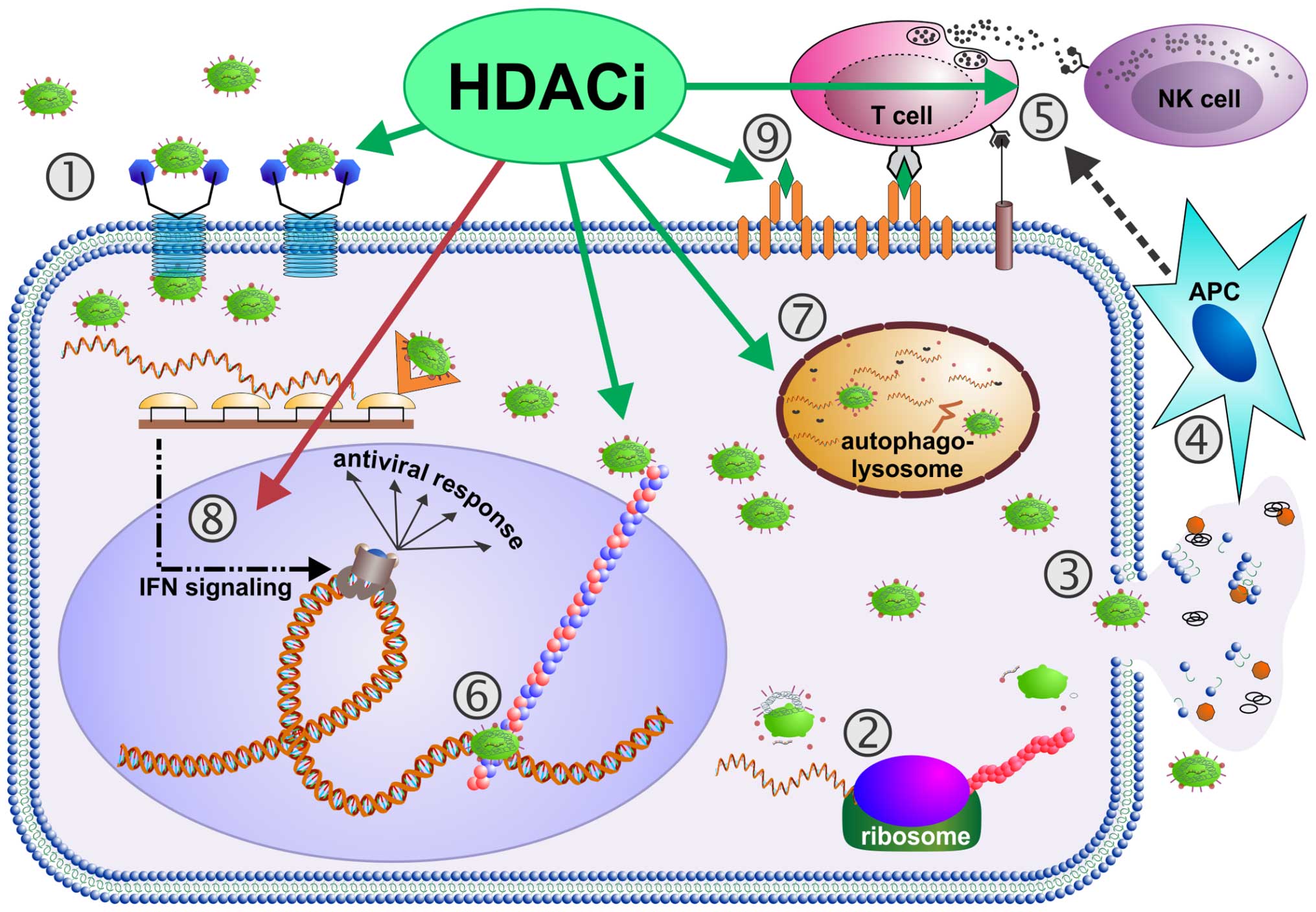
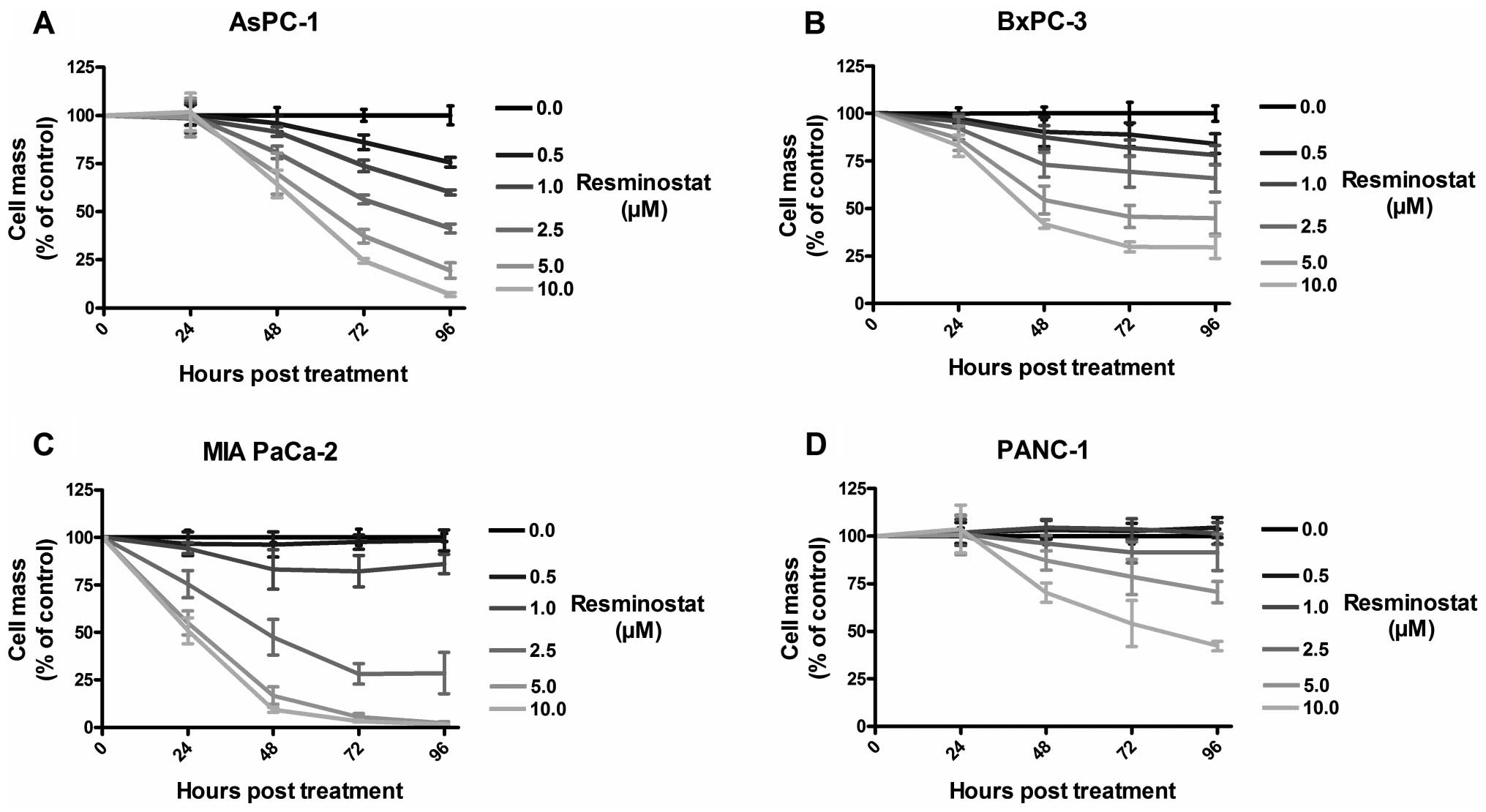
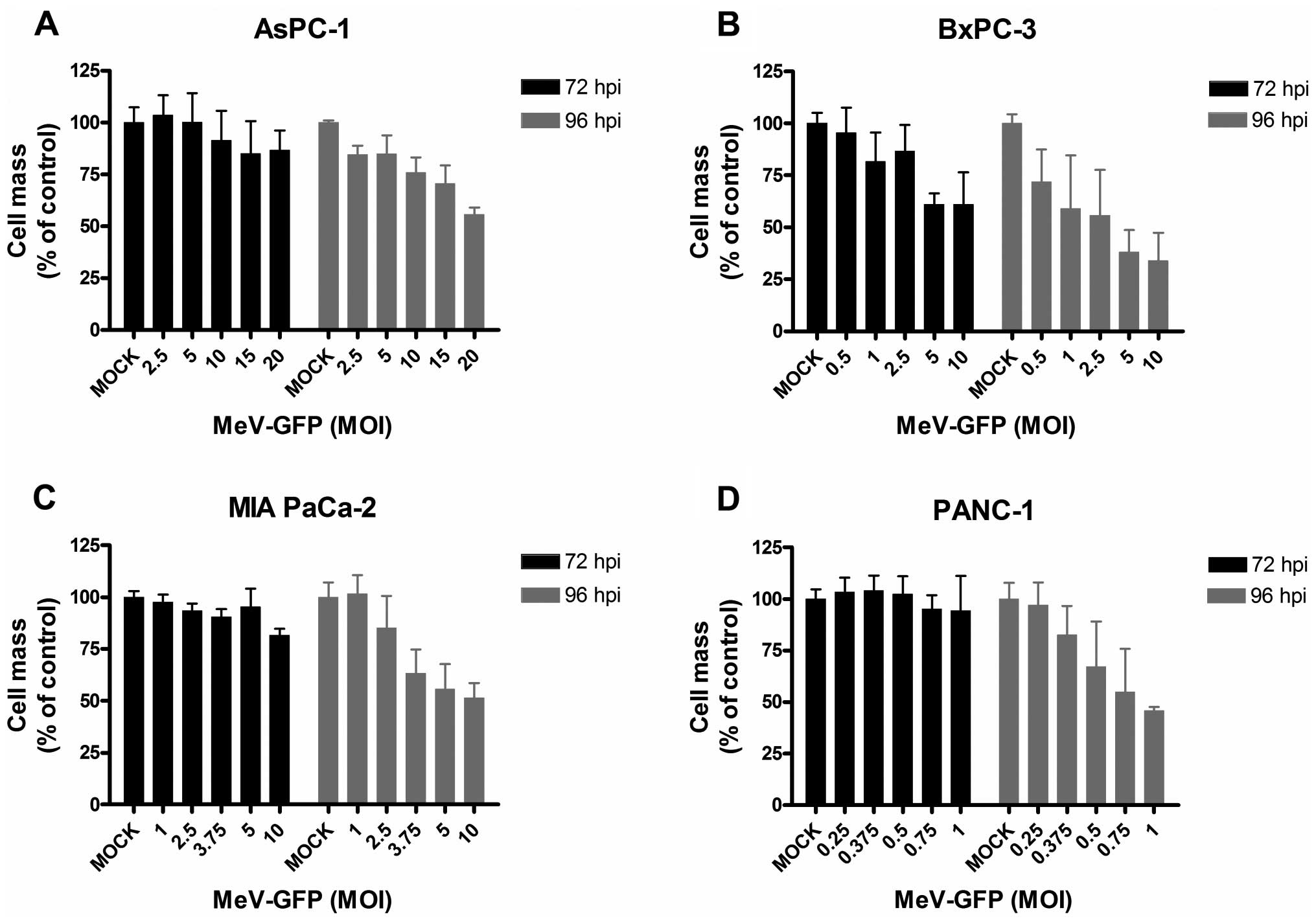
