Introduction
Rhabdomyosarcoma (RMS) is the most common soft
tissue sarcoma in childhood and young adulthood and accounts for
4–6% of all malignancies in this age group (1,2). RMS
protein expression profile is characteristic for striated muscle
tissue and includes desmin, myosin, sarcomeric actin and myoglobin.
Rhabdomyosarcoma cells commonly exhibit a characteristic sarcomeric
banding pattern, similar to skeletal muscle, due to the arrangement
of cytoskeletal elements in the cytoplasm. However, there are
numerous examples of RMS occurrence in organs with no striated
muscular elements such as gallbladder, prostate or urinary bladder.
This fact leads to conclusion, that various lines of RMS may
originate from primitive mesenchyme, which demonstrates tendency to
myogenesis (3). Clinical features
and molecular biology allow identifying two main subtypes of RMS
(embryonal and alveolar) and less frequent anaplastic
rhabdomyosarcoma. Embryonal rhabdomyosarcoma (ERMS) usually affects
infants and children before 5 years of age and is the most common
type of RMS. This subtype tends to occur in head and neck area,
however, it can be found also in or around urinal bladder, vagina
or prostate (4). Alveolar
rhabdomyosarcoma (ARMS) affects all age group equally, however, due
to the fact that ERMS is less common at older ages, there are
proportionally more cases of this type in older children and teens.
ARMS generally occurs in large muscles of the trunk, arms and legs.
ARMS in comparison to ERMS grows faster and requires more intense
treatment as it has worse prognosis (5).
Treatment for rhabdomyosarcoma consists of surgery,
chemotherapy and radiotherapy. It has to be noted, that surgery
might be difficult or even impossible due to localization of the
tumor. Chemotherapy is based on actinomycin D, cyclophosphamide,
doxorubicin, etoposide, ifosfamide or vincristine administration.
In addition to chemotherapy, radiation therapy can be implemented
to elevate treatment success rate (6). However, treatment of RMS is realized
as conventional therapy, scientists have put efforts into finding
new therapies such as immunotherapy using monoclonal antibodies
(7). Despite the fact that RMS may
be treated effectively, long-term survival chances decrease
dramatically when cancer recurrence occurs. That is the reason for
developing novel and more effective therapies against
rhabdomyosarcoma. Gene therapy based on suicide gene selectively
eradicating cancer cells could be the solution. The introduction of
herpes simplex virus thymidine kinase (HSV-TK) allows to
selectively eliminate cancer cells using antiviral medication,
ganciclovir (GCV), a synthetic analogue of 2′-deoxy-guanosine.
The HSV-TK converts non-toxic ganciclovir into a
toxic product and allows selective elimination of genetically
modified, TK-expressing, cells in vitro and in vivo.
Generally, thymidine kinase is the enzyme catalyzing the transfer
of the γ-phosphate from ATP to thymidine to produce dTMP. Unlike
the cellular thymidine kinase, herpes simplex virus thymidine
kinase is able to process various substrates including pyrimidines
and pyrimidine analogs (thymidine, deoxycytidine and AZT), as well
as purine analogs (acyclovir, ganciclovir, buciclovir and
penciclovir) (8). Ganciclovir
(9-[(1,3-dihydroxy-2-propoxy)methyl]guanine) is a potent inhibitor
of viruses of the Herpes family, including cytomegalovirus (CMV).
Ganciclovir is phosphorylated to ganciclovir monophosphate by
HSV-TK and further by other cellular kinases. The final product of
phosphorylation GCV is ganciclovir triphosphate, which is a
competitive inhibitor of deoxyguanosine triphosphate (dGTP) that
incorporates into DNA and, thus, inhibits DNA replication (8). Additionally, toxic GCV triphosphate
can be effectively distributed to adjacent cells resulting in
eradication of unmodified cells of the same type. This phenomenon
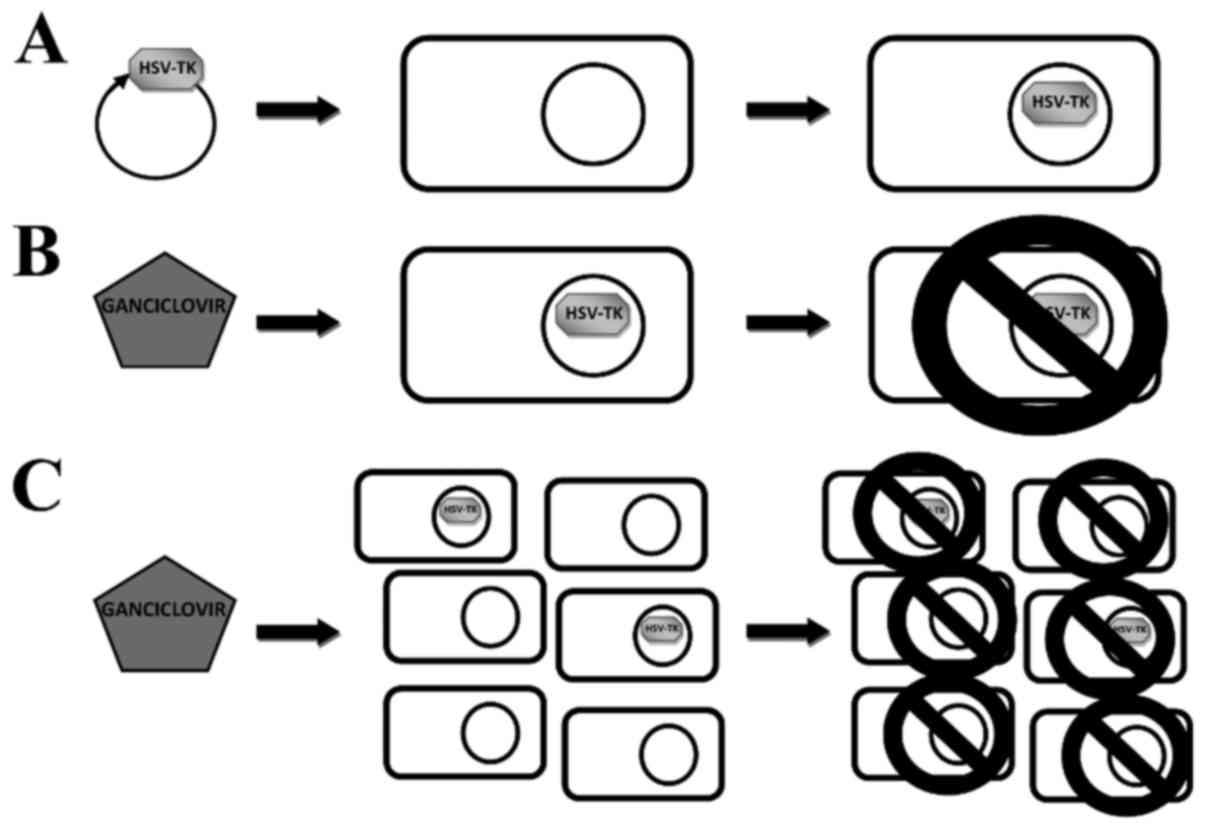
is known as bystander effect (Fig.
