Introduction
Surgery, radiotherapy, chemotherapy, endocrine
therapy, and molecular targeted therapy are extensively recommended
as combinatorial therapies in breast cancer, depending on cancer
stage and biomolecular subtype (1). Particularly, radiotherapy is the
indispensable treatment modality for loco-regional control after
breast conserving therapy and for eradicating cancer cells
remaining after surgery (2–4).
Besides radio-therapy, endocrine therapy such as aromatase
inhibitor or tamoxifen, is another important treatment option since
~70% of breast cancer patients are positive for estrogen receptor α
(ERα) (5). Generally, endocrine
therapy is administered for 3–5 years when breast cancer is
ERα-positive (6,7). However, despite the fact that
combined radiation and endocrine therapy is commonly used nowadays
(8–10), it has not been determined when
tamoxifen should be started with respect to radiotherapy to
maximize clinical benefits. In fact, clinician's opinion and
experience are the major determinants of whether concurrent or
sequential tamoxifen and radiotherapy are adopted due to a lack of
clear guidelines.
Some preclinical studies have shown that
pretreatment of breast cancer cells with tamoxifen interferes with
the effects of radiotherapy by arresting cells in the G0/G1 phase
(11–13). Moreover, concern has been expressed
about increased pulmonary and breast fibrosis associated with
concurrent tamoxifen and radiotherapy (14,15).
Thus, some clinicians may delay tamoxifen therapy until
radiotherapy has been completed to avoid possible toxicities.
However, others have suggested that pretreatment with tamoxifen
enhances the effect of radiation and does not alter the
radiosensitivity of breast cancer cells (16). In addition to preclinical studies,
several randomized trials have addressed the relative
effectivenesses of sequential and concurrent tamoxifen and
radiation therapy, but unfortunately, findings were contradictory
and no firm conclusions were drawn due, in part, to the small
numbers of patients enrolled (9,17,18).
Since the optimal scheduling for tamoxifen and
radio-therapy remain unclear, we attempted to identify an optimal
time for commencing tamoxifen treatment by analyzing tamoxifen
responses in MCF-7 cells at different times after irradiation. In
addition, we assessed the effect of tamoxifen in radioresistant
cells because tamoxifen has to be administered for several years
after radiotherapy and tumors may recur during tamoxifen treatment
due to the presence of radioresistant breast cancer cells.
Materials and methods
Cell culture
MCF-7 cells (a human breast cancer cell line) were
purchased from the Korean Cell Line Bank (Seoul, Korea). Cells were
cultured in DMEM (WelGENE, Daegu, Korea) supplemented with 10%
fetal bovine serum (Invitrogen Life Technologies, Carlsbad, CA,
USA), 1% antibiotic-antimycotic solution (WelGENE), and 10 μg/ml
insulin (WelGENE).
Irradiation and establishment of
radioresistant cell lines
MCF-7 cells (1×106) were seeded in a
75-cm2 culture flask, and irradiated using a 21 EX Linac
(Varian Medical Systems, Palo Alto, CA, USA) with 6 MV X-rays at a
rate of 3 Gy per minute. The field size was 25×25 cm and the beam
was delivered postero-anteriorly. Cells were irradiated with 5 Gy
and harvested (MCF-7-5 Gy) or maintained for a week, passaged at
80% confluence, and when in the growth phase irradiated with a
second fraction of 5 Gy (MCF-7-10 Gy). MCF-7-5 Gy and MCF-7-10 Gy
cells were harvested immediately after each 5 or 10 Gy irradiation
for further experiments. In addition, MCF-7-10 Gy cells were
maintained for 40 days to allow them time to recover (MCF-7-R1).
The same cycles of irradiation were repeated for a cumulative dose
of 20 Gy (MCF-7-R2) and 30 Gy (MCF-7-R3) over 5 months to establish
radioresistant MCF-7 cells.
Colony formation assay
Radioresistance was measured using a clonogenic cell
survival assay. MCF-7-R3 and MCF-7 control cells were seeded into
6-well plates at 300–1,200 cells/well and exposed to 2 or 4 Gy of
radiation. All cells were incubated for 10 days at 37°C in 5%
CO2; medium was replaced every 3 days. Colonies were
fixed with 4% formaldehyde and stained with 0.01% crystal violet.
Positive colonies, defined as groups of >50 cells, were counted
manually under a microscope (TS 100, Nikon, Japan). Plating
efficiencies of MCF-7-R3 and control cells were determined, and
survival fractions were calculated by counting colonies. The
experiments were performed in triplicate, and results are presented
as means ± SDs.
Cell viability assay
MCF-7 cells were maintained in phenol-red free DMEM
(WelGENE) supplemented with 10% charcoal-stripped FBS, 1%
antibiotic-antimycotic solution (WelGENE), and 10 μg/ml insulin for
1–2 days prior to the assay. Briefly, 5,000 cells/well were seeded
in 96-well plates, incubated at 37°C for 24 h, and treated with
tamoxifen at 5–20 μM (AG Scientific, San Diego, CA, USA). Cells
were then further incubated for 48 h and viabilities were
determined using an MTT assay (Sigma, St. Louis, MO, USA).
Absorbance at 570 nm was measured using a Multi-Detection
Microplate Reader (Molecular Devices, Sunnyvale, CA, USA).
Experiments were performed in triplicate, and results are presented
as means ± SDs.
Western blot analysis
MCF-7 cells were lysed with RIPA buffer [150 mM
NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 50 mM
Tris-HCl pH 7.5 and 2 mM EDTA]. Phosphatase and protease inhibitor
cocktail (GenDEPOT, Barker, TX, USA) were added to RIPA buffer
immediately before use. Total protein concentrations were measured
using bicinchoninic acid reagent (Sigma). Proteins were separated
in 8 or 10% SDS-PAGE gels and transferred to polyvinylidene
difluoride (PVDF) membranes at 100 V for 45 min, membranes were
blocked with 5% non-fat skim milk containing TBS-Tween (50 mM
Tris-HCl, 150 mM NaCl, 0.1% Tween-20) for 1 h at room temperature.