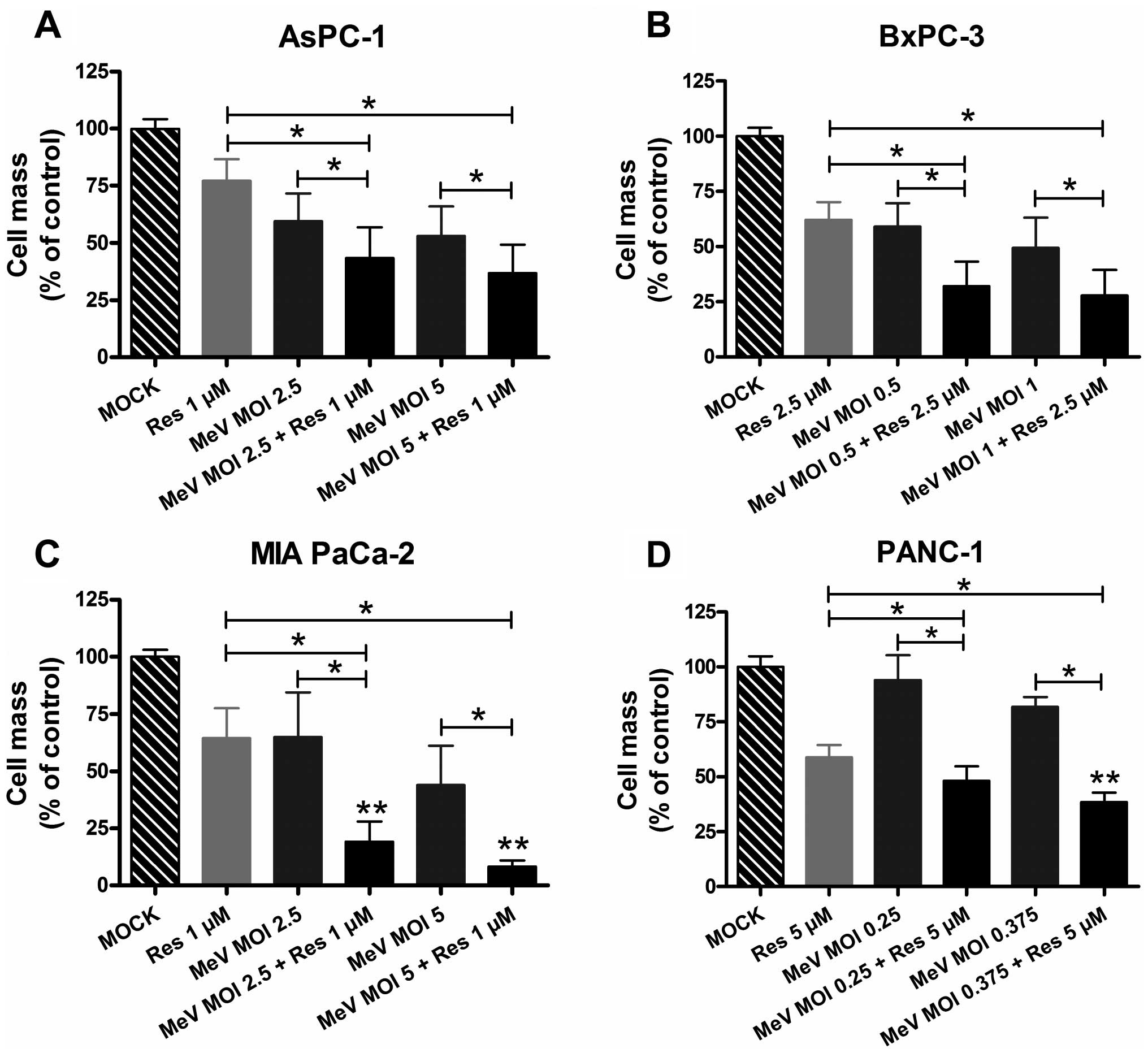
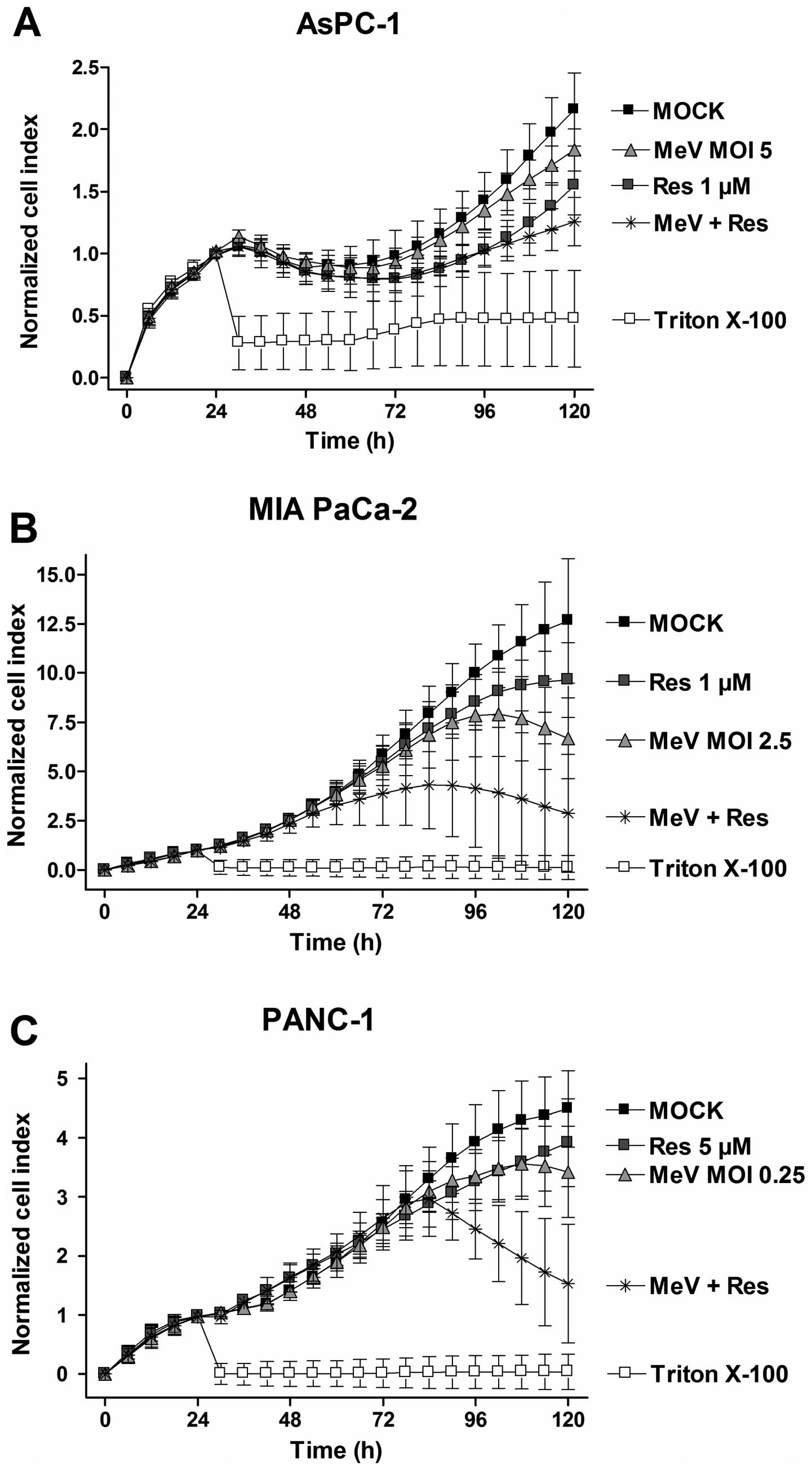
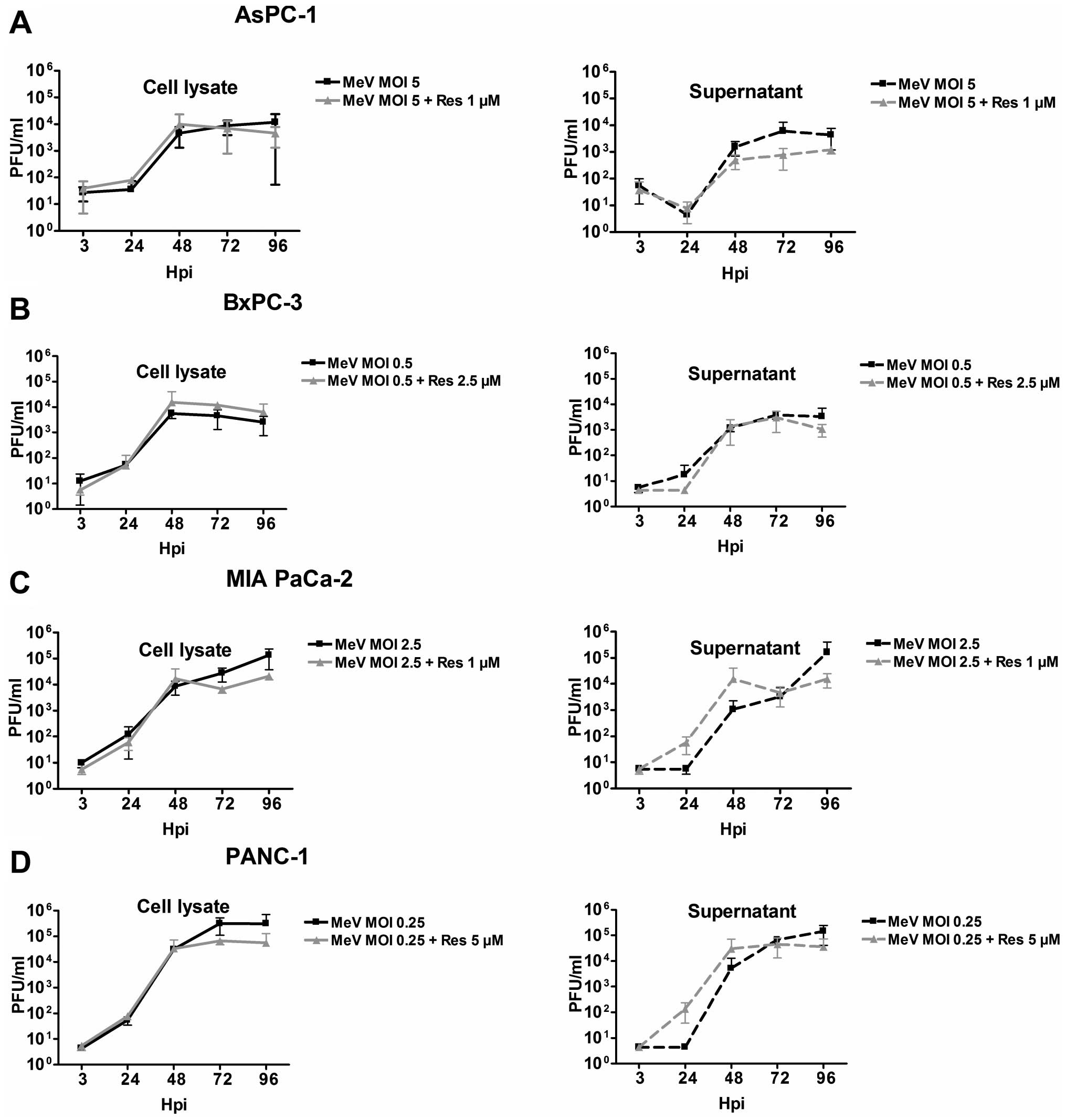
