Introduction
The estimated new cases and deaths of prostate
cancer (CaP) were 180,890 and 26,120, respectively, ranked first
and second among all cancer types in men according to the report
from American Cancer Society in 2016 (1). Since prostate cancer is an
age-related disease with higher incidence in elderly men, the
cumulative risk apparently shortens their life expectancy. Besides,
multiple therapies available for patients with prostate cancer at
advanced stages provide limited benefits (2). Both radical prostatectomy and
radiation therapy are effective in curing localized low grade
prostate cancer (3). Therefore,
early detection and treatment remains the best strategy in prostate
cancer management. PSA screen is non-specific and associated with
significant false positivity and low specificity. Conventional
imaging technologies are highly sensitive and specific in the
diagnosis of lesions in most solid organs but prostate cancer
remains an exception (4). Novel
molecular imaging techniques may provide distinct advantage in
solving this problem.
Near-infrared fluorescence (NIRF) imaging is a novel
imaging modality for biomedical imaging application (5). Using NIRF probes with maximal
absorption and emission wavelengths in the near infrared window,
this technique allows for the detection of tissue function,
metabolism, bio-distribution and for real-time monitoring of
various pathophysiological states (6). IR-780 iodide, a lipophilic cyanine
dye with peak emission at 780 nm, has been applied for cancer
imaging and therapy including photodynamic therapy (PDT) and
photothermal therapy (7). This dye
can accumulate selectively in cancer cells and be used to identify
cancerous lesions from normal tissues, probably through the
function of organic anion transporting peptides (OATP) and the
enhanced permeability and retention effect in cancer sites
(8,9). The cancer targeting ability could be
exploited for drug delivery to maximize therapeutic efficacy and
minimizing unwanted side effects. Besides, IR-780 iodide also
exhibits tumoricidal activity to drug-resistant lung cancer cells,
showing the potential as an ideal platform to construct theranostic
agents for cancer imaging and therapy synergistic with conventional
anticancer agents (8).
Docetaxel is a first-line chemotherapy drug for
castration resistant prostate cancer (10). We previously developed theranostic
NIRF conjugates using docetaxel as the active components (11). This theranostic compound retains
the excellent imaging ability of original NIRF dye as well as the
therapeutic effects of docetaxel, displaying huge potential in
prostate cancer diagnosis and therapy. Abiraterone is a
17α-hydroxylase/C17,20-lyase (CYP17) inhibitor that has been
approved for use in patients with castration-resistant prostate
cancer before or after docetaxel therapy (12–14).
It is also an antagonist to the androgen receptor and an inhibitor
of 3β-hydroxysteroid dehydrogenase, exerting anticancer effects
(15). We report on the
development of IR-780 dye-conjugated abiraterone and the testing
whether the new compound may be used in prostate cancer imaging and
therapy in in vitro and in vivo models. The results
suggested that the novel Abi-780 conjugates can be applied as
theranostic agents for both prostate cancer imaging and
therapy.
Materials and methods
Chemical reagents
Cyanine dye IR-780 iodide was purchased from
Sigma-Aldrich (St. Louis, MO, USA). Abiraterone, piperazine,
N,N-dimethylformamide (DMF), succinic anhydride,
N,N-diethylethanamin (Et3N), dichloromethane,
1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
(EDC·HCl) and 1-hydroxy-7-azabenzotriazole (HOAT) were purchased
from ACROS (Beijing, China).
Cell lines and cell culture
Human prostate cancer cell lines PC-3, DU-145, C4-2,
LNCaP and normal prostate epithelial cell line RWPE-1 were cultured
in RPMI-1640 (Invitrogen Life Technologies, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (Gibco-BRL, Carlsbad, CA,
USA) and penicillin (100 IU/ml)/streptomycin (100 μg/ml) in a
humidified incubator with 5% CO2 at 37°C.
Synthesis and characterization of
Abi-780
Abi-780 were synthesized through three steps.
Briefly, in step A, a mixture solution of IR-780 (0.677 g, 1.014
mmol) and piperazine (0.349 g, 4.056 mmol) in DMF was heated at
85°C under nitrogen. After completion of the reaction as indicated
by thin layer chromatography, products IR-780-piperazine conjugates
were purified using silica gel flash chromatography. Abiraterone
(1.0 g, 2.9 mmol), succinic anhydride (870.61 mg, 8.7 mmol) and
N,N-diethylethanamin (2.41 ml, 17.4 mmol) were added into
dry dichloromethane (30 ml) in another flask for step B. The crude
products were subsequently purified by column chromatography and
confirmed to obtain abiraterone-dimethyl succinate conjugates.
Finally, these two purified product were mixed at a ratio of 1:1
(0.24 mol), then incorporated with EDC·HCL (69.0 mg, 0.36 mmol),
HOAT (49.0 mg, 0.36 mmol) and dry dichloromethane for step C
reaction. The pure products of Abi-780 were purified by column
chromatography. Infrared spectrum, nuclear magnetic resonance
spectra (1H NMR and 13C NMR) and
high-resolution mass spectra (HRMS) were used for structural
characterization. Fluorescence spectroscopy was determined by F97
profluorescence spectrophotometer (Lengguang Tech, Shanghai,
China). NIR absorption spectroscopy was determined by using a
Shimadzu Spectrophotometer (UV-2550; Shimadzu Corp., Kyoto, Japan).
NIRF images were captured by the IVIS Lumina II imaging station
(Caliper Life Sciences, Hopkinton, MA, USA).
Uptake of Abi-780 in prostate cancer
cells
The cell staining procedures were described
previously (6). In brief, prostate
cancer cells and normal prostate epithelial cells were placed into
35-mm glass bottom petri dish (1×104 cells/well, NEST,
Shanghai, China) and cultured for 24 h. Then working solutions of
Abi-780 (20 μM) were added for 20 min at 37°C. Cells were gently
washed twice using phosphate-buffered saline (PBS) and then
incubated with 4′,6-diamidino-2-phenyindole (DAPI) for nuclei
staining at 37°C for 10 min. After repeated PBS washes and 10-min
fixation using 4% paraformaldehyde (Sigma-Aldrich), cells were
immediately observed by laser confocal microscopy with fixed
imaging parameters (Olympus FV1000, Tokyo, Japan), using 700 nm as
excitation wavelength and 780 nm as emission wavelength (9).
Subcellular localization of Abi-780 in prostate
cancer cells were detected pursuant to the previously established
protocols (6). Briefly,
commercially available probes Mito Tracker Green FM and Lyso
Tracker Green DND-26 (Molecular Probes, Camarillo, CA, USA) were
utilized to track cytoplasmic mitochondria and lysosomes. Following
DAPI staining, slides underwent staining using Mito Tracker 200 nM
for 30 min at 37°C, or 200 nM DND-26 for 60 min at 37°C. After
repeated PBS rinsing, cells were observed under the confocal laser
microscope. Emission/excitation lights for Mito and Lyso Tracker
was 490/516 and 554/576 nm, respectively. Images were merged for
co-localization analysis of Abi-780.
