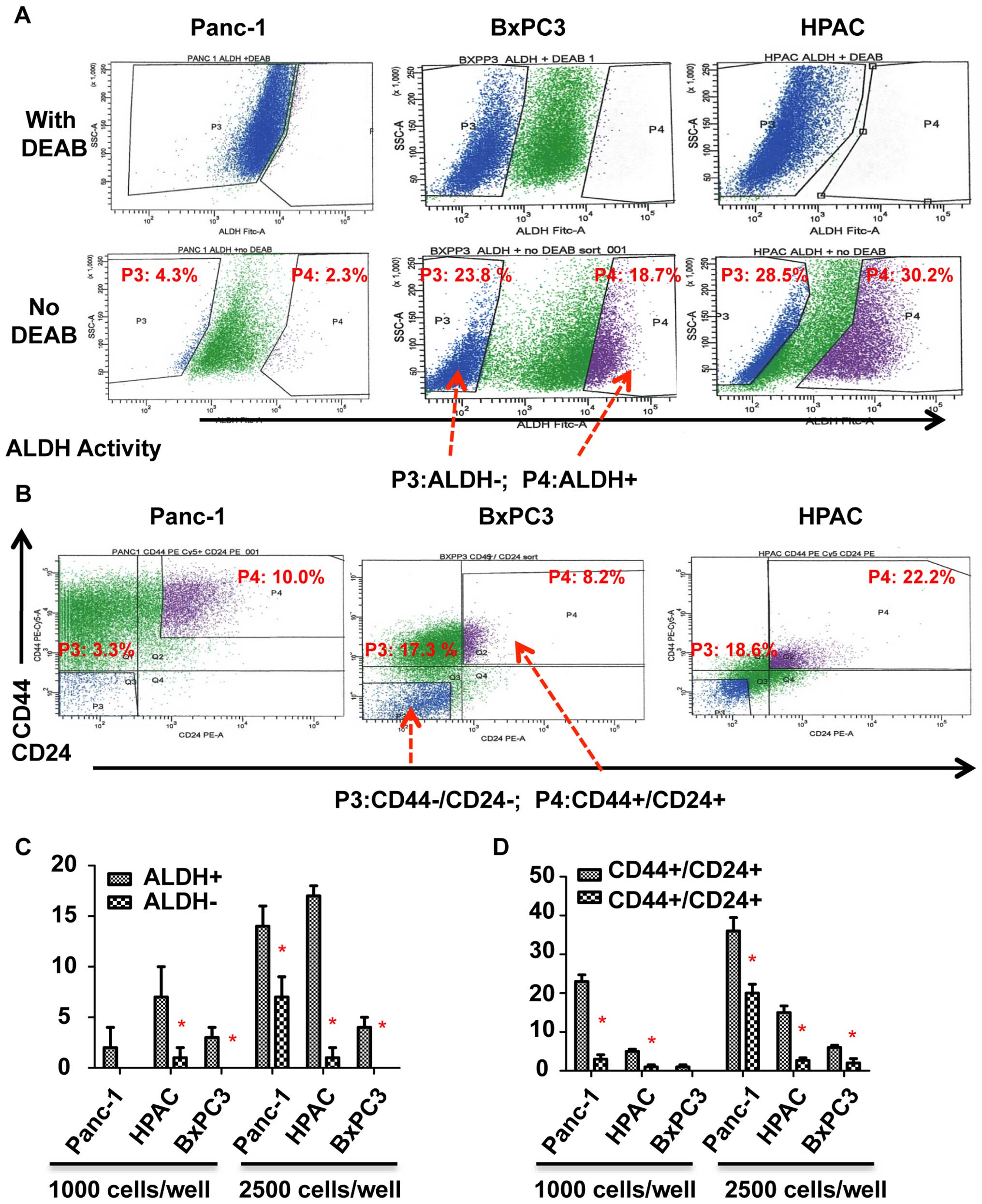
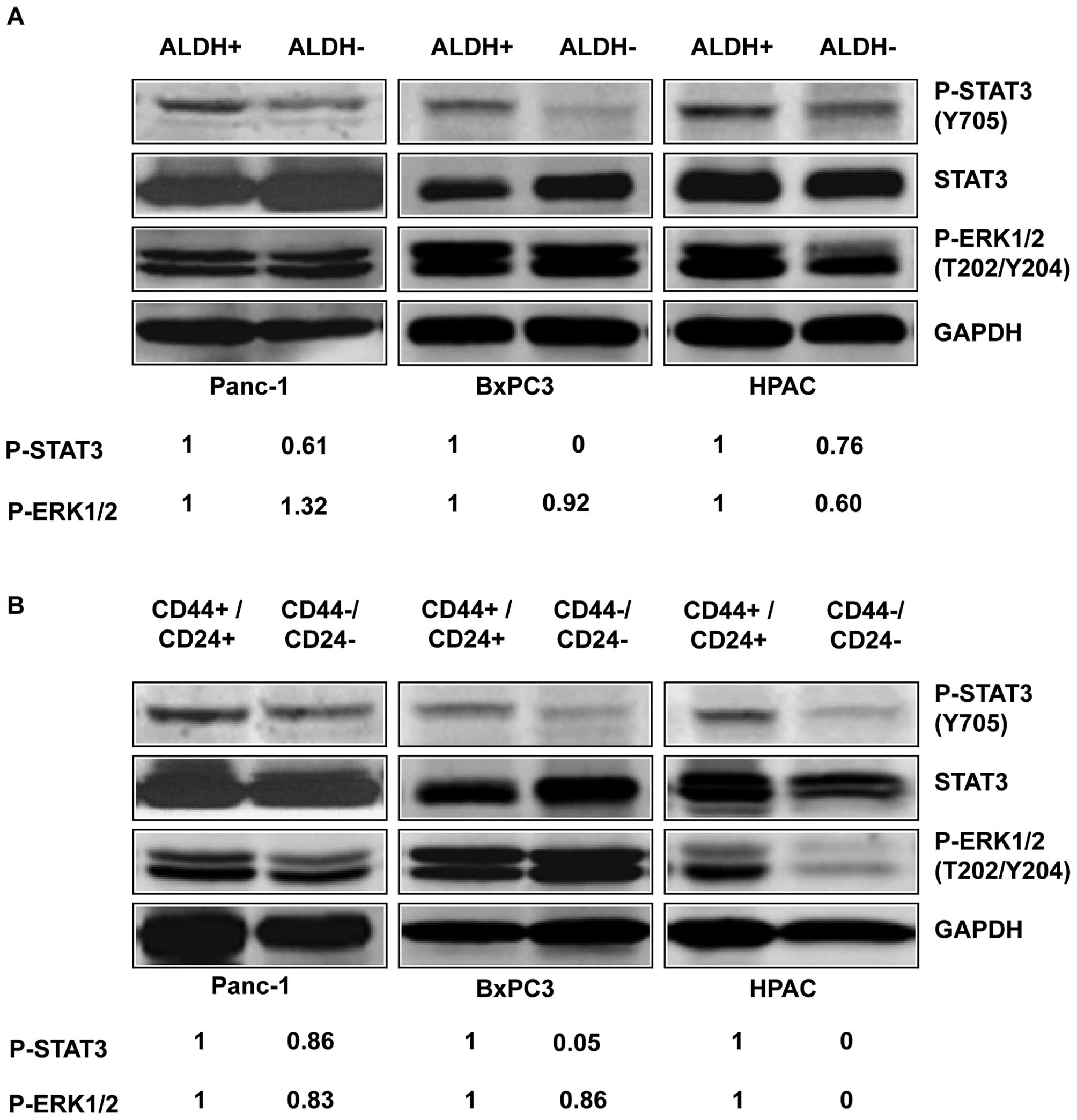
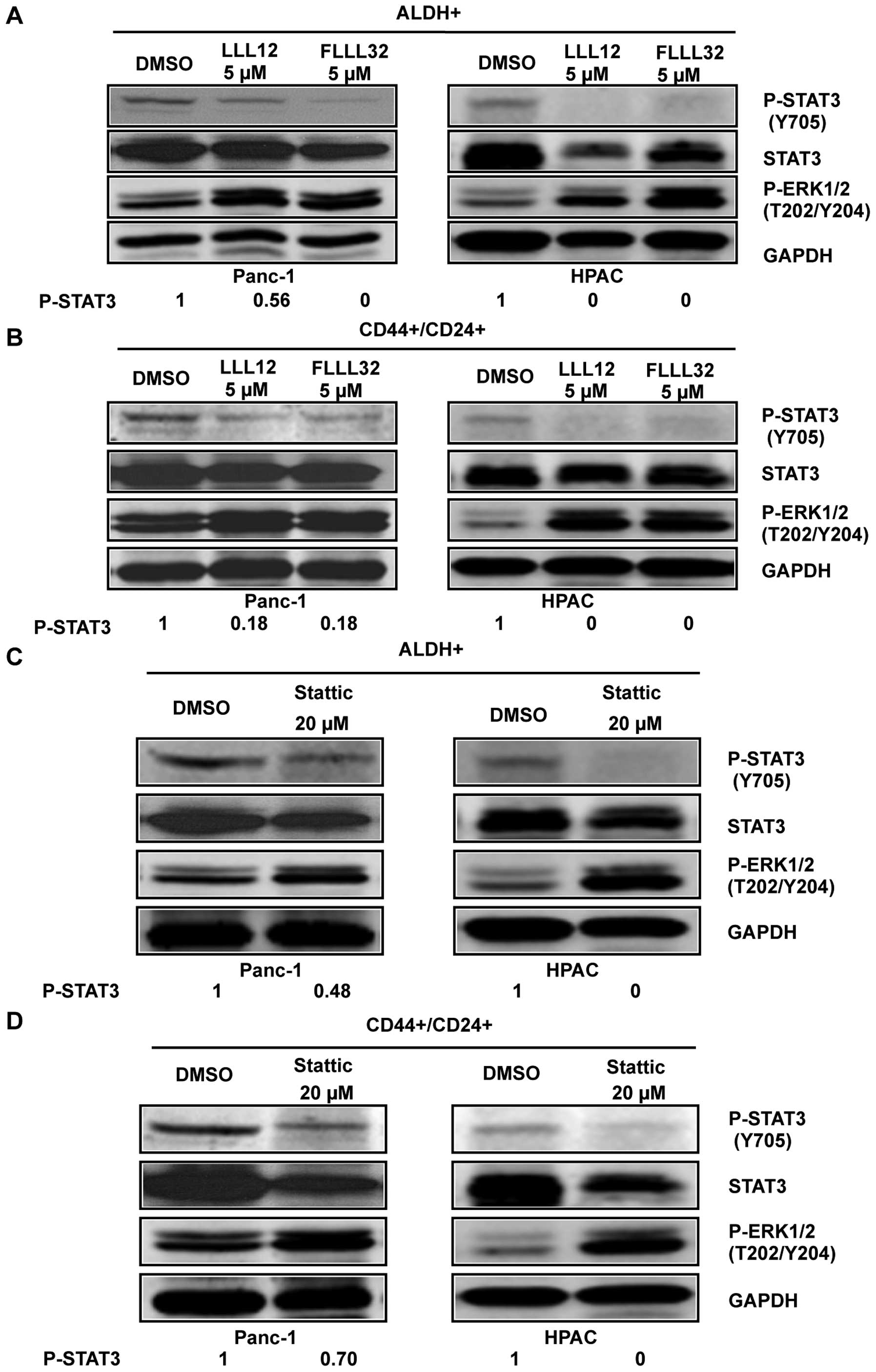
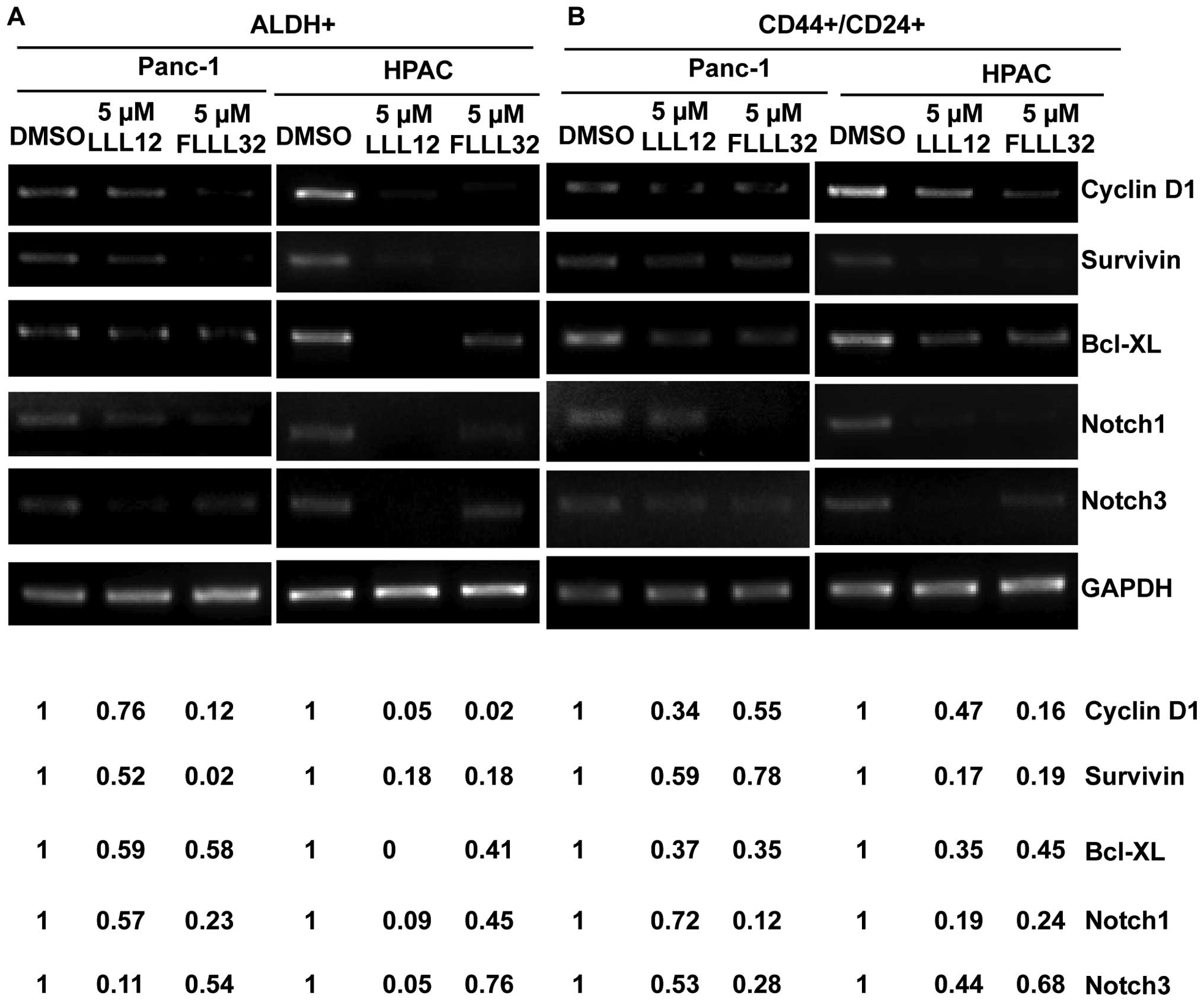
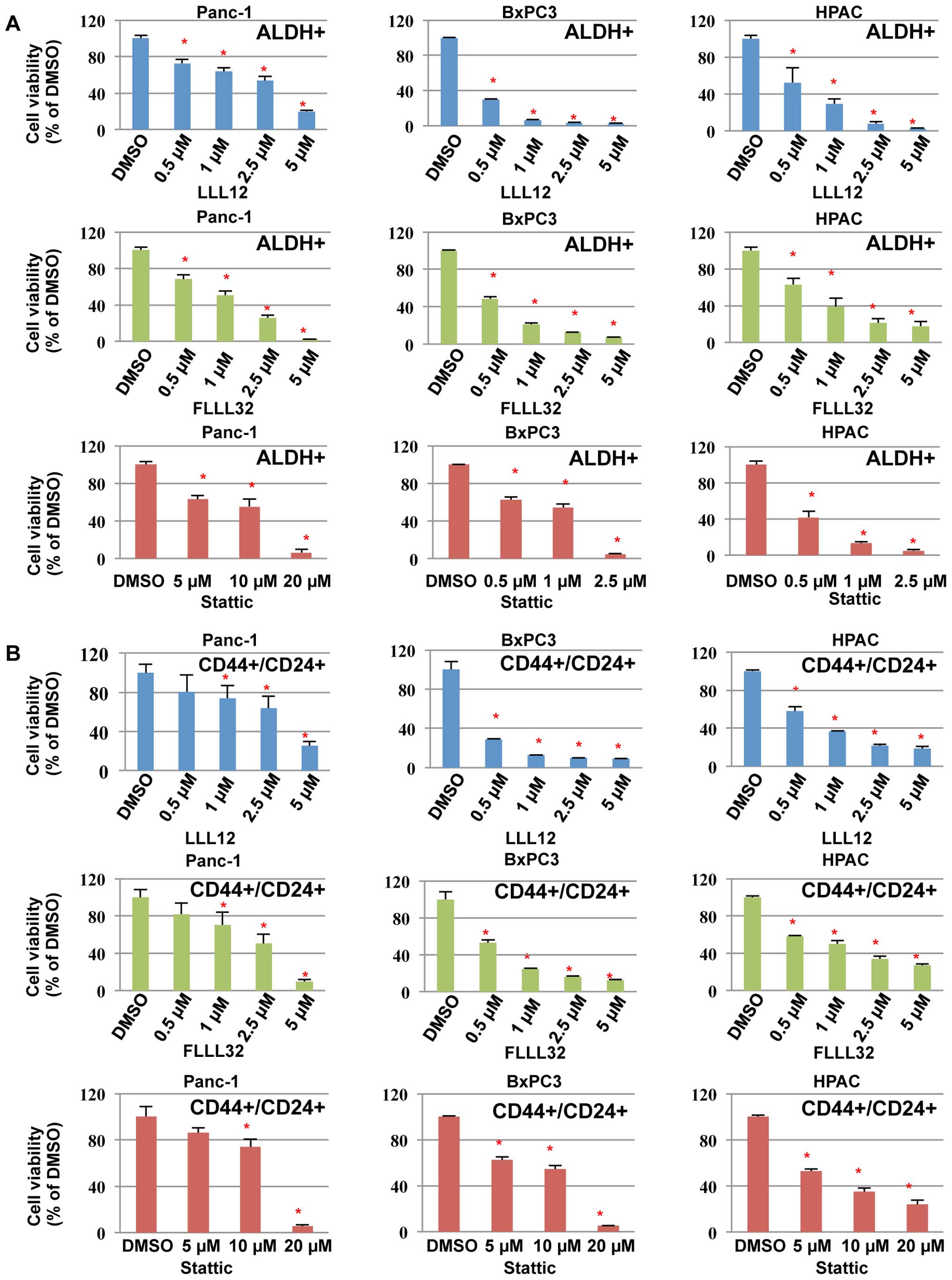
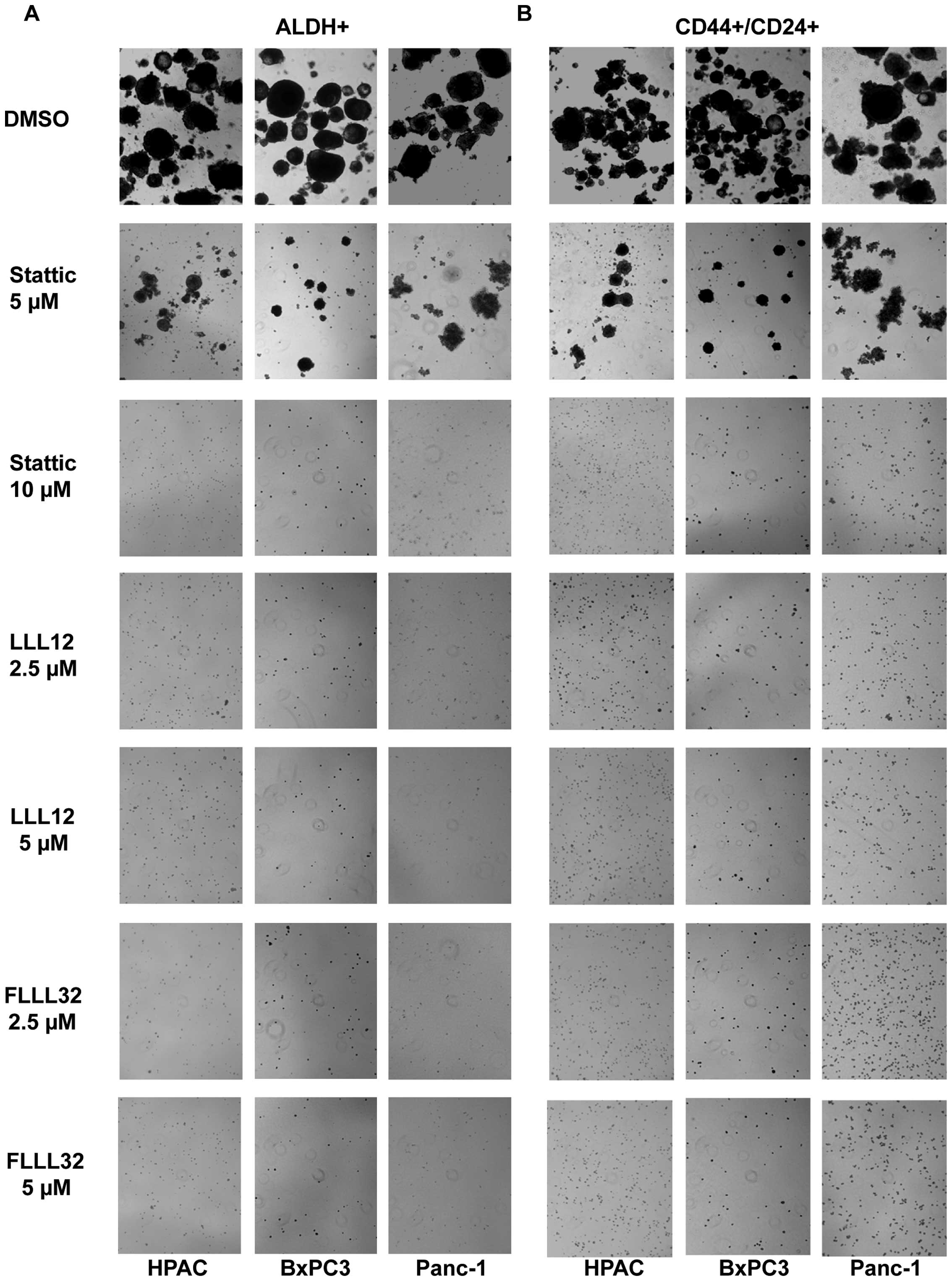
|
1
|
Society AC. Cancer Factor & Figures.
American Cancer Society; 2015, http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf.