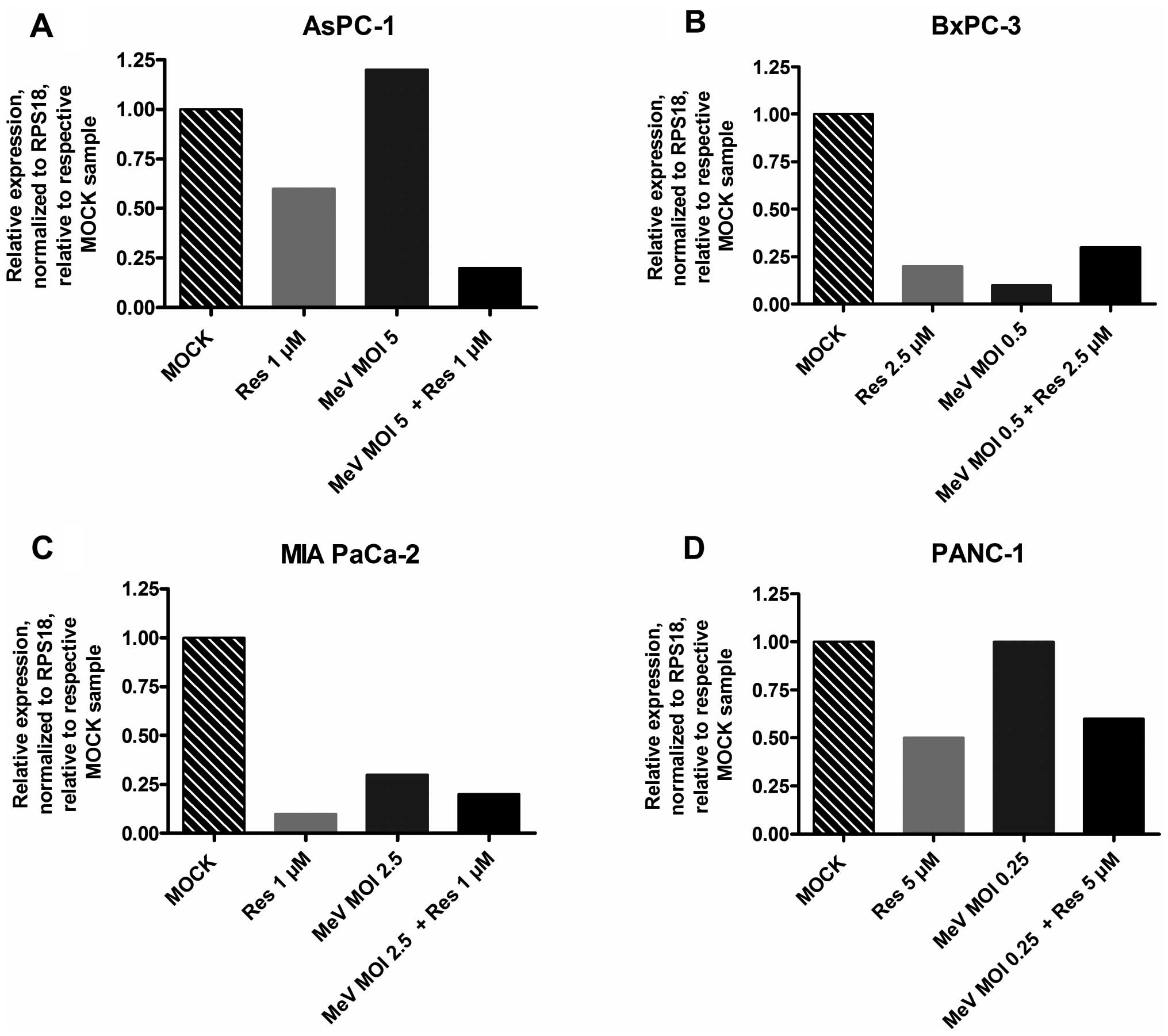
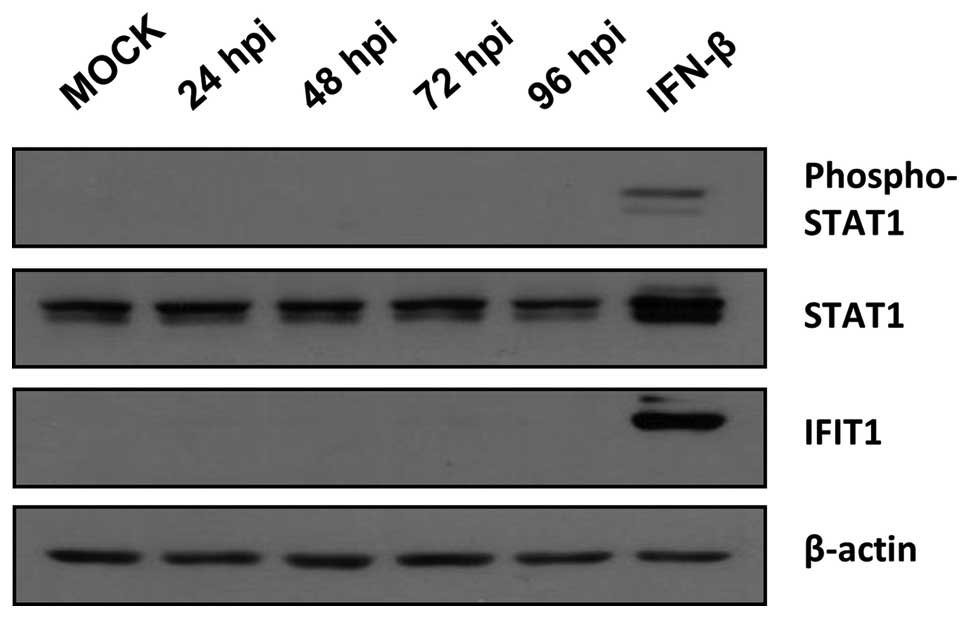
|
1
|
Russell SJ, Peng KW and Bell JC: Oncolytic
virotherapy. Nat Biotechnol. 30:658–670. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hammill AM, Conner J and Cripe TP:
Oncolytic virotherapy reaches adolescence. Pediatr Blood Cancer.