1). Rendering neighbor cells similarly susceptible to
ganciclovir is so efficient, that all tumor cells may be eliminated
with as few as 10% of malignant cells expressing HSV-TK gene
(9). The transport of activated
prodrug to adjacent cells is realized through gap junctions
(10). Gap junctions are clusters
of intercellular channels built from connexins (Cx) that allow
direct diffusion of ions and small molecules (up to 2 kDa) between
adjacent cells. Thus, they enable different biological functions
including sustaining homeostasis, metabolites exchange and
signaling (10–12).
Here, we present effective suicide gene therapy of
RMS cell line Rh30 with application of ganciclovir. In the present
study, tumor cells were efficiently eliminated both in vitro
and in vivo. We also proved strong gap junctional
intercellular communication (GJIC) in Rh30 cell line which
contributes to lethal bystander effect in this cell line.
Materials and methods
Cell culture conditions
Rh30 rhabdomyosarcoma cell line was maintained as a
monolayer culture in Dulbecco's modified Eagle's medium (DMEM) high
glucose, containing glucose in concentration of 4.5 g/l (Lonza,
Basel, Switzerland) supplemented with 10% v/v fetal bovine serum
(FBS; EURx, Gdańsk, Poland), 2 mM L-glutamine and 100 U/ml
penicillin and 100 µg/ml streptomycin (both from Thermo
Fisher Scientific, Waltham, MA, USA) antibiotics solution. The
cells were cultured at 37°C in a humidified atmosphere of 5%
CO2.
Generation and analysis of genetically
modified cells
Lentiviral particles were generated accordingly to
ViraPower protocol (Thermo Fisher Scientific) 9.5×106
HEK293T cells were seeded on T75 culture flask (DMEM high glucose,
10% FBS). Four hours after seeding, a transfection using calcium
orthophosphate (Ca3(PO4)2) was
performed. The transfection mixture was prepared as follows: 26–39
µg of plasmid DNA, 65 µl of 2.5 M CaCl2,
650 µl of 2X BBS (BES buffered saline, pH 7.2) (both from
Sigma-Aldrich, St. Louis, MO, USA) and 585 µl
H2O. The transfection mix was incubated for 20 min in
room temperature, then transferred into fresh culture medium
containing 25 µM chloroquine (Sigma-Aldrich). Medium
containing lentiviral particles was harvested 48 and 72 h after
transfection. Harvested medium was centrifuged (1750 g, 10 min,
4°C), filtered through 0.45 µm syringe filter and frozen at
−80°C. Two different transfection mixtures were prepared.
eGFP@pLenti6 expression plasmid and pLP1, pLP2, pVSVG (Thermo
Fisher Scientific) packaging plasmids were used to generate virus
introducing green fluorescent protein (GFP). The second mix
contained pLOX-gfp-iresTK expression plasmid with pCMV-dR8.2dvpr
and pVSVG packaging plasmids. pLOX-gfp-iresTK plasmid was a gift
from Didier Trono (Addgene plasmid #12243). The same copy number,
1.5×1012, of expression plasmids were used in both
mixes.
To determine the titer of lentiviral stocks HT1080
cells were infected. Four hours prior to transduction
5×104 cells were seeded on a 24-well plate. Various
volumes of harvested media (0.1–100 µl) were added to seeded
HT1080 cells. Forty-eight hours after the transduction the
percentages of modified (GFP-expressing) cells were assessed by
FACSCanto flow cytometer (Becton-Dickinson, Franklin Lakes, NJ,
USA) to calculate transduction units (TU) in 1 ml of each harvested
medium.
To obtain Rh30 modified cell lines, cells were
infected using generated lentiviral particles introducing GFP and
TK gene (Rh30TK cell line) or GFP only in the Rh30GFP control cell
line. Transduction was performed with MOI 5 (multiplicity of
infection) and 6 µg/ml polybrene (Sigma-Aldrich). Rh30GFP
and Rh30TK cell lines were purified using FACSAria II cell sorter
(Becton-Dickinson). The percentage of green fluorescent cells in
modified cell lines was measured by FACSCanto flow cytometer.
Suicide gene therapy in vitro
Rh30 (2.5×104) (WT, GFP and TK) cells
were seeded in the wells of a 24-well plate. Four hours after
seeding medium was changed for medium containing 0.1 µg/ml
ganciclovir or control fresh medium. Each well was prepared in
triplicate. On each of the following 5 days cells in each group
(WT, GFP and TK ± GCV) were collected and counted in Bürker's
chamber. On the final day of the experiment cells were also
photographed. In preliminary experiments 3 different GCV
concentrations were tested, 10, 1 and 0.1 µg/ml (data not
shown). Two different concentrations, 0.1 and 1 µg/ml, were
selected for further experiments (as being able to eliminate most
of the HSV-TK expressing cells within several days while not being
toxic to wild-type cells).
Gap-junctional intercellular
communication (GJIC) and bystander effect
GJIC was investigated by intercellular transfer of
calcein (13). Briefly, donor
cells were stained with calcein AM, green dye which may diffuse
through gap junctions, and DiI
(1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine perchlorate),
red dye staining cell membranes, not transferred by gap junctions
(both dyes from Thermo Fisher Scientific). Acceptor cells were
seeded 24 to 48 h before the experiment and cultured until reaching
80–90% confluency. Donor cells were harvested and incubated in
suspension in 1 mg/ml glucose solution containing 5 µM
calcein AM and 9 µM DiI for 30 min in 37°C in darkness.
During incubation cells were mixed several times. After triple wash
with PBS (Lonza) stained donor cells were seeded onto monolayer of
acceptor cells. After 90 min of incubation dye transfer was
examined with a fluorescent microscope.
Bystander effect defined as transfer of toxic
ganciclovir metabolite through gap junctions of neighboring cells
was analyzed by co-culture of Rh30TK and RH30WT cells. Mixtures of
these cells were prepared in such manner that between 0% (Rh30WT
only) and 80% (Rh30TK only) of HSV-TK-expressing cells were
present. Mixtures were seeded in duplicate. Twenty-four hours after
seeding half of the wells received 1 µg/ml ganciclovir. The
other half of the wells served as control. Cells were cultured for
8 days before analysis.
Xenografts and suicide gene therapy in
vivo
All animal experiments were conducted according to
the local ethics committee guidelines. Rh30 (WT, GFP and TK) cells
were subcutaneously injected into adult female NOD/SCID mice. Cells
(5×106) in 200 µl of PBS were injected per mouse.
Growth of tumors, and the health of the mice were monitored every
day. When tumors become measurable mice in each group were randomly
divided into two regimens: receiving 1 mg of ganciclovir in 0.5 ml
PBS (50 mg/kg of body weight) and receiving only 0.5 ml PBS. Both
groups were injected intraperitoneally, daily for 14 days. After
this period the mice were sacrificed, the tumors and the internal
organs (liver, kidney, spleen, lungs, brains and heart) were
isolated and fixed in 40% paraformaldehyde (Sigma-Aldrich). Tumors
were measured and weighed. Fixed tissues were imbedded in paraffin,
cut, stained with hematoxylin and eosin (H&E) and analyzed
histopathologically. Volume (v) of tumors was calculated with the
equation v = ½ ab2 where a and b are tumor sizes.
Statistical analysis
Statistical analysis of acquired data was performed
with STATISTICA 10.0 software (StatSoft, Inc., Tulsa, OK, USA).