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li C, Heidt DG, Dalerba P, Burant CF,
Zhang L, Adsay V, Wicha M, Clarke MF and Simeone DM: Identification
of pancreatic cancer stem cells. Cancer Res. 67:1030–1037. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei HJ, Yin T, Zhu Z, Shi PF, Tian Y and
Wang CY: Expression of CD44, CD24 and ESA in pancreatic
adenocarcinoma cell lines varies with local microenvironment.
Hepatobiliary Pancreat Dis Int. 10:428–434. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calò V, Migliavacca M, Bazan V, Macaluso
M, Buscemi M, Gebbia N and Russo A: STAT proteins: From normal
control of cellular events to tumorigenesis. J Cell Physiol.
197:157–168. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Germain D and Frank DA: Targeting the
cytoplasmic and nuclear functions of signal transducers and
activators of transcription 3 for cancer therapy. Clin Cancer Res.
13:5665–5669. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Turkson J and Jove R: STAT proteins: Novel
molecular targets for cancer drug discovery. Oncogene.
19:6613–6626. 2000. View Article : Google Scholar
|
|
8
|
Zhong Z, Wen Z and Darnell JE Jr: Stat3: A
STAT family member activated by tyrosine phosphorylation in
response to epidermal growth factor and interleukin-6. Science.
264:95–98. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sasse J, Hemmann U, Schwartz C,
Schniertshauer U, Heesel B, Landgraf C, Schneider-Mergener J,
Heinrich PC and Horn F: Mutational analysis of acute-phase response
factor/Stat3 activation and dimerization. Mol Cell Biol.
17:4677–4686. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Corvinus FM, Orth C, Moriggl R, Tsareva
SA, Wagner S, Pfitzner EB, Baus D, Kaufmann R, Huber LA, Zatloukal
K, et al: Persistent STAT3 activation in colon cancer is associated
with enhanced cell proliferation and tumor growth. Neoplasia.