55:1253–1263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bourke MG, Salwa S, Harrington KJ,
Kucharczyk MJ, Forde PF, de Kruijf M, Soden D, Tangney M, Collins
JK and O'Sullivan GC: The emerging role of viruses in the treatment
of solid tumours. Cancer Treat Rev. 37:618–632. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith TT, Roth JC, Friedman GK and
Gillespie GY: Oncolytic viral therapy: Targeting cancer stem cells.
Oncolytic Virother. 2014:21–33. 2014.PubMed/NCBI
|
|
5
|
Breitbach CJ, De Silva NS, Falls TJ, Aladl
U, Evgin L, Paterson J, Sun YY, Roy DG, Rintoul JL, Daneshmand M,
et al: Targeting tumor vasculature with an oncolytic virus. Mol
Ther. 19:886–894. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Andtbacka RH, Kaufman HL, Collichio F,
Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I,
Agarwala SS, et al: Talimogene laherparepvec improves durable
response rate in patients with advanced melanoma. J Clin Oncol.
33:2780–2788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ledford H: Cancer-fighting viruses win
approval. Nature. 526:622–623. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Russell SJ, Federspiel MJ, Peng KW, Tong
C, Dingli D, Morice WG, Lowe V, O'Connor MK, Kyle RA, Leung N, et
al: Remission of disseminated cancer after systemic oncolytic
virotherapy. Mayo Clin Proc. 89:926–933. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chiocca EA: The host response to cancer
virotherapy. Curr Opin Mol Ther. 10:38–45. 2008.PubMed/NCBI
|
|
10
|
Kim M, Zinn KR, Barnett BG, Sumerel LA,
Krasnykh V, Curiel DT and Douglas JT: The therapeutic efficacy of
adenoviral vectors for cancer gene therapy is limited by a low
level of primary adenovirus receptors on tumour cells. Eur J
Cancer. 38:1917–1926. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berchtold S, Lampe J, Weiland T, Smirnow
I, Schleicher S, Handgretinger R, Kopp HG, Reiser J, Stubenrauch F,
Mayer N, et al: Innate immune defense defines susceptibility of
sarcoma cells to measles vaccine virus-based oncolysis. J Virol.
87:3484–3501. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Escobar-Zarate D, Liu YP, Suksanpaisan L,
Russell SJ and Peng KW: Overcoming cancer cell resistance to VSV
oncolysis with JAK1/2 inhibitors. Cancer Gene Ther. 20:582–589.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Noll M, Berchtold S, Lampe J, Malek NP,
Bitzer M and Lauer UM: Primary resistance phenomena to oncolytic
measles vaccine viruses. Int J Oncol. 43:103–112. 2013.PubMed/NCBI
|
|
14
|
Nguyen TL, Wilson MG and Hiscott J:
Oncolytic viruses and histone deacetylase inhibitors - a
multi-pronged strategy to target tumor cells. Cytokine Growth
Factor Rev. 21:153–159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakashima H, Nguyen T and Chiocca EA:
Combining HDAC inhibitors with oncolytic virotherapy for cancer
therapy. Dovepress. 2015:183–191. 2015.
|
|
16
|
Khan O and La Thangue NB: HDAC inhibitors
in cancer biology: Emerging mechanisms and clinical applications.
Immunol Cell Biol. 90:85–94. 2012. View Article : Google Scholar
|
|
17
|
Bolden JE, Peart MJ and Johnstone RW:
Anticancer activities of histone deacetylase inhibitors. Nat Rev
Drug Discov. 5:769–784. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Bonifati S, Hristov G, Marttila T,
Valmary-Degano S, Stanzel S, Schnölzer M, Mougin C, Aprahamian M,
Grekova SP, et al: Synergistic combination of valproic acid and
oncolytic parvovirus H-1PV as a potential therapy against cervical
and pancreatic carcinomas. EMBO Mol Med. 5:1537–1555. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
MacTavish H, Diallo JS, Huang B, Stanford
M, Le Boeuf F, De Silva N, Cox J, Simmons JG, Guimond T, Falls T,
et al: Enhancement of vaccinia virus based oncolysis with histone
deacetylase inhibitors. PLoS One. 5:e144622010. View Article : Google Scholar
|
|
20
|
White MC and Frampton AR Jr: The histone
deacetylase inhibitor valproic acid enhances equine herpesvirus
type 1 (EHV-1)-mediated oncolysis of human glioma cells. Cancer
Gene Ther. 20:88–93. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bridle BW, Chen L, Lemay CG, Diallo JS,
Pol J, Nguyen A, Capretta A, He R, Bramson JL, Bell JC, et al: HDAC
inhibition suppresses primary immune responses, enhances secondary
immune responses, and abrogates autoimmunity during tumor
immunotherapy. Mol Ther. 21:887–894. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cody JJ, Markert JM and Hurst DR: Histone
deacetylase inhibitors improve the replication of oncolytic herpes
simplex virus in breast cancer cells. PLoS One. 9:e929192014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alvarez-Breckenridge CA, Yu J, Price R,
Wei M, Wang Y, Nowicki MO, Ha YP, Bergin S, Hwang C, Fernandez SA,
et al: The histone deacetylase inhibitor valproic acid lessens NK
cell action against oncolytic virus-infected glioblastoma cells by
inhibition of STAT5/T-BET signaling and generation of gamma
interferon. J Virol. 86:4566–4577. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ruf B, Berchtold S, Venturelli S, Burkard
M, Smirnow I, Prenzel T, Henning SW and Lauer UM: Combination of
the oral histone deacetylase inhibitor resminostat with oncolytic
measles vaccine virus as a new option for epi-virotherapeutic
treatment of hepatocellular carcinoma. Mol Ther Oncolytics.
2:150192015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nusinzon I and Horvath CM: Positive and
negative regulation of the innate antiviral response and beta
interferon gene expression by deacetylation. Mol Cell Biol.