In vitro antitumor effects
Viability assay
Cells were exposed to different concentrations of
Abi-780 (0, 2.5, 5, 10, 20, 40, 80 and 160 μM) to determine the
inhibitory effect of Abi-780 on cell proliferation using the CCK-8
assay kit (Beyotime, Beijing, China). Briefly, 100 μl LNCaP, C4-2
and RWPE-1 cells were placed into 96-well plate (5×103
cells/well) and cultured for 12 h. The cells were incubated with
different concentrations of Abi-780 dye for another 24 h. Then
cells were washed repeatedly using 100 μl fresh medium. After mixed
with 10 μl CCK-8 reagent at 37°C for 4 h, the absorbance was
determined using xMark™ microplate absorbance spectrophotometer at
450 nm (Bio-Rad, Hercules, CA, USA). Cell viability at different
Abi-780 concentrations were calculated as percentages of OD value
to that in control group without dye incubation.
Colony-formation assay
The influence of Abi-780 to the self-renewal of
prostate cancer cells was determined by colony-formation assay.
Cancer cells seeded in 6-well plates (200 cells/well) were
incubated with 20 μM abiraterone, IR-780 and Abi-780 for 24 h. Then
cells were washed with PBS and cultured in fresh medium. At day 10,
cells were fixed with 4% paraformaldehyde and stained using 0.1%
crystal violet solution (Beyotime) for 5 min. The number of
colonies (>50 cells) were counted.
Apoptosis assay by flow cytometry
Cells in a 6-well plate (1×105
cells/well) were incubated with 20 μM abiraterone, IR-780 and
Abi-780 for 24 h. No treatment was given in control group. Cells
were then harvested to detect apoptotic cells by flow cytometry
(Becton-Dickinson, Franklin Lakes, NJ, USA) using the Annexin
V-fluorescein isothiocyanate (FITC) and propidium iodide (PI)
staining kit (Beyotime) following the manufacturer's manual.
Apoptosis rate was defined as the percentage of Annexin V-positive
and PI-negative cells.
Migration/Invasion assay
Cultured LNCaP and C4-2 cells in 1% FBS media were
placed into the upper chambers (1×104/well) and
incubated with 20 μM abiraterone, IR-780 and Abi-780. No treatment
was given in control group. FBS (10%) was added into the lower
chamber to form a gradient prompting cell migration. For invasion
assay, Matrigel-coated inserts were used as in the upper chambers.
After incubation for 24 h, cells that migrated/invaded to the
bottom of upper chamber were fixed with 4% paraformaldehyde for 10
min and stained with 0.1% crystal violet solution (Beyotime). The
number of fixed cells was counted under Olympus DP-70 fluorescence
microscope.
In vivo NIRF imaging of Abi-780
Prostate cancer xenograft model
The animal experiments were permitted by the Ethics
Committee of the Fourth Military Medical University and performed
in accordance with Animal Care and Use Committee Guidelines of the
Fourth Military Medical University. All mice were obtained from the
Experimental Animal Center of the Fourth Military Medical
University. They were caged in specific-pathogen-free animal house
under normal light-dark cycle, free access to food and water. Human
prostate cancer LNCaP cells (100 μl) (1×107 cells/ml)
mixed with BD Matrigel™ matrix (BD Biosciences, San Jose, CA, USA)
at a ratio of 1:1 were inoculated subcutaneously into 27 athymic
nude mice according to the previously reported procedures (16). The mice were allocated for
subsequent studies when the diameter of tumor reached ~5 mm as
measured by a caliper.
Abi-780 dye for NIRF imaging of
prostate cancer
For NIRF imaging studies, Abi-780 dye were injected
i.p. (0.575 mg/kg) into 3 tumor-bearing mice. Twenty-four hours
later, the tumor-bearing mice were anesthetized using 2% isoflurane
in 100% oxygen with a delivery rate of 1.5 l/min. NIRF images of
the whole body and tissue bio-distribution were taken using the
IVIS Lumina II imaging station (Caliper Life Sciences). Then
subcutaneous xenografts were prepared as frozen sections and
paraffin-embedded tissue sections for histological analysis.
Briefly, for immediate laser confocal microscopy, frozen sections
(10 μm thick) were cut from tissues embedded in OCT medium (Sakura
Finetek, Torrance, CA, USA), stained by DAPI, and then observed
under laser confocal microscope. For hematoxylin and eosin
(H&E) staining, sections (5 μm thick) from paraffin-embedded
tissues after H&E staining were observed under a DP-70
microscope (Japan) by an expert pathologist (17).
In vivo antitumor assay
Twenty-four athymic nude mice bearing human prostate
cancer xenografts were randomly classified into four groups each
containing six mice: IR-780 group, i.p. injection of 100 μl IR-780
dye (3.34 mg/kg.d); Abi group, i.p. injection of 100 μl abiraterone
(3.5 mg/kg.d); Abi-780 group, i.p. injection of 100 μl Abi-780
(5.75 mg/kg.d); control group, 100 μl PBS was given. Tumor size was
monitored in the following four weeks. Tumor volumes were
calculated by the formula: tumor volume = (length ×
width2)/2. The whole body NIRF signal of mice from
treated groups was observed under the IVIS Lumina II Imaging
Station.
Toxicity studies
Twenty-four BALB/C mice (4–6 weeks old, 20–25 g)
were randomly allocated to three groups to evaluate the acute
toxicity of Abi-780. Daily i.p. injection of 100 μl Abi-780 dye at
a dose of 0.575, 5.75 and 57.5 mg/kg to 6 BALB/C mice in each group
was given for 30 days. Six mice in control group were injected with
100 μl normal saline. These mice were observed and weighed daily.
Thirty days later, mice were sacrificed and all tissues were
retrieved for histological evaluation by H&E staining.
Statistical analysis
Numerical data are described as mean ± standard
deviation (SD). SPSS software 16.0 (SPSS Co., Chicago, IL, USA) was
used for statistical analysis. The statistical significance of data
was determined by one-way ANOVA. Dunnett-t test was performed for
intragroup comparisons. P<0.05 was considered as statistically
different.