Blots were incubated with the following antibodies at 4°C
overnight; ERα, phosphorylated extracellular signal regulated
kinase 1/2 (p-ERK1/2), total-ERK1/2, phosphorylated protein kinase
B (p-AKT), total AKT, and β-actin (Cell Signaling Technology,
Beverly, MA, USA). Blots were incubated with horseradish
peroxidase-conjugated secondary anti-rabbit antibody (1:5,000;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 1 h at
room temperature, and developed using Luminescent Image Analyzer
LAS-4000 (Fujifilm, Tokyo, Japan).
RNA isolation and RT-PCR
Total RNA was isolated using the easy-BLUE™ Total
RNA Extraction kit (iNtRON Biotechnology. Inc., Sungnam, Korea) and
cDNA synthesis and RT-PCR (reverse transcriptase polymerase chain
reaction) were performed as previously described (19). The primer sequences used for the
RT-PCR were as follows: ERα, forward, 5′-TCCTGATGATTGGTCTCGTCT-3′;
reverse, 5′-ACATTTTCCCTGGTTCCTGTC-3′. GAPDH forward,
5′-ATCCCATCACCATCTTCCAG-3′; and reverse,
5′-TTCTAGACGGCAGGTCAGGT-3′. Densitometric analysis was performed
using Scion Image Software (Scion Corp., Frederick, MD, USA).
Flow cytometry
MCF-7 cells were trypsinized and washed with 2% FBS
in phosphate-buffered saline (PBS), incubated with CD24-PE and
CD44-FITC (BD Biosciences, San Diego, CA, USA) for 30 min on ice,
washed with 2% FBS in PBS, and resuspended in a final volume of 500
μl PBS buffer for analysis. Fluorescence-activated cell sorting
(FACS) was performed using a FACSCalibur II (BD Biosciences).
Unstained and single color-labeled samples were used to calibrate
the analyzer prior to each experiment.
Statistical analysis
All numerical data are expressed as mean values and
standard deviations. The Student's t-test was used to compare mean
values. P-values of <0.05 were considered statistically
significant, and the analysis was performed using SPSS version 18
software.
Results
Attenuated tamoxifen response in
concurrently irradiated MCF-7 cells
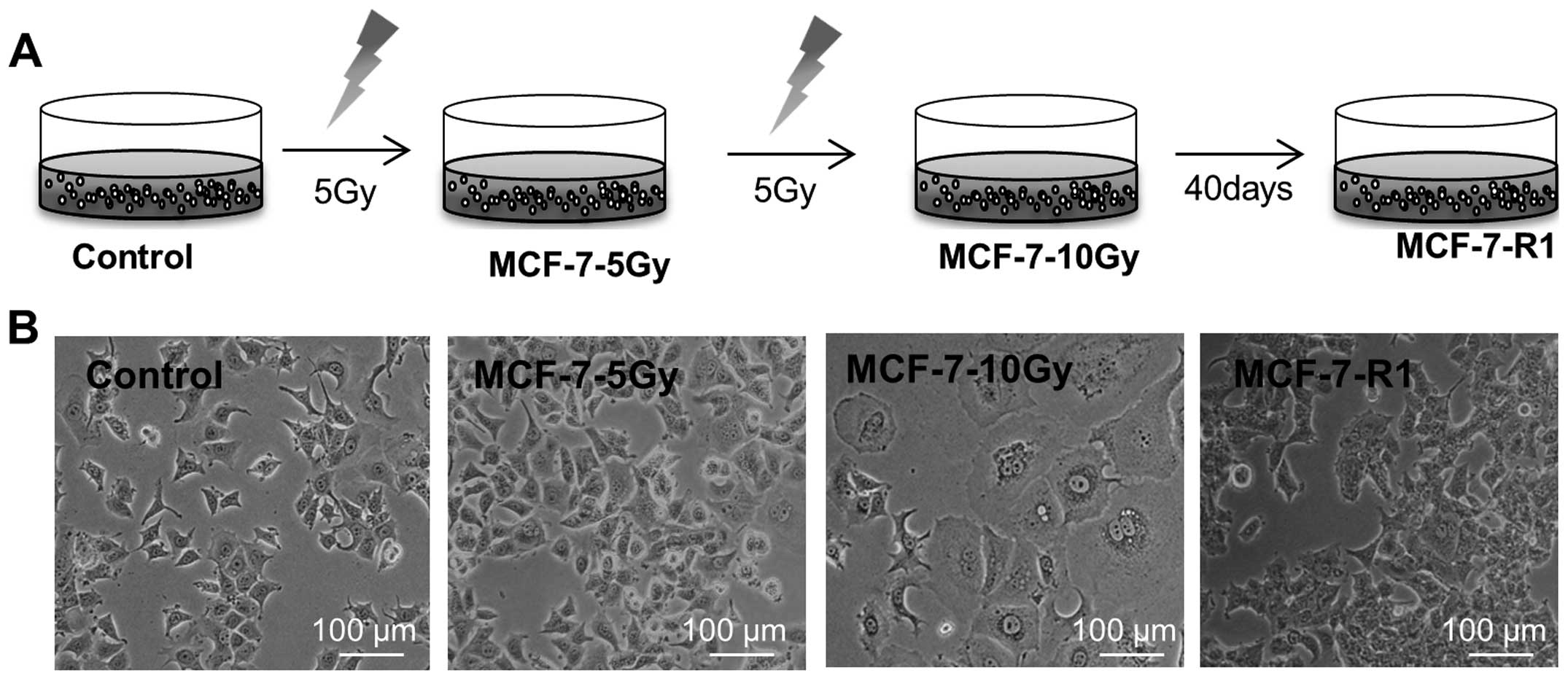
As schematically presented in Fig. 1A, MCF-7 cells were irradiated with
5 Gy (MCF-7-5 Gy), re-cultured for a week, and irradiated with a
second fraction of 5 Gy (MCF-7-10 Gy). Interestingly, initial
irradiation of cells with 5 Gy (MCF-7-5 Gy) exhibited mild effects
on cell viability and a change in cell morphology. However, a
second fraction of 5 Gy, resulting in a total dose of 10 Gy
(MCF-7-10 Gy), induced formation of giant cells with aberrant
nuclear morphology (Fig. 1B). The
formation of giant cells is normally followed by mitotic
catastrophe and cell death within a week. However, a few MCF-7-10
Gy cells survived and slowly recovered over 40–50 days (MCF-7-R1
cells). The overall morphology of MCF-7-R1 cells was similar to
that of controls (Fig. 1B). Since
mitotic catastrophe is the main form of cell death induced by
radiation, we considered MCF-7-5 Gy and MCF-7-10 Gy best
represented the clinical situation during radiotherapy, while
MCF-7-R1 better represented breast cancer soon after a course of
radiation therapy.