26:3106–3113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Génin P, Morin P and Civas A: Impairment
of interferon-induced IRF-7 gene expression due to inhibition of
ISGF3 formation by trichostatin A. J Virol. 77:7113–7119. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang X, Gao JS, Guan YJ, McLane KE, Yuan
ZL, Ramratnam B and Chin YE: Acetylation-dependent signal
transduction for type I interferon receptor. Cell. 131:93–105.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan ZL, Guan YJ, Chatterjee D and Chin
YE: Stat3 dimerization regulated by reversible acetylation of a
single lysine residue. Science. 307:269–273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang HM, Paulson M, Holko M, Rice CM,
Williams BR, Marié I and Levy DE: Induction of
interferon-stimulated gene expression and antiviral responses
require protein deacetylase activity. Proc Natl Acad Sci USA.
101:9578–9583. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Otsuki A, Patel A, Kasai K, Suzuki M,
Kurozumi K, Chiocca EA and Saeki Y: Histone deacetylase inhibitors
augment antitumor efficacy of herpes-based oncolytic viruses. Mol
Ther. 16:1546–1555. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu TC, Castelo-Branco P, Rabkin SD and
Martuza RL: Trichostatin A and oncolytic HSV combination therapy
shows enhanced antitumoral and antiangiogenic effects. Mol Ther.
16:1041–1047. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wunder T, Schmid K, Wicklein D, Groitl P,
Dobner T, Lange T, Anders M and Schumacher U: Expression of the
coxsackie adenovirus receptor in neuroendocrine lung cancers and
its implications for oncolytic adenoviral infection. Cancer Gene
Ther. 20:25–32. 2013. View Article : Google Scholar
|
|
33
|
Kasman L, Onicescu G and Voelkel-Johnson
C: Histone deacetylase inhibitors restore cell surface expression
of the coxsackie adenovirus receptor and enhance CMV promoter
activity in castration-resistant prostate cancer cells. Prostate
Cancer. 2012:1371632012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stiff A, Caserta E, Sborov DW, Nuovo GJ,
Mo X, Schlotter SY, Canella A, Smith E, Badway J, Old M, et al:
Histone deacetylase inhibitors enhance the therapeutic potential of
reovirus in multiple myeloma. Mol Cancer Ther. 15:830–841. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meng S, Xu J, Wu Y and Ding C: Targeting
autophagy to enhance oncolytic virus-based cancer therapy. Expert
Opin Biol Ther. 13:863–873. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shulak L, Beljanski V, Chiang C, Dutta SM,
Van Grevenynghe J, Belgnaoui SM, Nguyên TL, Di Lenardo T, Semmes
OJ, Lin R, et al: Histone deacetylase inhibitors potentiate
vesicular stomatitis virus oncolysis in prostate cancer cells by
modulating NF-κB-dependent autophagy. J Virol. 88:2927–2940. 2014.
View Article : Google Scholar :
|
|
37
|
Richetta C, Grégoire IP, Verlhac P, Azocar
O, Baguet J, Flacher M, Tangy F, Rabourdin-Combe C and Faure M:
Sustained autophagy contributes to measles virus infectivity. PLoS
Pathog. 9:e10035992013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dreux M and Chisari FV: Viruses and the
autophagy machinery. Cell Cycle. 9:1295–1307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nakashima H, Kaufmann JK, Wang PY, Nguyen
T, Speranza MC, Kasai K, Okemoto K, Otsuki A, Nakano I, Fernandez
S, et al: Histone deacetylase 6 inhibition enhances oncolytic viral
replication in glioma. J Clin Invest. 125:4269–4280. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guillerme JB, Gregoire M, Tangy F and
Fonteneau JF: Antitumor virotherapy by attenuated measles virus
(MV). Biology (Basel). 2:587–602. 2013.
|
|
41
|
Hutzen BRC and Studebaker AW: Advances in
the design and development of oncolytic measles viruses. Dovepress.
4:109–118. 2015.
|
|
42
|
Bitzer M, Horger M, Giannini EG, Ganten
TM, Wörns MA, Siveke JT, Dollinger mM, Gerken G, Scheulen ME, Wege
H, et al: Resminostat plus sorafenib as second-line therapy of
advanced hepatocellular carcinoma - The SHELTER Study. J Hepatol.
65:280–288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Russell SJ and Peng KW: Measles virus for
cancer therapy. Curr Top Microbiol Immunol. 330:213–241.
2009.PubMed/NCBI
|
|
44
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brunetto AT, Ang JE, Lal R, Olmos D,
Molife LR, Kristeleit R, Parker A, Casamayor I, Olaleye M, Mais A,
et al: First-inhuman, pharmacokinetic and pharmacodynamic phase I
study of Resminostat, an oral histone deacetylase inhibitor, in
patients with advanced solid tumors. Clin Cancer Res. 19:5494–5504.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kitazono S, Fujiwara Y, Nakamichi S,
Mizugaki H, Nokihara H, Yamamoto N, Yamada Y, Inukai E, Nakamura O
and Tamura T: A phase I study of resminostat in Japanese patients
with advanced solid tumors. Cancer Chemother Pharmacol.
75:1155–1161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Weiland T, Lampe J, Essmann F, Venturelli
S, Berger A, Bossow S, Berchtold S, Schulze-Osthoff K, Lauer UM and
Bitzer M: Enhanced killing of therapy-induced senescent tumor cells
by oncolytic measles vaccine viruses. Int J Cancer. 134:235–243.
2014. View Article : Google Scholar
|
|
48
|
Spearman C: The method of ‘right and wrong
cases’ (‘constant stimuli’) without gauss's formulae. Br J Psychol.
2:227–242. 1908.
|
|
49
|
Kärber G: Beitrag zur kollektiven
behandlung pharmakologischer reihenversuche. Naunyn Schmiedebergs
Arch Exp Pathol Pharmakol. 162:480–483. 1931.(In German).