Student's t-test or one-way ANOVA with post-hoc Tukey's test were
applied to verify statistically relevant differences between the
experimental groups. Data are presented as mean ± standard
deviation. P<0.05 indicates statistically significant
difference.
Results
Genetic modification of rhabdomyosarcoma
Rh30 cell line
In order to acquire rhabdomyosarcoma cells
expressing herpes simplex virus thymidine kinase (HSV-TK) we
performed stable lentiviral transduction of Rh30 cell line.
Lentiviral vectors were produced in HEK293T cells based on
Gateway® system. Cells were infected with multiplicity
of infection MOI 5. Two cell lines were generated: Rh30GFP,
expressing green fluorescent protein (GFP), and Rh30TK, expressing
GFP and HSV-TK. The latter was established thanks to application of
bicistronic lentiviral plasmid acquired from Addgene repository.
Two cistrons are separated by internal ribosome entry site (IRES)
which allows simultaneous expression of two transgenes. Lentiviral
plasmid coding GFP was cloned in our laboratory previously. In both
vectors expression of transgenes is regulated by strong promoter of
cytomegalovirus (CMV). As the used bicistronic vector does not
convey eukaryotic antibiotic resistance gene we performed
fluorescence-activated cell sorting for purification of transduced
cells. Hence we sorted GFP-positive cells and examined their purity
by flow cytometry. After two rounds of cell sorting we acquired two
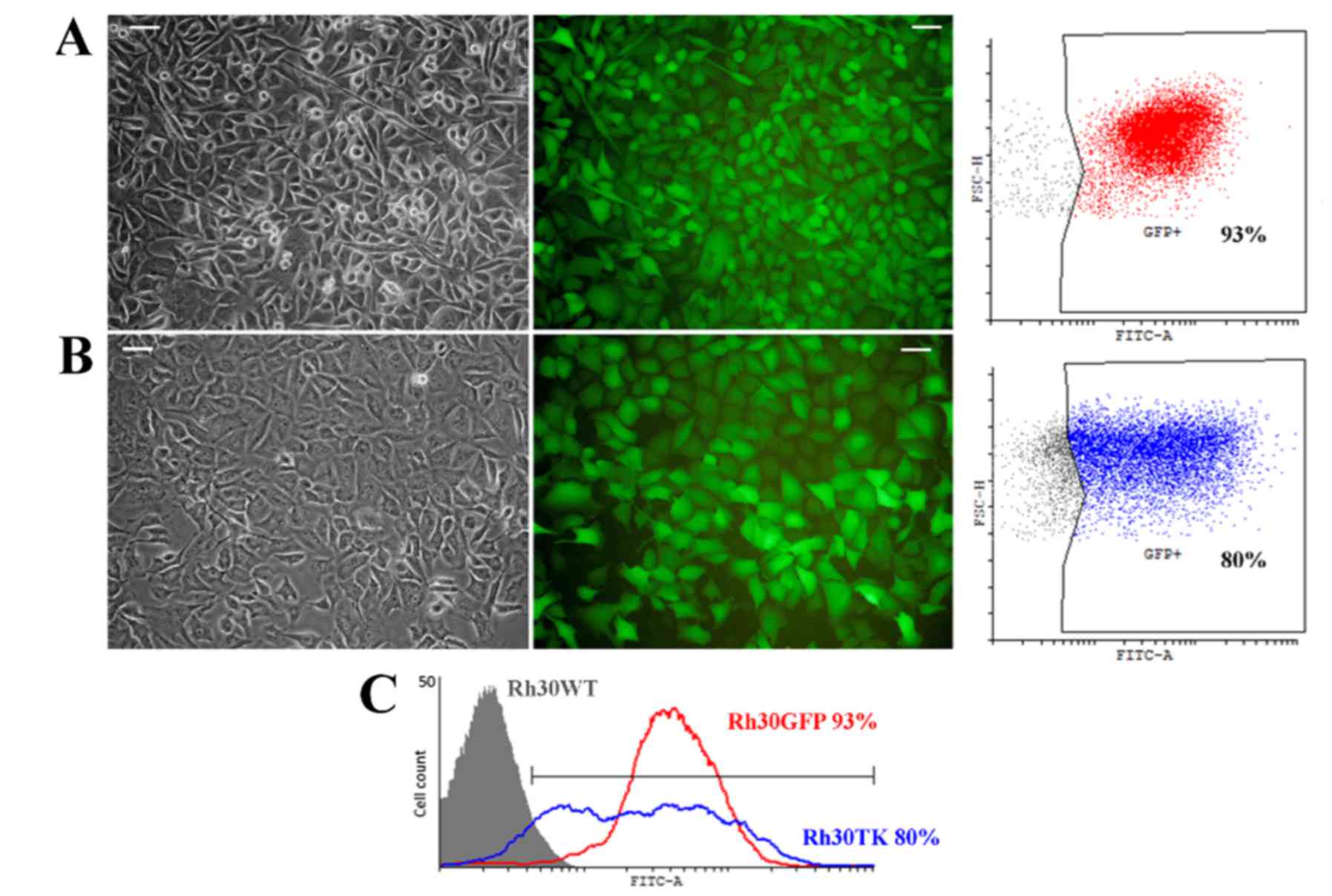
cell lines, named Rh30GFP (Fig.
2A) and Rh30TK (Fig. 2B), with
satisfactory purity, 93 and 80% of positive cells, respectively.
Genetically modified cells had strong GFP expression (visible in
microphotographs and histogram in Fig.
2) and typical morphology for rhabdomyosarcoma, although Rh30TK
cell line was characterized by gently altered morphology (visible
in Fig. 2B). Cells appeared to
have slightly larger size with fewer cells elongated compared to
wild-type cells (Rh30WT) and Rh30GFP cell line. We assumed that the
phenomenon was caused by presence of exogenous kinase (TK) which
might non-specifically phosphorylate multiple targets causing e.g.
change in morphology.