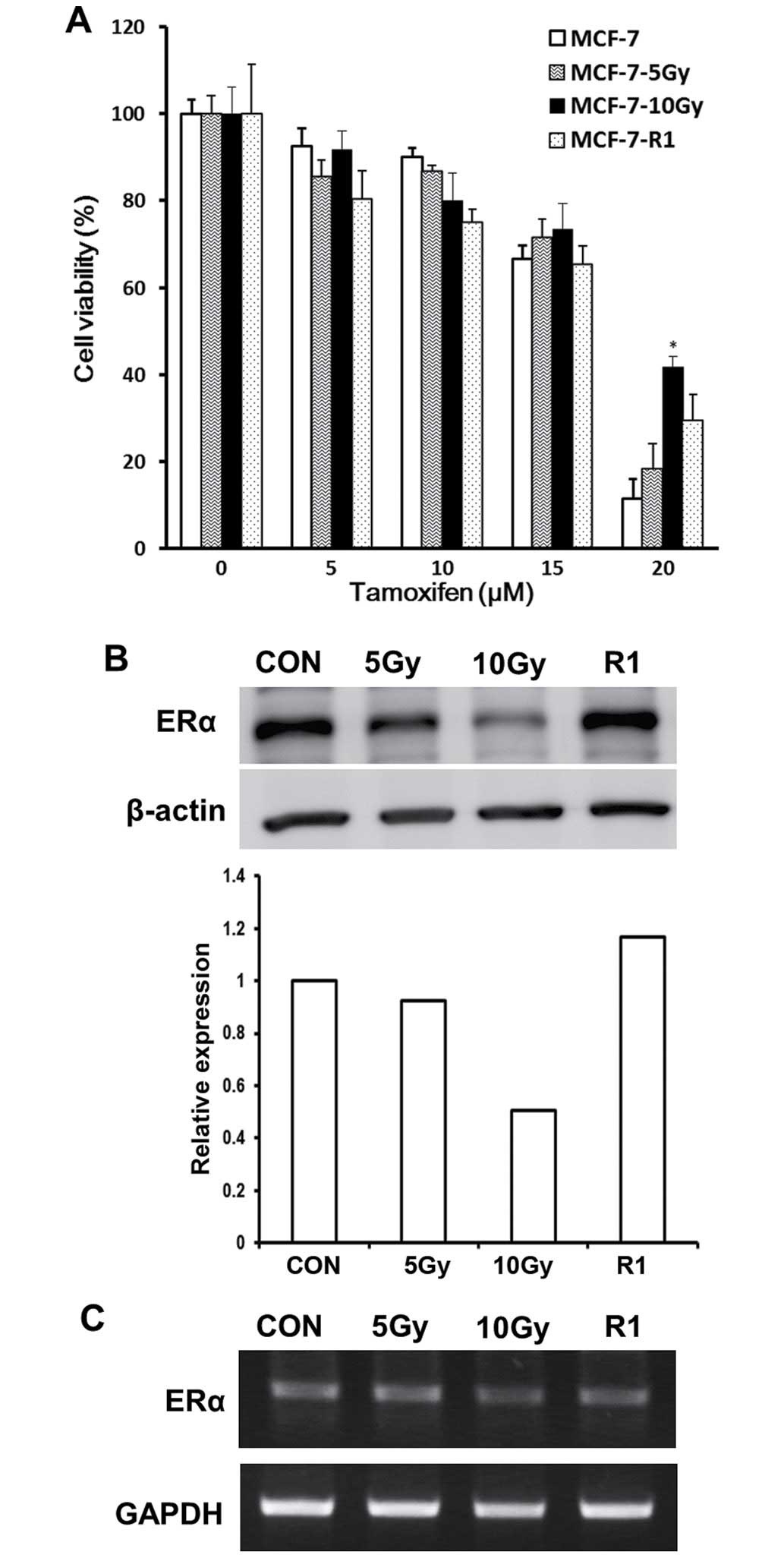
To evaluate the efficacy of concurrent and
sequential tamoxifen, we tested tamoxifen responses in MCF-7-5 Gy
and MCF-7-10 Gy (viewed as representative of concurrent treatment)
or in MCF-7-R1 (sequential treatment) by measuring cell viabilities
using the MTT assay. MTT assay was done with MCF-7 cells harvested
immediately after each 5 Gy (MCF-7-5 Gy) or 10 Gy (MCF-7-10 Gy)
irradiation or after subsequent culture of surviving MCF-7-10 Gy
cells for 40 days (MCF-7-R1). Although no significant difference in
survival response to tamoxifen was observed between MCF-7-5 Gy and
control cells, MCF-7-10 Gy cells exhibited an attenuated response
to tamoxifen; at a tamoxifen concentration of 20 μM they exhibited
~30% increase in cell viability versus control cells. However, the
efficacy of tamoxifen was restored in MCF-7-R1 cells (Fig. 2A). These observations suggest that
sequential tamoxifen treatment is more effective than concurrent
treatment.
