncoimmunology. 2:e258202013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ni D, Ma X, Li HZ, Gao Y, Li XT, Zhang Y,
Ai Q, Zhang P, Song EL, Huang QB, et al: Downregulation of FOXO3a
promotes tumor metastasis and is associated with metastasis-free
survival of patients with clear cell renal cell carcinoma. Clin
Cancer Res. 20:1779–1790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jia HY, Liu JL, Yuan MZ, Zhou CJ, Sun WD,
Zhao JJ, Wang J, Liu L and Luan Y: Regulation roles of MICA and
NKG2D in human renal cancer cells. Asian Pac J Cancer Prev.
16:3901–3905. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Subramani R, Gonzalez E, Nandy SB,
Arumugam A, Camacho F, Medel J, Alabi D and Lakshmanaswamy R:
Gedunin inhibits pancreatic cancer by altering sonic hedgehog
signaling pathway. Oncotarget. Mar 14–2016. View Article : Google Scholar : (Epub ahead of
print). PubMed/NCBI
|
|
23
|
Friese MA, Wischhusen J, Wick W, Weiler M,
Eisele G, Steinle A and Weller M: RNA interference targeting
transforming growth factor-beta enhances NKG2D-mediated antiglioma
immune response, inhibits glioma cell migration and invasiveness,
and abrogates tumorigenicity in vivo. Cancer Res. 64:7596–7603.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang F, Shao Y, Yang F, Liu M, Huang J,
Zhu K, Guo C, Luo J, Li W, Yang B, et al: Valproic acid upregulates
NKG2D ligand expression and enhances susceptibility of human renal
carcinoma cells to NK cell-mediated cytotoxicity. Arch Med Sci.
9:323–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mikami S, Mizuno R, Kosaka T, Saya H, Oya
M and Okada Y: Expression of TNF-α and CD44 is implicated in poor
prognosis, cancer cell invasion, metastasis and resistance to the
sunitinib treatment in clear cell renal cell carcinomas. Int J
Cancer. 136:1504–1514. 2015. View Article : Google Scholar
|
|
26
|
Hill B, De Melo J, Yan J, Kapoor A, He L,
Cutz JC, Feng X, Bakhtyar N and Tang D: Common reduction of the Raf
kinase inhibitory protein in clear cell renal cell carcinoma.
Oncotarget. 5:7406–7419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lawrie CH, Larrea E, Larrinaga G,
Goicoechea I, Arestin M, Fernandez-Mercado M, Hes O, Cáceres F,
Manterola L and López JI: Targeted next-generation sequencing and
non-coding RNA expression analysis of clear cell papillary renal
cell carcinoma suggests distinct pathological mechanisms from other
renal tumour subtypes. J Pathol. 232:32–42. 2014. View Article : Google Scholar
|
|
28
|
Zhang X, Yan L, Jiao W, Ren J, Xing N,
Zhang Y, Zang Y, Wang J and Xu Z: The clinical and biological
significance of MICA in clear cell renal cell carcinoma patients.
Tumour Biol. 37:2153–2159. 2016. View Article : Google Scholar
|
|
29
|
López-Soto A, Huergo-Zapico L, Galván JA,
Rodrigo L, de Herreros AG, Astudillo A and Gonzalez S:
Epithelial-mesenchymal transition induces an antitumor immune
response mediated by NKG2D receptor. J Immunol. 190:4408–4419.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eisele G, Wischhusen J, Mittelbronn M,
Meyermann R, Waldhauer I, Steinle A, Weller M and Friese MA: TGF-β
and metalloproteinases differentially suppress NKG2D ligand surface
expression on malignant glioma cells. Brain. 129:2416–2425. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kunigal S, Lakka SS, Joseph P, Estes N and
Rao JS: Matrix metalloproteinase-9 inhibition down-regulates
radiation-induced nuclear factor-kappa B activity leading to
apoptosis in breast tumors. Clin Cancer Res. 14:3617–3626. 2008.
View Article : Google Scholar : PubMed/NCBI
|