Genetically modified rhabdomyosarcoma
cells are highly vulnerable to ganciclovir
Our main goal was to evaluate the efficiency of
suicide gene therapy in rhabdomyosarcoma. We used generated cell
lines to assess vulnerability of HSV-TK-expressing rhabdomyosarcoma
cells to ganciclovir. In preliminary experiments we investigated
influence of different concentrations of ganciclovir (GCV) to
rhabdomyosarcoma cells and selected value which was totally safe
for unmodified cells, but was able to eradicate all
HSV-TK-expressing cells within several days (data not shown). Upon
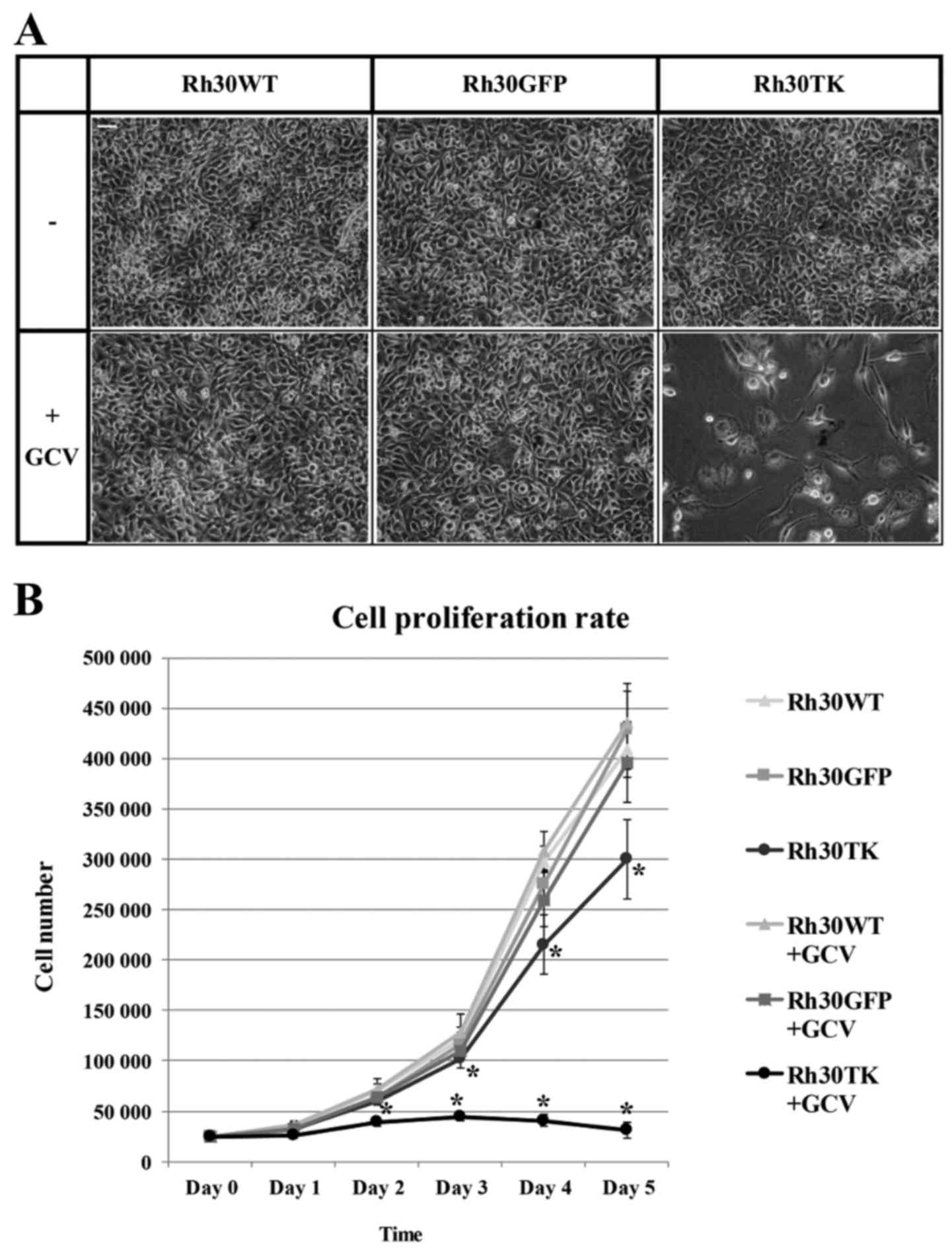
6 days of treatment of Rh30TK cells with 0.1 µg/ml
ganciclovir virtually all HSV-TK-positive cells were eliminated
(Fig. 3A) showing high level of
toxicity of GCV for this cell line. Rh30TK cells grew normally in
the absence of ganciclovir. Interestingly, this concentration was
totally non-toxic to other cell lines, Rh30GFP and wild-type Rh30WT
cells. Moreover, Rh30GFP cells expressed almost identical
proliferation rate as Rh30WT cells despite the presence or absence
of ganciclovir (Fig. 3B) proving
that applied concentration of ganciclovir is neutral for cells
lacking HSV-TK expression. Analysis of generated cell proliferation
rate revealed that Rh30TK cells (without GCV in medium) were
characterized by significantly slower growth rate compared to
control cell lines (Rh30WT and Rh30GFP). This observation was
associated with alterations in morphology and size of the HSV-TK
expressing cells and might be explained by non-specific
phosphorylation of signaling proteins involved in cell cycle, cell
proliferation and cell growth performed by HSV-TK (see Discussion).
Nevertheless, the data clearly proves that HSV-TK-expressing cells
are efficiently and selectively eliminated by ganciclovir in low
concentrations, which were non-toxic to wild-type cells. Thus,
introduction of TK to rhabdomyosarcoma cells sensitizes
rhabdomyosarcoma cells to GCV proving efficiency of applied suicide
gene therapy in vitro in rhabdomyosarcoma.
Gap-junctional intercellular
communication (GJIC) and toxic bystander effect in
rhabdomyosarcoma
In our in vitro model virtually all
HSV-TK-expressing cells were eliminated by ganciclovir treatment.
It has to be noted that the population of genetically modified
cells was very pure (reaching 80% of HSV-TK-positive Rh30 cells)
meaning that vast majority of cells in culture expressed thymidine
kinase. This might not be the case in experimental study concerning
clinical use of suicide therapy. In situ (genetic
modification of tumor cells directly in tumor) or ex vivo
(isolated tumor cells or tumor infiltrating cells are modified
in vitro and subsequently injected back to organism)
approaches imply limited efficiency of cell transduction and, thus,
considerably low percent of HSV-TK-expressing cells present in the
tumor. To assess whether our approach could be effective in such
situation we decided to examine the minimal proportion of Rh30TK
cells in co-culture necessary to effectively eradicate the whole
co-culture of cells. HSV-TK-lacking cells are eliminated in
co-culture thanks to ganciclovir toxic metabolite transfer through
gap junctions of adjacent cells (Fig.
1C).