The correlation between the efficacy of
tamoxifen and ERα expression
Since ERα is the major molecular target of
tamoxifen, we assessed the level of ERα expression in MCF-7-5 Gy,
MCF-7-10 Gy, and MCF-7-R1 cells to investigate whether ERα
expression is correlated with the tamoxifen response. Western blot
analysis revealed that ERα expression was gradually decreased in
MCF-7-5 Gy and remarkably lower in MCF-7-10 Gy cells (Fig. 2B), which also showed the most
attenuated response to tamoxifen (Fig.
2A). However, ERα expression and tamoxifen response (Fig. 2A) was recovered in MCF-7-R1 cells
(Fig. 2B). Furthermore, RT-PCR
analysis showed that the mRNA expression of ERα exhibited a similar
pattern observed in western blot analysis (Fig. 2C). Taken together, these results
show that response to tamoxifen was positively correlated with ERα
expression.
Establishment of radioresistant MCF-7
cells
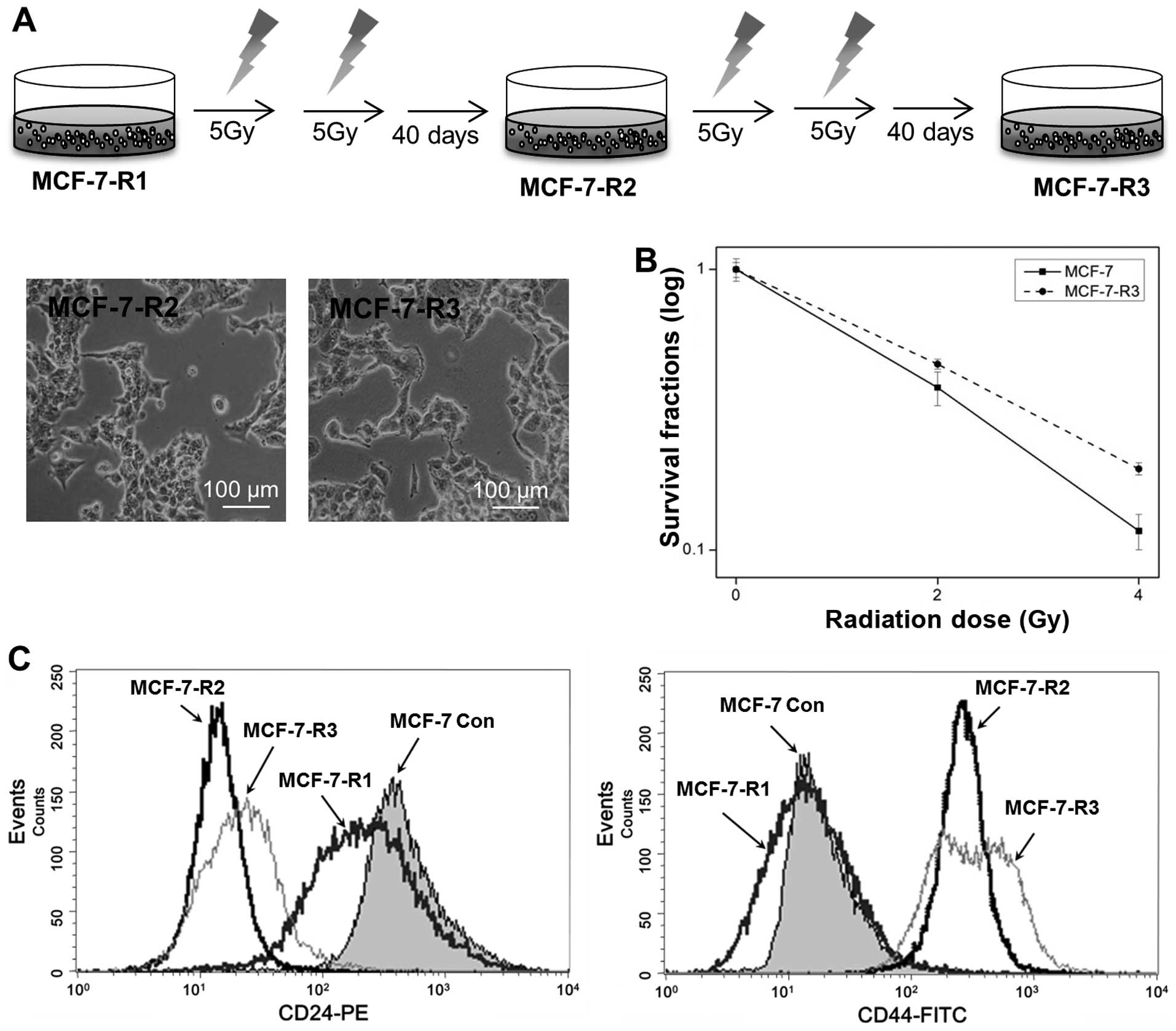
To establish radioresistant MCF-7 cells, MCF-7-R1
cells were further irradiated as described in Fig. 1A to cumulative doses of 20 and 30
Gy for MCF-7-R2 and MCF-7-R3, respectively (Fig. 3A). Interestingly, the more the
process was repeated, the lesser the recovery period was. To
confirm the radioresistance of MCF-7-R3, MCF-7 control and MCF-7-R3
cells were irradiated with 2–4 Gy and clonogenic survival assays
were performed. As shown in Fig.