7:545–555. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kusaba T, Nakayama T, Yamazumi K, Yakata
Y, Yoshizaki A, Nagayasu T and Sekine I: Expression of p-STAT3 in
human colorectal adenocarcinoma and adenoma; correlation with
clinicopathological factors. J Clin Pathol. 58:833–838. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma XT, Wang S, Ye YJ, Du RY, Cui ZR and
Somsouk M: Constitutive activation of Stat3 signaling pathway in
human colorectal carcinoma. World J Gastroenterol. 10:1569–1573.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao S, Venkatasubbarao K, Lazor JW,
Sperry J, Jin C, Cao L and Freeman JW: Inhibition of STAT3 Tyr705
phosphorylation by Smad4 suppresses transforming growth factor
beta-mediated invasion and metastasis in pancreatic cancer cells.
Cancer Res. 68:4221–4228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiu Z, Huang C, Sun J, Qiu W, Zhang J, Li
H, Jiang T, Huang K and Cao J: RNA interference-mediated signal
transducers and activators of transcription 3 gene silencing
inhibits invasion and metastasis of human pancreatic cancer cells.
Cancer Sci. 98:1099–1106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sahu RP and Srivastava SK: The role of
STAT-3 in the induction of apoptosis in pancreatic cancer cells by
benzyl isothiocyanate. J Natl Cancer Inst. 101:176–193. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin L, Hutzen B, Li PK, Ball S, Zuo M,
DeAngelis S, Foust E, Sobo M, Friedman L, Bhasin D, et al: A novel
small molecule, LLL12, inhibits STAT3 phosphorylation and
activities and exhibits potent growth-suppressive activity in human
cancer cells. Neoplasia. 12:39–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin L, Hutzen B, Zuo M, Ball S, Deangelis
S, Foust E, Pandit B, Ihnat MA, Shenoy SS, Kulp S, et al: Novel
STAT3 phosphorylation inhibitors exhibit potent growth-suppressive
activity in pancreatic and breast cancer cells. Cancer Res.
70:2445–2454. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schust J, Sperl B, Hollis A, Mayer TU and
Berg T: Stattic: A small-molecule inhibitor of STAT3 activation and
dimerization. Chem Biol. 13:1235–1242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar
|
|
20
|
Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li C
and Lin J: STAT3 is necessary for proliferation and survival in
colon cancer-initiating cells. Cancer Res. 71:7226–7237. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanei T, Morimoto K, Shimazu K, Kim SJ,
Tanji Y, Taguchi T, Tamaki Y and Noguchi S: Association of breast
cancer stem cells identified by aldehyde dehydrogenase 1 expression
with resistance to sequential paclitaxel and epirubicin-based
chemotherapy for breast cancers. Clin Cancer Res. 15:4234–4241.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaptein A, Paillard V and Saunders M:
Dominant negative stat3 mutant inhibits interleukin-6-induced
Jak-STAT signal transduction. J Biol Chem. 271:5961–5964. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grivennikov S and Karin M: Autocrine IL-6
signaling: A key event in tumorigenesis? Cancer Cell. 13:7–9. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dontu G, Jackson KW, McNicholas E,
Kawamura MJ, Abdallah WM and Wicha MS: Role of Notch signaling in
cell-fate determination of human mammary stem/progenitor cells.
Breast Cancer Res. 6:R605–R615. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tian X, Li J, Ma ZM, Zhao C, Wan DF and
Wen YM: Role of hepatitis B surface antigen in the development of
hepatocellular carcinoma: Regulation of lymphoid enhancer-binding
factor 1. J Exp Clin Cancer Res. 28:582009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Paydas S, Tanriverdi K, Yavuz S, Disel U,
Sahin B and Burgut R: Survivin and aven: Two distinct antiapoptotic
signals in acute leukemias. Ann Oncol. 14:1045–1050. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamaguchi H, Inokuchi K, Tarusawa M and
Dan K: Mutation of bcl-x gene in non-Hodgkin's lymphoma. Am J
Hematol. 69:74–76. 2002. View Article : Google Scholar : PubMed/NCBI
|