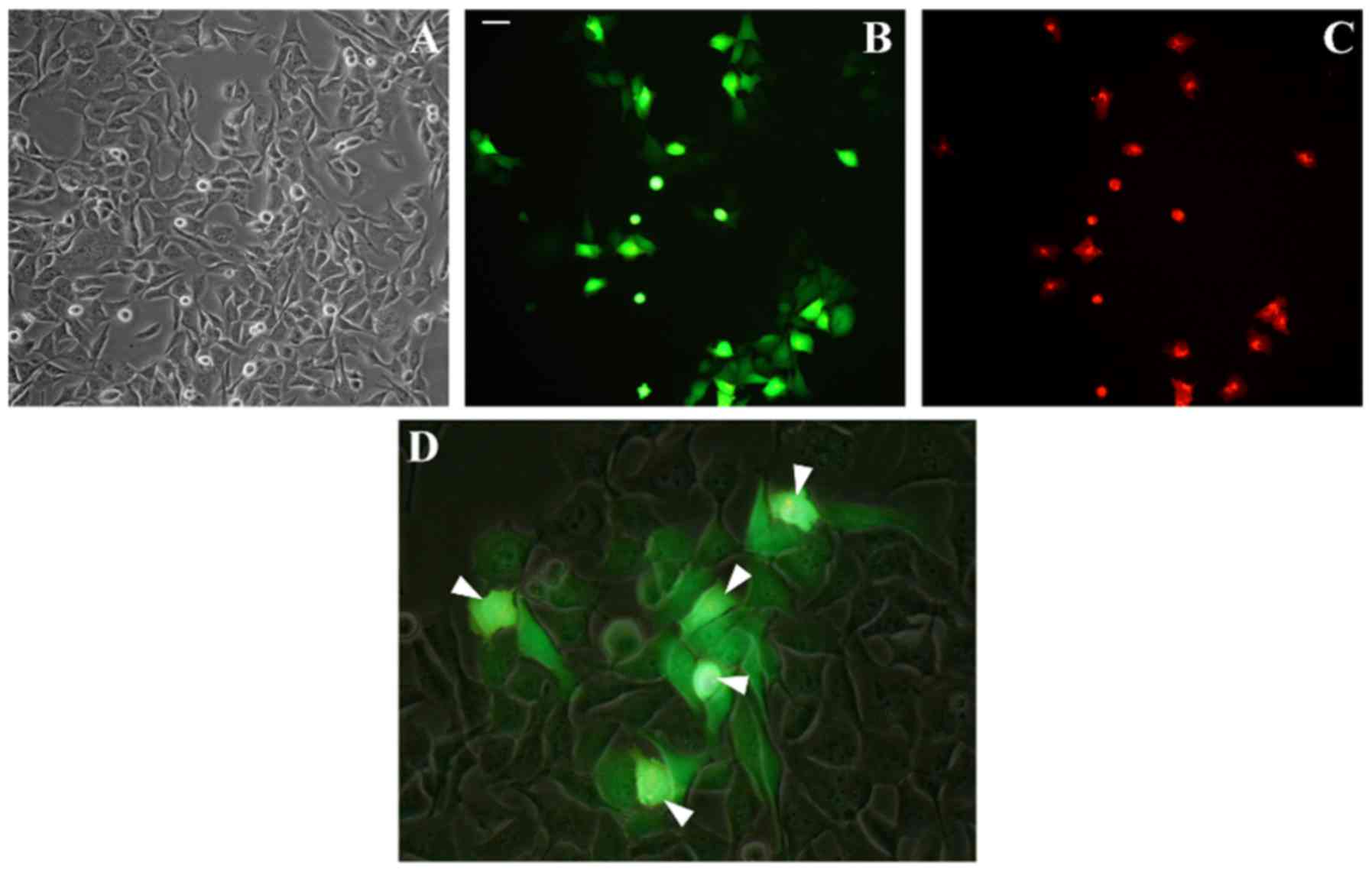
Firstly, we examined degree of gap junctional
intercellular communication (GJIC) in Rh30 cell line. In classic
experiment using double staining of donor cells with calcein (green
dye transferred by gap junctions) and DiI (red dye staining cell
membranes, not dissociating through gap junctions) we showed strong
GJIC in rhabdomyosarcoma Rh30 cell line (Fig. 4). Unstained acceptor cells
efficiently acquired calcein from stained donor cells showing
effective transfer of low mass molecules between rhabdomyosarcoma
cells through gap junctions.
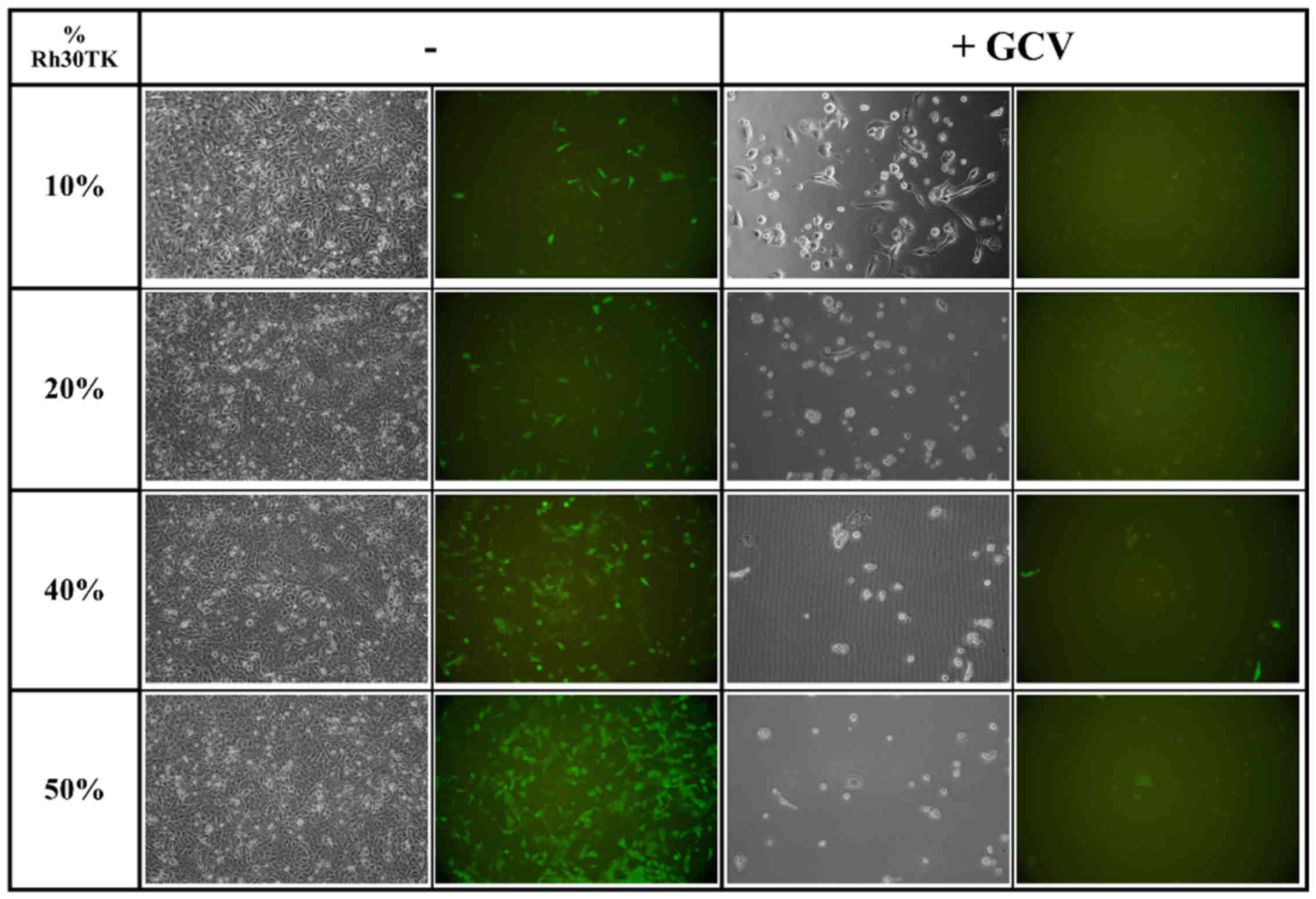
Knowing that GJIC is efficient in Rh30 cell line we
prepared mixtures of Rh30TK cells with Rh30WT containing between 10
and 80% of HSV-TK-expressing cells. After seeding and 8 days of
treatment with ganciclovir we observed almost complete elimination
of all cells in co-cultures containing 20% or more HSV-TK-positive
cells (Fig. 5). We observed no
toxicity in co-cultures without ganciclovir treatment. In mixture
containing 10% of cells we observed only single cells left proving
that small number of HSV-TK-expressing cells is sufficient to
eliminate whole population of cells in mixture and thus, strong
toxic bystander effect in rhabdomyosarcoma.