Results
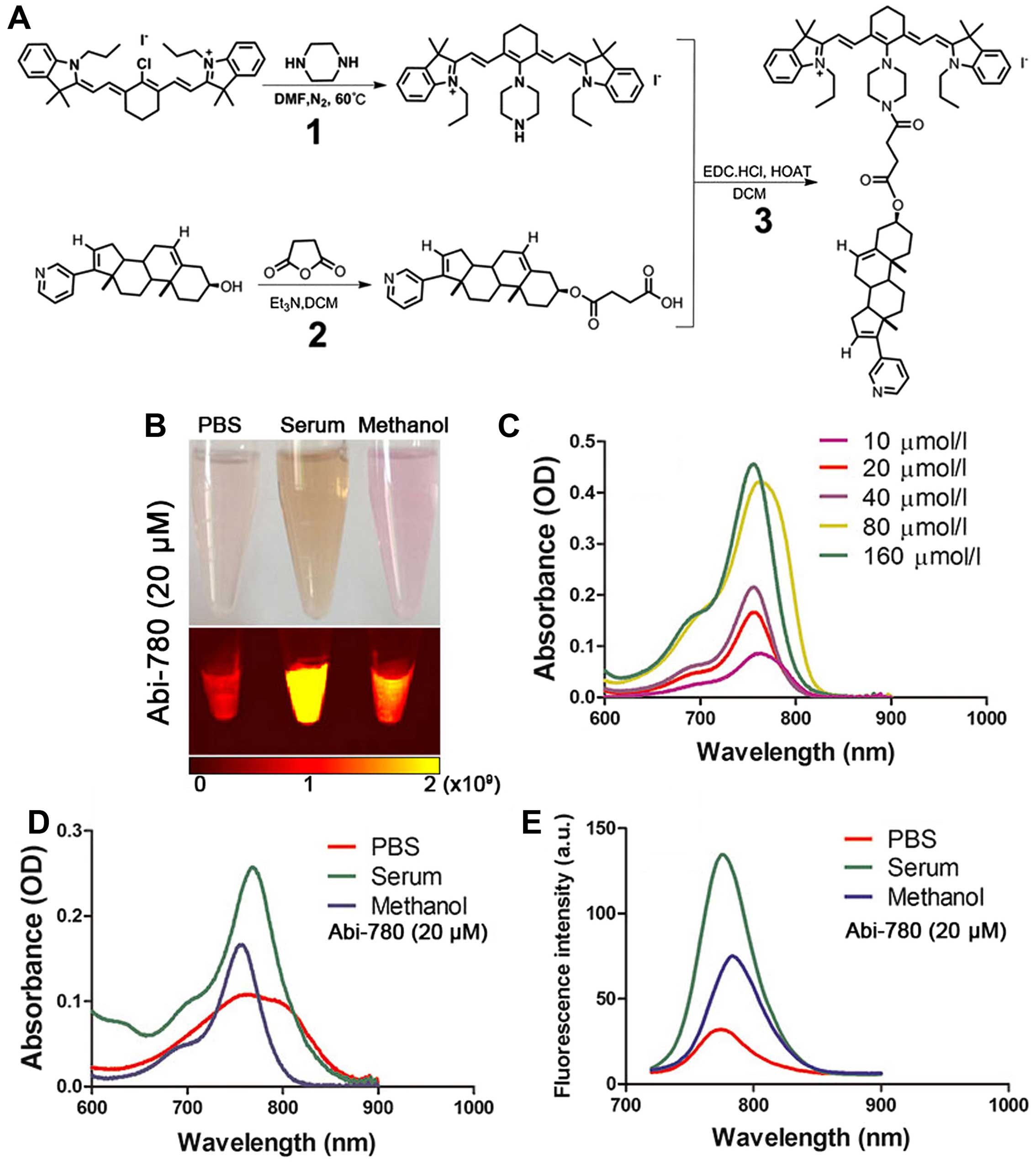
Synthesis and characterization of
Abi-780
We developed a novel route for the chemical
synthesis of Abi-780 (Fig. 1A).
After final purification procedure using silica gel flash
chromatography, 295 mg dark green products Abi-780 were
successfully obtained. The yielding rate was 59%. Molecular weight
of Abi-780 was 1149.4. The melting point was 125.6–128°C. Chemical
structure of Abi-780 conjugates was confirmed by IR, NMR and HRMS.
The data were listed as following: 1H NMR
(CDCl3, 400 MHz): δ: 8.63 (1H, s), 8.47 (1H, d, J=4.8
Hz), 7.75 (1H, s), 7.72 (1H, s), 7.66 (1H, d, J=8.8 Hz), 7.36 (1H,
s), 7.33 (2H, d, J=4.0 Hz), 7.15 (2H, t, J=7.4 Hz), 7.01 (2H, d,
J=7.6 Hz), 6.00 (1H, s), 5.934 (1H, s), 5.90 (1H, s), 5.43-5.42
(1H, m), 5.30 (1H, s), 4.69-4.67 (1H, m), 3.96 (5H, t, J=7.4 Hz),
3.89 (2H, s), 3.81 (2H, s), 3.61 (2H, s), 2.85 (2H, t, J=5.8 Hz),
2.73 (2H, t, J=5.8 Hz), 2.53 (4H, t, J=6.4 Hz), 2.41 (2H, d, J=7.2
Hz), 2.11-2.04 (4H, m), 1.92-1.85 (6H, m), 1.69 (12H,
s), 1.26 (19H, s), 1.09 (6H, d, J=8.8 Hz). 13C NMR
(CDCl3, 100 MHz): δ: 172.6; 171.5; 170.8; 169.8; 147.1;
142.7; 141.7; 140.4; 140.3; 129.8; 128.5; 125.7; 124.0; 123.4;
122.1; 109.8; 97.6; 74.1; 57.5; 50.3; 48.4; 47.4; 45.6; 38.1; 37.0;
36.9; 35.2; 31.9; 31.5; 30.4; 29.7; 29.1; 29.0; 28.2; 27.8; 25.3;
21.7; 20.8; 20.5; 19.3; 16.6; 11.8. IR (KBr): 3,425; 3,032; 2,962;
2,927; 2,854; 1,728; 1,651; 1,543; 1,504; 1,373; 1,253; 1,165;
1,095; 1,045; 1,003; 933; 798; 714; 517. HRMS (ESI+) for
C68H86N5O3: 1,020.6766
(measured), calculated as 1,020.6731. The optical properties of
Abi-780 were determined in different solvents including PBS, serum
and methanol (Fig. 1B). Absorbance
of Abi-780 in methanol was enhanced as the concentration increased
(Fig. 1C). The absorption peak was
at 760 nm in PBS, 768 nm in serum and 758 nm in methanol,
respectively (Fig. 1D). When 700
nm was set as excitation wavelength, the emission peak was at 774
nm in PBS, 783 nm in serum and 776 nm in methanol (Fig. 1E). The emission intensity was
relatively higher in serum than in PBS. The fluorescence
enhancement effect and stability of Abi-780 in serum made it
reliable for biomedical application.