3B, MCF-7-R3 exhibited radioresistance; survival fractions at 2
Gy for control and MCF-7-R3 cells were 0.37 and 0.46,
respectively.
Since cell morphology of MCF-7-R2 and R3 resembled
that of cells with an epithelial-mesenchymal transition phenotype,
exhibiting fibroblast and mesenchymal characteristics (Fig. 3A), we analyzed the expression of
cell-surface proteins, CD44 and CD24, in MCF-7-R2 and R3 cells by
flow cytometry. The majority of MCF-7 control cells were of the
CD24+/CD44− phenotype and MCF-7-R1 cells also
exhibited this phenotype. However, the expression of CD44 was
increased in MCF-7-R2 and R3 cells and CD24 was barely expressed
(Fig. 3C), which suggested that
cell characteristics were completely changed in radioresistant
cells.
Radioresistant MCF-7 cells were also
tamoxifen resistant with no change in ERα expression
Generally, tamoxifen treatment is recommended for
3–5 years in ERα-positive breast cancer patients after radiotherapy
(6,7), and thus, there is a risk of tumor
recurrence during the long tamoxifen treatment period. This
suggests that the efficacy of tamoxifen in radioresistant cells
needs to be assessed to maximize the clinical benefits of tamoxifen
in cases that recur after radiotherapy. To evaluate the efficacy of
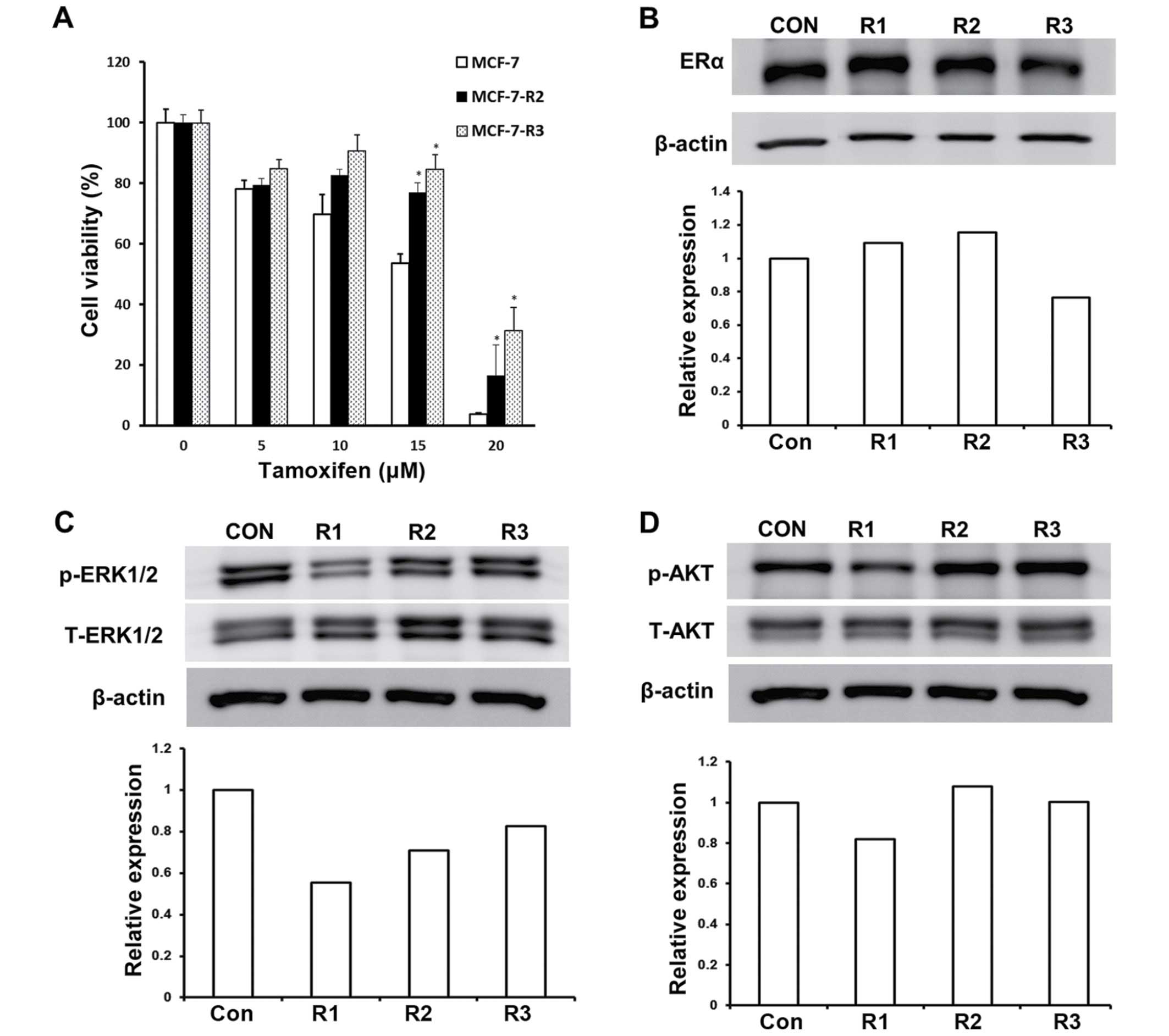
tamoxifen in radioresistant cells, MCF-7-R2 and MCF-7-R3 cells were
exposed to different concentrations of tamoxifen and cell
viabilities were determined. As shown in Fig. 4A, MCF-7-R2 and MCF-7-R3 cells were
less sensitive to tamoxifen; they exhibited >30% increased
viability than the control cells. Furthermore, the resistances of
MCF-7-R2 and -R3 cells to tamoxifen was greater at the higher
concentrations tested (Fig. 4A).