Suicide gene therapy in vivo is an
efficient model
Efficient ganciclovir-induced elimination of
HSV-TK-expressing cells in vitro and strong toxic bystander
effect in Rh30 cell line are promising for clinical application of
suicide gene therapy. To further confirm this notion we performed
pre-clinical in vivo study in a xenograft model. After
formation of measurable tumors in NOD/SCID mice we started daily
intraperitoneal administration of ganciclovir in experimental
groups and saline in control groups. Administration of 50 mg/kg
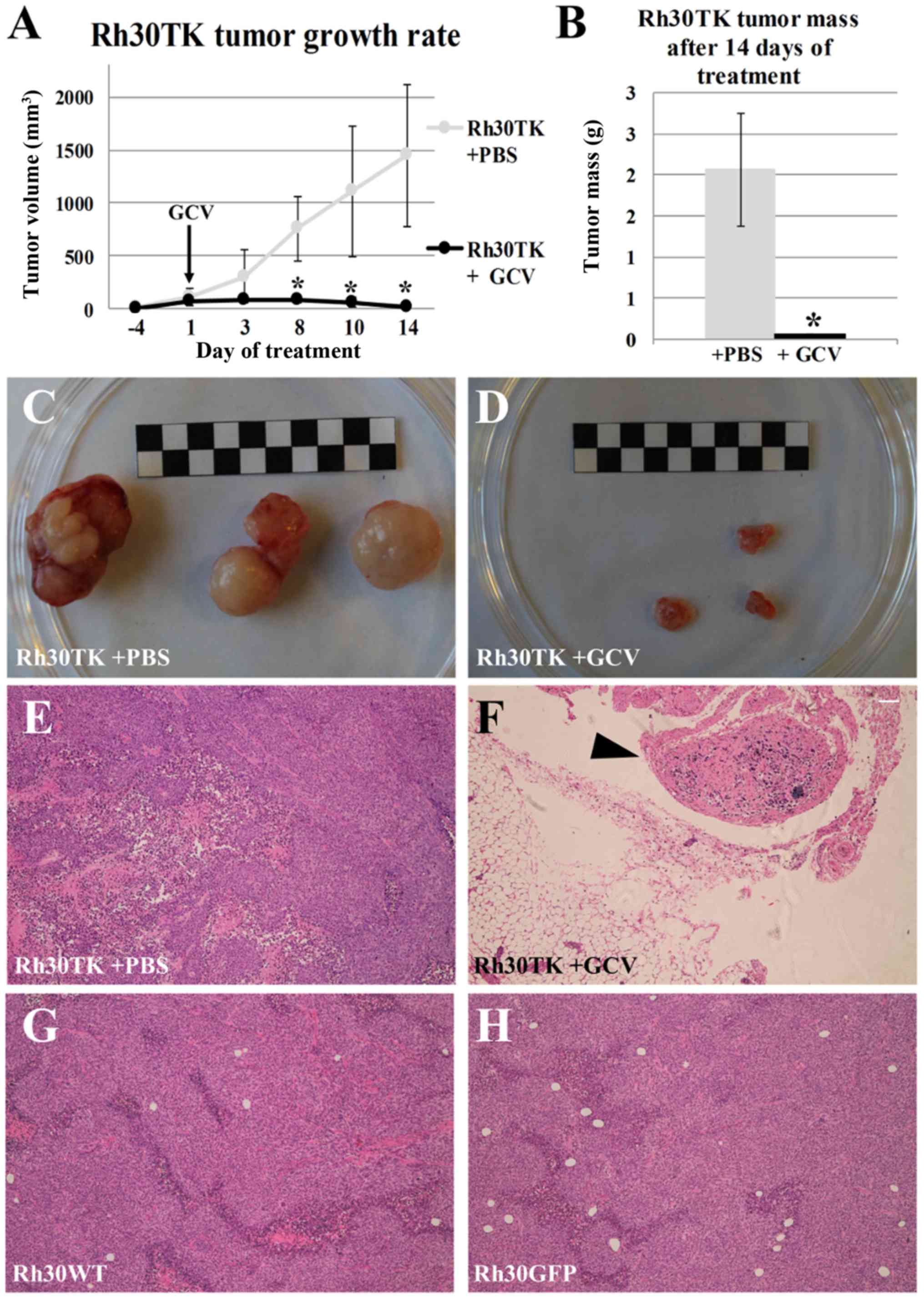
ganciclovir caused almost complete eradication of tumor formed by
Rh30TK cells within 14 days of treatment (Fig. 6). In all other groups tumors
developed at a rate comparable to wild-type cells. After 14 days
mice were sacrificed and the tumors as well as the organs were
isolated for histological analysis. It is noteworthy that in the
course of daily systemic ganciclovir administration no multi-organ
toxicity was observed (data not shown) suggesting that applied
dosage of ganciclovir is neutral to healthy (not expressing HSV-TK)
tissues and organs within applied time course. Histological
analysis of isolated 'tumors' from Rh30TK+ganciclovir group
revealed that isolated tissues were actually lymph nodes with only
single tumor cells (Fig. 6F). This
observation proves efficient elimination of HSV-TK-expressing tumor
cells in the in vivo model and encourages further
pre-clinical research, which can bring suicide gene therapy concept
to clinical applications.
Discussion
Suicide gene therapy using herpes simplex virus
thymidine kinase (HSV-TK) expression combined with ganciclovir
(GCV) exposure is a well-known model in vitro. Our results
show high efficacy of Rh30 rhabdomyosarcoma cell line elimination
by suicide gene therapy involving HSV-TK and GCV. Virtually all
HSV-TK expressing cells are eliminated within only 8 days of low
dose (0.1 µg/ml) ganciclovir treatment. Genetic modification
of cells with HSV-TK gene is claimed to have no impact on cell
viability and proliferation capacities (14). Surprisingly, we observed
significantly slower growth of Rh30TK cells without ganciclovir
addition (Fig. 3). Additionally,
no growth inhibition has been observed for control Rh30GFP cell
line that proliferated as wild-type Rh30 suggesting that genetic
modification is not responsible for decrease in proliferation rate
of Rh30TK cell line. That decrease may be explained by HSV-TK
ability to process wide range of substrates contrary to cellular
thymidine kinases. Although, no ganciclovir was present in culture
medium, the variety of other chemical compounds both in medium and
within the cell could be potentially processed by HSV thymidine
kinase, thus, an unspecified product of HSV thymidine kinase
activity could interfere with cell cycle signaling pathway. Among
many substrates of HSV-TK, some of them may be proteins involved in
cellular signaling pathways (cyclins). Such deregulation could lead
to observed decreased proliferation rate and changes in
size/morphology of HSV-TK-expressing cells. Nevertheless, it has to
be noted, that without GCV supplementation, Rh30TK cell line was
continuously growing in contrast to Rh30TK cells in the presence of
prodrug as shown on Fig. 3.
Obtained data showed efficient gap junction
intercellular communication (GJIC) in Rh30 cell line. The
co-culture of Rh30 unmodified cells with HSV-TK+
modified cells using different cell ratios were performed to
evaluate the efficiency of toxic bystander effect. The lowest ratio
of HSV-TK-expressing cells to wild-type cells which was 1:4 (20% of
Rh30TK cells in co-culture) was sufficient to eliminate all cells
in culture after ganciclovir addition, however, co-culture
containing only 10% of HSV-TK+ cells probably could lead
to the same effect if ganciclovir administration time was longer
than 8 days. Pictures of 10% Rh30TK co-culture after 8 days showed
that only few cells survived and severe cytotoxic effect was
visible. Nevertheless, similar results of bystander effect in
different cancer cell lines were claimed by researchers in numerous
publications (9,15–18).