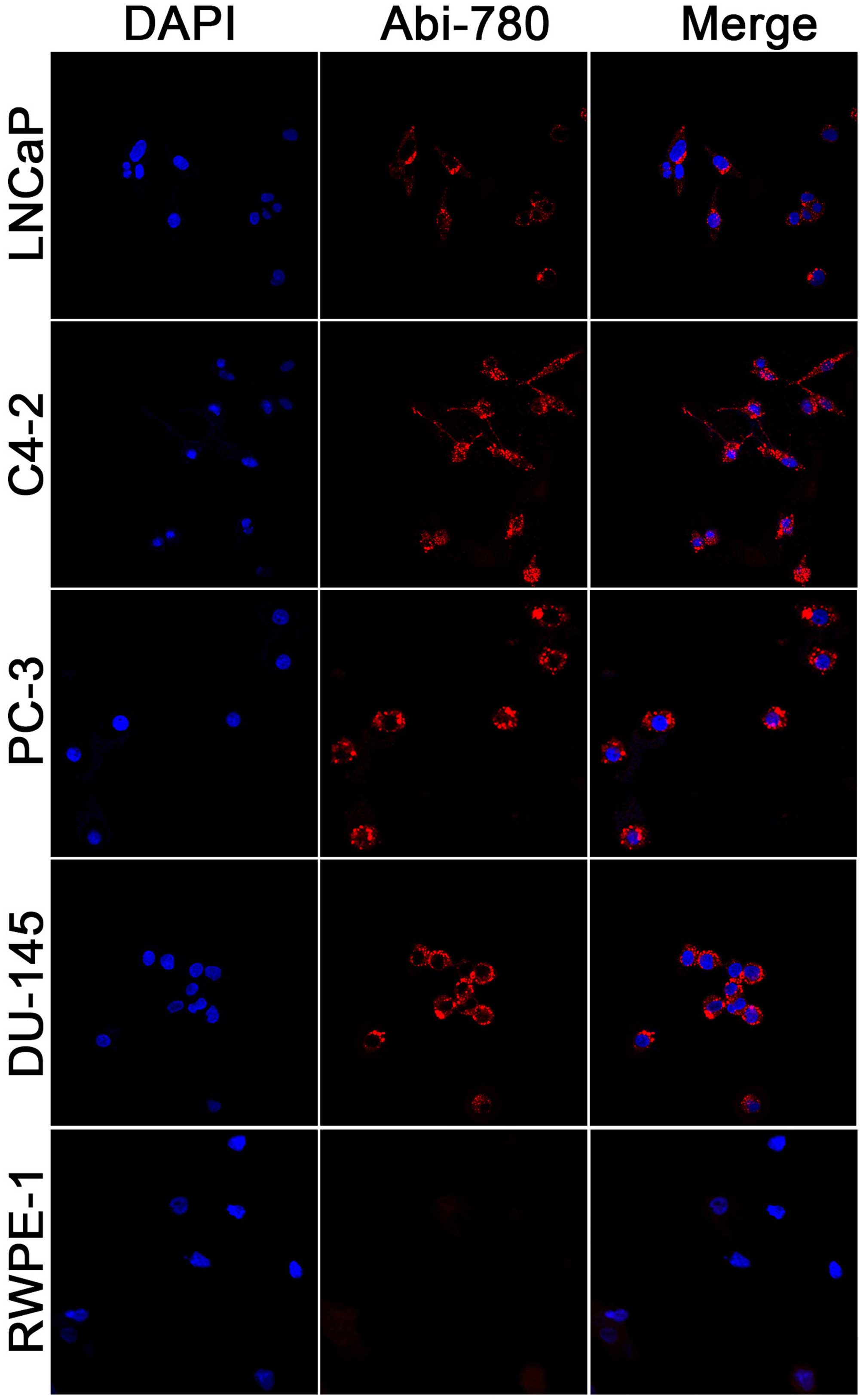
In vitro cellular uptake study
Selective uptake and accumulation of
IR-780 dye in prostate cancer cells
PC-3, DU-145, LNCaP, C4-2 and RWPE-1 were stained by
20 μM Abi-780 to determine whether the NIRF signal varied in
prostate cancer cells and normal prostate epithelial cells.
Selective uptake and accumulation of NIRF dyes were found in
prostate cancer cells but not in normal prostate epithelial cells
(Fig. 2). These results
demonstrated that the new conjugates maintained the excellent
optical property of IR-780, and hence may be useful for molecularly
targeted imaging.
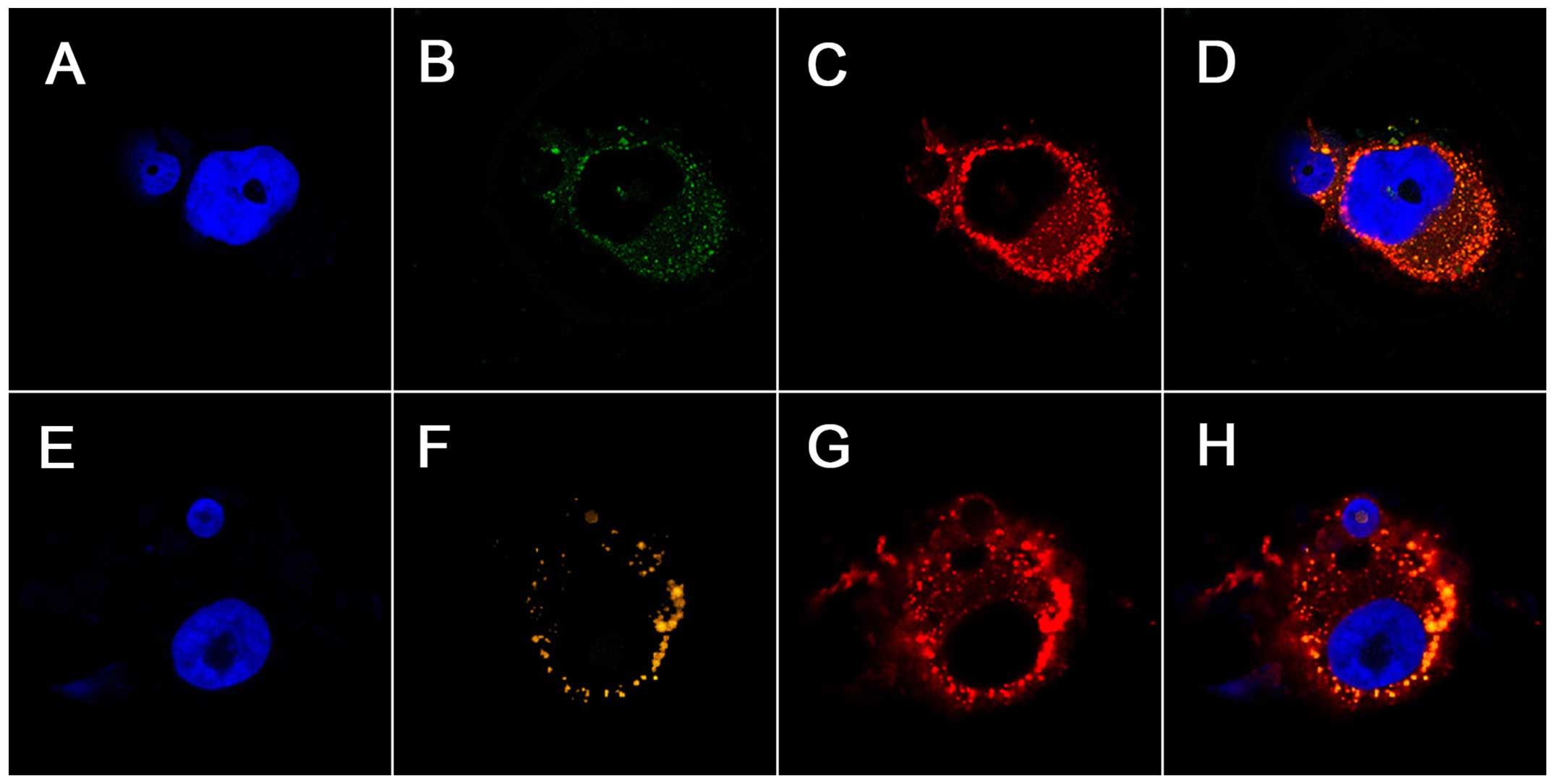
Previous studies have documented the preferential
accumulation of IR-780 dye in the mitochondria of drug-resistant
lung cancer cells (18). We
explored the subcellular co-localization of Abi-780 in prostate
cancer cells PC-3. Cells and organelles were clearly delineated by
Abi-780, Lyso-tracker and Mito-tracker dye (Fig. 3). The merged images revealed that a
substantial portion of these dyes accumulated in the mitochondria
and lysosomes of prostate cancer cells. These results confirmed
that Abi-780 maintained the unique property of IR-780 for membrane
transport, which could be utilized for selective delivery of
anticancer drugs into cancer cells.