Since attenuated tamoxifen response in concurrently irradiated
MCF-7 cells was found to be positively correlated with ERα
expression (Fig. 2), we measured
the expression of ERα in MCF-7-R2 and -R3 cells. Interestingly, the
expression of ERα was not altered in these MCF-7-R2 and -R3 cells
(Fig. 4B), which implies that the
ERα signaling pathway was not involved in the tamoxifen resistance
exhibited by MCF-7-R2 and -R3 cells.
Enhanced AKT activation in radioresistant
MCF-7 cells
To understand the mechanism underlying cellular
resistance to tamoxifen, we investigated signaling pathways in
MCF-7-R2 and -R3 cells. We first evaluated molecules associated
with the non-genomic ERα signaling pathway including EGFR, HER2,
mitogen-activated protein kinase/extracellular-signal-regulated
kinases (MAPK/ERK), and AKT (20–23).
No changes in phosphorylated HER2 and phosphorylated EGFR levels
were observed in MCF-7-R2 and -R3 cells (data not shown).
Phosphorylated ERK1/2 levels were depressed in MCF-7-R1 cells but
recovered in MCF-7-R2 and MCF-7-R3 cells (Fig. 4C), suggesting that phosphorylated
ERK1/2 is associated with response to tamoxifen in concurrently
irradiated MCF-7-R1 cells, but not in MCF-7-R2 and MCF-7-R3 cells.
On the other hand, phosphorylated AKT levels were enhanced in
MCF-7-R2 and MCF-7-R3 cells versus control cells (Fig. 4D), implying that constitutive AKT
activation in radioresistant cells promotes resistance to
tamoxifen.
Discussion
In this study, we tried to identify the optimal time
to start tamoxifen treatment in ERα-positive breast cancer patients
to maximize clinical benefits in combination with radiotherapy.
Some previous studies have evaluated the relative effectiveness of
sequential and concurrent tamoxifen treatment in this context, but
the results obtained were inconsistent (11–13,16).
Furthermore, the majority of preclinical studies did not consider
the timing of tamoxifen administration relative to radiotherapy,
and only analyzed the radiosensitivity of breast cancer after
tamoxifen treatment. However, we considered the timing and
sequencing of tamoxifen should be applied to reflect the clinical
situation. Thus, in this study, we classified irradiated MCF-7
cells according to the course of clinical treatment by treating
them with tamoxifen during, immediately and several months after
radiotherapy.
In clinical practice, breast radiation is most
commonly given 5 days a week for ~5 or 6 weeks. During the course
of radiotherapy, DNA is damaged in tumor cells and this triggers
mitotic catastrophe, which is considered to be the major mechanism
of cell death induced by radiation in solid tumors (24,25).
It is known that mitotic death is caused by aberrant mitosis and
subsequent giant cell formation (26,27).
In this study, we observed the formation of giant cells when MCF-7
cells were irradiated with a second dose of 5 Gy (MCF-7-10 Gy).
However, after the formation of giant cells followed by mitotic
catastrophe, a few cells survived and returned to the normal cell
cycle (MCF-7-R1). Thus, we considered that MCF-7-5 Gy and MCF-7-10
Gy cells represented the clinical situation during radiotherapy,
and MCF-7-R1 cells, which survived mitotic catastrophe, represented
breast cancer cells soon after radiotherapy. When we evaluated the
efficacy of tamoxifen at different times after irradiation,
attenuated tamoxifen response was observed in MCF-7-10 Gy cells,
but not in MCF-7-R1 cells, which suggests that tamoxifen is
ineffective when aberrant mitosis had occurred by radiation.
Furthermore, we found the expression of ERα was diminished when
giant cells were formed, presuming that radiation-induced aberrant
mitosis reduces ERα expression, resulting in the decreased response
to tamoxifen. Taken together, our data suggested that sequential
tamoxifen treatment following radiotherapy would be optimal instead
of the concurrent treatment.
As mentioned above, patients generally receive
radio-therapy for 5–6 weeks, whereas tamoxifen is recommended for
3–5 years in ERα-positive breast cancer patients after
radio-therapy (6,7). To investigate the efficacy of
tamoxifen on recurred tumors due to surviving radioresistant cells,
we established the radioresistant MCF-7 cell lines, MCF-7-R2 and
MCF-7-R3. Interestingly, MCF-7-R3 cells exhibited resistance to
tamoxifen without exhibiting aberrant mitosis or reduction in ERα
expression. Although the expression of ERα was not altered in
radioresistant MCF-7-R2 and MCF-7-R3, they were distinguishable
from parental MCF-7 cells or MCF-7-R1 by the upregulation of CD44
and downregulation of CD24. CD44 and CD24 are cell surface
glycoproteins that participate in cell-matrix and cell-cell
interactions (28,29). Furthermore, a subset of
CD24−/CD44+ cells were found to be cancer
stem cells in human breast cancer (30). We observed that normal control and
MCF-7-R1 cells were of the CD24+/CD44−
subtype, whereas radioresistant MCF-7-R2 and MCF-7-R3 cells
exhibited the CD24−/CD44+ subtype even after
3 months, implying that the changes of cell characteristics may
contribute to the resistance to both radiation and tamoxifen
treatment.
Several studies have shown that PI3K/AKT, HER2, and
MAPK/ERK signaling are associated with radioresistance (31–36).