Bystander effect is desired as a method of
unmodified cell eradication. On the other hand, it may be
responsible for killing adjacent non-malignant cells, as toxic
product of GCV conversion is diffused to cells surrounding modified
cells. Thus, removal of tumor in suicide gene therapy procedure is
associated with harm to non-malignant cells existing in tumor
niche. However, it has to be noted, that similar side-effects occur
also in conventional therapies in cancer treatment. Surgical
intervention affects tissues surrounding cancer cells, which are
partially resected with tumor. Analogous imprecision of action can
be observed in radiation therapy where severe side-effects can
occur. Moreover, another conventional treatment, chemotherapy, is
connected with systemic toxicity and substantial distress for
patient. Hence, it has to be considered if suicide gene therapy can
be a method of reducing collateral damage, especially as a part of
combined treatment to shorten the time of healing. Our in
vivo experiments revealed small remaining connective tissues
and no other tissues in sites of injection with HSV-TK+
rhabdomyosarcoma cells were affected, however, this model is far
from clinical condition.
It has to be mentioned, that there are two
mechanisms of bystander effects. One is based on gap junction
intercellular communication (GJIC), the other one is gap
junction-independent. Gap junction-independent bystander effect may
include transfer of apoptotic vesicles, exocytosis of cytotoxic
factors from the GCV-treated HSV-TK+ cells and enhanced
cellular immune response in vivo, although it is likely that
a synergism of these mechanisms occurs in a living organism
(10). Conducted experiments
proved both strong gap-junctional intercellular communication
(GJIC) and efficient, toxic bystander effect in examined Rh30 cell
line, which confirm literature data concerning this
rhabdomyosarcoma cell line (19).
Such results are very promising from the perspective of clinical
application of suicide therapy as in vivo not only genetic
modification efficacy is limiting, but also distribution of
ganciclovir might be restricted.
The efforts to use suicide gene therapy in clinical
application have met incomplete success. Preclinical and clinical
studies using adeno- and retroviral vectors revealed relatively
poor responses due to insufficient gene transfer and inefficient
gene expression within tumors. The aim of in vivo approach
in suicide gene therapy is to introduce suicide gene accurately to
cancer cells. This can be achieved by in situ transfection
or transduction, however, the efficiency of these methods remains
relatively low in comparison to in vitro gene introduction.
The ideal situation for suicide gene therapy would be to deliver
HSV-TK gene as a vector targeting tumor systemically for the
treatment of metastatic diseases. The promising concept is to use a
virus genetically modified to carry a ligand for receptor present
on the cancer cell surface (such as high affinity laminin receptor)
(20). Nevertheless, introduction
of suicide gene to cancer cells exclusively is still a demanding
issue and a main obstacle in bringing suicide gene therapy to the
clinic.
Efficient distribution of toxic product after
ganciclovir administration was achieved, additionally, thanks to
strong GJIC, we were able to eradicate Rh30 cells in co-culture
in vitro. Interestingly, literature describes very low
intercellular communication in the RD, another rhabdomyosarcoma
cell line (21). Even in cells
characterized by inefficient GJIC suicide gene therapy approach can
be applied when combined with enhancement of GJIC by introduction
of connexin transgene (22). The
type of Cx expressed (by transfection or otherwise) does not appear
to be crucial for the bystander effect because similar results were
obtained with HeLa cells expressing various Cx (15). The most widely introduced Cx's, due
to theirs versatility, are Cx26 or Cx43, as they assure efficient
communication between cells obligatory for gap-junction dependent
bystander effect to exist (23–25).
However, another genetic modification complicates the procedure
and, what is more important, is generally connected with higher
toxicity level and cellular stress. Contrary to RD cell line, there
was no need to upregulate connexins in examined Rh30 cell line.
Different nature of these two cell lines, embryonal for RD and
alveolar for Rh30 may be the possible explanation for the
phenomenon of different connexin levels. Considering this, the
perspective of usefulness of suicide gene therapy may depend on
diagnosed rhabdomyosarcoma type.
Suicide gene therapy is also promising concept for
eradication of cells resistant to conventional anticancer
treatment. Cancer stem cells are believed to be resistant to chemo-
and radio-therapy, and thus, responsible for recurrence of cancer
after treatment (26). In suicide
gene therapy approach this obstacle is overcome by efficient toxic
bystander effect.
In our experiments only 14 days of GCV systemic
administration was satisfactory for rhabdomyosarcoma xenografted
tumors to be eliminated. The analysis of eradicated Rh30TK tumors
discovered no existence of rhabdomyosarcoma cell aggregates,
examined residues contained solely connective tissue and lymph
nodes. Samples of organs, such as brain, liver, spleen and kidney
were collected to assess possible in vivo toxicity of
ganciclovir. The histopathological examination revealed no evidence
of systemic toxic influence of ganciclovir. GCV action and
side-effects are well known due to its widespread usefulness in
antiviral therapy. Treatment with GCV is associated with a range of
serious hematological adverse effects (27). In the present study, no toxic
effects on various organ systems can be explained by low dose of
GCV, short time required to eliminate growing tumors and short time
of whole experiment in consequence. It is possible that multi-organ
toxicity can affect animals if administration of GCV for longer
period of time had been performed. On the other hand, adverse
effects unable to be verified in a mouse model, but affecting human
(fever, sweating, nausea, abdominal pain, headache, confusion,
hallucination or seizures) have to be of concern during potential
suicide gene therapy (27).
Our experiments were not planned to test in
vivo elimination of Rh30 cell population containing low
proportion of HSV-TK-positive cells. These data were only obtained
in vitro and confirmed high efficiency of HSV-TK suicide
gene strategy to eliminate all cancer cells even if modified cells
were limited to 10% of the cell mixture. Mouse model was designed
to evaluate the ability of this approach to rapid tumor growth
inhibition and eradication of rhabdomyosarcoma cells by systemic
(intraperitoneal) administration of ganciclovir. Despite this,
collected data suggest that elimination of HSV-TK modified and
wild-type cell mixture should be effective in a living organism as
well. There are numerous data related to suicide gene therapy,
indicating no considerable alterations in gap junction
intercellular communication between in vitro and in
vivo approaches (28). As we
confirmed stable and efficient GJIC in investigated Rh30
rhabdomyosarcoma cell line in vitro, similar effects in a
mouse model are expected.
In conclusion, we showed that suicide gene therapy
involving HSV-TK + GCV in rhabdomyosarcoma can be very effective.
Almost all tumor cells are eradicated in vitro when
HSV-TK-expressing cells comprise 20% of the population. These
results prove high efficiency of the therapy. Importantly, our
approach is also effective in vivo, where we observed almost
complete remission of tumors upon only 14 days of systemic
administration of GCV. These results are very promising for future
clinical application of suicide therapy. Although more research
concerning effect of prolonged exposure to GCV and efficiency of
tumor elimination upon low percentage of HSV-TK-expressing cells
present within the tumor are necessary to establish security and
efficiency of such treatment, suicide gene therapy is very
promising concept in future clinical studies concerning
rhabdomyosarcoma.