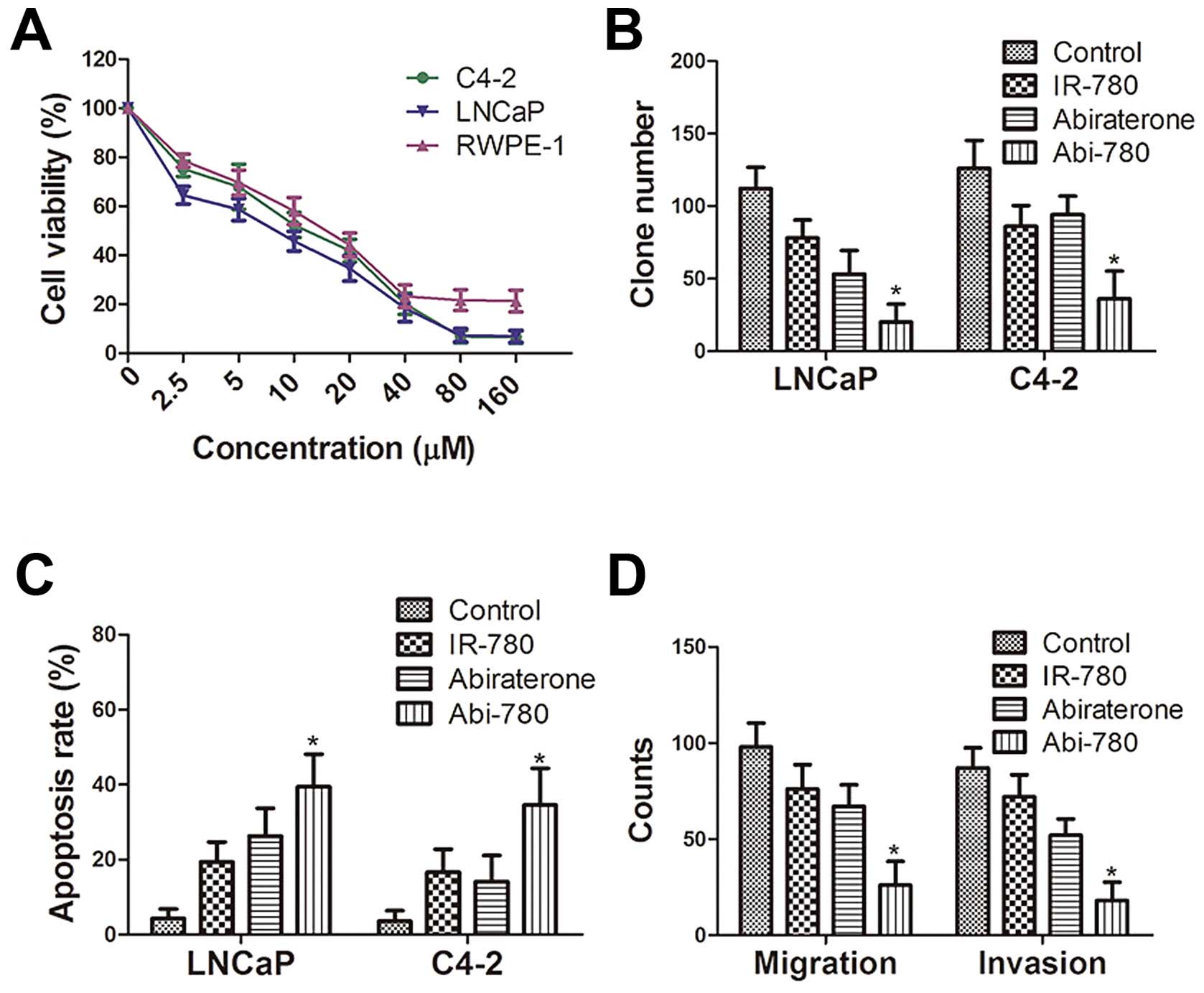
In vitro antitumor effect
Dose-dependent inhibition of cultured
prostate cells by Abi-780 dye
The impact of Abi-780 on cell proliferation was
evaluated in human prostate cancer cells LNCaP, C4-2 and normal
human prostate epithelial cells RWPE-1. A dose-dependent inhibition
on cell proliferation was revealed by MTT assay (Fig. 4A). Abi-780 at 20 μM also decreased
the colony-forming ability of both LNCaP and C4-2 cells in
comparison with that of control group (P<0.05, Fig. 4B). As revealed in apoptotic assays,
Abi-780 significantly increased the percentage of apoptotic cells
in both LNCaP and C4-2 cells compared to that of control group
(P<0.05, Fig. 4C). Furthermore,
Abi-780 apparently reduced the migration and invasion potential of
both LNCaP and C4-2 cells in comparison with those of control group
(P<0.05, P<0.05, Fig. 4D).
It was also revealed that IR-780 alone had a moderate anticancer
ability on prostate cancer cells. Although abiraterone alone
effectively inhibited the growth of LNCaP cells, it had limited
impact on androgen independent C4-2 cells, a metastatic subline
from LNCaP cells, thus, suggesting a synergized tumor killing
ability of Abi-780 in prostate cancer cells.
In vivo NIRF imaging
NIRF imaging of prostate cancer in
mouse models using IR-780 dye
To validate the feasibility of using Abi-780 for
live imaging, Abi-780 were i.p. injected into athymic nude mice
bearing subcutaneous prostate cancer xenografts. The tumor
xenografts were clearly demarcated in these mice 24 h after Abi-780
administration (Fig. 5A). The
ex vivo bio-distribution study confirmed the selective
accumulation of Abi-780 in prostate cancer tissues (Fig. 5B). The moderate signal detected in
gallbladder and only a weak signal in other organs confirmed that
Abi-780 was metabolized mainly through the liver. Moreover, strong
NIRF signal could also be detected in frozen sections of xenografts
(Fig. 5C–E). H&E staining
confirmed the existence of prostate cancer in these xenografts
(Fig. 5F). The strong NIRF signal
only detected in cancer tissues demonstrated the feasibility of
Abi-780 for prostate cancer imaging.