Ahmed et al reported that total ERK1/2 is slightly increased
and phosphorylated ERK1/2 is decreased in radio-resistant MCF-7
cells (31). Chang et al
found that the PI3K/AKT/mTOR signaling pathway is activated in
radioresistant prostate cancer cells (36). In this study, we evaluated the
expression of AKT, ERK1/2, HER2, and EGFR in radioresistant cells,
and found that phosphorylated AKT was increased in MCF-7-R2 and
MCF-7-R3 cells, but phosphorylated HER2 and EGFR were not (data not
shown). Since activation of the PI3K-AKT signaling pathway is
associated with the radioresistance of many cancers by increasing
the rate of DNA repair (37), and
AKT is related to the non-genomic ERα pathway (38), our data suggest that constitutive
AKT activation may contribute to the resistance shown by MCF-7-R2
and -R3 cells to radiation and tamoxifen.
The main goal of this study was to propose an
optimal schedule for tamoxifen and radiotherapy. Obviously,
extensive clinical studies on a large number of patients are the
best way to address this issue, but this clinical approach is
demanding in terms of time and money. Although this study was
conducted in vitro using breast cancer cells, our
experimental scheme considered the timing and sequencing of
tamoxifen administration so as to reflect the clinical situation.
Based on the tamoxifen response and the status of ERα expression
shown by irradiated and non-irradiated MCF-7 cells, our findings
propose that tamoxifen treatment after radiotherapy is a better
treatment option than concurrent treatment. However, the observed
reduced efficacy of tamoxifen on radioresistant cells, which showed
normal ERα expression, suggests that an additional targeted
therapy, such as, AKT inhibitor therapy, is required to improve
radioresistant breast cancer response to tamoxifen.
Acknowledgements
This study was supported by a grant from the Basic
Science Research Program, the National Research Foundation of Korea
(NRF), funded by the Ministry of Education, Science and Technology,
Republic of Korea (grant no. NRF-2015R1D1A1A01059738).
Abbreviations:
|
HER2
|
human epidermal growth factor receptor
2
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
EGFR
|
epidermal growth factor receptor
|
|
PI3K
|
phosphoinositol-3 kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
AKT
|
protein kinase B
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
References
|
1
|
National Comprehensive Cancer Network.
Clinical Practice Guidelines in Oncology. 2014, http://www.nccn.org.
|
|
2
|
Komoike Y, Akiyama F, Iino Y, Ikeda T,
Akashi-Tanaka S, Ohsumi S, Kusama M, Sano M, Shin E, Suemasu K, et
al: Ipsilateral breast tumor recurrence (IBTR) after
breast-conserving treatment for early breast cancer: Risk factors
and impact on distant metastases. Cancer. 106:35–41. 2006.
View Article : Google Scholar
|
|
3
|
Arriagada R, Lê MG, Contesso G,
Guinebretière JM, Rochard F and Spielmann M: Predictive factors for
local recurrence in 2006 patients with surgically resected small
breast cancer. Ann Oncol. 13:1404–1413. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ha B, Suh HS, Lee J, Lee KJ, Lee R and
Moon BI: Long-term results of forward intensity-modulated radiation
therapy for patients with early-stage breast cancer. Radiat Oncol
J. 31:191–198. 2013. View Article : Google Scholar
|
|
5
|
Masood S: Estrogen and progesterone
receptors in cytology: A comprehensive review. Diagn Cytopathol.
8:475–491. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fisher B, Dignam J, Bryant J and Wolmark
N: Five versus more than five years of tamoxifen for lymph
node-negative breast cancer: Updated findings from the National
Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J
Natl Cancer Inst. 93:684–690. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Azria D, Pelegrin A, Dubois JB, Mirimanoff
RO and Ozsahin M: Radiation therapy and tamoxifen: concurrent or
sequential? It's no longer the question! J Clin Oncol.
23:4239–4241; author reply 4241–4232. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harris EE, Christensen VJ, Hwang WT, Fox K
and Solin LJ: Impact of concurrent versus sequential tamoxifen with
radiation therapy in early-stage breast cancer patients undergoing
breast conservation treatment. J Clin Oncol. 23:11–16. 2005.
View Article : Google Scholar
|
|
10
|
Whelan T and Levine M: Radiation therapy
and tamoxifen: Concurrent or sequential? That is the question. J
Clin Oncol. 23:1–4. 2005. View Article : Google Scholar
|
|
11
|
Osborne CK, Boldt DH, Clark GM and Trent
JM: Effects of tamoxifen on human breast cancer cell cycle
kinetics: Accumulation of cells in early G1 phase. Cancer Res.
43:3583–3585. 1983.PubMed/NCBI
|
|
12
|
Paulsen GH, Strickert T, Marthinsen AB and
Lundgren S: Changes in radiation sensitivity and steroid receptor
content induced by hormonal agents and ionizing radiation in breast
cancer cells in vitro. Acta Oncol. 35:1011–1019. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wazer DE, Tercilla OF, Lin PS and
Schmidt-Ullrich R: Modulation in the radiosensitivity of MCF-7
human breast carcinoma cells by 17B-estradiol and tamoxifen. Br J
Radiol. 62:1079–1083. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bentzen SM, Skoczylas JZ, Overgaard M and
Overgaard J: Radiotherapy-related lung fibrosis enhanced by
tamoxifen. J Natl Cancer Inst. 88:918–922. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koc M, Polat P and Suma S: Effects of
tamoxifen on pulmonary fibrosis after cobalt-60 radiotherapy in
breast cancer patients. Radiother Oncol. 64:171–175. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ellis PA, Saccani-Jotti G, Clarke R,
Johnston SR, Anderson E, Howell A, A'Hern R, Salter J, Detre S,
Nicholson R, et al: Induction of apoptosis by tamoxifen and ICI
182780 in primary breast cancer. Int J Cancer. 72:608–613. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahn PH, Vu HT, Lannin D, Obedian E,
DiGiovanna MP, Burtness B and Haffty BG: Sequence of radiotherapy
with tamoxifen in conservatively managed breast cancer does not
affect local relapse rates. J Clin Oncol. 23:17–23. 2005.