Abbreviations:
|
Cx
|
connexin
|
|
GCV
|
ganciclovir
|
|
GFP
|
green fluorescent protein
|
|
GJIC
|
gap junctional intercellular
communication
|
|
HSV-TK
|
herpes simplex virus thymidine
kinase
|
|
RMS
|
rhabdomyosarcoma
|
Acknowledgments
Authors would like to acknowledge the contribution
of Dr Grażyna Drabik and Elżbieta Trześniowska-Popiel in
histopathological analysis as well as Dr Kazimierz Węglarczyk and
Dr Rafał Szatanek in cell sorting. The present study was supported
by the National Science Centre grant no. N407 048538 and KNOW -
Leading National Research Centre 2012–2017.
References
|
1
|
O'Brien D, Jacob AG, Qualman SJ and
Chandler DS: Advances in pediatric rhabdomyosarcoma
characterization and disease model development. Histol Histopathol.
27:13–22. 2012.
|
|
2
|
Ognjanovic S, Linabery AM, Charbonneau B
and Ross JA: Trends in childhood rhabdomyosarcoma incidence and
survival in the United States, 1975–2005. Cancer. 115:4218–4226.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geltzeiler M, Li G, Abraham J and Keller
C: The case for primary salivary rhabdomyosarcoma. Front Oncol.
5:742015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kashi VP, Hatley ME and Galindo RL:
Probing for a deeper understanding of rhabdomyosarcoma: Insights
from complementary model systems. Nat Rev Cancer. 15:426–439. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parham DM and Ellison DA:
Rhabdomyosarcomas in adults and children: an update. Arch Pathol
Lab Med. 130:1454–1465. 2006.PubMed/NCBI
|
|
6
|
Malempati S and Hawkins DS:
Rhabdomyosarcoma: Review of the Children's Oncology Group (COG)
Soft-Tissue Sarcoma Committee experience and rationale for current
COG studies. Pediatr Blood Cancer. 59:5–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kashima K, Watanabe M, Sato Y, Hata J,
Ishii N and Aoki Y: Inhibition of metastasis of rhabdomyosarcoma by
a novel neutralizing antibody to CXC chemokine receptor-4. Cancer
Sci. 105:1343–1350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kokoris MS and Black ME: Characterization
of herpes simplex virus type 1 thymidine kinase mutants engineered
for improved ganciclovir or acyclovir activity. Protein Sci.
11:2267–2272. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nicholas TW, Read SB, Burrows FJ and Kruse
CA: Suicide gene therapy with Herpes simplex virus thymidine kinase
and ganciclovir is enhanced with connexins to improve gap junctions
and bystander effects. Histol Histopathol. 18:495–507.
2003.PubMed/NCBI
|
|
10
|
Andrade-Rozental AF, Rozental R,
Hopperstad MG, Wu JK, Vrionis FD and Spray DC: Gap junctions: The
'kiss of death' and the 'kiss of life'. Brain Res Brain Res Rev.
32:308–315. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peiris TH and Oviedo NJ: Gap junction
proteins: Master regulators of the planarian stem cell response to
tissue maintenance and injury. Biochim Biophys Acta. 1828:109–117.
2013. View Article : Google Scholar
|
|
12
|
Nielsen MS, Axelsen LN, Sorgen PL, Verma
V, Delmar M and Holstein-Rathlou NH: Gap junctions. Compr Physiol.
2:1981–2035. 2012.
|
|
13
|
Czyż J, Irmer U, Schulz G, Mindermann A
and Hülser DF: Gap-junctional coupling measured by flow cytometry.
Exp Cell Res. 255:40–46. 2000. View Article : Google Scholar
|
|
14
|
Di Ianni M, Di Florio S, Venditti G,
Falzetti F, Mannoni P, Martelli MF and Tabilio A: T lymphocyte
transduction with herpes simplex virus-thymidine kinase (HSV-tk)
gene: Comparison of four different infection protocols. J
Hematother Stem Cell Res. 8:645–652. 1999. View Article : Google Scholar
|
|
15
|
Mesnil M and Yamasaki H: Bystander effect
in herpes simplex virus-thymidine kinase/ganciclovir cancer gene
therapy: Role of gap-junctional intercellular communication. Cancer
Res. 60:3989–3999. 2000.PubMed/NCBI
|
|
16
|
You Y: Targets in Gene Therapy. InTech.
2011, http://dx.doi.org/10.5772/1012.
|
|
17
|
Neschadim A, Wang JC, Lavie A and Medin
JA: Bystander killing of malignant cells via the delivery of
engineered thymidine-active deoxycytidine kinase for suicide gene
therapy of cancer. Cancer Gene Ther. 19:320–327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Veldwijk MR, Berlinghoff S, Laufs S,
Hengge UR, Zeller WJ, Wenz F and Fruehauf S: Suicide gene therapy
of sarcoma cell lines using recombinant adeno-associated virus 2
vectors. Cancer Gene Ther. 11:577–584. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wildner O, Morris JC, Vahanian NN, Ford H
Jr, Ramsey WJ and Blaese RM: Adenoviral vectors capable of
replication improve the efficacy of HSVtk/GCV suicide gene therapy
of cancer. Gene Ther. 6:57–62. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tseng JC, Zanzonico PB, Levin B, Finn R,
Larson SM and Meruelo D: Tumor-specific in vivo transfection with
HSV-1 thymidine kinase gene using a Sindbis viral vector as a basis
for prodrug ganciclovir activation and PET. J Nucl Med.
47:1136–1143. 2006.PubMed/NCBI
|
|
21
|
Lin Z, Zhang Z, Han Y, Naus CC, Yu KR and
Holtzer H: Functional expression of gap junction gene Cx43 and the
myogenic differentiation of rhabdomyosarcoma cells. Sci China B.
38:305–312. 1995.PubMed/NCBI
|
|
22
|
Mesnil M: Connexins and cancer. Biol Cell.
94:493–500. 2002. View Article : Google Scholar
|
|
23
|
Garcia-Rodríguez L, Pérez-Torras S, Carrió
M, Cascante A, García-Ribas I, Mazo A and Fillat C: Connexin-26 is
a key factor mediating gemcitabine bystander effect. Mol Cancer
Ther. 10:505–517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sanson M, Marcaud V, Robin E, Valéry C,
Sturtz F and Zalc B: Connexin 43-mediated bystander effect in two
rat glioma cell models. Cancer Gene Ther. 9:149–155. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tanaka M, Fraizer GC, De La Cerda J,
Cristiano RJ, Liebert M and Grossman HB: Connexin 26 enhances the
bystander effect in HSVtk/GCV gene therapy for human bladder cancer
by adenovirus/PLL/DNA gene delivery. Gene Ther. 8:139–148. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ciurea ME, Georgescu AM, Purcaru SO,
Artene SA, Emami GH, Boldeanu MV, Tache DE and Dricu A: Cancer stem
cells: Biological functions and therapeutically targeting. Int J
Mol Sci. 15:8169–8185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
CYTOVENE® Product Monograph.
http://www.rochecanada.com/en/products/pharmaceuticals/consumerinformation/cytovene.html.
|
|
28
|
van Dillen IJ, Mulder NH, Vaalburg W, de
Vries EFJ and Hospers GAP: Influence of the bystander effect on
HSV-tk/GCV gene therapy. A review Curr Gene Ther. 2:307–322. 2002.
View Article : Google Scholar
|