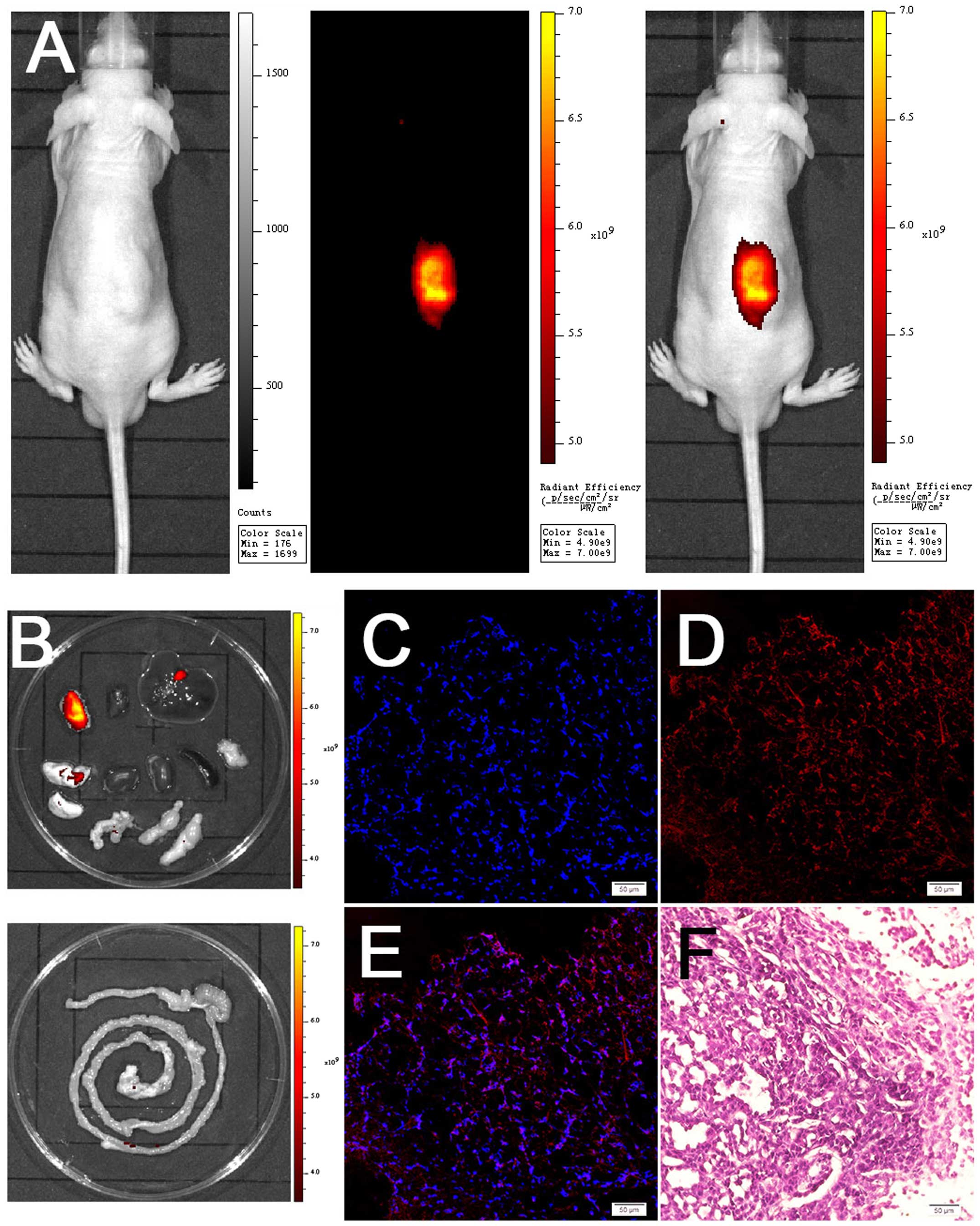 | Figure 5Near-infrared fluorescence (NIRF)
imaging of subcutaneous prostate cancer xenografts using Abi-780.
(A) From left to right, bright light field, NIRF field and merged
image. (B) Bio-distribution studies of Abi-780 (from upper left to
down right, tumor, heart, liver, lung, kindey, spleen, pancreas,
bladder and seminal vesicle, testis, intestines). (C) DAPI staining
of frozen sections. (D) NIRF signal from frozen sections. (E)
Merged image of C and D, showing the accumulation of Abi-780 in
cancer cells. (F) H&E staining of prostate cancer xenografts
(magnification, ×200). |
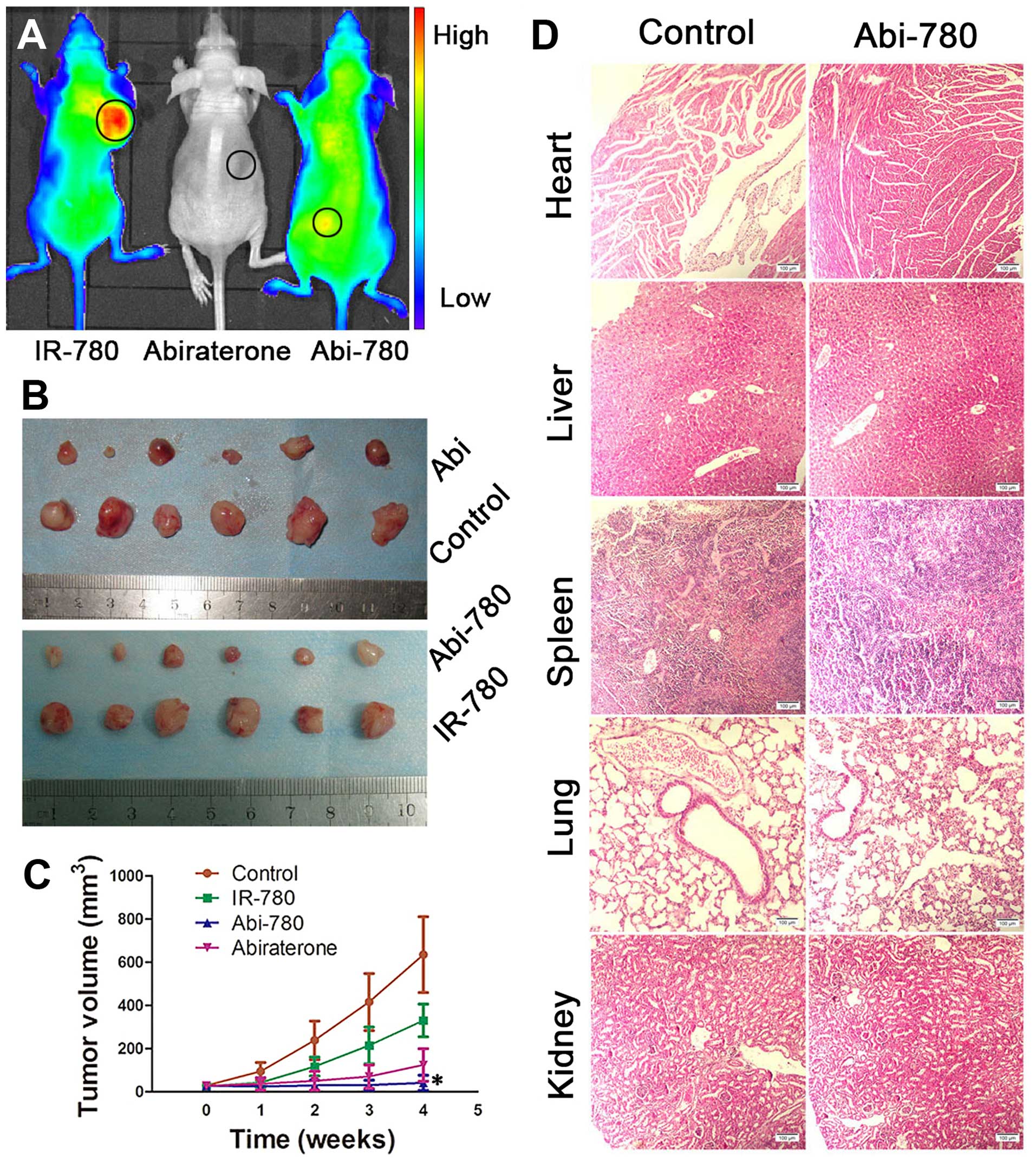
In vivo antitumor effect
The in vivo antitumor effect was investigated
using LNCaP tumor xenograft model. Athymic nude mice bearing
prostate cancer xenografts received i.p. injection of IR-780,
abiraterone or Abi-780. Tumor volumes were measured to assess the
inhibitory effect on prostate cancer. The whole body NIRF signal of
mice from three treated groups at the end-point confirmed
inhibitory effect of Abi-780 on prostate cancer xenografts
(Fig. 6A). Abi-780 showed a more
pronounced tumor inhibition effect than other treatments
(P<0.05) (Fig. 6B and C). These
results confirmed the dual function of Abi-780 for cancer imaging
and therapy. In addition, we applied a 10-fold higher dose in mice
to observe the toxicity of Abi-780. Mice with ≤57.5 mg/kg daily
i.p. injection of Abi-780 experienced no death or significant
weight loss. Histopathological analysis of the main organs
harvested from these mice displayed no apparent abnormalities in
comparison with those from normal mice (Fig. 6D).