View Article : Google Scholar
|
|
50
|
Walewski J, Pszkiewicz-Kozik E, Warzewska
A, Borsaru G, Moicean A, Hellmann A, Mayer J, Hauns B, Mais A,
Henning SW, et al: Final results of the phase II SAPHIRE trial of
resminostat (4SC-201) in patients with relapsed/refractory Hodgkin
lymphoma. Presented at 53rd ASH Annual Meeting and Exposition.
(abstract 2675); 2011, http://www.4sc.de/wp-content/uploads/SAPHIRE-Poster-ASH-San-Diego-2011.pdf.
|
|
51
|
Abassi YA, Xi B, Zhang W, Ye P, Kirstein
SL, Gaylord MR, Feinstein SC, Wang X and Xu X: Kinetic cell-based
morphological screening: Prediction of mechanism of compound action
and off-target effects. Chem Biol. 16:712–723. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Weiland T, Berger A, Essmann F, Lauer UM,
Bitzer M and Venturelli S: Kinetic tracking of therapy-induced
senescence using the real-time cell analyzer single plate system.
Assay Drug Dev Technol. 10:289–295. 2012. View Article : Google Scholar
|
|
53
|
Stojdl DF, Lichty BD, tenOever BR,
Paterson JM, Power AT, Knowles S, Marius R, Reynard J, Poliquin L,
Atkins H, et al: VSV strains with defects in their ability to
shutdown innate immunity are potent systemic anti-cancer agents.
Cancer Cell. 4:263–275. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Abend A and Kehat I: Histone deacetylases
as therapeutic targets - from cancer to cardiac disease. Pharmacol
Ther. 147:55–62. 2015. View Article : Google Scholar
|
|
55
|
Mottamal M, Zheng S, Huang TL and Wang G:
Histone deacetylase inhibitors in clinical studies as templates for
new anticancer agents. Molecules. 20:3898–3941. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Feng W, Zhang B, Cai D and Zou X:
Therapeutic potential of histone deacetylase inhibitors in
pancreatic cancer. Cancer Lett. 347:183–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xu C, Li H, Su C and Li Z: Viral therapy
for pancreatic cancer: Tackle the bad guys with poison. Cancer
Lett. 333:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Honda K, Takaoka A and Taniguchi T: Type I
interferon [corrected] gene induction by the interferon regulatory
factor family of transcription factors. Immunity. 25:349–360. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nguyên TL, Abdelbary H, Arguello M,
Breitbach C, Leveille S, Diallo JS, Yasmeen A, Bismar TA, Kirn D,
Falls T, et al: Chemical targeting of the innate antiviral response
by histone deacetylase inhibitors renders refractory cancers
sensitive to viral oncolysis. Proc Natl Acad Sci USA.
105:14981–14986. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fuertes MB, Kacha AK, Kline J, Woo SR,
Kranz DM, Murphy KM and Gajewski TF: Host type I IFN signals are
required for antitumor CD8+ T cell responses through
CD8{alpha}+ dendritic cells. J Exp Med. 208:2005–2016.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Prestwich RJ, Errington F, Diaz RM, Pandha
HS, Harrington KJ, Melcher AA and Vile RG: The case of oncolytic
viruses versus the immune system: Waiting on the judgment of
Solomon. Hum Gene Ther. 20:1119–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Katsura T, Iwai S, Ota Y, Shimizu H, Ikuta
K and Yura Y: The effects of trichostatin A on the oncolytic
ability of herpes simplex virus for oral squamous cell carcinoma
cells. Cancer Gene Ther. 16:237–245. 2009.
|
|
63
|
Murrow L and Debnath J: Autophagy as a
stress-response and quality-control mechanism: Implications for
cell injury and human disease. Annu Rev Pathol. 8:105–137. 2013.
View Article : Google Scholar
|
|
64
|
Yang S, Wang X, Contino G, Liesa M, Sahin
E, Ying H, Bause A, Li Y, Stommel JM, Dell'antonio G, et al:
Pancreatic cancers require autophagy for tumor growth. Genes Dev.
25:717–729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
White E and DiPaola RS: The double-edged
sword of autophagy modulation in cancer. Clin Cancer Res.
15:5308–5316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gammoh N, Lam D, Puente C, Ganley I, Marks
PA and Jiang X: Role of autophagy in histone deacetylase
inhibitor-induced apoptotic and nonapoptotic cell death. Proc Natl
Acad Sci USA. 109:6561–6565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hristov G, Krämer M, Li J, El-Andaloussi
N, Mora R, Daeffler L, Zentgraf H, Rommelaere J and Marchini A:
Through its nonstructural protein NS1, parvovirus H-1 induces
apoptosis via accumulation of reactive oxygen species. J Virol.
84:5909–5922. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kaufman HL, Kohlhapp FJ and Zloza A:
Oncolytic viruses: A new class of immunotherapy drugs. Nat Rev Drug
Discov. 14:642–662. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gujar S, Dielschneider R, Clements D,
Helson E, Shmulevitz M, Marcato P, Pan D, Pan L, Ahn D-G, Alawadhi
A, et al: Multifaceted therapeutic targeting of ovarian peritoneal
carcinomatosis through virus-induced immunomodulation. Mol Ther.
21:338–347. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Moehler MH, Zeidler M, Wilsberg V,
Cornelis JJ, Woelfel T, Rommelaere J, Galle PR and Heike M:
Parvovirus H-1-induced tumor cell death enhances human immune
response in vitro via increased phagocytosis, maturation, and
cross-presentation by dendritic cells. Hum Gene Ther. 16:996–1005.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kroesen M, Gielen P, Brok IC, Armandari I,
Hoogerbrugge PM and Adema GJ: HDAC inhibitors and immunotherapy; a
double edged sword? Oncotarget. 5:6558–6572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Setiadi AF, Omilusik K, David MD, Seipp
RP, Hartikainen J, Gopaul R, Choi KB and Jefferies WA: Epigenetic
enhancement of antigen processing and presentation promotes immune
recognition of tumors. Cancer Res. 68:9601–9607. 2008. View Article : Google Scholar : PubMed/NCBI
|