View Article : Google Scholar
|
|
18
|
Pierce LJ, Hutchins LF, Green SR, Lew DL,
Gralow JR, Livingston RB, Osborne CK and Albain KS: Sequencing of
tamoxifen and radiotherapy after breast-conserving surgery in
early-stage breast cancer. J Clin Oncol. 23:24–29. 2005. View Article : Google Scholar
|
|
19
|
Chun SY, Kwon YS, Nam KS and Kim S:
Lapatinib enhances the cytotoxic effects of doxorubicin in MCF-7
tumorspheres by inhibiting the drug efflux function of ABC
transporters. Biomed Pharmacother. 72:37–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shou J, Massarweh S, Osborne CK, Wakeling
AE, Ali S, Weiss H and Schiff R: Mechanisms of tamoxifen
resistance: Increased estrogen receptor-HER2/neu cross-talk in
ER/HER2-positive breast cancer. J Natl Cancer Inst. 96:926–935.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haynes MP, Sinha D, Russell KS, Collinge
M, Fulton D, Morales-Ruiz M, Sessa WC and Bender JR: Membrane
estrogen receptor engagement activates endothelial nitric oxide
synthase via the PI3-kinase-Akt pathway in human endothelial cells.
Circ Res. 87:677–682. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Santen RJ, Song RX, McPherson R, Kumar R,
Adam L, Jeng MH and Yue W: The role of mitogen-activated protein
(MAP) kinase in breast cancer. J Steroid Biochem Mol Biol.
80:239–256. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kato S, Endoh H, Masuhiro Y, Kitamoto T,
Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H,
et al: Activation of the estrogen receptor through phosphorylation
by mitogen-activated protein kinase. Science. 270:1491–1494. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rupnow BA and Knox SJ: The role of
radiation-induced apoptosis as a determinant of tumor responses to
radiation therapy. Apoptosis. 4:115–143. 1999. View Article : Google Scholar
|
|
25
|
Verheij M: Clinical biomarkers and imaging
for radiotherapy-induced cell death. Cancer Metastasis Rev.
27:471–480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Galluzzi L, Maiuri MC, Vitale I, Zischka
H, Castedo M, Zitvogel L and Kroemer G: Cell death modalities:
Classification and pathophysiological implications. Cell Death
Differ. 14:1237–1243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eriksson D, Löfroth PO, Johansson L,
Riklund KA and Stigbrand T: Cell cycle disturbances and mitotic
catastrophes in HeLa Hep2 cells following 2.5 to 10 Gy of ionizing
radiation. Clin Cancer Res. 13:S5501–S5508. 2007. View Article : Google Scholar
|
|
28
|
Kristiansen G, Winzer KJ, Mayordomo E,
Bellach J, Schlüns K, Denkert C, Dahl E, Pilarsky C, Altevogt P,
Guski H, et al: CD24 expression is a new prognostic marker in
breast cancer. Clin Cancer Res. 9:4906–4913. 2003.PubMed/NCBI
|
|
29
|
Lee HJ, Choe G, Jheon S, Sung SW, Lee CT
and Chung JH: CD24, a novel cancer biomarker, predicting
disease-free survival of non-small cell lung carcinomas: A
retrospective study of prognostic factor analysis from the
viewpoint of forthcoming (seventh) new TNM classification. J Thorac
Oncol. 5:649–657. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ahmed KM, Dong S, Fan M and Li JJ: Nuclear
factor-kappaB p65 inhibits mitogen-activated protein kinase
signaling pathway in radioresistant breast cancer cells. Mol Cancer
Res. 4:945–955. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kunigal S, Lakka SS, Joseph P, Estes N and
Rao JS: Matrix metalloproteinase-9 inhibition down-regulates
radiation-induced nuclear factor-kappa B activity leading to
apoptosis in breast tumors. Clin Cancer Res. 14:3617–3626. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duru N, Fan M, Candas D, Menaa C, Liu HC,
Nantajit D, Wen Y, Xiao K, Eldridge A, Chromy BA, et al:
HER2-associated radioresistance of breast cancer stem cells
isolated from HER2-negative breast cancer cells. Clin Cancer Res.
18:6634–6647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
No M, Choi EJ and Kim IA: Targeting HER2
signaling pathway for radiosensitization: Alternative strategy for
therapeutic resistance. Cancer Biol Ther. 8:2351–2361. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin F, Luo J, Gao W, Wu J, Shao Z, Wang Z,
Meng J, Ou Z and Yang G: COX-2 promotes breast cancer cell
radioresistance via p38/MAPK-mediated cellular anti-apoptosis and
invasiveness. Tumour Biol. 34:2817–2826. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang L, Graham PH, Hao J, Ni J, Bucci J,
Cozzi PJ, Kearsley JH and Li Y: Acquisition of
epithelial-mesenchymal transition and cancer stem cell phenotypes
is associated with activation of the PI3K/Akt/mTOR pathway in
prostate cancer radioresistance. Cell Death Dis. 4:e8752013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Clark AS, West K, Streicher S and Dennis
PA: Constitutive and inducible Akt activity promotes resistance to
chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol
Cancer Ther. 1:707–717. 2002.PubMed/NCBI
|
|
38
|
Guo RX, Wei LH, Tu Z, Sun PM, Wang JL,
Zhao D, Li XP and Tang JM: 17 beta-estradiol activates PI3K/Akt
signaling pathway by estrogen receptor (ER)-dependent and
ER-independent mechanisms in endometrial cancer cells. J Steroid
Biochem Mol Biol. 99:9–18. 2006. View Article : Google Scholar : PubMed/NCBI
|