Discussion
Radical prostatectomy is the only surgical treatment
showing cancer-specific survival benefit for localized prostate
cancer (3). Besides, radiation
therapy, chemotherapy and androgen deprivation therapy are
considered in high-risk and/or metastatic patients. However, there
exist concerns about the iatrogenic trauma from invasive surgery
and toxic effects from irradiation and chemotherapeutic agents.
Therefore, in recent years, growing interests have been focused on
developing novel multifunctional agents that can simultaneously
realize cancer diagnosis and personalized therapy without severe
side effects, offering a new concept in the management of cancer.
NIRF imaging technology is a promising imaging modality that has
attracted extensive attention over the last few years (5,19).
Using excellent NIRF probes, deep penetration of NIRF imaging is up
to 10 cm into tissues without safety concerns about radiation
exposure (20,21). These NIRF probes could also be
easily modified as drug delivery carriers to construct theranostic
agents with potent antitumor activity. Although conventional NIRF
probes are vulnerable for photobleaching and self-aggregation,
IR-780 iodide displays excellent optical properties, cancer
targeted imaging capability as well as remarkable tumoricidal
activity in drug-resistant lung cancer cells (6,21).
When nitrogen mustard was combined to IR-780 iodide, the new
compound displayed potent antitumor effect toward cancer cells. The
improved cancer targeting capability reduced severe side effects of
nitrogen mustard (22).
Abiraterone is a CYP17 inhibitor that improves
overall survival in prostate cancer patients with or without prior
docetaxel therapy (23). Besides
CYP17 inhibition, abiraterone is also an antagonist to the androgen
receptor and can inhibit 3β-hydroxysteroid dehydrogenase (15,24),
and contribute to its antitumor effects on prostate cancer cells
which express high levels of androgen receptor and synthesize their
own androgens (25). However,
daily use of a relatively high-dose abiraterone for prostate cancer
patients is associated with increased incidence of adverse effects
and toxicities (13,26).
We introduced the abiraterone moiety to the
structure of IR-780 to minimize the side effects of abiraterone,
and in the meantime, to obtain novel theranostic agents for
prostate cancer imaging and therapy. The new compound maintained
the preferential accumulation of IR-780 in cancer cells, requisite
for cancer targeted NIRF imaging. Abi-780 exerted a synergized
tumoricidal activity against prostate cancer cells in comparison
with IR-780 or abiraterone alone, showing great potential as ideal
theranostic agents to realize cancer imaging and therapy
simultaneously. Abiraterone is a potent inhibitor of androgen
synthesis. Here we showed that Abi-780 could effectively inhibit
clone formation and induce apoptosis of LNCaP cells, but it had
little impact on androgen-independent C4-2 cells when used alone,
in accordance with previous studies from another group (27,28).
Another distinct advantage of theranostic agents
Abi-780 is the facile real-time monitoring of therapeutic effects.
Fluorescence imaging via visible light is often applied to observe
the metabolism and bio-distribution of target of interest in live
animals. This imaging procedure is simple to use and relatively
sensitive (29). However, the
depth of tissue penetration is limited by visible light. Moreover,
it is impossible to realize consistent monitoring of pre-labeled
targets owing to the high extinction coefficient and
photo-bleaching of fluorescent dyes. Of note, NIRF dye-based
multifunctional agent Abi-780 is capable of long-time in
vivo monitoring (9). High
specificity in prostate cancer cell imaging using Abi-780 dye might
also be exploited in accurate quantification of live circulating
tumor cells in prostate cancer patients (30). Theranostic agent Abi-780 makes
feasible non-invasive prostate cancer imaging, therapy and
real-time monitoring of therapeutic effects.
In conclusion, Abi-780 can selectively accumulate in
prostate cancer cells and exert strong tumor-killing ability
against prostate cancer, which could be applied as potent
theranostic agents for simultaneous NIRF imaging and therapy of
prostate cancer. These could also extend to sensitive and reliable
noninvasive cancer imaging during surgical operations. The lack of
detailed anatomical information might limit the application of
Abi-780 mediated NIRF imaging, which can be solved by the
multimodal imaging that combine NIRF technology and other
conventional imaging modalities. NIRF technology using the
armamentarium of excellent NIRF dyes will pave the way for the
development of newer theranostic agents, a promising field of
cancer targeted imaging and personalized therapy.
Acknowledgements
We acknowledge support from Scientifi Innovative
Project of Shaanxi Province no. 2012KTCL03-03.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cai QY, Yu P, Besch-Williford C, Smith CJ,
Sieckman GL, Hoffman TJ and Ma L: Near-infrared fluorescence
imaging of gastrin releasing peptide receptor targeting in prostate
cancer lymph node metastases. Prostate. 73:842–854. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al; European Association of Urology. EAU guidelines
on prostate cancer. part 1: Screening, diagnosis, and local
treatment with curative intent-update 2013. Eur Urol. 65:124–137.
2014. View Article : Google Scholar
|
|
4
|
Osborne JR, Akhtar NH, Vallabhajosula S,
Anand A, Deh K and Tagawa ST: Prostate-specific membrane
antigen-based imaging. Urol Oncol. 31:144–154. 2013. View Article : Google Scholar
|
|
5
|
Yi X, Wang F, Qin W, Yang X and Yuan J:
Near-infrared fluorescent probes in cancer imaging and therapy: An
emerging field. Int J Nanomed. 9:1347–1365. 2014. View Article : Google Scholar
|
|
6
|
Yang X, Shi C, Tong R, Qian W, Zhau HZ,
Wang R, Zhu G, Cheng J, Yang VW, Cheng T, et al: Near IR
heptamethine cyanine dye-mediated cancer imaging. Clin Cancer Res.
16:2833–2844. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang C, Cheng H, Yuan A, Tang X, Wu J and
Hu Y: Hydrophobic IR780 encapsulated in biodegradable human serum
albumin nanoparticles for photothermal and photodynamic therapy.
Acta Biomater. 14:61–69. 2015. View Article : Google Scholar
|
|
8
|
Wang Y, Liu T, Zhang E, Luo S, Tan X and
Shi C: Preferential accumulation of the near infrared heptamethine
dye IR-780 in the mitochondria of drug-resistant lung cancer cells.
Biomaterials. 35:4116–4124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang X, Shao C, Wang R, Chu CY, Hu P,
Master V, Osunkoya AO, Kim HL, Zhau HE and Chung LW: Optical
imaging of kidney cancer with novel near infrared heptamethine
carbocyanine fluorescent dyes. J Urol. 189:702–710. 2013.
View Article : Google Scholar
|
|
10
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al; European Association of Urology. EAU guidelines
on prostate cancer. Part II: Treatment of advanced, relapsing, and
castration-resistant prostate cancer. Eur Urol. 65:467–479. 2014.
View Article : Google Scholar
|
|
11
|
Hahn C, Song SH, Oh CH and Berini P:
Single-mode lasers and parity-time symmetry broken gratings based
on active dielectric-loaded long-range surface plasmon polariton
waveguides. Opt Express. 23:19922–19931. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Attard G, Reid AH and de Bono JS:
Abiraterone acetate is well tolerated without concomitant use of
corticosteroids. J Clin Oncol. 28:e560–561; author reply e562.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ryan CJ, Smith MR, Fong L, Rosenberg JE,
Kantoff P, Raynaud F, Martins V, Lee G, Kheoh T, Kim J, et al:
Phase I clinical trial of the CYP17 inhibitor abiraterone acetate
demonstrating clinical activity in patients with
castration-resistant prostate cancer who received prior
ketoconazole therapy. J Clin Oncol. 28:1481–1488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Attard G, Reid AH, A'Hern R, Parker C,
Oommen NB, Folkerd E, Messiou C, Molife LR, Maier G, Thompson E, et
al: Selective inhibition of CYP17 with abiraterone acetate is
highly active in the treatment of castration-resistant prostate
cancer. J Clin Oncol. 27:3742–3748. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yin L and Hu Q: CYP17 inhibitors -
abiraterone, C17,20-lyase inhibitors and multi-targeting agents.
Nat Rev Urol. 11:32–42. 2014. View Article : Google Scholar
|
|
16
|
Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao
C, Murphy CF, Yang H, Zhau HE, Balian G and Chung LW: Establishing
human prostate cancer cell xenografts in bone: Induction of
osteoblastic reaction by prostate-specific antigen-producing tumors
in athymic and SCID/bg mice using LNCaP and lineage-derived
metastatic sublines. Int J Cancer. 77:887–894. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yi X, Zhang G and Yuan J: Renoprotective
role of fenoldopam pretreatment through hypoxia-inducible
factor-1alpha and heme oxygenase-1 expressions in rat kidney
transplantation. Transplant Proc. 45:517–522. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang C, Liu T, Su Y, Luo S, Zhu Y, Tan X,
Fan S, Zhang L, Zhou Y, Cheng T, et al: A near-infrared fluorescent
heptamethine indocyanine dye with preferential tumor accumulation
for in vivo imaging. Biomaterials. 31:6612–6617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu W, Peck EM, Hendzel KD and Smith BD:
Sensitive structural control of macrocycle threading by a
fluorescent squaraine dye flanked by polymer chains. Org Lett.
17:5268–5271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hellebust A and Richards-Kortum R:
Advances in molecular imaging: Targeted optical contrast agents for
cancer diagnostics. Nanomedicine (Lond). 7:429–445. 2012.
View Article : Google Scholar
|
|
21
|
Yue C, Liu P, Zheng M, Zhao P, Wang Y, Ma
Y and Cai L: IR-780 dye loaded tumor targeting theranostic
nanoparticles for NIR imaging and photothermal therapy.
Biomaterials. 34:6853–6861. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang E, Luo S, Tan X and Shi C:
Mechanistic study of IR-780 dye as a potential tumor targeting and
drug delivery agent. Biomaterials. 35:771–778. 2014. View Article : Google Scholar
|
|
23
|
Sternberg CN, Castellano D, Daugaard G,
Géczi L, Hotte SJ, Mainwaring PN, Saad F, Souza C, Tay MH, Garrido
JM, et al; Abiraterone Global EAP Investigators. Abiraterone
acetate for patients with metastatic castration-resistant prostate
cancer progressing after chemotherapy: Final analysis of a
multi-centre, open-label, early-access protocol trial. Lancet
Oncol. 15:1263–1268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Richards J, Lim AC, Hay CW, Taylor AE,
Wingate A, Nowakowska K, Pezaro C, Carreira S, Goodall J, Arlt W,
et al: Interactions of abiraterone, eplerenone, and prednisolone
with wild-type and mutant androgen receptor: A rationale for
increasing abiraterone exposure or combining with MDV3100. Cancer
Res. 72:2176–2182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Antonarakis ES, Lu C, Wang H, Luber B,
Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, et
al: AR-V7 and resistance to enzalutamide and abiraterone in
prostate cancer. N Engl J Med. 371:1028–1038. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matsubara N, Uemura H, Satoh T, Suzuki H,
Nishiyama T, Uemura H, Hashine K, Imanaka K, Ozono S and Akaza H: A
phase 2 trial of abiraterone acetate in Japanese men with
metastatic castration-resistant prostate cancer and without prior
chemotherapy (JPN-201 study). Jpn J Clin Oncol. 44:1216–1226. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Murga JD, Moorji SM, Han AQ, Magargal WW,
DiPippo VA and Olson WC: Synergistic co-targeting of
prostate-specific membrane antigen and androgen receptor in
prostate cancer. Prostate. 75:242–254. 2015. View Article : Google Scholar
|
|
28
|
Kosaka T, Miyajima A, Yasumizu Y, Miyazaki
Y, Kikuchi E and Oya M: Limited in vitro efficacy of CYP17A1
inhibition on human castration resistant prostate cancer. Steroids.
92:39–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shan L: Near-infrared fluorescence
1,1-dioctadecyl-3,3,3,3-tetra-methylindotricarbocyanine iodide
(DiR)-labeled macrophages for cell imaging. Molecular Imaging and
Contrast Agent Database. MICAD; Bethesda, MD: 2004
|
|
30
|
Shao C, Liao CP, Hu P, Chu CY, Zhang L,
Bui MH, Ng CS, Josephson DY, Knudsen B, Tighiouart M, et al:
Detection of live circulating tumor cells by a class of
near-infrared heptamethine carbocyanine dyes in patients with
localized and metastatic prostate cancer. PLoS One. 9:e889672014.
View Article : Google Scholar : PubMed/NCBI
|